Abstract
Background
Although men on active surveillance for prostate cancer (PCa) may benefit from intervention with 5α-reductase inhibitors (5-ARIs), it has not been resolved whether 5-ARIs are effective for delaying disease progression and, if so, whether specific patients are more likely to benefit.
Objective
To identify molecular features predictive of patient response to 5-ARIs.
Design, setting, and participants
Nkx3.1 mutant mice, a model of early-stage PCa, were treated with the 5-ARI finasteride, and histopathological and molecular analyses were performed. Cross-species computational analyses were used to compare expression profiles for treated mice with those of patients who had received 5-ARIs before prostatectomy.
Intervention
Finasteride administered to Nkx3.1 mutant mice. 5-ARI-treated patient specimens obtained retrospectively.
Outcome measurements and statistical analysis
Endpoints in mice included histopathology, immunohistochemistry, and molecular profiling. GraphPad Prism software, R-studio, and Matlab were used for statistical and data analyses.
Results and limitations
Finasteride treatment of Nkx3.1 mutant mice resulted in a significant reduction in prostatic intraepithelial neoplasia (PIN), as evident from histopathological and expression profiling analyses. Cross-species computational analysis comparing finasteride-treated mice with two independent 5-ARI–treated patient cohorts showed that reduced NKX3.1 expression is predictive of response to 5-ARI. A limitation of the study is that these retrospective human cohorts have relatively few patients with limited clinical outcome data. Future prospective clinical trials are needed to validate whether stratifying patients on the basis of NKX3.1 expression improves the benefit of 5-ARIs during active surveillance.
Conclusions
This co-clinical study implicates NKX3.1 status as a predictor of response to 5-ARIs, and suggests that molecular features, including NKX3.1 expression, may help to identify PCa patients most likely to benefit from 5-ARIs during active surveillance.
Patient summary
The aim of precision cancer prevention is to tailor interventions on the basis of individualized patient characteristics. We propose that patients with low NKX3.1 expression are optimal candidates for intervention with 5α-reductase inhibitors as an adjunct to active surveillance.
Keywords: 5α-Reductase inhibitors, Active surveillance, Chemoprevention, Dutasteride, Finasteride, NKX3.1, Precision cancer prevention, Prostate cancer
1. Introduction
Widespread screening combined with the rise in the aging population has led to increased diagnosis of prostate cancer (PCa), but the majority of men diagnosed are at low risk of disease progression [1,2]. Rather than undergoing invasive procedures, many men are opting for active surveillance, whereby treatment is delayed until signs of overt progression [2,3]. It would be advantageous to better understand the risk of disease progression at the individual patient level and to identify molecular features predictive of response for specific interventions, which is the premise of precision cancer prevention [4].
Because of the critical dependence of PCa on the androgen receptor (AR) [5,6], many interventions for PCa target AR signaling or androgen biosynthesis [7]. Among these are agents that target 5α-reductase, which catalyzes the conversion of testosterone to dihydrotestosterone (DHT) [8]. Although 5α-reductase inhibitors (5-ARIs), including finasteride and dutasteride, have been evaluated in prospective clinical trials for both primary prevention [9–12] and secondary prevention for patients on active surveillance [13,14], their benefits in these settings remain controversial.
We performed co-clinical analyses to evaluate the phenotypic and molecular consequences of finasteride treatment in a genetically engineered mouse model (GEMM) that is analogous to low-risk PCa in humans [15,16]. Using cross-species computational analyses to compare finasteride treatment of GEMMs with retrospective cohorts of 5-ARI–treated patients, we show that reduced expression of NKX3.1 is a predictor of response to 5-ARIs. We propose that expression of NKX3.1 be evaluated as a means of stratifying men on active surveillance as candidates for intervention with 5-ARIs.
2. Patients and methods
2.1. Preclinical analyses
Experiments using animals were performed according to protocols approved by the Institutional Animal Care and Use Committee at Columbia University Medical Center. Nkx3.1 wild-type (Nkx3.1+/+) and germline homozygous mutant (Nkx3.1−/−) mice, and Pten germline heterozygous mutant mice (Pten+/−) have been described previously [16,17]. Finasteride (Kemprotec, Carnforth, UK) was dissolved in ethanol at a concentration of 20 mg/ml and diluted using sterile phosphate-buffered saline to a working stock of 1 mg/ml. Cohorts of mice were randomly assigned to treatment with finasteride or vehicle. At sacrifice, prostate tissues were fixed in 10% formalin and paraffin-embedded or snap-frozen in liquid nitrogen. Histopathological grading was performed according to the classification of Park et al [18]. Immunohistochemical staining was as previously described [19]; images were captured using an Olympus VS120 whole-slide scanning microscope. Levels of steroids in serum were determined by extraction with hexane/dichloromethane (3:2 v/v) followed by purification using a XEVO TQS tandem mass spectrometer (Waters, Milford, MA, USA) with a detection limit of 10 pg/ml. Quantitative real-time polymerase chain reaction (PCR) was carried out on RNA prepared using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) with a QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany) [19]. RNA sequencing was performed using a MagMAX-96 total RNA isolation kit (Life Technologies) as previously described [19]. Raw counts for RNA sequencing (RNAseq) data were normalized and the variance was stabilized using the DESeq2 package (Bioconductor) in R-studio 0.99.902, R v3.3.0.
