Abstract
Background
Auditory complaints following mild traumatic brain injury are common, but few studies have addressed the role of auditory temporal processing in speech recognition complaints.
Purpose
In this study, deficits understanding speech in a background of speech noise following MTBI were evaluated with the goal of comparing the relative contributions of auditory and non-auditory factors.
Research Design
A matched-groups design was used in which a group of listeners with a history of MTBI were compared to a group matched in age and pure-tone thresholds, as well as a control group of young listeners with normal hearing.
Study Sample
Thirty-three listeners participated in the study, including thirteen in the MTBI group (mean age 46.7 years), eleven in the Matched group (mean age 49 years), and nine in the YNH group (mean age 20.8 years).
Data Collection and Analysis
Speech-in-noise deficits were evaluated using subjective measures as well as monaural word (WIN) and sentence (QuickSIN) tasks, and a binaural spatial release task. Performance on these measures was compared to psychophysical tasks evaluating monaural and binaural temporal fine structure tasks and spectral resolution. Cognitive measures of attention, processing speed, and working memory were evaluated as a possible difference between MTBI and Matched groups contributing to speech-in-noise deficits.
Results
A high proportion of listeners in the MTBI group reported difficulty understanding speech in noise (84%) compared to the Matched group (9.1%), and listeners who reported difficulty were more likely to have abnormal results on objective measures of speech in noise. No significant group differences were found between the MTBI and Matched listeners on any of the measures reported, but the number of abnormal tests differed across groups. Regression analysis revealed that a combination of auditory and auditory processing factors contributed to monaural speech-in-noise scores, but the benefit of spatial separation was related to a combination of working memory and peripheral auditory factors across all listeners in the study.
Conclusions
The results of this study are consistent with previous findings that a subset of listeners with MTBI have objective auditory deficits. Speech-in-noise performance was related to a combination of auditory and non-auditory factors, confirming the important role of audiology in MTBI rehabilitation. Further research is needed to evaluate the prevalence and causal relationship of auditory deficits following MTBI.
Keywords: Hearing Impairment, Traumatic Brain Injury, Post-Concussive Syndrome, Auditory Processing Disorder
Traumatic brain injury (TBI) affects 1.4 million people in the United States each year (CDC, 2006). An estimated 75% of reported injuries are mild (Finkelstein et al., 2006). Traditionally it was thought that in the majority of mild traumatic brain injury (MTBI) cases, cognitive and neurosensory sequellae were minor and spontaneously recovered, but recent evidence contradicts that viewpoint (Hoffer et al., 2013). Many patients continue to experience symptoms long after an initial recovery period, a disorder called post-concussive syndrome. Many of those individuals report persistent auditory complaints (Cockrell and Gregory, 1992; Jury and Flynn, 2001; Bergemalm and Borg, 2001; Oleksiak et al., 2012). Moreover, while many symptoms tend to improve over time, auditory complaints are the least likely to recover decades after the injury (Hoofien et al., 2001).
The exact nature of auditory complaints is a source of discussion, but a number of studies report deficits in auditory function persisting even in the absence of auditory threshold elevation (Cockrell and Gregory, 1992; Nölle et al., 2004; Musiek et al., 2004; Bergemalm and Lyxell, 2005; Flood et al., 2005; Turgeon et al., 2011; Oleksiak et al., 2012; Gallun et al., 2012; Saunders et al., 2015). It is unclear whether those deficits were due to peripheral auditory, central auditory, or non-auditory cognitive factors. For example, abnormal results on tests of dichotic listening (e.g., Turgeon et al., 2011; Gallun et al., 2012; Saunders et al., 2015) can result from corpus callosum damage (Musiek et al., 2004) that is not auditory-specific. While the auditory literature presents impaired performance on complex speech tasks as evidence for auditory processing disorder, the neuropsychology literature uses deficits on complex speech tasks as evidence of various cognitive impairments. Numerous studies have shown long-term deficits in cognitive function after MTBI, including processing speed (e.g., Dean and Sterr, 2013), working memory (e.g., Vanderploeg et al., 2005), attention (e.g., Mangels et al., 2002), and information processing (e.g., O’Jile et al., 2006). No doubt both cognitive and auditory factors contribute to impaired performance on speech tasks, but few studies have closely examined the auditory system deficits following TBI.
For listeners with a history of MTBI, reported difficulty understanding speech in noise may be related to degraded temporal fine structure (TFS) processing. Neurons in the auditory system respond to sound with sub-millisecond temporal resolution that is an order of magnitude more precise than other sensory systems (Frisina, 2001; Wang, 2007). This precise encoding may be disrupted by diffuse axonal injury and associated demyelination and neuronal loss. Impaired TFS processing may result in a loss of ability to take advantage of TFS cues in speech, including the ability to benefit from interaural cues associated with spatial segregation of talkers, which may lead to reduced speech understanding in complex listening environments. Although existing data on listeners with MTBI suggest auditory processing impairment (Bergemalm and Lyxell, 2005; Turgeon et al., 2011), the complex nature of tasks included in commonly employed batteries used to screen for central auditory processing disorder (CAPD) is problematic, in that the demonstrated deficits may be related to cognitive factors–such as memory and attention–that are not specific to the auditory domain. It remains unclear whether auditory complaints resulting from MTBI are psychogenic, are a consequence of domain-general cognitive deficits, or are in fact due to impairments specific to the auditory system.
Two studies have evaluated the relationship between auditory and cognitive function after TBI. Bergemalm and Lyxell (2005) reported a correlation between cognition (including processing speed, working memory, and information processing) and tests of auditory processing. In that study, auditory test results were combined across distorted (interrupted) speech, clinical psychophysics (interaural phase), and auditory brainstem response amplitudes and latencies. Auditory results were correlated with cognition across TBI and non-TBI matched controls, but the details of the analysis were not reported. Krause and colleagues (2014) reported a significant correlation between speech in two-talker background and standardized measures of processing speed, as well as a correlation between processing speed and subjective assessment of listening effort while performing various speech-in-noise tasks. Both groups consisted of listeners varying in age across a span of 35 years (18–55), with normal pure-tone thresholds, so the correlation may have been related to the effects of aging on both processing speed (Salthouse, 2000) and speech understanding in noise (Humes and Dubno, 2010). Aging, like TBI, is not a monolithic condition with associated auditory and cognitive deficits, but is correlated with deficits in specific domains. By measuring the cognitive correlates directly, their contribution to deficits in the auditory domain can be evaluated. Accordingly, we still lack data as to whether both auditory and non-auditory factors are directly related to difficulty understanding speech in noise after TBI.
A final issue of interest is the growing body of data suggesting a link between MTBI and auditory processing deficits among individuals who experienced MTBI as a result of military combat, predominantly blast exposure (Gallun et al., 2012; Oleksiak et al, 2012; Saunders et al., 2015). A majority of those individuals also experienced acoustic trauma and a high level of long-term noise exposure. At present, there are few similarly complete data sets that can be used to examine the consequences of non-blast MTBI, such as caused by falls, sports concussions, or motor vehicle collisions. Turgeon and colleagues (2011) tested a small group of college athletes with sports-related concussions, and found that three out of five had deficits on two or more tasks taken from a CAPD screening battery, despite normal pure-tone thresholds. Across blast-exposed and non-blast MTBI, some of the non-speech abilities most commonly found to be impaired have been auditory temporal processing and binaural integration, suggesting a possible deficit in ability to process TFS cues. Such deficits, if present, might reasonably be expected to have a downstream impact on speech understanding.
To address these issues, cognition and auditory processing abilities should be carefully measured and their respective relationships to performance on complex speech tasks tested. In the present study, the role of auditory processing in speech-in-noise deficits following MTBI was evaluated using an extensive battery of tests designed to differentiate the relative contributions of peripheral auditory, auditory processing, and non-auditory cognitive factors. Participants were recruited from a community population whose TBIs were uncomplicated by blast exposure and other potential peripheral damage from military service.
Methods
Participants
Thirty-three listeners participated in the experiment, divided into three groups for which candidacy was determined based on medical history, age, and pure-tone thresholds. Thirteen listeners (aged 25–71 years, mean 46.7 years) were included in the MTBI group based on a history of uncomplicated mild traumatic brain injury for which the acute recovery was complete and any residual auditory or cognitive deficits were chronic, long-term symptoms. MTBI group participation was determined according to Diagnostic and Statistical Manual of Mental Disorders, 5th ed., defined as patient report of an insult the head resulting in a period of confusion or disorientation, posttraumatic amnesia of any duration, and loss of consciousness less than thirty minutes (American Psychiatric Association, 2013). Recovery from acute symptoms typically occurs within two months of MTBI, and persistent symptoms, including persistent auditory complaints, beyond two months are considered chronic symptoms (Hall et al., 2005). MTBI group histories are summarized in Table 1. Persistent symptoms, including auditory complaints, were not required for inclusion in the MTBI group.
Table 1.
