Abstract
The current study examined whether and which specific contents of patients’ memory for cognitive therapy (CT) were associated with treatment adherence and outcome. Data were drawn from a pilot RCT of forty-eight depressed adults, who received either CT plus Memory Support Intervention (CT + Memory Support) or CT-as-usual. Patients’ memory for treatment was measured using the Patient Recall Task and responses were coded into cognitive behavioral therapy (CBT) codes, such as CBT Model and Cognitive Restructuring, and non-CBT codes, such as individual coping strategies and no code. Treatment adherence was measured using therapist and patient ratings during treatment. Depression outcomes included treatment response, remission, and recurrence. Total number of CBT codes recalled was not significantly different comparing CT + Memory Support to CT-as-usual. Total CBT codes recalled were positively associated with adherence, while non-CBT codes recalled were negatively associated with adherence. Treatment responders (vs. non-responders) exhibited a significant increase in their recall of Cognitive Restructuring from session 7 to posttreatment. Greater recall of Cognitive Restructuring was marginally significantly associated with remission. Greater total number of CBT codes recalled (particularly CBT Model) was associated with non-recurrence of depression. Results highlight the important relationships between patients’ memory for treatment and treatment adherence and outcome.
Keywords: patient recall, memory, cognitive therapy, cognitive restructuring, transdiagnostic, depression
Converging evidence across disciplines suggests that patients have poor memory for the content of treatment, which negatively affects adherence to treatment recommendations and clinical outcome. The medical literature has documented poor patient recall for treatment recommendations and health behavior advice (e.g., Bober, Hoke, Duda, & Tung, 2007; Flocke & Stange, 2004; Jansen et al., 2008; Kravitz et al., 1993). Importantly, poor patient recall for medical information is associated with low adherence to treatment recommendations (e.g., Flocke & Stange, 2004; Kravitz et al., 1993; Pickney & Arnason, 2005; Tosteson et al., 2003; Vermeire, Hearnshaw, Van Royen, & Denekens, 2001) and suboptimal clinical outcomes (Bearden et al., 2006; Cohen, Forbes, Mann, & Blanchard, 2006; Martínez-Arán et al., 2004; Polak, Witteveen, Reitsma, & Olff, 2012).
A small but emerging literature has also documented that patient recall for the contents of evidence based psychological treatments (EBPTs) such as cognitive behavioral therapy (CBT) is not optimal and can even be inaccurate. Although several studies have reported that worse baseline verbal memory functioning may be associated with poor treatment response to CBT (Nijdam, De Vries, Gersons, & Olff, 2015; Scott et al., 2017; Wild & Gur, 2008), only a few studies have documented a link between memory for psychological treatment contents and clinical outcome. Among individuals with chronic insomnia, recall of treatment recommendations in CBT for insomnia was around 13%–33% after completing the treatment, although in this study greater recall of treatment recommendations did not predict improvement in insomnia outcomes (Chambers, 1991). In a more recent study, patients’ immediate recall of session contents was only 20–37% among individuals with comorbid bipolar disorder and insomnia receiving CBT for insomnia, and greater recall predicted better clinical outcome (Lee & Harvey, 2015). Another study showed that more than half of the thoughts about, and application of, treatment contents were inaccurate among depressed individuals during the week following a computer-delivered CBT learning module (Gumport et al., 2015). One goal of the current study is to add evidence to this emerging literature on patients’ memory for psychological treatment contents.
These converging lines of research lead to the hypothesis that deriving strategies to improve patients’ memory for treatment may be a novel pathway to improving treatment adherence and outcome. Indeed, a recent pilot randomized controlled trial (RCT) provided initial evidence supporting the use of Memory Support Intervention (MSI) as an adjunctive treatment to enhance patients’ memory for treatment and to improve treatment outcome in the context of cognitive therapy (CT) for depression (Harvey et al., 2016). The current version of the MSI is comprised of eight memory support (MS) strategies, including attention recruitment, categorization, evaluation, application, repetition, practice remembering, cue-based reminders, and praise recall (for more detail see Supplemental Material A). These MS strategies are integrated into treatment-as-usual by treatment providers with the goal of enhancing patients’ memory for treatment contents (Harvey et al., 2014, 2016). Note that the MSI is not intended to directly enhance patients’ memory functioning per se. In this pilot study, MSI was integrated into standard CT for depression (CT + Memory Support) and was compared to standard CT (CT-as-usual). Results suggested that the MSI exerted promising effects on patient recall of treatment contents and treatment outcomes (Harvey et al., 2016).
In this emerging program of research, patients’ memory for treatment is measured using the Patient Recall Task, a free recall task administered at mid-treatment (session 7), posttreatment, and 6-month follow-up (Lee & Harvey, 2015). In this task, participants are asked to list as many treatment points as they can remember from their therapy sessions and to indicate the treatment points from the most recent session. A treatment point is defined as “a main idea, principle, or experience that the treatment provider wants the patient to remember or implement as part of the treatment” (Lee & Harvey, 2015). Responses to this task (i.e., freely recalled treatment points) are then scored to obtain the total number of distinct treatment points recalled. To date, the total number of distinct treatment points recalled, regardless of the specific contents, have been examined in relation to the use of memory support, treatment adherence, and treatment outcome. Briefly, greater patient recall of cumulative treatment contents at mid-treatment was associated with better treatment adherence (Dong, Lee, & Harvey, 2017a) and greater recall of the most recent session was associated with clinical outcomes including treatment responses, remission, and recurrence (Harvey et al., 2016). However, the specific contents of patient recall have not been examined.
To address this gap, the current study examined the qualitative features of patient memory for treatment as well as their relationship to treatment adherence during treatment and outcome at posttreatment and 6-month follow-up. We used data from the NIMH-funded pilot RCT of adults with Major Depressive Disorder (MDD) receiving CT for depression, where participants were randomly allocated to receive 14 sessions of CT + Memory Support or CT-as-usual. Patients’ memory for treatment was assessed using the Patient Recall Task at session 7, posttreatment, and 6-month follow-up. We manually coded all patients’ written responses to the Patient Recall Task into specific CBT concepts and skills (CBT codes), which include: Behavioral Activation, CBT Model, Cognitive Restructuring, Self-Monitoring, Thinking Traps, other CBT techniques (e.g., relaxation, assertive communication, problem solving). These CBT codes were derived from standard treatment manuals of CT for depression and aim to capture treatment contents delivered in this pilot RCT. Patient recall responses that were inconsistent with the CBT contents were assigned non-CBT codes, including Individual Coping strategies and No Code. We then examined the relationships between these patient recall codes and treatment adherence and outcome.
This study has three aims. The first aim was to examine the effects of time (from session 7 to posttreatment and 6-month follow-up) and treatment condition on patient memory for treatment as indexed by the total number of CBT codes recalled (i.e., sum of all specific CBT codes recalled). We hypothesized that the total number of CBT codes recalled would be highest at posttreatment and that the CT + Memory Support condition would have greater total CBT codes than CT-as-usual. The second aim was to examine whether patient recall is associated with treatment adherence. We hypothesized that greater recall of CBT codes (i.e., Behavioral Activation, CBT Model, Cognitive Restructuring, Self-Monitoring, Thinking Traps, other CBT techniques) would be associated with better treatment adherence, and that greater recall of non-CBT codes (i.e., Individual Coping and No Code) would be associated with worse treatment adherence. The third aim was to examine whether patient recall is associated with depression outcome. We hypothesized that greater recall of CBT codes would be associated with better depression outcome (e.g., treatment response and remission at posttreatment, no recurrence at 6-month follow-up), whereas non-CBT codes would not be associated with depression outcome.
Methods
Participants
The participants were forty-eight adults who participated in a NIMH-funded randomized control trial for CT for depression. This study was approved by Committee for the Protection of Human Subjects at the University of California, Berkeley. Written informed consent was obtained and all participants gave this consent willingly. Details of the study are reported elsewhere (Harvey et al., 2016). Table 1 presents the demographic variables of the sample.
Table 1.