2.2. Patient cohorts
Patient specimens were obtained following protocols approved by the institutional review board of Weill Cornell Medicine (WCM) and Fred Hutchinson Cancer Research Center (FHCRC). Two independent retrospective patient cohorts were used for training and testing/validation (Table 1). The WCM cohort (n = 9) included patients with clinically localized PCa who had been receiving finasteride or dutasteride before prostatectomy. The FHCRC cohort (n = 15) included samples collected as part of the multicenter ARI40010 study [20] from patients who had received dutasteride for 4 mo before prostatectomy. Immunohistochemistry was performed on paraffin-embedded tissues with a rabbit polyclonal NKX3.1 antibody (Biocare Medical, Pacheco, CA, USA) using a Leica Bond III automated stainer (Leica Biosystems, Wetzlar, Germany), and quantified using HALO software (Indica Labs, Corrales, NM, USA).
Table 1.
Characteristics of the patients from the 5-ARI cohorts
| WCM cohort (training) | FHCRC (validation) | |
|---|---|---|
| Patients (n) | ||
| 5-ARI treated | 9 | 10 a |
| Untreated | 18 | 5 |
| Sample collection period | 2012–2015 | 2003–2005 |
| Age (yr) | 64 (59.5–68.5) b | 61 (46–73) c |
| PSA range (ng/ml) | 2.39–14.5 | 2.5–10 |
| Pathological Gleason score | 6 (6–9) d | |
| Treated patients | 3 + 3 (n = 4), 3 + 4 (n = 4), 4 + 3 (n = 1) | |
| Untreated patients | 3 + 4 (n = 7), 4 + 3 (n = 8), 4 + 4 (n = 3) | |
5-ARI = 5α-reductase inhibitor; WCM = Weill Cornell Medicine; FHCRC = Fred Hutchinson Cancer Research Center; PSA = prostate-specific antigen.
Of the ten patients, five received a low (0.5 mg) and five a high ARI dose (3.5 mg).
Median (interquartile range).
Mean (range).
Median (range).
2.3. Statistical analysis
Independent groups were compared using a two-tailed two-sample Welch t test assuming that variances between the populations were not equal. When two phenotypes were compared (ie, finasteride- vs vehicle-treated samples), the Welch t test was applied to estimate the difference in RNAseq counts (ie, differential expression) between these phenotypes for each gene. Differential expression signatures were thus defined as the list of genes ranked by t values from a two-tailed two-sample t test comparing the finasteride- and vehicle-treated samples. For comparison with human gene signatures, mouse genes were mapped to human orthologs using the homoloGene database (www.ncbi.nlm.nih.gov/homologene).
Pathway enrichment was assessed using gene set enrichment analysis (GSEA) [21] to query pathways collected from the C2 database, in which normalized enrichment scores (NES) and p values were estimated using 1000 gene permutations. Master regulator (MR) analysis was performed using the Master Regulator Inference algorithm (MARINa) by interrogating a human PCa interactome [22] using “humanized” mouse signatures and human signatures. The statistical significance of each MR was estimated using 1000 gene permutations, and p < 0.05 was considered significant. To estimate clinical relevance, we used a Cox proportional hazards model for univariate analysis and Kaplan-Meier survival analysis for multivariate analysis, performed with the surv and coxph functions from the survcomp package (Bioconductor; www.bioconductor.org). Before the Kaplan-Meier analysis, k-means clustering was used to segregate patients into two groups corresponding to low or high MR activity. The p values were estimated using a Wald test for the Cox proportional hazards model, and a log-rank test for Kaplan-Meier survival analysis. Significance for statistical tests was assumed at p < 0.05. Statistical analysis was performed using GraphPad Prism software (Version 6.0), R-studio (0.99.902, R v3.3.0), and Matlab (2012a).
2.4. Accession numbers
Raw and normalized expression profiling data are publicly available through the Gene Expression Omnibus database via accession number GSE92999.
