Abstract
Purpose
Tumor-derived cell-free DNA (cfDNA) in plasma can be used for molecular testing and provide an attractive alternative to tumor tissue. Commonly used PCR-based technologies can test for limited number of alterations at the time. Therefore, novel ultrasensitive technologies capable of testing for a broad spectrum of molecular alterations are needed to further personalized cancer therapy.
Experimental Design
We developed a highly sensitive ultra-deep next-generation sequencing (NGS) assay using reagents from TruSeq Nano library preparation and Nextera Rapid Capture target enrichment kits to generate plasma cfDNA sequencing libraries for mutational analysis in 61 cancer-related genes using common bioinformatics tools. The results were retrospectively compared to molecular testing of archival primary or metastatic tumor tissue obtained at different points of clinical care.
Results
In a study of 55 patients with advanced cancer, the ultra-deep NGS assay detected 82% (complete detection) to 87% (complete and partial detection) of the aberrations identified in discordantly collected corresponding archival tumor tissue. Patients with a low variant allele frequency (VAF) of mutant cfDNA survived longer than those with a high VAF did (P=0.018). In patients undergoing systemic therapy, radiological response was positively associated with changes in cfDNA VAF (P=0.02), and compared with unchanged/increased mutant cfDNA VAF, decreased cfDNA VAF was associated with longer time to treatment failure (TTF; P=0.03).
Conclusions
Ultra-deep NGS assay has good sensitivity compared to conventional clinical mutation testing of archival specimens. A high VAF in mutant cfDNA corresponded with shorter survival. Changes in VAF of mutated cfDNA were associated with TTF.
Keywords: cell-free DNA, ultra-deep next-generation sequencing, liquid biopsy, cancer, plasma
INTRODUCTION
Cell-free DNA (cfDNA) in plasma is a rich resource for the detection of genomic variants in cancer patients, which can be used as a minimally invasive approach for diagnostic and monitoring purposes (1). As tumor DNA comprises a small fraction of total cfDNA (typically ≤1%), highly sensitive and accurate techniques are required for cancer mutation detection in cfDNA (1). Polymerase chain reaction (PCR)-based technologies, such as droplet digital PCR (ddPCR), BEAMing (beads, emulsions, amplification, and magnetics) PCR, or other quantitative allele-specific PCR methods can detect a low frequency of molecular aberrations in cfDNA, but these approaches cannot sample many target sites and are hampered by the complexities of determining optimal PCR primer sequences, primer combinations, and amplification conditions (2–5). Next-generation sequencing (NGS)-based assays, which are commonly used for tumor tissue testing, are not sufficiently sensitive for plasma cfDNA profiling owing to low tumor DNA fractions in the circulatory system (1). However, the use of targeted panels that can be sequenced to extremely high depth can overcome this issue. Therefore, we developed an assay that employs commercially available reagents to detect somatic variants in 61 common cancer-related genes (Supplementary Table 1). The accuracy of this assay was assessed using spike-in experiments of samples with known mutational profiles, and the approach was then applied to a cohort of patients with advanced cancers.
METHODS
Patients
The study enrolled patients with progressing advanced cancers who were referred to ‘s Department of Investigational Cancer Therapeutics for experimental therapies from October 2010 to June 2015 and whose tumor mutation status was known from clinical testing of their formalin-fixed, paraffin-embedded (FFPE) specimens. Patients had the option of providing longitudinally collected plasma samples during the course of their therapy. The study was conducted in accordance with MD Anderson’s Institutional Review Board guidelines.
Tumor Tissue Analyses
Archival FFPE specimens of patients’ primary and metastatic tumors obtained from routine diagnostic and/or therapeutic procedures were tested for aberrations in common cancer-related genes in a Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory. DNA was extracted from microdissected FFPE tumor sections and analyzed with a PCR-based DNA sequencing method, mass spectrometric detection (MassARRAY, Sequenom, San Diego, CA), or NGS (Ion Torrent, Thermo Fisher Scientific, Waltham, MA). We also used the commercially available FoundationOne panel (Foundation Medicine) for molecular aberrations in ≥182 cancer-related genes. The lower limit of detection for these technologies is approximately 5–10% VAF and is influenced by clonal heterogeneity and the presence of normal tissue.
