Abstract
Depression often is characterized by inflexible autonomic and metacognitive processes that interfere with effective self-regulation. However, few studies have integrated these factors to improve the prediction of which individuals are at greatest risk for depression. Among 134 undergraduates, we evaluated whether parasympathetic inflexibility (a lack of reduction in respiratory sinus arrhythmia) in response to a sadness induction involving loss would prospectively predict symptoms of depression across four waves of follow-up over twelve weeks. Furthermore, we evaluated whether metacognitive components of perseverative cognition (PC) and decentering (identified by a principal component analysis) would moderate this relationship in opposite directions. Multilevel modeling demonstrated that the relationship between parasympathetic inflexibility and prospective symptoms of depression was exacerbated by PC, but attenuated by decentering. Furthermore, individuals with parasympathetic inflexibility, PC, and low decentering were at greatest risk for symptoms of depression across follow-up. These results support the utility of integrating autonomic and metacognitive risk factors to identify individuals at risk for depression.
Keywords: depression, respiratory sinus arrhythmia, risk factors, flexibility, rumination, worry, decentering
Major Depressive Disorder (MDD) is the most common mental disorder (Kessler, Chiu, Demler, Merikangas, & Walters, 2005). It is associated with tremendous impairment and considerable comorbidity with other psychiatric conditions, resulting in major personal, economic, and societal costs (Kessler et al., 2006; Kessler & Wang, 2009). Given these debilitating effects, research has aimed to identify possible mechanisms and risk factors for MDD that might serve as targets for prevention or treatment (Alloy et al., 2017). Broadly, MDD is associated with a loss of biological and behavioral flexibility (Kashdan & Rottenberg, 2010; Stange, Alloy, & Fresco, in press). Specifically, MDD is characterized by inflexible physiological responses (Bylsma, Salomon, Taylor-Clift, Morris, & Rottenberg, 2014), difficulty disengaging from perseverative thinking processes such as rumination, and with mentally distancing oneself from one’s negative thinking (Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008; Fresco et al., 2007a; Bernstein et al., 2015). Furthermore, healthy individuals who are inflexible in response to changes in environmental or emotional context also may be susceptible to developing depression (Stange et al., in press). However, not all individuals who are inflexible in one domain necessarily develop depression, suggesting that the identification of risk factors in isolation may be an overly simplistic representation of risk. Thus, research has sought to integrate biological and behavioral factors that confer risk for depression, and examine how they might interact, in the service of improving the prediction of which individuals are at greatest risk for depression.
One biological index of flexibility relevant to depression is parasympathetic nervous system activity, which can facilitate adaptive behavioral and emotional responses to meet contextual demands (Beauchaine, 2001; Kashdan & Rottenberg, 2010; Porges, 2007; Thayer & Lane, 2009). Parasympathetic flexibility can be measured as the extent to which individuals show contextually-appropriate changes in parasympathetic activity across different environmental or emotional cues. One index of parasympathetic flexibility is respiratory sinus arrhythmia (RSA), a measure of variability in heart rate that occurs over the respiration cycle. During periods of rest, the medial prefrontal cortex (mPFC) typically exerts inhibitory control over the amygdala, indirectly enhancing cardiac control via the vagus nerve, and resulting in elevated resting RSA (Thayer & Lane, 2009; Thayer, Åhs, Fredrikson, Sollers, & Wager, 2012). However, during periods of emotional or environmental challenge (e.g., stressors, sadness, attention to salient stimuli), the parasympathetic nervous system typically withdraws its inhibitory control over heart rate, which results in reductions in RSA, allowing the body to mobilize resources needed to flexibly respond to the challenge (Beauchaine, 2001).
RSA has been proposed as a biological index of the capacity for effective emotion regulation (Beauchaine & Thayer, 2015; Thayer et al., 2012). Indeed, extensive literature has documented that lower levels of RSA at rest are associated with maladaptive emotion regulation and MDD (Kemp et al., 2010; Rottenberg, 2007). However, recent work has suggested that RSA reactivity (vagal withdrawal) in response to sadness might be an index of regulatory flexibility that is particularly relevant to understanding depression and depression risk (for recent reviews, see Hamilton & Alloy, 2016; Stange et al., in press). Indeed, MDD appears to be characterized by a lack of RSA reactivity in response to sadness (Bylsma et al., 2014; Rottenberg, Clift, Bolden, & Salomon, 2007a), and individuals who have lower RSA reactivity (or vagal withdrawal) may be at risk for the onset of symptoms of depression and a poorer course of MDD (Panaite et al., 2016; Rottenberg et al., 2005; Stange et al., 2017). However, few such prospective studies have been conducted to evaluate the extent to which low RSA reactivity confers risk for future depression. Furthermore, beyond identifying parasympathetic inflexibility as a general risk factor, there is a need to identify contexts – such as other known risk factors – which could work synergistically with the parasympathetic nervous system in risk for depression (e.g., Aldao, 2013; Stange et al., in press).
Perseverative cognition (PC; Brosschot, Gerin, & Thayer, 2005; Brosschot, Gerin, & Thayer, 2006; Ottaviani et al., 2016) is one factor that may worsen the role of parasympathetic inflexibility in conferring risk for depression. PC refers to metacognitive capacities characterized by repetitive, negatively-valenced, and self-referential mental activity (Watkins, 2008; Mennin & Fresco, 2013; Ottaviani et al., 2016). Although some forms of self-referential thought can promote concrete processing and adaptive engagement with current circumstances (e.g., Mennin & Fresco, 2013; Morin, 2017), PC involves an abstract level of construal that may exacerbate negative affective states (e.g., Segerstrom et al., 2000; Watkins, 2008). Two exemplars of PC that have received considerable empirical attention are depressive rumination, a way of responding to distress that involves repetitively and passively focusing on symptoms of distress and on the possible causes and consequences of these symptoms (Nolen-Hoeksema et al., 2008), and worry, a relatively uncontrollable and negatively-valenced chain of thoughts and images representing an attempt to engage in mental problem-solving of an issue of uncertain outcome (Borkovec, Robinson, Pruzinsky, & DePree, 1983). Although differing in content (e.g., loss vs. threat) and temporal orientation (e.g., past vs. future orientation), rumination and worry are correlated and have many similarities, including mental activity that is self-referential and perseverative, primarily diffuse and abstract in thinking style, and both have been associated with cognitive inflexibility and difficulty disengaging attention from negative stimuli (e.g., Fresco et al., 2002; Nolen-Hoeksema et al., 2008; Watkins, 2008). Importantly, PC also confers risk for emotional disorders such as MDD (Abela & Hankin, 2011; Aldao, Nolen-Hoeksema, & Schweizer, 2010; Marchetti, Koster, Klinger, & Alloy, 2016; Nolen-Hoeksema et al., 2008; Olatunji et al., 2013; Roelofs, Huibers, Peeters, Arntz, & van Os, 2008; Spasojevic & Alloy, 2001; Stange et al., 2016). Furthermore, the cognitive representation of stressors embodied in PC may cause a “fight-or-flight” action response, triggering withdrawal of parasympathetic activity that persists during times when it may not be necessary or adaptive for a response to environmental demands (Brosschot, 2010; Brosschot et al., 2006, 2007; LeMoult, Yoon, & Joormann, 2016; Ottaviani, Medea, Lonigro, Tarvainen, & Couyoumdjian, 2015). Therefore, individuals who engage in PC who also demonstrate inflexible parasympathetic responses to sadness might have a “double load” of risk for depression in that both sets of characteristics represent inappropriate responses to contextual demands and may interfere with effective self-regulation.
In contrast with PC, decentering is a form of metacognition defined as the ability to “step outside of one’s immediate experience, thereby changing the very nature of that experience” (Safran & Segal, 1990, p. 117). Decentering refers to a set of characteristics involving three related metacognitive processes (Bernstein et al., 2015): meta-awareness, an awareness of one’s subjective experience in consciousness, such as feeling and thinking (e.g., “I am having the thought that I am stupid” rather than “I am stupid”); disidentification from internal experience, the experience of internal states as being separate from one’s self (e.g., “I am having a feeling of sadness” rather than “I am sad”); and reduced effects of thought content on other mental processes, such as attention and emotion. Interestingly, decentering is inversely associated with PC (Fresco et al., 2007a; Kaiser, Andrews-Hanna, Metcalf, & Dimidjian, 2015), and experimental studies have demonstrated that low decentering mediates the relationship between rumination and negative thinking in depression (Lo et al., 2014). Decentering is attenuated in individuals with MDD relative to healthy individuals (Fresco et al., 2007a) and predicts better outcomes in psychotherapy (Teasdale et al., 2001, 2002; Fresco et al., 2007b). As meta-awareness is an integral component of mindfulness and decentering (Bernstein et al., 2015), mindfulness-based treatments such as mindfulness-based cognitive therapy (MBCT; Segal, Williams, & Teasdale, 2012) present one avenue of improving the ability to engage in decentering. Furthermore, mindfulness treatments that target meta-awareness also may improve parasympathetic flexibility (Delgado-Pastor, Perakakis, Subramanya, Telles, & Vila, 2013; Krygier et al., 2013), suggesting that decentering and parasympathetic flexibility may work hand-in-hand to facilitate self-regulation and protect against depression. In contrast, it also is possible that decentering would protect against the depressogenic effects of parasympathetic inflexibility. However, few studies have evaluated contextual factors such as decentering or PC that may moderate the degree to which parasympathetic inflexibility confers risk for depression.
