Abstract
Phenolic compounds represent a class of environmental chemicals with potentially endocrine-disrupting capabilities. We investigated longitudinal associations between childhood exposure to phenols, from both manmade and natural sources, and subsequent measures of adiposity among girls enrolled in the Breast Cancer and the Environment Research Program between 2004 and 2007. Baseline (ages 6–8 years) urinary concentrations were obtained for creatinine and phenol metabolites: enterolactone, genistein, daidzein, benzophenone-3, bisphenol A, the sum of parabens (methyl, ethyl, and propyl parabens), 2,5-dichlorophenol, and triclosan. Body mass index (weight (kg)/height (m)2), waist circumference, and percent body fat were measured at annual or semiannual examinations through 2015 (n = 1,017). Linear mixed-effects regression was used to estimate how baseline concentrations of phenols (tertile groups) were related to changes in girls’ adiposity measurements from ages 7 through 15 years. Enterolactone was inversely associated with body mass index, waist circumference, and percent body fat, while 2,5-dichlorophenol was positively associated with these measurements. A nonmonotonic association was observed for triclosan and girls’ adiposity; however, it was due to effect modification by baseline overweight status. Triclosan was positively associated with adiposity only among overweight girls. These results suggest that exposure to specific phenols during childhood may influence adiposity through adolescence.
Keywords: adiposity, child, longitudinal studies, phenol
Phenolic compounds represent a class of environmental chemicals with similar chemical structures that may have endocrine-disrupting capabilities (1). They are found in manmade sources, such as pesticides and personal care products, as well as natural sources, such as fruits and vegetables. A wide range of biological activities have been identified for phenols, with both adverse and beneficial associations being reported in relation to metabolic health-related outcomes, including obesity (2–5). For example, 2,5-dichlorophenol is a metabolite of 1,4-dichlorobenzene, a widely used chemical found in pesticides, mothballs, and deodorizers. In cross-sectional studies of children, urinary concentrations of 2,5-dichlorophenol are positively associated with obesity (6, 7). Bisphenol A (BPA) is a chemical used in the manufacturing of polycarbonate plastic and epoxy resins, found in plastic food packaging and the linings of metal cans, respectively. There is evidence to support potentially obesogenic actions of BPA in rodents (8), but findings in humans have been inconsistent (9). In contrast, enterolactone, genistein, and daidzein are phytoestrogens obtained naturally from dietary sources. Enterolactone is a lignan derived from grains and vegetables, while genistein and daidzein are isoflavones derived mainly from soybeans and other legumes. Numerous health benefits are reported for phytoestrogens, including a decreased risk of obesity (5, 10).
There has been limited study of the associations between exposure to phenols during childhood and adiposity in children, with the majority of evidence coming from cross-sectional studies. With the exception of BPA, there have been no longitudinal studies examining childhood exposures to these chemicals or their precursors. In a previous longitudinal study (11), we observed a positive association between exposure to low-molecular-weight phthalates, some of which are also endocrine-disrupting chemicals, and body mass index (BMI) and waist circumference during childhood. Though specific periods during which children are most susceptible to chemical exposures are unknown, late childhood/early adolescence may be a stage of particular vulnerability because of the intense developmental changes (i.e., hormonal and body composition changes) associated with it. Our objective in the current study was to investigate associations between urinary concentrations of 10 phenol biomarkers in elementary-school-aged girls and subsequent changes in adiposity measurements during an approximate 8-year follow-up period.
METHODS
Data were collected as part of the puberty cohort studies of the Breast Cancer and Environment Research Program, funded by the National Institute of Environmental Health Sciences and the National Cancer Institute. Data collection occurred at 3 sites: 1) Icahn School of Medicine at Mount Sinai (Mount Sinai), with recruitment in the East Harlem neighborhood of New York, New York; 2) Cincinnati Children's Hospital/University of Cincinnati (Cincinnati), with recruitment from schools in the Cincinnati, Ohio, metropolitan area and the Breast Cancer Registry of Greater Cincinnati; and 3) Kaiser Permanente Northern California (Kaiser), with recruitment in the San Francisco Bay Area (San Francisco, California). Eligibility included age (6–8 years), female sex, and no underlying endocrine medical conditions; additionally, at Mount Sinai, only girls of black or Hispanic race/ethnicity were eligible. The study and recruitment process are described elsewhere (12). The study protocol was approved by the institutional review board at each site and the Centers for Disease Control and Prevention. A total of 1,239 girls aged 6–8 years were enrolled in 2004–2007 and had baseline data collected. For this analysis, 1,017 (82.1%) girls who had baseline urinary biomarker measurements and at least 3 anthropometric measurements taken during the study period (baseline through 2015) were included; the last visit occurred when girls were aged 15.6 years, on average (range, 12.8–18.4 years).
Urinary phenol measurements
At baseline, girls provided an early-morning (Cincinnati) or casual (Mount Sinai and Kaiser) spot urine sample. Samples were analyzed at the Centers for Disease Control and Prevention; laboratory analytical methods and quality control procedures have been published previously (13). Urinary concentrations of creatinine and 10 phenol metabolites—benzophenone-3, BPA, methyl-, ethyl-, and propyl-parabens, 2,5-dichlorophenol, triclosan, enterolactone, genistein, and daidzein—were obtained. Concentrations of paraben metabolites were summed based on molecular weight, expressed as propyl paraben (molecular weight 180.2 g/mol). All phenols were detected in over 80% of the samples except for butyl paraben, which was detected in 48% of the samples. Values below the limit of detection were imputed as limit of detection/√2 (13, 14). Urinary phenol concentrations were corrected for creatinine concentration (μg/g creatinine) to account for urine dilution. Girls with unusually low (<10 mg/dL; n = 12) or high (>300 mg/dL; n = 1) creatinine levels were excluded (among included girls, the median creatinine level was 91.5 mg/dL).
To address whether results were affected by the method of adjusting for dilution, we determined that extreme metabolite concentrations were not due to creatinine concentration alone and that low creatinine values were distributed across the range of biomarker concentrations. We repeated analyses while excluding girls with low creatinine values (<50 mg/dL), as well as using phenol concentrations without correction for creatinine. For 2,5-dichlorophenol and triclosan, associations were similar or greater in magnitude and precision compared with those using creatinine-corrected concentrations. For enterolactone, the observed differences in adiposity measures for medium versus low concentrations were strengthened while observed differences for high versus low concentrations were attenuated; as a result, associations for medium/high versus low concentrations remained but were attenuated. Overall, in analyses excluding low creatinine values and in those without creatinine correction, effect estimates for high versus low concentrations were attenuated by approximately 5%–20% and 25%–50%, respectively, compared with those with creatinine correction.
Anthropometric and covariate assessments
Data on weight, standing height, and umbilical waist circumference were collected at baseline and at yearly follow-up visits (measurements were taken biannually at the Cincinnati site) by trained interviewers using a standard protocol adapted from the National Health and Nutrition Examination Survey (15). The median number of measurements for each girl during the follow-up period was 9 (range, 3–15). Children wore light clothing and no shoes. All measurements were taken twice and averaged for analyses. Measurements were taken a third time and averaged only if the absolute difference between the previous 2 measurements exceeded the tolerance level. BMI was calculated as weight (in kilograms) divided by squared height (in meters). Percentage of body fat was determined using bioelectrical impedance analysis (Tanita Corporation of America, Inc., Arlington Heights, Illinois). BMI, waist circumference, and percent body fat were considered because they are distinct, indirect assessments of adiposity. BMI and percent body fat are different methods used to estimate overall body fatness, while waist circumference estimates central adiposity (or visceral fat) (16), and all are predictive of metabolism-related adverse health outcomes (17, 18).
