Abstract
As the buccal route of administration has the ability to avoid the GI tract and first pass effect by directing the absorption towards the cheek area, the bioavailability of BCS class III drugs can be increased through this route. Only a handful of studies have been conducted using oleic acid as a permeation enhancer in any transbuccal drug delivery system. Therefore the objective of this novel study was to develop a buccal tablet using two concentrations of oleic acid for a model BCS class III drug via hot-melt extrusion technology and to investigate the effects of oleic acid on the physicochemical properties of the tablet. The model drug selected was ondansetron hydrochloride. Formulations consisting of the polymers (hydroxypropyl methylcellulose and polyethylene oxide) and two concentrations of oleic acid were prepared by hot-melt extrusion techniques. A melting point depression of the drug was obtained in the extruded granules as seen by the DSC thermograms. The ex-vivo permeation studies showed a greater permeation of the drug in the formulation containing 10% oleic acid (F2) as compared to the formulation containing 20% oleic acid (F1), although not statistically significant. The in-vitro bioadhesion studies, swelling studies and surface pH measurements of the tablets were also conducted. In conclusion, permeation studies exhibited the potential of oleic acid as a buccal permeation enhancer as a significant permeation of the drug was obtained in the formulations. Hot-melt extrusion technology was successfully employed to formulate buccal tablets of ondansetron hydrochloride.
Keywords: oleic acid, ondansetron hydrochloride, permeation enhancer, buccal tablet, hot-melt extrusion technology
Introduction
Although the traditional oral route of drug administration is preferred due to its ease of administration and patient compliance, disadvantages such as the hepatic first pass metabolism and enzymatic degradation inside the GI tract could prevent the successful delivery of drugs through this route. Some of the major enzymes present in the liver such as CYP 3A4 and CYP 3A5 metabolize several drugs after absorption through the digestive system, thereby allowing only a small amount of drug to enter the systemic circulation. Drugs that are administered through the oral cavity enter the systemic circulation without any deviations through the internal jugular vein. By doing so, the bioavailability of the drugs can be improved as the drugs can bypass the first pass metabolism. Moreover, when drugs are delivered through the buccal route, they can also avoid degradation by digestive enzymes (1, 2).
Ondansetron hydrochloride (OND), a selective serotonin 5-HT3 receptor antagonist is prescribed to reduce nausea and vomiting that comes with cancer chemotherapy and radiotherapy (3). Its low permeability can be attributed to the extensive first pass hepatic metabolism by the cytochrome P450 enzyme system and therefore it has a poor bioavailability of 60% (4). The drug having a half-life of 3–5 hours and a small molecular weight of 365.86 Da makes it a perfect candidate for administration through the buccal route (5).
One of the drawbacks of transbuccal drug delivery systems is the membrane permeability barrier. Some of the solutions proposed include drug derivatives, drug saturated systems, physical approaches and chemical penetration enhancers. Of these options, the chemical penetration enhancers have been a popular choice for investigators (6). Some of the characteristics of a penetration enhancer include a fast absorbing enhancing effect, reversible action and the enhancer should be non-toxic and not irritating to the buccal tissues (7, 8). Examples of penetration enhancers that can be used include surfactants, bile salts, polyols and fatty acids.
Several studies have been conducted demonstrating the use of oleic acid as a permeation enhancer in transdermal drug delivery systems (9–13). A few studies have also been conducted using oleic acid as a buccal permeation enhancer. Morishita et al., studied the effect of the permeation enhancers (oleic acid and other unsaturated fatty acids) on the release of the drug as well as the hypoglycemic effect of the drug on rat buccal mucosa (14). Lee et al. and Birudaraj et al., studied the combined effect of oleic acid and propylene glycol on the permeation of a model peptide and drug respectively (15, 16). To date, there are no known studies conducted that combine oleic acid and a BCS class III drug (OND). Furthermore, no studies have utilized these formulations to produce a buccal tablet via hot-melt extrusion. Hot-melt extrusion (HME) technology is defined as the process of pumping in raw materials that comprise of the drug, the polymer and the excipients to obtain a product of uniform shape when subjected to a rotating screw at high temperatures (17). Over the conventional methods of pharmaceutical manufacturing, HME provides better efficiency and short times in obtaining the final product, effective in delivering drugs to the patient as well as eliminates the use of solvents. Hence, HME has been recognized as a suitable alternative over the conventional methods of manufacturing tablets, films, implants, etc. which can be delivered through the oral, transdermal and the transmucosal routes (18, 19). The novel aim of this study was to formulate a BCS class III drug (OND) into a buccal tablet comprising of two concentrations of oleic acid via the hot-melt extrusion technology and to investigate the effect of the concentration of the fatty acid on the physicochemical characteristics of the tablet.
Materials and Methods
Materials
Ondansetron hydrochloride (OND) was purchased from A Chemtek Inc. (Worcester, MA). Hydroxypropyl methylcellulose (HPMC E5LV) was purchased from Dow Chemical Company (Midland, MI). Polyethylene oxide (PEO, Sentry Polyox WSR N10 LEO NF) was a kind gift from Colorcon (North Wales, PA). Microcrystalline cellulose (MCC, Avicel NF) was a gift from FMC Biopolymer (Brussels). Guar gum was purchased from Hercules Inc. (Wilmington, DE). Magnesium stearate was a gift from Alfa Aesar (Lancashire). Oleic acid was purchased from Fisher Scientific (Fair Lawn, NJ). The other reagents used in this study were of analytical grade.
