Abstract
Exosomes have an evolving role in paracrine and autocrine signaling, which is enhanced because these lipid vesicles are quite stable and can deliver miRNA, DNA, protein and other molecules to cells throughout the body. Most cell types release exosomes, and exosomes are found in all biological fluids, making them accessible biomarkers. Significantly, exosomes can carry a biologically potent cargo, which can alter the phenotype of recipient cells. In the cardiovascular system exosomes have been primarily studied for their role in mediating the beneficial effects of mesenchymal stem cells after myocardial injury. Exosomes released by cardiac cells in disease states, such as myocardial ischemia, can potentially have important pathophysiologic effects on other cardiac cells as well as on distant organs.
Keywords: Exosome, microvesicle, mesenchymal stem cell, cardioprotection, cardiac injury, biomarker
1. Introduction
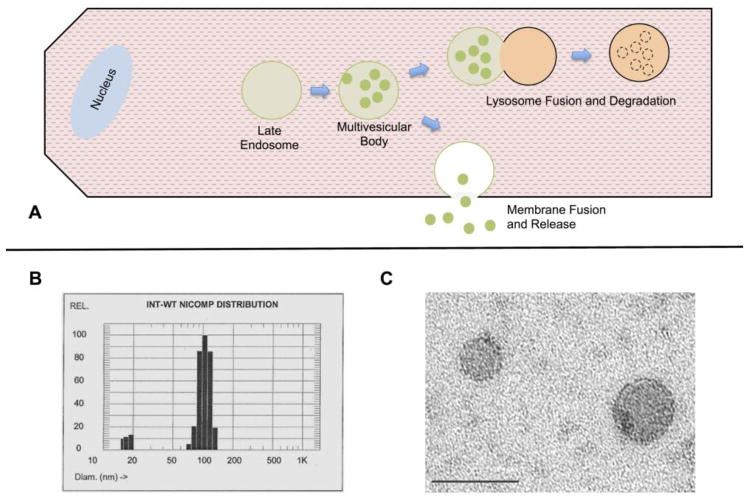
Exosomes are a subset of extracellular microvesicles (Table I) first identified as part of reticulocyte maturation, where exosomes are a vehicle for removing unneeded proteins and membrane, as the reticulocyte transforms into a mature erythrocyte [1, 2]. Electron micrograph studies of reticulocyte externalization of the transferrin receptor demonstrated the presence of small lipid vesicles, which were termed exosomes. Exosomes are the product of a series of steps involving the multivesicular body (MVB). MVBs form in cells by invagination of the cell membrane. Subsequently, exosomes are formed by an invagination of the MVB’s, resulting in preservation of the original cell membrane orientation in the intraluminal vesicle (ILV) membranes. MVBs either fuse with the lysosome for protein degradation or with the plasma membrane, releasing ILVs into the extracellular environment as 30–100nm microvesicles, exosomes[3] (Fig. 1). The regulation of this switch between the lysosome vs. release into the extracellular space remains undefined. Recently, exosomes have been identified as a mechanism of intercellular signaling through the delivery of proteins and nucleotides, which alter recipient cells. Exosomes were first identified to have a pathological role by Skog who found that glioblastoma tumor cells release exosomes, which can be taken up by normal neural cells, changing their phenotype to a more cancerous one [4]. Glioblastoma exosomes transferred mRNA to the normal neural cells, and this mRNA was subsequently translated [4]. Furthermore, proteomic analysis of glioblastoma exosomes, found they were enriched in angiogenic proteins, and after uptake they were able to stimulate endothelial cell tubule formation. Another example of potential exosome mediated intercellular signaling involves viral transmission. Viral infection is associated with increased exosome production, and it has been postulated that exosomes could mediate immune evasion by transmitting virus particles between cells[5, 6]. Exosomes have been investigated in many biological fluids, and found to serve a number of roles from pathological to protective.
Figure 1.
A) Overview of exosome production. Invagination of the multivesicular body (MVB) forms intraluminal vesicles (ILVs). The MVB can either fuse with the lysosome for degradation or fuse with the cell membrane to release the ILVs as exosomes. B) Particle sizing of exosomes from rat cardiac myocytes treated with brief hypoxia/reoxygenation. Exosomes in solution have a peak hydrodynamic radius in the 100nm range. C) Electron micrograph of exosomes isolated from rat plasma, bar is 100nm.
1.1 Pathways to Exosome Generation
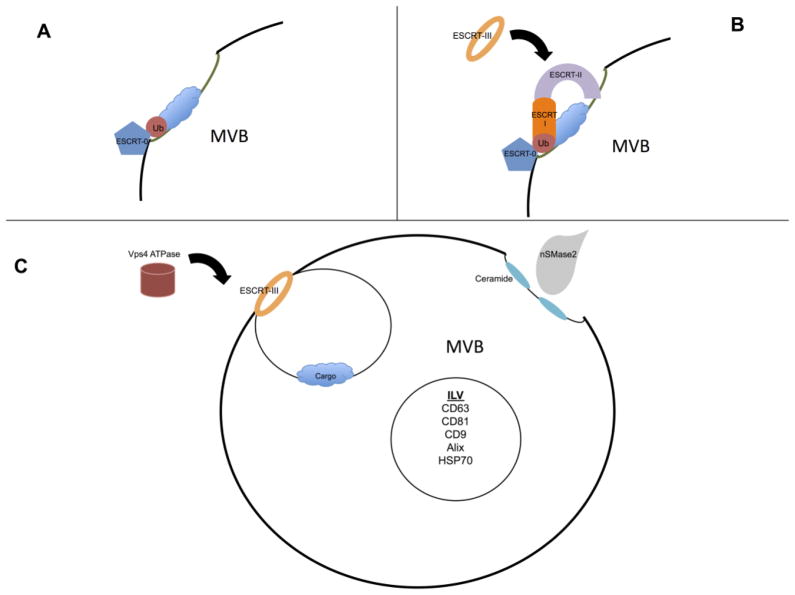
The MVBs are late endosomes that mature from invaginations of the cellular plasma membrane. The formation and packaging of ILVs is still not fully understood, as exosomes do not contain random cytosolic proteins, but are enriched in certain proteins and nucleic acids relative to their parent cells [4, 7–9]. One mechanism of exosome packing is through the endosomal sorting complexes required for transport (ESCRT) machinery (Fig. 2). ESCRT-0 is responsible for binding ubiquitinated cargo and localizing to the endosomal membrane via interactions with phosphatidylinositol-3-phosphate (PI3P), as well as clathrin binding domains (Fig. 2A). The ESCRT-0 components are then able to recruit the ESCRT-I complex by interaction with the tumor susceptibility gene 101 subunit (Tsg101). The ESCRT-I assembly can then recruit ESCRT-II to the limiting endosomal membrane. ESCRT-II is able to recruit and activate the ESCRT-III machinery (Fig. 2B). ESCRT-II connects the ESCRT-I and ESCRT-III machinery by having one end of the complex, the vacuolar protein sorting-associated protein (Vps) 36 subunit, interact with ESCRT-I and ubiquitin, while the other end (two Vps25 subunits) bind the Vps20 subunit of ESCRT-III [10]. Activation of ESCRT-III results in the formation of Snf7 oligomers that cause budding of the vesicle into the endosome lumen (Fig. 2C); then ALG-2 interacting protein-X (ALIX) is recruited to stabilize ESCRT-III and deubiquitinate the proteins. After budding, the ATPase Vps4 is needed to provide energy to allow for disassembly and recycling of the ESCRT machinery [11–13]. There are other non-ubquitinated/ESCRT pathways including ceramide and lipid rafts. Inhibition of neutral sphingomyelinase (N-Smase2), which is responsible for ceramide production, blocks exosome production[14–16]. Ceramide is essential to create curvature of the endosomal membrane, thus facilitating ILV budding[14] (Fig 2C).
