Abstract
Background
Individuals with stroke fall frequently, and no exercise intervention has been shown to prevent falls post-stroke. Perturbation-based balance training (PBT), which involves practicing reactions to instability, shows promise for preventing falls in older adults and individuals with Parkinson disease. This study aimed to determine if PBT during in-patient stroke rehabilitation can prevent falls after discharge into the community.
Methods
Individuals with sub-acute stroke completed PBT as part of routine in-patient rehabilitation (n=31). Participants reported falls experienced in daily life for up to 6 months post-discharge. Fall rates were compared to a matched historical control group (HIS) who did not complete PBT during in-patient rehabilitation.
Results
Five of 31 PBT participants, compared to 15/31 HIS participants, reported at least one fall. PBT participants reported 10 falls (0.84 falls per person per year), whereas HIS participants reported 31 falls (2.0 falls per person per year). When controlled for follow-up duration and motor impairment, fall rates were lower in the PBT group than the HIS group (rate ratio: 0.36 [0.15, 0.79]; p=0.016).
Conclusions
These findings suggest that PBT is promising for reducing falls post-stroke. While this was not a randomized controlled trial, this study may provide sufficient evidence for implementing PBT in stroke rehabilitation practice.
INTRODUCTION
Falls are a frequent medical complication during all stages of stroke recovery.1 The risk of falling2 and fall-related injury3 is more than twice as high for people with stroke compared to similarly-aged people without stroke. Individuals who have recently been discharged home after in-patient stroke rehabilitation are particularly vulnerable to falling.1,4–6 Those who fall soon after discharge from in-patient rehabilitation have worse functional recovery at 6-months post-discharge than those who do not fall,7 possibly because the fall leads to fear and self-imposed activity restriction. Current treatment approaches likely do not adequately prepare individuals with stroke for the challenges they will face after discharge home to their ‘normal’ lives.8–10
Physical exercise, particularly balance training, reduces fall risk among older adults.11 However, traditional approaches to balance training do not prevent falls post-stroke.8,9 Falls happen due to failure to recover from a loss of balance.12 Therefore, balance training that improves balance reactions might help to prevent falls. Perturbation-based balance training (PBT), which involves exposing individuals to repeated postural perturbations,13,14 is a novel exercise intervention that aims to improve control of balance reactions. Preliminary studies suggest that PBT almost halves fall rates among healthy older adults, older people with various diagnoses (including chronic stroke), and people with Parkinson disease.15
This study aimed to determine the effect of PBT on fall occurrence after discharge home from in-patient stroke rehabilitation. Secondary objectives were to determine effects of PBT on balance confidence, functional balance and mobility, and participation in daily physical activity. We hypothesized that, compared to a historical control group, the PBT group would: report lower rates of falls and greater physical activity participation in the 6 months post-discharge; and have greater improvements in balance confidence and balance and mobility function from admission to discharge from in-patient rehabilitation. We also report on the characteristics of falls after PBT.
METHODS
Study design
This study involved a prospective cohort study with comparison to a matched historical control group. In 2013, physiotherapists at our institution began to implement PBT as part of routine care for appropriate patients with sub-acute stroke. This prevented us from undertaking a randomized controlled trial (RCT), as it would not have been ethical to allocate participants to a no-PBT control group when the intervention is part of routine care. Thus, we prospectively recruited individuals who completed PBT during in-patient rehabilitation, and compared fall rates for this group to a matched historical control group who did not complete PBT, but who tracked fall-events post-discharge within a previous observational study.16 The study was approved by the institution’s Research Ethics Board, and participants provided written informed consent.
Participants
Individuals with sub-acute stroke receiving in-patient rehabilitation at the Toronto Rehabilitation Institute were invited to participate. Participants were eligible if they: 1) could stand independently for at least 30s; 2) could walk with or without a gait aid (but without assistance of another person) for at least 10m; 3) completed and tolerated a reactive balance control assessment17 during in-patient rehabilitation; and 4) were discharged to their own homes. The historical cohort were recruited between October 2010 and March 2013, and the prospective cohort were recruited between September 2015 and July 2016 (Figure 1).