Hot-Melt Extrusion (HME)
OND, HPMC and PEO and oleic acid were sieved through US #35 mesh screen and blended using a V-shell blender (Maxiblend, Globe Pharma, New Brunswick, NJ). The blends were processed in a co-rotating twin screw extruder with a modified screw design without an extrusion die (Process 11, Thermo Fisher Scientific, Karlsruhe). The first mixing zone located at the third zone consisted of 60° elements that would ensure a uniformly powdered mixture. The second mixing zone situated at the sixth zone consisted of 90° elements that would provide a homogenous mixing to the blend. The physical blend was manually fed into the extruder and the screw speed was set at 50 rpm. The temperature of all the zones were heated to 60°C. Milling of the extrudates was performed on a comminuting mill (Fitzpatrick, Model “L1A”; Elmhurst, IL) and further sieved using the US #35 and US #40 sieves. The extrudates retained had particle sizes ranging between 300 to 425 μm. The retained extrudates were stored in foil-lined polyethylene bags until further analysis.
Characterization of HME Granules
Differential Scanning Calorimetry (DSC)
DSC studies were conducted on a PerkinElmer Diamond differential scanning calorimeter (DSC) with a Pyris Software. Samples of the pure drug and extrudates were prepared by securely fastening 2~4 mg of the material into aluminum pans. The samples were heated from 30~240°C at a heating rate of 5°C/min. The DSC studies were conducted under an inert nitrogen atmosphere with a flow rate of 20 ml/min.
X-ray Diffraction (XRD)
XRD diffraction studies were performed for the pure drug, polymers and extrudates on a Bruker D8 Advance (Bruker, Billerica, MA) that has a Cu-source and theta-2-theta diffractometer furnished with a Lynx-eye position sensitive detector. A voltage of 40 kV and a current of 30 mA was selected for the generator. The samples were spread over a Si sample holder. The scan was conducted over a range of 2θangles from 2° to 35° with a 0.05° step size at 3s per step.
Tablet Compression
The extrudates were mixed with guar gum, MCC and magnesium stearate prior to compression. Tablets were punched by a ten-station Piccola classic tablet press (SMI, Lebanon, NJ) using an 8.0 mm flat round punch and a compression force of 200 kg/cm2. A rotational speed of 10 rpm (production speed of 6000 tablets/hr) was used.
Method of Analysis
Drug content was determined using a HPLC system (Waters, Milford, MA) having a dual λ absorbance detector and a Waters C18 (4.6 × 150 mm; 5 μm particles) column. The mobile phase consisted of methanol and 10 mM PBS at pH 7.4 in the ratio of 65:35 (%v/v). A steady flow rate of 1ml/min was set with the injection volume at 10 μL. The drug was detected at a wavelength of 306 nm (20).
Ex-vivo Permeation
Freshly slaughtered porcine mucosa was obtained from domestic pigs (Smokehouse meats, Pontotoc, MS) and the excess tissue was cut. Franz diffusion cells (Logan Instruments, Somerset, NJ) were used to conduct the ex-vivo permeation studies. The mucosa was placed between the receptor and the donor compartment and clamped with membrane holders. The receptor compartment consisted of 5 ml of PBS at pH 7.4 that simulated systemic conditions and the donor compartment comprised of the buccal tablet with PBS at pH 6.6 that imitated the salivary conditions. The cells were maintained at 37°C with constant stirring. At predetermined time intervals over a span of 6 hours, 0.5 ml of the sample was taken from the receptor compartment and replaced with PBS 7.4 of the same volume. The samples withdrawn were then subjected to analysis using the HPLC system (21).
In-vitro Drug Release
The in-vitro dissolution studies were conducted as per the United States Pharmacopoeia XIII rotating paddle method. The dissolution media consisted of 150 ml of PBS at pH 6.6. The study was conducted at a temperature of 37°C with a paddle speed of 50 rpm (5). At regular time intervals for 6 hours, 1 ml of the sample was withdrawn (and filtered using a 10μm filter) and replaced with PBS of the same volume. The samples withdrawn were then analyzed through the HPLC system.
Statistical Data Analysis
Levels of statistical significance were assessed using an unpaired t-test between the formulations. If the difference (p-value) obtained was less than 0.05, the formulations were considered to be significantly different. The values obtained were a mean of three readings.
In-vitro Bioadhesion
Mucoadhesion experiments were performed on a texture analyzer (Model TA.XT2i, Texture Technologies Corp./Stable Micro Systems) with a 5 kg load cell. The mucosa was placed on the base of the analyzer with the membrane facing upwards. The tablet to be tested was attached using a double sided tape to the base of a flat end cylindrical probe fixed to the arm of the texture analyzer. A speed of 0.1 mm/sec was used to lower the tablet until it touched the mucosa. For a time of 120 seconds, a contact force of 0.5 N was applied. After which the tablet was removed from the mucosa at a speed of 5 mm/sec. The tack and work of adhesion for each of the formulation was measured (22).
Tablet Swelling Index
Tablets were weighed and placed in 5ml of PBS 6.6. At regular time intervals, the tablets were removed and excess liquid was blotted and reweighed again (23). The percentage swelling index was calculated using the following equation:
where W1 and W2 are the weights of the tablet before placing it in phosphate buffer and at regular time intervals respectively.
Surface pH
Tablets were placed in 5 ml of PBS 6.6 for a time period of 2 hours after which the pH of the tablets was measured by the pH meter (Mettler Toledo, Columbus, OH) (24).