At a neurobiological level, the role of the default mode network (DMN) in parasympathetic flexibility, PC, and decentering provides reason to suspect potentially synergistic relationships between these characteristics. For example, Thayer et al. (2012) have proposed that RSA represents an index of how well top-down appraisals of stimulus threat shape parasympathetic responses to the environment. Modulation of RSA with these appraisals is thought to occur via the mPFC, a key node in the default mode network. In addition to modulating RSA, the mPFC (and the DMN more generally) appears to play a role in processing the degree to which beliefs are self-relevant, and for internally-focused thought that is perceived as self-relevant, such as PC (Whitfield Gabrieli & Ford, 2012; Marchetti et al., 2012; Hamilton et al., 2011). Relatedly, a recent neuroanatomical and processing model (Paulus & Stein, 2010) for emotional disorders posited that individuals at risk for anxiety and depression exhibit a reduced signal to noise ratio of interoceptive afferents via the mPFC, such that bodily signals are excessively perceived as having motivational significance. As a consequence, compensatory recruitment of cognitive control regions (e.g., dorsal anterior cingulate, dorsolateral prefrontal cortex) is required to effectively discriminate between relevant and irrelevant sensations. This increased cognitive activity may result in processes such as worry and rumination, which are aimed at improving predictions of the relevance of bodily sensations (Paulus & Stein, 2010). Ironically, however, PC may serve to further tax mental resources, impeding disengagement from PC or distancing oneself from one’s thoughts (Whitfield Gabrieli & Ford, 2012; Marchetti, Koster, Sonuga-Barke, & De Raedt, 2012; Bernstein et al., 2015). In contrast, it is possible that decentering would improve the signal to noise ratio of interoceptive afferents, thus reducing the likelihood of PC and facilitating healthy parasympathetic activity.
Consistent with these hypotheses, MBCT may reduce excessive connectivity within the default mode network (Farb et al., 2007, 2011; Taylor et al., 2013), possibly reflecting strengthened present-moment awareness (e.g., Fresco et al., 2007b). Similarly, Fresco et al. (2017) examined the associations between pretreatment neural patterns of intrinsic functional connectivity in hubs within the DMN to treatment-related changes in worry and decentering in generalized anxiety disorder patients (with and without MDD) receiving emotion regulation therapy (e.g., Fresco, Mennin, Heimberg, & Ritter, 2013; Mennin, Fresco, Ritter, & Heimberg, 2015). Findings revealed that treatment linked gains in decentering, as well as reductions in worry were predicted by reduced functional connectivity between the anterior mPFC and a cluster in occipital lobe, as well as by greater connectivity between DMN hubs and regions of the salience network. Although we did not evaluate the DMN in the present study, it is possible that parasympathetic flexibility, PC, and decentering reflect related measures of a self-regulatory system that could work together synergistically to contribute risk for, or protection against, depression. For example, individuals who demonstrate parasympathetic inflexibility, high levels of PC, and low decentering could have particular dysfunction in the DMN, and hence, might be especially at risk for depression. In contrast, individuals with at least one protective factor (e.g., high decentering in the context of parasympathetic inflexibility and PC) might be able to step back from excessive internal processing, facilitating re-engagement with the world, and therefore, might be at relatively lower risk for depression.
In the present study, we conducted the first prospective test of the integration of parasympathetic and metacognitive factors in risk for depression. We evaluated parasympathetic and metacognitive factors at baseline, and symptoms of depression at five waves across a twelve-week follow-up period. We expected that less RSA withdrawal (less parasympathetic flexibility) in response to a sad mood induction would predict higher levels of depressive symptoms across follow-up, after accounting for baseline symptoms. Furthermore, we hypothesized that PC and decentering would moderate this relationship in different directions, such that PC would exacerbate the relationship between parasympathetic inflexibility and depression, whereas decentering would protect against this relationship.
Method
Participants and Procedure
Participants were undergraduate students at Temple University ranging in age from 18 to 65. For inclusion, participants had normal or corrected-to-normal vision and were fluent in English. Participants were recruited from undergraduate psychology classes using the department’s online listing of studies and from the diverse student body via flyers posted around campus. Students received psychology course credit and/or were compensated in cash for participation. All participants completed informed consent approved by the University’s Institutional Review Board. The sample included 178 participants (Mage = 21.94, SD = 5.71) and was 57.9% female, 9.0% Hispanic or Latino, 20.2% African American/Black, 64.0% Caucasian/White, 12.4% Asian, 0.6% Pacific Islander, 1.1% Native American, and 6.2% “other” race.
At Wave 1, participants completed a laboratory assessment that involved completing questionnaires in an online survey and watching videos while heart rate and respiration were assessed. Every three weeks after Wave 1, participants completed follow-up assessments remotely that included measures of current symptoms of depression experienced in the prior three weeks (Waves 2–5). To qualify for inclusion in the present multi-wave analyses (see Data Analysis), participants were required to have completed at least two of the four possible follow-up assessments, as using fewer than two observations could result in unreliable estimates of levels of depressive symptoms across follow-up. This yielded a final sample size of 134. Participants included in the present analyses did not differ from participants excluded from analyses (n = 44) on any study variables or demographic characteristics (ps > .08).
Measures
RSA Reactivity
At the Wave 1 assessment, participants were seated comfortably in a small assessment room and the experimenter attached cardiovascular sensors. After a five-minute rest period to become acclimated to the room and sensors, participants watched a series of video clips, which were presented on a desktop computer approximately 24 inches in front of them. Film selection was based on criteria recommended by Rottenberg et al. (2007b). To establish physiological parameters during a neutral baseline film, participants first viewed a two-minute nature film clip (a documentary on Denali National Park), followed by a film depicting a boy who is distraught at the death of his father (the movie The Champ), which has been demonstrated to elicit sadness (Rottenberg et al., 2007b). Participants completed a brief self-report measure of affect before and after each film.
Electrocardiogram (ECG) and respiration signals were assessed with a three-lead electrocardiogram and BioPac BioHarness, and were continuously recorded on a PC with AcqKnowledge 4.3 software (equipment and software from Biopac Instruments Inc., Goleta, CA), sampled at 1000Hz. Cloth base disposable Ag/AgCl electrodes were placed in a modified Lead-II configuration on the chest. ECG signals were amplified with a Biopac MP150 with an ECG100 amplifier. We measured respiration rate (RR) with an RSP100C amplifier with a TSD100C respiratory transducer, which was placed around the chest, crossing under the armpits and on top of the breastbone. Respiration data were high-pass filtered and visually inspected for artifacts and corrected when needed, following established procedures outlined elsewhere (Grossman et al., 1990; Rottenberg et al., 2007b). HR data were manually visually inspected for artifacts with the aid of a channel that computed momentary inter-beat interval, and artifacts were manually adjusted as necessary (< 1% of heartbeats required adjustment). RSA was calculated using the well-validated peak-valley method (Grossman et al., 1990); the maximum heart rate during the expiration window of respiration was subtracted from the minimum heart rate during the inspiration window of respiration. RSA was computed in milliseconds with higher values reflecting greater vagal tone (or parasympathetic activity). Average RSA was computed separately for each film epoch (neutral film, sad film). To assess RSA reactivity, analyses evaluated the effect of RSA during the sad film after controlling for RSA during the neutral film; thus, higher levels of RSA during the sad film represented residual change in RSA, with higher scores reflecting smaller decreases in RSA (less vagal withdrawal, less parasympathetic flexibility) during the sad film relative to the neutral film.
Beck Depression Inventory (BDI-II; Beck et al., 1996)
The BDI-II assessed the severity of cognitive, affective, and somatic symptoms of depression during the previous three weeks. It is the most commonly-used self-report measure of depressive symptoms and has demonstrated good internal consistency and validity in undergraduate samples (Storch et al., 2004; Dozois et al., 1998). In the present study, αs = .87–.90 at Waves 1–5.
Ruminative Response Scale (RRS; Treynor, Gonzalez, & Nolen-Hoeksema, 2003)
The RRS is a 10-item self-report measure that assesses two components of rumination: brooding and reflective pondering. The measure consists of five brooding items (e.g., “think about a recent situation, wishing it had gone better”) and five reflection items (e.g., “analyze recent events to try to understand why you are depressed”), which are scored on a Likert scale ranging from 1 (almost never) to 4 (almost always). It has excellent internal consistency and validity (Armey et al,. 2009; Treynor et al., 2003). In the present study, α = .74 for brooding, and α = .76 for reflective pondering.
Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990)
The PSWQ is a 16-item inventory designed to assess trait worry and to capture the generality, excessiveness, and uncontrollability characteristics of pathological worry (e.g., “my worries overwhelm me” and “I worry all the time”). Items are rated on a 1 (not at all typical of me) to 5 (very typical of me) Likert scale. Among samples of college undergraduates, the PSWQ has repeatedly demonstrated good internal consistency and good test-retest reliability over intervals as long as ten weeks (Meyer et al., 1990; Fresco et al., 2002). In this sample, the PSWQ demonstrated excellent internal consistency (α = .95).
Experiences Questionnaire (EQ; Fresco et al., 2007a)
The decentering subscale of the EQ is an 11-item self-report instrument that assesses the ability to mentally distance oneself from one’s thoughts and feelings. Participants respond to items (e.g., “I can observe unpleasant feelings without being drawn into them”) on a scale ranging from 1 (never) to 5 (all the time). Items are summed, with higher scores representing greater decentering. The measure has demonstrated good validity and internal consistency (Fresco et al., 2007a,b; Teasdale et al., 2002; Bernstein et al., 2015); in the present study, α = .88.
Metacognitive Awareness Questionnaire (MAQ)
The MAQ is a nine-item self-report instrument that assesses the degree to which individuals see that their negative thoughts and feelings when sad might not reflect actual realities, an important component of decentering (Teasdale et al., 2001). Individuals respond to items (e.g., “when I get low, I remind myself that I may be seeing things as more negative than they really are”) on a Likert scale ranging from 1 (totally disagree) to 7 (totally agree). Higher scores represent greater problems with metacognitive awareness, or lower levels of decentering. The MAQ has demonstrated adequate internal consistency (Teasdale et al., 2001). In the present study, α = .64.
Positive and Negative Affect Scale (PANAS), Brief Version (Mackinnon et al., 1999)
The PANAS – Brief version is a brief, 10-item version of the original self-report measure (Watson, Clark, & Tellegen, 1988) that assesses current emotions and affective experiences. It was administered as a manipulation check before and after each of the film and rest periods in the study to evaluate shifts in affect. Participants indicate the extent to which a number of different affective words describe their current state. It contains two subscales, each with five items: positive affect (inspired, alert, excited, enthusiastic, determined) and negative affect (afraid, upset, nervous, scared, distressed). The PANAS is a commonly-used measure of affect in experimental studies, and it has excellent validity and reliability (Crawford & Henry, 2004; Watson et al., 1988). In the present study, the PANAS had excellent internal consistency across the experimental periods (before and after each film): positive affect α = .84–.91, negative affect α = .90–.97.
Data Analysis
Given the nested structure of the data (multiple observations of depressive symptoms within each person), multilevel modeling (MLM) was used (Raudenbush & Bryk, 2002). MLM is advantageous in terms of maximizing data usage because it can flexibly handle cases with missing data, so participants with missing data (e.g., participants who miss a follow-up visit) are not eliminated from the data analyses. To avoid problems with unreliable estimates of levels of depressive symptoms across follow-up as might occur with only one measurement of BDI, participants were included in analyses if they had completed at least two of the four possible waves of follow-up (Waves 2–5). Analyses were conducted with the Mplus 6.12 statistical software package (Muthen & Muthen, 2011), which allowed for use of full information maximum likelihood (FIML) estimation of data (Enders & Bandalos, 2001), using maximum likelihood estimation with robust standard errors.
To reduce the number of component cognitive measures, we first conducted a principal component analysis (PCA), hypothesizing that the cognitive measures would load onto components representing PC and decentering. Next, we used MLM to evaluate whether the relationship between low RSA reactivity to the sad film and higher levels of prospective depressive symptoms would be moderated by the components and by the original cognitive measures. Significant interactions were probed at +/− 1 standard deviation from the mean of the moderator (Aiken & West, 1991). For ease of interpretation, Level 2 variables (RSA, the components, and the cognitive measures) were Z-standardized at the between-subjects level. Depressive symptoms at Wave 1 served as a Level 2 covariate, so that results are interpreted as Wave 1 variables predicting future levels of depressive symptoms beyond the influence of baseline symptoms. Each of the primary analyses included an interaction term between RSA during the sad film and one of the components, RSA during the neutral film and one of the components, and the main effects of these variables. Thus, rather than computing difference scores representing change in RSA from the neutral to sad films, the value of RSA during the sad film is interpreted as residual change in RSA after accounting for RSA during the neutral film. An example MLM equation is provided in the Appendix.
Results
Preliminary Analyses
First, we conducted manipulation checks to evaluate the extent to which the sad film induced the intended emotions. As expected, compared to the neutral baseline film, there was an increase in negative affect following the sad film (t = −9.87, p < .001, d = .59), as well as a decrease in positive affect (t = 10.36, p < .001, d = .53). As expected, participants showed decreases in RSA (t = 4.52, p < .001, d = .19) from the neutral film to the sad film.
Overall, participants had relatively low symptoms of depression (Wave 1 BDI Mean = 8.57, SD = 7.47; Wave 2 BDI Mean = 7.37, SD = 7.34; Wave 3 BDI Mean = 6.37, SD = 7.46; Wave 4 BDI Mean = 6.12, SD = 7.42; Wave 5 BDI Mean = 4.84, SD = 5.90). Including the baseline assessment, a total of 592 observations were completed (Wave 1 n = 134; Wave 2 n = 122; Wave 3 n = 122; Wave 4 n = 112; Wave 5 n = 102). Number of assessments completed was not related to any study variables except for Wave 1 compensation type (cash vs. course credit); individuals who received cash for Wave 1 completed more assessments (M = 4.86) than participants who received course credit (M = 4.37), t = 4.04, p < .01.1
The intra-class correlation for an empty model predicting BDI was .651, indicating that 65.1% of the variance in depressive symptoms occurred at the between-subjects level (Level 2), whereas 34.9% of the variance occurred at the within-subjects level (Level 1).
Principal Component Analysis
Given that the cognitive measures were correlated with one another as expected, we performed a PCA with varimax rotation to reduce the number of variables in the primary analyses. We did not restrict the number of components identified, which were selected based on having eigenvalues ≥ 1. The PCA yielded a two-component solution. Component 1 had an eigenvalue of 2.28 and accounted for 45.59% of the variance, whereas Component 2 had an eigenvalue of 1.12 and accounted for an additional 22.32% of the variance. The rotated component matrix and coefficient matrix (loadings), displayed in Table 2, suggested that Component 1 was characterized primarily by brooding, reflective pondering, and worry, which we labeled as the perseverative cognition (PC) component. Component 2 appeared to be characterized primarily by the two decentering measures, and thus was labeled as the decentering component. For interpretability, we reverse-scored Component 2 so that higher levels represent higher levels of decentering.
Table 2.
Rotated Component Matrix and Coefficient Matrix (Loadings) for Two-Component Solution Identified by Principal Component Analysis
| Rotated Component Matrix | Coefficient Matrix | |||
|---|---|---|---|---|
|
| ||||
| Component: | 1 | 2 | 1 | 2 |
| Brooding | .83 | .26 | .39 | .06 |
| Reflective Pondering | .79 | −.33 | .47 | −.40 |
| Worry | .77 | .35 | .34 | .15 |
| Decentering (EQ) | −.40 | −.67 | −.09 | −.47 |
| Decentering (MAQ) | −.06 | .77 | −.17 | .63 |
Note. EQ = experiences questionnaire;
MAQ = metacognitive awareness questionnaire.
Correlations among study variables are presented in Table 1. Most of the cognitive variables were correlated with one another, whereas most were not correlated with RSA reactivity. Surprisingly, PC was not significantly correlated with RSA reactivity; however, higher reflective pondering was associated with less vagal withdrawal (less RSA reactivity) during the sad film.
Table 1.
Correlations Among Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 BDI (Wave 1) | – | .55*** | .42*** | .45*** | −.48*** | .19* | .56*** | −.27** | .11 |
| 2 RRS Brooding | – | .49*** | .61*** | −.41*** | .17 | .82*** | −.25** | .09 | |
| 3 RRS Reflective Pondering | – | .42*** | −.13 | −.04 | .80*** | .28*** | .19* | ||
| 4 PSWQ Worry | – | −.50*** | .04 | .81*** | −.28** | −.04 | |||
| 5 EQ Decentering | – | −.26** | −.44*** | .70*** | .09 | ||||
| 6 MAQ Decentering | – | −.08 | −.79*** | .00 | |||||
| 7 Perseverative Cognition (Component 1) | – | .00 | .10 | ||||||
| 8 Decentering (Component 2) | – | .12 | |||||||
| 9 ΔRSA (Residual) | – |
p < .001,
p < .01,
p < .05.