Demographic Information of the Study Sample (N = 48)
| Characteristic |
Total (N = 48) |
CT – as usual (N=23) |
CT+Memory Support (N= 25) |
|||
|---|---|---|---|---|---|---|
| M or N | % or SD | M or N | % or SD | M or N | % or SD | |
| Female | 29 | 60.42 | 17 | 73.90 | 12 | 48.00 |
| Ethnicity | ||||||
| Hispanic or Latino | 8 | 16.67 | 3 | 13.04 | 5 | 20.00 |
| Not Hispanic or Latino | 37 | 77.08 | 17 | 73.91 | 20 | 80.00 |
| Declined to answer | 3 | 6.25 | 3 | 13.04 | 0 | 0.00 |
| Race | ||||||
| American Indian/Alaska Native | 1 | 2.08 | 0 | 0.00 | 1 | 4.00 |
| Asian | 4 | 8.33 | 3 | 13.04 | 1 | 4.00 |
| African American | 2 | 4.17 | 1 | 4.35 | 1 | 4.00 |
| Caucasian | 36 | 75.00 | 16 | 69.57 | 20 | 80.00 |
| Bi – racial/Multi – racial | 1 | 2.08 | 1 | 4.35 | 0 | 0.00 |
| Declined to answer | 4 | 8.33 | 2 | 8.70 | 2 | 8.00 |
| Marital Status | ||||||
| Single | 23 | 47.92 | 11 | 47.83 | 12 | 48.00 |
| Married/Partnered | 18 | 37.50 | 8 | 34.78 | 10 | 40.00 |
| Divorced/Separated/Widow | 6 | 12.50 | 3 | 13.05 | 3 | 12.00 |
| Declined to answer | 1 | 2.08 | 1 | 4.35 | 0 | 0.00 |
| Employed | ||||||
| Full – time | 13 | 27.08 | 6 | 26.09 | 7 | 28.00 |
| Part – time | 13 | 27.08 | 4 | 17.39 | 9 | 36.00 |
| Unemployed | 15 | 31.25 | 8 | 34.78 | 7 | 28.00 |
| Retired | 3 | 6.25 | 2 | 8.70 | 1 | 4.00 |
| Declined to answer | 4 | 8.33 | 3 | 13.05 | 1 | 4.00 |
| Income | ||||||
| <$20,000 | 17 | 35.42 | 7 | 30.40 | 10 | 40.00 |
| $20,000 – $35,000 | 6 | 12.50 | 2 | 8.70 | 4 | 16.00 |
| $35,000 – $50,000 | 11 | 22.92 | 7 | 30.40 | 4 | 16.00 |
| $50,000 – $60,000 | 6 | 12.50 | 1 | 4.30 | 5 | 20.00 |
| >$60,000 | 3 | 6.25 | 2 | 8.70 | 1 | 4.00 |
| Refused/Did not know | 8 | 10.42 | 4 | 16.70 | 1 | 4.00 |
| Comorbidity, Medical | 23 | 47.92 | 11 | 45.80 | 12 | 50.00 |
| Comorbidity, Psychiatric | 26 | 54.17 | 10 | 43.50 | 16 | 64.00 |
| Mood Medication | 20 | 41.67 | 11 | 44.00 | 9 | 39.10 |
| Age (years) | 44.27 | 10.97 | 44.65 | 12.17 | 43.92 | 9.98 |
| Education (years) | 16.93 | 2.86 | 16.26 | 2.03 | 15.40 | 1.68 |
Note. M = Mean. N = no. of observations. SD = Standard Deviation. Two randomized treatment conditions did not differ on these variables (data reported elsewhere in Harvey et al., 2016).
Participants were screened and selected via an in-person assessment. Participants were included in the study if they: (a) met diagnostic criteria for major depressive disorder (MDD), first episode, recurrent, or chronic, based on DSM-IV-TR criteria (American Psychological Association, 2000); (b) had a score that is equal to or higher than 24 on the Inventory of Depressive Symptomatology – Self Report (IDS-SR; Rush, Gullion, Basco, Jarrett, & Trivedi, 1996); (c) were older than 18 years old; (d) took no or stable medication that had a minimal effect on memory in the past eight weeks; and (e) were able and willing to provide written consent; (f) had an IQ equal to or above 80.
Exclusion criteria included (a) history of certain psychiatric disorders (i.e., bipolar affective disorder, psychosis, schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder, psychotic organic brain syndrome; antisocial, borderline, or schizotypal personality disorder), (b) diagnosis of current non-psychotic Axis I disorder, which is primary and requires treatment not from the present study; (c) substance dependence in the past six months; (d) evidence of any medical disorder or condition that could be a causal factor for depression onset or stopping treatment; (h) current suicidal risk sufficient to preclude participation in cognitive behavioral therapy.
Procedures
Study Procedures
After assessment of eligibility, patients were randomized to either cognitive therapy with memory support (CT + Memory Support) or cognitive therapy as usual (CT-as-usual). Participants in both conditions received individual 60-minute treatment sessions for 14 weeks with a therapist holding a master’s or doctoral degree in psychology. Each treatment session was videotaped. Therapists in both conditions followed an identical protocol and handouts used were of the same quality and quantity. The only exception was that providers in the CT + Memory Support condition were trained in using the eight memory support strategies including attention recruitment, categorization, evaluation, application, repetition, practice remembering, cue-based reminders, and praise recall (Harvey et al., 2014) and were instructed to incorporate these strategies as much as possible when applicable in treatment.
Note that memory support strategies are designed to intertwine into the treatment-as-usual and are not specific components that take extra time to implement. In other words, adding MS strategies only changes how treatment contents are delivered rather than what is being delivered in each session. For a sample script of how therapists intertwine MS into CT-as-usual, please refer to the supplemental material of the main pilot RCT report (Harvey et al., 2016). There were no significant group differences between CT+Memory Support and CT-as-usual on Cognitive Therapy Rating Scale (CTRS) or Credibility/Expectancy Questionnaire (CEQ) scores, suggesting that the Memory Support Intervention can be intertwined into CT-as-usual without compromising the treatment delivered or patient expectation of improvement (Harvey et al., 2016).
It is also worth noting that there was a baseline level of MS in CT-as-usual, which is significantly lower than the high levels of MS in CT + Memory Support (Dong, Lee, & Harvey, 2017b; Harvey et al., 2016). Specifically, total amount of MS used, the number of different MS categories used, the number of bundles of MS strategies used, as well as 6 out of 8 individual MS strategies (all except for the two most infrequently used MS strategies: categorization and cue-based reminder) were significantly higher in CT+Memory Support than in CT-as-usual with medium to large effect sizes, suggesting that the Memory Support Intervention effectively increases the amount of MS delivered (Dong, Lee, & Harvey, 2017; Harvey et al., 2016). There was also variability in terms of the how MS strategies were implemented across participants (see Table 2 for summary statistics in Dong, Lee, & Harvey, 2017). Briefly, in the CT + Memory Support condition, repetition (M = 6.34 [instances per session], SD = 2.93), attention recruitment (M = 4.33, SD = 1.85), and practice remembering (M = 3.35, SD = 2.37) were used most frequently, while categorization (M = 0.25, SD = 0.36), cue-based reminder (M = 0.45, SD = 0.34), and praise recall (M = 0.56, SD = 0.66) were used least frequently.
Table 2.
Descriptive Statistics of Patient Recall Codes at Session 7, Posttreatment, and 6-month Follow-up for CT+Memory Support and CT-as-usual
| Patient Recall Codes | Total Sample | CT+Memory Support | CT-as-usual | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S7 (n=42) | Post (n=38) | 6FU (n=39) | S7 (n=23) | Post (n=21) | 6FU (n=21) | S7 (n=19) | Post (n=17) | 6FU (n=18) | |
|
| |||||||||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Total CBT codes | 7.24 (3.63) | 8.71 (4.43) | 7.72 (4.10) | 7.74 (4.08) | 9.62 (4.96) | 8.33 (4.15) | 6.63 (3.00) | 7.59 (3.20) | 7.00 (4.03) |
| BA | 0.40 (0.66) | 0.71 (1.11) | 0.72 (1.21) | 0.43 (0.73) | 0.90 (1.34) | 0.76 (1.45) | 0.37 (0.60) | 0.47 (0.72) | 0.67 (0.91) |
| CBT Model | 1.33 (1.34) | 1.34 (1.26) | 1.13 (1.00) | 1.65 (1.43) | 1.38 (1.16) | 1.19 (0.93) | 0.95 (1.13) | 1.29 (1.40) | 1.06 (1.11) |
| CR | 2.67 (1.71) | 3.03 (1.73) | 2.56 (1.74) | 2.96 (1.97) | 3.14 (1.74) | 2.43 (1.91) | 2.32 (1.29) | 2.88 (1.76) | 2.72 (1.56) |
| SM | 0.43 (0.91) | 0.47 (0.51) | 0.41 (0.79) | 0.17 (0.65) | 0.33 (0.48) | 0.24 (0.54) | 0.74 (1.10) | 0.65 (0.49) | 0.61 (0.98) |
| TT | 1.00 (1.40) | 0.92 (1.36) | 1.10 (1.80) | 1.22 (1.65) | 1.05 (1.63) | 1.38 (1.94) | 0.74 (0.99) | 0.76 (0.97) | 0.78 (1.63) |
| Other CBT techniques | 1.40 (1.71) | 2.24 (2.17) | 1.79 (1.96) | 1.30 (1.26) | 2.81 (2.42) | 2.33 (2.18) | 1.53 (2.17) | 1.53 (1.62) | 1.17 (1.50) |
| IC | 0.90 (1.28) | 1.29 (2.15) | 1.67 (1.95) | 0.74 (1.18) | 1.81 (2.68) | 1.81 (2.20) | 1.11 (1.41) | 0.65 (1.00) | 1.50 (1.65) |
| No code | 0.24 (0.53) | 0.32 (0.77) | 0.36 (1.11) | 0.36 (0.66) | 0.43 (0.98) | 0.52 (1.44) | 0.05 (0.23) | 0.18(0.39) | 0.17 (0.51) |
Note. S7 = session 7. Post = posttreatment. 6FU = 6-month follow-up. BA = Behavioral Activation. CBT Model = Cognitive Behavioral Therapy Model. CR = Cognitive Restructuring. SM = Self-Monitoring. TT = Thinking Trap. IC = Individual Coping. The differences in sample size at session 7, post, and follow-up were due to missing data.