Audiometric and traumatic injury history data for individual MTBI participants.
| ID | MTBI History | Time since most recent TBI (years) |
Medical diagnosis | Audiological care |
|---|---|---|---|---|
| 363 | 3 falls | 2 | yes, treated for lacerations | none |
| 368 | multiple sports | 10 | no | none |
| 375 | motor vehicle accident (MVA) | 6 | yes, treated for lacerations | audiogram WNL |
| 389 | 3 falls | 3 | yes, aphasia treated by SLP | none |
| 398 | multiple sports | 11 | no | none |
| 402 | multiple sports, MVA | 17 | yes, treated for lacerations free refer | none |
| 149 | multiple sports, MVA | 1 | yes, CT, monitored for possible hemmhorage | audiogram & CAPD abnormal, hearing aid (left) |
| 427 | multiple sports, MVA | 11 | yes, CT, neuropsych. eval. WNL | audiogram WNL |
| 438 | MVA | 14 | yes, treated for lacerations | none |
| 436 | MVA | 46 | yes, motor and speech treatment | none |
| 441 | fall | 2 | yes, neuropsych. eval. | hearing screening WNL |
| 448 | sports | 2 | yes, CT; memory loss, balance and word retrieval, treatment for PT, OT, SLP | none |
| 356 | multiple sports | 15 | no | none |
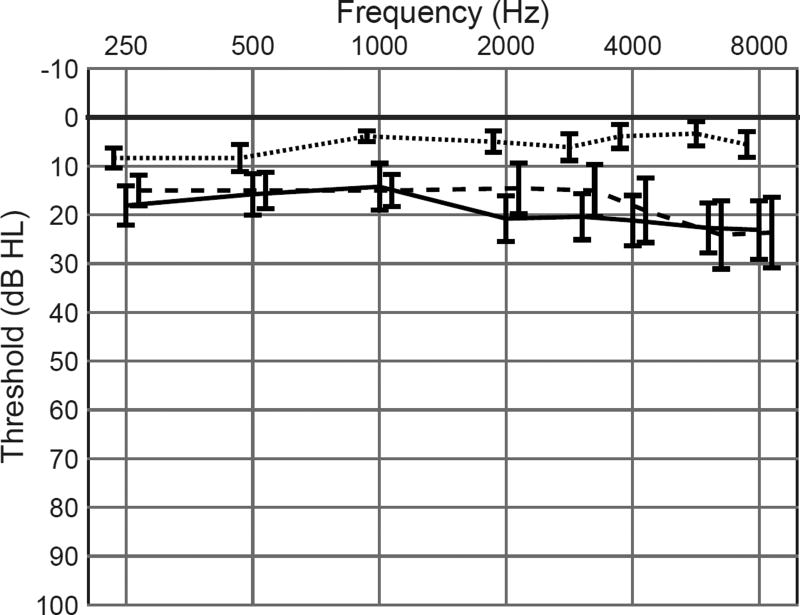
In addition to the MTBI group, two groups of control listeners were recruited. Those individuals had no history of TBI or other neurological disorder. A control group consisting of nine young listeners with normal pure-tone thresholds (YNH; aged 18–24 years, mean 20.8 years) established normal performance across the various measures in the study. To facilitate a matched-groups design, a second control group was recruited on the basis of age and pure-tone thresholds to match the listeners in the MTBI group. This group (Matched) consisted of eleven participants (aged 27–70 years, mean 49 years). Group mean and standard deviation audiograms are shown in Figure 1. Listeners recruited for the MTBI group were included over a broad range of age and a small range of hearing loss, as shown in Figure 1. Matched group participants were recruited such that there was no significant group difference in pure tone thresholds or age compared to the MTBI group. This resulted in a Matched group that does not reflect the age and audiometric distribution that would be found in a random sample of the population; accordingly, matched group data should not be interpreted as reflecting the typical healthy population, but rather as an experimental comparison for interpretation of the MTBI results.
Figure 1.
Group mean audiograms for the young, matched, and MTBI groups. The young group is represented by the dotted line, the matched group is represented by the dashed line, and the MTBI group is represented by the solid line. The standard deviation around each point is marked with a vertical bar.
Listeners in all groups completed a basic auditory assessment including otoscopy, tympanometry, pure-tone audiometry, speech reception thresholds (SRT), and word recognition in quiet (WR) using the NU-6 word lists presented via compact disk recording (Auditec, St. Louis, MO). Audiometric data are summarized in Table 2. Listeners with abnormal tympanometry or conductive hearing loss were excluded from participation, as defined by a gap in pure-tone air- and bone-conduction thresholds greater than 10 dB at two or more frequencies (Roup et al., 1998).
Table 2.
Summary characteristics for the young group, and individual Matched and MTBI data. (Values in boldface type indicate abnormal results relative to published normative data).
| ID | Marker | Age | Difficulty … in quiet? | Difficulty … in noise? | PTA (dB HL) | SRT (dB HL) | WR (%) | QSIN (dB SNR) | WIN (dB SNR) | Spatial Benefit | Abnormal Tests | Attention | Processing Speed | Working Memory |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young | 18–24 (20.8) | 0% yes, 100% no | 0% yes, 100% no | −2–17 (6.4) | −5–15 (4.7) | 92–100 (97) | −0.25–4.5 (1.03) | 2–5.6 (4.5) | 2.0–10 (6.75) | 0–3 (1.2) | −1.2–1.5 (0) | −1.3–1.7 (0) | −2.1–1.2 (0) | |
| Match | 27–70 (49.0) | 0% yes, 100% no | 9% yes, 91% no | 0–50 (15.2) | 0–45 (13.2) | 73–100 (95) | −2.25–11 (2.18) | 3.6–14.8 (6.5) | 1.0–9.0 (6.55) | 0–8 (2.2) | −1.1–1.6 (0.0) | −6.5–0.2 (−2.5) | −2.1–1.1 (−0.2) | |
| 9 | ✶ | 38 | no | no | 12 / 8 | 10 / 5 | 100 / 96 | 2.0 / 3.0 | 5.2 / 5.2 | 7.5 | 1 | 0.31 | −2.57 | 0.04 |
| 364 | + | 63 | no | no | 13 / 13 | 10 / 10 | 96 / 96 | 1.5 / 5.0 | 2.0 / 6.0 | 6 | 3 | 0.45 | −6.48 | −0.13 |
| 377 | ○ | 65 | no | no | 17 / 13 | 15 / 15 | 92 / 100 | 3.0 / 3.5 | 6.0 / 4.4 | 7.5 | 3 | −0.59 | −2.17 | −0.13 |
| 382 | ● | 56 | no | no | 18 / 15 | 15 / 10 | 96 / 96 | 0.0 / 0.0 | 7.6 / 3.6 | 6 | 3 | −0.22 | −2.17 | −0.57 |
| 290 | x | 35 | no | no | 2 / 3 | 0 / 0 | 100 / 100 | −1.5 / −3.0 | 6.8 / 6.0 | 7 | 1 | 1.61 | −2.17 | −2.13 |
| 95 | ▢ | 46 | no | no | 8 / 3 | 5 / 5 | 96 / 100 | 0.0 / −2.0 | 4.4 / 2.8 | 9 | 0 | 1.15 | −1.39 | 0.73 |
| 453 | △ | 46 | no | no | 18 / 15 | 10 / 10 | 100 / 96 | 0.5 / 1.0 | 3.6 / 6.0 | 8 | 0 | −0.08 | −1.00 | 1.08 |
| 354 | ◇ | 27 | no | no | 0 / 3 | 0 / 10 | 100 / 100 | 2.5 / 2.5 | 7.6 / 5.2 | 7.5 | 1 | −0.56 | 0.17 | 0.04 |
| 361 | ▽ | 28 | no | no | 10 / 17 | 10 / 15 | 96 / 96 | 1.5 / 2.0 | 6.0 / 6.8 | 1 | 2 | −0.60 | −2.96 | −0.74 |
| 324 | ▷ | 70 | no | yes | 45 / 50 | 40 / 45 | 70 / 76 | 10.0 / 12.0 | 13.2 / 16.4 | 2 | 7 | −1.09 | −3.35 | −0.22 |
| 270 | ◁ | 65 | no | no | 30 / 22 | 30 / 20 | 92 / 100 | 0.5 / 4.0 | 8.4 / 10.0 | 5 | 2 | −0.14 | −2.96 | −0.05 |
| MTBI | 25–71 (46.7) | 0% yes, 100% no | 85% yes, 15% no | −2–50 (15.8) | 0–30 (14.2) | 76–100 (95) | 0.25–7.75 (2.62) | 3.2–14.4 (7.7) | −1.5–10.5 (3.92) | 0–8 (3.2) | −0.7–1.2 (0.0) | −6.5–0.6 (−3.1) | −1.3–0.8 (−0.3) | |
| 363 | ✶ | 60 | no | yes | 3 / 5 | 5 / 10 | 92 / 100 | 1.0 / 5.0 | 5.2 / 6.0 | 2.5 | 2 | 0.09 | −3.35 | −0.48 |
| 368 | + | 32 | no | yes | 5 / 5 | 5 / 5 | 96 / 96 | 2.0 / 0.0 | 6.0 / 5.2 | 8 | 0 | −0.01 | −1.39 | 0.30 |
| 375 | ○ | 26 | no | yes | 5 / 12 | 5 / 5 | 100 / 100 | 0.0 / 3.0 | 6.8 / 9.2 | 5 | 2 | −0.67 | −0.61 | 0.13 |
| 389 | ● | 62 | no | yes | 25 / 33 | 20 / 35 | 100 / 100 | 1.5 / 1.5 | 7.6 / 6.0 | −0.5 | 3 | 0.64 | −4.13 | −0.57 |
| 398 | x | 28 | no | no | 7 / 3 | 0 / 0 | 96 / 100 | 0.0 / 0.5 | 5.2 / 4.4 | 9 | 0 | −0.37 | −2.17 | 0.30 |
| 402 | ▢ | 34 | no | no | 3 / −2 | 5 / 5 | 100 / 100 | 1.0 / 1.5 | 3.6 / 2.8 | 10.5 | 0 | −0.67 | −2.17 | 0.82 |
| 149 | △ | 25 | no | yes | 10 / 12 | 15 / 15 | 96 / 92 | 5.0 / 5.0 | 8.4 / 2.8 | 5.5 | 4 | 0.20 | −1.78 | 0.38 |
| 427 | ◇ | 56 | no | yes | 5 / 7 | 5 / 10 | 100 / 88 | 4.0 / 2.0 | 10.0 / 9.2 | 2 | 7 | −0.45 | −2.57 | −0.74 |
| 438 | ▽ | 51 | no | yes | 23 / 40 | 15 / 35 | 100 / 92 | 1.0 / 4.5 | 11.6 / 17.2 | 2 | 7 | −0.73 | −2.57 | −0.22 |
| 436 | ▷ | 69 | no | yes | 20 / 17 | 20 / 15 | 96 / 96 | 4.0 / 4.5 | 6.8 / 11.6 | 3 | 4 | 0.85 | −5.70 | −1.26 |
| 441 | ◁ | 52 | no | yes | 47 / 50 | 25 / 35 | 80 / 72 | 9.0 / 6.5 | 14.0 / 13.2 | −1.5 | 6 | 0.25 | −6.48 | −0.91 |
| 448 | ☆ | 71 | no | yes | 15 / 15 | 15 / 15 | 92 / 96 | 1.5 / 2.0 | 9.2 / 6.8 | 0.5 | 4 | −0.31 | −4.52 | −0.74 |
| 356 | ✡ | 41 | no | yes | 23 / 23 | 25 / 25 | 100 / 96 | 1.0 / 1.0 | 6.0 / 4.4 | 5 | 0 | 1.15 | −3.35 | −0.39 |
Testing was completed in two or three sessions each lasting no longer than two hours. Participants were recruited from the Northwestern University campus and Evanston, Illinois area by flyers and word-of-mouth. Informed consent was obtained prior to participation and compensation was provided at an hourly rate. Institutional review board approval was obtained for all recruitment, informed consent, and testing materials and procedures.
Subjective impairment