Note. ΔRSA = Respiratory Sinus Arrhythmia during the sad film, controlling for RSA during the neutral film; thus, higher ΔRSA represents higher RSA during sad film relative to neutral film (i.e., smaller decreases or less contextually-appropriate “reactivity” of RSA).
Do Cognitive Factors Moderate the Relationship between RSA and Prospective Symptoms of Depression?2
Results of MLM analyses using components yielded by the PCA are presented in Table 3. There was a significant interaction between PC (Component 1) and RSA, such that lower RSA reactivity predicted significantly higher prospective depressive symptoms among individuals with greater levels of PC (t = 2.42, p = .02), but not among individuals with lower levels of PC (t = −1.29, p = .20; Figure 1a). In addition, there was a significant interaction between decentering (Component 2) and RSA, such that lower RSA reactivity predicted significantly higher prospective depressive symptoms among individuals with lower levels of decentering (t = 2.70, p < .01), but not among individuals with higher levels of decentering (t = −1.40, p = .16; Figure 1b).
Table 3.
Interactions between Parasympathetic Responses to Sadness and Metacognitive Components Predicting Changes in Depressive Symptoms over Follow-up
| Model | Predictor | B | SE | p | ΔR2 | f2 |
|---|---|---|---|---|---|---|
| 1 | Component 1 | .68 | 2.13 | |||
| RSA | 0.72 | 0.66 | .28 | .01 | .03 | |
| Perseverative Cognition (PC) | 1.16 | 0.41 | <.01 | .06 | .19 | |
| RSA × PC | 1.92 | 0.76 | .01 | .05 | .16 | |
| 2 | Component 2 | .64 | 1.78 | |||
| RSA | 0.57 | 0.85 | .50 | <.01 | <.01 | |
| Decentering | 0.30 | 0.43 | .48 | <.01 | <.01 | |
| RSA × Decentering | 2.73 | 1.10 | .01 | .04 | .11 | |
| 3 | Combined Component Model | .72 | 2.57 | |||
| RSA | 0.04 | 0.68 | .95 | <.01 | <.01 | |
| Perseverative Cognition (PC) | 1.39 | 0.40 | .001 | .08 | .26 | |
| Decentering | −0.55 | 0.39 | .15 | .01 | .04 | |
| RSA × PC | 2.10 | 0.65 | .001 | .07 | .25 | |
| RSA × Decentering | −3.04 | 0.94 | .001 | .07 | .25 | |
| 4 | Three-Way Interaction Component Model | .74 | 2.85 | |||
| RSA | 0.62 | 0.64 | .40 | .01 | .04 | |
| Perseverative Cognition (PC) | 1.60 | 0.42 | <.001 | .10 | .38 | |
| Decentering | −0.73 | 0.36 | .04 | .03 | .12 | |
| RSA × PC | 1.18 | 0.65 | .07 | .02 | .08 | |
| RSA × Decentering | −2.11 | 0.90 | .02 | .04 | .15 | |
| PC × Decentering | −0.25 | 0.58 | .56 | <.01 | <.01 | |
| PC × Decentering × RSA | −3.00 | 1.26 | .02 | .04 | .15 | |
Note. RSA = Respiratory Sinus Arrhythmia. R2 for full model represents the proportion of variance explained by the model relative to an unrestricted (empty) model containing no predictors (Kreft & de Leeuw, 1998; Singer, 1998). ΔR2 = partial R2 of given predictor (proportion of variance in depression symptoms predicted) after accounting for covariates. f2 represents Cohen’s f2, a measure of effect size. In all models, RSA represents the value during the sad film, while controlling for RSA during the neutral film, so that the RSA values displayed represent residual change “reactivity” scores, with higher RSA representing less reactivity (smaller decreases in RSA) relative to neutral film (e.g., in Model 1, covariates are RSA during neutral film and interaction between RSA during neutral film and PC).
Figure 1.
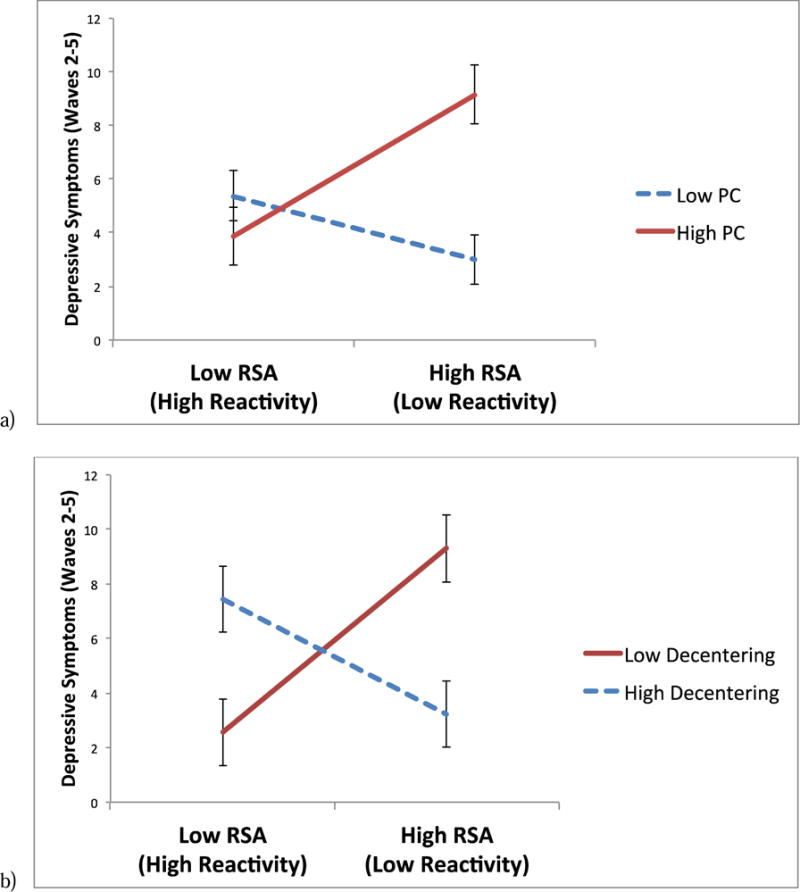
Two-way interactions between respiratory sinus arrhythmia (RSA) reactivity to sad film and (a) perseverative cognition (PC) and (b) decentering, predicting symptoms of depression at waves 2–5, controlling for symptoms of depression at wave 1 (error bars represent standard errors of the relationship between RSA reactivity and symptoms of depression, for each level of the moderator).
Next, to evaluate the relative strength of PC and decentering as moderators, we ran a combined component model that included RSA, PC, decentering, and the RSA × PC and RSA × Decentering interaction terms (Table 3). Both interactions remained significant. Finally, we evaluated possible synergistic effects of high PC and low decentering on RSA, by testing whether decentering would moderate the RSA × PC interaction. This model was similar to the combined model described above, with the addition of a PC × Decentering interaction term, and the three-way PC × Decentering × RSA interaction term. As hypothesized, the three-way interaction term was significant (Figure 2). Thus, we proceeded to test the RSA × PC two-way interaction term at high and low levels of decentering (Aiken & West, 1991). At high levels of decentering, the two-way RSA × PC interaction was not significant (t = −1.26, p = .21). However, at low levels of decentering, the RSA × PC interaction was significant (t = 3.02, p < .005), so we further decomposed this two-way interaction term at low levels of decentering. Among individuals low in decentering who also had low PC, RSA reactivity did not predict prospective levels of depressive symptoms (t = −0.77, p = .44), suggesting a possible protective effect of decentering on the risk conferred by PC and low RSA reactivity. In addition, supportive of the hypothesized synergistic effects of PC and low decentering, among individuals low in decentering who also had high PC, RSA reactivity predicted significantly higher prospective depressive symptoms (t = 4.71, p < .001).
Figure 2.
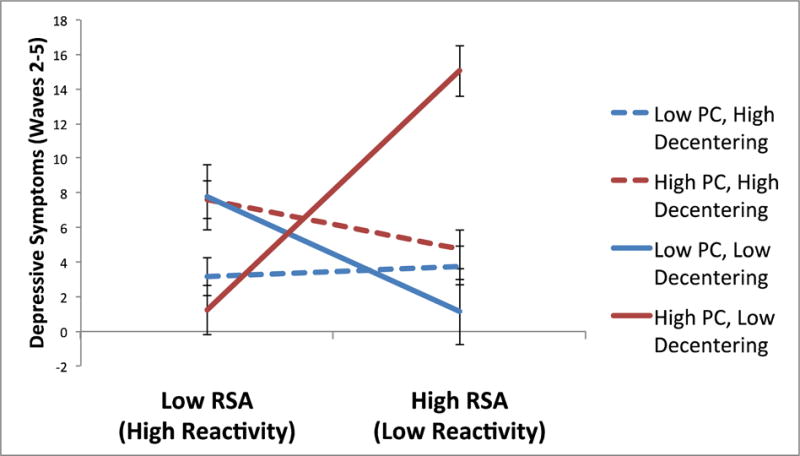
Three-way interaction between respiratory sinus arrhythmia (RSA) reactivity to sad film, perseverative cognition (PC), and decentering, predicting symptoms of depression at waves 2–5, controlling for symptoms of depression at wave 1 (error bars represent standard errors of the relationship between RSA reactivity and symptoms of depression, for each level of the moderators).