Figure 1. Participant flowchart.
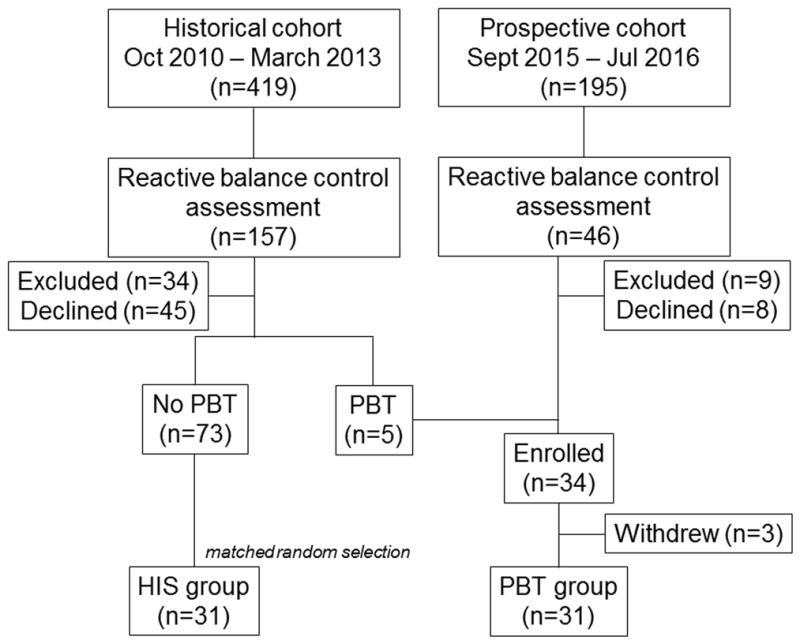
Participants in the historical cohort were excluded due to insufficient English language ability (n=11), cognitive impairment (n=4), living too far from the hospital (n=2), and not discharged home (n=17); participants in the prospective cohort were excluded as they did not do PBT (n=6), had cognitive impairment (n=1), or were not discharged home (n=2). Twenty-nine participants were recruited from the prospective cohort, and added to 5 historical cohort participants who completed PBT to form the PBT group. Of these, 3 withdrew without completing any falls monitoring, leaving 31 PBT participants for inclusion in the final analysis. From the 73 historical cohort participants who did not complete PBT, 31 were randomly selected to be matched to the PBT participants using the procedure described in the text.
There were 31 individuals in the PBT group. A matched sample of participants in the historical control group (HIS) was selected from the 73 eligible individuals in the historical cohort who participated in the previous study and did not complete PBT during in-patient rehabilitation. The groups were matched on Berg Balance Scale (BBS)18 scores on admission to rehabilitation and time post-stroke at discharge from rehabilitation. The median BBS score (38/56) and time post-stroke (50 days) within the PBT group were calculated. Participants were then classified into one of four subgroups: 1) high BBS (>38) and high time post-stroke (>50 days); 2) high BBS and low time post-stroke; 3) low BBS and high time post-stroke; and 4) low BBS and low time post-stroke. For each participant in the PBT group, a HIS participant from the same sub-group was randomly selected to create matched groups. There were more PBT participants in the high BBS and high time post-stroke sub-group than were available in this sub-group for the historical cohort, such that one PBT participant could not be matched to an individual in the historical cohort using this method; this participant was matched to the un-matched individual in the historical cohort with the closest BBS score and time post-stroke.
Intervention
In-patients at the Toronto Rehabilitation Institute typically receive one hour of individualized physiotherapy per day, five days per week. For participants in the PBT group, a portion (~30 minutes/day) of participants’ regular physiotherapy was replaced with PBT. The number and frequency of PBT sessions depended upon participant tolerance and functional level, length of stay, and participant rehabilitation goals and preferences. Participant-specific training goals were determined based on an initial assessment of reactive balance control;17 e.g., if a participant showed difficulty executing reactive steps with the paretic limb on the initial assessment, the training would focus on practicing executing stepping with the paretic limb.