Plasma collection and cfDNA isolation
Whole blood was collected in ethylenediaminetetraacetic acid–containing tubes and centrifuged and spun twice within 2 hours to yield plasma. For each sample, cfDNA was extracted from 1–3 mL of plasma using the QIAamp Circulating Nucleic Acid kit (cat# 55114, Qiagen, Valencia, CA). Plasma was incubated with ACL buffer (containing carrier RNA) and Proteinase K for 60 minutes.
Library Preparation and NGS
cfDNA yield in the 100–400 base pair range was determined using High-sensitivity DNA reagents (cat# 5067-4626, Agilent Technologies, Santa Clara) on a 2100 Bioanalyzer instrument (Agilent Technologies, Santa Clara, CA). Libraries were prepared using adapter TruSeq Nano reagents (cat# FC-121-4003, Illumina, San Diego, CA) with minor changes. No upfront fragmentation of the cfDNA was performed. To minimize DNA sample loss, the bead-based sample cleanup after end-repair reactions was eliminated prior to cfDNA A-tailing. A detailed library preparation protocol and comparison to the standard Illumnina TruSeq Nano protocol is provided in Supplementary File 1.
Adapter-ligated cfDNA was PCR-amplified, and libraries were enriched for genomic target regions of interest by probe hybridization. For target enrichment of the generated sequencing libraries, 80-mer biotinylated DNA probes tiling 61 genes with relevance to a broad range of cancer types were designed using DesignStudio (Ilumina, San Diego, CA) (Supplementary Table 1). cfDNA libraries were enriched for desired genomic content using these biotinylated probes with modifications to the Nextera Rapid Capture Enrichment protocol (cat# FC-140-1007, Illumina, San Diego, CA). For library enrichment, we employed the oligo blocker Capture Target Buffer 3 (cat# 15048799, Illumina, San Diego, CA) instead of enrichment hybridization buffer. Enrichment wash solution was substituted with Enhanced enrichment wash solution (cat# 15065792, kit# FC-144-1000, Illumina, San Diego, CA).
The analytical sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the assay were determined by spiking in DNA of NCI-H1975, a non-small cell lung carcinoma (NSCLC) cell line (ATCC CRL-5908) with 4–5 validated mutations covered in our panel (EGFR heterozygous c.2369C>T p.T790M, EGFR heterozygous c.2573T>G p.L858R, PIK3CA heterozygous c.353G>A p.G118D, TP53 homozygous c.818G>A p.R273H, NF1 heterozygous c.846G>A ) at 0.5% input mass in 30 ng of wild-type NA12878 genomic DNA (Coriell Institute, Camden, NJ). This model therefore represents assay analytical performance in the context of 0.25% and 0.5% VAF (heterozygous and homozygous variants respectively). DNA were sonicated with an S series sonicator (Covaris, Woburn, MA) and size-selected using Pippin Prep (Sage Science, Beverly, MA) to mimic a size of ~170 base pairs. For analytical studies, 4 independent experiments with technical replicates of each condition were tested in each experiment.
All samples were sequenced on a HiSeq 2500 sequencer (Illumina, San Diego, CA) in high-throughput mode, typically with 4–6 samples sequenced on a single flow cell, using a 100 base-pair, dual-indexed, paired-end sequencing configuration.
For mutation analysis, Fastq files containing raw sequencing reads were generated using bcl2fastq Conversion Software 2.17.1.13 (Illumina, San Diego, CA). Fastq files were aligned to a human reference genome (UCSC hg19) using BWA-MEM (v0.7.10-r789) with default parameters. Alignments were converted to BAM format using Samtools (v1.2). Alignment coordinates of raw read alignments were adjusted to include soft-clipped bases. Raw reads that shared identical start and end coordinates were considered to be PCR duplicates and were grouped together for error correction. Grouped reads were aligned to one another, and a consensus sequence was generated by taking the most prevalent base at each position. Consensus base qualities were generated at each position by summing the base qualities that agreed with the consensus and subtracting those that disagreed. Consensus base qualities were capped at 90. For variant calling, error-corrected consensus sequences were re-aligned to the hg19 genome using BWA-MEM. Mpileup files were generated using Samtools with the parameters “-Q30 -q 15 -B -A -d10000000.” Variant calls were generated using VarScan (v2.3.9) with parameters “--min-var-freq 0.0 --min-reads2 1 --strand-filter 1 --p-value 1 --min-freq-for-hom 1.” Variants belonging to dbSNP or ClinVar were annotated using SnpEff (v4.1b).