As a secondary step, to investigate which measures might be driving the interactive effects between RSA and the two components identified in the PCA, we evaluated interactions between RSA and brooding, reflective pondering, worry, and decentering (on the EQ and MAQ), in five separate multilevel models (Table 4). Each of these five cognitive variables significantly moderated the relationship between RSA and prospective depressive symptoms. As expected, the form of these interactions was such that lower RSA reactivity predicted higher prospective depressive symptoms among individuals with higher levels of brooding (t = 2.69, p < .01), reflective pondering (t = 2.26, p = .02), or worry (t = 2.19, p = .03), or lower levels of decentering (EQ: t = 2.58, p = .01; MAQ: t = 2.33, p = .02); however, lower RSA reactivity did not predict prospective depressive symptoms among individuals with lower levels of brooding (t = −1.58, p = .11), reflective pondering (t = −0.34, p = .73), or worry (t = −0.93, p = .35), or higher levels of decentering (EQ: t = −1.32, p = .19; MAQ: t = −0.98, p = .33). Thus, the interactions between RSA and the components identified by the PCA did not seem to be driven by only a subset of the measures that comprised the components.
Table 4.
Interactions between Parasympathetic Responses to Sadness and Individual Metacognitive Variables Predicting Changes in Depressive Symptoms over Follow-up
| Model | Predictor | B | SE | p | ΔR2 | f2 |
|---|---|---|---|---|---|---|
| 5 | Brooding | .68 | 2.13 | |||
| RSA | 0.77 | 0.62 | .22 | .01 | .03 | |
| Brooding | 1.22 | 0.43 | .005 | .06 | .19 | |
| RSA × Brooding | 2.00 | 0.68 | <.01 | .06 | .19 | |
| 6 | Reflective Pondering | .64 | 1.78 | |||
| RSA | 1.20 | 0.73 | .10 | .02 | .06 | |
| Reflective Pondering | 0.63 | 0.34 | .06 | .03 | .08 | |
| RSA × Reflective Pondering | 1.46 | 0.66 | .03 | .04 | .11 | |
| 7 | Worry | .70 | 2.33 | |||
| RSA | 0.60 | 0.84 | .47 | <.01 | <.01 | |
| Worry | 0.77 | 0.38 | .04 | .03 | .10 | |
| RSA × Worry | 1.69 | 0.73 | .02 | .04 | .13 | |
| 8 | Decentering (EQ) | .68 | 2.13 | |||
| RSA | −0.08 | 0.98 | .94 | <.01 | <.01 | |
| Decentering | −0.82 | 0.43 | .06 | .03 | .09 | |
| RSA × Decentering | −2.63 | 1.28 | .04 | .03 | .09 | |
| 9 | Decentering (MAQ) | .66 | 1.94 | |||
| RSA | 1.06 | 0.74 | .15 | .02 | .06 | |
| Decentering | −0.17 | 0.41 | .68 | <.01 | <.01 | |
| RSA × Decentering | 2.13 | 1.00 | .03 | .03 | .09 |
Note. RSA = Respiratory Sinus Arrhythmia. R2 for full model represents the proportion of variance explained by the model relative to an unrestricted (empty) model containing no predictors (Kreft & de Leeuw, 1998; Singer, 1998). ΔR2 = partial R2 of given predictor (proportion of variance in depression symptoms predicted) after accounting for covariates. f2 represents Cohen’s f2, a measure of effect size. In all models, RSA represents the value during the sad film, while controlling for RSA during the neutral film, so that the RSA values displayed represent residual change “reactivity” scores, with higher RSA representing less reactivity (smaller decreases in RSA) relative to neutral film (e.g., in Model 5, covariates are RSA during neutral film and interaction between RSA during neutral film and brooding).
In these models, the main effects of PC appeared to account for a larger proportion of variance (R2) and had larger effect sizes (f2) than the main effects of decentering and RSA; the main effect of PC also was a significant predictor of prospective depressive symptoms in each of the models involving PC. In contrast, decentering and RSA reactivity individually predicted relatively less variance and were less consistently significant as main effects.
Discussion
In the present study, we integrated multiple components of metacognition with an established biological correlate and possible risk factor for depression in a multi-wave study. Parasympathetic inflexibility (less withdrawal of RSA in the context of a sad film) was associated with greater prospective symptoms of depression across twelve weeks of follow-up. However, this effect was moderated by two principal components of metacognition: perseverative cognition (PC), which appeared to exacerbate the relationship between parasympathetic inflexibility and future symptoms of depression, and decentering, which attenuated the relationship between parasympathetic inflexibility and symptoms of depression. Furthermore, in a fully integrated model, decentering also moderated the interaction between parasympathetic inflexibility and PC predicting depression, such that individuals with greater parasympathetic inflexibility, greater brooding, and lower decentering were at greatest risk for depressive symptoms across follow-up. Together, these results suggest that integrating these components enables the identification of potentially synergistic relationships between cognitive and physiological risk factors for depression, and may lead to greater specificity in identifying individuals at risk compared to when evaluating these factors in isolation.
Individuals who frequently engage in PC may exhibit chronic activation of a stress response, with numerous maladaptive downstream consequences for physiological, cognitive, and behavioral flexibility (Brosschot et al., 2006, 2007, 2010; LeMoult, Yoon, & Joormann, 2016; Ottaviani et al., 2015, 2016). When combined with a lack of parasympathetic flexibility in response to a sad film, which may represent difficulty appropriately engaging with relevant stimuli, individuals may have a “double load” of difficulty adapting to situational demands. This physiological and cognitive burden could interfere with effective self-regulation (e.g., Yaroslavsky et al., 2016) and make them more susceptible to experiencing symptoms of depression when adaptation becomes necessary. Although the study did not evaluate neural networks, it is possible that difficulty with flexibly disengaging from self-referential thinking and attending to task-relevant stimuli are driven in part by dysfunctional activity within the default mode network. Excessive connectivity within the default mode network, or a lack of connectivity between the default mode and cognitive control network, may interfere with the ability to flexibly balance orientation toward internally- and externally-generated demands (Marchetti et al., 2012; Mennin & Fresco, 2013; Whitfield-Gabrieli & Ford, 2012). This could lend itself to excessive PC and to inflexible parasympathetic responses to sad stimuli, and could interfere with the effective regulation of negative affect. Interestingly, each of the scales comprising PC – brooding, pondering, and worry – appeared to have similar effects in terms of exacerbating the relationship between parasympathetic inflexibility and future symptoms of depression. This suggests that the specific content of PC may be less important than the perseverative nature of the cognition itself. In conjunction with parasympathetic inflexibility, these factors could lead synergistically to difficulty engaging with the environment, which could have a variety of undesirable consequences. For example, given the difficulty they may experience with mentally engaging with the environment, individuals with both risk factors might withdraw from social situations in which positive reinforcement otherwise might be available; for some, this could lead to a downward spiral into depression.
In contrast with PC, the decentering component (and each of the scales comprising the component) appeared to protect against the effects of parasympathetic inflexibility on future symptoms of depression. Furthermore, decentering also attenuated the risk conferred by the synergistic effects of parasympathetic inflexibility and PC. This suggests that even if individuals have two important risk factors that prevent the flexible and effective engagement with the environment, the ability to distance oneself from one’s thinking could be powerful enough to prevent this risk from spiraling into depression. These results are consistent with the idea that having a meta-awareness of one’s thoughts and the ability to distance oneself from them may reduce the emotional impact of perseverative thinking processes (Fresco et al., 2007a,b). By having greater space between one’s thoughts and one’s behavior, decentering may allow for behavioral and emotional responses that are less automatic and more deliberate, allowing one to notice unpleasant perseverative thoughts while being less reactive to them, and thereby facilitating self-regulation and protecting against depression risk (e.g., Kang, Gruber, & Gray, 2013). In contrast, in the present study, individuals who were most at risk for depression were those who had all three risk factors – parasympathetic inflexibility, high levels of PC, and low levels of decentering. Lacking the ability to decenter in the context of the other risk factors might mean that individuals have difficulty stepping back and disengaging from PC, which, in conjunction with parasympathetic inflexibility may make them less able to adapt to a variety of situations. This could lead individuals to miss important contextual information (e.g., awareness of one’s negative affect, that a current regulatory strategy has not been effective, or of opportunities for reward in the environment) that might signal that a change would be useful (e.g., Aldao, Sheppes, & Gross, 2015; Bonanno & Burton, 2013; Cheng, 2001; Gross, 2015; Kato, 2012; Stange et al., in press).
