Abstract
In 2030, elderly people will represent 20% of the United States population. Even now, chronic cardiac diseases, especially heart failure with preserved systolic function (HFpEF), are the most expensive DRGs for Medicare. Progressive interstitial fibrosis in the aging heart is well recognized as an important component of HFpEF. Our recent studies suggested an important pathophysiologic role for reduced TGF-β receptor 1 (TGFβR1) signaling in mesenchymal stem cells (MSCs) and their mesenchymal fibroblast progeny in the development of interstitial fibrosis.
This report arises from our previous studies, which suggest that an inflammatory phenotype exists in these mesenchymal fibroblasts as a result of a reduced TGF-β-Smad-dependent pathway but upregulated farnesyltransferase (FTase)-Ras-Erk signaling. In this report we provide evidence for a therapeutic approach that downregulates Erk activation through an adenosine monophosphate-activated kinase (AMPK) pathway. Aging C57BL/6J mice were treated with AICAR (an AMPK activator) for a 30-day period. This treatment suppressed excessive monocyte chemoattractant protein-1 (MCP-1) generation, which diminished leukocyte infiltration and in consequence suppressed the formation of macrophage-derived myeloid fibroblasts. Interestingly, the number of mesenchymal fibroblasts was also reduced. In addition, we observed changes in extracellular matrix (ECM) deposition, specifically that collagen type I and the alternatively spliced variant of fibronectin (EDA) expressions were reduced. These data suggest that the upregulation of AMPK activity is a potential therapeutic approach to fibrosis in the aging heart.
Keywords: fibrosis, fibroblast, heart, macrophage, AMPK
1. INTRODUCTION
We have previously described an acute model of cardiac interstitial fibrosis induced by MCP-1 generation resulting in the accumulation of myeloid fibroblasts [1]. The myeloid fibroblast formation was rapidly reduced when MCP-1 was suppressed by TGF-β1 [2].
In contrast to acute models of interstitial fibrosis, the aging mouse (C57BL/6J) developed progressive fibrosis and cardiac dysfunction that was associated with an increasing number of myeloid fibroblasts and increasing MCP-1 and IL-13 synthesis [3]. The age-dependent increase in MCP-1 correlated with an increase in interstitial fibrosis and associated hemodynamic abnormalities [3].
In the aging mouse, we found a defect in endogenous MSCs resulting in marked reduction of TGFβR1 expression and reduced TGF-β responsiveness [4]. Because of reduced responsiveness to TGF-β, these fibroblasts make inflammatory chemokines and cytokines (usually suppressed by TGF-β) [5] that causes an induction of the myeloid fibroblasts [6]. This results in continuous ongoing inflammatory fibrosis in the old heart [6]. In addition, MSCs lack the brake on stem cell differentiation maintained by TGF-β [7] and enter into a differentiation cycle more readily [8], which causes the increased number of mesenchymal fibroblasts found in the aged heart [9]. Finally, despite impaired TGF-β signaling, the mesenchymal fibroblast continued to make collagen via an upregulated FTase-Ras-Erk pathway [9].
We have previously demonstrated that the use of the AMPK agonist, AICAR, increased TGF-β responsiveness to MSCs and their fibroblast progeny in vitro [4] and in a myocardial infarction model in the aging mouse [10]. Therefore, we postulated that AICAR would suppress the cellular phenotype described above via amplified TGF-β signaling. Our data suggest that AICAR treatment reduces the number of myeloid and mesenchymal fibroblasts. Interestingly, in the mesenchymal fibroblasts derived from the aging heart, upregulation of MCP-1 (that is necessary for myeloid fibroblast formation) and some ECM proteins depends on Erk activation, which is reduced by AICAR treatment. The purpose of this study was to evaluate AICAR treatment as a potential therapeutic target in age-dependent cardiac fibrosis.