Qualitative Coding Procedures
Transcription and segmentation of text
Participants’ written responses to the Patient Recall Task were first transcribed verbatim. Three participant responses used diagrams to depict the CBT model; these were transcribed as brief sentences describing the main idea and keywords noted in the diagram. Each response was subsequently segmented into a codeable unit. Following prior research (Foa, Molnar, & Cashman, 1995; Harvey & Bryant, 1999), a codeable unit was defined as a clause containing only one thought, action, or idea. After the coding was completed, codeable units were examined and combined if the adjacent codeable units were assigned the same code and were about the same treatment point or idea. For instance, if a codeable unit was an elaboration of the idea expressed in the previous codeable unit, the two codeable units would be combined.
Development of Codebook and Coding Procedures
Codes were developed based on two sources: 1) core CBT treatment contents described in Cognitive Behavior Therapy: Basics and Beyond (Beck, 2011) and 2) core CBT treatment handouts and materials for the therapist training workshop conducted for this study. Following the initial creation of the codebook, three stages of coding were conducted. During the first stage of coding, two expert coders (AGH and LD) began an iterative process of coding, reliability assessment, codebook modification, and recoding (as described in Hruschka et al., 2004), with the goal of establishing more than 80% agreement between the two coders on a random sample of fourteen patient recall task responses (equivalent to 10% of the sample). For example, a participant’s entire response to the Patient Recall Task at session 7 is counted as one of the responses for initial coding. During the second stage of coding, each research assistant (XZ and SLO) received training in CBT and began coding to establish over 80% agreement with an expert coder (LD) for 10% of the sample. This was also an iterative process of coding, assessing reliability, discussing discrepancies in code assignment, revising the codebook, and recoding, until the acceptable agreement was reached. During the third stage, the two research assistants coded the entire sample and reliability coefficients were assessed at the end of the coding. All coders were blinded to demographic information, treatment condition and outcome measures during all stages of the coding procedure. Discrepancies were resolved through discussion between the two research assistants and two expert coders and final codes were generated upon consensus.
The final codebook (see Supplemental Material B) was used in coding and includes the following CBT codes:
Behavioral Activation: describing the process of identifying and increasing pleasure/mastery experiences to improve mood.
CBT Model: identifying the CBT components of or interplay among thoughts, feeling, actions, and physiology, or describing how the CBT model can be applied to a real-life situation.
Cognitive Restructuring: identifying, challenging, or modifying thoughts or beliefs.
Self-Monitoring: identifying the use of self-monitoring technique, such as observing/recording emotional response, dysfunctional thought, and/or problem behavior.
Thinking Traps: identifying common cognitive errors, such as black-and-white thinking, overgeneralization, personalization, and catastrophizing.
Other CBT techniques: describing any other CBT techniques, including relaxation, self-care, relapse prevention, psychoeducation, behavioral experiment, assertive communication and problem solving.
Individual Coping Strategies: describing highly-individualized techniques or coping strategies that are not given by the CBT therapists or handouts but are meaningful and helpful to the participants.
No Code: given when the recalled point is not a treatment point, or there was insufficient context to make sense of it.
Initially, Behavioral Experiment, Assertive Communication, and Problem Solving were also assigned as separate codes. Due to low prevalence of these codes, these three codes were merged into the code “Other CBT techniques” for data analysis.
One summary variable “total CBT codes” was derived by taking the sum of all CBT codes (i.e., Behavioral Activation, CBT Model, Cognitive Restructuring, Self-Monitoring, Thinking Traps, and Other CBT techniques) for data analysis. Note that the “total CBT codes” is different from the total number of distinct treatment points recalled (i.e., the product of the scoring of the Patient Recall Task), as the qualitative coding allowed for recalling the same treatment points more than once. For example, in the current study, a participant can have multiple instances of Cognitive Restructuring from his/her response to the Patient Recall Task administered at a given time point, indicating greater memory of the specific content of Cognitive Restructuring. However, for the scoring of the Patient Recall Task, if a patient recalled the same treatment point more than once, only one point is awarded to the group of repeated points (Lee, Worrell, & Harvey, 2015). Also note that Aim 1 in the current study and in Harvey et al. (2016) both examined the effects of treatment condition (CT + Memory Support vs. CT-as-usual) on patient recall. There are important differences in the patient recall variables used in the two studies. Although the patient recall variables in both studies were derived from the same Patient Recall Task, they were coded or scored using different, separately-developed coding schema or rubric. In the current study, responses to the Patient Recall Tasks were manually coded for specific CT contents using the coding scheme (see Supplemental Material B). In Harvey et al. (2016), responses to the Patient Recall Tasks were scored for the total number of distinct treatment points according to a rubric. Hence, the current study examined the total amount of recall for each specific CT content rather than the total number of distinct treatment points remembered (granted that the two should be positively correlated).
Inter-coder reliability
Inter-coder reliability was assessed using several indices: percent agreement, Kappa, and Brenna-Prediger Coefficient (KABAK; Brennan & Prediger, 1981). In the current study, percent agreement was 84.61%, Kappa was 0.81, and KABAK was 0.82, suggesting satisfactory agreement among coders. A Kappa above 0.70 represents good agreement based on Peat (2001, p.228).
Measures
Patient Recall Task
The Patient Recall Task was administered to the patients at session 7, posttreatment and at the 6-month follow-up. This is a free recall task in which the patient is given instructions to write down as many treatment or therapy points as they can remember since the beginning of the treatment, during the entirety of a 10-minute period (Lee & Harvey, 2015; Lee, Worrell, & Harvey, 2015). Patients’ responses were in the form of a list and were portrayed by words, sentences, and diagrams. The instructions for the task follows:
“Take a moment to think back to all the treatment sessions you’ve had with us so far. In the space provided below (use back of sheet if needed), please list as many distinct ‘therapy points’ as you can recall since the start of your treatment. A ‘therapy point’ is an insight, skill, or strategy that you think is important for you to remember and/or implement as part of your treatment. Make sure to only include points that are broad in scope (i.e., points that you would want to remember years from now). You have 10 minutes for this task. Please take the entire 10 minutes so that you record every single point you remember.”
The Patient Recall Task shows adequate inter-rater reliability (r = 0.92, p < 0.001) between two independent raters’ coding for accurately recalled treatment points, and adequate predictive validity of treatment outcome (r’s = 0.34-.69, p’s < 0.001–.15) (Lee et al., 2015).
Binary Mood Outcomes
Binary mood outcomes response and remission were derived using Inventory of Depressive Symptoms – Self Report (IDS-SR) scores measured at pretreatment and posttreatment. IDS-SR is a 30-item questionnaire rated on a 4-point scale to assess severity of depression, with higher score indicating more severe symptoms (Rush et al., 1996). This measure has satisfactory reliability and validity (Rush et al., 1996). In the current study, the IDS-SR was administered to measure depression at baseline (α = 0.78), posttreatment (α = 0.89), and 6-months follow-up (α = 0.92).
Applying criteria from the American College of Neuropsychopharmacology (ACNP) (Rush et al., 2006), response occurred with a decrease of 50% in IDS-SR score from baseline to posttreatment, remission occurred with IDS-SR score of 14 or less at posttreatment, and recurrence was defined as a return to moderate or severe depression following recovery (remission that has been sustained for ≥ 4 months). Recurrence was established using a combination of the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2002) and the Longitudinal Interval Follow-up Evaluation (LIFE; Keller, 1987).
Note that the IDS-SR was a primary outcome for the parent study (Harvey et al., 2016). However, we decided to use response and remission that were derived using IDS-SR scores at pretreatment and posttreatment in the current study because the assessment time points of IDS-SR and patient recall did not match to allow for multilevel modeling (i.e., IDS-SR was assessed at pretreatment, posttreatment, and follow-up; patient recall was assessed at session 7, posttreatment, and follow-up). Relapse, defined as greater than or equal to 26 on the IDS-SR at 6-month follow-up for participants who had remitted, was also originally included in the parent study (Harvey et al., 2016) but was not included in the current analyses due to low sample size (3 out of 7 who had remitted at posttreatment met criteria for relapse in CT + Memory Support; 0 out of 3 met criteria for relapse in CT-as-usual).
Treatment Adherence Rating Scale – Therapist- and Patient-Report
Patient adherence to treatment was measured using a rating scale developed for this study. All items were rated during weekly treatment sessions by both therapists and patients on a scale of 0% to 100% with 10% increments. The items were derived based on Lichstein, Riedel, & Grieve’s (1994) treatment implementation model. This model posits that ensuring treatment receipt (i.e., treatment was comprehended and accepted by the patient as intended) and out-of-session enactment (i.e., treatment recommendations/homework are practiced out of session as intended) are prerequisite steps to infer treatment effectiveness (Lichstein et al., 1994).