Content Assay of Drug in Extrudates
Twenty tablets were crushed using a mortar and pestle. An equivalent weight of 200 mg was dissolved in methanol. The sample was suitably diluted and then subjected to HPLC analysis.
Evaluation of Buccal Tablets
Weight
Ten tablets from each formulation were weighed using an electronic balance and the average weight was calculated.
Thickness
The thickness of 10 tablets selected from each formulation were measured using a Vernier caliper and the average values were calculated.
Hardness
Three tablets from each formulation were subjected to the hardness tester (Hardness tester, Schleuniger) and the mean values were calculated.
Friability
A dual scooping projection Vander Kamp friabilator (Vankel Industries Inc., Chatham, NJ) was used to conduct the friability test. Twenty tablets each were chosen from the two batches and were weighed before placing them in the apparatus. A rotating speed of 25 rpm and a time period of 4 minutes was set. The tablets were weighed again and the % friability loss was calculated using the following equation:
Results and Discussion
Hot-Melt Extrusion (HME)
The composition of the buccal tablets and the control tablet is shown in Table 1. HPMC and PEO provided the mucoadhesion and swelling to the tablet. Oleic acid was selected as a penetration enhancer for the drug. Guar gum was incorporated into the formulation to aid in the mucoadhesion as well as in the swelling of the polymers. MCC was selected as a diluent and magnesium stearate was used as a lubricant. Process parameters such as the temperature, screw speed and screw configuration were optimized based on preliminary studies conducted. The modified screw design consisting of two mixing zones (Figure 1) that ensured the breakdown of agglomerates if any and to provide a homogenous mixing inside the extruder. The temperature set for all the zones was below the degradation temperature of oleic acid (>80°C). The process parameters produced a minimum torque (2~3%).
Table 1.
Composition of the control tablet and the buccal tablets (in percentile).
| Formulation | OND | HPMC | PEO | Oleic acid | Guar gum | MCC | Magnesium stearate |
|---|---|---|---|---|---|---|---|
| Control | 20 | 25.92 | 14.08 | - | 10 | 29.9 | 0.1 |
| F1 | EX1 | 10 | 29.9 | 0.1 | |||
| 20 | 12.96 | 7.04 | 20 | ||||
| F2 | EX2 | 10 | 29.9 | 0.1 | |||
| 20 | 19.44 | 10.56 | 10 | ||||
Figure 1.
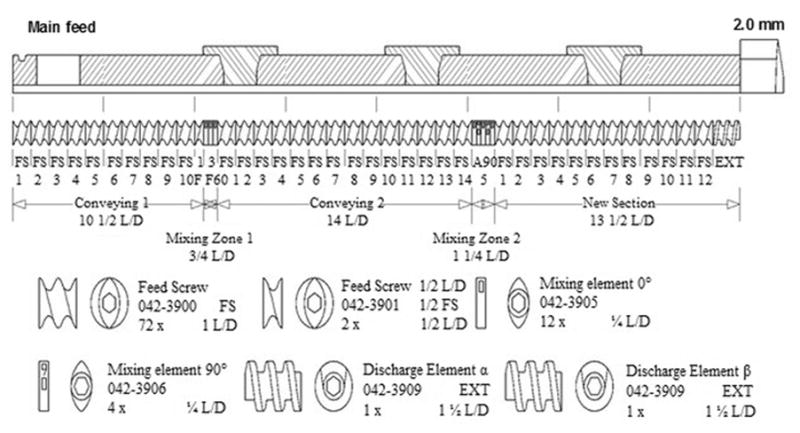
Modified Screw Configuration of the twin-screw extruder used in this study.
Physicochemical Properties of HME Granules
DSC Thermogram
DSC studies were carried out to determine the compatibility of the drug with the polymers and other excipients used (Figure 2). The pure drug gave a melting peak of 186.94°C. Both of the formulations presented a crystalline form of the drug. The extruded formulations reduced the melting point of the drug with EX1 exhibiting a larger reduction (165.74°C) than EX2 (175.49°C), due to the presence of a higher concentration of oleic acid that acted as a plasticizer. DSC studies of the pure polymers were also conducted (data not shown). PEO gave a peak of 68.73°C whereas an amorphous form of HPMC was obtained. A second peak (at 44°C for EX1 and 55°C for EX2) was also present in the formulations. Additional DSC studies on the formulations without PEO were conducted. For these formulations, no peak in the range of 40–70°C were obtained (data not shown). Therefore, it could be concluded that the second peak obtained for the formulations could be attributed to the presence of PEO.
Figure 2.
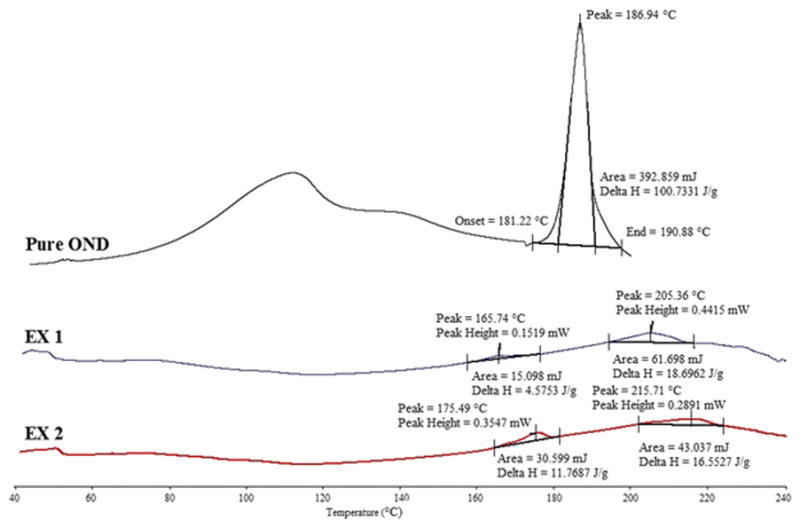
DSC results of pure drug and formulations.