Figure 2.
A) ESCRT-0 binds ubiquitinated cargo and localizes to the MVB membrane. B) Subsequently ESCRT-I and ESCRT-II are recruited to the limiting endosomal membrane. The ends of ESCRT-II are then able to recruit and activate the ESCRT-III machinery. C) Activation of ESCRT-III causes budding of the vesicle into the endosome lumen. The ATPase vacuolar protein sorting-associated protein 4 (Vps4) is needed to provide energy to allow for disassembly and recycling of the ESCRT machinery. In a non-ESCRT related pathway, nSMase2 produces ceramide at the endosomal membrane, which results in membrane curvature and ILV budding.
1.2 Exosome Fate: Release vs. Degradation
While there are multiple routes to MVB biogenesis and exosome release, it is unknown how and if they play a role in the eventual fate of MVBs, potentially regulating the exosome release versus exosome-lysosome fusion leading to degradation of the exosomal content. MVBs are trafficked to the plasma membrane with the help of the Rab GTPase family of proteins[17–19]; Rab5 and Rab7 are common markers of early and late endosomes respectively, while inhibition of Rab27 GTPases has been shown to block exosome secretion [18]. Silencing of Rab27a and Rab27b resulted in inhibition of docking with the cellular membrane and redistribution to the perinuclear space [18]. Rab27 overexpressing tumors show increased invasive properties in metastasis linked to output of exosomes to establish pre-metastatic niches [20–22]. Membrane fusion of the MVB is carried out by interactions of the vesicular soluble N-ethylmaleimide-sensitive factor-attachment protein receptors (SNAREs) with the intracellular target SNAREs allowing for release of exosomes[23, 24].
1.3 Mechanisms of Exosome Uptake
Studies of exosome uptake indicate multiple different mechanisms of uptake, which may be due to both dissimilarities in recipient cells and in exosome composition[25]. A common mechanism of internalization is via endocytosis. Exosome uptake occurs rapidly and in an energy-requiring manner [26–28]. Inhibition of endocytic pathways by actin filament depolymerization, significantly reduces exosome uptake, but does not fully prevent it [29, 30]. Both caveolin-mediated and clathrin-mediated endocytosis are implicated as methods of entry, but inhibition of endocytosis does not fully abrogate exosome internalization [26, 30–33]. As a singular method of exosome uptake has not been agreed upon, blocking such general internalization methods would not be specific or effective therapeutic targets. However exosomes can still interact with cells via receptor-ligand interactions on the exosome and cell membranes. Understanding what receptors are activated by exosomes in specific disease states may indicate specific therapeutic targets.
1.4 Exosomes and Intercellular Signaling
While the mechanism(s) of exosome uptake is still being investigated, there is evidence of exosomes affecting recipient cells as a novel form of intercellular signaling. Exosomes labeled with lipophilic dyes, such as PKH67 or DiI, which stain the exosome membrane, are visible inside recipient cells with fluorescence microscopy[34–36]; uptake in vivo has also been observed after injection of fluorescently labeled exosomes into small animals [37, 38]. Exosomes have also been imaged inside recipient cells by transmission electron microscopy[39–41]. To show that exosome content is released in the receiving cells, exosomes loaded with luciferin triggered bioluminescence in luciferase expressing cells [32]. Study of the signaling activity of transferred exosomes found that mRNA and miRNA carried by exosomes was functional in recipient cells [4, 7, 42]. This transfer of active RNA and protein has been shown to alter the phenotype of the recipient cells[4]. In addition, exosomes are able to carry active receptors between cells in vivo [43] including immune cell activation by antigen presentation and transfer of functional MHC complexes [44, 45]. The sum of these findings provides convincing evidence that exosomes are not only taken up by cells, but their content incorporates into the cell modifying its function and phenotype.
1.5 Exosome Purification
There are a number of methods for exosome isolation with varying strengths and weaknesses (Table 2). The most common approach is through differential ultracentrifugation, in which conditioned cell media or biological fluid is processed through progressively increasing centrifugation steps to remove contaminating cell debris, before finally pelleting the exosomes by ultra-centrifuge. This purification method can be modified by flotation in a sucrose gradient; exosomes have a density range of 1.13–1.19 g/ml[46]. A less time intensive method uses ultrafiltration to concentrate the conditioned media after cell debris have been removed. Size exclusion chromatography is a popular method for purifying exosomes and removing contaminating proteins from biological samples [47, 48]. Protein affinity columns are also effective for capturing exosomes from samples, but may result in a selection bias, as they will only bind vesicles that present the target protein[49]. An alternative to these labor-intensive protocols is the commercially available precipitation reagents that will allow exosomes to be pelleted out of solution using a tabletop centrifuge[50]; however, they will not produce high purity exosomes if the starting samples have high levels of contaminating proteins or cellular debris[49]. For best results, differential ultracentrifugation can be combined with another exosome purification protocol such as size exclusion chromatography or sucrose gradient fractionation. However, for studies looking for a specific exosome cellular population, it may be best to first select for targets by immunoprecipitation.
Table 2.
Exosome Isolation Methods
| Method | Process | Pro | Con |
|---|---|---|---|
| Differential Centrifugation | Centrifugation steps to remove contaminants (500–10,000xg); followed by pelleting of exosomes (100,000xg) Further processing by sucrose density gradient |
Clearance of contaminating debris and large vesicles Effectively pellets exosomes from media |
Labor intensive Non-specific, heterogenous vesicle population Varying pelleting efficiency (k-factor) between protocols |
| Precipitation (e.g. ExoQuick) | Reagent mixed with exosome containing media or serum; forces vesicles out of solution to be pelleted by low speed centrifugation | Quick, only basic equipment needed | Non-specific, increased contamination from lipid/protein/debris Reagent may interfere in downstream protocols |
| Size Exclusion Chromatography | Serum or media added to column containing size exclusion beads; Exosomes collected in early flow throw. Pelleted by ultracentrifugation |
Removes contaminating protein and small molecules Directly apply biological fluid samples |
Labor intensive May require secondary isolation method |
| Affinity Capture | Pull down of exosomes using antibodies that identify specific surface markers | Specific, removes unwanted protein and other vesicles | Requires previous analysis for surface maker Selection bias from total population |
2. Cardiac Exosomes
The understanding of the role of exosomes in the heart and cardiovascular disease has increased, with much of the focus on therapeutic uses. While investigating the effects of extracellular HSP60 (exHSP60) on cardiac myocytes, it was found that the exHSP60 was contained in exosomes released by isolated adult cardiac myocyte’s in culture [51, 52]. Further study observed that the content of cardiac exosomes changed based on the type of cellular stress. Rat cardiac myocytes were treated with either hypoxia/reoxygenation, too mild to cause necrosis, or ethanol at levels seen with the consumption of alcohol. Each of these treatments results in the generation of reactive oxygen species[52]. Interestingly, ethanol exposure caused greater release of exosomes, compared to hypoxia/reoxygenation. Ethanol exposure was transient, as the ethanol would evaporate or be metabolized in the cell culture plate maintained in the incubator, as ethanol was only given at the beginning of the experiment. Intriguingly, inhibiting ROS formation, which required a combination of three different antioxidants, markedly decreased exosome formation [52]. Proteomic analysis of exosomes produced after hypoxia/reoxygenation vs. ethanol (ROS mediated) demonstrated that the protein content differed significantly for the two treatments, as well as from previous studies on different cell types and stressors [52].