For gene-amplification calling, we employed an algorithm to call copy number variants (CNV) in tumor samples. The robustness of the CNV detection algorithm was validated by the identification of amplifications in DNA samples harboring known amplifications using an enrichment-based ligation assay similar to that used in this study. The presence of amplifications in these samples was reconfirmed using ddPCR (personal communication, Matt Friedenberg, Oncology, Illumina, San Diego, CA). In our study, a training dataset was established from sample data from 44 healthy individuals to learn the baseline coverage behavior, and then step-wise normalization was performed on target regions to reduce baseline effect, followed by GC bias removal through loess regression in test samples. The algorithm calculates a fold change for each gene by comparing the median normalized value across its target regions to the genome median. For further stringency in CNV calling, we determined the CNV for gene regions with >15 genomic bins, for which the corrected copy number fold-change was >3 standard deviations from the test population’s cancer samples.
Statistical Analysis
Agreement rates between the mutation analyses of the FFPE specimens and plasma cfDNA were assessed using common genes and their loci queried by both tissue and cfDNA platforms. Overall survival (OS) was defined as the time from the date of study entry to the date of death or last follow-up. The Royal Marsden Hospital (RMH) score was calculated on the basis of lactate dehydrogenase levels (greater than the upper limit of normal vs. normal), albumin levels (<3.5 g/ml vs. ≥3.5 g/ml), and number of metastatic sites (>2 sites vs. ≤2 sites). Time to treatment failure (TTF) was defined as the time from the date of systemic therapy initiation to the date of removal from the treatment. The Kaplan-Meier method was used to estimate OS and TTF, and a log-rank test was used to compare OS and TTF among patient subgroups. Cox proportional hazards regression models were fit to assess the association between patient characteristics and OS. All tests were two-sided, and P values <0.05 were considered statistically significant. All statistical analyses were performed with the GraphPad (GraphPad Software, Inc., La Jolla, CA) or SPSS 23 (SPSS, Chicago, IL) software programs.
RESULTS
Analytical performance
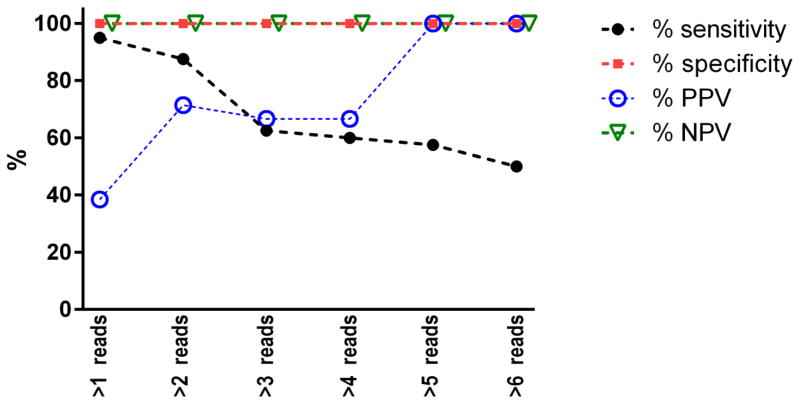
To determine the analytical performance of the assay, we spiked the DNA of the NCI-H1975 cell line, which harbors well-characterized variants, into a wild-type genomic DNA background. After processing these samples using our cfDNA library preparation protocol, we were able to determine the analytical sensitivity, specificity, PPV, and NPV of the assay at different variant sequence read thresholds. A threshold of ≥2 unique variant sequence reads (denoted by non-identical genome alignment coordinates) yielded an analytical sensitivity of 95% and specificity of >99.99% for known variants at a variant allele frequency (VAF) of 0.25% (Fig. 1). To optimize assay sensitivity, we evaluated all clinical samples using a threshold of ≥2 unique variant sequence reads.
Figure 1. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the assay.
With a requirement of >1 unique sequence reads (denoted by non-identical genome alignment coordinates), the sensitivity and specificity of the assay were 95% and >99.99%, respectively, for known variants at a variant allele frequency of 0.25%.