2. METHODS
2.1 Animals
14–21 month-old male C57BL/6J mice were obtained from the National Institute of Aging and aged if necessary. Animals were injected with AICAR (Toronto Research Chemical, 0.5 mg/g of body weight) or saline every day for 7 days or three times a week for 1 month. All animals were treated in accordance with the guidelines of the Baylor College of Medicine Animal Care and Research Advisory Committee.
2.2 Cell isolation
Hearts were cut into 1 mm3 pieces and digested with Liberase TH Roche Diagnostics, Indianapolis, IN). The resulting non-myocyte cells were immediately used for flow cytometry analysis [6] or for tissue culture.
2.3 Tissue culture
Cells were cultured as previously described [4]. Quiescence was initiated 24 h before each experiment by switching the medium to low glucose DMEM without FBS.
2.4 Flow cytometry
Cell viability was determined by Calcein AM (Thermo Fisher Scientific). Cells were labeled as described before [6].
2.5 Immunofluorescence staining
Paraffin-embedded mouse heart sections were stained with anti-EDA antibody (#F6140, Sigma, St. Louis, MO) or collagen type 1a (Rockland Immunochemicals) followed by the secondary antibody conjugated to fluorescein or DyLight 549 (#115-506-062, Jackson ImmunoResearch).
2.6 Quantitative PCR (qPCR)
Gene expression was measured by the comparative ΔΔCT method to calculate the amount of target mRNA normalized to an endogenous reference (Hprt or 18S) as previously described [6].
Primer sequences
Col3a1: sense 5′-GATGAGGAGCCACTAGACTG-3′ and antisense 5′-GCCATCAGGAAGCACAGG-3′; MCP-1: sense 5′-TCTGGGCCTGCTGTTCACA-3′ and antisense 5′-GGCGTTAACTGCATCTGGCT-3′, FN: sense 5′-TTCAAGTGTGATCCCCATGAAG-3′ and antisense 5′-CAGGTCTACGGCAGTTGTCA-3′; EDA: sense 5′-CAAACTGCAGTGACC-3′ and antisense 5′-CATGAGTCCTGACAC-3′; Hprt: sense 5′-GCCCCAAAATGGTTAAGGTT-3′, antisense 5′-TTGCGCTCATCTTAGGCTTT-3′; 18S sense 5′-CGGACAGGATTGACAGATTG -3′, antisense 5′-CAAATCGCTCCACCAACTAA -3′. FN primers can detect all seven fibronectin variants, which include plasma and cellular fibronectin).
2.7 Western Blot
For western blot analysis cultured cell or whole heart lysates were used as described before [4, 9]. The following primary antibodies obtained from Cell Signaling (Danvers, MA) were used: anti-AMPK (#2603), anti-pAMPK (Thr172, #2535), anti-Erk (#4695), anti-pErk (Thr202/204, #4370). Secondary antibody anti-rabbit IR Dye 800CW was purchased from Licor (#926-32211).