For the therapist version, at the end of each weekly treatment session, the therapist rated the patient’s treatment receipt on two items: 1) understanding of the content of the session (no understanding to excellent understanding), and 2) patient acceptance/agreement with the content of the session (did not accept/agree to full acceptance/agreement). They also rated the patient’s treatment enactment during the past week on three items: 1) homework assignment completion for the past week (did not complete to fully completed); 2) overall the extent to which patient adhered to the instructions/recommendations of the treatment during the past week (no adherence to perfect adherence); and 3) overall the extent to which the patient mastered the skills learned in therapy in the past week (no mastery to perfect mastery).
For the patient version, at the beginning of each weekly treatment session, the patient rated his/her adherence on four items that intend to measure treatment enactment: 1) completed the practice exercises outside of session this past week (did not complete to fully completed); 2) followed the instructions/recommendations of the treatment this past week (not at all to completely); 3) mastered the skills learned in therapy this past week (no at all to completely); and 4) used the skills learned in therapy this past week (never to at every opportunity). At the end of each weekly treatment session, the patient rated two adherence items that intend to measure treatment receipt: 1) understanding the content of this session (no understanding to excellent understanding); and 2) accepting/agreeing with the content of this session (did not accept/agree to full acceptance/agreement).
Two summary scores (treatment receipt and enactment) were generated for therapist and patient ratings, respectively. The internal consistency for these adherence scales were excellent: Cronbach’s α’s = 0.87 and 0.89 for the therapist ratings of treatment receipt and enactment, and α’s = 0.84 and 0.87 for the patient ratings of treatment receipt and enactment, respectively.
Data Analysis
All data analyses were conducted using Stata 14 (StataCorp, 2015). A significance level of 0.05 was used throughout. Multilevel modeling (or hierarchical linear modeling) using maximum likelihood estimation was conducted to examine the relationship between patient recall codes on treatment adherence measured at session 7 and posttreatment (Aim 2). The fixed part of the model included continuous patient recall variables (e.g., total CBT codes, total non-CBT codes), two indicators for time periods (posttreatment, 6-month follow-up, with session 7 as the reference), and two patient recall by time period interaction terms. Interaction terms were added because the levels of patient recall codes were not constant across time periods, and were only retained if approached statistical significance at 0.10. The random part of the model included a random intercept and slope of time (in days) since entry into the study, assumed to have a bivariate normal distribution with zero means and unstructured covariance matrix.
Multilevel modeling was also used to examine whether the trajectories of change in the patient recall codes were different for treatment responders/remitters versus treatment non-responders or non-remitters (Aim 3). The fixed part of the model included a binary treatment response or remission variable, two indicators for time periods (posttreatment, 6-month follow-up, with session 7 as the reference), and two response/remission by time period interaction terms.
Aims 2 and 3 analyses were conducted combining the two conditions. The rationale for this decision is threefold: 1) the treatment contents were the same for two conditions, 2) the only difference between the two conditions was the level of memory support provided: high levels in CT + Memory Support and low, but non-zero levels in CT-as-usual, and 3) greater statistical power would be achieved.
Results
Descriptive statistics
Table 1 presents the basic demographic variables for the whole sample, CT + Memory Support, and CT-as-usual. Table 2 presents the descriptive statistics of each patient recall code at session 7, posttreatment, and 6-month follow-up for the whole sample and each condition (i.e., CT + Memory Support, and CT-as-usual). Table 3 presents the descriptive statistics of all other study variables, including treatment adherence and IDS-SR scores at each time point.
Table 3.
Descriptive Statistics of Study Variables
| Variable | N | M | SD | Min | Max |
|---|---|---|---|---|---|
| Treatment Adherence- Therapist Rating | |||||
| Treatment Enactment | |||||
| S7 | 40 | 69.17 | 18.47 | 0 | 100 |
| Post | 35 | 74.10 | 16.55 | 26.67 | 100 |
| Treatment Receipt | |||||
| S7 | 40 | 79.88 | 17.78 | 0 | 100 |
| Post | 35 | 84.43 | 16.26 | 10 | 100 |
| Treatment Adherence-Patient Rating | |||||
| Treatment Enactment | |||||
| S7 | 42 | 59.40 | 22.27 | 0 | 100 |
| Post | 39 | 68.72 | 21.55 | 7.5 | 97.5 |
| Treatment Receipt | |||||
| S7 | 40 | 88.38 | 12.53 | 45 | 100 |
| Post | 38 | 93.55 | 9.29 | 65 | 100 |
| IDS-SR scores | |||||
| Pre | 48 | 41.20 | 9.23 | 26 | 61 |
| Post | 42 | 22.29 | 11.56 | 1 | 46 |
| 6FU | 41 | 23.12 | 13.30 | 3 | 55 |
Note. S7 = session 7. Post = posttreatment. 6FU = 6-month follow-up.
Aim 1
We examined whether total numbers of CBT codes were significantly higher in CT + Memory Support than CT-as-usual. Multilevel modeling indicated that although CT + Memory Support recalled a greater number of total CBT codes than CT-as-usual (b = 1.23, SE = 1.04, p = 0.24), this difference did not reach statistical significance after controlling for the effect of time (session 7, posttreatment, 6-month follow-up). For both treatment conditions, there was a significant increase in the mean of the total CBT code from session 7 to posttreatment (b = 1.30, SE = 0.53, p = 0.01), and a non-significant decrease from posttreatment to 6-month follow-up (b = 0.30, SE = 0.52, p = 0.58). Treatment condition by time interactions were not significant.
Aim 2
As shown in Table 4a, higher total number of CBT codes recalled was significantly associated with better patient (b = 2.19, p = 0.02) and therapist ratings (b = 2.49, p < 0.001) of treatment enactment, and this relationship was stronger at session 7 than at posttreatment (code by time interaction b = −1.94, p = 0.05 for patient rating and b = −1.89, p = 0.03 for therapist rating). Total CBT codes did not significantly predict patient or therapist ratings of treatment receipt.
Table 4a.
CBT Codes Predicting Treatment Adherence (Treatment Receipt and Enactment)
| Total CBT code | BA | CBT Model | CR | SM | TT | Other CBT Techniques | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | |
| Therapist Ratings | |||||||||||||||||||||
| Treatment Receipt | |||||||||||||||||||||
| time (Post vs. S7) | 2.94 | 4.02 | 0.47 | 3.62 | 4.02 | 0.37 | 4.17 | 3.80 | 0.27 | 3.11 | 4.04 | 0.44 | −0.75 | 4.82 | 0.88 | 4.27 | 3.89 | 0.27 | 4.20 | 4.06 | 0.30 |
| code | 0.78 | 0.52 | 0.14 | 1.56 | 2.26 | 0.49 | 3.23 | 1.52 | 0.03 | 2.11 | 1.17 | 0.07 | −9.28 | 2.90 | <0.001 | 1.88 | 1.50 | 0.21 | −0.15 | 1.08 | 0.89 |
| code by time | 10.32 | 6.19 | 0.10 | ||||||||||||||||||
| Treatment Enactment | |||||||||||||||||||||
| time (Post vs. S7) | 21.52 | 7.87 | 0.01 | 12.04 | 4.18 | <0.001 | 8.02 | 3.50 | 0.02 | 6.61 | 3.62 | 0.07 | 1.62 | 4.59 | 0.72 | 8.10 | 3.65 | 0.03 | 7.59 | 3.81 | 0.05 |
| code | 2.49 | 0.71 | <0.001 | 9.57 | 4.07 | 0.02 | 3.41 | 1.57 | 0.03 | 3.15 | 1.17 | 0.01 | −8.70 | 2.86 | 0.002 | 3.17 | 1.51 | 0.04 | 0.28 | 1.10 | 0.80 |
| code by time | −1.89 | 0.86 | 0.03 | −9.14 | 4.26 | 0.03 | 12.88 | 6.07 | 0.03 | ||||||||||||
| Patient Ratings | |||||||||||||||||||||
| Treatment Receipt | |||||||||||||||||||||
| time (Post vs. S7) | −2.06 | 1.84 | 0.26 | −1.87 | 1.78 | 0.29 | −1.60 | 1.75 | 0.36 | −2.01 | 1.76 | 0.25 | −1.58 | 1.71 | 0.36 | −1.17 | 1.66 | 0.48 | −0.84 | 1.64 | 0.61 |
| code | 0.46 | 0.36 | 0.20 | 1.01 | 1.50 | 0.50 | 0.44 | 0.99 | 0.66 | 1.65 | 0.73 | 0.02 | −1.12 | 1.52 | 0.46 | 2.47 | 0.91 | 0.01 | −1.26 | 0.61 | 0.04 |
| Treatment Enactment | |||||||||||||||||||||
| time (Post vs. S7) | 25.08 | 8.91 | 0.01 | 10.65 | 4.04 | 0.01 | 10.65 | 3.97 | 0.01 | 9.73 | 3.79 | 0.01 | 10.63 | 3.93 | 0.01 | 11.23 | 4.04 | 0.01 | 11.40 | 3.96 | <0.001 |
| code | 2.19 | 0.91 | 0.02 | −0.02 | 2.96 | 1.00 | 1.12 | 1.99 | 0.57 | 4.04 | 1.44 | 0.01 | −1.18 | 3.28 | 0.72 | 2.80 | 1.87 | 0.13 | −1.22 | 1.30 | 0.35 |
| code by time | −1.94 | 1.00 | 0.05 | ||||||||||||||||||
Note. BA = Behavioral Activation. CBT Model = Cognitive Behavioral Therapy Model. CR = Cognitive Restructuring. SM = Self-Monitoring. TT = Thinking Trap. S7 = session 7. Post = posttreatment. 6FU = 6-month follow-up.