Testing of clinical samples of plasma cfDNA
To validate the assay, we tested 165 plasma cfDNA samples collected from 55 patients with advanced cancers who had results from clinical molecular testing of archival tumor tissue (Table 1). The median patient age was 58 years (range, 30–82 years). Most patients were white (n=50; 91%) and men (n=30; 55%). The most common tumor types were colorectal cancer (n=14; 25%), non-small cell lung cancer (NSCLC; n=11; 20%), and breast cancer (n=5; 9%) (Table 1). The median time from tissue to plasma sampling was 19.5 months (range, 1.0–114.5 months). For 39 patients (71%), archival tumor tissue was tested using an Ion Torrent targeted NGS assay (Thermo Fisher Scientific Inc., Waltham, MA), and for 10 patients (18%), archival tumor tissue was tested using a FoundationOne targeted NGS assay (Foundation Medicine, Cambridge, MA). Among the 55 patients, 49 had 92 molecular aberrations in archival tumor tissue, and 6 had no molecular aberrations in archival tumor tissue (Table 1 and Supplementary File 2).
Table 1.
Characteristics of the 55 patients enrolled in the study.
| Characteristics | Number of patients (%) | |
|---|---|---|
| Age, years – median (range) | 58 (30–82) | |
| Gender | Male | 30 (55) |
| Female | 25 (45) | |
| Ethnicity | White | 50 (91) |
| Hispanic | 2 (4) | |
| African-American | 2 (4) | |
| Asian | 1 (2) | |
| Cancer type | Colorectal cancer | 14 (25) |
| Non-small cell lung cancer | 11 (20) | |
| Breast cancer | 5 (9) | |
| Cholangiocarcinoma | 4 (7) | |
| Melanoma | 4 (7) | |
| Thyroid carcinoma | 3 (5) | |
| Pancreatic cancer | 3 (5) | |
| Ovarian cancer | 3 (5) | |
| Appendiceal carcinoma | 2 (4) | |
| Other cancers | 6 (11) | |
| Molecular testing of tumor tissue | Ion Torrent NGS 46 genes | 13 (24) |
| Ion Torrent NGS 50 genes | 26 (47) | |
| Foundation One targeted NGS | 10 (18) | |
| Other NGS | 2 (4) | |
| PCR | 3 (5) | |
| Sequenom MassArray | 1 (2) | |
| Molecular aberrations in tumor tissue | Patients with molecular aberration | 49 (89) |
| Patients without molecular aberration | 6 (11) | |
| Total number of molecular aberrations | 92 | |
Abbreviations: NGS, next generation sequencing
The average amount of cfDNA isolated from 3 mL of plasma was 47.1 ng (range, 3–586 ng). Libraries from cfDNA were sequenced with an average of 97 ± 54 × 106 pass-filter reads per sample, yielding a mean coverage of 1,456 unique reads (standard error, 74 unique reads) per target site. When requiring ≥2 uniquely mapped reads at a locus to detect a variant, this coverage depth allows for the detection of variants down to a VAF of 0.33% with 95% confidence.
To enable robust variant calling from cfDNA sequencing data, we created a normal baseline from 44 healthy individuals. By ascertaining VAF distributions at each target site in these healthy individuals, we were able to retain somatic variants with statistically different VAFs relative to the normal baseline (P ≤ 0.01). To improve sensitivity, we also removed variants supported by only 1 uniquely mapped read as well as synonymous variants and those in untranslated regions from further consideration. Furthermore, to exclude germline mutations, we removed variants in the dbSNP database (build 142) that had ≥20% minor allele frequency (MAF) from further analyses.(6) In this manner, we identified a median of 26 mutations per sample. With the requirement of more uniquely mapped reads to call a variant, there was a significant drop in the number of variants detected per sample, with a median of only 3 variants per sample supported by at least 6 uniquely mapped reads (Supplementary Fig. 1).
Among the 55 baseline plasma cfDNA samples, 45 (82%) had molecular profiles identical to those of archival tumor tissue (complete detection), and 3 (5%) had molecular profiles that overlapped but were not identical to those of archival tumor tissue (partial detection), resulting in an aggregate complete and partial detection rate of 87% (Table 2). The sensitivity of plasma cfDNA testing was 80% (95% confidence interval [CI], 0.66–0.90) for complete detection and 86% (95% CI, 0.73–0.94) for complete and partial detection. Of the 14 variants not detected in cfDNA, 8 (57%) had unknown therapeutic impact and 6 (43%) had proven or possible therapeutic impact (Table 3).
Table 2.
Concordance between ultra-deep next-generation sequencing of plasma cell-free (cfDNA) and clinical molecular testing of archival tumor tissue for the 55 patients enrolled in the study.
| Type of agreement between plasma cfDNA and tumor tissue | Number of patients (%) |
|---|---|
| Complete detection | 45 (82%) |
| Partial detection | 3 (5%) |
| Aggregate complete and partial detection | 48 (87%) |
| Complete disagreement | 7 (13%) |
Table 3.