PBT included tasks to induce external and internal perturbations. External perturbations were administered through external forces (e.g., push or pull from the physiotherapist), whereas internal perturbations consisted of rapid voluntary movements (e.g., kicking a soccer ball) that could cause a loss of balance. As participants progressed in their balance recovery capabilities, difficulty was increased by shifting task requirements along a continuum from stable to mobile, and from predictable to unpredictable,19 and by increasing perturbation magnitude and/or imposing sensory or environmental challenges.
Outcome measures
The primary outcome was occurrence of falls (“an event that results in a person coming to rest unintentionally on the ground or other lower level”20) in the 6 months post-discharge. Participants were provided with stamped, addressed postcards to mail to the research team fortnightly. Each postcard contained a calendar for the fortnight, and participants were asked to check a box if they experienced a fall on each day. A research assistant called participants who did not return the postcard within 2 weeks to determine if any falls occurred. The research assistant also contacted participants reporting a fall to complete a short questionnaire regarding the cause and consequences of the fall. Participants reported physical activities using the Physical Activity Scale for Individuals with Physical Disabilities (PASIPD)21 at approximately 2-, 4- and 6-months post-discharge.
Age, sex, time post-stroke, type of stroke, lesion location, and Chedoke-McMaster Stroke Assessment (CMSA)22 foot and leg scores were extracted from participants’ hospital charts to describe the study cohort. The National Institutes of Health Stroke Scale (NIH-SS)23 was scored upon study enrolment as a cohort descriptor. Clinical assessment scores at admission and discharge were also extracted from patient charts: BBS18 (functional balance measure), Clinical Outcome Variables Scale24 (COVS; functional mobility measure) and the Activities-Specific Balance Confidence questionnaire25 (ABC; measure of balance confidence during daily activities).
Statistical analysis
Baseline cohort descriptors were compared between groups using Mann-Whitney U and chi-square tests. Logistic and Poisson regression were used to compare the proportion of fallers and fall rates between the PBT and HIS groups, respectively; duration of the follow-up monitoring period and CMSA leg scores were used as covariates in both analyses. Fisher’s exact test was used to compare characteristics of falls between groups. Analysis of covariance was used to compare secondary outcomes (ABC, BBS, and COVS scores) between groups at discharge controlling for admission scores; discharge scores were rank-transformed prior to analysis as the measures are ordinal. PASIPD scores were averaged to create one score per person, and were compared between groups using Mann-Whitney U tests. Alpha was 0.05 for all analyses.
RESULTS
Participant characteristics are displayed in Table 1. There were no between-group differences on demographic or clinical measures, although there was a trend toward higher CMSA leg scores in the PBT group than the HIS group. Participants in the PBT group completed 1–12 sessions of PBT during their regularly scheduled physiotherapy sessions (median: 6 sessions). No adverse events directly related to PBT were reported.
Table 1. Participant characteristics.
Values are means with standard deviations in parentheses (continuous variables) or counts (categorical variables). The p-value is for the Mann-Whitney U test or chi-square test comparing groups.
| HIS | PBT | p-value | |
|---|---|---|---|
| Age (years) | 60.1 (15.3) | 58.8 (9.6) | 0.35 |
| Sex (number) | |||
| Men | 22 | 24 | 0.56 |
| Women | 9 | 7 | |
| Time post-stroke (days) | 53.6 (21.0) | 53.4 (19.2) | 0.92 |
| Affected hemisphere (number) | |||
| Right | 10 | 13 | 0.21 |
| Left | 16 | 17 | |
| Both | 5 | 1 | |
| BBS (score) | 29.1 (18.5) | 32.5 (16.8) | 0.51 |
| NIH-SS (score) | 3.4 (2.8) | 3.2 (2.4) | 0.88 |
| CMSA leg (score)* | 4.0 (1.3) | 4.7 (1.3) | 0.053 |
| CMSA foot (score)* | 3.9 (1.5) | 4.1 (1.5) | 0.45 |
Data missing for two HIS participants for this variable.