X-Ray Diffraction
Figure 3 represents the X-Ray diffraction patterns of the pure drug, pure polymers, EX1 and EX2. OND is a crystalline drug and exhibited a strong peak at 6°. Both the formulations exhibited an identical diffractogram to the drug peak thereby supporting the results obtained from the DSC experiments stating that the drug present in the formulations after extrusion were crystalline in nature. Strong peaks were present at 19° and 24° for the polymer PEO whereas the diffractogram for pure HPMC showed no peaks.
Figure 3.
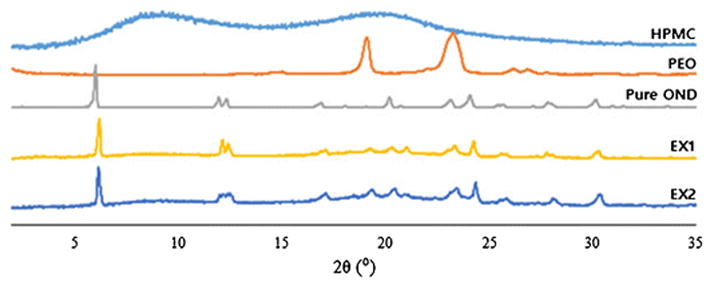
XRD diffractograms for pure drug, polymers and formulations.
Ex-vivo Permeation Studies
Model animals used for permeation studies include dogs, rabbits and monkeys, however pigs are more frequently used as their nonkeratinized oral mucosa closely resembles that to the human buccal epithelium. The control which did not have any permeation enhancer permeated only 0.0095 ± 0.0013 mg/cm2. As extremely small amounts of drug were permeated from the control, the data is not shown. From Figure 6, F1 having a 20 % oleic acid content permeated 1.42 ± 0.11 mg/cm2 of drug whereas F2 having a 10 % oleic acid content permeated 1.83 ± 0.16 mg/cm2 of drug. The potential of oleic acid to act as a permeation enhancer lies in its cis double bond that hinders the formation of well-ordered compact structures and as a result enhances the lipid fluidity. In addition, oleic acid also disrupts the polar head group as well as the hydrophobic region of the membrane lipids that are present in the human buccal epithelial cells (25, 26). In transdermal drug delivery systems, the mechanism of penetration by oleic acid is still being studied, but it has been proposed that when oleic acid permeates the membrane, it perturbs the multi-lamellar structure and therefore gives rise to pathways that are open to permeation in these domains. As the concentration of oleic acid increases, a distinct phase is created within the stratum corneum lipids and hence can no longer disrupt the endogenous lipid domains. Therefore, the amount of drug permeating through the membrane decreases with increase in oleic acid concentration (27–31). This phenomenon of change in functional behavior of oleic acid which is dependent on the concentration could be extended to transbuccal drug delivery system as can be seen in Figure 4 (The unpaired t-test revealed a p-value of 0.054 indicating “approached” acceptable levels of statistical significance for the formulations).
Figure 6.
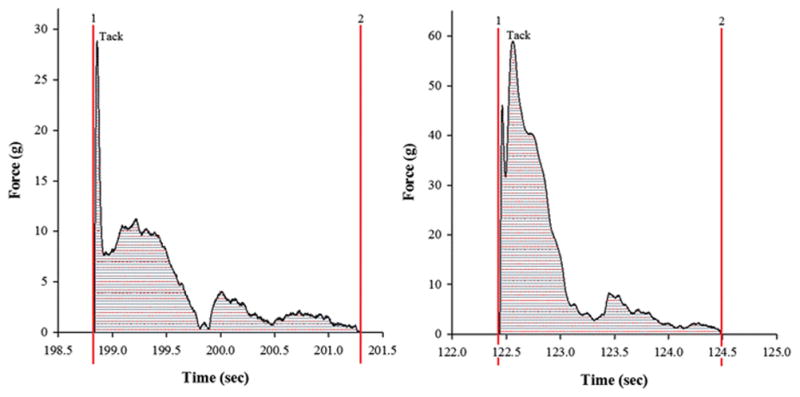
Force vs. Distance profile for (A) F1 and (B) F2 (The figures have been enlarged to depict the area under the curve which represents the work of adhesion). Each value represents the mean ± S.D. (n=3).
Figure 4.