Partial and complete disagreements. Alterations with proven or possible therapeutic impact are in bold.
| Patient number | Tumor type | Aberration in tissue | Aberration in cfDNA( VAF%) |
|---|---|---|---|
| Partial disagreements | |||
| 46 | NSCLC | KRASG12V | KRASG12V (0.18%) |
| TP53R248Q | Not detected | ||
| TP53C238S | Not detected | ||
| 86 | Breast cancer | ERBB2D769H | ERBB2D769H (0.34%) |
| IDH2R172K | Not detected | ||
| 102 | NSCLC | KRASG12C | KRASG12C (0.31%) |
| NOTCH1L2457V | Not detected | ||
| Complete disagreement | |||
| 44 | NSCLC | KRASG12D | Not detected |
| 47 | Urothelial carcinoma | TP53E286* | Not detected |
| 54 | Appendiceal carcinoma | KRASG12D | Not detected |
| GNASR201H | Not detected | ||
| 55 | NSCLC | BRAFD594G | Not detected |
| TP53H193L | Not detected | ||
| 73 | NSCLC | KRASG12C | Not detected |
| 80 | Breast cancer | PIK3CAH1047R | Not detected |
| TP53K120* | Not detected | ||
| 111 | Breast cancer | AKT1E17K | Not detected |
Abbreviations: NSCLC, non-small cell lung cancer; cfDNA, cell-free DNA; VAF, variant allele frequency
All reported variants in cfDNA are listed in Supplementary File 2. Some of these variants were not reported in archival tumor tissue, and their presence in plasma cfDNA could represent tumor heterogeneity, DNA release from sites not sampled by biopsy, or clonal evolution of the tumor. Therefore, we identified potentially clinically significant genomic variants in cfDNA that had not been characterized in the baseline tumor tissue in 14 patients (Table 4).
Table 4.
Potentially clinically significant molecular aberrations in plasma cell-free (cfDNA) not reported in the archival tumor tissue and the clinical context.
| Patient number | Cancer | Molecular profile in tumor tissue | Molecular profile in plasma cfDNA (VAF) | Possible clinical relevance |
|---|---|---|---|---|
| 24 | Colorectal cancer | None | KRASG12C (2.54%) | Presence of KRASG12C and PTENR130Q mutations plausibly explains prior resistance to EGFR antibody |
| PTENR130Q (0.21%) | ||||
| 25 | Melanoma | BRAFV600E | BRAFV600E (1.99%) | Emergence of IDH1R132H mutation on therapy with BRAF inhibitor |
| TP53P278S | TP53P278S (1.00%) | |||
| TP53R110C | TP53R110C (0.59%) | |||
| IDH1R132H (0.22%) | ||||
| 26 | Melanoma | BRAFV600E | KRASQ61L (26.67%) | Disappearance of BRAFV600E and emergence of PIK3CAE545K on therapy with BRAF and MET inhibitors |
| PIK3CAE545K (1.38%) | ||||
| 33 | Anaplastic thyroid cancer | BRAFV600E | BRAFV600E (2.45%) | Presence of inactivating mutation BRAFD594G increases signaling through MAPK pathway and plausible explain progression to BRAF and MEK inhibitors |
| TP53K132M | TP53K132M (4.48%) | |||
| BRAFD594G (0.13%) | ||||
| 38 | Colorectal cancer | KRASG12D | KRASG12D (7.89%) | PIK3CAE545A mutation offers additional target for cancer therapy selection |
| PIK3CAE545A (0.66%) | ||||
| 51 | Colorectal cancer | KRASG12D | KRASG12D (42.66%) | Emergence of EGFRL858M and HRASQ61H mutations at progression on panRAF inhibitor |
| EGFRL858M (0.05%) | ||||
| HRASQ61H (0.05%) | ||||
| 52 | Colorectal cancer | None | KRASG12C (0.53%) | KRASG12C mutation offers target for cancer therapy selection (resistance to EGFR antibodies) |
| 73 | Non-small cell lung cancer | KRASG12C | PIK3CAE545K (0.18%) | PIK3CAE545K offers additional target for cancer therapy selection |
| 77 | Head & neck squamous cancer | PIK3CAE545K | PIK3CAE545K (1.06%) | BRAFD594G and KRASG13V mutations can plausibly explain resistance to mTOR inhibitor based therapy |