Listeners were asked to assess their hearing ability subjectively using an interview and structured questionnaire. Each listener was asked, “Do you have difficulty understanding speech in a quiet room?”, and, “Do you have difficulty understanding speech in a noisy room?” In addition to these questions, the short form of the Speech, Spatial, and Qualities of Hearing scale (SSQ12) was used to evaluate the perception of sound clarity and quality in various listening situations (Noble et al., 2013).
Speech in speech background
Monaural speech in multi-talker background
Monaural speech recognition in a background of multi-talker noise was evaluated using clinical measures of speech-in-noise deficits. The purpose of this testing was twofold: 1. To evaluate a possible group difference in speech-in-noise abilities between MTBI and Matched groups, and 2. To evaluate whether individual listeners’ reported difficulty understanding speech in noise was consistent with signal to noise ratio (SNR) loss on tests with known psychometric properties.
Sentence recognition in four-talker noise was measured using the Quick Speech in Noise test (QuickSIN; Etymotic Research, Elk Grove Village, IL). The QuickSIN consists of lists of six low-context sentences spoken by a female talker in a background of four-talker babble with a descending SNR. Each listener completed two test lists monaurally in each ear from the reduced set of lists shown to estimate SNR loss consistently in normal and impaired listeners (McArdle and Wilson, 2006).
Word recognition in four-talker noise was evaluated using the Words-in-Noise test (WIN; Wilson and Burks, 2005). WIN words and a preceding carrier phrase are spoken by a male talker in a background of four-talker babble with a descending SNR. Listeners completed one list of thrity-five words monaurally in each ear. Both the QuickSIN and WIN tests are scored by the number of keywords correctly repeated by the listener. This score is converted to an SNR loss representing the dB increase in signal relative to the noise necessary to perform at an SNR consistent young listeners with normal pure-tone thresholds. Details about the physical test environment remained consistent across auditory tasks and are described below.
Binaural speech in two-talker background
To evaluate listeners’ ability to benefit from spatial separation of sound sources (spatial release from masking [SRM]), a measure developed by Gallun and colleagues (2013) was used. The SRM test consists of three structured sentences presented simultaneously in a spatial environment simulated under headphones. Sentences were from the coordinate response measure corpus (Bolia et al., 2000), matching the formula, “ready <call sign>, go to <color> <number> now,” where <call sign> was a label indicating the target sentence and the listener must identify the matching <color> <number> on a response grid. Male talkers were used and the target was always identified by the call sign, “Charlie.” The target was located at 0° azimuth in all conditions and the masker talkers were either collocated or spatially separated at ±45° azimuth. Previous results showed that the difference between collocated and ±45° spatial separation was most sensitive to group differences in age and hearing loss (Gallun et al., 2013).
Presentation of the SRM task under headphones (ER2; Etymotic Research) required combining target and masker signals after convolution with a generic head-related impulse response that preserved interaural timing cues, but obscured interaural level cues dependent on individual listener head and pinna morphology. The target talker was presented at a fixed level of 50 dB sensation level relative to the listener’s SRTs, and the target-to-masker ratio (TMR) of the maskers was varied in 2-dB increments from −10 to +10 dB TMR. Each listener completed two sets of 20 practice trials in the collocated and spatially separated conditions, which were presented in descending order of TMR. The test included two sets of 20 test trials in the collocated condition, presented in random order of TMR, followed by two sets of 20 trials in the spatially-separated condition in random order of TMR. Spatial release was defined as the difference in 50% TMR between collocated and spatially-separated conditions expressed in dB. The score represents the benefit the listener received from the virtual spatial separation of the talkers, relying primarily on temporal interaural cues.
Psychophysics
Monaural temporal fine structure difference limen (TFS)
Monaural temporal fine structure perception was evaluated using methods derived from Moore and Sek (2009). In this task, listeners were presented with a sequence of tones consisting of a harmonic complex with a missing fundamental composed of the tenth through nineteenth harmonic of a 100 Hz fundamental. The target interval contained a shift in the frequency of each harmonic by a fixed frequency, resulting in inconsistent envelope and fine structure information in the signal. The temporal envelope cue derived from the spacing of harmonics remained unchanged but the temporal fine structure and place-pitch cue indicated a deviation from the standard (Oxenham et al., 2009). The tone complexes were presented at 65 dB SPL, and a threshold equalizing noise was presented at −15 dB relative to the tone complex. Stimuli were 400 ms duration including a 25 ms raised cosine ramp at onset and offset. A random starting phase for each tone in the complex was selected each trial. Threshold for the detection of an increment in frequency was tracked using a two-down, one-up procedure for ten reversals (Levitt, 1971). The last four reversals were averaged to compute threshold. Listeners completed at least one familiarization track and test tracks were completed until performance stabilized. The reported threshold was the average of the best two tracks completed by each listener. Testing was completed monaurally in the right ear using ER-3A headphones (Etymotic Research, Elk Grove Village, Illinois). A two-cue, two-alternative forced choice (2C2AFC; Bernstein and Trahoitis, 1982) method was used to reduce the memory load of the psychophysical procedure by presenting the standard before and after each target.
Monaural spectral ripple reversal detection (SRR)
Spectral ripple reversal detection (SRR) was used as a gross measure of auditory spectral resolution (Won et al., 2007). A relationship between spectral resolution and speech-in-noise deficits after MTBI could provide evidence of a peripheral rather than central cause. Stimuli were generated using a bank of sinusoids with 16 Hz spacing and random phase between 350 to 6000 Hz. Sinusoidal spectral modulation was applied on a log frequency axis. The signal duration was 500 ms and included 50 ms raised cosine onset and offset ramps. The task was a three-alternative forced choice task in which the target interval was spectrally modulated with a phase difference of 180° compared to the standard. The spectral modulation rate expressed as number of ripple periods in one octave was varied to find the highest rate at which the listener could detect a reversal. Thresholds were tracked to 70.7% correct (Levitt, 1971). Tracks consisted of fourteen reversals, with the last four reversals averaged to compute thresholds. Presentation level was set at “loud but comfortable” using the Contour Test (Cox et al., 1997), a loudness judgment task in which listeners rated the loudness of ascending intensity noise stimuli. During the SRR test, the presentation level of each signal was roved ±6 dB. Listeners completed two tracks, with additional tracks added if the thresholds were not in agreement. Thresholds from the best two tracks were averaged to give the final threshold score.