3. RESULTS
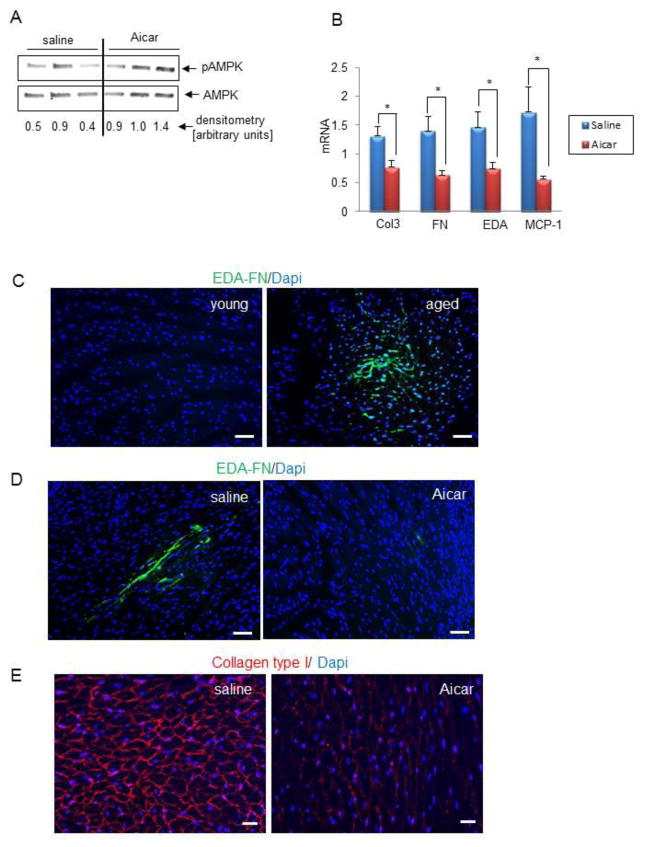
Figure 1A demonstrates that in vivo AICAR treatment results in a two fold increase in AMPK activation. Upregulation of the AMPK pathway signaling caused a suppression of MCP-1, fibronectin (FN) and its alternatively spliced isoform EDA, as well as collagen type III (Col 3) (Figure 1B). Figure 1C demonstrates the presence of alternatively spliced fibronectin (EDA) by immunofluorescence staining, in the aging but not in the young heart. EDA is a marker usually associated with inflammatory injury [11]. Since EDA is usually transiently expressed, we examined the effect of 7 days (short term) of AICAR injection on the aged heart phenotype. After 7 days, AICAR substantially decreased the presence of EDA in the aged heart (Figure 1D). Deposition of collagen type I into ECM fibrils depends on fibronectin. Because 7 days of treatment was enough for EDA to disappear from the aging heart, we treated mice for 7 days with AICAR but analyzed hearts in regards to collagen remodeling after 30 days, since changes in collagen degradation may take longer. Even short term AICAR treatment caused a reduction in collagen deposition (Figure 1E), when compared with saline injected mice.
Figure 1. AICAR treatment reduces levels of injury markers and fibrosis.
A. Western blot analysis of phosphorylated AMPK in heart lysates isolated from saline and AICAR-injected mice. B, QPCR analysis of RNA isolated from whole hearts derived from 21 month-old mice injected with saline or AICAR for 4 weeks. Results are represented as mean ± SEM. * denotes p<0.05. C, EDA is present in the aged uninjured heart as shown by immunofluorescence staining. Young denotes 3 month-old and aged denotes 24 month-old mice Scale bar = 50 μm. D, 7 days AICAR treatment reduces the presence of EDA in the aged heart. 14 month-old mice were injected with saline or AICAR for 7 days. Heart sections were stained with anti-EDA antibody (green) or Dapi (blue). Scale bar = 50 μm. E, 14 month-old mice were injected with saline or AICAR for 7 days and 30 days after the last injection hearts were analyzed using anti-collagen type I antibody (red) or Dapi (blue). Scale bar = 50μm. N=3 (for A, C), 5 (for D and E), and for B, N = 8, 4 for saline and AICAR treated mice respectively.