BBS=Berg Balance Scale; CMSA=Chedoke-McMaster Stroke Assessment
The mean falls monitoring duration was 151 days for the PBT group, and 180 days for the HIS group. Fewer PBT participants than HIS participants reported at least one fall in the six months post-discharge (PBT: 5/31 participants; HIS: 15/31; odds ratio: 0.21 [0.063, 0.67], p=0.0090; Table 2). After controlling for monitoring duration and CMSA leg scores, the effect of group on odds of falling at least once was no longer statistically significant (odds ratio: 0.27 [0.071, 1.02], p=0.054). PBT participants reported 10 falls (0.84 falls/person-year), whereas HIS participants reported 31 falls (2.0 falls/person-year). Fall rates were significantly lower in the PBT group compared to the HIS group when group alone was included in the model (rate ratio: 0.32 [0.15, 0.63]; p=0.0019) and when controlling for monitoring duration and CMSA leg scores (rate ratio: 0.36 [0.15, 0.79]; p=0.016).
Table 2. Falls after discharge.
Values are point estimates for odds (risk of falling at least once) or rate ratios (fall rates) with 95% confidence intervals in brackets. Odds and rate ratios for the model including group alone, and the model including group, monitoring duration, and Chedoke-McMaster Stroke Assessment (CMSA) leg scores (multivariate model) are presented. Two HIS participants were excluded from the multivariate model as CMSA leg scores were missing for these participants.
| Odds ratio | p-value | |
|---|---|---|
| Group | ||
| HIS | 1 | |
| PBT | 0.21 [0.063, 0.67] | 0.0090 |
|
| ||
| Group | ||
| HIS | 1 | |
| PBT | 0.27 [0.071, 1.02] | 0.054 |
| Monitoring duration (days) | 1.01 [0.98, 1.03] | 0.51 |
| CMSA leg score | 0.64 [0.40, 1.04] | 0.074 |
|
| ||
| Rate ratio | ||
|
| ||
| Group | ||
| HIS | 1 | |
| PBT | 0.32 [0.15, 0.63] | 0.0019 |
|
| ||
| Group | ||
| HIS | 1 | |
| PBT | 0.35 [0.15, 0.79] | 0.016 |
| Monitoring duration (days) | 1.00 [0.99, 1.01] | 0.96 |
| CMSA leg score | 0.77 [0.61, 0.96] | 0.022 |
Characteristics of falls in both groups are presented in Table 3. There were significant differences between groups in activity at the time of the fall, location of the fall, and fall-related injuries. PBT participants were more likely than HIS participants to fall when engaged in challenging activities (e.g., walking on stairs versus transferring; p=0.0009), and to fall outdoors (p=0.013). PBT participants were also more likely than HIS participants to suffer an injury as a result of the fall (p=0.026), although the majority of injuries were minor (e.g., bruises or small lacerations). PBT participants reported trying to stop themselves from falling by executing a reactive step for 50% of falls, whereas HIS participants only executed a reactive step for 16% of falls; however, this difference was not statistically significant (p=0.13).
Table 3. Characteristics of falls in daily life.
Values are counts with percentage in parentheses. The p-value is for Fisher’s exact test, comparing fall characteristics between groups; ‘do not recall’ responses were excluded from the analysis. Note that three reported falls were excluded; for two (1 PBT and 1 HIS) the participant was asleep and fell out of bed, and for one (HIS) the participant fell due to a loss of consciousness.