Although the primary results of interest involved interactions between these putative risk factors, each of the models involving PC also involved a significant main effect of PC on prospective symptoms of depression, which accounted for a relatively greater proportion of variance in symptoms of depression compared to either decentering or RSA. This pattern of results may indicate that PC may be the strongest risk factor for depression, and hence, arguably the most important to target clinically. However, our results suggest that the degree to which PC confers risk for depression may be exacerbated by the absence of decentering and by the presence of parasympathetic inflexibility. These results could help refine existing models of risk to more specifically identify which individuals with PC are at greatest risk for depression. These results also can inform treatments for individuals carrying these multiple risk factors, who may benefit from treatments that plausibly influence each of these factors (e.g., mindfulness-based approaches) or for adjunctive treatments that target these multiple risk factors separately (e.g., adding RSA biofeedback to treatments targeting PC and decentering). In addition, it was surprising that PC was not associated with decreases in RSA in our study, given that experimental studies have documented that experimentally-induced “state” PC is associated with acute decreases in RSA (Ottaviani et al., 2016). One possible explanation may be that the current study measured “trait” PC, as opposed to “state” PC that participants were using during the sad film; we also did not experimentally manipulate PC by instructing individuals to ruminate or worry, which could help to account for differences between studies.
Although the present study involved a non-clinical sample, these results have several important clinical implications. First, decentering and related processes such as mindfulness are involved in several treatments for depression (Mennin, Ellard, Fresco, & Gross, 2013), including MBCT (Bieling et al., 2012; Fresco et al., 2007b; Segal et al., 2012), emotion regulation therapy (ERT; Mennin & Fresco, 2013b; Mennin et al., 2015), acceptance and commitment therapy (Hayes, Luoma, Bond, Masuda, & Lillis, 2006), dialectical behavior therapy (Linehan, 2014), and metacognitive therapy (Wells et al., 2012). It is possible that training individuals in decentering would reduce the tendency to engage in PC or would improve parasympathetic flexibility. For example, some prior studies have demonstrated that mindfulness training reduces PC (Chambers, Lo, & Allen, 2008; Feldman, Greeson, & Senville, 2010; Jain et al., 2007), improves intrinsic connectivity between the DMN and hubs in the cognitive control network (Creswell et al., 2016; King et al., 2016), and may improve resting levels of parasympathetic activity (e.g., Krygier et al., 2013). Alternatively, even if training in mindfulness or decentering does not directly alter these risk factors, the results of this study suggest that improving decentering could de-automatize emotional reactions to PC, and might reduce the risk conferred by PC and parasympathetic inflexibility. In addition, several existing treatments explicitly target PC, including rumination-focused CBT (Watkins et al., 2011; Jacobs et al., 2016), ERT (Mennin & Fresco, 2013b; Mennin et al., 2015), metacognitive therapy (Wells, 2012), and cognitive control training (Siegle et al., 2014). It is possible that these treatments also could be tailored for prevention to apply to individuals who are at risk, but do not have a history of depression. Indeed, a recent study showed that targeting PC with cognitive-behavioral training may be effective in preventing subsequent depression and anxiety, even in non-clinical, never-depressed samples (Topper et al., 2017). Finally, biofeedback is an intervention tool that holds promise for helping individuals to improve parasympathetic activity in response to different stimuli (Karavidas et al., 2007; Siepmann et al., 2008). Emerging wearable technologies also have enabled the use of momentary assessment of parasympathetic functioning (Valenza et al., 2014, 2015). It is possible that in the future, these tools could be used to provide biofeedback in ecologically valid contexts, perhaps in combination with interventions to implement decentering or reduce PC during periods of extended vagal withdrawal.
Despite the strengths of integrating measures of parasympathetic flexibility with various metacognitive characteristics, several limitations of the present study must be noted. First, we evaluated PC and decentering as traits, but not “state” levels of these constructs in response to the sad film; thus, we are unable to determine whether people were engaging in these strategies during the film or how state levels of these strategies would affect RSA responses to the film. It is possible that evaluating “state” PC or decentering would yield significant relationships with parasympathetic flexibility that were not observed in the present study, and that might suggest mediational relationships between these constructs. Second, although the sadness induction in response to the film is a validated and commonly-used procedure (Rottenberg et al., 2007b), the ecological validity of the task for responses to sadness or other types of stimuli outside of the lab is not clear. In addition, the study sample was composed of university students; thus, the results cannot necessarily be extended to the general public or to clinical populations.
We also examined fluctuations in symptoms of depression rather than clinically-significant depressive episodes. Although evaluating depression on a dimensional level may improve potential power to detect nuanced effects and is consistent with evidence supporting the dimensional structure of depression (e.g., Liu, 2016), the clinical significance of the present results is not immediately clear (although results with RSA reactivity in clinical samples have paralleled those in the present study; e.g., Fraguas et al., 2007; Panaite et al., 2016; Rottenberg, Kasch, Gross, & Gotlib, 2002; Rottenberg, Salomon, Gross, & Gotlib, 2005). Future studies also should consider evaluating depression severity and perseverative cognition using interviewer-based methods that may be less susceptible to reporter bias than self-report scales. Although the present study integrated psychophysiological and self-report scales in evaluating depression risk, in the future, researchers should consider using additional methods of assessment to reduce shared variance between constructs that is attributable primarily to the method of assessment. Next, individuals who chose to receive cash (as opposed to course credit) completed more follow-up assessments; thus, it is possible that the results are more applicable to these individuals who had fewer follow-up data points estimated, although it is not clear how this one difference would have influenced the results given that these individuals did not differ on any other study measures. It also is possible that the relatively low internal consistency of the self-report measures, particularly the MAQ, obscured or attenuated some relationships that truly exist, despite the use of the principal component analysis to reduce the noise associated with any given measure. Finally, although the present study evaluated risk for depression, RSA, PC, and decentering represent promising transdiagnostic factors that may be implicated in other types of psychopathology, including anxiety disorders (Beauchaine & Thayer, 2015; Bernstein et al., 2015; Nolen-Hoeksema et al., 2008; Watkins, 2008).
In conclusion, the present study represents the first prospective test of the integration of parasympathetic and metacognitive factors in risk for depression. The results supported an integrated model, suggesting that perseverative cognition may exacerbate the effects of parasympathetic inflexibility on future symptoms of depression, whereas decentering may protect against these effects. These results suggest the presence of synergistic relationships between cognitive and physiological factors in conferring risk for depression, and that integrating these factors may lead to an improved ability to identify individuals who are at risk.
Depression often is characterized by inflexible autonomic and metacognitive processes.
Few studies have integrated these factors to improve the prediction of risk for depression.
The relationship between parasympathetic inflexibility and prospective depression was exacerbated by perseverative cognition (PC), but attenuated by decentering.
Individuals with parasympathetic inflexibility, PC, and low decentering were at greatest risk.
These results support the utility of integrating autonomic and metacognitive risk factors to identify individuals at risk for depression.
Acknowledgments
This work was supported by grants to Jonathan P. Stange from the National Institute of Mental Health (F31MH099761), the Association for Psychological Science, the American Psychological Foundation, and the American Psychological Association. Jonathan P. Stange was supported by NIMH grant 5T32MH067631-12. Jessica L. Hamilton was supported by National Research Service Award F31MH106184 from NIMH. Lauren B. Alloy was supported by NIMH Grant MH101168. David M. Fresco was supported by NHLBI Grant R01HL119977 and NINR Grant P30NR015326.
Appendix A
Structure of hierarchical linear models predicting prospective symptoms of depression, using Model 1 (Table 3) as an example.
-
Level 1 (Within-Subjects) Model:
BDIti(t) = π0i + eti
-
Level 2 (Between-Subjects) Model:
π0i = β00 + β01*(BDIt1i) + β02*(RSAneutrali) + β03*(RSAsadi) + β04*(PCi) + β05*(RSAneutrali)*(PCi) + β06*(RSAsadi)*(PCi) + r0i
-
Legend:
BDIt1i = Beck Depression Inventory score at Wave 1
RSAneutrali = RSA during neutral film
RSAsadi = RSA during sad film
PCi = Perseverative cognition component score
π0i = Regression coefficient for Level 2 predictors of BDI at Waves 2–5
eti = Level 1 error term
β00 = Level 2 intercept of BDI at Waves 2–5
β01 = Regression coefficient of BDI at Wave 1 predicting BDI at Waves 2–5
β02 = Regression coefficient of RSA during neutral film predicting BDI at Waves 2–5
β03 = Regression coefficient of RSA during sad film predicting BDI at Waves 2–5
β04 = Regression coefficient of PC predicting BDI at Waves 2–5
β05 = Regression coefficient for interaction between RSA during neutral film and PC predicting BDI at Waves 2–5
β06 = Regression coefficient for interaction between RSA during sad film and PC predicting BDI at Waves 2–5
r0i = Level 2 residual for intercept of BDI at Waves 2–5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
However, these groups did not differ on any other study measures.