Data regarding sociodemographic and other characteristics were provided by the girls’ caregivers (usually mothers) via self-administered (Cincinnati) or interviewer-administered questionnaires in English or Spanish. Race/ethnicity was identified hierarchically as black, Hispanic, white, or Asian. Caregivers’ highest attained educational level was used as a measure of socioeconomic status.
Statistical analysis
Statistical analyses were performed using Stata 13 (StataCorp LP, College Station, Texas). Unadjusted geometric mean baseline urinary biomarker concentrations of phenols were calculated according to selected characteristics of the population. Phenol levels were first analyzed in quintiles of creatinine-corrected concentrations (μg/g creatinine) for assessment of dose response and were collapsed into tertiles (designated as low, medium, and high concentrations) for final models.
Linear mixed-effects models (19–21) with an unstructured correlation matrix were used to assess the relationship between baseline urinary creatinine-corrected phenol concentrations (tertiles) and the girls’ BMI, waist circumference (cm), and percent body fat (%) trajectories from ages 7 (baseline) through 15 years. We used this age range because of the smaller number of girls with adiposity measurements collected at younger and older ages. Models included phenol concentration tertiles, age (at examination, centered and estimated to the nearest tenth of a year), age squared (to allow for nonlinearity), a term for interaction between age and phenol concentration tertiles, a term for interaction between age squared and phenol concentration tertiles, and a term for interaction between race/ethnicity and age (to allow for differences in girls’ adiposity measures over time by race/ethnicity). These models were used to generate predicted differences (and 95% confidence intervals) in the anthropometric outcomes, comparing tertiles of phenol concentration at each integer age using the pwcompare command. Additional adjustment for site, caregiver education, and early puberty (defined as breast stage 2 at <9.4 years) (22) did not substantially alter the magnitude or precision of the results. We also examined whether growth trajectories differed by child overweight status at baseline, dichotomized at the 85th percentile of BMI-for-age, by including the 3-way interaction term overweight status × phenol concentration tertile × age in each model.
RESULTS
Individual analyses were conducted for baseline urinary concentrations of each phenol (including the sum of parabens) and the selected adiposity measurements. Urinary concentrations of enterolactone, 2,5-dichlorophenol, and triclosan were consistently associated with the adiposity measurements throughout the follow-up period, and results for these phenols are presented here. Results for the remaining phenols are provided in Web Tables 1–5 (available at https://academic.oup.com/aje).
The sociodemographic characteristics of this population have been described previously (1, 12, 22). Table 1 shows the unadjusted geometric mean urinary concentrations of enterolactone, genistein, daidzein, 2,5-dichlorophenol, and triclosan according to sociodemographic characteristics. As shown here and as described previously (13), concentrations differed across categories of each of the characteristics (site, age at baseline, caregiver education, race/ethnicity, and baseline overweight status), with few exceptions.
Table 1.
Baseline Geometric Mean Enterolactone, 2,5-Dichlorophenol, and Triclosan Concentrations (μg/g Creatinine) According to Selected Characteristics Among Girls in the Breast Cancer and Environment Research Program, 2004–2015
| Characteristic | Enterolactone (n = 995) | 2,5-Dichlorophenol (n = 1,017) | Triclosan (n = 1,005) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Girls | GM | 95% CI | No. of Girls | GM | 95% CI | No. of Girls | GM | 95% CI | |
| Study sitea | |||||||||
| Mount Sinai | 309 | 331.7 | 289.9, 379.6 | 310 | 87.6 | 74.3, 103.2 | 310 | 15.8 | 13.0, 19.1 |
| Cincinnati | 299 | 419.9 | 371.8, 474.3 | 309 | 9.9 | 8.2, 11.9 | 309 | 29.1 | 24.6, 34.5 |
| Kaiser | 387 | 660.9 | 586.0, 745.4 | 398 | 5.1 | 4.4, 5.9 | 386 | 13.1 | 11.3, 15.2 |
| Age, years | |||||||||
| 6–6.9 | 262 | 427.4 | 373.3, 489.5 | 263 | 19.9 | 15.5, 25.6 | 263 | 16.9 | 14.0, 20.4 |
| 7–7.9 | 518 | 537.9 | 484.9, 596.8 | 534 | 10.1 | 8.6, 11.8 | 522 | 17.4 | 15.2, 20.1 |
| ≥8 | 215 | 364.8 | 308.3, 431.6 | 220 | 26.4 | 20.6, 33.7 | 220 | 19.4 | 15.7, 24.1 |
| Caregiver educationb | |||||||||
| High school or less | 475 | 362.1 | 325.0, 403.5 | 488 | 30.6 | 25.7, 36.3 | 482 | 17.2 | 14.9, 19.8 |
| College | 325 | 575.8 | 506.9, 654.2 | 334 | 8.6 | 7.0, 10.5 | 329 | 18.0 | 15.2, 21.4 |
| Graduate/professional | 171 | 636.9 | 536.8, 755.7 | 171 | 5.3 | 4.3, 6.6 | 170 | 17.6 | 13.7, 22.6 |
| Race/ethnicity | |||||||||
| Black | 289 | 468.3 | 410.9, 533.7 | 304 | 31.0 | 25.0, 38.4 | 300 | 18.9 | 16.0, 22.4 |
| Hispanic | 293 | 353.2 | 306.7, 406.7 | 297 | 37.3 | 30.4, 45.8 | 293 | 15.0 | 12.3, 18.2 |
| Asian | 52 | 465.3 | 301.9, 717.1 | 52 | 6.2 | 3.9, 10.0 | 52 | 14.5 | 9.4, 22.4 |
| White | 361 | 580.0 | 515.7, 652.2 | 364 | 4.3 | 3.7, 4.9 | 360 | 19.8 | 16.7, 23.4 |
| Baseline BMIc percentile | |||||||||
| ≤50th | 337 | 554.4 | 490.1, 627.2 | 343 | 11.5 | 9.4, 14.1 | 337 | 21.0 | 17.6, 25.0 |
| 50.01th–84.99th | 340 | 529.5 | 469.0, 597.9 | 349 | 13.5 | 11.1, 16.4 | 346 | 16.3 | 13.9, 19.2 |
| ≥85th | 318 | 337.1 | 293.5, 387.2 | 325 | 21.4 | 17.2, 26.7 | 322 | 16.2 | 13.6, 19.3 |
Abbreviations: BMI, body mass index; CI, confidence interval; GM, geometric mean.
a Data collection occurred at 3 sites: 1) Icahn School of Medicine at Mount Sinai, New York, New York (Mount Sinai); 2) Cincinnati Children's Hospital/University of Cincinnati, Cincinnati, Ohio (Cincinnati); and 3) Kaiser Permanente Northern California, San Francisco, California (Kaiser).
b Information on caregiver education was missing for 24 girls.
c Weight (kg)/height (m)2.