| HIS | PBT | p-value | |
|---|---|---|---|
| Cause of fall | |||
| Slip | 12 (38.7) | 2 (20) | 0.73 |
| Trip | 9 (29.0) | 3 (30) | |
| Incorrect weight transfer* | 10 (32.3) | 5 (50) | |
| Activity at the time of the fall | |||
| Sitting | 1 (3.2) | 1 (10) | 0.0009 |
| Standing | 2 (6.5) | 0 (0) | |
| Walking on level surface | 9 (29.0) | 2 (20) | |
| Walking up stairs | 0 (0) | 5 (50) | |
| Transferring | 9 (29.0) | 0 (0) | |
| Turning/reaching/bending | 6 (19.4) | 1 (10) | |
| Walking on a moving surface† | 1 (3.2) | 1 (10) | |
| Other‡ | 2 (6.5) | 0 (0) | |
| Where did the fall occur | |||
| Indoors | 26 (83.9) | 4 (40) | 0.013 |
| Outdoors | 5 (16.1) | 6 (60) | |
| Action to try to prevent the fall | |||
| Do not recall | 4 (12.9) | 1 (10) | |
| None | 9 (29.0) | 2 (20) | 0.13 |
| Step | 5 (16.1) | 5 (50) | |
| Other§ | 6 (19.4) | 1 (10) | |
| Assistance required to get up from fall | |||
| Do not recall | 6 (19.4) | 0 (0) | |
| No | 19 (61.3) | 4 (40) | >0.99 |
| Yes | 6 (19.4) | 6 (60) | |
| Injuries | |||
| Do not recall | 5 (16.1) | 0 (0) | |
| None | 19 (61.3) | 3 (30) | 0.026|| |
| Cuts or bruises | 3 (9.7) | 6 (60) | |
| Joint sprain | 3 (9.7) | 1 (10) | |
| Bump on head | 1 (3.2) | 0 (0) | |
| Medical assistance required after fall | |||
| Do not recall | 5 (16.1) | 0 (0) | |
| No injuries | 19 (61.3) | 3 (30) | |
| Injured but did not seek treatment | 5 (16.1) | 6 (60) | >0.99¶ |
| Saw family physician | 0 (0) | 1 (10) | |
| Treated in hospital emergency room | 2 (6.5) | 0 (0) | |
Incorrect weight transfer is defined as “self-induced shifting of body weight causing the centre of gravity to move outside the base of support”, e.g., missed step, missed seat when sitting down, turning too quickly35
Escalator (1 fall) and treadmill (1 fall).
Stepping backwards (1 fall), and loading a car (1 fall).
Attempted to control fall by sitting or kneeling (4 falls), leaned onto furniture (1 fall), used an ‘in-place’ response (2 falls), or an reach-to-grasp response (8 falls).
Analysis compared no injury to any injury, ignoring the type of injury.
Analysis compared number of individuals who sought any medical assistance to those who did not seek medical assistance, ignoring the type of assistance sought. ‘Do not recall’ and ‘no injuries’ responses were excluded from this analysis.
PBT participants did not show greater improvement in balance confidence (ABC), functional balance (BBS), or functional mobility (COVS) than HIS participants (p-values>0.24; Table 4). There was no difference between groups on self-reported physical activity after discharge (PBT mean PASIPD score: 9.5, standard deviation: 5.8; HIS mean PASIPD score: 9.8; standard deviation: 5.1; p=0.67).
Table 4.
Change in balance confidence, functional balance, and mobility from admission to discharge. Values are means with standard deviations in parentheses. The p-value is for the ANCOVA comparing groups at discharge from rehabilitation, controlling for admission values.
| HIS | PBT | p-value | |||||
|---|---|---|---|---|---|---|---|
| n | Admission | Discharge | n | Admission | Discharge | ||
| ABC (%) | 17 | 74.2 (16.1) | 81.3 (15.8) | 15 | 63.1 (21.4) | 82.2 (12.5) | 0.29 |
| BBS (score) | 31 | 29.1 (18.5) | 50.7 (4.8) | 28 | 30.8 (16.6) | 52.1 (3.8) | 0.24 |
| COVS (score) | 31 | 58.6 (17.3) | 80.5 (6.8) | 21 | 57.0 (15.0) | 79.9 (7.6) | 0.96 |
ABC=Activities-specific Balance Confidence scale; BBS=Berg Balance Scale; COVS=Clinical Outcome Variables Scale.