3. Results
3.1. Finasteride abrogates PIN in a GEMM of early-stage PCa
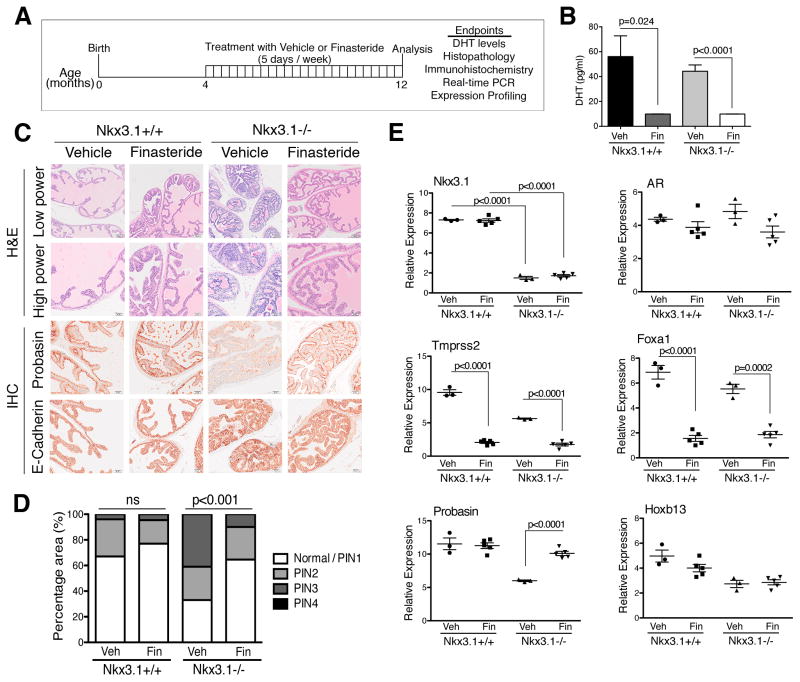
Nkx3.1 mutant mice develop PIN as a consequence of aging, and share conserved molecular features with early-stage human PCa [15,16,23]. We reasoned that this GEMM could provide information on the efficacy of finasteride in delaying progression of early-stage PCa and on the molecular programs associated with such a response. Cohorts of littermate wild-type (Nkx3.1+/+) and homozygous mutant (Nkx3.1−/−) mice were randomly assigned to the vehicle or finasteride arms at age 4 mo (Fig. 1A), which is before the onset of PIN in these mice [16]. Treatment (10 mg/kg/d, five times weekly [24]) was continued for up to 12 mo, by which point untreated Nkx3.1−/− mice display PIN [15,16,23]. At the conclusion of the study, mice were euthanized to assess DHT levels (Fig. 1B), histopathological phenotype (Fig. 1C,D), and expression of markers of prostate differentiation, cancer initiation, and androgen response (Figs. 1E and 2A).
Fig. 1.
Finasteride abrogates prostatic intraepithelial neoplasia (PIN) in a genetically engineered mouse model of prostate cancer. (A) Preclinical trial design. Cohorts of Nkx3.1+/+ and Nkx3.1−/− mice aged 4 mo were randomly assigned to treatment with finasteride (1 mg/ml in phosphate-buffered saline) or vehicle once a day on a schedule of 5 d/wk for 8 mo, until the mice were aged 12 mo. At the conclusion of the study, mice were sacrificed and analysis of the endpoints indicated was performed. PCR = polymerase chain reaction. (B) Levels of dihydrotestosterone (DHT) in serum as indicated (n = 5 per group); p values represent comparisons between bracketed groups and were estimated using a two-tailed two-sample t test. Veh = vehicle; Fin = finasteride. (C) Histological analysis. Shown are representative images of hematoxylin and eosin (H&E) staining and immunohistochemical (IHC) staining of anterior prostate from Nkx3.1+/+ and Nkx3.1−/− mice treated with finasteride or vehicle, as indicated (n = 25 per group). Antibodies were as previously reported [19]. Scale bars represent 100 μm (H&E low power) or 50 μm (H&E high power and IHC staining). (D) Summary of PIN phenotype following treatment. Shown is the percentage area of prostatic tissue that is normal/PIN1, PIN2, PIN3, and PIN4 following treatment with finasteride in Nkx3.1+/+ (n = 5 per group) and Nkx3.1−/− (n = 15 per group) mice; p values were estimated using a two-tailed two-sample t test. ns = not significant. (E) Quantitative real-time PCR was carried out using total RNA from Nkx3.1+/+ and Nkx3.1−/− prostate treated with vehicle or finasteride, as indicated. Analyses were performed in triplicate and normalized to GAPDH; p values were estimated using a two-tailed two-sample t test.
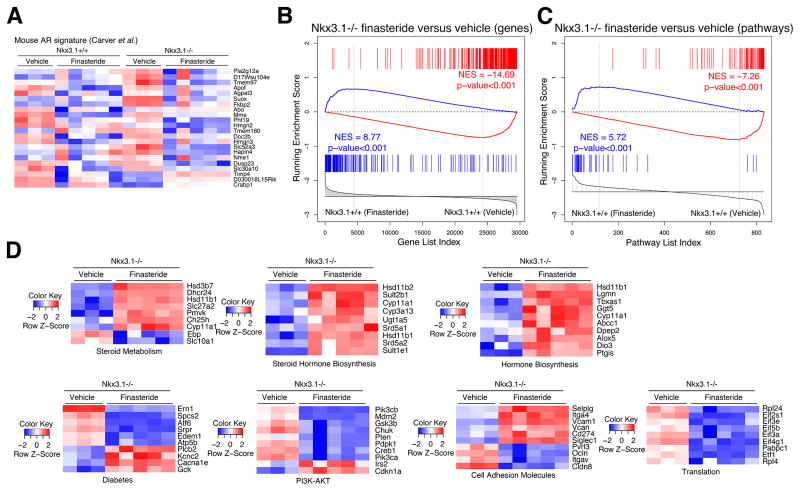
Fig. 2.