Enterolactone was inversely associated with changes in girls’ adiposity measurements (Table 2). At baseline, differences in adiposity measurements between tertiles of enterolactone (medium vs. low, high vs. low, and high vs. medium) were observed, which increased through ages 12–13 years and leveled off thereafter. When comparing girls with high creatinine-corrected concentrations with those with low concentrations, predicted differences in BMI ranged from −0.76 (95% confidence interval (CI): −1.28, −0.25) at age 7 years to −1.85 (95% CI: −2.58, −1.12) at age 13 years; differences in waist circumference ranged from −2.37 cm (95% CI: −3.93, −0.80) at age 7 years to −4.71 cm (95% CI: −6.71, −2.70) at age 14 years; and differences in percent body fat ranged from −2.05% (95% CI: −3.48, −0.62) at age 7 years to −3.61% (95% CI: −4.90, −2.32) at age 11 years.
Table 2.
Predicted Differencesa in Body Mass Index, Waist Circumference, and Percent Body Fat From Age 7 Years to Age 15 Years According to Tertile (Low, Medium, or Highb) of Enterolactone Concentration Among Girls (n = 995) in the Breast Cancer and Environment Research Program, 2004–2015
| Age (years) and Tertile Comparison | Predicted Difference | |||||
|---|---|---|---|---|---|---|
| Body Mass Indexc | Waist Circumference, cm | % Body Fat | ||||
| Difference | 95% CI | Difference | 95% CI | Difference | 95% CI | |
| 7 | ||||||
| Medium vs. low | −0.40 | −0.92, 0.12 | −1.23 | −2.78, 0.32 | −1.51 | −2.94, −0.07 |
| High vs. low | −0.76 | −1.28, −0.25 | −2.37 | −3.93, −0.80 | −2.05 | −3.48, −0.62 |
| High vs. medium | −0.36 | −0.88, 0.15 | −1.14 | −2.68, 0.41 | −0.54 | −1.96, 0.88 |
| 8 | ||||||
| Medium vs. low | −0.55 | −1.07, −0.03 | −1.53 | −2.92, −0.13 | −1.60 | −2.96, −0.24 |
| High vs. low | −1.09 | −1.61, −0.57 | −3.00 | −4.40, −1.61 | −2.71 | −4.06, −1.36 |
| High vs. medium | −0.55 | −1.06, −0.03 | −1.47 | −2.85, −0.09 | −1.11 | −2.45, 0.23 |
| 9 | ||||||
| Medium vs. low | −0.65 | −1.20, −0.11 | −1.77 | −3.18, −0.36 | −1.65 | −2.97, −0.34 |
| High vs. low | −1.36 | −1.91, −0.82 | −3.53 | −4.94, −2.13 | −3.19 | −4.50, −1.88 |
| High vs. medium | −0.71 | −1.25, −0.17 | −1.76 | −3.16, −0.36 | −1.54 | −2.84, −0.24 |
| 10 | ||||||
| Medium vs. low | −0.72 | −1.30, −0.13 | −1.96 | −3.47, −0.44 | −1.67 | −2.97, −0.38 |
| High vs. low | −1.57 | −2.15, −0.99 | −3.97 | −5.48, −2.46 | −3.49 | −4.78, −2.20 |
| High vs. medium | −0.85 | −1.43, −0.28 | −2.01 | −3.51, −0.51 | −1.82 | −3.10, −0.54 |
| 11 | ||||||
| Medium vs. low | −0.75 | −1.38, −0.12 | −2.09 | −3.73, −0.45 | −1.65 | −2.94, −0.36 |
| High vs. low | −1.72 | −2.35, −1.10 | −4.30 | −5.93, −2.67 | −3.61 | −4.90, −2.32 |
| High vs. medium | −0.98 | −1.60, −0.35 | −2.21 | −3.84, −0.59 | −1.96 | −3.24, −0.68 |
| 12 | ||||||
| Medium vs. low | −0.74 | −1.42, −0.06 | −2.16 | −3.92, −0.41 | −1.59 | −2.89, −0.30 |
| High vs. low | −1.82 | −2.49, −1.14 | −4.54 | −6.28, −2.79 | −3.55 | −4.84, −2.26 |
| High vs. medium | −1.08 | −1.75, −0.41 | −2.37 | −4.11, −0.64 | −1.95 | −3.24, −0.67 |
| 13 | ||||||
| Medium vs. low | −0.69 | −1.42, 0.04 | −2.18 | −4.05, −0.31 | −1.50 | −2.81, −0.19 |
| High vs. low | −1.85 | −2.58, −1.12 | −4.67 | −6.53, −2.81 | −3.30 | −4.61, −1.99 |
| High vs. medium | −1.16 | −1.88, −0.44 | −2.49 | −4.34, −0.64 | −1.80 | −3.10, −0.50 |
| 14 | ||||||
| Medium vs. low | −0.61 | −1.39, 0.18 | −2.14 | −4.15, −0.13 | −1.36 | −2.71, −0.02 |
| High vs. low | −1.82 | −2.61, −1.04 | −4.71 | −6.71, −2.70 | −2.88 | −4.22, −1.53 |
| High vs. medium | −1.22 | −2.00, −0.44 | −2.56 | −4.55, −0.57 | −1.51 | −2.84, −0.18 |
| 15 | ||||||
| Medium vs. low | −0.48 | −1.34, 0.38 | −2.05 | −4.27, 0.17 | −1.19 | −2.60, 0.22 |
| High vs. low | −1.74 | −2.59, −0.89 | −4.64 | −6.85, −2.43 | −2.27 | −3.67, −0.86 |
| High vs. medium | −1.26 | −2.11, −0.41 | −2.59 | −4.79, −0.39 | −1.07 | −2.47, 0.32 |
Abbreviation: CI, confidence interval.
a Models included enterolactone concentration tertiles, age, age squared, a term for interaction between age and enterolactone tertiles, a term for interaction between age squared and enterolactone tertiles, and a term for interaction between race/ethnicity and age.
b Tertile cutpoints (median) for enterolactone concentration: low, 158 μg/g creatinine; medium, 504 μg/g creatinine; high, 1,350 μg/g creatinine.
c Weight (kg)/height (m)2.
Baseline urinary concentrations of 2,5-dichlorophenol were positively associated with changes in girls’ BMI, waist circumference, and percent body fat (Table 3). Differences in adiposity measurements were observed between tertiles (medium vs. low and high vs. low) beginning at age 8–9 years, which consistently increased through approximately age 13 years. When comparing girls with high concentrations of 2,5-dichlorophenol with those with low concentrations, predicted differences in BMI ranged from 0.82 (95% CI: 0.20, 1.44) at age 9 years to 1.57 (95% CI: 0.74, 2.40) at age 13 years; differences in waist circumference ranged from 2.15 cm (95% CI: 0.57, 3.73) at age 8 years to 5.67 cm (95% CI: 3.16, 8.17) at age 15 years; and differences in percent body fat ranged from 2.20% (95% CI: 0.66, 3.74) at age 8 years to 3.39% (95% CI: 1.92, 4.86) at age 12 years.
Table 3.