DISCUSSION
People with stroke fall frequently, with particularly high rates of falling apparent soon after discharge from rehabilitation.5,6 Previous balance training-focused interventions, whereby the goal is for participants to maintain stability during voluntary movement, have failed to demonstrate reduced fall rates post-stroke.8,9 PBT is a novel balance-training intervention focused on improving control of reactions to instability. We found that individuals who completed PBT during in-patient stroke rehabilitation reported fewer falls in the 6-months post-discharge than individuals who did not complete PBT. Fall rates following PBT (0.84 falls/person-year) were lower than those reported in previous studies of community-dwelling individuals with stroke (1.4–5 falls/person-year).6
While the results of this study suggest that PBT administered during stroke rehabilitation may be beneficial for preventing falls after discharge, it is important to recognise that this study was not an RCT. Changes in clinical practice (other than adoption of PBT) or patient characteristics over time may have influenced the results of this study. However, the two groups were similar at admission to rehabilitation, and identical inclusion/exclusion criteria and data collection procedures were applied for both cohorts. Well-designed RCTs provide the highest level of evidence (Level I) for effectiveness of interventions, whereas historically controlled studies only provide Level IV evidence.26 However, historically controlled studies are time- and cost-effective alternatives to RCTs, and such a study design was appropriate for our setting given that an RCT was not possible. Furthermore, Level IV evidence is sufficient to recommend PBT in practice provided the results are not inconsistent with other studies.27 Results of the current study are consistent with reduced fall rates after PBT observed in other studies among healthy older adults, older adults with mixed diagnoses (including chronic stroke), and individuals with Parkinson disease.15 We are in the process of conducting an RCT to determine if PBT can reduce falls in chronic stroke.19
The number of PBT sessions varied among participants, based on factors such as participant tolerance, length of stay, and rehabilitation goals. Nine participants completed just one session of PBT. Previous studies completed among healthy older adults suggest that a single session of PBT can produce lasting improvements in reactive balance control28 and reduce falls in daily life for up to a year post-training.29 Therefore, it is possible that participants in the current study benefitted from a single session of PBT. The optimal dose of PBT will need to be determined in future studies.
Examination of fall characteristics suggest that PBT participants fell under more challenging circumstances than HIS participants (i.e., when engaged in more challenging activities, such as walking up stairs versus walking on even ground, and when outdoors versus indoors). Fall occurrence can be considered as an interaction between the person, environment, and task.30 That is, individuals with relatively unimpaired balance will typically fall when engaged in challenging tasks (e.g., sports participation) and/or challenging environments (e.g., walking on ice),31 whereas individuals with impaired balance will fall in less challenging situations.32 It is likely not possible to completely prevent all falls, but most important to prevent those falls that occur in more routine situations. Therefore, evaluation of interventions aiming to prevent falls should not only consider the number of falls, but also the characteristics of falls to determine if there is a shift from those occurring in routine to more challenging situations. PBT participants suffered fall-related injuries more frequently than HIS participants; it is noteworthy that, while the number of falls was lower in the PBT than the HIS group, the number of injurious falls was identical between groups. It is possible that the increased frequency of injuries is directly related to the fact that PBT participants fell in more challenging situations; for example, in the current study, 7/11 outdoor falls resulted in an injury.
We expected that reduced fall rates following PBT would be accompanied by improved functional balance and mobility, improved balance confidence, and increased participation in physical activity. The results do not support our hypotheses; the PBT group did not show greater improvements in BBS, COVS, or ABC than the HIS group, and the PBT group did not report more daily physical activity than the HIS group. It is possible that measures used in the current study do not capture the specific features of balance control that are likely to improve with PBT. For example, the BBS does not include items to assess reactive balance control. Other measures, such as the Balance Evaluation Systems Test,33 might better capture the specific training effects of PBT on balance control.