Finasteride leads to reversal of the molecular phenotype of Nkx3.1 mutant mice. (A) Heat map depicting gene expression levels of AR-regulated genes reported by Carver et al [27] comparing vehicle- or finasteride-treated Nkx3.1+/+ or Nkx3.1−/− prostate, as indicated. (B) Gene set enrichment analysis (GSEA) comparing a reference gene expression signature from prostates of Nkx3.1+/+ finasteride-treated mice (n = 5) versus Nkx3.1+/+ vehicle-treated mice (n = 3) with a query signature (p < 10−7) of Nkx3.1−/− finasteride-treated mice (n = 5) versus Nkx3.1−/− vehicle-treated mice (n = 3). (C) GSEA comparing a reference pathway signature from prostates of Nkx3.1+/+ finasteride-treated mice (n = 5) versus Nkx3.1+/+ vehicle-treated mice (n = 3) with a query pathway signature (top 50 differentially changed pathways) of Nkx3.1−/− finasteride-treated mice (n = 5) versus Nkx3.1−/− vehicle-treated mice (n = 3). (D) Heat maps depicting expression levels of selected leading edge genes from the pathways in (C) from the C2 database, comparing Nkx3.1−/− mice treated with vehicle or finasteride.
Finasteride was highly effective in inhibiting 5α-reductase, as evidenced by the nearly complete inhibition of serum DHT levels in both Nkx3.1+/+ and Nkx3.1−/− mice (n = 5 per group; p = 0.024 and p ≤ 0.0001, respectively; Fig. 1B). In addition, several AR-regulated genes, including Tmprss2 and Foxa1, were downregulated to a similar extent in Nkx3.1+/+ and Nkx3.1−/− prostates (n = 5 per group; p < 0.001; Fig. 1E), whereas expression levels of AR itself and a non-AR regulated gene, Hoxb13, were not affected (n = 5 per group; Fig. 1E). Notably, expression of Nkx3.1, a known target of AR, was not affected in finasteride-treated Nkx3.1+/+ mice, reflecting the complexity of the relationship between AR and NKX3.1 expression [25,26].
However, finasteride had a profound effect on the histological phenotype of Nkx3.1−/− but not Nkx3.1+/+ prostates (Fig. 1C,D). Specifically, the PIN phenotype of Nkx3.1−/− prostates was significantly abrogated following treatment, while the normal prostate histology of Nkx3.1+/+ mice was virtually unaffected (n = 25–30 per group, p < 0.001; Fig. 1C,D). Notably, abrogation of the PIN phenotype was not observed in finasteride-treated Pten heterozygous mutant mice (Pten+/−), which develop PIN but are wild type for Nkx3.1 (Supplementary Fig. 1). The striking reversal of the PIN phenotype in finasteride-treated Nkx3.1−/− mice was accompanied by increased expression of markers of prostate differentiation, including E-cadherin and probasin (n = 5 per group; p < 0.0001; Fig. 1C,E). RNA sequencing comparing prostate tissues from vehicle- and finasteride-treated Nkx3.1+/+ or Nkx3.1−/− mice further indicated the similar inhibition of 5α-reductase in Nkx3.1+/+ and Nkx3.1−/− prostates, as evidenced from their comparable reduction in expression of AR-regulated genes (p < 0.001; Fig. 2A) [27]. However, GSEA comparing a differential gene expression signature between finasteride- and vehicle-treated Nkx3.1−/− prostates with a comparable signature from Nkx3.1+/+ prostates revealed a strong difference after treatment. Specifically, genes downregulated in finasteride-treated Nkx3.1−/− prostates were upregulated in Nkx3.1+/+ prostates (NES = 8.77; p < 0.001; Fig. 2B); conversely, genes upregulated in finasteride-treated Nkx3.1−/− prostates were downregulated in Nkx3.1+/+ prostates (NES = 14.69; p < 0.001; Fig. 2B).