2.1 Exosomes in Cardiac Signaling
The effect of exosomes on cardiac cells depends on the source, as well as the stimulus for release, which will influence exosome content (Table 3). In a study on the benefits of exercise on diabetic cardiovascular complications, exosomes isolated from the serum of diabetic mice adapted to exercise showed enhanced expression of miR-29b and -455. Treatment with mimics of these miRNAs decreased expression of matrix metalloprotease 9 (MMP-9), a protein linked to adverse cardiac remodeling in response to stress [53]. Exosomes released during diabetic cardiomyopathy carry miR-320, which downregulates expression of HSP20 [54]. Decreasing levels of heat shock proteins is thought to be a major cause of cellular dysfunction. Using a streptozotocin (STZ) diabetes induced mouse model, it was found that overexpression of HSP20 significantly reduced cardiac dysfunction and triggered greater exosome production; these exosomes carried higher levels of HSP20, survivin, and p-Akt [54]. In vitro treatment of cardiac myocytes and endothelial cells with these exosomes reduced oxidative stress; in vivo treatment decreased adverse cardiac remodeling in the STZ mouse model [54]. Pressure overload in mice led to the release of exosomes containing angiotensin II type I receptor (AT1R). The primary source of AT1R containing exosomes was cardiac myocytes[43]. AT1R enriched exosomes were injected into the tail vein of AT1R-KO mice and were sufficient to confer blood pressure responsiveness to angiotensin II by remote transfer and expression of AT1Rs on endothelial and smooth muscle cells[43]. This elegant study demonstrates that exosomes are capable of transferring biochemically functional cell receptors in between cells.
Table 3.
Summary of Cardiac Exosome Functions
| Cell Type | Model | Content | Results |
|---|---|---|---|
| Cardiac Progenitor Cells | Human | miR-210, 132, 146a, 181[70] | Inhibit apoptosis Promote angiogenesis [70] |
| Hypoxia Rat CPC | Upregulated miR- 292, 210,103, 17, 199a, 20a, 15b[71] | Decreased fibrosis Increased angiogenesis [71] |
|
| Cardiac Myocytes | Type 2 Diabetic Rat Model | Enriched in miR- 320 [80] | Diabetic cardiac myocyte derived exosomes inhibited cardiac endothelial cell proliferation [80] |
| Cardiac pressure overload in Mice | AT1R [43] | Functional transfer of AT1R to endothelial and smooth muscle cells Confer blood pressure responsiveness to Angiotensin II [43] |
|
| Cardiac Fibroblast | Mouse | miR-21* [34] | Cardiac myocyte hypertrophy [34] |
| Cardiosphere Derived Cells | Human | Highly enriched in miR-146a [88] | Pro angiogenic In vivo injection in mouse MI model [88]
|
Somewhat surprisingly circulating plasma exosomes from healthy rats have been reported to be cardioprotective when administered prior to ischemia/reperfusion injury[55]. Exosomes were administered by tail vein injection before LAD occlusion; the resulting infarct size was significantly reduced relative to vehicle[55]. Tracking of the exosomes by microscopy did not show any uptake in primary cardiac myocytes, rather protection appeared to be mediated by a receptor-ligand interaction. Others have reported that inducible HSP70 (HSP72) on the exosome surface can interact with toll-like receptor (TLR4) to activate HSP27, leading to cardioprotection; treatment of exosomes with a neutralizing HSP70 antibody negated the cardioprotection of primary cardiac myocytes from hypoxia-reoxygenation injury[55]. Generally, TLR4 activation is thought to be detrimental in heart disease [56–58]. Investigation of TLR4 in heart failure found increased TLR4 expression in cardiac myocytes, and activation of TLR4 induced a significant production of pro-inflammatory cytokines, thus further study is still needed to determine what aspects of HSP70-TLR4 interaction are resulting in cardioprotection [55, 59–63].
2.2 Exosomes in Ischemic Conditioning
Investigation of remote ischemic conditioning (RIC) found that rats treated with RIC for 4 weeks following myocardial infarction demonstrated a better ejection fraction and less severe LV remodeling, compared to untreated rats[64]. Exosomes isolated from RIC treated rats’ serum had increased levels of miR-29a, a negative regulator of tissue fibrosis; the miR29a expression levels were also significantly higher in the marginal area of infarction after RIC treatment[64]. However, other investigators reported that after RIC miR-144, which is linked to cardioprotection, was significantly increased in the exosome-free plasma associated with Argonaute-2; the exosomal fraction only showed an increase in the miR-144 precursor hairpin[65]. The composition of serum and plasma is complex with a very large range of proteins and other molecules. Careful study is needed to elucidate the exact contribution of exosomes released after RIC or circulating in normal plasma to determine exactly what is delivered as treatment and whether these effects are consistently reproducible.
2.3 Cardiac Progenitor Cell (CPC) Derived Exosomes
Cardiac progenitor cells have been identified as releasing cardioprotective exosomes. CPCs are a small, heterogeneous population of stem-like cells found in the adult heart that are thought to help replenish cardiac myocyte populations, though their specific role in cardiac injury is still being investigated[66–69]. Analysis of conditioned media from human CPCs showed the peak concentration of released extracellular vesicles, using nanoparticle tracking analysis, to be in the size range of exosomes (50–100nm); flow cytometry showed expression of exosome markers CD63, CD9, and CD81[70]. These exosomes contained miRNAs known to inhibit apoptosis and promote angiogenesis[70]. Exosomes isolated from mouse CPC conditioned media were efficiently taken up in vitro by H9C2 cardio myoblasts[36]. The CPC-derived exosomes inhibited caspase 3/7 activation in H9C2 cells in response to oxidative stress with hydrogen peroxide [36]. While increased angiogenesis and apoptosis inhibition may increase the risk of tumor formation, these single treatments at the time of acute injury are primarily focused on preventing tissue damage and cell death. In vivo administration of CPC-derived exosomes in a mouse ischemia-reperfusion injury model resulted in a significant decrease in cardiac myocyte apoptosis relative to control [36]. Hypoxia treated CPCs were found to have upregulation of 11 secreted miRNAs, 7 of which were contained in CPC released exosomes. Injection of the myocardium with CPC exosomes produced after hypoxia resulted in improved cardiac function, and reduced fibrosis in rats in both in vivo and in vitro ischemia-reperfusion models [71]. Analysis of the hypoxic CPC-derived, exosomal miRNAs identified additional miRNA clusters that may be of therapeutic value for future study [71]. Study of exosomes from pediatric cardiac progenitor cells found that donor age and cellular hypoxia altered the efficacy of exosome treatment. Athymic rats were treated with human CPC exosomes after ischemia-reperfusion injury. Twenty-eight days after injury it was observed that exosomes derived from neonatal normoxic and hypoxic CPCs, as well as infant and child hypoxic CPCs, showed significantly improved ejection fraction [72]. However, only rats treated with exosomes from hypoxic CPCs showed a significant decrease in fibrosis and increased angiogenesis [72]. Further study of the role of age in cardiac progenitor cells found that neonatal CPCs (nCPC) had greater in vitro proliferative capacity [73]. When implanted into infarcted myocardium, nCPCs were more effective at recovering cardiac function relative to adult CPCs [73]. Treatment with nCPC total conditioned medium was even more potent than either implanting nCPCs or treatment with nCPC exosomes alone [73].