Previous studies have used equipment such as programmable treadmills34 or custom moving platforms13 to provide perturbations in PBT. The PBT program evaluated in this study requires minimal equipment. No equipment was required to administer the perturbations as this was done manually by a push or pull from the physiotherapist or via ‘internal’ perturbations. The only equipment needed is a safety harness attached to an overhead support to ensure patient safety during training by preventing a fall to the floor. Most physiotherapy practices will already have body-weight support systems and/or ceiling lift tracks that could be adapted for this purpose; thus, PBT can likely be easily implemented in clinical practice.
To conclude, these findings suggest that perturbation-based balance training, delivered during in-patient rehabilitation, can reduce the rate of falls in daily life post-discharge among individuals with stroke. The training program used in this study was readily implemented in routine practice with equipment that is commonly available in neurorehabilitation settings. Future work should identify the minimum dose of training required for participants to benefit.
Acknowledgments
Grant support: This study was supported by the Heart and Stroke Foundation Canadian Partnership for Stroke Recovery. The authors acknowledge the support of the Toronto Rehabilitation Institute; equipment and space have been funded with grants from the Canada Foundation for Innovation, Ontario Innovation Trust, and the Ministry of Research and Innovation. Avril Mansfield is supported by a New Investigator Award from the Canadian Institutes of Health Research (MSH-141983). Vincent DePaul was supported by a Focus on Stroke Post-Doctoral Fellowship from the Heart and Stroke Foundation of Canada
References
- 1.Batchelor FA, Mackintosh SF, Said CM, Hill KD. Falls after stroke. Int J Stroke. 2012;7(6):482–490. doi: 10.1111/j.1747-4949.2012.00796.x. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe Y. Fear of falling among stroke survivors after discharge from inpatient rehabilitation. Int J Rehabil Res. 2005;28:149–152. doi: 10.1097/00004356-200506000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen L, Engstad T, Jacobson BK. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke. 2002;33:542–547. doi: 10.1161/hs0202.102375. [DOI] [PubMed] [Google Scholar]
- 4.Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ. 1995;311:83–86. doi: 10.1136/bmj.311.6997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim JY, Jung SH, Kim W-S, Paik N-J. Incidence and risk factors of poststroke falls after discharge from inpatient rehabilitation. PM R. 2012;4(12):945–953. doi: 10.1016/j.pmrj.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Weerdesteyn V, de Niet M, van Duijhoven HJR, Geurts ACH. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45(8):1195–1213. [PubMed] [Google Scholar]
- 7.Wong JS, Brooks D, Inness EL, Mansfield A. The impact of falls on motor and cognitive ability after discharge from in-patient stroke rehabilitation. J Stroke Cerebrovasc Dis. 2016;27(7):1613–1621. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batchelor F, Hill K, Mackintosh S, Said C. What works in falls prevention after stroke? a systematic review and meta-analysis. Stroke. 2010;41(8):1715–1722. doi: 10.1161/STROKEAHA.109.570390. [DOI] [PubMed] [Google Scholar]
- 9.Verheyden GS, Weerdesteyn V, Pickering RM, et al. Interventions for preventing falls in people after stroke. Cochrane Database Syst Rev. 2013;31(5):CD008728. doi: 10.1002/14651858.CD008728.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackintosh SFH, Hill K, Dodd KJ, Goldie P. Falls and injury prevention should be part of every stroke rehabilitation plan. Clin Rehabil. 2005;19:441–451. doi: 10.1191/0269215505cr796oa. [DOI] [PubMed] [Google Scholar]
- 11.Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med. 2016 doi: 10.1136/bjsports-2016-096547. [DOI] [PubMed] [Google Scholar]
- 12.Maki BE, McIlroy WE. Postural control in the older adult. Clin Geriatr Med. 1996;12(4):635–658. [PubMed] [Google Scholar]