Predicted Differencesa in Body Mass Index, Waist Circumference, and Percent Body Fat From Age 7 Years to Age 15 Years According to Tertile (Low, Medium, or Highb) of 2,5-Dichlorophenol Concentration Among Girls (n = 1,017) in the Breast Cancer and Environment Research Program, 2004–2015
| Age (years) and Tertile Comparison | Predicted Difference | |||||
|---|---|---|---|---|---|---|
| Body Mass Indexc | Waist Circumference, cm | % Body Fat | ||||
| Difference | 95% CI | Difference | 95% CI | Difference | 95% CI | |
| 7 | ||||||
| Medium vs. low | 0.13 | −0.43, 0.62 | 0.58 | −1.02, 2.18 | 1.22 | −0.36, 2.54 |
| High vs. low | −0.08 | −0.66, 0.51 | 1.09 | −0.64, 2.81 | 1.35 | −0.28, 2.97 |
| High vs. medium | −0.21 | −0.74, 0.32 | 0.51 | −1.07, 2.10 | 0.13 | −1.33, 1.59 |
| 8 | ||||||
| Medium vs. low | 0.39 | −0.15, 0.94 | 1.31 | −0.13, 2.76 | 1.66 | 0.25, 3.08 |
| High vs. low | 0.41 | −0.18, 1.01 | 2.15 | 0.57, 3.73 | 2.20 | 0.66, 3.74 |
| High vs. medium | 0.02 | −0.51, 0.55 | 0.84 | −0.59, 2.27 | 0.54 | −0.85, 1.92 |
| 9 | ||||||
| Medium vs. low | 0.62 | 0.05, 1.19 | 1.91 | 0.45, 3.38 | 2.00 | 0.63, 3.37 |
| High vs. low | 0.82 | 0.20, 1.44 | 3.07 | 1.47, 4.68 | 2.83 | 1.33, 4.32 |
| High vs. medium | 0.20 | −0.35, 0.76 | 1.16 | −0.28, 2.61 | 0.83 | −0.51, 2.17 |
| 10 | ||||||
| Medium vs. low | 0.79 | 0.18, 1.40 | 2.38 | 0.81, 3.95 | 2.24 | 0.88, 3.59 |
| High vs. low | 1.14 | 0.47, 1.80 | 3.85 | 2.14, 5.56 | 3.24 | 1.76, 4.71 |
| High vs. medium | 0.34 | −0.25, 0.94 | 1.48 | −0.06, 3.02 | 1.00 | −0.32, 2.32 |
| 11 | ||||||
| Medium vs. low | 0.93 | 0.27, 1.58 | 2.70 | 1.01, 4.40 | 2.37 | 1.02, 3.71 |
| High vs. low | 1.37 | 0.65, 2.08 | 4.50 | 2.65, 6.34 | 3.42 | 1.96, 4.89 |
| High vs. medium | 0.44 | −0.20, 1.08 | 1.79 | 0.13, 3.45 | 1.06 | −0.26, 2.37 |
| 12 | ||||||
| Medium vs. low | 1.02 | 0.31, 1.72 | 2.90 | 1.09, 4.71 | 2.39 | 1.04, 3.74 |
| High vs. low | 1.51 | 0.74, 2.28 | 5.00 | 3.02, 6.97 | 3.39 | 1.92, 4.86 |
| High vs. medium | 0.50 | −0.19, 1.19 | 2.10 | 0.32, 3.88 | 1.00 | −0.32, 2.31 |
| 13 | ||||||
| Medium vs. low | 1.06 | 0.30, 1.82 | 2.96 | 1.03, 4.89 | 2.31 | 0.95, 3.68 |
| High vs. low | 1.57 | 0.74, 2.40 | 5.36 | 3.25, 7.47 | 3.13 | 1.64, 4.62 |
| High vs. medium | 0.51 | −0.23, 1.25 | 2.40 | 0.51, 4.30 | 0.82 | −0.52, 2.15 |
| 14 | ||||||
| Medium vs. low | 1.07 | 0.24, 1.89 | 2.88 | 0.80, 4.96 | 2.13 | 0.73, 3.53 |
| High vs. low | 1.55 | 0.65, 2.44 | 5.58 | 3.30, 7.86 | 2.65 | 1.12, 4.18 |
| High vs. medium | 0.48 | −0.32, 1.28 | 2.70 | 0.66, 4.74 | 0.52 | −0.85, 1.89 |
| 15 | ||||||
| Medium vs. low | 1.02 | 0.13, 1.91 | 2.67 | 0.37, 4.97 | 1.84 | 0.38, 3.31 |
| High vs. low | 1.43 | 0.46, 2.40 | 5.67 | 3.16, 8.17 | 1.95 | 0.35, 3.55 |
| High vs. medium | 0.41 | −0.46, 1.28 | 2.99 | 0.75, 5.24 | 0.11 | −1.32, 1.54 |
Abbreviation: CI, confidence interval.
a Models included 2,5-dichlorophenol concentration tertiles, age, age squared, a term for interaction between age and 2,5-dichlorophenol tertiles, a term for interaction between age squared and 2,5-dichlorophenol tertiles, and a term for interaction between race/ethnicity and age.
b Tertile cutpoints (median) for 2,5-dichlorophenol concentration: low, 2.3 μg/g creatinine; medium, 12 μg/g creatinine; high, 103 μg/g creatinine.
c Weight (kg)/height (m)2.
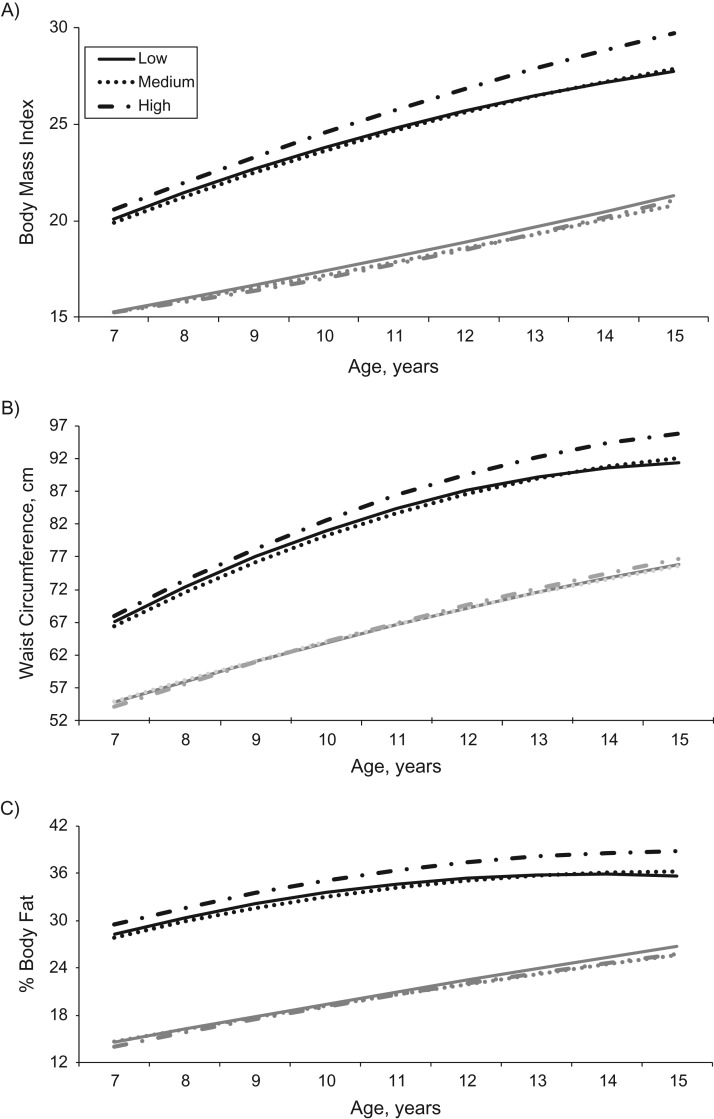
A nonmonotonic association was observed for baseline triclosan concentrations with adiposity measurements. Girls with medium concentrations had greater BMI, waist circumference, and percent body fat during the study period than girls with low and high concentrations. However, baseline overweight status modified this association. Among overweight girls, triclosan was positively associated with adiposity measurements. Differences in adiposity measures were observed for overweight girls with high triclosan concentrations compared with those with medium or low concentrations beginning at age 8–9 years and increasing through age 15 years (Table 4, Figure 1). Predicted differences in BMI, waist circumference, and percent body fat (comparing high concentrations with medium concentrations or high concentrations with low concentrations) ranged from 0.5 to 2.0, from 1.0 cm to 4.4 cm, and from 1.2% to 3.1% from age 8 years to age 15 years, respectively (Table 4). Triclosan was not associated with adiposity measurements among girls who were not overweight at baseline (Web Table 6).