2.4 Exosomes: The Dark Side
While the study of exosomes and their effect on cardiac function has mostly focused on protection from injury, there is some data suggesting exosomes can have pathological effects during cardiac stress. As discussed, HSP60 is released from cardiac myocytes in exosomes. Analysis of the isolated exosomes indicated that they were remarkably stable under both physiological and pathophysiological conditions[52]. If released from the exosomes, exHSP60 can be detrimental to cardiac myocytes via activation of TLR4[61]; there is evidence of other stress related proteins released in exosomes, but there is still debate about their adverse effects when extracellular [60, 62, 74–77]. Co-culture of adult murine cardiac myocytes with cardiac fibroblasts (CF) resulted in the development of cardiac myocyte hypertrophy[78, 79]. Analysis of CF conditioned media found exosomes enriched in miR-21*[34]; these “star” passenger strands normally undergo degradation within the cell. Further study found that the sorbin and SH3 domain-containing protein 2 (SORBS2) as well as the PDZ and LIM domain 5 (PDLIM5) protein were targets for miR-21* from exosomes. Knockdown of either of these genes leads to cardiac myocyte hypertrophy[34]; these results suggest that the cardiac hypertrophy phenotype induced by cardiac fibroblasts may be due to downregulation of SORBS2 and PDLIM5 by miR-21* carried by exosomes[34]. Moreover, angiotensin II (Ang II) treatment of cardiac fibroblasts results in increased exosome release. Exposure of isolated, neonatal rat cardiac myocytes to the Ang II CF-derived exosomes resulted in activation of the renin angiotensin system, inducing hypertrophy in an autocrine manner[35]. Treatment of adult mouse cardiac fibroblasts with GW4869 and dimethyl amiloride, which inhibit exosome formation, prevented Ang II exosome release and markedly reduced Ang II induced myocardial hypertrophy and cardiac fibrosis[35]. These findings support a role for cardiac fibroblast exosomes in the adverse cardiac remodeling that occurs in response to stress. Exosomes have also been found to have a potential role in other cardiac diseases, including diabetic cardiomyopathy. Co-culture of cardiac endothelial cells and cardiac myocytes derived from type 2 diabetic Goto-kakizaki rats demonstrated inhibition of cardiac endothelial cell proliferation, while co-culture with control cardiac myocytes increased endothelial cell proliferation[80]. Both effects were negated when cardiac myocytes were treated with GW4869, a neutral sphingomyelinase inhibitor known to block exosome production, supporting that these changes are exosome mediated [80]. Additional experiments demonstrated that diabetic cardiac myocytes transferred exosomes enriched in miR-320 to endothelial cells, resulting in an inhibition of endothelial cell proliferation and tube formation; once again, treatment with GW4869 blocked the transfer of miR-320 to endothelial cells[80]. Thus exosomes released by cardiac myocytes appear to impair angiogenesis in diabetes. These varied studies support a pathologic role for exosomes in the progression of chronic systemic diseases, including, but not limited to, diabetes, hypertension and pathologic cardiac remodeling. Further study is needed to elucidate how exosomes released by cardiac myocytes as well as cardiac fibroblasts and endothelial cells in cardiovascular disease states affect the progression of cardiovascular disease.
3. Stem Cell Exosomes in Cardiac Treatment
3.1 MSC and Hematopoietic Stem Cells
While it was hoped that stem cells would regenerate damaged heart tissue, it appears that much of their benefit comes from paracrine factors, particularly exosomes (Table 4) [81, 82]. In a mouse ischemia/reperfusion injury model, it was found that treatment with MSC derived exosomes prior to reperfusion resulted in a significant decrease in infarct size[83]. In vivo treatment of mice with MSC-derived exosomes before reperfusion showed an elevation in bioenergetics and a decrease in oxidative stress; 30 minutes after reperfusion, exosome treated mice had significantly increased ATP/ADP and NADH/NAD+ ratios [84]. Furthermore, there was a marked decrease in local and systemic inflammation 24 hours after exosome treatment [84]. It has also been observed that treatment with mouse embryonic stem cell (ESC) derived exosomes resulted in better cardiac function after myocardial infarction in mice [85]. Treatment with ESC-derived exosomes augmented resident CPCs in the infarcted heart, resulting in increased CPC proliferation and survival [85]. Analysis of the exosome contents found enrichment in the miR290–295 cluster, which are expressed in ESCs and thought to regulate cell cycle transition[85]. After ischemic injury, therapeutic transplantation of human CD34+ stem cells improved cardiac function and increased neovascularization[86]. Investigation of therapeutic paracrine signaling in CD34+ stem cells found that the proangiogenic effects could be attributed to the release of exosomes. In vitro treatment of cultured human umbilical vein endothelial cells (HUVECs) with CD34+ stem cell derived exosomes resulted in increased tubule formation; treatment with exosome depleted CD34+ stem cell conditioned media had no significant effect on the HUVECs [87]. In vivo, CD34+ exosomes in a Matrigel-plug were subcutaneously injected into mice and induced endothelial vessel growth in the plug [87]. Moreover, implantation of CD34+ exosome in the cornea of mice caused significantly greater vessel growth relative to treatment with CD34+ depleted mononuclear cell exosomes[87]. The specific angiogenic signals carried by exosomes are still being defined. Work to date suggests that stem cell derived exosomes have therapeutic potential for treating cardiovascular disease. This actually is advantageous, as purification, storage and delivery of exosomes can be much simpler than the preparation and delivery of stem cells. Furthermore, exosomes’ content can potentially be manipulated after isolation before use in treatment.
Table 4.
Summary of Therapeutic Exosome Studies
| Cells/Treatment | Model | Result |
|---|---|---|
| Mesenchymal Stem Cells | Mouse Ischemia-Reperfusion | Elevated bioenergetics Decreased oxidative stress Decreased inflammation [83, 84] |
| CD34+ stem cells | Treatment of HUVECs | Increased tubule formation [87] |
| In vivo implantation in mouse cornea | Induced endothelial vessel growth [87] | |
| Embryonic Stem Cells | Mouse AMI | Increase survival of resident CPCs Enhanced neovascularization Cardiac myocyte survival Reduced fibrosis [85] |
| Cardiosphere Derived Cells | Mouse MI | Decreased scar mass Increased viable mass Lower pro-inflammatory cytokines [88] |
| Pig MI | Decreased infarct size Improved LVEF Decreased fibrosis and hypertrophy [91] |
|
| Remote Ischemic Conditioning | Rat MI | Increased ejection fraction Lesser LV remodeling [64, 65] |
3.2 Cardiosphere Derived Exosomes
Investigation of cardiosphere-derived cells (CDCs) has also linked their regenerative properties to the release of exosomes. Conditioned media from CDCs was enriched with RNA, the majority of which was contained within exosomes [88]. Co-culture of human CDCs with HUVECS was found to have proangiogenic effects that were not observed when exosome production was disrupted by nSmase2 knockdown in CDCs [89]. Injection of CDC derived exosomes in an acute MI mouse model mimicked the same benefits seen with injection of CDCs themselves [88]; the exosome treated hearts showed decreased scar mass and increased viable mass, as well as lower levels of pro-inflammatory cytokines, which may be a result of previously reported reduction in inflammatory macrophages by CDCs [90]. Injection of exosomes 21 days after MI, when scar formation would already have progressed, showed regenerative possibilities, as the hearts had decreased scar mass with increased viable mass, and increased ejection fraction after this treatment [88]. Benefit from such delayed treatment raises the possibility of improving cardiac function in patients after infarction has already occurred, which is important, as cardiac injury in humans is not infrequently diagnosed after the critical window for reperfusion has passed. Analysis of CDC derived exosomes found that they were enriched in miR-146a [70, 88]; in addition, exosome treated cells also showed enrichment in miR-146a [88]. Knockout of miR-146a in mice results in impaired heart function, while exosomes deficient in miR-146a conferred less protection from oxidant stress; analysis of hearts treated with miR-146a showed a reduction in inflammatory cytokines consistent with blunted TLR signaling, as well as suppression of NOX4 and SMAD4, which is a key mediator of the TGF-Beta pathway [88]. MiR-146a alone was able to reproduce some of the benefits of CDC-derived exosomes in a chronic MI mouse model, such as increased viable mass, but did not show decreased scar mass or significant functional benefits [88].