Interaural phase difference detection (IPD)
Low-frequency temporal fine structure was evaluated by measuring detection thresholds for a difference in interaural phase in a 500 Hz tone presented to each ear (Hopkins and Moore, 2010). Bergemalm and Lyxell used a clinical IPD task with 500 Hz stimuli to demonstrate central auditory dysfunction following TBI (2005). In this task, tones were presented via ER-3A headphones at an RMS level of 80 dB SPL. In the standard interval the relative phase of the tones was 0°, and the phase difference was varied in the target interval. An 2-down, 1-up adaptive tracking procedure was used to estimate threshold in a 2C2AFC task. Each interval had a duration of 400 ms including a 25 ms raised cosine envelope at onset and offset. Listeners completed at least one familiarization track and repeated tracks until performance stabilized. Each track consisted of ten reversals, of which the final four were averaged to determine threshold. The best two thresholds were averaged to obtain each listener’s final threshold.
Interaural coherence (IC)
Detection thresholds for a decrease in interaural coherence were measured to assess temporal coding in the auditory system (Whitmer et al., 2012). Two independent white noise sources were added and subtracted with variable weighting factors to generate stimuli for the right and left ears with a given IC (Hartmann and Cho, 2011). The standard interval had a fixed IC of unity. IC in the target interval was adaptively decreased in a 2-down, 1-up track to estimate threshold. A 2C2AFC task was used. Stimuli were presented over ER-3A headphones at a root mean squared level of 65 dB SPL. Each interval had a duration of 400 ms including a 25 ms ramp. The bandwidth of the noise stimuli was 22 kHz, but the signal was attenuated above 4 kHz at −20 dB per octave by the headphone frequency response.
Familiarization consisted of at least one track followed by two or more test tracks. Due to difficulty many listeners had performing the IC task, a demonstration program was made available for listeners whose tracks failed to converge after two attempts. The demonstration included a switch to turn on and off a 400 ms gated noise signal and a slider controlling IC. Tracks consisted of ten reversals, and the final four reversals were averaged to give a threshold. Listeners’ best two track thresholds were averaged to give their reported threshold.
Test environment
Auditory tests were performed in a double-walled, sound-attenuating booth. Listeners were seated at a table with a computer monitor, keyboard, and mouse. Tests were implemented in MATLAB (Mathworks, Natick, Massachusetts) and Pure Data (Puckette, 1996).
Cognition
Cognitive ability was assessed with three tasks measuring attention, processing speed, and working memory. The primary goal of cognitive testing was to rule out differences in cognition between the Matched group listeners with no history of head injury and those in the MTBI group who may have suffered global cognitive deficits resulting from their injury. Tests were selected to assess cognition using the visual modality in order to determine the relationship between non-auditory cognitive factors and the auditory abilities evaluated in this study. The Trail Making Test evaluated executive attention by timing the participants in the completion of a task requiring them to mark consecutive circles labeled numerically or with alternating numbers and letters (Reitan, 1958). A digit-symbol coding task evaluated processing speed by timing the participants in a symbol coding task completed using a paper and pencil (Wechsler, 1945). A computer-based reading span test was used to evaluate visual working memory in which participants were asked to recall keywords while reading and evaluating the semantic validity of unrelated sentences (Shah and Miyake, 1996).
Statistical analyses
Matched-groups design
A matched-groups design was adopted in this study to evaluate potential group differences in auditory processing. Given that little is known about the long-term effects of TBI on the perception of basic auditory cues, and that a recent study cast doubt on the idea that TBI results in impaired speech understanding in noise (Krause et al., 2014), the present study was designed to explore the role of auditory and cognitive processes affecting speech. Aging, as well as age-typical elevation in pure-tone thresholds, are known to affect speech understanding in noise, mediated by a combination of auditory and cognitive factors. In this study, listeners with a history of TBI were recruited across a wide range of age and an age-typical range of pure-tone thresholds. By comparing this group to a matched group of listeners with no history of neurological insult spanning an equivalent range of age and pure-tone thresholds, any additional deficit resulting from TBI beyond age and pure-tone thresholds could be demonstrated. The number of abnormal test results for listeners in the TBI group is often reported in studies that include a battery of auditory tests (Nölle et al., 2004; Bergemalm and Lyxell, 2005; Turgeon et al., 2011; Gallun et al., 2012; Saunders et al., 2015). In this study the number of listeners with abnormal results were computed based on normative data when available, and based on YNH group data otherwise. The purpose of counting abnormal results is to compare the rate of abnormal results between MTBI and Matched groups, and to facilitate comparison with previous reports.
Statistical methods
Group comparisons presented in this study include repeated-measures ANOVA in which three groups are compared, MTBI, YNH, and Matched, across within-subjects factors of speech-in-noise performance, auditory processing, and cognition. In order to evaluate the role of auditory and cognitive factors in deficits understanding speech in noise, stepwise linear regression was used including auditory and cognitive predictors of speech-in-noise performance.
Results
Auditory and cognitive measures
Subjective impairment
Each participant was asked if they had difficulty understanding speech in a quiet or noisy room. In the young group, all of the listeners responded “no” to both questions. None of the listeners in the matched group responded that they had difficulty in quiet, but one (1/11) said they had difficulty in noise. This listener had audiometry consistent with mild-to-moderate sensorineural hearing loss. Two other listeners in the matched group had borderline or mild sensorineural hearing loss but denied difficulty in noise. In the MTBI group, all of the listeners answered “no” when asked about difficulty in quiet, but eleven (11/13) answered “yes” to difficulty in noise. Details about individual listener responses in the MTBI group are listed in Table 2.
Speech in speech background
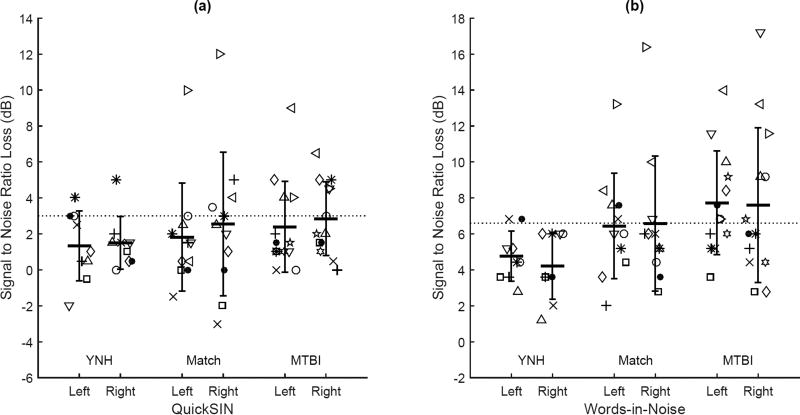
The QuickSIN and WIN tests were used to evaluate potential MTBI group differences in monaural speech understanding. Figure 2(a) shows the QuickSIN and Figure 2(b) shows the WIN mean and standard deviation for the three groups in each ear as well as individual subject data, with a horizontal line representing the cutoff for normal performance. Individual data for the MTBI group are listed alongside audiometric data in Table 2. In the YNH group, one listener (1/9) performed outside of the normal range of 3 dB SNR loss on the QuickSIN in both ears. Four listeners (4/11) were outside of the normal range in the Matched group in at least one ear, and six (6/13) listeners were outside of normal in the MTBI group in at least one ear. On the WIN test, none of the listeners in the YNH group tested outside of the normal range of 6.6 dB SNR loss in either ear. Six in the Matched group (6/9) were outside of the normal range, and eight in the MTBI group (8/13) in at least one ear on the WIN.
Figure 2.
Individual thresholds for the speech-in-noise tests for young, matched, and MTBI group listeners. Listeners in each group are represented by a unique symbol. For the Match and MTBI groups, symbols correspond to those in Table 1. Horizontal bars represent group means and error bars represent the standard deviation. The horizontal dashed line represents the cutoff above which speech recognition in noise is considered abnormal in each test.
Continuous QuickSIN and WIN thresholds for each subject were entered into an Analysis of Variance (ANOVA) to evaluate potential group differences using a within-subjects factor of SNR loss score and a between-subjects factor of group. A significant effect of group was found for WIN SNR loss score in the left ear only (F[2, 23.436] = 3.491, p = 0.043, ηp2 = 0.189). Post-hoc tests of group mean differences for WIN left ear threshold showed that the YNH group mean SNR loss was significantly better than the MTBI group (Mean difference = −2.968 dB, p = 0.013), but not the Matched group (p = 0.159); no difference was found between the Matched and MTBI groups (p = 0.235). This finding is consistent with the fact that the groups contain predominantly normal hearing listeners, but the Matched and MTBI group mean audiograms were slightly elevated relative to the YNH group. Previous reports of listeners with a history of TBI did not find differences relative to controls on speech tasks with colocated, multi-talker background (Begemalm and Lyxell, 2005; Krause et al., 2014).