Table 4.
Predicted Differencesa in Body Mass Index, Waist Circumference, and Percent Body Fat From Age 7 Years to Age 15 Years According to Tertile (Low, Medium, or Highb) of Triclosan Concentration Among Overweight Girls (BMI ≥85th Percentile; n = 322) in the Breast Cancer and Environment Research Program, 2004–2015
| Age (years) and Tertile Comparison | Predicted Difference | |||||
|---|---|---|---|---|---|---|
| Body Mass Indexc | Waist Circumference, cm | % Body Fat | ||||
| Difference | 95% CI | Difference | 95% CI | Difference | 95% CI | |
| 7 | ||||||
| Medium vs. low | −0.23 | −1.01, 0.55 | −0.66 | −2.77, 1.45 | −0.47 | −2.16, 1.21 |
| High vs. low | 0.46 | −0.29, 1.21 | 0.85 | −1.19, 2.88 | 1.16 | −0.46, 2.78 |
| High vs. medium | 0.69 | −0.12, 1.50 | 1.51 | −0.68, 3.70 | 1.63 | −0.12, 3.38 |
| 8 | ||||||
| Medium vs. low | −0.22 | −1.00, 0.55 | −0.80 | −2.83, 1.23 | −0.57 | −2.11, 0.98 |
| High vs. low | 0.51 | −0.24, 1.25 | 1.04 | −0.92, 2.99 | 1.22 | −0.27, 2.71 |
| High vs. medium | 0.73 | −0.07, 1.54 | 1.84 | −0.27, 3.94 | 1.79 | 0.18, 3.39 |
| 9 | ||||||
| Medium vs. low | −0.21 | −1.03, 0.62 | −0.85 | −2.96, 1.26 | −0.60 | −2.09, 0.89 |
| High vs. low | 0.60 | −0.20, 1.39 | 1.30 | −0.73, 3.33 | 1.33 | −0.10, 2.77 |
| High vs. medium | 0.80 | −0.05, 1.66 | 2.15 | −0.04, 4.34 | 1.93 | 0.38, 3.48 |
| 10 | ||||||
| Medium vs. low | −0.18 | −1.09, 0.72 | −0.81 | −3.10, 1.47 | −0.56 | −2.06, 0.93 |
| High vs. low | 0.72 | −0.15, 1.60 | 1.64 | −0.56, 3.84 | 1.50 | 0.06, 2.94 |
| High vs. medium | 0.91 | −0.04, 1.85 | 2.45 | 0.07, 4.83 | 2.06 | 0.51, 3.62 |
| 11 | ||||||
| Medium vs. low | −0.14 | −1.15, 0.86 | −0.69 | −3.20, 1.82 | −0.46 | −2.00, 1.08 |
| High vs. low | 0.89 | −0.08, 1.86 | 2.05 | −0.37, 4.47 | 1.72 | 0.24, 3.20 |
| High vs. medium | 1.04 | −0.01, 2.09 | 2.74 | 0.12, 5.35 | 2.18 | 0.58, 3.79 |
| 12 | ||||||
| Medium vs. low | −0.10 | −1.22, 1.02 | −0.48 | −3.25, 2.29 | −0.30 | −1.91, 1.31 |
| High vs. low | 1.10 | 0.02, 2.18 | 2.53 | −0.14, 5.20 | 1.99 | 0.44, 3.54 |
| High vs. medium | 1.20 | 0.03, 2.37 | 3.01 | 0.12, 5.89 | 2.29 | 0.61, 3.97 |
| 13 | ||||||
| Medium vs. low | −0.04 | −1.28, 1.21 | −0.18 | −3.23, 2.88 | −0.07 | −1.78, 1.63 |
| High vs. low | 1.35 | 0.15, 2.55 | 3.09 | 0.15, 6.03 | 2.31 | 0.67, 3.96 |
| High vs. medium | 1.39 | 0.09, 2.69 | 3.27 | 0.08, 6.45 | 2.39 | 0.61, 4.17 |
| 14 | ||||||
| Medium vs. low | 0.03 | −1.35, 1.41 | 0.21 | −3.17, 3.58 | 0.22 | −1.62, 2.05 |
| High vs. low | 1.64 | 0.31, 2.97 | 3.72 | 0.47, 6.97 | 2.69 | 0.92, 4.46 |
| High vs. medium | 1.61 | 0.17, 3.05 | 3.51 | −0.01, 7.03 | 2.47 | 0.56, 4.39 |
| 15 | ||||||
| Medium vs. low | 0.11 | −1.42, 1.64 | 0.69 | −3.06, 4.43 | 0.57 | −1.45, 2.59 |
| High vs. low | 1.97 | 0.50, 3.45 | 4.43 | 0.81, 8.04 | 3.12 | 1.17, 5.07 |
| High vs. medium | 1.86 | 0.27, 3.46 | 3.74 | −0.17, 7.65 | 2.55 | 0.44, 4.65 |
Abbreviation: CI, confidence interval.
a Models included triclosan concentration tertiles, age, age squared, a term for interaction between age and triclosan tertiles, a term for interaction between age squared and triclosan tertiles, and a term for interaction between race/ethnicity and age.
b Tertile cutpoints (median) for triclosan concentration: low, 3.7 μg/g creatinine; medium, 15 μg/g creatinine; high, 86 μg/g creatinine.
c Weight (kg)/height (m)2.
Figure 1.
Predicted changes in body mass index (weight (kg)/height (m)2) (A), waist circumference (B), and percent body fat (C) from age 7 years to age 15 years, by tertile (low, medium, or high) of triclosan concentration, among girls who were overweight (body mass index ≥85th percentile; black lines) and girls who were normal-weight (body mass index <85th percentile; gray lines) at baseline (n = 1,005), Breast Cancer and the Environment Research Program, 2004–2015.