We further investigated the molecular consequences of finasteride treatment through GSEA comparison of biological pathways affected after treatment in Nkx3.1−/− and Nkx3.1+/+ prostates, which also revealed strong reversal in both upregulated and downregulated leading edges (NES = 5.72 and 7.26, respectively; p < 0.001; Fig. 2C). The pathways significantly reverted in the finasteride- versus vehicle-treated Nkx3.1−/− mice include those associated with steroid hormone signaling, such as REACTOME steroid metabolic signaling (p = 0.002), and cancer initiation or progression, including REACTOME translation (p ≤ 0.001) and REACTOME PI3K-AKT signaling (p ≤ 0.001) (Fig. 2D). Taken together, these histopathological and molecular studies demonstrate that the Nkx3.1−/− prostate is profoundly affected by finasteride, which is evident from the striking abrogation of the PIN phenotype and accompanied by global reversion of the molecular phenotype towards that of normal prostate.
3.2. Molecular signature of response to 5α-reductase inhibition in human PCa
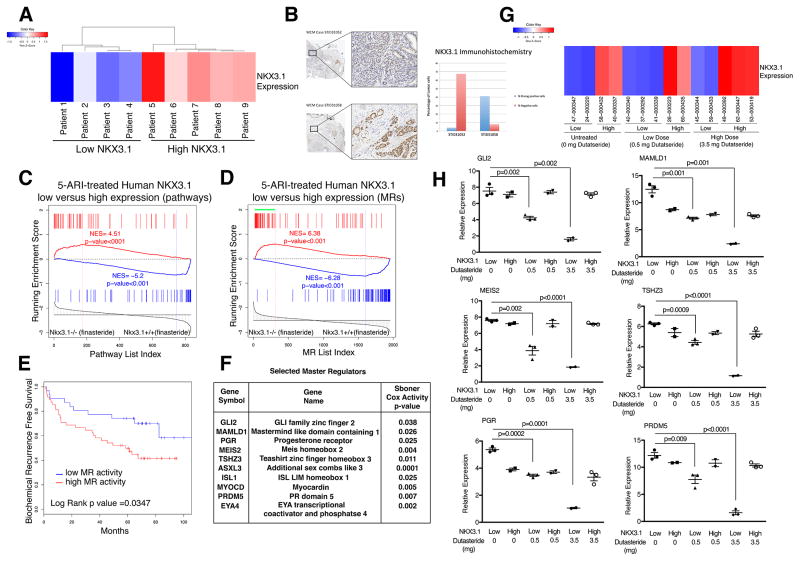
We investigated whether the molecular signature of finasteride response in GEMMs may help to predict patient response for human PCa. We performed expression profiling using a cohort of PCa patients who had received 5-ARIs before prostatectomy (n = 9, WCM cohort; Table 1). These tumor samples segregated into low or high levels of NKX3.1 mRNA expression (Fig. 3A), which was confirmed at the protein level by immunohistochemistry (Fig. 3B). Working from the presumption that low NKX3.1 expressers should be analogous to Nkx3.1−/− mice and high NKX3.1 expressers to Nkx3.1+/+ mice, we compared gene signatures from 5-ARI-treated low versus high NKX3.1-expressing human tumors with finasteride-treated Nkx3.1−/− versus Nkx3.1+/+ prostates. Specifically, we performed cross-species GSEA to compare biological pathways affected in finasteride-treated Nkx3.1−/− versus Nkx3.1+/+ prostates with pathways affected in 5-ARI-treated low versus high NKX3.1-expressing human tumors. The findings revealed strong enrichment of pathways in both the upregulated and downregulated leading edges (NES = 4.51 and 5.2, respectively; p < 0.001; Fig. 3C), indicative of conservation of response to 5-ARI treatment in mouse and human prostates that is contingent on the status of NKX3.1 expression.
Fig. 3.
Cross-species analysis identifies a 5α-reductase inhibitor (5-ARI) response signature with prognostic value. (A) Heat map indicating relative NKX3.1 expression levels in 5-ARI–treated human prostates from the Weill Cornell Medicine (WCM) cohort (Table 1). (B) Immunohistochemistry images at low (top) and high (bottom) magnification of representative prostate cancer cases with weak (STID31052) and strong (STID31058) NKX3.1 protein expression. The adjacent bar plot represents automated image analysis of the percentage of cells with strong (blue) or negative (red) staining (original magnification, 20× and 200×). (C) Gene set enrichment analysis (GSEA) comparing a reference mouse pathway signature from prostates of Nkx3.1−/− finasteride-treated mice (n = 5) versus Nkx3.1+/+ finasteride-treated mice (n = 5) with a query human pathway signature (top 50 differentially changed pathways) of 5-ARI–treated low-NKX3.1 (n = 4) versus high-NKX3.1 (n = 5) human prostates. (D) GSEA comparing a reference mouse master regulator (MR) signature from prostates of Nkx3.1−/− finasteride-treated mice (n = 5) versus Nkx3.1+/+ finasteride-treated mice (n = 5) with a query human MR signature (p < 0.001) of 5-ARI–treated low-NKX3.1 (n = 4) versus high-NKX3.1 (n = 5) human prostates. The green bar indicates positively enriched MRs in the leading edge. (E) Kaplan-Meier survival analysis on the basis of activity levels of MRs of the 5-ARI response signature from (C) using biochemical recurrence (BCR)-free survival as the endpoint as reported by Glinsky et al [30]. The p value was estimated using a log-rank test to determine the difference in outcomes between patients with higher MR activity levels (red) and those with lower MR activity levels (blue). (F) Selected MRs with function and Cox p value indicated. The Cox proportional hazards model was based on MR activity levels in the data set described by Sboner et al [31], using prostate cancer–specific survival as the endpoint. The Cox p value was estimated using a Wald test. (G) Heat map indicating relative NKX3.1 expression levels from 5-ARI–treated human prostates from the Fred Hutchinson Cancer Research Center (FHCRC) cohort including prostate samples with no treatment, low dutasteride treatment (0.5 mg), and high dutasteride treatment (3.5 mg) (Table 1). (H) Validation of selected MRs in the FHCRC cohort by qualitative real-time polymerase chain reaction. Experiments were performed using five samples per group and carried out; values are normalized to GAPDH and p values were estimated using two-tailed two-sample t test.