3.3 CDC Exosomes in Large Animal MI Models
The efficacy of CDC derived exosomes was investigated in a large animal acute myocardial infarction (AMI) model. Human CDC exosomes were isolated from conditioned CDC culture media and delivered by intra-myocardial injection into the LV wall 30 minutes after coronary artery reperfusion in a pig model of MI [91]. Intra-myocardial delivery of exosomes decreased infarct size and improved left ventricular ejection fraction (LVEF); importantly, intra-myocardial exosome delivery had much greater myocardial retention compared to intracoronary delivery [91]. While intracoronary and intravenous delivery of exosomes is effective in small animals such as mice and rats, it did not work in this larger animal model. Further basic studies of intravenous or intracoronary delivery are necessary to optimize exosome delivery for clinical application; clinical studies using CDCs have seen effective intracoronary delivery, but there are substantial differences between CDCs and exosomes [92]. Increased animal size may require significantly increased exosome dosage and/or greater time for exosome circulation and uptake. Uptake of exosomes from the coronaries, which have rapid passage of blood, would be expected to be less efficient than intra-myocardial injection. Cardiac histology showed decreased fibrosis and decreased cardiomyocyte hypertrophy. The presence of allogenic exosomes did not appear to promote a significant inflammatory response, but exosome treated animals did have higher levels of allo-antibodies [91].
The ability of CDCs to provide lasting therapeutic benefit may be due in part to the ability of CDC exosomes to alter the bioactivity of surrounding tissue [88, 93, 94]. There is evidence that fibroblasts treated with CDC exosomes switch to secreting therapeutic paracrine factors. CDC derived exosomes dramatically changed fibroblast’s secretome and miRNA profile, including in the subsequently secreted fibroblast exosomes [94]. Intra-myocardial injection of exosome-primed fibroblasts in a rat MI model resulted in a significant angiogenic effect and reduction in scar mass, relative to injection with unprimed fibroblasts [94]. Similarly, pre-treatment of cardiac stem cells (CSCs) with MSC derived exosomes resulted in an altered miRNA expression profile, and transplantation of MSC exosome primed CSCs resulted in a better cardiac outcome [67]. In an acute MI rat model, 28 days after MI, the group treated with exosome-primed CSCs had significantly reduced ventricular fibrosis and a higher ejection fraction relative to control groups, as well as those treated with standard CSCs [67]. Fluorescence microscopy demonstrated that exosome primed CSCs had a larger number of engrafted cells that co-localized with capillaries; overall, the animals treated with exosome primed CSCs had a greater density of capillaries and arterioles relative to those treated with control CSCs [67]. As not all neovascularization was associated with engrafted CSCs, it is still likely that much of the therapeutic benefits of CSCs come from paracrine factors rather than CSC differentiation. This conversion of bioactivity may indicate an approach by which exosomes’ therapeutic effect can be amplified, and helps explain why stem cell derived exosomes have persisting benefits after treatment.
4. Exosomes in other Disease States
Outside of the therapeutic focus on exosomes, there is mounting evidence that exosomes from tumors can alter the microenvironment to promote tumor growth and malignancy. In some of the very first work on exosomes and disease, Skog found that glioblastoma exosomes were carrying active RNA and proangiogenic proteins [4]. Uptake of glioblastoma exosomes altered the phenotype of the recipient normal neural cell to a more cancerous phenotype. When added to a glioma cell line, glioblastoma exosomes also significantly stimulated cellular proliferation [4]. Uptake of glioblastoma exosomes promoted a pro-angiogenic phenotype in normal brain endothelial cells [4]. Exosomes from melanoma cells cause a similar increase in angiogenic signals [95, 96]. In addition to glioblastoma, exosomes from other solid tissue cancers also have the ability to promote cancerous growth and metastases [97–99]. Prostate cancer derived exosomes traffic oncogenic proteins and miRNAs to induce neoplastic transformation of adipose-derived stem cells [100]. Likewise breast cancer cell-derived exosomes contained RISC-associated pre-miRNAs that altered the transcriptome and promoted tumorigenesis in endothelial cells [101]. In other work, tumor-derived exosomes transferred multi-drug resistance associated proteins and miRNAs, which in vivo would increase resistance to chemotherapy [102]. Exosomes contribute to drug efflux by exporting drugs from tumor cells [103]; in addition, they may interfere with therapeutic antibodies, as the epitopes on the exosomes can bind the therapeutic antibody [104, 105]. Exosomes have also been identified as a method of cell-to-cell transmission in certain neurogenerative diseases [106] and prion diseases [107].
4.1 Exosomes in the Immune Responses
Immune cell derived exosomes have been shown to aid in antigen presentation and immune cell regulation. B-cell and dendritic cell exosomes display functional MHC-I and II. Exosomes secreted by mature dendritic cells can transfer MHC-I protein complexes to neighboring dendritic cells to amplify the immune response and prime T lymphocytes [44,45]. However exosomes from immature dendritic cells can have an immunosuppressive effect. A combination of rapamycin and immature dendritic cell-derived exosomes was found to induce tolerance of cardiac allograft in mice [108]. Furthermore, in rats donor-derived exosomes combined with short-term treatment by an immunosuppressive agent induced donor-specific cardiac allograft tolerance and a significant delay in rejection [109]. Thus, exosomes modulate the immune response, which has the potential to be applied therapeutically.
5. Exosomes As Biomarkers
Exosomes are found in almost all bodily fluids including blood, urine and saliva, making them readily available for investigation [110–114]. While exosomes are often initially identified by their size profile, there are a number of proteins commonly enriched in exosomes to distinguish them from the overlapping extracellular vesicles (Table 1). These proteins include tetraspanins, such as CD63 and CD81, as well as heat shock proteins, including heat shock cognate 70 (HSC70) and heat shock protein 90 (HSP90). While these may be common markers, one important aspect of exosomes is that their internal cargo differs depending on the cell type of origin and the stimulus for exosome production [52, 115]. Along with their complement of proteins, exosome populations are enriched with certain nucleic acids [4, 42, 116]. Analysis of exosome miRNA showed that certain motifs were over represented, and sumoylated heterogeneous nuclear ribonucleoproteins (hnRNPs) recognized these 3′ motifs to control loading of miRNA into exosomes [117]. While miRNA are likely to have the greatest impact on recipient cells, exosomes carry a range of other RNA species. Sequencing of the RNA content of circulating exosomes found significant amounts of mRNA, tRNA, rRNA as well as lesser amounts of piRNA and snoRNA [118, 119].
Table 1.