For specific CBT codes, higher recall of the CBT Model code was significantly associated with better therapist ratings of treatment receipt (b = 3.23, p = 0.03) and enactment (b = 3.41, p = 0.03), although recall of CBT Model did not significantly predict patient ratings of treatment adherence. Higher recall of Cognitive Restructuring predicted better patient ratings of treatment receipt (b = 1.65, p = 0.02) and enactment (b = 4.04, p = 0.01) as well as therapist ratings of treatment receipt (b = 2.11, p = 0.07) and enactment (b = 3.15, p = 0.01) either significantly or at trend level. Higher Behavioral Activation significantly predicted better therapist ratings of treatment enactment (b = 9.57, p = 0.02), and this relationship was significantly stronger at session 7 than at posttreatment (code by time: b = −9.14, p = 0.03). While higher recall of Self-Monitoring at session 7 predicted worse therapist-ratings of treatment receipt (b = −9.28, p < 0.001) and enactment (b = −8.70, p = 0.002), at posttreatment higher recall of Self-Monitoring predicted better treatment enactment (code by time: b = 12.88, p = 0.03) and receipt (code by time: b = 10.32, p = 0.10) either significantly or at trend level. Higher recall of Thinking Traps significantly predicted patient ratings of treatment receipt (b = 2.47, p = 0.01) and therapist ratings of treatment enactment (b = 3.17, p = 0.04). Higher recall of other CBT techniques was associated with lower patient ratings of treatment receipt (b = −1.26, p = 0.04), but did not predict other treatment adherence variables.
As shown in Table 4b, higher Individual Coping predicted worse therapist ratings of treatment receipt (b = −2.08, p = 0.07) and enactment (b = −1.96, p = 0.08) at trend level. Higher No Code predicted worse therapist ratings of treatment receipt (b = −5.05, p = 0.07) and enactment (b = −8.35, p = 0. 004) either significantly or at trend level.
Table 4b.
Non-CBT Codes Predicting Treatment Adherence (Treatment Receipt and Enactment)
| Individual Coping | No code | |||||
|---|---|---|---|---|---|---|
| Coef. | SE | p | Coef. | SE | p | |
| Patient Ratings | ||||||
| Treatment Receipt | ||||||
| intercept | 93.15 | 3.08 | <0.001 | 92.83 | 3.13 | <0.001 |
| time (post vs. s7) | −1.44 | 1.66 | 0.39 | −1.52 | 1.75 | 0.38 |
| code | −0.71 | 0.64 | 0.27 | −1.18 | 1.72 | 0.50 |
| Treatment Enactment | ||||||
| intercept | 48.34 | 6.63 | <0.001 | 48.70 | 6.61 | <0.001 |
| time (post vs. s7) | 10.76 | 3.95 | 0.01 | 10.88 | 3.96 | 0.01 |
| code | −0.53 | 1.39 | 0.70 | −4.07 | 3.57 | 0.25 |
| Therapist Ratings | ||||||
| Treatment Receipt | ||||||
| intercept | 77.20 | 6.05 | <0.001 | 77.14 | 6.29 | <0.001 |
| time (post vs. s7) | 4.96 | 3.78 | 0.19 | 4.40 | 3.97 | 0.27 |
| code | −2.08 | 1.13 | 0.07 | −5.50 | 2.98 | 0.07 |
| Treatment Enactment | ||||||
| intercept | ||||||
| time (post vs. s7) | 61.21 | 5.65 | <0.001 | 61.87 | 5.69 | <0.001 |
| code | 8.80 | 3.41 | 0.01 | 8.45 | 3.50 | 0.02 |
| code × time | −1.96 | 1.13 | 0.08 | −8.35 | 2.87 | 0.004 |
Note. BA = Behavioral Activation. CBT Model = Cognitive Behavioral Therapy Model. CR = Cognitive Restructuring. SM = Self-Monitoring. TT = Thinking Trap. IC = Individual Coping. S7 = session 7. Post = posttreatment. 6FU = 6-month follow-up.
Aim 3
Treatment response
As evident in Table 5a, changes from session 7, posttreatment, to 6-month follow-up for total CBT codes recalled, as well as all specific patient recall codes except for Cognitive Restructuring, were not significantly different in comparing treatment responders to non-responders. For Cognitive Restructuring, Table 5a and Figure 1 show that the interaction between treatment response and time for the recall of Cognitive Restructuring was significant from session 7 to posttreatment and marginally significant from session 7 to 6-month follow-up. Specifically, while treatment responders and non-responders had the same levels of Cognitive Restructuring recalled at session 7 (b = 0.01, p = 0.99), treatment responders exhibited significant increase in their recall of Cognitive Restructuring relative to non-responders from session 7 to posttreatment (b = 1.83, p = 0.002) and had greater reduction in the recall of Cognitive Restructuring codes from posttreatment to 6-month follow-up relative to non-responders (b = 1.08, p = 0.06).
Table 5a.
CBT and Non-CBT Codes Predicted by Treatment Response
| Responder vs. Non-responder at S7 | Responders vs. Non-responders for post vs. S7 change | Responders vs. Non-responders for FU vs. S7 change | Responders vs. Non-responders for FU vs. Post change | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | |
| Total CBT codes | 0.12 | 1.18 | 0.92 | 1.58 | 1.07 | 0.14 | 1.18 | 1.06 | 0.27 | −0.40 | 1.06 | 0.71 |
| BA | −0.14 | 0.30 | 0.65 | 0.003 | 0.33 | 0.99 | −0.16 | 0.33 | 0.64 | −0.16 | 0.33 | 0.63 |
| CBT Model | 0.20 | 0.40 | 0.61 | 0.05 | 0.39 | 0.90 | −0.19 | 0.39 | 0.63 | −0.23 | 0.39 | 0.55 |
| CR | 0.01 | 0.52 | 0.99 | 1.83 | 0.58 | 0.002 | 0.75 | 0.58 | 0.19 | −1.08 | 0.58 | 0.06 |
| SM | −0.53 | 0.24 | 0.03 | 0.48 | 0.33 | 0.15 | 0.70 | 0.33 | 0.03 | 0.22 | 0.33 | 0.51 |
| TT | 0.17 | 0.44 | 0.69 | −0.15 | 0.53 | 0.78 | 0.33 | 0.53 | 0.54 | 0.47 | 0.53 | 0.37 |
| Other CBT technique | 0.66 | 0.65 | 0.32 | −0.54 | 0.70 | 0.44 | −0.13 | 0.70 | 0.85 | 0.41 | 0.70 | 0.55 |
| IC | 0.10 | 0.53 | 0.85 | 0.22 | 0.62 | 0.72 | −0.55 | 0.62 | 0.37 | −0.77 | 0.62 | 0.21 |
| No code | −0.24 | 0.29 | 0.40 | 0.20 | 0.38 | 0.60 | 0.23 | 0.38 | 0.54 | 0.03 | 0.38 | 0.94 |
Note. BA = Behavioral Activation. CBT Model = Cognitive Behavioral Therapy Model. CR = Cognitive Restructuring. SM = Self-Monitoring. TT = Thinking Trap. IC = Individual Coping. S7 = session 7. Post = posttreatment. 6FU = 6-month follow-up.
Figure 1.
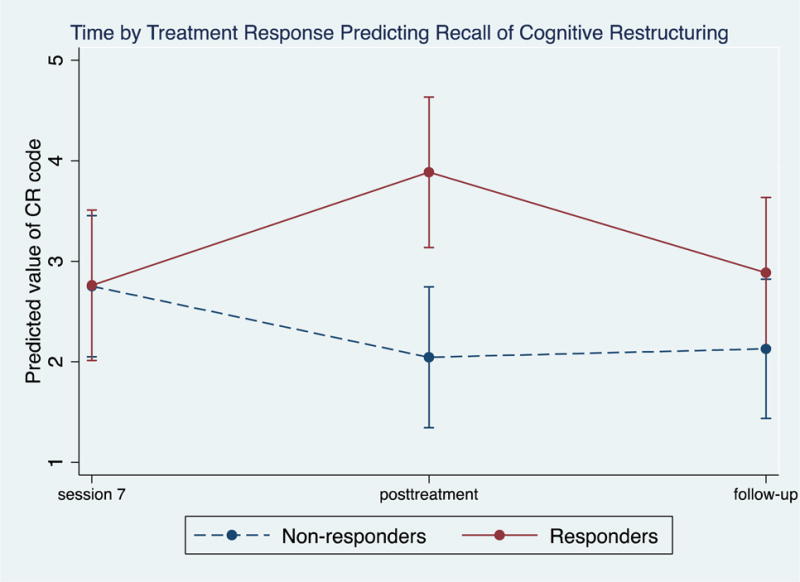
Levels of Cognitive Restructuring at Session 7, Posttreatment, and 6-month follow-up Comparing Treatment Responders versus Non-Responders
As also shown in Table 5a, although treatment non-responders had significantly higher recall of Self-Monitoring at session 7 (b = −0.53, p = 0.03), treatment responders had a significant increase in Self-Monitoring from session 7 to 6-month follow-up relative to treatment non-responders (b = 0.70, p = 0.03).