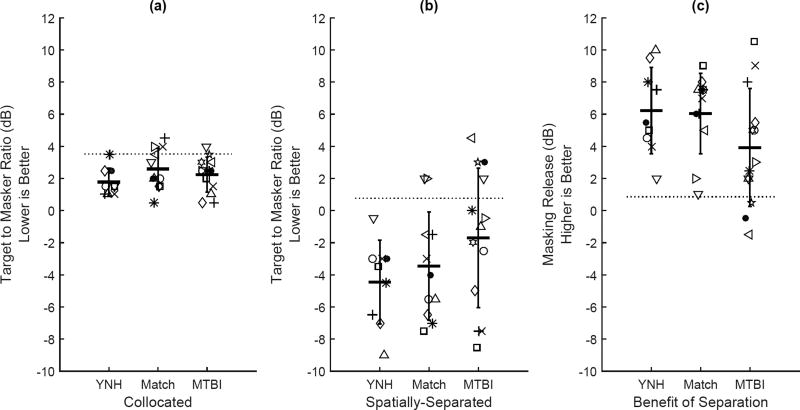
The ability of listeners to benefit from a spatial separation of target and masker talkers was evaluated using the SRM. Individual and group mean SRM data are shown in Figure 3. Scores from the collocated and spatially-separated conditions are shown separately and the difference is shown as the benefit of spatial separation. The YNH group mean and standard deviation thresholds were 1.78 (SD=0.87) dB in the collocated condition and −4.44 (2.60) dB in the spatially separated condition, with a benefit of spatial separation of 6.22 (SD=2.68) dB. These results are consistent with previous results for young listeners with normal pure-tone thresholds (Gallun et al., 2013). Using YNH data to define normal performance as the mean plus two standard deviations, the number of Matched and MTBI group listeners with performance outside the normal range were counted. In the Matched group, three listeners in the collocated condition and an additional listener in the spatially-separated condition had abnormal results (4/11), but none in the Matched group showed an impaired benefit of spatial separation. In the MTBI group, one listener in the collocated condition and three listeners in the spatially-separated condition were outside of the normal range (4/13), and three of these listeners had reduced benefit of spatial separation based on YNH group norms.
Figure 3.
Individual results for the SRM task presented in collocated (left panel) and spatially separated (center panel) noise conditions. Each point represents the dB target to masker ratio at the listeners’ estimated 50% threshold. The benefit of spatial separation in dB is shown in the right panel. Listeners in each group are represented by a unique symbol, and the symbols correspond to those used in Table 1. Group mean scores are shown with horizontal bars and vertical bars show the SD. The dashed line represents the cutoff for abnormal performance as defined by two SD worse than the YNH mean.
A two-way ANOVA was performed to evaluate the benefit of spatial separation on the SRM task and to determine group differences in spatial benefit. Within-subjects factors of colocated and spatially-separated performance, a between-subjects factor of group, and group-by-spatial separation interaction were included in the model. A significant effect of spatial separation was found (F[1, 7.821] = 10.707, p = 0.003, ηp2 = 0.284) indicating that listeners were able to benefit from the spatial separation of the talkers. Group differences were not significant (F[2, 2.188] = 2.996, p = 0.067, ηp2 = 0.182), nor was the interaction of group and spatial separation (F[2, 1.420] = 1.944, p = 0.163, ηp2 = 0.126). This is consistent with previous studies that have found no group effects comparing listeners with TBI to age-matched peers on complex auditory tasks (Bergemalm and Lyxell, 2005). However, these data are also consistent with the finding that impairments are often very heterogeneous across individuals rather than consisting of a common deficit shown by all group members (Gallun et al., 2012). For this reason, individual patterns of impairment may be more indicative of the types of dysfunction observed clinically than are group differences.
In order to evaluate whether subjective claims of difficulty in noise were supported by objective speech-in-noise scores, a repeated-measures ANOVA was performed on the pooled data from the MTBI and Matched groups, with a between-subjects factor of stated speech-in-noise deficit as defined by listener responses to the question of having difficulty understanding speech in noise, and within-subjects factors of the QuickSIN, WIN, SRM collocated, and SRM spatial-separated scores. A small but significant effect of stated speech-in-noise deficit was found (F[1, 14.716] = 7.270, p = 0.013, ηp2 = 0.248) and a significant interaction between stated deficit and speech score (F[3, 4.263] = 3.345, p = 0.024, ηp2 = 0.132). This result shows that listeners reporting difficulty understanding speech in noise in the MTBI group (11/13), combined with the single listener reporting difficulty in the Matched group (1/9), obtained significantly worse performance on objective measures of speech understanding compared to those who reported no difficulty. This finding, like that of the reduced SRM reported above for several members of the MTBI group, is consistent with the idea that not all cases of MTBI are expected to result in long-term auditory symptoms but objective deficits are more likely among those reporting symptoms.
Psychophysics
A repeated-measures ANOVA was used to evaluate the between-subjects factor of group across the within-subjects factors of the psychophysical tasks. Greehouse-Geisser correction of the degrees of freedom and mean squared error was used due to violation of the sphericity assumption. A significant effect of task (F[1.457, 2504.396] = 8.419, p = 0.002, ηp2 = 0.245) was found, but no significant group difference or group and task interaction. For each task, YNH mean and standard deviation performance was calculated and compared to existing studies, and the number of Matched and MTBI group listeners falling outside the mean plus two standard deviations was counted.
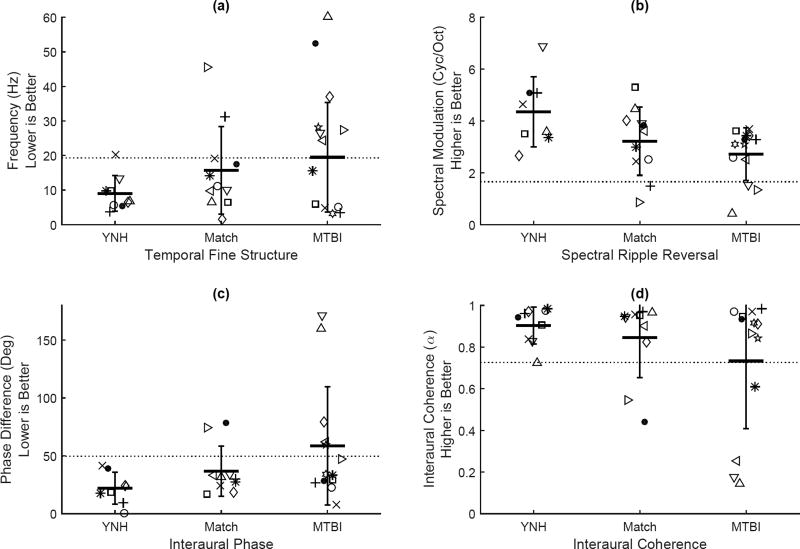
Mean and standard deviation TFS thresholds for the YNH group were 9.02 (SD=5.15) Hz. This is consistent with thresholds obtained using similar stimuli for young listeners with normal pure-tone thresholds (Moore et al., 2006). Two listeners (2/11) in the Matched group and seven listeners (7/13) in the MTBI group had thresholds outside of the normal range. This is again supportive of the finding among blast-related auditory processing dysfunction (Gallun et al., 2012) that group differences may be small or non-existent even when substantial numbers of participants are indicating impairment by performing outside the normal range. Individual and group mean and standard deviation TFS thresholds are shown in Figure 4(a). Horizontal lines indicate normal performance based on YNH scores.
Figure 4.
Individual results of the four psychoacoustic tests for each group. Listeners are represented by a different symbol in each group and the symbols correspond to those used in Table 1. Group mean and SD are shown with horizontal bars. Horizontal dashed line represents normal limits as defined by 2 SD worse than YNH mean thresholds.
SRR thresholds were reported in terms of the number of cycles of spectral modulation per octave, with higher numbers indicating better performance, shown in Figure 4(b). Mean and standard deviation SRR thresholds for the YNH group were 4.35 (SD=1.35) cycles per octave. Thresholds were consistent with young listeners with normal pure-tone thresholds tested using the same paradigm reported previously (Souza et al., 2014). Thresholds could not be obtained for one listener in the YNH group, reducing the total number of subjects included in YNH group to eight. Two listeners (2/11) in the Matched group and three listeners (3/13) in the MTBI group had SRR thresholds outside of the normal range of two SD above the YNH mean.
Thresholds for IPD were reported in terms of the interaural difference in phase in degrees. Mean and standard deviation thresholds for the YNH group were 21.89 (SD=13.84) degrees. YNH thresholds were slightly elevated relative to thresholds for young listeners with normal pure-tone thresholds obtained using comparable methods (Strelcyk and Dau, 2009; Hopkins and Moore, 2011). This may have been due to differences in the amount of training listeners received, or to differences in the duration and presentation of stimuli. A single listener in the YNH group was unable to perform the task after multiple attempts and reinstruction, leaving a remaining eight listeners in the YNH group. Three listeners (3/11) in the Matched group and five listeners (5/13) in the MTBI group were outside of the normal range established by the mean and SD of the YNH group. Figure 4(c) shows individual as well as group mean and SD thresholds on the IPD task.