Shedding Vesicle Characteristics
| Particle | Size (nm) | Source | Common markers |
|---|---|---|---|
| Exosome | 30–100 | Internal budding of multivesicular bodies; released by fusion of MVB with cell membrane | Tetraspanins (CD63, CD81, CD9) TGS 101 Alix HSP 70 |
| Shedding Microvesicle | 100–1000 | Budding from cellular membrane | CD40 Selectin Intergrin |
| Apoptotic body | 1000–5000 | Blebbing from apoptotic cell membrane | Fragmented DNA Annexin V |
5.1 Exosomes as Biomarkers and Prognostic Markers in Cardiovascular Disease
As exosomal content appears to be selectively packaged with protein and nucleotides that reflect the source cell’s status, this makes it possible to use exosomes as biomarkers for pathophysiological conditions. Cardiovascular disease is the number one cause of death in the United States. Those that survive an acute coronary syndrome (ACS) are then at an increased risk of future cardiovascular events. The standard biomarker for an ACS diagnosis is an elevated level of high sensitivity cardiac troponin in circulation. Initial studies found increased levels of miR-1,-499, and -21 in the plasma of ACS patients with negative troponin levels or with symptom onset less than 3 hours, and had increased diagnostic value when combined with troponin levels[120]; however, the use of circulating miRNA biomarkers is bottlenecked by the time required for their quantification by qPCR. Analysis of patients with stable coronary artery disease found that an increase in miRNA-126 and miRNA-199a in circulating microvesicles was associated with a reduced risk for future major adverse cardiovascular events [121]. Serum collected 2 to 3 weeks after acute myocardial infarction had increased p-53 responsive miRNAs (miR-194, miR34a), predominantly in exosomes; patients with these increased miRNA levels were more likely to later develop ischemic heart failure [122]. This analysis was done on serum samples frozen for over a year and then thawed, which can potentially rupture lipid vesicles, so we cannot be certain that the miRNA was within exosomes; nonetheless, the findings are important with regard to early identification of patients likely to develop heart failure and also possible contributing mechanisms. Exosomes isolated from the plasma of patients with carotid atherosclerosis contained exosomes that were enriched in Galectin-3 relative to control patients; furthermore, Galectin-3 concentrations above the median in patients with peripheral artery disease were associated with an increased risk of cardiovascular mortality [123]. While more investigation is necessary, analysis of the content of peripheral blood exosomes provides a potentially promising assay for predicting the course of disease and the risk of developing heart failure.
6. Future Directions
Exosomes show great promise for providing new insights into the pathophysiology of signaling in cardiovascular disease progression, as well as for application in diagnosis and therapeutics. As mechanisms of exosome packaging are better understood, it has become possible to effectively engineer exosomes from cells for therapeutic purposes. There are currently phase I trials using autologous dendritic cell-derived exosomes that have been loaded with tumor antigens in the hopes of eliciting an immune response to inhibit tumor growth[124, 125]. There is also the possibility of creating artificial exosomes in which synthetic liposomes are combined with natural exosome components to act as delivery mechanisms for therapeutics [126].
Exosomes are an important component of stem cell paracrine signaling and are a focal point of stem cell-based therapies. Although stem cells fail to implant and regenerate damaged heart tissue, as originally hoped, they may be effective as factories for the production of cell-free therapeutic treatments, including the emergency treatment of acute MI. Already it has been reported that modification of CD34+ stem cells with angiogenic sonic hedgehog (SHH), which is packaged in exosomes, increases their therapeutic potential[127]. MSCs overexpressing GATA4 have been shown to deliver anti-apoptotic miRNAs and promote cardiac functional recovery [128]. Thus, genetically modified stem cells could be used to generate therapeutic exosomes.
Key hurdles remain in the investigation of exosomes. Researchers need to agree upon standards for exosomes isolation and analysis, as the differing methods may have effects on downstream applications [49]. Moreover the selection of extracellular vesicles that are isolated can ‘muddy the waters’ if there isn’t a strict definition kept between each extracellular vesicle sub-population (size, protein markers). Shedding vesicles have a wide size range (20–1000 nM) and overlap with many different vesicle types. The more general isolation methods such as precipitation will pull down contaminating proteins, including albumin and gamma globulins, and particles, such as apoptotic bodies with exosomes, while increased specificity could limit the population of exosomes that is analyzed. Many of the proteins commonly used to characterize exosomes are not exosome-specific but rather those that are commonly seen to be enriched in exosome isolations; thus, multiple markers should be identified with their relative proportion, or enrichment compared to their source cells [129]. Furthermore, when studying the functional activity of exosomes, stringent negative controls should be put in place to remove background interference [129]. Optimization and standardization will allow for effective definition of exosomes in research and clinical use and progress in the field.
The use of exosomes as biomarkers is still in the early stages of study, but holds promise. Exosome profiles for different disease states such as myocardial injury and tumor growth still need further investigation to establish them as effective diagnostic tools; with circulating miRNAs being the leading candidate. Databases such as ExoCarta (www.exocarta.org), Vesiclepedia (www.microvesicles.org), and EVpedia (www.evpedia.info) are repositories for thousands of proteins, mRNAs, and miRNAs that have been identified in exosomes from different species and tissues by different labs. Their effectiveness as early diagnostic markers would need to be compared to the standard clinical biomarkers, and speed of generating results and cost would be key factors as to whether they can be applied in the clinical setting.
Exosome biology holds great promise in understanding a novel method of intercellular signaling in both disease progression and therapeutic treatment. Exosomes from damaged cardiac cells, as well as tumors, can have deleterious effects on the surrounding tissue by the transfer of functional proteins and RNA. On the other hand, exosomes are part of effective therapeutic paracrine signaling responsible for the benefits of stem cell treatments. Exosome treatments show great benefits in therapeutic treatment of cardiomyopathy. However, the field is still developing and requires further study and stringent guidelines to ensure proper understanding of the effects mediated by exosomes. Overcoming these issues will allow for greater control of exosome production and signaling, hopefully leading to breakthroughs in treatment. Future studies will continue to drive forward in developing exosome therapeutics for the treatment of heart disease as well as other diseases.
Highlights.
Exosomes transfer active nucleic acids and proteins between cells and can alter cellular phenotypes
Therapeutic benefits of stem cells in cardiac treatment mediated by exosomes
Exosomes are promising as prognostic markers in cardiovascular disease
Exosomes can be potentially manipulated to deliver targeted cargo to cells
Acknowledgments
Funding
This work was supported by HL077281 (AAK), HL079071(AAK), and a VA Merit Award all to A. A. Knowlton.