All results were consistent when controlling for other factors that may influence parasympathetic activity, including age, race, body mass index, and use of antidepressants and other psychiatric medications (Kemp et al., 2010).
Contributor Information
Jonathan P. Stange, University of Illinois at Chicago
Jessica L. Hamilton, Temple University
David M. Fresco, Kent State University
Lauren B. Alloy, Temple University
References
- Abela JR, Hankin BL. Rumination as a vulnerability factor to depression during the transition from early to middle adolescence: a multiwave longitudinal study. Journal of Abnormal Psychology. 2011;120(2):259–271. doi: 10.1037/a0022796. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Aldao A. The future of emotion regulation research: Capturing context. Perspectives on Psychological Science. 2013;8(2):155–172. doi: 10.1177/1745691612459518. [DOI] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010;30(2):217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Aldao A, Sheppes G, Gross JJ. Emotion regulation flexibility. Cognitive Therapy and Research. 2015;39(3):263–278. [Google Scholar]
- Alloy LB, Salk R, Stange JP, Abramson LY. Cognitive vulnerability and unipolar depression. In: DeRubeis RJ, Strunk DR, editors. The Oxford Handbook of Mood Disorders. New York: Oxford University Press; 2017. pp. 142–153. [Google Scholar]
- Armey M, Fresco DM, Moore M, Mennin D, Turk C, Heimberg RG, … &, Alloy LB. Brooding and pondering: Isolating the active ingredients of depressive rumination with exploratory factor analysis and structural equation modeling. Assessment. 2009;16(4):315–327. doi: 10.1177/1073191109340388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Thayer JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology. 2015;98(2):338–350. doi: 10.1016/j.ijpsycho.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Hadash Y, Lichtash Y, Tanay G, Shepherd K, Fresco DM. Decentering and related constructs: A critical review and metacognitive processes model. Perspectives on Psychological Science. 2015;10(5):599–617. doi: 10.1177/1745691615594577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieling PJ, Hawley LL, Bloch RT, Corcoran KM, Levitan RD, Young LT, Segal ZV. Treatment-specific changes in decentering following mindfulness-based cognitive therapy versus antidepressant medication or placebo for prevention of depressive relapse. Journal of Consulting and Clinical Psychology. 2012;80(3):365–372. doi: 10.1037/a0027483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA, Burton CL. Regulatory flexibility: An individual differences perspective on coping and emotion regulation. Perspectives on Psychological Science. 2013;8(6):591–612. doi: 10.1177/1745691613504116. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Robinson E, Pruzinsky T, DePree JA. Preliminary exploration of worry: Some characteristics and processes. Behaviour Research and Therapy. 1983;21(1):9–16. doi: 10.1016/0005-7967(83)90121-3. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences. 2011;108(50):20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF. Markers of chronic stress: Prolonged physiological activation and (un) conscious perseverative cognition. Neuroscience & Biobehavioral Reviews. 2010;35(1):46–50. doi: 10.1016/j.neubiorev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60(2):113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Pieper S, Thayer JF. Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrinology. 2005;30(10):1043–1049. doi: 10.1016/j.psyneuen.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Van Dijk E, Thayer JF. Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. International Journal of Psychophysiology. 2007;63(1):39–47. doi: 10.1016/j.ijpsycho.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Salomon K, Taylor-Clift A, Morris BH, Rottenberg J. Respiratory sinus arrhythmia reactivity in current and remitted major depressive disorder. Psychosomatic Medicine. 2014;76(1):66–73. doi: 10.1097/PSY.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R, Lo BCY, Allen NB. The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cognitive Therapy and Research. 2008;32(3):303–322. [Google Scholar]
- Cheng C. Assessing coping flexibility in real-life and laboratory settings: a multimethod approach. Journal of Personality and Social Psychology. 2001;80(5):814–833. doi: 10.1037//0022-3514.80.5.814. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43(3):245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Taren AA, Lindsay EK, Greco CM, Gianaros PJ, Fairgrieve A, Ferris JL. Alterations in resting-state functional connectivity link mindfulness meditation with reduced interleukin-6: A randomized controlled trial. Biological Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Delgado-Pastor LC, Perakakis P, Subramanya P, Telles S, Vila J. Mindfulness (Vipassana) meditation: Effects on P3b event-related potential and heart rate variability. International Journal of Psychophysiology. 2013;90(2):207–214. doi: 10.1016/j.ijpsycho.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment. 1998;10(2):83–89. [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8(3):430–457. [Google Scholar]
- Farb N, Segal Z, Anderson A. Towards a neuroimaging biomarker of depression vulnerability. Translational Neuroscience. 2011;2(4):281–292. [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2(4):313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman G, Greeson J, Senville J. Differential effects of mindful breathing, progressive muscle relaxation, and loving-kindness meditation on decentering and negative reactions to repetitive thoughts. Behaviour Research and Therapy. 2010;48(10):1002–1011. doi: 10.1016/j.brat.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraguas R, Jr, Marci C, Fava M, Iosifescu DV, Bankier B, Loh R, Dougherty DD. Autonomic reactivity to induced emotion as potential predictor of response to antidepressant treatment. Psychiatry Research. 2007;151(1):169–172. doi: 10.1016/j.psychres.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Fresco DM, Frankel AN, Mennin DS, Turk CL, Heimberg RG. Distinct and overlapping features of rumination and worry: The relationship of cognitive production to negative affective states. Cognitive Therapy and Research. 2002;26(2):179–188. [Google Scholar]
- Fresco DM, Mennin DS, Heimberg RG, Ritter M. Emotion Regulation Therapy for Generalized Anxiety Disorder. Cognitive and Behavioral Practice. 2013;20(3):282–300. doi: 10.1016/j.cbpra.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco DM, Moore MT, van Dulmen MH, Segal ZV, Ma SH, Teasdale JD, Williams JMG. Initial psychometric properties of the experiences questionnaire: validation of a self-report measure of decentering. Behavior Therapy. 2007a;38(3):234–246. doi: 10.1016/j.beth.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Fresco DM, Roy AK, Adelsberg S, Seeley S, García-Lesy E, Liston C, Mennin DS. Distinct functional connectivities predict clinical response with Emotion Regulation Therapy. Frontiers of Human Neuroscience. 2017 doi: 10.3389/fnhum.2017.00086. http://dx.doi.org/10.3389/fnhum.2017.00086. [DOI] [PMC free article] [PubMed]
- Fresco DM, Segal ZV, Buis T, Kennedy S. Relationship of posttreatment decentering and cognitive reactivity to relapse in major depression. Journal of Consulting and Clinical Psychology. 2007b;75(3):447–455. doi: 10.1037/0022-006X.75.3.447. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Current status and future prospects. Psychological Inquiry. 2015;26(1):1–26. [Google Scholar]
- Grossman P, Beek JV, Wientjes C. A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology. 1990;27(6):702–714. doi: 10.1111/j.1469-8986.1990.tb03198.x. [DOI] [PubMed] [Google Scholar]
- Hamilton JL, Alloy LB. Atypical reactivity of heart rate variability to stress and depression: Systematic review of the literature and directions for future research. Clinical Psychology Review. 2016;50:67–79. doi: 10.1016/j.cpr.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: Model, processes and outcomes. Behaviour Research and Therapy. 2006;44(1):1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Jacobs RH, Watkins ER, Peters AT, Feldhaus CG, Barba A, Carbray J, Langenecker SA. Targeting Ruminative Thinking in Adolescents at Risk for Depressive Relapse: Rumination-Focused Cognitive Behavior Therapy in a Pilot Randomized Controlled Trial with Resting State fMRI. PloS one. 2016;11(11):e0163952. doi: 10.1371/journal.pone.0163952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Shapiro SL, Swanick S, Roesch SC, Mills PJ, Bell I, Schwartz GE. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Annals of Behavioral Medicine. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Metcalf CA, Dimidjian S. Dwell or decenter? Rumination and decentering predict working memory updating after interpersonal criticism. Cognitive Therapy and Research. 2015;39(6):744–753. [Google Scholar]
- Kang Y, Gruber J, Gray JR. Mindfulness and de-automatization. Emotion Review. 2013;5(2):192–201. [Google Scholar]
- Kato T. Development of the Coping Flexibility Scale: evidence for the coping flexibility hypothesis. Journal of Counseling Psychology. 2012;59(2):262–273. doi: 10.1037/a0027770. [DOI] [PubMed] [Google Scholar]
- Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, Hassett A. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback. 2007;32(1):19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review. 2010;30(7):865–878. doi: 10.1016/j.cpr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biological Psychiatry. 2010;67(11):1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RM, Wang PS. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. American Journal of Psychiatry. 2006;163(9):1561–1568. doi: 10.1176/appi.ajp.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Wang PS. Epidemiology of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd. New York: Guilford; 2009. pp. 5–22. [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, Liberzon I. Altered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depression and Anxiety. 2016;33(4):289–299. doi: 10.1002/da.22481. [DOI] [PubMed] [Google Scholar]