IC thresholds are reported in terms of the alpha parameter in the stimulus generation algorithm (Hartmann and Cho, 2011), which can range from zero (no interaural coherence) to one (total interaural coherence). In this task, a higher alpha indicates better performance. Mean and standard deviation thresholds for the YNH group were 0.904 (SD=0.0889). Thresholds for the best performers in the YNH group were consistent with previous studies, but several listeners increased the group variance by performing poorly on the task. Based on the mean and SD of the YNH group, a single listener (1/11) in the Matched group and four listeners (4/13) in the MTBI group were considered impaired. Individual and group mean and SD IC thresholds are shown in Figure 4(d).
Cognition
Potential group differences in cognition were evaluated with a repeated-measures ANOVA using the within-subjects factors of the cognitive measures. A significant group difference (F[2, 29.379] = 5.163, p = 0.012, ηp2 = 0.269) was found, as well as a significant effect of cognitive measure (F[1.572, 21.869] = 284.593, p < 0.0001, ηp2 = 0.910), but no interaction between group and measure. Greehouse-Geisser correction of the degrees of freedom and mean squared error was used due to violation of the sphericity assumption. A Shapiro-Wilk test of normality found no significant violation of the normality assumption, so post-hoc tests were performed with Bonferroni correction to evaluate group differences across the cognitive measures. No significant group differences were found on any of the three cognitive measures between the MTBI and Matched groups This result is consistent with the low incidence and small effect size affecting group differences in cognition in cases of long-term MTBI (Tellier et al., 2009). Individual effects of cognition on performance were evaluated through correlational analyses, reported below.
Prediction of speech-in-noise and spatial release deficits
The primary purpose of this study was to evaluate the relative contribution of auditory dysfunction to difficulty speech understanding in noise and in competing speech for listeners with a history of MTBI, and the potential contribution of other, non-auditory cognitive factors. To address this question, peripheral auditory, central auditory, and cognitive measures were evaluated in three groups of listeners, an MTBI group, a YNH control group, and a Matched control group consisting of age- and pure-tone threshold-matched controls. Stepwise linear regression models were created for each of the speech tasks using the following predictor variables: age, mean pure-tone thresholds at 500, 1000, and 2000 Hz in both ears (PTA), TFS, SRR, IPD, IC, working memory, processing speed, and attention. The stepwise regression identified the predictor variable that accounted for the largest proportion of the variance, then continued to find variables that accounted for residual variance in an iterative process until the remaining variables could not account for a significant portion of the residual variance.
The regression analysis for QuickSIN scores found two factors accounting for 50.4% of the variance (p < 0.0001), PTA, which accounted for 39.2% of the variance and SRR, which accounted for an additional 11.2% of the variance. As SRR is a measure of spectral resolution in the auditory system, it is thought to represent peripheral auditory function, as is the case for PTA. Regression analysis for WIN scores revealed a model that included two factors accounting for a total of 79.5% of the variance. The first factor included in the model was PTA, accounting for 58.9% of the variance in WIN scores, and the second factor was IPD, which accounted for an additional 20.6% of the variance. IPD relies on interaural comparison of phase, which requires phase locking in the cochlea to be maintained until a binaural comparison is made in the lower auditory brainstem. IPD sensitivity can thus be considered a measure of both TFS and of binaural processing.
The regression model for SRM, computed from the difference in collocated and spatially-separated scores to represent the benefit of a spatial separation of talkers, included two factors accounting for a total of 68.8% of the variance. The first factor included in the model, accounting for 53.6% of the variance, was working memory, and the second factor was PTA, accounting for an additional 15.2% of the variance. None of the auditory processing variables entered into the model provided a significant improvement in the variance explained. This result suggests that the SRM task was sensitive to different listener characteristics than the monaural speech-in-noise tasks, and that cognition, specifically working memory, was the determining factor in listener performance. The fact that the role of working memory was observed for the difference between colocated and spatially separated was somewhat surprising, given that the difference score was used in order to remove any shared factors in the colocated and spatially separated conditions. This suggests that those who benefit most from spatial separation are those who have the greatest working memory capacity to make use of the spatial difference.
Discussion
MTBI affects millions of people every year, and there is increasing evidence that a majority suffer persistent neurosensory symptoms (Hoffer et al., 2013). Common among post-concussive symptoms are auditory complaints, which may be present and untreated in a majority of cases (Oleksiak et al., 2012), but data remain scarce for civilian populations who lack the auditory comorbidities associated with military service. The impairments that have been found in people with auditory complaints after blast and non-blast injuries include auditory processing deficits that may be associated with elevated pure-tone thresholds (Lew et al., 2007; Oleksiak et al., 2012; Gallun et al., 2012; Saunders et al., 2015).
In the present study, many more listeners in the MTBI group reported difficulty understanding speech in noise than their age- and pure-tone threshold-matched peers. Group differences in objective monaural and binaural speech tasks were not significant. Because not all listeners in the MTBI group had auditory complaints, and because the rate of long-term auditory symptoms after MTBI is likely between 16% (Cockrell and Gregory, 1992) and 87.5% (Oleksiak et al., 2012), there was no reason to expect that all of our MTBI participants would demonstrate auditory dysfunction. However, when listeners in the MTBI and Matched groups were re-categorized by their stated difficulty in noise, significant objective speech-in-noise and spatial release from masking differences were found.
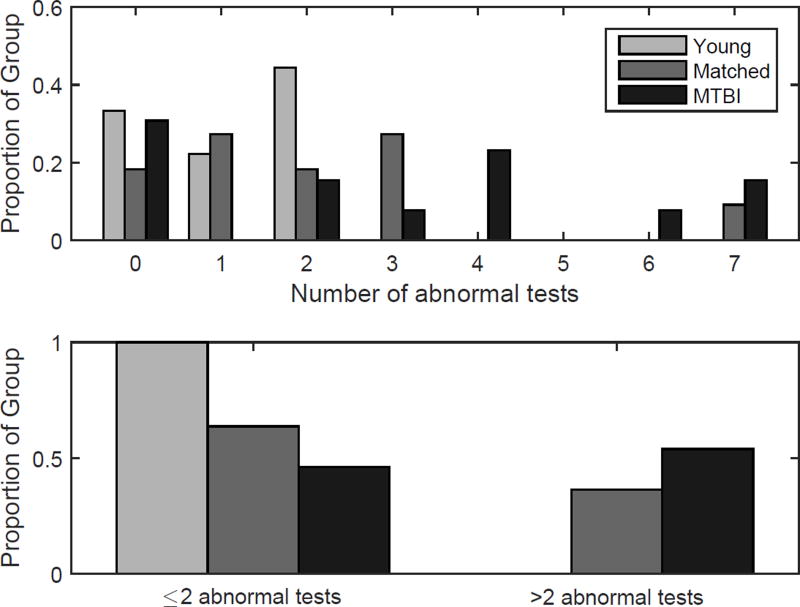
An alternative way to view the effects of MTBI on auditory function is to observe patterns of abnormal auditory test results among listeners and compare the rate of abnormal results across groups (Gallun et al., 2012). The number of abnormal results for each listener were sorted into bins in Figure 5. Included in the chart were the three speech tests, where a score outside the normal range in either ear was counted as one abnormal result; and the four psychophysical tasks. The sum of abnormal results was then converted to a proportion to facilitate comparison across groups. In the MTBI group, 7/13 (62%) had two or more abnormal results on the speech in noise tasks, and one or more abnormal result in the psychophysical tasks. Contrast this with the young group, in which only one listener (11%) had abnormal results on more than two tests; and with the matched group, in which four listeners (36%) had more than two abnormal results. Moreover, abnormal results in the matched group were dominated by the single listener with mild-to-moderate sensorineural hearing loss (the most loss of that group) who had abnormal results on eight of the tests. This listener was recruited to match a single participant in the MTBI group with similar age and audiometric profile. Although limited conclusions can be made from this type of analysis, the pattern of abnormal results across groups is consistent with a higher rate of auditory dysfunction in listeners with a history of MTBI than matched controls. The proportions are also very similar to those reported by Gallun et al. (2012) despite the use of different tests and a patient group with and without MTBI diagnoses who had all reported exposure to multiple high-intensity explosions during their military service. It should be noted that in both of those studies, the proportions were also similar to each other, despite the fact that Gallun et al. (2012) tested patients within six months of blast exposure and Gallun et al. (in press) tested a different group of patients exposed between four and ten years earlier, comparable to the present study.
Figure 5.