Given this conservation, we performed cross-species computational analyses of the NKX3.1 low and high mouse and human treatment groups to identify MRs of treatment response. The rationale follows from our previous work showing that computational analyses of preclinical data from GEMMs can predict treatment response in human PCa [22,28], and from the broader conceptual framework that GEMMs can be used to infer patient response for precision prevention [29].
First, we used MARINa to identify MRs of 5-ARI response for the human and mouse treatment groups by interrogating a human PCa interactome with corresponding human and mouse signatures (ie, 5-ARI–treated low vs high NKX3.1-expressing human prostate and finasteride-treated Nkx3.1−/− vs Nkx3.1+/+ prostate) [22,28]. We then used cross-species GSEA to compare the 5-ARI–responsive “human” and “mouse” MRs, which revealed strong conservation of the signatures in both the upregulated and downregulated leading edges (NES = 6.38 and 6.28, respectively; p < 0.001; Fig. 3D).
On the basis of this conservation of the 5-ARI–treated mouse and human prostate contingent on NKX3.1 expression, combined with the striking phenotypic consequences of finasteride treatment in Nkx3.1−/− prostates (Fig. 2), we reasoned that patients with low NKX3.1 expression may be optimal candidates for intervention with 5-ARIs. Indeed, we found that a signature comprising activated MRs from the positive leading edge in low NKX3.1–5-ARI–treated prostates was associated with adverse patient outcomes when tested on an independent cohort of PCa patients described by Glinsky et al [30]. In particular, Kaplan-Meier survival analysis comparing a high versus low level of activity of the MR signature segregated the patients into worse and better outcomes, respectively, on the basis of biochemical recurrence–free survival (p = 0.035; Fig. 3E). Furthermore, a subset of the MRs were associated with adverse outcome, as evidenced by univariate analyses using a Cox proportional hazards model on a second cohort described by Sboner et al [31] in which the clinical endpoint was death due to PCa (p values ranging from 0.04 to 0.0001; Fig. 3F). We further validated these findings using a cohort of patients who had received no treatment or a lower (0.5 mg) or higher (3.5 mg) dose of dutasteride daily for 4 mo in a neoadjuvant trial (n = 15, FHCRC cohort; Table 1 [20]). Similar to the WCM cohort, these patients could be grouped on the basis of low or high NKX3.1 expression (Fig. 3G). Notably, six of the MRs associated with adverse outcome (as Fig. 3F) were independently validated in the FHCRC cohort (Fig. 3H). In particular, dutasteride treatment resulted in reduced expression of these MRs in the low but not the high NKX3.1 patient samples. Taken together, these findings suggest that patients with low NKX3.1 expression are more likely to benefit from 5-ARI intervention, and further suggest that these treatment-response MRs can be used as a readout to assess the efficacy of such treatment.