Benzophenone-3 and the sum of parabens were not associated with the adiposity measurements (Web Table 1). Associations were observed for BPA (Web Table 2) and daidzein (Web Table 3); however, they were not consistent across the adiposity measurements or throughout the follow-up period. Compared with low concentrations of daidzein, medium and high concentrations were associated with greater adiposity beginning at approximately 10 years of age and throughout the follow-up period. A similar pattern of associations was observed for genistein (Web Table 4). High concentrations of BPA were associated with lower percent body fat but not BMI or waist circumference, beginning at approximately 9 years of age and throughout the follow-up period.
We further examined joint exposure to enterolactone, 2,5-dichlorophenol, and triclosan. First, results of models for the individual chemicals were mutually adjusted for the other chemicals. In each of these analyses, associations were attenuated but not appreciably different from those observed in unadjusted models. Second, we created categories of combined tertiles of exposure to 2,5-dichlorophenol and enterolactone: low 2,5-dichlorophenol/high enterolactone (n = 155; reference group), high 2,5-dichlorophenol/low enterolactone (n = 123), and remaining combinations of low, medium, and high 2,5-dichlorophenol/enterolactone (n = 716). The results suggested a generally additive association; therefore, the chemicals were acting independently of each other rather than synergistically (Web Table 7). We also created categories of combined exposure to enterolactone, 2,5-dichlorophenol, and triclosan, but sample sizes were small. Only 4% of the sample had high 2,5-dichlorophenol/high triclosan/low enterolactone, and only 6% of the sample had low 2,5-dichlorophenol/low triclosan/high enterolactone.
DISCUSSION
We found that baseline urinary concentrations of enterolactone and 2,5-dichlorophenol at approximately 7 years of age were associated with changes in adiposity measurements over an 8-year follow-up period in a multiethnic population of girls. Differences in measurements between high and low tertiles of phenol concentrations tended to increase and become stronger from baseline through approximately age 12–13 years, and leveled off thereafter. Enterolactone was inversely associated with changes in BMI, waist circumference, and percent body fat, while 2,5-dichlorophenol was positively associated with changes in these measurements. Triclosan was also positively associated with adiposity measurements, but only among girls who were overweight at baseline. BPA was inversely associated with percent body fat, while daidzein and genistein were positively associated with the adiposity measures; however, these associations were relatively small and were not consistent across the adiposity measures or throughout the follow-up period. Benzophenone-3 and the sum of parabens were not associated with adiposity measurements.
There are plausible biological mechanisms that warrant the study of phenols in relation to adiposity, mainly that some phenols are endocrine disruptors. Endocrine-disrupting chemicals may alter metabolism via estrogenic, antiestrogenic, or antiandrogenic action and/or by interfering with thyroid or other hormone functions (2). Phytoestrogens, which include enterolactone, dadzein, and genistein, may act as antiadipogens by binding to and modifying the regulation of estrogen receptors and peroxisome proliferator-activated receptors, both of which are involved in lipid metabolism (5). Enterolactone, specifically, can inhibit aromatase activity (23), which may also influence body fat. In children, high urinary enterolactone concentrations (defined as >90th percentile (24) or highest quartile (25)), but not daidzein or genistein concentrations (25), were associated with decreased odds of overweight and obesity (24, 25). In our study, daidzein was associated with small increases in adiposity, and a similar pattern was observed for genistein, though many associations did not reach statistical significance. In rodents, diets with high amounts of soy are associated with reduced body fat, which is mainly attributed to genistein (26). In humans, although there is evidence for both daidzien and genistein having beneficial effects on metabolic risk factors (i.e., cholesterol levels), their associations with body fat are not as apparent (27). Soy products may need to be consumed in large quantities or in supplement form to influence body composition (5, 27), which is difficult to achieve among children, especially in the United States. Additionally, enterolactone may alter the gut microbiome. Enterolactone is produced during the metabolism of dietary lignans, such as seeds, grains, and some fruits and vegetables, by specific microorganisms in the gut. Higher proportions of these specific microorganisms, which may influence energy absorption, expenditure, and storage, are associated with a decreased risk of obesity (28).
2,5-Dichlorophenol likely acts as a thyroid agonist. In a study carried out among Flemish adolescents, 2,5-dichlorophenol was negatively correlated with free thyroxine (T4) levels but was positively associated with thyroid-stimulating hormone (29). In cross-sectional analyses of data from the National Health and Nutrition Examination Survey (2003–2010), increasing concentrations of 2,5-dichlorophenol were associated with greater BMI z scores, waist circumference, and likelihood of obesity in children and adolescents (6, 7). Observed associations were stronger among adolescents (ages 12–19 years) than among younger children (ages 6–11 years) (7). In the current study, differences in adiposity measurements between high and low tertiles of 2,5-dichlorophenol were apparent beginning at age 8 years and continuing through approximately age 13 years.
Similar to 2,5-dichlorophenol, triclosan may also interfere with thyroid function. Previous studies in rats (30) and obese women (31) have shown decreases in thyroxine levels with exposure to triclosan. In adolescents, triclosan exposure was associated with increased serum total triiodothyonine (T3) levels (32). Investigators in cross-sectional analyses have reported null (7, 33) and negative (34) associations with adiposity among children. We observed a positive association, but only among girls who were already overweight at baseline, when body burdens were measured. This interaction was not investigated in the previous studies. It is plausible that thyroid function is impaired in obesity and influences susceptibility to triclosan exposures, since obese individuals tend to have lower serum thyroxine levels but higher levels of triiodothyonine and thyroid-stimulating hormone compared with their normal-weight counterparts (35). Other explanations include obesity-related variations in physiology and the metabolism of triclosan (and other chemicals) (36), as well as reverse causality.
We found primarily null associations of BPA and parabens with the adiposity measurements, with the exception of a small inverse association between BPA and percent body fat. Both BPA and parabens can promote adipogenesis (8, 37, 38) by altering levels of thyroid hormones (32, 39) and by having estrogenic and antiandrogenic properties (40, 41). To our knowledge, only 1 study has examined the relationship between parabens and obesity, finding no difference in concentrations between nonobese and obese children (33). Results from previous studies of childhood BPA exposures are mixed. Cross-sectional studies have found positive (42–45) and null (33, 45) associations, as well as sex- and age-specific associations (46), while prospective studies have found mostly null associations (45, 47, 48). Though our findings are consistent with those of other prospective studies, urinary concentrations of BPA were low in our study population (as well as others (45)), and we may have lacked adequate variation or power to detect associations among girls with higher exposures.
In general, differences in adiposity measurements between tertiles (high vs. low) of enterolactone, 2,5-dichlorophenol, and triclosan increased from ages 7–9 years through ages 12–13 years, leveling off thereafter. This pattern suggests that childhood phenol exposures influence adiposity but associations are muted (or diminished) as the child enters adolescence, when other factors, such as puberty, may play a role. In a previous analysis (1), a sharp increase in the trajectory of BMI percentile was observed among lean (BMI <71st percentile) girls during the pubertal window at approximately age 12 years, while BMI percentile remained constant among heavier girls (BMI ≥71st percentile). This observation among heavier girls may also explain the pattern observed for triclosan and adiposity measurements in the current study; rather than leveling off, differences in adiposity measurements consistently increased through age 15 years among overweight girls. However, we cannot exclude the possibility of residual confounding, particularly from exposures incurred during the fetal period. Given that exposures in the prenatal and postnatal environments are likely correlated, some of the observed associations for phenols and postnatal adiposity may be at least partially confounded by prenatal exposures to these chemicals (45, 49).