Remission
As shown in Table 5b, relative to non-remitters, treatment remitters had marginally significant increase in the recall of Cognitive Restructuring codes from session 7 to posttreatment (b = 1.20, p = 0.07) and from session 7 to 6-month follow-up (b = 1.23, p = 0.06). No other results related to remission reached statistical significance.
Table 5b.
CBT and Non-CBT Codes Predicted by Remission
| Remitter vs. Non-remitter at S7 | Remitters vs. Non-remitters for Post vs. S7 change | Remitters vs. Non-remitters for FU vs. S7 change | Remitters vs. Non-remitters for FU vs. Post change | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | |
| Total CBT codes | −1.12 | 1.33 | 0.40 | 1.62 | 1.17 | 0.17 | 1.37 | 1.16 | 0.24 | −0.25 | 1.16 | 0.83 |
| BA | −0.07 | 0.33 | 0.83 | −0.07 | 0.33 | 0.83 | −0.19 | 0.36 | 0.61 | 0.02 | 0.36 | 0.96 |
| CBT Model | 0.11 | 0.45 | 0.81 | 0.002 | 0.43 | 1.00 | −0.35 | 0.43 | 0.41 | −0.35 | 0.43 | 0.41 |
| CR | −0.70 | 0.61 | 0.25 | 1.20 | 0.66 | 0.07 | 1.23 | 0.66 | 0.06 | 0.02 | 0.66 | 0.97 |
| SM | −0.28 | 0.27 | 0.30 | 0.34 | 0.37 | 0.36 | 0.01 | 0.37 | 0.98 | −0.33 | 0.37 | 0.37 |
| TT | 0.63 | 0.50 | 0.21 | −0.32 | 0.58 | 0.58 | 0.13 | 0.58 | 0.82 | 0.45 | 0.58 | 0.44 |
| Other CBT technique | −0.30 | 0.73 | 0.68 | 0.68 | 0.77 | 0.38 | 0.64 | 0.77 | 0.40 | −0.04 | 0.77 | 0.96 |
| IC | 0.01 | 0.60 | 0.98 | 0.43 | 0.68 | 0.53 | −0.61 | 0.68 | 0.37 | −1.04 | 0.68 | 0.12 |
| No code | −0.09 | 0.32 | 0.77 | 0.31 | 0.42 | 0.46 | −0.31 | 0.42 | 0.46 | −0.31 | 0.42 | 0.46 |
Note. BA = Behavioral Activation. CBT Model = Cognitive Behavioral Therapy Model. CR = Cognitive Restructuring. SM = Self-Monitoring. TT = Thinking Trap. IC = Individual Coping. S7 = session 7. Post = posttreatment. 6FU = 6-month follow-up.
Recurrence
As shown in Table 5c, although participants who experienced a recurrence of depression at 6-month follow-up initially had significantly higher recall of total CBT codes at session 7, participants who did not experience recurrence at 6-month follow-up exhibited a significant increase in total CBT codes from session 7 to posttreatment (b = −2.67, p = 0.03), as well as a marginally significant increase in total CBT codes recalled from session 7 to 6-month follow-up (b = −2.24, p = 0.06). Similarly, although participants who experienced recurrence had higher recall of CBT Model at session 7, those who did not experience recurrence exhibited significantly increase in CBT Model recalled from session 7 to posttreatment (b = −0.91, p = 0.04) and to 6-month follow-up (b = −0.88, p = 0.04). For Behavioral Activation, participants who experienced recurrence at 6-month follow-up exhibited a significant increase in their recall of Behavioral Activation from posttreatment to 6-month follow-up (b = 0.82, p = 0.02). In addition, those who did not experience recurrence exhibited significant increase in No Code from session 7 to posttreatment (b = −0.66, p = 0.04), while those who had recurrence exhibited a significant increase in No Code from posttreatment to 6-month follow-up (b = 0.70, p = 0.02). No other treatment condition by time terms were significant.
Table 5c.
Patient Recall Codes Predicted by Treatment Recurrence
| Recurrence vs. Non-recurrence at S7 | Recurrence vs. Non-recurrence for Post vs. S7 change | Recurrence vs. Non-recurrence for FU vs. S7 change | Recurrence vs. Non-recurrence for FU vs. Post change | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | |
| Total CBT codes | 3.36 | 1.42 | 0.02 | −2.67 | 1.22 | 0.03 | −2.24 | 1.19 | 0.06 | 0.43 | 1.19 | 0.72 |
| BA | −0.53 | 0.38 | 0.16 | −0.25 | 0.35 | 0.48 | 0.58 | 0.34 | 0.09 | 0.82 | 0.34 | 0.02 |
| CBT Model | 1.21 | 0.45 | 0.01 | −0.91 | 0.44 | 0.04 | −0.88 | 0.43 | 0.04 | 0.03 | 0.43 | 0.94 |
| CR | 1.61 | 0.66 | 0.01 | −0.60 | 0.77 | 0.44 | −0.90 | 0.75 | 0.24 | −0.30 | 0.75 | 0.69 |
| SM | −0.21 | 0.30 | 0.49 | 0.29 | 0.41 | 0.48 | 0.28 | 0.40 | 0.50 | −0.01 | 0.40 | 0.97 |
| TT | 0.41 | 0.56 | 0.46 | 0.41 | 0.56 | 0.46 | −0.44 | 0.69 | 0.52 | 0.20 | 0.69 | 0.77 |
| Other CBT technique | 0.03 | 0.81 | 0.97 | −0.51 | 0.85 | 0.55 | −0.92 | 0.83 | 0.27 | −0.41 | 0.83 | 0.62 |
| IC | −0.16 | 0.71 | 0.82 | −0.66 | 0.87 | 0.45 | −0.87 | 0.86 | 0.31 | −0.21 | 0.85 | 0.81 |
| No code | 0.03 | 0.24 | 0.90 | −0.66 | 0.32 | 0.04 | 0.04 | 0.31 | 0.90 | 0.70 | 0.31 | 0.02 |
Note. BA = Behavioral Activation. CBT Model = Cognitive Behavioral Therapy Model. CR = Cognitive Restructuring. SM = Self-Monitoring. TT = Thinking Trap. IC = Individual Coping. S7 = session 7. Post = posttreatment. 6FU = 6-month follow-up.
Discussion
The present study examined patients’ memory for treatment in adults with MDD who received CT for depression. The first aim was to examine the effects of treatment condition and time on qualitative patient recall codes. Although the effects of treatment condition did not reach statistical significance, participants in CT + Memory Support condition recalled a greater total number of CBT codes than those in CT-as-usual. This result is consistent with the main report of this pilot RCT, which found that participants in CT + Memory Support recalled more distinct treatment points than those in CT-as-usual, with an effect size in the small-to-medium range but not reaching statistical significance (Harvey et al., 2016). In addition, the total number of CBT codes was highest at posttreatment than session 7 or 6-month follow-up, and there was no significant treatment condition by time interaction on total number of CBT codes recalled.
The lack of significant between-condition difference on the patient recall of total CBT code may be due to the small sample size in this pilot RCT. Another explanation is that the free-recall format of the Patient Recall Task may be a conservative measure of patients’ memory for treatment and too difficult for participants. Indeed, classic cognitive experiments show that relative to cued-recall or recognition tasks free-recall tasks result in less information recalled (Hart, 1967; Tulving & Pearlstone, 1966). Future studies of patients’ memory for treatment should consider exploring the use of recognition or cued-recall task of treatment contents and other indices of learning to better capture the effects of memory support on learning and memory (Gumport, Williams, & Harvey, 2015). In addition, given that the Patient Recall Task instructed participants to list statements that are broad in scope, it is possible that some participants may have listed fewer points to be succinct and general. This would result in an underestimation of the total number of patient recall codes. Nevertheless, from observation, most participants listed many statements given the free-recall format of the task.
The second aim was to examine whether and which patient recall codes were associated with treatment adherence. Although a few did not reach statistical significance, most regression coefficients were in the expected direction such that higher recall of CBT codes (e.g., Behavioral Activation, CBT Model, Cognitive Restructuring, Thinking Traps) predicted better treatment adherence from session 7 to posttreatment. In addition, non-CBT codes (IC and No Code) were either significantly or marginally significantly associated with worse treatment adherence. These are consistent with our hypothesis. Nevertheless, there were a few exceptions, including the negative association between other CBT technique and patient ratings of treatment receipt and the negative association between Self-Monitoring and therapist ratings of treatment receipt at session 7. Overall, our results suggest that patients’ memory for treatment is differentially associated with treatment adherence, such that treatment-consistent memories (e.g., CBT codes) are positively associated with adherence but memories that are not directly related to CT treatment contents are negatively associated with adherence.