- Krygier JR, Heathers JA, Shahrestani S, Abbott M, Gross JJ, Kemp AH. Mindfulness meditation, well-being, and heart rate variability: a preliminary investigation into the impact of intensive Vipassana meditation. International Journal of Psychophysiology. 2013;89(3):305–313. doi: 10.1016/j.ijpsycho.2013.06.017. [DOI] [PubMed] [Google Scholar]
- LeMoult J, Yoon KL, Joormann J. Rumination and cognitive distraction in major Depressive Disorder: an examination of respiratory sinus arrhythmia. Journal of Psychopathology and Behavioral Assessment. 2015 doi: 10.1007/s10862-015-9510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM. DBT® skills training manual. Guilford Publications; 2014. [Google Scholar]
- Liu RT. Taxometric evidence of a dimensional latent structure for depression in an epidemiological sample of children and adolescence. Psychological Medicine. 2016;46:1265–1275. doi: 10.1017/S0033291715002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CS, Ho SM, Nicky KK, Siu BP. Decentering mediates the effect of ruminative and experiential self-focus on negative thinking in depression. Cognitive Therapy and Research. 2014;38(4):389–396. [Google Scholar]
- Mackinnon A, Jorm AF, Christensen H, Korten AE, Jacomb PA, Rodgers B. A short form of the Positive and Negative Affect Schedule: Evaluation of factorial validity and invariance across demographic variables in a community sample. Personality and Individual Differences. 1999;27(3):405–416. [Google Scholar]
- Marchetti I, Koster EH, Klinger E, Alloy LB. Spontaneous thought and vulnerability to mood disorders: The dark side of the wandering mind. Clinical Psychological Science. 2016;4(5):835–857. doi: 10.1177/2167702615622383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychology Review. 2012;22(3):229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Ellard KK, Fresco DM, Gross JJ. United we stand: Emphasizing commonalities across cognitive-behavioral therapies. Behavior Therapy. 2013;44(2):234–248. doi: 10.1016/j.beth.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS, Fresco DM. What, me worry and ruminate about DSM-5 and RDoC? The importance of targeting negative self-referential processing. Clinical Psychology: Science and Practice. 2013;20(3):258–267. doi: 10.1111/cpsp.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS, Fresco DM, Ritter M, Heimberg RG. An open trial of emotion regulation therapy for generalized anxiety disorder and co-occurring depression. Depression and Anxiety. 2015;32(8):614–623. doi: 10.1002/da.22377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State worry questionnaire. Behaviour Research and Therapy. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Morin A. Toward a Glossary of Self-related Terms. Frontiers in Psychology. 2017;8(2002):97–9. doi: 10.3389/fpsyg.2017.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Sixth. Los Angeles, CA: Muthén & Muthén; 1998–2011. [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Naragon-Gainey K, Wolitzky-Taylor KB. Specificity of Rumination in Anxiety and Depression: A Multimodal Meta‐Analysis. Clinical Psychology Science and Practice. 2013;20(3):225–257. [Google Scholar]
- Ottaviani C, Medea B, Lonigro A, Tarvainen M, Couyoumdjian A. Cognitive rigidity is mirrored by autonomic inflexibility in daily life perseverative cognition. Biological Psychology. 2015;107:24–30. doi: 10.1016/j.biopsycho.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, Brosschot JF. Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin. 2016;142(3):231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Panaite V, Hindash AC, Bylsma LM, Small BJ, Salomon K, Rottenberg J. Respiratory sinus arrhythmia reactivity to a sad film predicts depression symptom improvement and symptomatic trajectory. International Journal of Psychophysiology. 2016;99:108–113. doi: 10.1016/j.ijpsycho.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Xiao-Feng L. Effects of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychological Methods. 2001;6(4):387–401. [PubMed] [Google Scholar]
- Roelofs J, Huibers M, Peeters F, Arntz A, van Os J. Rumination and worrying as possible mediators in the relation between neuroticism and symptoms of depression and anxiety in clinically depressed individuals. Behaviour Research and Therapy. 2008;46(12):1283–1289. doi: 10.1016/j.brat.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biological Psychology. 2007;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007a;44(3):450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Ray RD, Gross JJ. Emotion elicitation using films. In: Coan JA, Allen JJB, editors. Handbook of emotion elicitation and assessment. New York: Oxford University Press; 2007b. [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42(3):277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2(2):135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42(3):277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Safran JD, Segal ZV. Cognitive therapy: An interpersonal process perspective. New York: Basic; 1990. [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression. Guilford Press; 2012. [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Raichle ME. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Price RB, Jones NP, Ghinassi F, Painter T, Thase ME. You gotta work at it pupillary indices of task focus are prognostic for response to a neurocognitive intervention for rumination in depression. Clinical Psychological Science. 2014;2(4):455–471. [Google Scholar]
- Spasojević J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1(1):25–37. doi: 10.1037/1528-3542.1.1.25. [DOI] [PubMed] [Google Scholar]
- Stange JP, Alloy LB, Fresco DM. Inflexibility and vulnerability to depression: A systematic qualitative review. Clinical Psychology: Science and Practice. doi: 10.1111/cpsp.12201. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Connolly SL, Burke TA, Hamilton JL, Hamlat EJ, Abramson LY, Alloy LB. Inflexible cognition predicts first onset of major depression in adolescence. Depression and Anxiety. 2016;33(11):1005–1012. doi: 10.1002/da.22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Hamilton JL, Olino TM, Fresco DM, Alloy LB. Autonomic reactivity and vulnerability to depression: A multi-wave study. Emotion. 2017;17(4):602–615. doi: 10.1037/emo0000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory-Second Edition in a sample of college students. Depression and Anxiety. 2004;19(3):187–189. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- Taylor VA, Daneault V, Grant J, Scavone G, Breton E, Roffe-Vidal S, Beauregard M. Impact of meditation training on the default mode network during a restful state. Social Cognitive and Affective Neuroscience. 2013;8(1):4–14. doi: 10.1093/scan/nsr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: empirical evidence. Journal of Consulting and Clinical Psychology. 2002;70(2):275–287. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Scott J, Moore RG, Hayhurst H, Pope M, Paykel ES. How does cognitive therapy prevent relapse in residual depression? Evidence from a controlled trial. Journal of Consulting and Clinical Psychology. 2001;69(3):347–357. doi: 10.1037//0022-006x.69.3.347. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Review. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Topper M, Emmelkamp PM, Watkins E, Ehring T. Prevention of anxiety disorders and depression by targeting excessive worry and rumination in adolescents and young adults: A randomized controlled trial. Behaviour Research and Therapy. 2017;90:123–136. doi: 10.1016/j.brat.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27(3):247–259. [Google Scholar]
- Valenza G, Citi L, Gentili C, Lanatá A, Scilingo EP, Barbieri R. Characterization of depressive states in bipolar patients using wearable textile technology and instantaneous heart rate variability assessment. IEEE Journal of Biomedical and Health Informatics. 2015;19(1):263–274. doi: 10.1109/JBHI.2014.2307584. [DOI] [PubMed] [Google Scholar]
- Valenza G, Nardelli M, Lanata A, Gentili C, Bertschy G, Paradiso R, Scilingo EP. Wearable monitoring for mood recognition in bipolar disorder based on history-dependent long-term heart rate variability analysis. IEEE Journal of Biomedical and Health Informatics. 2014;18(5):1625–1635. doi: 10.1109/JBHI.2013.2290382. [DOI] [PubMed] [Google Scholar]
- Watkins ER. Constructive and unconstructive repetitive thought. Psychological Bulletin. 2008;134(2):163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins ER, Mullan E, Wingrove J, Rimes K, Steiner H, Bathurst N, Scott J. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. The British Journal of Psychiatry. 2011;199(4):317–322. doi: 10.1192/bjp.bp.110.090282. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wells A, Fisher P, Myers S, Wheatley J, Patel T, Brewin CR. Metacognitive therapy in treatment-resistant depression: A platform trial. Behaviour Research and Therapy. 2012;50(6):367–373. doi: 10.1016/j.brat.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Bylsma LM, Jennings JR, George C, Baji I, Kiss E. Parasympathetic nervous system activity predicts mood repair use and its effectiveness among adolescents with and without histories of major depression. Journal of Abnormal Psychology. 2016;125(3):323–336. doi: 10.1037/abn0000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Kovacs M. Atypical patterns of respiratory sinus arrhythmia index an endophenotype for depression. Development and Psychopathology. 2014;26(4pt2):1337–1352. doi: 10.1017/S0954579414001060. [DOI] [PMC free article] [PubMed] [Google Scholar]