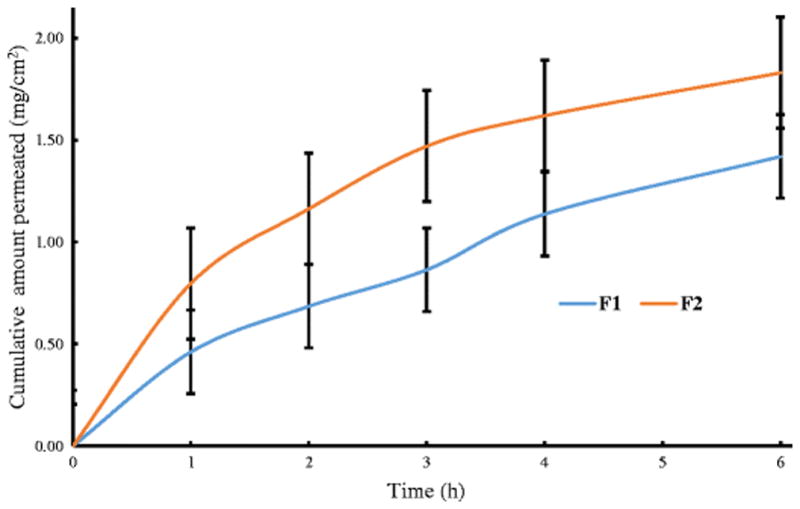
Cumulative amount of OND permeated vs. time from buccal tablets. Each value represents the mean ± S.D. (n=3).
In-vitro Drug Release Studies
The in-vitro dissolution profiles for the formulations are shown in Figure 5. The release profile was enhanced with an increase in oleic acid content. Similar results were observed by Hu et al. who attributed their results to oleic acid outer layers that favored lipophilic drugs and the release mechanism was explained by matrix erosion or diffusion mechanisms (32). A higher concentration of oleic acid in F1 exhibited a drug release of 85% as compared to the 57% drug release shown in F2. This was due to the crystalline nature of the drug obtained in the formulations that hindered the release of the drug. The incomplete release of the drug could be explained by the concentrations of HPMC and PEO used. By increasing the molecular weight of the polymer, the viscosity of the gel formed on the tablets increases and therefore slows down the release of the drug. Even though the molecular weights of the polymers used were low, by increasing the concentration of the polymers in the formulations, the results of the drug release obtained would be similar to that obtained by using high molecular weight polymers at lower concentrations (33, 34). KinetDS software was employed to determine the mechanism of drug release. The drug data was analyzed using the zero order, first order, Korsmeyer-Peppas and Higuchi kinetic equations and the regression coefficient (R2) values were obtained. The formulations fit best into the Korsmeyer-Peppas equation (R2 = 0.9970 and n = 0.53 for F1 and 0.9945 and n = 0.35 for F2). The Korsmeyer-Peppas equation is given by:
Figure 5.
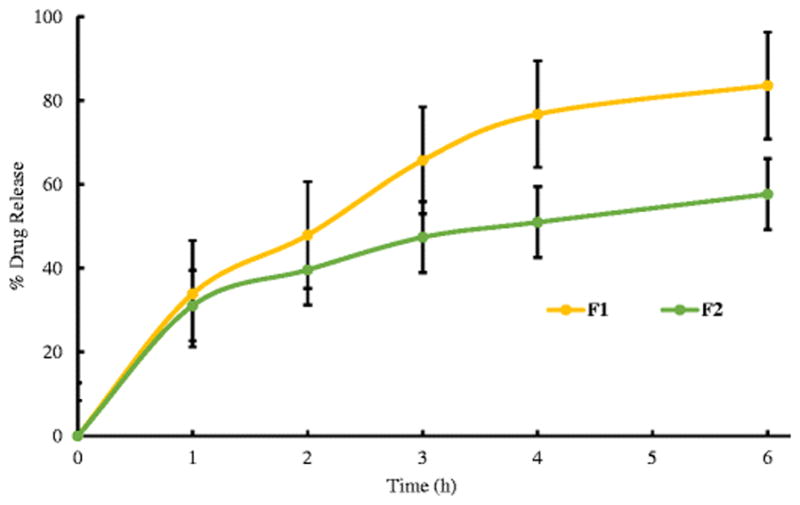
In vitro drug release profiles of the formulations. Each value represents the mean ± S.D. (n=3).
Where, Qt is the drug released in time t, Q∞is the drug release after an infinite time, Kkp is the release constant that consists of the structural and geometrical characteristics of the tablets and n is the release exponent that specifies the mechanism of the drug release (35). As the release constant obtained for F1 was > 0.5, F1 followed the non-Fickian release mechanism (drug release followed a diffusion and a matrix erosion mechanism) and as F2 had a release constant < 0.5, F2 followed a Fickian release mechanism (drug release was controlled by a diffusion controlled mechanism) (36). The results from the t-test revealed a p-value of 0.0088 for the formulations. As the p-value was less than 0.05, the two formulations differences were statistically significant for the in-vitro drug release studies.
In-vitro Bioadhesion Studies
Duchene et al., described the general mechanism behind mucoadhesive polymers in three stages. A close contact between the mucoadhesive incorporated into the formulation and the mucus comprised of the first stage. In the second stage, the mucoadhesive macromolecules swell and penetrate inside the mucus leading to a physical entanglement. These macromolecules then interact with each other through secondary non-covalent bonds making up the third stage (37). With increase in hydration over time, HPMC forms a gel that is strong enough to adhere to the surface prolonging the contact time. But its high glass transition temperature (> 200°C) decreases its flexibility and therefore the polymer provides moderate mucoadhesivity (38). PEO is known to have an extensive and rapid penetration into the substrate due to its linear flexible chains that have a high segmental mobility. This penetration gives rise to a close contact between the polymer and the substrate and therefore increases the bio adhesion (39). Figure 6 shows a force vs. time plot for both the formulations where area under the curve stands for work of adhesion. The peak of detachment obtained for F1 was 27.71 ± 1.23 g and for F2 was 58.08 ± 1.29 g. The work of adhesion obtained for F1 and F2 were 8.26 ± 2.14 g.sec and 15.83 ± 1.55 g.sec respectively. The peak detachment force relies on the hydrogen bonds that are developed between the mucoadhesive polymer and the mucosa. Physical entanglement can also be associated with detachment forces as it promotes interlocking of chains which are a result of the diffusion between the polymer chains and mucus glycoproteins. Increase in swelling of the polymer can lead to an intense physical entanglement thereby resulting in a wider force-time curve and therefore enhancing the work of adhesion (23).