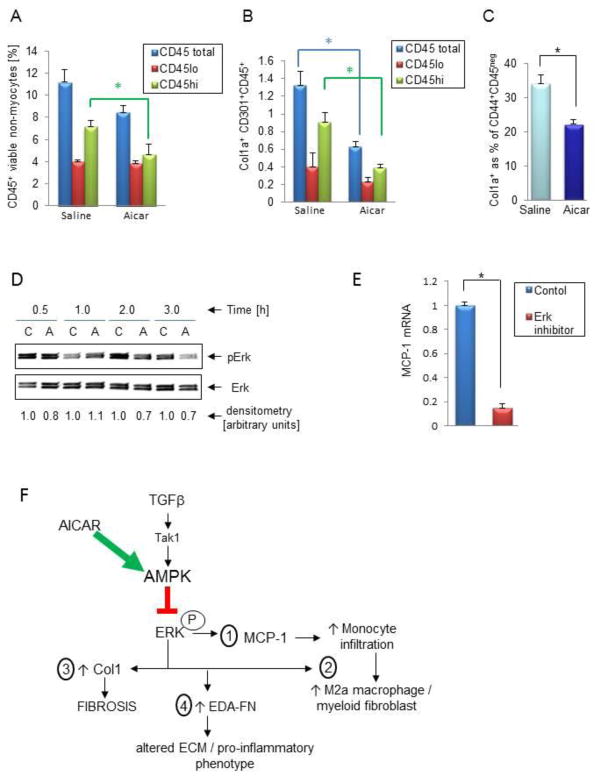
We have shown before [6] that the expression level of the CD45 pan leukocyte marker can distinguish between resident (CD45lo) and infiltrating (CD45hi) leukocytes. In Figure 2A we demonstrate that AICAR treatment reduced the number of infiltrating leukocytes (CD45hi) while having no effect on resident leukocytes (CD45lo), consistent with reduced MCP-1 expression (Figure 1B). Similarly, we demonstrated that monocyte-derived alternatively activated macrophages (M2a)/myeloid fibroblasts (Col1a+CD301+CD45hi) were suppressed by AICAR treatment (Figure 2B).
Figure 2. AICAR treatment reduces the number of fibroblasts of two developmental origins in the aged mouse heart.
A, Flow cytometry analysis of calcein+ (viable) non-myocytes isolated from 21 month-old hearts using CD45-PE antibody. The graph depicts quantification of all CD45+ cells that have low (CD45lo), high (CD45hi) or total expression of CD45. B, Analysis of the contribution of resident (CD45lo) and infiltrating (CD45hi) leukocytes to the M2a macrophage/myeloid fibroblast pool (Col1a+CD301+CD45+) by flow cytometry. C, The percentage of mesenchymal fibroblasts (as collagen producing cells within the CD44+CD45− pool) is reduced with AICAR treatment as quantified by flow cytometry analysis. D, AICAR treatment reduces Erk phosphorylation. Quiescent mesenchymal fibroblasts derived from 24–30 month-old hearts were treated with 0.5 mM of AICAR for indicated period of time. C denotes control, A denotes AICAR. E, QPCR analysis of MCP-1 expression in quiescent mesenchymal fibroblasts derived from 24–30 month-old hearts subjected to 1 μM PD0325901 (Erk inhibitor) for 24 h. * denotes p<0.05. F, Schema (see Conclusions). Results are represented as mean ± SEM. N = 6 and 12 (for A, B); N= 6 and 4 (for C) for saline and AICAR treated mice respectively and N=4 (for D and E).
We have shown before that the lack of TGF-β responsiveness in MSCs allows their augmented differentiation into mesenchymal fibroblasts. In Figure 2C, we demonstrate that activation of AMPK and possible amplification of TGF-β signaling (TGF-β is available in the aging heart) causes reduction of the number of mesenchymal fibroblasts (Col1a+CD44+CD45neg) as well. There was no significant difference in the total amount of MSCs between the saline and AICAR treated animals (data not shown).
Since the FTase-Ras-Erk pathway is highly operant in the aging mesenchymal fibroblasts [9], we hypothesized that AMPK may exert its effects at least partially via downregulation of the Erk pathway. We found that when mesenchymal fibroblasts derived from aged hearts were treated with 0.5 mM of AICAR, phosphorylated Erk was reduced by 30% (Figure 2D). Moreover, since other crucial players in aging fibroblasts such as MCP-1 (Figure 2E), Col1 [9] and EDA [12] can also be controlled by the Erk pathway, we hypothesized that Erk activation is a critical mediator of age-dependent fibrosis that is downregulated by AICAR treatment.