Abbreviations
- CDC
Cardiosphere-derived cell
- CF
Cardiac fibroblast
- CPC
Cardiac progenitor cell
- CSC
Cardiac stem cell
- ESC
Embryonic stem cell
- ESCRT
Endosomal sorting complexes required for transport
- HSP
Heat shock protein
- ILV
Intraluminal vesicle
- MHC
Major histocompatibility complex
- miR
microRNA
- MSC
Mesenchymal stem cell
- MVB
Multivesicular body
- N-Smase2
Neutral sphingomyelinase2
- RIC
Remote ischemic conditioning
- ROS
Reactive oxygen species
- Vps
Vacuolar protein sorting-associated protein
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942–8. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329–39. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140(1):13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 4.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meckes DG, Raab-Traub N. Microvesicles and Viral Infection. Journal of Virology. 2011;85(24):12844–12854. doi: 10.1128/JVI.05853-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pegtel DM, van de Garde MDB, Middeldorp JM. Viral miRNAs exploiting the endosomal-exosomal pathway for intercellular cross-talk and immune evasion. Biochimica Et Biophysica Acta-Gene Regulatory Mechanisms. 2011;1809(11–12):715–721. doi: 10.1016/j.bbagrm.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–7. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 10.Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–62. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 11.Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. Journal of Cell Science. 2013;126(24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 13.Henne WM, Buchkovich NJ, Emr SD. The ESCRT Pathway. Developmental Cell. 2011;21(1):77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 15.Guo BB, Bellingham SA, Hill AF. The Neutral Sphingomyelinase Pathway Regulates Packaging of the Prion Protein into Exosomes. Journal of Biological Chemistry. 2015;290(6):3455–3467. doi: 10.1074/jbc.M114.605253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature Communications. 2011:2. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–32. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. sup pp 1–13. [DOI] [PubMed] [Google Scholar]
- 19.Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115(Pt 12):2505–15. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 20.Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, Lambert J, Van Belle S, Cocquyt V, Gespach C, Bracke M, Seabra MC, Gahl WA, De Wever O, Westbroek W. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J Natl Cancer Inst. 2010;102(12):866–80. doi: 10.1093/jnci/djq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, Hendrix A, Lamy P, Dagnaes-Hansen F, Rasmussen MH, Bui KH, Fristrup N, Christensen EI, Nordentoft I, Morth JP, et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014;74(20):5758–71. doi: 10.1158/0008-5472.CAN-13-3512. [DOI] [PubMed] [Google Scholar]
- 22.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Thery C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–30. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 23.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–43. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 24.Fader CM, Sanchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochimica Et Biophysica Acta-Molecular Cell Research. 2009;1793(12):1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular Internalization of Exosomes Occurs Through Phagocytosis. Traffic. 2010;11(5):675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 27.Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. Bmc Cancer. 2011:11. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronquist KG, Sanchez C, Dubois L, Chioureas D, Fonseca P, Larsson A, Ullen A, Yachnin J, Ronquist G, Panaretakis T. Energy-requiring uptake of prostasomes and PC3 cell-derived exosomes into non-malignant and malignant cells. Journal of Extracellular Vesicles. 2016:5. doi: 10.3402/jev.v5.29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan MLG, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang ZL, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barres C, Blanc L, Bette-Bobillo P, Andre S, Mamoun R, Gabius HJ, Vidal M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115(3):696–705. doi: 10.1182/blood-2009-07-231449. [DOI] [PubMed] [Google Scholar]
- 31.Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes Derived from Epstein-Barr Virus-Infected Cells Are Internalized via Caveola-Dependent Endocytosis and Promote Phenotypic Modulation in Target Cells. Journal of Virology. 2013;87(18):10334–10347. doi: 10.1128/JVI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK, Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124(Pt 3):447–58. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 34.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124(5):2136–46. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol. 2015;89(Pt B):268–79. doi: 10.1016/j.yjmcc.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431(3):566–71. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Vos KE, Abels ER, Zhang X, Lai C, Carrizosa E, Oakley D, Prabhakar S, Mardini O, Crommentuijn MH, Skog J, Krichevsky AM, Stemmer-Rachamimov A, Mempel TR, El Khoury J, Hickman SE, et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro Oncol. 2016;18(1):58–69. doi: 10.1093/neuonc/nov244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Gao W, Yuan J, Wu C, Yao K, Zhang L, Ma L, Zhu J, Zou Y, Ge J. Exosomes derived from dendritic cells improve cardiac function via activation of CD4(+) T lymphocytes after myocardial infarction. J Mol Cell Cardiol. 2016;91:123–33. doi: 10.1016/j.yjmcc.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 39.Barile L, Gherghiceanu M, Popescu LM, Moccetti T, Vassalli G. Ultrastructural evidence of exosome secretion by progenitor cells in adult mouse myocardium and adult human cardiospheres. J Biomed Biotechnol. 2012;2012:354605. doi: 10.1155/2012/354605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Morgelin M, Belting M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288(24):17713–24. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, Genoud C, Martin K, Pizzato N, Voshol J, Morrissey DV, Andaloussi SE, Wood MJ, Meisner-Kober NC. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213(2):173–84. doi: 10.1083/jcb.201506084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waldenstrom A, Genneback N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012;7(4):e34653. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating Exosomes Induced by Cardiac Pressure Overload Contain Functional Angiotensin II Type 1 Receptors. Circulation. 2015;131(24):2120–30. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, Tursz T, Amigorena S, Angevin E, Zitvogel L. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172(4):2126–36. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 45.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156–62. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 46.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 47.Boing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malik ZA, Liu TT, Knowlton AA. Cardiac Myocyte Exosome Isolation. Methods Mol Biol. 2016;1448:237–48. doi: 10.1007/978-1-4939-3753-0_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 50.Taylor DD, Zacharias W, Gercel-Taylor C. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol Biol. 2011;728:235–46. doi: 10.1007/978-1-61779-068-3_15. [DOI] [PubMed] [Google Scholar]
- 51.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292(6):H3052–6. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 52.Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, Knowlton AA. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol. 2013;304(7):H954–65. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaturvedi P, Kalani A, Medina I, Familtseva A, Tyagi SC. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J Cell Mol Med. 2015;19(9):2153–61. doi: 10.1111/jcmm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Gu H, Huang W, Peng J, Li Y, Yang L, Qin D, Essandoh K, Wang Y, Peng T, Fan GC. Hsp20-Mediated Activation of Exosome Biogenesis in Cardiomyocytes Improves Cardiac Function and Angiogenesis in Diabetic Mice. Diabetes. 2016;65(10):3111–28. doi: 10.2337/db15-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65(15):1525–36. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 56.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109(6):784–9. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 57.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg. 2004;128(2):170–9. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 58.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114(1 Suppl):I270–4. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 59.Liu L, Wang Y, Cao ZY, Wang MM, Liu XM, Gao T, Hu QK, Yuan WJ, Lin L. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J Cell Mol Med. 2015;19(12):2728–40. doi: 10.1111/jcmm.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res. 2009;105(12):1186–95. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heiserman JP, Chen L, Kim BS, Kim SC, Tran AL, Siebenborn N, Knowlton AA. TLR4 mutation and HSP60-induced cell death in adult mouse cardiac myocytes. Cell Stress Chaperones. 2015;20(3):527–35. doi: 10.1007/s12192-015-0577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian J, Guo X, Liu XM, Liu L, Weng QF, Dong SJ, Knowlton AA, Yuan WJ, Lin L. Extracellular HSP60 induces inflammation through activating and up-regulating TLRs in cardiomyocytes. Cardiovasc Res. 2013;98(3):391–401. doi: 10.1093/cvr/cvt047. [DOI] [PubMed] [Google Scholar]