Swelling Studies
Generally when a buccal tablet comes into contact with the medium (e.g. water or PBS), the medium penetrates into the polymer matrix and the swollen gel like polymer allows the drug to diffuse out. The mechanism in which the drug diffuses out is based on the swelling behavior of the polymers involved in the formulation (40). Upon contact with any bodily fluids, the hydrophilic HPMC matrix hydrates starting from its outer boundary and works its way towards the center creating a gel like layer that surrounds the drug. The diffusion of the drug through the matrix to the media is based on the thickness of this newly formed layer (41). A similar swelling mechanism is also observed in PEO (42). Khullar et al. demonstrated that with the addition of guar gum, the rate of water uptake into the matrix increases and the gum swells when it absorbs water (43). From Figure 7, the formulations showed an increase in the % swelling index for up to 2 hours. Beyond 2 hours, a decrease in the % swelling index was observed. On prolonged exposure to the media, the polymer keeps hydrating until a “disentanglement concentration” is reached. At this point, the polymer chains would disentangle and separate themselves from the gel matrix. As a result, the polymer then undergoes swelling, dissolution and diffusion from the matrix at the same time resulting in the erosion of the polymer (44).
Figure 7.
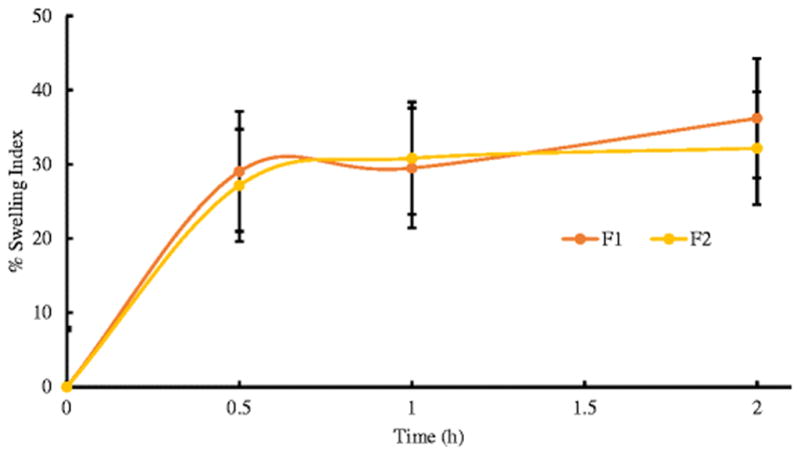
Swelling study profiles for the formulations. Each value represents the mean ± S.D. (n=3).
Surface pH
Surface pH of the buccal dosage forms is measured as an acidic or an alkaline pH would cause irritation to the mucosa. The pH obtained for F1 was 6.01 ± 0.14 and that of F2 was 5.64 ± 0.12. The pH obtained for the formulations were considered safe as they were in an acceptable salivary pH range of 5.5 to 7.0.
Evaluation of Buccal Tablet
The punched tablets were evaluated for weight, thickness, friability, hardness and uniformity of drug content. The results are presented in Table 2. The weight and thickness variation were found to be small in the range of 190.96 to 193.44 mg (F1), 200.08 to 201.08 mg (F2) and 0.138 to 0.142 mm (F1), 0.128 to 0.132 mm (F2), respectively. The obtained friability loss (0.14% and 0.19% for F1 and F2 respectively was less than 1%) which indicated that the tablets possessed an excellent mechanical strength that could endure external impacts. The drug content of both the tablets were uniform as seen in Table 2 and within the acceptable limits.
Table 2.
Physical Properties of the buccal tablets.
| Formulation | Weight (mg) | Thickness (mm) | Friability loss (%) | Hardness (N) | Uniformity of drug content (%) |
|---|---|---|---|---|---|
| F1 | 192.20±1.24 | 0.140±0.002 | 0.14 | 24.83±0.21 | 101.29±1.03 |
| F2 | 200.58±0.50 | 0.130±0.002 | 0.19 | 25.15±0.06 | 102.7±1.91 |
Conclusions
Mucoadhesive buccal HME tablets were successfully developed and evaluated. From the ex-vivo permeation studies, both of the formulations showed a significant permeation of the drug over the control indicating the ability of oleic acid to work as a permeation enhancer in transbuccal drug delivery systems. The in-vitro drug release studies in which F1 released 85% of the drug was explained by the presence of the crystalline form of the drug present after extrusion as well as the concentration of the polymers used. Also no significant increase in the drug release was obtained after six hours. The combination of the polymers PEO and HPMC along with the addition of guar gum provided substantial adhesion to the buccal tablets. In conclusion, HME technology could be employed to prepare mucoadhesive buccal tablets. Moreover, the incorporation of oleic acid as a permeation enhancer also significantly enhanced the buccal permeation of ondansetron hydrochloride.
Acknowledgments
This work was partially supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH. The authors also thank the Pii Center for Pharmaceutical Technology for its contributions to this project.
Footnotes
Declaration of interest
The authors report no declaration of interest.