The proportion of listeners who performed in the abnormal range for a given number of tests. Abnormal was defined by clinical normative data for the QuickSIN and WIN tests, and two SD worse than the YNH mean for all other tests. The top panel shows the proportion of listeners in integer bins, and the lower panel shows the proportion who had abnormal results on two or fewer tests versus more than two tests. Groups are separated by lightness, with the young group represented by the light grey bars, the matched group in medium grey bars, and the MTBI group in dark grey bars. Note that none of the listeners in the YNH group were abnormal on greater than two tests.
Predictors of speech deficits
Results of the regression analysis suggest that different underlying factors contribute to monaural and binaural speech-in-speech and speech-in-noise tasks. In the monaural tasks, variance was best explained by peripheral auditory factors. Furthermore, the factors that best accounted for the residual variance in both tests were auditory processing tasks that are thought to primarily reflect function at the auditory periphery. Despite the variance in age among study participants, aging and cognitive factors that are known to vary with age were not significant predictors of QuickSIN or WIN performance. The conclusion is that suprathreshold deficits associated with mild and subclinical elevation in pure-tone thresholds are the most important factors affecting monaural speech in noise tasks. Because individual pure-tone thresholds prior to the injury are not known in the present study, it is impossible to determine with certainty that MTBI contributed to cochlear damage in these cases, versus threshold elevation secondary to other factors.
Pure-tone thresholds are known to predict QuickSIN and WIN performance (e.g., McArdle, Wilson, and Burks, 2005), though numerous studies have shown that other factors – including a history of traumatic injury – mediate this relationship (e.g., Killion and Niquette 2000, Wilson, 2003). In the present study, mild and sub-clinical pure-tone threshold elevation likely dominated comparisons; future studies should address this issue by determining the relationship between MTBI and pure-tone threshold elevation in subjects with pre-injury audiometric data (i.e. military service members), and by evaluating speech-in-noise in after MTBI in listeners that meet strict criteria for normal cochlear function.
The role of cognition in SRM spatial benefit was much greater than the monaural speech tasks. Working memory was the factor that accounted for the greatest amount of variance in the benefit of a spatial separation of talkers. This finding was consistent with the relationship between working memory and performance on similar coordinate-response measure tasks (Gygi and Shafiro, 2012).
Clinical implications
With regard to the myriad potential effects of MTBI, audiologists can do more than assess sensorineural hearing loss. It is only with a comprehensive assessment of the auditory system – including speech in noise and psychophysical measures of auditory perception – that auditory complaints following MTBI can be fully enumerated. Failing to quantify impairment in one part of the auditory system can have serious negative consequences for a patient in that their perceived disability is never validated and, in some cases, results in the loss of financial damages or disability compensation.
In addition to providing auditory assessment, audiology can take a role in rehabilitation by addressing the communication needs of the person with MTBI. Whether the source of a brain injured individual’s communication difficulty lies in their auditory system or in domain-general or psychological functions, audiologists can provide counselling and technology that facilitate participation in activities of daily living and rehabilitation services. Even in the absence of a complete understanding of the effects of MTBI on the auditory system, a patient’s activity limitations can be addressed using tools such as aural rehabilitation and hearing assistive devices. Anecdotal and case reports indicate that audiologists are currently fitting people who have hearing complaints after MTBI with low-gain hearing aids even in the absence of elevated pure-tone thresholds (Hoover et al., 2014), however there is currently no data available on the prevalence of this treatment nor is the practice supported by published clinical guidelines (Hoover et al., 2015).
Future studies should attempt to revise the set of tests performed on patients with MTBI because existing CAPD tests do not specifically address the impairments likely to result from MTBI. A better understanding of the effects of MTBI on speech recognition, particularly in noisy environments listeners find difficult, and of the auditory processing and cognitive deficits that underlie these difficulties will help improve assessment and rehabilitation after MTBI. Audiology should reassess its role in MTBI care, and can facilitate treatment by addressing the short-term and long-term communication needs of people with post-concussive auditory dysfunction. Rehabilitation after MTBI, including rehabilitation of non-auditory symptoms that rely on auditory communication for assessment and service delivery, may be improved with appropriate diagnosis and treatment of auditory complaints.
Acknowledgments
Work was supported by NIH grants R01 DC60014 and R01 DC12289 (to P. Souza)
Abbreviations
- 2C2AFC
two-cue, two-alternative forced choice
- ANOVA
analysis of variance
- CAPD
central auditory processing disorder
- IC
interaural coherence
- IPD
interaural phase difference
- MTBI
mild traumatic brain injury
- PTA
pure-tone average
- QuickSIN
Quick Speech in Noise test
- SD
standard deviation
- SNR
signal to noise ratio
- SRM
spatial release from masking
- SRR
spectral ripple reversal
- SRT
speech reception thresholds
- TBI
traumatic brain injury
- TFS
temporal fine structure
- TMR
target to masker ratio
- WIN
Words-in-Noise test
- dB
decibel
- YNH
young listeners with normal pure-tone thresholds
Footnotes
Some data reported in this manuscript were presented at the AudiologyNOW! convention, March 2013, Orlando, FL; and at the Acoustical Society of America, May 2014, Providence, RI.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- Bergemalm P-O, Borg E. Long-term objective and subjective audiologic consequences of closed head injury. Acta Otolaryngol. 2001;121(6):724–734. doi: 10.1080/00016480152583674. [DOI] [PubMed] [Google Scholar]
- Bergemalm PO, Lyxell B. Appearances are deceptive? long-term cognitive and central auditory sequelae from closed head injury. Int J Audiol. 2005;44(1):39–49. doi: 10.1080/14992020400022546. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Detection of interaural delay in high-frequency noise. J Acoust Soc Am. 1982;71(1):147–152. doi: 10.1121/1.409973. [DOI] [PubMed] [Google Scholar]
- Bolia RS, Nelson WT, Ericson MA, Simpson BD. A speech corpus for multitalker communications research. J Acoust Soc Am. 2000;107:1065. doi: 10.1121/1.428288. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Incidence rates of hospitalization related to traumatic brain injury – 12 states, 2002. Morb Mortal Wkly Rep. 2006;55(8):201–204. [PubMed] [Google Scholar]
- Cockrell JL, Gregory SA. Audiological deficits in brain-injured children and adolescents. Brain Inj. 1992;6(3):261–266. doi: 10.3109/02699059209029667. [DOI] [PubMed] [Google Scholar]
- Cox RM, Alexander GC, Taylor IM, Gray GA. The Contour Test of loudness perception. Ear Hear. 1997;18:388–400. doi: 10.1097/00003446-199710000-00004. [DOI] [PubMed] [Google Scholar]
- Dean PJ, Sterr A. Long-term effects of mild traumatic brain injury on cognitive performance. Front Hum Neurosci. 2013;12:7–30. doi: 10.3389/fnhum.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein EA, Corso PS, Miller TR. The Incidence and Economic Burden of Injuries in the United States. Oxford: Oxford University Press; 2006. [Google Scholar]
- Flood GM, Dumas HM, Haley SM. Central auditory processing and social functioning following brain injury in children. Brain Injury. 2005;19(12):1019–1026. doi: 10.1080/02699050500110223. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hear Res. 2001;158(1):1–27. doi: 10.1016/s0378-5955(01)00296-9. [DOI] [PubMed] [Google Scholar]
- Gallun FJ, Diedesch AC, Kampel SD, Jakien KM. Independent impacts of age and hearing loss on spatial release in a complex auditory environment. Front Neurosci. 2013;7 doi: 10.3389/fnins.2013.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallun FJ, Diedesch AC, Kubli LR, Walden TC, Folmer RL, Lewis MS, McDermott DJ, Fausti SA, Leek MR. Performance on tests of central auditory processing by individuals exposed to high-intensity blasts. J Rehabil Res Dev. 2012;49(7):1005–1024. doi: 10.1682/jrrd.2012.03.0038. [DOI] [PubMed] [Google Scholar]