4. Discussion
The rise in diagnoses of low-risk, early-stage PCa has led to a surge in active surveillance monitoring in lieu of more invasive procedures. Indeed, since PCa is characteristically indolent, simply delaying progression may be highly advantageous for many patients. In this context, 5-ARIs have been investigated as an adjunct to active surveillance [13,14]; however, considering their potential adverse side effects and variable response, it would be valuable to identify the subset(s) of patients who are most likely to benefit from 5-ARI intervention. Using co-clinical analyses of GEMMs and human PCa to investigate the phenotypic and molecular contexts in which 5-ARIs may be most effective, we found that prostate tumors with low NKX3.1 expression are more likely to respond to and benefit from 5-ARI treatment. NKX3.1 is a prostate-specific homeobox gene that is essential for proper prostate development, differentiation, specification, and stem cell function [15,16,19,23,32]; conversely, loss of NKX3.1 function leads to defects in prostate differentiation, as well as preinvasive prostate phenotypes that share molecular conservation with early-stage human PCa [15,16,19,23]. Notably, NKX3.1 is localized to chromosomal region 8p21, which undergoes deletion in a majority (>60%) of PCas at early stages, while NKX3.1 expression is reduced in cancer progression and coincident with cancer initiation [6,16,33,34]. Thus, given the biological functions of NKX3.1, combined with the prevalence and significance of its loss of expression in early-stage PCa, a substantial number of men may benefit from assessment of NKX3.1 expression as a way of selecting for 5-ARI intervention. However, this will need to be tested in prospective clinical trials in which patients on active surveillance are evaluated to determine whether NKX3.1 expression correlates with response to 5-ARI intervention and whether such intervention delays progression. We further propose that the efficacy of 5-ARI intervention can be monitored using the MR signature of treatment response identified here. Our study also highlights the importance of incorporating precision medicine for cancer prevention; precision medicine has been widely adopted, albeit primarily for cancer therapeutics [35]. In particular, our findings suggest that the varied response to 5-ARIs among PCa patients on active surveillance may reflect differences in levels of NKX3.1 expression, which can be readily evaluated at the mRNA or protein level. Moreover, the co-clinical paradigm, with the aim of integrating analyses of GEMMs and human clinical studies to provide information on patient care [36], has largely been limited to therapeutic studies until now. Our study highlights the importance of using a co-clinical approach for cancer prevention. We envision that future studies investigating the long-term benefit of 5-ARIs for patients on active surveillance, and more generally other interventions, can be guided by co-clinical studies in a precision prevention paradigm [29].
5. Conclusions
Our results suggest that patients with low NKX3.1 expression are likely to benefit from intervention with 5-ARIs, and that the beneficial consequences can be evaluated using a molecular signature of treatment response.
Supplementary Material
Acknowledgments
Funding/Support and role of the sponsor: Aditya Dutta was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, grant UL1 TR000040. Christopher E. Barbieri received funding from the National Cancer Institute (K08CA187417-01), the Prostate Cancer Foundation, a Urology Care Foundation Rising Star in Urology Research Award, and a Damon Runyon Cancer Research Foundation MetLife Foundation Family Clinical Investigator award. Elahe A. Mostaghel was supported in part by Pacific Northwest Prostate Cancer SPORE grant P50 CA097186. Antonina Mitrofanova is a recipient of a Prostate Cancer Foundation Young Investigator Award. Cory Abate-Shen had access to the HICCC facilities supported by the Cancer Center Support Grant (P30 CA013696-40) and the National Center for Advancing Translational Sciences (UL1TR001873), and received support from the National Cancer Institute (U01 CA141535-05 and P01 CA154293-03) and an American Cancer Society Research Professorship supported in part by a generous gift from the F.M. Kirby Foundation. The sponsors played a role in manuscript approval.
We are grateful to Edward Gelmann M.D. for helping to obtain the patient cohorts for this study and for helpful discussions. Immunohistochemistry was performed at the Translational Research Program, Department of Pathology and Laboratory Medicine at Weill Cornell Medicine. RNA sequencing was carried out at the J.P. Sulzberger Columbia Genome Center at Columbia University Medical Center (mouse data) and the Genomics core facility at Weill Cornell Medicine (human data). Antonina Mitrofanova acknowledges access to the HPC facilities and support of the computational biology scientists of the Office of Advanced Research Computing (OARC) at Rutgers University.
Footnotes
Author contributions: Cory Abate-Shen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dutta, Mitrofanova, Abate-Shen.
Acquisition of data: Dutta, Panja, Virk, Kim, Zott, Cremers, Golombos, Liu, Mosquera.
Analysis and interpretation of data: Dutta, Panja, Virk, Zott, Cremers, Mosquera, Barbieri, Mitrofanova, Abate-Shen.
Drafting of the manuscript: Dutta, Mitrofanova, Abate-Shen.
Critical revision of the manuscript for important intellectual content: Golombos, Mosquera, Mostaghel, Barbieri.
Statistical analysis: Dutta, Golombos, Mosquera, Mitrofanova.
Obtaining funding: Dutta, Mostaghel, Barbieri, Mitrofanova, Abate-Shen.
Administrative, technical, or material support: Mostaghel.
Supervision: Barbieri, Mitrofanova, Abate-Shen.
Other: None.