References
- 1.Verma S, Kaul M, Rawat A, Saini S. An overview on buccal drug delivery system. IJPSR. 2011;1303:1321. [Google Scholar]
- 2.Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci. 1998 Jan 1;1(1):15–30. [PubMed] [Google Scholar]
- 3.Cho E, Gwak H, Chun I. Formulation and evaluation of ondansetron nasal delivery systems. Int J Pharm. 2008;349:101–7. doi: 10.1016/j.ijpharm.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Gwak H, Oh I, Chun I. Transdermal Delivery of Ondansetron Hydrochloride: Effects of Vehicles and Penetration Enhancers. Drug Dev Ind Pharm. 2004;30(2):187–94. doi: 10.1081/ddc-120028714. [DOI] [PubMed] [Google Scholar]
- 5.Kumar P, Prasad R, Pramod M, Reddy P. Effect of permeation enhancer on ex-vivo permeation of Ondansetron HCl buccal tablets. IJPSR. 2011;2(11):2841–5. [Google Scholar]
- 6.Nicolazzo JA, Reed BL, Finnin BC. Buccal penetration enhancers—How do they really work? J Control Release. 2005;105:1–15. doi: 10.1016/j.jconrel.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Aungst BJ. Permeability and metabolism as barriers to transmucosal delivery of peptides and proteins. Drugs Pharm Sci. 1994;62:323–43. [Google Scholar]
- 8.Hassan N, Ahad A, Ali M, Ali J. Chemical permeation enhancers for transbuccal drug delivery. Expert Opin Drug Del. 2010;7:97–112. doi: 10.1517/17425240903338758. [DOI] [PubMed] [Google Scholar]
- 9.Maurya A, Murthy SN. Pretreatment with skin permeability enhancers: importance of duration and composition on the delivery of diclofenac sodium. J Pharm Sci. 2014;103:1497–503. doi: 10.1002/jps.23938. [DOI] [PubMed] [Google Scholar]
- 10.Yamane MA, Williams AC, Barry BW. Effects of terpenes and oleic acid as skin penetration enhancers towards 5-fluorouracil as assessed with time; permeation, partitioning and differential scanning calorimetry. Int J Pharm. 1995;116:237–51. [Google Scholar]
- 11.Gao S, Singh J. Effect of oleic acid/ethanol and oleic acid/propylene glycol on the in vitro percutaneous absorption of 5-fluorouracil and tamoxifen and the macroscopic barrier property of porcine epidermis. Int J Pharm. 1998;165:45–55. [Google Scholar]
- 12.Aungst BJ, Rogers NJ, Shefter E. Enhancement of naloxone penetration through human skin in vitro using fatty acids, fatty alcohols, surfactants, sulfoxides and amides. Int J Pharm. 1986;33:225–34. [Google Scholar]
- 13.Tanojo H, Boelsma E, Junginger HE, Ponec M, Boddéc HE. In vivo human skin permeability enhancement by oleic acid: a laser Doppler velocimetry study. J Control Release. 1999;58:97–104. doi: 10.1016/s0168-3659(98)00144-8. [DOI] [PubMed] [Google Scholar]
- 14.Morishita M, Barichello JM, Takayama K, Chiba Y, Tokiwa S, Nagai T. Pluronic® F-127 gels incorporating highly purified unsaturated fatty acids for buccal delivery of insulin. Int J Pharm. 2001;212(2):289–93. doi: 10.1016/s0378-5173(00)00615-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Kellaway IW. Combined effect of oleic acid and polyethylene glycol 200 on buccal permeation of [D-Ala 2, D-Leu 5] enkephalin from a cubic phase of glyceryl monooleate. Int J Pharm. 2000;204:137–44. doi: 10.1016/s0378-5173(00)00490-7. [DOI] [PubMed] [Google Scholar]
- 16.Birudaraj R, Berner B, Shen S, Li X. Buccal permeation of buspirone: mechanistic studies on transport pathways. J Pharm Sci. 2005;94:70–8. doi: 10.1002/jps.20208. [DOI] [PubMed] [Google Scholar]
- 17.Crowley M, Zhang F, Repka M, et al. Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug Dev Ind Pharm. 2007;33(9):909–26. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- 18.Patil H, Tiwari RV, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2016;17(1):20–42. doi: 10.1208/s12249-015-0360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari RV, Patil H, Repka MA. Contribution of hot-melt extrusion technology to advance drug delivery in the 1st century. Expert Opin Drug Del. 2016;13:451–64. doi: 10.1517/17425247.2016.1126246. [DOI] [PubMed] [Google Scholar]
- 20.Hu L, Damaj B, Martin R, Michniak-Kohn B. Enhanced in vitro transbuccal drug delivery of ondansetron HCl. Int J Pharm. 2011;404(1):66–74. doi: 10.1016/j.ijpharm.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 21.Jones E, Ojewole E, Pillay V, et al. Monolayered multipolymeric buccal films with drug and polymers of opposing solubilities for ARV therapy: Physico-mechanical evaluation and molecular mechanics modelling. Int J Pharm. 2013;455(1):197–212. doi: 10.1016/j.ijpharm.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Boyapally H, Nukala R, Bhujbal P, Douroumis D. Controlled release from directly compressible theophylline buccal tablets. Colloid Surface B. 2010;77(2):227–33. doi: 10.1016/j.colsurfb.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Hassan N, Khar R, Ali M, Javed Ali J. Development and Evaluation of Buccal Bioadhesive Tablet of an Anti-emetic Agent Ondansetron. AAPS PharmSciTech. 2009;10:1085–92. doi: 10.1208/s12249-009-9304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganem-Quintanar A, Guerrero D, Rieg F, Bur P. Ex vivo oral mucosal permeation of lidocaine hydrochloride with sucrose fatty acid esters as absorption enhancers. Int J Pharm. 1998;173:203–10. [Google Scholar]