Reproducibility of phenol concentrations throughout the study period must be considered when interpreting the reported findings. We were limited to single, baseline urine measurements of phenols. Studies suggest that levels of these chemicals are reasonably stable over time for the purpose of ranking and have acceptable intraindividual variability over more than a year (50–52). In the current study, reproducibility of phenol concentrations (intraclass correlations) among girls with 3 samples collected during a 3-year period was low to moderate; specifically, intraclass correlation coefficients were 0.27, 0.48, and 0.69 for triclosan, enterolactone, and 2,5-dichlorophenol, respectively. Surrogate analysis (53, 54) was also conducted using tertiles of the average concentrations of all 3 samples. The median value of each tertile increased monotonically from the lowest tertile to the highest, supporting the use of a single spot urine sample to categorize girls into low, medium, and high exposures over a 3-year period (Susan Teitelbaum, Icahn School of Medicine at Mount Sinai, unpublished data, 2016). Given this information, we do not know whether the associations between specific phenols and adiposity measures in girls observed through early adolescence resulted from consistent exposure to these chemicals throughout the study period or if these associations simply persisted from ages closer to the time when baseline measurements were taken (ages 7–10 years). Misclassification, particularly at later ages, could also have attenuated the observed associations, and, as mentioned above, reverse causality is possible.
This study uniquely included prospectively collected girls’ BMI, waist circumference, and percent body fat measurements for assessment of longitudinal changes in adiposity through adolescence in relation to childhood phenol exposures. With the exception of BPA, only cross-sectional studies, if any, exist for the other phenols. In general, our findings are consistent with those from previous studies: Concentrations of enterolactone and 2,5-dichlorophenol were associated with measurable differences in girls’ BMI, waist circumference, and percent body fat that persisted through early adolescence. Triclosan was also associated with adiposity measurements, but only among girls who were already overweight. The observed differences in adiposity measurements are similar to or greater than those reported in studies of dietary exposures in children (55–59). Given the current limited investigation but relative consistency of these associations across studies, further examination of physiological mechanisms and timing of exposures, specifically multiple exposures and windows of susceptibility, is encouraged.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Public Health Nutrition, College of Global Public Health, New York University, New York, New York (Andrea L. Deierlein); Department of Preventive Medicine, Icahn School of Medicine at Mount Sinai, New York, New York (Ashley Pajak, Maida Galvez, Mary S. Wolff, Susan L. Teitelbaum); Department of Environmental Health, College of Medicine, University of Cincinnati, Cincinnati, Ohio (Susan M. Pinney); Division of Laboratory Sciences, Centers for Disease Control and Prevention, Atlanta, Georgia (Michael Rybak, Antonia M. Calafat); Division of Research, Kaiser Permanente, Oakland, California (Lawrence H. Kushi); Division of Adolescent Medicine, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio (Frank M. Biro); and Division of Environmental and Occupational Disease Control, California Department of Public Health, Richmond, California (Gayle C. Windham).
This work was supported by the National Institute of Environmental Health Sciences (grant R00ES023474); awards from the National Institute of Environmental Health Sciences, the National Cancer Institute, the Environmental Protection Agency, the National Institutes of Health, and the Department of Health and Human Services for the Breast Cancer and the Environment Research Program (awards U01ES012770, U01ES012771, U01ES012800, U01ES012801, U01ES019435, U01ES019453, U01ES019454, U01ES019457, R827039, P01ES009584, P30ES006096, and P30ES023515); the National Center for Research Resources (grant CSTA-UL1RR029887); the New York State Empire Clinical Research Investigator Program; a Pediatric Environmental Health Fellowship (grant HD049311); and the Avon Foundation.
We thank the collaborators at the study centers involved in this research, including Jessica Montana, Dr. Nancy Mervish, Dr. Cheryl Stein, Rochelle Osborne, Lisa Boguski, Dr. Joel Forman, and Dr. Barbara Brenner (Mount Sinai); Gayle Greenberg, Peggy Monroe, and Dr. Bob Bornschein (Cincinnati); and Dr. Robert Hiatt, Dr. Louise Greenspan, Dr. Julie Deardorff, and Janice Barlow (Kaiser). We also thank Daniel L. Parker, Xiaoyun Ye, Amber Bishop, and Tao Jia for measurement of the phenol metabolites.
This research was presented at the 28th Annual Conference of the International Society for Environmental Epidemiology, Rome, Italy, September 1–4, 2016.
The findings and conclusions of this research are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Cancer Institute, the National Institutes of Health, the Centers for Disease Control and Prevention, or the California Department of Public Health.
Conflict of interest: none declared.
REFERENCES
- 1. Wolff MS, Teitelbaum SL, McGovern K, et al. . Environmental phenols and pubertal development in girls. Environ Int. 2015;84:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gore AC, Chappell VA, Fenton SE, et al. . EDC-2: the Endocrine Society's second Scientific Statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peterson J, Dwyer J, Adlercreutz H, et al. . Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr Rev. 2010;68(10):571–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. [DOI] [PubMed] [Google Scholar]
- 5. Orgaard A, Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med (Maywood). 2008;233(9):1066–1080. [DOI] [PubMed] [Google Scholar]
- 6. Twum C, Wei Y. The association between urinary concentrations of dichlorophenol pesticides and obesity in children. Rev Environ Health. 2011;26(3):215–219. [DOI] [PubMed] [Google Scholar]
- 7. Buser MC, Murray HE, Scinicariello F. Association of urinary phenols with increased body weight measures and obesity in children and adolescents. J Pediatr. 2014;165(4):744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohlstein JF, Strong AL, McLachlan JA, et al. . Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J Mol Endocrinol. 2014;53(3):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LaKind JS, Goodman M, Mattison DR. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit Rev Toxicol. 2014;44(2):121–150. [DOI] [PubMed] [Google Scholar]
- 10. Jeng HAC, Kantaria K, Beydoun HA. Urinary phytoestrogens in relation to metabolic disturbances among children and adolescents. J Environ Sci Health B. 2015;50(2):121–127. [DOI] [PubMed] [Google Scholar]
- 11. Deierlein AL, Wolff MS, Pajak A, et al. . Longitudinal associations of phthalate exposures during childhood and body size measurements in young girls. Epidemiology. 2016;27(4):492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biro FM, Galvez MP, Greenspan LC, et al. . Pubertal assessment methods and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010;126(3):e583–e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolff MS, Teitelbaum SL, Pinney SM, et al. . Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ Health Perspect. 2010;118(7):1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolff MS, Teitelbaum SL, McGovern K, et al. . Phthalate exposure and pubertal development in a longitudinal study of US girls. Hum Reprod. 2014;29(7):1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. . CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 16. Brambilla P, Bedogni G, Moreno LA, et al. . Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes. 2006;30(1):23–30. [DOI] [PubMed] [Google Scholar]
- 17. L'Allemand-Jander D. Clinical diagnosis of metabolic and cardiovascular risks in overweight children: early development of chronic diseases in the obese child. Int J Obes (Lond). 2010;34(suppl 2):S32–S36. [DOI] [PubMed] [Google Scholar]