| BRAFD594G (0.11%) | ||||
| KRASG13V (0.12%) | ||||
| 86 | Breast cancer | ERBB2D769H | ERBB2D769H (0.03%) | KRASG12D, PIK3CAE545K and PIK3CAE542K mutations emerged at progression to ERBB2 and mTOR inhibitors |
| IDH2R172K | KRASG12D (0.04%) | |||
| PIK3CAE545K (1.21%) | ||||
| PIK3CAE542K (0.46%) | ||||
| 87 | Colorectal cancer | None | KRASQ61H (2.78%) | Presence of KRASQ61H mutation plausibly explains prior resistance to EGFR antibody |
| 94 | Cholangio-carcinoma | TP53C141* | PIK3CAE542K (0.39%) | PIK3CAE545K and IDH1R132L mutations offer additional target for cancer therapy selection |
| IDH1R132L (0.39%) | ||||
| 95 | Non-small cell lung cancer | None | PIK3CAE545Q (0.33%) | PIK3CAE545Q and KRASG12A mutations offer additional target for cancer therapy selection |
| KRASG12A (1.44%) | ||||
| 112 | Non-small cell lung cancer | BRAFV600E | BRAFV600E (4.73%) | IDH2R172G mutation emerged at progression to BRAF inhibitor |
| IDH2R172G (0.12%) |
Although the assay was not specifically designed or analytically validated to detect gene amplification, we used it to perform a CNV analysis of the cfDNA samples. Of the 55 patients, 10 (18%) had undergone CLIA–certified laboratory testing for amplifications in tumor tissue. Of these 10 patients, 3 had 4 CNV events, 2 of which were confirmed in cfDNA. Furthermore, 2 patients for whom tissue CNV testing had not been performed had an additional 3 gene amplifications in cfDNA (Supplementary Table 2).
cfDNA and survival
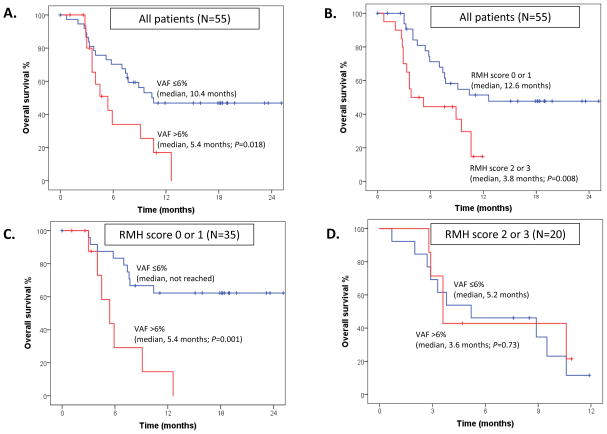
To determine whether the abundance of mutant plasma cfDNA was associated with survival, we first calculated the aggregate cfDNA VAF for all variants from the 55 plasma samples collected at baseline. We then divided the 55 patients into two groups according to the aggregate VAF (VAF ≤6% vs. VAF >6%). These thresholds were selected based on a 5% trimmed mean value of mutant cfDNA and were deemed to be representative to minimize the bias from samples with no mutated cfDNA detected. The median OS duration of the 38 patients with a mutant cfDNA VAF of ≤6% (10.4 months; 95% CI, not estimated) was significantly longer than that of 17 patients with a mutated cfDNA VAF of >6% (5.4 months; 95% CI, 3.0–7.8 months; P=0.018) (Fig. 2a).
Figure 2. Overall survival (OS) and variant allele frequency (VAF) or Royal Marsden Hospital (RMH) score.
A. The median OS duration of the 38 patients with a mutant cell-free DNA (cfDNA) VAF of ≤6% (blue; 10.4 months; 95% confidence interval [CI], not estimated) was longer than that of the 17 patients with a mutated cfDNA VAF of >6% (red; 5.4 months; 95% CI, 3.0–7.8 months; P=0.018). B. The median OS duration of the 35 patients with an RMH score of 0 or 1 (blue; 12.6 months; 95% CI, not estimated) was significantly longer than that of the 20 patients with an RMH score of 2 or 3 (red; 3.8 months; 95% CI, 0.5–7.1 months; P=0.008). C. Among 35 patients with favorable RMH scores (0 or 1), the median OS duration of 25 patients with a mutated cfDNA VAF of ≤6% (blue; not reached) was longer than that of 10 patients with a mutated cfDNA VAF of >6% (red; 5.4 months; 95% CI, 3.1–7.7 months; P=0.001). D. Among 20 patients with unfavorable RMH scores (2 or 3), the median OS duration of 13 patients with a mutated cfDNA VAF of ≤6% (blue; 5.2 months; 95% CI, 0–10.8 months) was longer than that of 7 patients with a mutated cfDNA VAF of >6% (red; 3.6 months; 95% CI, 2.7–4.5 months) but this difference was not significant (P=0.73).