- 25.Boelsma E, Tanojo H, Boddé HE, Ponec M. Assessment of the potential irritancy of oleic acid on human skin: evaluation in vitro and in vivo. Toxicol In Vitro. 1996;10:729–42. doi: 10.1016/s0887-2333(96)00053-7. [DOI] [PubMed] [Google Scholar]
- 26.Tanojo H, Bos-van Geest A, Bouwstra JA, Junginger HE, Boodé HE. In vitro human skin barrier perturbation by oleic acid: thermal analysis and freeze fracture electron microscopy studies. Thermochim Acta. 1997;293:77–85. [Google Scholar]
- 27.Niazy EM. Influence of oleic acid and other permeation promoters on transdermal delivery of dihydroergotamine through rabbit skin. Int J Pharm. 1991;67:97–100. [Google Scholar]
- 28.Naik A, Pechtold LA, Potts RO, Guy RH. Mechanism of oleic acid-induced skin penetration enhancement in vivo in humans. J Control Release. 1995;37:299–306. [Google Scholar]
- 29.Larrucea E, Arellano A, Santoyo S, Ygartua P. Combined effect of oleic acid and propylene glycol on the percutaneous penetration of tenoxicam and its retention in the skin. Eur J Pharm Biopharm. 2001;52(2):113–9. doi: 10.1016/s0939-6411(01)00158-8. [DOI] [PubMed] [Google Scholar]
- 30.Mak VH, Potts RO, Guy RH. Oleic acid concentration and effect in human stratum corneum: non-invasive determination by attenuated total reflectance infrared spectroscopy in vivo. J Control Release. 1990;12:67–75. [Google Scholar]
- 31.Ongpipattanakul B, Burnette RR, Potts RO, Francoeur ML. Evidence that oleic acid exists in a separate phase within stratum corneum lipids. Pharm Res. 1991;8(3):350–4. doi: 10.1023/a:1015845632280. [DOI] [PubMed] [Google Scholar]
- 32.Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloid Surface B. 2005;45(3):167–73. doi: 10.1016/j.colsurfb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Dow TC. Using methocel cellulose ethers for controlled release of drugs in hydrophilic matrix systems. The Dow Chemical Company; 2000. [Accessed 12-05-16]. http://www.colorcon.com/literature/marketing/mr/Extended%20Release/METHOCEL/English/hydroph_matrix_broch.PDF. [Google Scholar]
- 34.POLYOX Water-Soluble Resins, Unique Resins for Binding, Lubricity, Adhesion and Emollient Performance. The DOW Chemical Company; [Accessed 12-05-16]. http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_094e/0901b8038094e22f.pdf?filepath=/pdfs/noreg/326-00001.pdf&fromPage=GetDoc. [Google Scholar]
- 35.Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- 36.Patil H, Tiwari RV, Upadhye SB, Vladyka RS, Repka MA. Formulation and development of pH-independent/dependent sustained release matrix tablets of ondansetron HCl by a continuous twin-screw melt granulation process. Int J Pharm. 2015;496:33–41. doi: 10.1016/j.ijpharm.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Duchěne D, Touchard F, Peppas NA. Pharmaceutical and medical aspects of bioadhesive systems for drug administration. Drug Dev Ind Pharm. 1988;14:283–318. [Google Scholar]
- 38.Karavas E, Georgarakis, Bikiaris D. Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties for adjusting drug release in predictable pulsatile chronotherapeutics. Eur J Pharm Biopharm. 2006;64:115–26. doi: 10.1016/j.ejpb.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Prodduturi S, Urman K, Otaigbe J, Repka M. Stabilization of Hot-Melt Extrusion Formulations Containing Solid Solutions Using Polymer Blends. AAPS PharmSciTech. 2007;8(2):E152–61. doi: 10.1208/pt0804087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langer R, Peppas N. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials. 1981;2(4):201–14. doi: 10.1016/0142-9612(81)90059-4. [DOI] [PubMed] [Google Scholar]
- 41.Tritt-Goc J, Piślewski N. Magnetic resonance imaging study of the swelling kinetics of hydroxypropylmethylcellulose (HPMC) in water. J Control Release. 2002;80:79–86. doi: 10.1016/s0168-3659(01)00556-9. [DOI] [PubMed] [Google Scholar]
- 42.Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optimal performance. Pharm Sci Technol To. 2000;3(6):198–204. doi: 10.1016/s1461-5347(00)00269-8. [DOI] [PubMed] [Google Scholar]
- 43.Khullar P, Khar RK, Agarwal SP. Evaluation of guar gum in the preparation of sustained-release matrix tablets. Drug Dev Ind Pharm. 1998;24(11):1095–9. doi: 10.3109/03639049809089955. [DOI] [PubMed] [Google Scholar]
- 44.Ju RT, Nixon PR, Patel MV, Tong DM. Drug release from hydrophilic matrices. 2. A mathematical model based on the polymer disentanglement concentration and the diffusion layer. J Pharm Sci. 1995;84(12):1464–77. doi: 10.1002/jps.2600841214. [DOI] [PubMed] [Google Scholar]