- Gallun FJ, Lewis MS, Folmer RL, Diedesch AC, Kubli LR, McDermott DJ, Leek MR. Implications of blast exposure for central auditory function: A review. J Rehabil Res Dev. 2012;49(7):1059–1074. doi: 10.1682/jrrd.2010.09.0166. [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BC. Auditory filter shapes in subjects with unilateral and bilateral cochlear impairments. J Acoust Soc Am. 1986;79(4):1020–1033. doi: 10.1121/1.393374. [DOI] [PubMed] [Google Scholar]
- Gygi B, Shafiro V. Spatial and temporal factors in a multitalker dual listening task. Acta Acustica united with Acustica. 2012;98(1):142–157. [Google Scholar]
- Hall RC, Hall RC, Chapman MJ. Definition, diagnosis, and forensic implications of postconcussional syndrome. Psychosomatics. 2005;46(3):195–202. doi: 10.1176/appi.psy.46.3.195. [DOI] [PubMed] [Google Scholar]
- Hartmann W, Cho Y. Generating partially correlated noise — A comparison of methods. J Acoust Soc Am. 2011;130:292–301. doi: 10.1121/1.3596475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer ME, Balaban C, Nicholas R, Marcus D, Murphy S, Gottshall K. Neurosensory Sequelae of Mild Traumatic Brain Injury. Psychiatric Annals. 2013;43(7):318–323. [Google Scholar]
- Hoofien D, Gilboa A, Vakil E, Donovick PJ. Traumatic brain injury (TBI) 10? 20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj. 2001;15(3):189–209. doi: 10.1080/026990501300005659. [DOI] [PubMed] [Google Scholar]
- Hoover EC, Souza PE, Gallun FJ. Degraded temporal processing after traumatic brain injury. J Acoust Soc Am. 2014;135(4):2166–2166. [Google Scholar]
- Hoover EC, Souza PE, Gallun FJ. Competing Views on Abnormal Auditory Results After Mild Traumatic Brain Injury. SIG-6 Perspect Hear Hear Dis Res Diagn. 2015;19(1):12–21. [Google Scholar]
- Hopkins K, Moore BC. Moderate cochlear hearing loss leads to a reduced ability to use temporal fine structure information. J Acoust Soc Am. 2007;122(2):1055–1068. doi: 10.1121/1.2749457. [DOI] [PubMed] [Google Scholar]
- Hopkins K, Moore BC. Development of a fast method for measuring sensitivity to temporal fine structure information at low frequencies. Int J Audiol. 2010;49(12):940–946. doi: 10.3109/14992027.2010.512613. [DOI] [PubMed] [Google Scholar]
- Hopkins K, Moore BC. The effects of age and cochlear hearing loss on temporal fine structure sensitivity, frequency selectivity, and speech reception in noise. J Acoust Soc Am. 2011;130:334–349. doi: 10.1121/1.3585848. [DOI] [PubMed] [Google Scholar]
- Humes LE, Dubno JR. Factors affecting speech understanding in older adults. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The Aging Auditory System. New York, NY: Springer; 2010. pp. 211–257. [Google Scholar]
- Jury MA, Flynn MC. Auditory and vestibular sequelae to traumatic brain injury: a pilot study. New Zealand Med J. 2001;114(1134):286–288. [PubMed] [Google Scholar]
- Killion MC, Niquette PA. What can the pure-tone audiogram tell us about a patient's SNR loss? The Hearing Journal. 2000;53(3):46–48. [Google Scholar]
- Krause MO, Kennedy MR, Nelson PB. Masking release, processing speed and listening effort in adults with traumatic brain injury. Brain Inj. 2014;28(11):1473–1484. doi: 10.3109/02699052.2014.920520. [DOI] [PubMed] [Google Scholar]
- Lew HL, Jerger JF, Guillory SB, Henry JA. Auditory dysfunction in traumatic brain injury. J Rehabil Res Dev. 2007;44(7):921. doi: 10.1682/jrrd.2007.09.0140. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down procedures in psychoacoustics. J Acoust Soc Am. 1971;49:467. [PubMed] [Google Scholar]
- Mangels JA, Craik FI, Levine B, Schwartz ML, Stuss DT. Effects of divided attention on episodic memory in chronic traumatic brain injury: a function of severity and strategy. Neuropsychologia. 2002;40(3):2369–2385. doi: 10.1016/s0028-3932(02)00084-2. [DOI] [PubMed] [Google Scholar]
- McArdle RA, Wilson RH. Homogeneity of the 18 QuickSIN™ lists. J Am Acad Audiol. 2006;17(3):157–167. doi: 10.3766/jaaa.17.3.2. [DOI] [PubMed] [Google Scholar]
- McArdle RA, Wilson RH, Burks CA. Speech recognition in multitalker babble using digits, words, and sentences. J Am Acad Audiol. 2005;16(9):726–739. doi: 10.3766/jaaa.16.9.9. [DOI] [PubMed] [Google Scholar]
- Moore BC, Glasberg BR, Flanagan HJ, Adams J. Frequency discrimination of complex tones; assessing the role of component resolvability and temporal fine structure. J Acoust Soc Am. 2006;119(1):480–490. doi: 10.1121/1.2139070. [DOI] [PubMed] [Google Scholar]
- Moore BC, Sek A. Development of a fast method for determining sensitivity to temporal fine structure. Int J Audiol. 2009;48(4):161–171. doi: 10.1080/14992020802475235. [DOI] [PubMed] [Google Scholar]
- Musiek FE, Baran JA, Shinn J. Assessment and remediation of an auditory processing disorder associated with head trauma. J Am Acad Audiol. 2004;15(2):117–132. doi: 10.3766/jaaa.15.2.3. [DOI] [PubMed] [Google Scholar]
- Noble W, Jensen NS, Naylor G, Bhullar N, Akeroyd MA. A short form of the Speech, Spatial and Qualities of Hearing scale suitable for clinical use: The SSQ12. Int J Audiol. 2013;52(6):409–12. doi: 10.3109/14992027.2013.781278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nölle C, Todt I, Seidl RO, Ernst A. Pathophysiological changes of the central auditory pathway after blunt trauma of the head. Neurotrauma. 2004;21(3):251–258. doi: 10.1089/089771504322972040. [DOI] [PubMed] [Google Scholar]
- O’Jile JR, Ryan LM, Betz B, Parks-Levy J, Hilsabeck RC, Rhudy JL, Gouvier WD. Information processing following mild head injury. Arch Clin Neuropsychol. 2006;21(4):293–296. doi: 10.1016/j.acn.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Oleksiak M, Smith BM, St Andre JR, Caughlan CM, Steiner M. Audiological issues and hearing loss among Veterans with mild traumatic brain injury. J Rehabil Res Dev. 2012;49(7):995–1003. doi: 10.1682/jrrd.2011.01.0001. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Micheyl C, Keebler MV. Can temporal fine structure represent the fundamental frequency of unresolved harmonics? J Acoust Soc Am. 2009;125(4):2189–2199. doi: 10.1121/1.3089220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckette M. Proceedings, Second Intercollege Computer Music Concerts. Tachikawa, Japan: Kunitachi College of Music; 1996. Pure Data: another integrated computer music environment; pp. 37–41. [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8(3):271–276. [Google Scholar]
- Roup CM, Wiley TL, Safady SH, Stoppenbach DT. Tympanometric screening norms for adults. Am J Audiol. 1998;7:55–60. doi: 10.1044/1059-0889(1998/014). [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54(1):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Saunders GH, Frederick MT, Arnold M, Silverman S, Chisolm TH, Myers P. Auditory difficulties in blast-exposed veterans with clinically normal hearing. J Rehabil Res Dev. 2015;52(3):343–360. doi: 10.1682/JRRD.2014.11.0275. [DOI] [PubMed] [Google Scholar]
- Shah P, Miyake A. The separability of working memory resources for spatial thinking and language processing: an individual differences approach. J Exp Psychol Gen. 1996;125(1):4. doi: 10.1037//0096-3445.125.1.4. [DOI] [PubMed] [Google Scholar]
- Souza PE, Blackburn MC, Hoover EC, Gallun FJ. Characterizing severe hearing loss. American Auditory Society; Scottsdale, AZ: 2014. Mar, [Google Scholar]
- Strelcyk O, Dau T. Relations between frequency selectivity, temporal fine-structure processing, and speech reception in impaired hearing. J Acoust Soc Am. 2009;125(5):3328–3345. doi: 10.1121/1.3097469. [DOI] [PubMed] [Google Scholar]
- Tellier A, Marshall SC, Wilson KG, Smith A, Perugini M, Stiell IG. The heterogeneity of mild traumatic brain injury: Where do we stand? Brain Inj. 2009;23(11):879–887. doi: 10.1080/02699050903200555. [DOI] [PubMed] [Google Scholar]
- Turgeon C, Champoux F, Lepore F, Leclerc S, Ellemberg D. Auditory processing after sport-related concussions. Ear Hear. 2011;32(5):667–670. doi: 10.1097/AUD.0b013e31821209d6. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Curtiss G, Belanger HG. Long-term neuropsychological outcomes following mild traumatic brain injury. J Int Neuropsychol. 2005;11(3):228–236. doi: 10.1017/S1355617705050289. [DOI] [PubMed] [Google Scholar]
- Wang X. Neural coding strategies in auditory cortex. Hear Res. 2007;229(1):81–93. doi: 10.1016/j.heares.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Wechsler D. A standardized memory scale for clinical use. J Psychol. 1945;19(1):87–95. [Google Scholar]
- Whitmer WM, Seeber BU, Akeroyd MA. Apparent auditory source width insensitivity in older hearing-impaired individuals. J Acoust Soc Am. 2012;132:369. doi: 10.1121/1.4728200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RH. Development of a speech-in-multitalker-babble paradigm to assess word-recognition performance. J Am Acad Audiol. 2003;14(9):453–470. [PubMed] [Google Scholar]
- Wilson RH, Burks CA. Use of 35 words for evaluation of hearing loss in signal-to-babble ratio: A clinic protocol. J Rehabil Res Dev. 2005;42(6):839. doi: 10.1682/jrrd.2005.01.0009. [DOI] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Rubinstein JT. Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. J Assoc Res Otolaryngol. 2007;8(3):384–392. doi: 10.1007/s10162-007-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]