The third aim was to examine whether and which patient recall codes were associated with depression outcome. Although most CBT codes were not significantly associated with treatment response and remission, we did observe a significant association between recall of Cognitive Restructuring and treatment response such that treatment responders, relative to non-responders, exhibited a significant surge from session 7 to posttreatment in their recall of cognitive restructuring contents. Similarly, there was a marginally significant association between recall of Cognitive Restructuring and treatment remission. The positive results appear to be specific to Cognitive Restructuring, suggesting that simply being able to recall the CBT model, behavioral activation, self-monitoring, thinking traps, or other CBT techniques (e.g., relaxation) might not be enough to produce symptom change. Being better able to recall contents and process of cognitive restructuring may be more related to treatment response and possibly remission. Overall, these findings replicate and extend prior research showing that patient recall of treatment contents, specifically contents related to cognitive restructuring, is associated with clinical outcome in CT for depression.
Cognitive Restructuring code was assigned when a patient recall response described evaluating, challenging, and/or changing the contents of existing thoughts and beliefs. The ability to recall Cognitive Restructuring related contents may reflect patients’ ability to perform cognitive restructuring techniques, which is hypothesized as a key mechanism of change in CT for depression (Beck et al., 1979). This result is consistent with several lines of prior literature. First, in a sample of depressed adults, patients’ comprehension and usage of CT skills at session 7 and posttreatment predicted greater probability of treatment response to CT (Jarrett, Vittengl, Clark, & Thase, 2011). Second, greater therapist adherence to CT technique (e.g., encouraging clients to evaluate thoughts and beliefs) was predictive of symptom reductions in early sessions. Also, this result extends the previous literature that established a link between patient recall and clinical outcome (Harvey et al., 2016; Lee & Harvey, 2015) and suggests that in CT for depression patient recall of Cognitive Restructuring seems to be most predictive of clinical outcome.
For the outcome of recurrence of depression at 6-month follow-up, those who did not experience recurrence of depression exhibited a significant increase of total CBT codes from session 7 to posttreatment, and a marginally significant increase from session 7 to 6-month follow-up. A similar pattern was also found for CBT Model and recurrence. Interestingly, those who had recurrence exhibited an increase of BA from posttreatment to 6-month follow-up; this pattern is also found for no code and recurrence. It is likely that the recurrence of symptoms triggered the memory of Behavioral Activation and perhaps other treatment-inconsistent memories (i.e., No Code). In general, these results suggest that recall of total CBT codes (particularly CBT Model) were positively associated with better recurrence outcome, but the recall of Behavioral Activation appears to be an exception.
This study has several strengths, including the use of both qualitative and quantitative methods, repeated measures of all study variables, and longitudinal and randomization design. There are several limitations that should be considered when interpreting the current results. First, no causality between predictors (patient recall) and outcomes (adherence and depression outcomes) should be assumed. For example, it is possible that treatment responders may have better memory functioning at posttreatment than non-responders. Causal relationships between patient recall and treatment adherence or outcome need to be established in future research. Second, this study is based on a pilot RCT with a small sample size. It is purposefully underpowered for hypothesis testing, as the rationale for a pilot study is to test for feasibility and an initial signal prior to launching a fully powered large scale clinical trial (Anderson & Prentice, 1999; Craig et al., 2008). Future larger-scale studies should attempt to replicate the current findings. Relatedly, the lack of a significant association between some of the patient recall codes and outcome may be due to the small sample size and reduced power. Again, future larger-scale studies should examine these constructs.
Third, in the pilot study, therapists in the CT+Memory Support condition were trained to use as many MS strategies as possible to deliver each treatment point. The pilot study was used to derive the optimal dose and type of memory support so this information was not available during the conduct of the pilot study. There was also significant within-condition variability in the implementation of MS strategies. Future studies should consider giving more systematic guidance on the amount and type of memory support to provide, and to further clarify the impact of the within-condition variability of MS strategies on patient recall, adherence, and outcome. Fourth, the generalizability of the current findings may be limited by the exclusion of participants with other severe mental disorders, current suicide risk, or current substance use problems. Further examination of the utility of the Memory Support Intervention as well as patients’ memory for treatment in a broader range of psychiatric conditions as well as treatments should be carefully devised and conducted. Finally, it is possible that therapists in CT + Memory Support may be biased in their ratings of patient adherence. Future studies should also incorporate objective measures of treatment adherence, and examine the possible impact of adherence on treatment outcome as well as whether adherence to therapy mediates the link between patients’ memory for treatment and treatment outcome.
It is important for future studies to replicate the current findings on patients’ poor memory for treatment in individuals with other mental disorders, as memory impairment is a problem across diagnostic categories including anxiety disorders (Airaksinen, Larsson, & Forsell, 2005; Castaneda, Tuulio-Henriksson, Marttunen, Suvisaari, & Lönnqvist, 2008; Moon, Yang, & Jeong, 2015; Zlomuzica et al., 2014), post-traumatic stress disorders (Isaac, Cushway, & Jones, 2006; Johnsen & Asbjørnsen, 2008), and schizophrenia (Varga, Magnusson, Flekkøy, David, & Opjordsmoen, 2007). It is also important to extend the current findings to other evidence-based psychological treatments. Additional future directions include to consider if there are additional MS types that should be added to the Memory Support Intervention (e.g., recognition), and to examine potential mechanisms that underlie the link between poor patients’ memory for treatment and clinical outcome.
In sum, the current study examined the qualitative features of patient recall of CT as they relate to treatment adherence and outcome. To the best of our knowledge, this study is among the first to investigate the construct of patient recall of treatment contents in EBPTs and to incorporate a qualitative method. Our results are consistent with results from other studies on the learning and memory processes of CT (Gumport et al., 2015; Harvey et al., 2016; Jarrett et al., 2011; Lee & Harvey, 2015) and on the mechanism of change in CT (Strunk, Brotman, & DeRubeis, 2010). Together these results provide indirect support for the cognitive model of depression and the CT approach described by Beck et al. (1979). This study also suggests that patients’ memory for treatment, as indicated by patient recall of treatment contents, may be an important process variable to investigate in future research on CT or other EBPTs.
Supplementary Material
Highlights.
Patients’ memory for treatment is poor and is associated with worse outcome
No significant between-condition difference on patient recall of total CBT-related contents
Patient recall of treatment-consistent contents was associated with greater adherence
Patient recall of specific cognitive therapy contents was associated with better outcome
Patients’ memory for treatment is potentially a novel target for treatment improvement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: Evidence of episodic memory dysfunction. Journal of Psychiatric Research. 2005;39(2):207–214. doi: 10.1016/j.jpsychires.2004.06.001. http://doi.org/10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Prentice RL. Individually randomized intervention trials for disease prevention and control. Statistical Methods in Medical Research. 1999;8(4):287–309. doi: 10.1177/096228029900800403. http://doi.org/10.1177/096228029900800403. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Glahn DC, Monkul ES, Barrett J, Najt P, Villarreal V, Soares JC. Patterns of memory impairment in bipolar disorder and unipolar major depression. Psychiatry Research. 2006;142(2–3):139–150. doi: 10.1016/j.psychres.2005.08.010. http://doi.org/10.1016/j.psychres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy of depression. New York, NY: Guilford Press; 1979. [Google Scholar]
- Bober SL, Hoke LA, Duda RB, Tung NM. Recommendation recall and satisfaction after attending breast/ovarian cancer risk counseling. Journal of Genetic Counseling. 2007;16(6):755–762. doi: 10.1007/s10897-007-9109-0. http://doi.org/10.1007/s10897-007-9109-0. [DOI] [PubMed] [Google Scholar]
- Brennan RL, Prediger DJ. Coefficient Kappa: Some Uses, Misuses, and Alternatives. Educational and Psychological Measurement. 1981;41(3):687–699. http://doi.org/10.1177/001316448104100307. [Google Scholar]
- Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lönnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. Journal of Affective Disorders. 2008;106(1–2):1–27. doi: 10.1016/j.jad.2007.06.006. http://doi.org/10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Chambers MJ. Patient recall of recommendations in the behavioural treatment of insomnia. Sleep Research. 1991;20:222. [Google Scholar]
- Cohen AS, Forbes CB, Mann MC, Blanchard JJ. Specific cognitive deficits and differential domains of social functioning impairment in schizophrenia. Schizophrenia Research. 2006;81(2–3):227–238. doi: 10.1016/j.schres.2005.09.007. http://doi.org/10.1016/j.schres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. http://doi.org/10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Lee JY, Harvey AG. Do improved patient recall and the provision of memory support enhance treatment adherence? Journal of Behavior Therapy and Experimental Psychiatry. 2017a;54:219–228. doi: 10.1016/j.jbtep.2016.08.017. http://doi.org/10.1016/j.jbtep.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Lee JY, Harvey AG. Memory support strategies and bundles: A pathway to improving cognitive therapy for depression? Journal of Consulting and Clinical Psychology. 2017b;85(3):187–199. doi: 10.1037/ccp0000167. http://doi.org/10.1037/ccp0000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV–TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York, NY: Biometrics Research; 2002. [Google Scholar]
- Flocke SA, Stange KC. Direct observation and patient recall of health behavior advice. Preventive Medicine. 2004;38(3):343–349. doi: 10.1016/j.ypmed.2003.11.004. http://doi.org/10.1016/j.ypmed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Foa EB, Molnar C, Cashman L. Change in rape narratives during exposure therapy for posttraumatic stress disorder. Journal of Traumatic Stress. 1995;8(4):675–690. doi: 10.1007/BF02102894. http://doi.org/10.1007/BF02102894. [DOI] [PubMed] [Google Scholar]
- Gumport NB, Williams JJ, Harvey AG. Learning cognitive behavior therapy. Journal of Behavior Therapy and Experimental Psychiatry. 2015;48:164–169. doi: 10.1016/j.jbtep.2015.03.015. http://doi.org/10.1016/j.jbtep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JT. Memory and the memory-monitoring process. Journal of Verbal Learning and Verbal Behavior. 1967;6(5):685–691. http://doi.org/10.1016/S0022-5371(67)80072-0. [Google Scholar]
- Harvey AG, Bryant RA. A qualitative investigation of the organization of traumatic memories. British Journal of Clinical Psychology. 1999;38(4):401–405. doi: 10.1348/014466599162999. http://doi.org/10.1348/014466599162999. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Lee J, Smith RL, Gumport NB, Hollon SD, Rabe-Hesketh S, Abrons D. Improving outcome for mental disorders by enhancing memory for treatment. Behaviour Research and Therapy. 2016;81:35–46. doi: 10.1016/j.brat.2016.03.007. http://doi.org/10.1016/j.brat.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Lee J, Williams J, Hollon SD, Walker MP, Thompson MA, Smith R. Improving outcome of psychosocial treatments by enhancing memory and learning. Perspectives on Psychological Science. 2014;9(2):161–179. doi: 10.1177/1745691614521781. http://doi.org/10.1177/1745691614521781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruschka DJ, Schwartz D, St John DC, Picone-Decaro E, Jenkins RA, Carey JW. Reliability in Coding Open-Ended Data: Lessons Learned from HIV Behavioral Research. Field Methods. 2004;16(3):307–331. http://doi.org/10.1177/1525822X04266540. [Google Scholar]
- Isaac CL, Cushway D, Jones GV. Is posttraumatic stress disorder associated with specific deficits in episodic memory? Clinical Psychology Review. 2006;26(8):939–955. doi: 10.1016/j.cpr.2005.12.004. http://doi.org/10.1016/j.cpr.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Jansen J, van Weert J, van der Meulen N, van Dulmen S, Heeren T, Bensing J. Recall in older cancer patients: measuring memory for medical information. The Gerontologist. 2008;48(2):149–157. doi: 10.1093/geront/48.2.149. http://doi.org/48/2/149 [pii] [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Vittengl JR, Clark LA, Thase ME. Skills of Cognitive Therapy (SoCT): A new measure of patients’ comprehension and use. Psychological Assessment. 2011;23(3):578–586. doi: 10.1037/a0022485. http://doi.org/10.1037/a0022485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen GE, Asbjørnsen AE. Consistent impaired verbal memory in PTSD: A meta-analysis. Journal of Affective Disorders. 2008;111(1):74–82. doi: 10.1016/j.jad.2008.02.007. http://doi.org/10.1016/j.jad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Keller MB. The Longitudinal Interval Follow-up Evaluation. Archives of General Psychiatry. 1987;44(6):540. doi: 10.1001/archpsyc.1987.01800180050009. http://doi.org/10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kravitz RL, Hays RD, Sherbourne CD, DiMatteo MR, Rogers WH, Ordway L, Greenfield S. Recall of recommendations and adherence to advice among patients with chronic medical conditions. Archives of Internal Medicine. 1993;153(16):1869–78. http://doi.org/10.1001/archinte.1993.00410160029002. [PubMed] [Google Scholar]
- Lee JY, Harvey AG. Memory for therapy in bipolar disorder and comorbid insomnia. Journal of Consulting and Clinical Psychology. 2015;83(1):92–102. doi: 10.1037/a0037911. http://doi.org/10.1037/a0037911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Worrell FC, Harvey AG. The Development and Validation of the Memory Support Rating Scale. Psychological Assessment. 2015 doi: 10.1037/pas0000219. http://doi.org/10.1037/pas0000219. [DOI] [PMC free article] [PubMed]
- Lichstein KL, Riedel BW, Grieve R. Fair tests of clinical trials: A treatment implementation model. Advances in Behaviour Research and Therapy. 1994;16(1):1–29. http://doi.org/10.1016/0146-6402(94)90001-9. [Google Scholar]
- Martínez-Arán A, Vieta E, Reinares M, Colom F, Torrent C, Sánchez-Moreno J, Salamero M. Cognitive Function Across Manic or Hypomanic, Depressed, and Euthymic States in Bipolar Disorder. American Journal of Psychiatry. 2004;161(2):262–270. doi: 10.1176/appi.ajp.161.2.262. http://doi.org/10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- Moon CM, Yang JC, Jeong GW. Explicit verbal memory impairments associated with brain functional deficits and morphological alterations in patients with generalized anxiety disorder. Journal of Affective Disorders. 2015;186:328–36. doi: 10.1016/j.jad.2015.07.038. http://doi.org/10.1016/j.jad.2015.07.038. [DOI] [PubMed] [Google Scholar]
- Nijdam MJ, De Vries GJ, Gersons BPR, Olff M. Response to psychotherapy for posttraumatic stress disorder: The role of pretreatment verbal memory performance. Journal of Clinical Psychiatry. 2015;76(8):e1023–e1028. doi: 10.4088/JCP.14m09438. http://doi.org/10.4088/JCP.14m09438. [DOI] [PubMed] [Google Scholar]
- Pickney CS, Arnason JA. Correlation between patient recall of bone densitometry results and subsequent treatment adherence. Osteoporosis International. 2005;16(9):1156–1160. doi: 10.1007/s00198-004-1818-8. http://doi.org/10.1007/s00198-004-1818-8. [DOI] [PubMed] [Google Scholar]
- Polak AR, Witteveen AB, Reitsma JB, Olff M. The role of executive function in posttraumatic stress disorder: A systematic review. Journal of Affective Disorders. 2012;141(1):11–21. doi: 10.1016/j.jad.2012.01.001. http://doi.org/10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine. 1996;26(3):477–486. doi: 10.1017/s0033291700035558. http://doi.org/10.1017/S0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Schatzberg AF. Report by the ACNP Task Force on Response and Remission in Major Depressive Disorder. Neuropsychopharmacology. 2006;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. http://doi.org/10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Scott JC, Harb G, Brownlow JA, Greene J, Gur RC, Ross RJ. Verbal memory functioning moderates psychotherapy treatment response for PTSD-Related nightmares. Behaviour Research and Therapy. 2017;91:24–32. doi: 10.1016/j.brat.2017.01.004. http://doi.org/10.1016/j.brat.2017.01.004. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- Strunk DR, Brotman MA, DeRubeis RJ. The process of change in cognitive therapy for depression: Predictors of early inter-session symptom gains. Behaviour Research and Therapy. 2010;48(7):599–606. doi: 10.1016/j.brat.2010.03.011. http://doi.org/10.1016/j.brat.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson ANA, Grove MR, Hammond CS, Moncur MM, Ray GT, Hebert GM, Ettinger B. Early discontinuation of treatment for osteoporosis. American Journal of Medicine. 2003;115(3):209–216. doi: 10.1016/s0002-9343(03)00362-0. http://doi.org/10.1016/S0002-9343(03)00362-0. [DOI] [PubMed] [Google Scholar]
- Tulving E, Pearlstone Z. Availability versus accessibility of information in memory for words. Journal of Verbal Learning and Verbal Behavior. 1966;5(4):381–391. http://doi.org/10.1016/S0022-5371(66)80048-8. [Google Scholar]
- Varga M, Magnusson A, Flekkøy K, David AS, Opjordsmoen S. Clinical and neuropsychological correlates of insight in schizophrenia and bipolar I disorder: does diagnosis matter? Comprehensive Psychiatry. 2007;48(6):583–591. doi: 10.1016/j.comppsych.2007.06.003. http://doi.org/10.1016/j.comppsych.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. Journal of Clinical Pharmacy and Therapeutics. 2001;26(5):331–342. doi: 10.1046/j.1365-2710.2001.00363.x. http://doi.org/10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- Wild J, Gur RC. Verbal memory and treatment response in post-traumatic stress disorder. The British Journal of Psychiatry : The Journal of Mental Science. 2008;193(3):254–5. doi: 10.1192/bjp.bp.107.045922. http://doi.org/10.1192/bjp.bp.107.045922. [DOI] [PubMed] [Google Scholar]
- Zlomuzica A, Dere D, Machulska A, Adolph D, Dere E, Margraf J. Episodic Memories in Anxiety Disorders: Clinical Implications. Frontiers in Behavioral Neuroscience. 2014 Apr;8:1–19. doi: 10.3389/fnbeh.2014.00131. http://doi.org/10.3389/fnbeh.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