Financial disclosures: Cory Abate-Shen certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith RA, Andrews K, Brooks D, et al. Cancer screening in the United States, 2016: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2016;66:96–114. doi: 10.3322/caac.21336. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146–51. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29:3669–76. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 4.Rebbeck TR. Precision prevention of cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:2713–5. doi: 10.1158/1055-9965.EPI-14-1058. [DOI] [PubMed] [Google Scholar]
- 5.Culig Z, Santer FR. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 2014;33:413–27. doi: 10.1007/s10555-013-9474-0. [DOI] [PubMed] [Google Scholar]
- 6.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–11. doi: 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azzouni F, Mohler J. Role of 5α-reductase inhibitors in prostate cancer prevention and treatment. Urology. 2012;79:1197–205. doi: 10.1016/j.urology.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 10.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 11.Thompson IM, Jr, Goodman PJ, Tangen CM, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013;369:603–10. doi: 10.1056/NEJMoa1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson D, Garmo H, Bill-Axelson A, Mucci L, Holmberg L, Stattin P. Use of 5α-reductase inhibitors for lower urinary tract symptoms and risk of prostate cancer in Swedish men: nationwide, population based case-control study. BMJ. 2013;346:f3406. doi: 10.1136/bmj.f3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finelli A, Trottier G, Lawrentschuk N, et al. Impact of 5α-reductase inhibitors on men followed by active surveillance for prostate cancer. Eur Urol. 2011;59:509–14. doi: 10.1016/j.eururo.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Fleshner NE, Lucia MS, Egerdie B, et al. Dutasteride in localised prostate cancer management: the REDEEM randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:1103–11. doi: 10.1016/S0140-6736(11)61619-X. [DOI] [PubMed] [Google Scholar]
- 15.Irshad S, Bansal M, Castillo-Martin M, et al. A molecular signature predictive of indolent prostate cancer. Sci Transl Med. 2013;5:202ra122. doi: 10.1126/scitranslmed.3006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, et al. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999;13:966–77. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MJ, Cardiff RD, Desai N, et al. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci U S A. 2002;99:2884–9. doi: 10.1073/pnas.042688999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Walls JE, Galvez JJ, et al. Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol. 2002;161:727–35. doi: 10.1016/S0002-9440(10)64228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta A, Le Magnen C, Mitrofanova A, Ouyang X, Califano A, Abate-Shen C. Identification of an NKX3.1-G9a-UTY transcriptional regulatory network that controls prostate differentiation. Science. 2016;352:1576–80. doi: 10.1126/science.aad9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gleave M, Qian J, Andreou C, et al. The effects of the dual 5α-reductase inhibitor dutasteride on localized prostate cancer—results from a 4-month pre-radical prostatectomy study. Prostate. 2006;66:1674–85. doi: 10.1002/pros.20499. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aytes A, Mitrofanova A, Lefebvre C, et al. Cross-species regulatory network analysis identifies a synergistic interaction between FOXM1 and CENPF that drives prostate cancer malignancy. Cancer Cell. 2014;25:638–51. doi: 10.1016/j.ccr.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, et al. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 2002;62:2999–3004. [PubMed] [Google Scholar]
- 24.Opoku-Acheampong AB, Nelsen MK, Unis D, Lindshield BL. The effect of finasteride and dutasteride on the growth of WPE1-NA22 prostate cancer xenografts in nude mice. PLoS One. 2012;7:e29068. doi: 10.1371/journal.pone.0029068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei Q, Jiao J, Xin L, et al. NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell. 2006;9:367–78. doi: 10.1016/j.ccr.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Tan PY, Chang CW, Chng KR, Wansa KD, Sung WK, Cheung E. Integration of regulatory networks by NKX3-1 promotes androgen-dependent prostate cancer survival. Mol Cell Biol. 2012;32:399–414. doi: 10.1128/MCB.05958-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitrofanova A, Aytes A, Zou M, Shen MM, Abate-Shen C, Califano A. Predicting drug response in human prostate cancer from preclinical analysis of in vivo mouse models. Cell Rep. 2015;12:2060–71. doi: 10.1016/j.celrep.2015.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Magnen C, Dutta A, Abate-Shen C. Optimizing mouse models for precision cancer prevention. Nat Rev Cancer. 2016;16:187–96. doi: 10.1038/nrc.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glinsky GV, Glinskii AB, Stephenson AJ, Hoffman RM, Gerald WL. Gene expression profiling predicts clinical outcome of prostate cancer. J Clin Invest. 2004;113:913–23. doi: 10.1172/JCI20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sboner A, Demichelis F, Calza S, et al. Molecular sampling of prostate cancer: a dilemma for predicting disease progression. BMC Med Genomics. 2010;3:8. doi: 10.1186/1755-8794-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Kruithof-de Julio M, Economides KD, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–25. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowen C, Bubendorf L, Voeller HJ, et al. Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res. 2000;60:6111–5. [PubMed] [Google Scholar]
- 35.Friedman AA, Letai A, Fisher DE, Flaherty KT. Precision medicine for cancer with next-generation functional diagnostics. Nat Rev Cancer. 2015;15:747–56. doi: 10.1038/nrc4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nardella C, Lunardi A, Patnaik A, Cantley LC, Pandolfi PP. The APL paradigm and the “co-clinical trial” project. Cancer Discov. 2011;1:108–16. doi: 10.1158/2159-8290.CD-11-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.