- 18. Moreno L, Pineda I, Rodriguez G, et al. . Waist circumference for the screening of the metabolic syndrome in children. Acta Paediatr. 2002;91(12):1307–1312. [DOI] [PubMed] [Google Scholar]
- 19. Harville DA. Maximum likelihood approaches to variance component estimation and to related problems. J Am Stat Assoc. 1977;72(358):320–338. [Google Scholar]
- 20. Ware JH. Linear models for the analysis of longitudinal studies. Am Stat. 1985;39(2):95–101. [Google Scholar]
- 21. Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 22. Biro FM, Greenspan LC, Galvez MP, et al. . Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mäkelä TH, Wähälä KT, Hase TA. Synthesis of enterolactone and enterodiol precursors as potential inhibitors of human estrogen synthetase (aromatase). Steroids. 2000;65(8):437–441. [DOI] [PubMed] [Google Scholar]
- 24. Frankenfeld C. Relationship of obesity and high urinary enterolignan concentrations in 6806 children and adults: analysis of National Health and Nutrition Examination Survey data. Eur J Clin Nutr. 2013;67(8):887–889. [DOI] [PubMed] [Google Scholar]
- 25. Xu C, Liu Q, Zhang Q, et al. . Urinary enterolactone is associated with obesity and metabolic alteration in men in the US National Health and Nutrition Examination Survey 2001–10. Br J Nutr. 2015;113(4):683–690. [DOI] [PubMed] [Google Scholar]
- 26. Cederroth CR, Vinciguerra M, Kühne F, et al. . A phytoestrogen-rich diet increases energy expenditure and decreases adiposity in mice. Environ Health Perspect. 2007;115:1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amiot M, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev. 2016;17(7):573–586. [DOI] [PubMed] [Google Scholar]
- 28. Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem. 2009;20(10):743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Croes K, Den Hond E, Bruckers L, et al. . Endocrine actions of pesticides measured in the Flemish Environment and Health Studies (FLEHS I and II). Environ Sci Pollut Res Int. 2015;22(19):14589–14599. [DOI] [PubMed] [Google Scholar]
- 30. Witorsch RJ. Critical analysis of endocrine disruptive activity of triclosan and its relevance to human exposure through the use of personal care products. Crit Rev Toxicol. 2014;44(6):535–555. [DOI] [PubMed] [Google Scholar]
- 31. Geens T, Dirtu AC, Dirinck E, et al. . Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroid hormones and weight loss in overweight and obese individuals. Environ Int. 2015;76:98–105. [DOI] [PubMed] [Google Scholar]
- 32. Koeppe ES, Ferguson KK, Colacino JA, et al. . Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci Total Environ. 2013;445-446:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xue J, Wu Q, Sakthivel S, et al. . Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ Res. 2015;137:120–128. [DOI] [PubMed] [Google Scholar]
- 34. Li S, Zhao J, Wang G, et al. . Urinary triclosan concentrations are inversely associated with body mass index and waist circumference in the US general population: experience in NHANES 2003–2010. Int J Hyg Environ Health. 2015;218(4):401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Longhi S, Radetti G. Thyroid function and obesity. J Clin Res Pediatr Endocrinol. 2013;5(suppl 1):40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt Sinai J Med. 2011;78(1):22–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu P, Chen X, Whitener RJ, et al. . Effects of parabens on adipocyte differentiation. Toxicol Sci. 2013;131(1):56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miyawaki J, Sakayama K, Kato H, et al. . Perinatal and postnatal exposure to bisphenol A increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb. 2007;14(5):245–252. [DOI] [PubMed] [Google Scholar]
- 39. Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in US adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ Health Perspect. 2011;119(10):1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boberg J, Taxvig C, Christiansen S, et al. . Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol. 2010;30(2):301–312. [DOI] [PubMed] [Google Scholar]
- 41. Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol. 2008;28(5):561–678. [DOI] [PubMed] [Google Scholar]
- 42. Eng DS, Lee JM, Gebremariam A, et al. . Bisphenol A and chronic disease risk factors in US children. Pediatrics. 2013;132(3):e637–e645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bhandari R, Xiao J, Shankar A. Urinary bisphenol A and obesity in US children. Am J Epidemiol. 2013;177(11):1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308(11):1113–1121. [DOI] [PubMed] [Google Scholar]
- 45. Harley KG, Schall RA, Chevrier J, et al. . Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect. 2013;121(4):514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li D, Miao M, Zhou Z, et al. . Urine bisphenol-A level in relation to obesity and overweight in school-age children. PLoS One. 2013;8(6):e65399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Braun JM, Lanphear BP, Calafat AM, et al. . Early-life bisphenol A exposure and child body mass index: a prospective cohort study. Environ Health Perspect. 2014;122(11):1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maserejian NN, Trachtenberg FL, Wheaton OB, et al. . Changes in urinary bisphenol A concentrations associated with placement of dental composite restorations in children and adolescents. J Am Dent Assoc. 2016;147(8):620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buckley JP, Herring AH, Wolff MS, et al. . Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children's Environmental Health Study. Environ Int. 2016;91:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Engel LS, Buckley JP, Gong Y, et al. . Predictors and variability of repeat measurements of urinary phenols and parabens in a cohort of Shanghai women and men. Environ Health Perspect. 2014;122(7):733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mouritsen A, Frederiksen H, Sørensen K, et al. . Urinary phthalates from 168 girls and boys measured twice a year during a 5-year period: associations with adrenal androgen levels and puberty. J Clin Endocrinol Metab. 2013;98(9):3755–3764. [DOI] [PubMed] [Google Scholar]
- 52. Calafat AM, Longnecker MP, Koch HM, et al. . Optimal exposure biomarkers for nonpersistent chemicals in environmental epidemiology. Environ Health Perspect. 2015;123(7):A166–A168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mervish N, Blount B, Valentin-Blasini L, et al. . Temporal variability in urinary concentrations of perchlorate, nitrate, thiocyanate and iodide among children. J Expo Sci Environ Epidemiol. 2012;22(2):212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mervish NA, Pajak A, Teitelbaum SL, et al. . Thyroid antagonists (perchlorate, thiocyanate, and nitrate) and childhood growth in a longitudinal study of US girls. Environ Health Perspect. 2016;124(4):542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schwingshackl L, Hobl LP, Hoffman G. Effects of low glycemic index/low glycemic load vs. high glycemic index/high glycemic load diets on overweight/obesity and associated risk factors in children and adolescents: a systematic review and meta-analysis. Nutr J. 2015;14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tucker LA, Seljaas GT, Hager RL. Body fat percentage of children varies according to their diet composition. J Am Diet Assoc. 1997;97(9):981–986. [DOI] [PubMed] [Google Scholar]
- 57. Lu L, Xun P, Wan Y, et al. . Long-term association between dairy consumption and risk of childhood obesity: a systematic review and meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2016;70(4):414–423. [DOI] [PubMed] [Google Scholar]
- 58. Moreno LA, Bel-Serrat S, Santaliestra-Pasias A, et al. . Dairy products, yogurt consumption, and cardiometabolic risk in children and adolescents. Nutr Rev. 2015;73(suppl 1):8–14. [DOI] [PubMed] [Google Scholar]
- 59. Laverty AA, Magee L, Monteiro CA, et al. . Sugar and artificially sweetened beverage consumption and adiposity changes: national longitudinal study. Int J Behav Nutr Phys Act. 2015;12:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.