4. CONCLUSIONS
Figure 2F summarizes the results presented in the context of our previous [4, 10] and current work on the effect of amplifying TGF-β signaling via AICAR-dependent activation of AMPK in defective mesenchymal fibroblasts. AICAR-dependent AMPK activation inhibits the Erk pathway and suppresses MCP-1-stimulated monocyte infiltration (1) and therefore M2a macrophage polarization/myeloid fibroblast formation (2). It also reduces collagen levels (3). Finally, AICAR therapy inhibits the augmented production of native FN and its alternatively spliced EDA variant (4).
Others have found that AMPK may protect against fibrosis in various organs via a number of proposed direct and indirect mechanisms (see recent review) [13, 14]. The current paper suggests that AICAR-dependent treatment may be a potential clinical approach to cardiac fibrosis in aging. On this last note we have not observed adverse effects from AICAR treatment of aged mice in this study nor in our previous studies [10]. AICAR has also been used clinically with no severe adverse effects [15].
HIGHLIGHTS.
Inflammation based remodeling
Role of Erk pathway in inflammation and fibrosis
AICAR-induced AMPK decreases Erk activation
AMPK-dependent suppression of the inflammatory fibroblast
Acknowledgments
This work was supported by NIH grant R01HL089792, The Medallion Foundation and The Hankamer Foundation.
GLOSSARY
- FN
Fibronectin
- MSC
mesenchymal stem cell
- TGF-β
transforming growth factor-β
- TGFβR1
TGF-β receptor 1
- MCP-1
monocyte chemoattractant protein-1
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, et al. Of mice and dogs: Species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–77. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML. Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol. 2011;50:248–56. doi: 10.1016/j.yjmcc.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cieslik KA, Trial J, Entman ML. Defective myofibroblast formation from mesenchymal stem cells in the aging murine heart rescue by activation of the AMPK pathway. Am J Pathol. 2011;179:1792–806. doi: 10.1016/j.ajpath.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cieslik KA, Trial J, Crawford JR, Taffet GE, Entman ML. Adverse fibrosis in the aging heart depends on signaling between myeloid and mesenchymal cells; role of inflammatory fibroblasts. J Mol Cell Cardiol. 2014;70:56–63. doi: 10.1016/j.yjmcc.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trial J, Pena Heredia C, Taffet GE, Entman ML, Cieslik KA. Dissecting the Role of Myeloid and Mesenchymal Fibroblasts in Age-dependent Cardiac Fibrosis. Basic Res Cardiol. 2017;112:34. doi: 10.1007/s00395-017-0623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 9.Cieslik KA, Trial J, Carlson S, Taffet GE, Entman ML. Aberrant differentiation of fibroblast progenitors contributes to fibrosis in the aged murine heart: role of elevated circulating insulin levels. FASEB J. 2013;27:1761–71. doi: 10.1096/fj.12-220145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cieslik KA, Taffet GE, Crawford JR, Trial J, Mejia OP, Entman ML. AICAR-dependent AMPK activation improves scar formation in the aged heart in a murine model of reperfused myocardial infarction. J Mol Cell Cardiol. 2013;63:26–36. doi: 10.1016/j.yjmcc.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, et al. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–92. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ayoubi AM, Zheng H, Liu Y, Bai T, Eblen ST. Mitogen-activated protein kinase phosphorylation of splicing factor 45 (SPF45) regulates SPF45 alternative splicing site utilization, proliferation, and cell adhesion. Mol Cell Biol. 2012;32:2880–93. doi: 10.1128/MCB.06327-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang S, Li T, Yang Z, Yi W, Di S, Sun Y, et al. AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res Rev. 2017;38:18–27. doi: 10.1016/j.arr.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, et al. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008;52:918–24. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman MF, Ferguson TB, White JA, Ambrosio G, Koglin J, Nussmeier NA, et al. Effect of adenosine-regulating agent acadesine on morbidity and mortality associated with coronary artery bypass grafting: the RED-CABG randomized controlled trial. JAMA. 2012;308:157–64. doi: 10.1001/jama.2012.7633. [DOI] [PubMed] [Google Scholar]