- 63.Kim SC, Ghanem A, Stapel H, Tiemann K, Knuefermann P, Hoeft A, Meyer R, Grohe C, Knowlton AA, Baumgarten G. Toll-like receptor 4 deficiency: smaller infarcts, but no gain in function. BMC Physiol. 2007;7:5. doi: 10.1186/1472-6793-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, Tanaka M, Osada-Oka M, Shimada K, Miura K, Yoshiyama M, Iwao H. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol. 2015;178:239–46. doi: 10.1016/j.ijcard.2014.10.144. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, Wei C, Hu PZ, Kharbanda RK, Redington AN. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Research in Cardiology. 2014;109(5) doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 66.Le T, Chong J. Cardiac progenitor cells for heart repair. Cell Death Discov. 2016;2:16052. doi: 10.1038/cddiscovery.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. J Am Heart Assoc. 2016;5(1) doi: 10.1161/JAHA.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128(2):122–31. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 70.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103(4):530–41. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 71.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116(2):255–63. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agarwal U, George A, Bhutani S, Ghosh-Choudhary S, Maxwell JT, Brown ME, Mehta Y, Platt MO, Liang Y, Sahoo S, Davis ME. Experimental, Systems, and Computational Approaches to Understanding the MicroRNA-Mediated Reparative Potential of Cardiac Progenitor Cell-Derived Exosomes From Pediatric Patients. Circ Res. 2017;120(4):701–712. doi: 10.1161/CIRCRESAHA.116.309935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma S, Mishra R, Bigham GE, Wehman B, Khan MM, Xu H, Saha P, Goo YA, Datla SR, Chen L, Tulapurkar ME, Taylor BS, Yang P, Karathanasis S, Goodlett DR, et al. A Deep Proteome Analysis Identifies the Complete Secretome as the Functional Unit of Human Cardiac Progenitor Cells. Circ Res. 2017;120(5):816–834. doi: 10.1161/CIRCRESAHA.116.309782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu X, Deng L, Wang D, Li N, Chen X, Cheng X, Yuan J, Gao X, Liao M, Wang M, Liao Y. Mechanism of TNF-alpha autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1alpha, presented by exosomes. J Mol Cell Cardiol. 2012;53(6):848–57. doi: 10.1016/j.yjmcc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277(17):15028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 76.Wachstein J, Tischer S, Figueiredo C, Limbourg A, Falk C, Immenschuh S, Blasczyk R, Eiz-Vesper B. HSP70 enhances immunosuppressive function of CD4(+)CD25(+)FoxP3(+) T regulatory cells and cytotoxicity in CD4(+)CD25(−) T cells. PLoS One. 2012;7(12):e51747. doi: 10.1371/journal.pone.0051747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghosh AK, Sinha D, Mukherjee S, Biswas R, Biswas T. LPS stimulates and Hsp70 down-regulates TLR4 to orchestrate differential cytokine response of culture-differentiated innate memory CD8(+) T cells. Cytokine. 2015;73(1):44–52. doi: 10.1016/j.cyto.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 78.Fredj S, Bescond J, Louault C, Potreau D. Interactions between cardiac cells enhance cardiomyocyte hypertrophy and increase fibroblast proliferation. J Cell Physiol. 2005;202(3):891–9. doi: 10.1002/jcp.20197. [DOI] [PubMed] [Google Scholar]
- 79.LaFramboise WA, Scalise D, Stoodley P, Graner SR, Guthrie RD, Magovern JA, Becich MJ. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am J Physiol Cell Physiol. 2007;292(5):C1799–808. doi: 10.1152/ajpcell.00166.2006. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–50. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10(3):244–58. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallina C, Turinetto V, Giachino C. A New Paradigm in Cardiac Regeneration: The Mesenchymal Stem Cell Secretome. Stem Cells Int. 2015;2015:765846. doi: 10.1155/2015/765846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–12. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 85.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117(1):52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114(20):2163–9. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 87.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109(7):724–8. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2(5):606–19. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lang JK, Young RF, Ashraf H, Canty JM. Inhibiting Extracellular Vesicle Release from Human Cardiosphere Derived Cells with Lentiviral Knockdown of nSMase2 Differentially Effects Proliferation and Apoptosis in Cardiomyocytes, Fibroblasts and Endothelial Cells In Vitro. Plos One. 2016;11(11) doi: 10.1371/journal.pone.0165926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marban E. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 2015;125(8):3147–62. doi: 10.1172/JCI81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marban L, Ghaleh B, Marban E. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125(1):100–12. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tseliou E, Fouad J, Reich H, Slipczuk L, de Couto G, Aminzadeh M, Middleton R, Valle J, Weixin L, Marban E. Fibroblasts Rendered Antifibrotic, Antiapoptotic, and Angiogenic by Priming With Cardiosphere-Derived Extracellular Membrane Vesicles. J Am Coll Cardiol. 2015;66(6):599–611. doi: 10.1016/j.jacc.2015.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71(11):3792–801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 96.Hood JL, Pan H, Lanza GM, Wickline SA I Consortium for Translational Research in Advanced, and Nanomedicine. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89(11):1317–28. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Atay S, Banskota S, Crow J, Sethi G, Rink L, Godwin AK. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A. 2014;111(2):711–6. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clayton A. Cancer cells use exosomes as tools to manipulate immunity and the microenvironment. Oncoimmunology. 2012;1(1):78–80. doi: 10.4161/onci.1.1.17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, Fang Z, Rezk BM, Moparty K, Sikka SC, Sartor O, Abdel-Mageed AB. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32(4):983–97. doi: 10.1002/stem.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–21. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O’Driscoll L. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7(12):e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4(10):1595–604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 104.Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, Weinhage T, Menck K, Hupfeld T, Koch R, Trumper L, Wulf GG. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della Mina P, Menard S, Filipazzi P, Rivoltini L, Tagliabue E, Pupa SM. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. Journal of Cellular Physiology. 2012;227(2):658–667. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- 106.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103(30):11172–7. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101(26):9683–8. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li X, Li JJ, Yang JY, Wang DS, Zhao W, Song WJ, Li WM, Wang JF, Han W, Zhang ZC, Yu Y, Cao DY, Dou KF. Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PLoS One. 2012;7(8):e44045. doi: 10.1371/journal.pone.0044045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peche H, Renaudin K, Beriou G, Merieau E, Amigorena S, Cuturi MC. Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am J Transplant. 2006;6(7):1541–50. doi: 10.1111/j.1600-6143.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 110.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–87. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 111.Lasser C, Alikhani VS, Ekstrom K, Eldh M, Paredes PT, Bossios A, Sjostrand M, Gabrielsson S, Lotvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–78. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 113.Cheruvanky A, Zhou H, Pisitkun T, Kopp JB, Knepper MA, Yuen PS, Star RA. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Renal Physiol. 2007;292(5):F1657–61. doi: 10.1152/ajprenal.00434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Keller S, Ridinger J, Rupp AK, Janssen JWG, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. Journal of Translational Medicine. 2011:9. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 116.Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9(21):4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 117.Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sanchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, Kohli M, Thibodeau SN, Boardman L, Wang L. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li M, Zeringer E, Barta T, Schageman J, Cheng A, Vlassov AV. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc Lond B Biol Sci. 2014;369(1652) doi: 10.1098/rstb.2013.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oerlemans MI, Mosterd A, Dekker MS, de Vrey EA, van Mil A, Pasterkamp G, Doevendans PA, Hoes AW, Sluijter JP. Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol Med. 2012;4(11):1176–85. doi: 10.1002/emmm.201201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, Schmitz T, Dolf A, Endl E, Franklin BS, Sinning JM, Vasa-Nicotera M, Nickenig G, Werner N. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc. 2014;3(6):e001249. doi: 10.1161/JAHA.114.001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, Kitamura T, Hamasaki T, Nanto S, Kawahara Y, Komuro I. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res. 2013;113(3):322–6. doi: 10.1161/CIRCRESAHA.113.301209. [DOI] [PubMed] [Google Scholar]
- 123.Madrigal-Matute J, Lindholt JS, Fernandez-Garcia CE, Benito-Martin A, Burillo E, Zalba G, Beloqui O, Llamas-Granda P, Ortiz A, Egido J, Blanco-Colio LM, Martin-Ventura JL. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J Am Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.114.000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3(1):10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3(1):9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525–41. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, Kamide CE, Liu T, Gupta R, Sahoo S, Misener S, Kishore R, Losordo DW. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012;111(3):312–21. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349–60. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]