- 13.Mansfield A, Peters AL, Liu BA, Maki BE. A perturbation-based balance training program for older adults: study protocol for a randomised controlled trial. BMC Geriatr. 2007;7(1):12. doi: 10.1186/1471-2318-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansfield A, Peters AL, Liu BA, Maki BE. Effect of a perturbation-based balance-training program on compensatory stepping and grasping reactions in older adults: a randomized controlled trial. Phys Ther. 2010;90(4):476–491. doi: 10.2522/ptj.20090070. [DOI] [PubMed] [Google Scholar]
- 15.Mansfield A, Wong JS, Bryce J, Knorr S, Patterson KK. Does perturbation-based balance training prevent falls? A review and meta-analysis of preliminary randomized controlled trials. Phys Ther. 2015;95(5):700–709. doi: 10.2522/ptj.20140090. [DOI] [PubMed] [Google Scholar]
- 16.Mansfield A, Wong JS, McIlroy WE, et al. Do measures of reactive balance control predict falls in people with stroke returning to the community? Physiotherapy. 2015;101(4):373–380. doi: 10.1016/j.physio.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Inness EL, Mansfield A, Biasin L, Brunton K, Bayley M, McIlroy WE. Clinical implementation of a reactive balance control assessment in a sub-acute stroke patient population using a ‘lean-and-release’ methodology. Gait Posture. 2015;41(2):529–534. doi: 10.1016/j.gaitpost.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Berg K, Wood-Dauphinée S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 1989;41:304–311. [Google Scholar]
- 19.Mansfield A, Aqui A, Centen A, et al. Perturbation training to promote safe independent mobility post-stroke: study protocol for a randomized controlled trial. BMC Neurol. 2015;15:87. doi: 10.1186/s12883-015-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil. 2002;83:165–170. doi: 10.1053/apmr.2002.28030. [DOI] [PubMed] [Google Scholar]
- 21.van der Ploeg HP, Streppel KR, van der Beek AJ, van der Woude LH, Vollenbroek-Hutten M, van Mechelen W. The Physical Activity Scale for Individuals with Physical Disabilities: test-retest reliability and comparison with an accelerometer. J Phys Act Health. 2007;4(1):96–100. doi: 10.1123/jpah.4.1.96. [DOI] [PubMed] [Google Scholar]
- 22.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH Stroke Scale. Arch Neurol. 1989;46(6):660–662. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 24.Seaby L, Torrance G. Reliability of a physiotherapy functional assessment used in a rehabilitation setting. Physiother Can. 1989;41:264–270. [Google Scholar]
- 25.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 26.Howick J, Chalmers I, Glasziou P, et al. [Accessed February 15, 2015];The Oxford Levels of Evidence 2. http://www.cebm.net/index.aspx?o=5653.
- 27.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–310. doi: 10.1097/PRS.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatt T, Yang F, Pai Y-C. Learning to resist gait-slip falls: long-term retention in community-dwelling older adults. Arch Phys Med Rehabil. 2012;93:557–564. doi: 10.1016/j.apmr.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pai Y-C, Bhatt T, Yang F, Wang E. Perturbation training can reduce community-dwelling older adults’ annual fall risk: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2014;69(12):1586–1594. doi: 10.1093/gerona/glu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shumway-Cook A, Woollacott MH. Motor control: translating research into clinical practice. 3. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 31.Heijnen MJH, Rietdyk S. Falls in young adults: perceived causes and environmental factors assessed with a daily online survey. Hum Mov Sci. 2016;46:86–95. doi: 10.1016/j.humov.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Speechley M, Tinetti M. Falls and injuries in frail and vigorous community elderly persons. J Am Geriatr Soc. 1991;39:46–52. doi: 10.1111/j.1532-5415.1991.tb05905.x. [DOI] [PubMed] [Google Scholar]
- 33.Horak FB, Wrisley DM, Frank J. The balance evaluation systems test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89:484–498. doi: 10.2522/ptj.20080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lurie JD, Zagaria AB, Pidgeon DM, Forman JL, Spratt KF. Pilot comparative effectiveness study of surface perturbation treadmill training to prevent falls in older adults. BMC Geriatr. 2013;13:49. doi: 10.1186/1471-2318-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinovitch SN, Feldman F, Yang Y, et al. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet. 2013;381(9860):47–54. doi: 10.1016/S0140-6736(12)61263-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