Next, we analyzed the prognostic impact of mutant cfDNA VAF on OS using a multi co-variable analysis that included patients’ RMH prognostic scores. (RMH score is a prospectively validated tool used to predict the OS of patients with advanced cancers who are referred for early-phase clinical trials; scores of 0 or 1 are associated with longer OS than scores of 2 or 3) (7). As expected, the median OS duration of the 35 patients with an RMH score of 0 or 1 (12.6 months; 95% CI, not estimated) was significantly longer than that of the 20 patients with an RMH score of 2 or 3 (3.8 months; 95% CI, 0.5–7.1 months; P=0.008) (Fig. 2b). A multi-variable analysis revealed that, compared with an RMH score of 2 or 3, an RMH score of 0 or 1 was associated with longer OS duration (hazard ratio [HR], 0.45; 95% CI, 0.22–0.94; P=0.033) and that, compared with a mutated cfDNA MAF of >6%, a mutated cfDNA MAF of ≤6% demonstrated a trend to longer OS (HR, 0.50; 95% CI, 0.24–1.06; P=0.07).
Finally, we analyzed the association between the VAF of mutated cfDNA and OS for patients with favorable RMH scores (0 or 1) and for those with unfavorable RMH scores (2 or 3). Among the 35 patients with favorable RMH scores, the median OS duration of 25 patients with a mutated cfDNA VAF of ≤6% (not reached) was significantly longer than that of 10 patients with a mutated cfDNA VAF of >6% (5.4 months; 95% CI, 3.1–7.7 months; P=0.001) (Fig. 2c). Among the 20 patients with unfavorable RMH scores, the median OS duration of 13 patients with a mutated cfDNA VAF of ≤6% (5.2 months; 95% CI, 0–10.8 months) was longer than that of 7 patients with a mutated cfDNA VAF of >6% (3.6 months, 95% CI, 2.7–4.5 months), but this difference was not significant (P=0.73) (Fig. 2d). We found no association between mutated cfDNA VAF (≤6% vs. >6%) and tumor burden (≤2 metastatic sites vs. >2 metastatic sites as per RMH score; P=0.53).
Serial monitoring of mutated plasma cfDNA
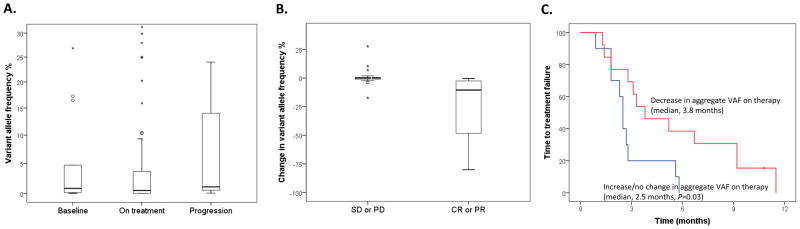
Longitudinal serial plasma specimens obtained before and during systemic therapy were available for 19 patients who received a total of 23 systemic therapies. The median number of cfDNA samples per patient was 4 (range, 2–11 samples). The median aggregate VAF of mutated cfDNA at baseline (0.82%), during therapy (0.48%), and at disease progression (1.06%) did not differ significantly (P=0.15) (Fig. 3a). A decrease in the aggregate VAF of mutated cfDNA was associated with the best response to therapy (complete response [CR] or partial response [PR] vs. stable disease [SD] or progressive disease [PD]) on imaging studies as per Response Evaluation Criteria in Solid Tumors (RECIST; median change, −10.51% for CR/PR vs. 0% for SD/PD; P=0.02) (Fig. 3b) (8). Of the 23 diverse systemic cancer therapies these patients received, 13 decreased the VAF of mutated cfDNA and 10 caused no change or increased the VAF of mutated cfDNA. The median TTF of the patients with a decrease in aggregate VAF mutant DNA (3.8 months; 95% CI, 1.3–6.3 months) was significantly longer than that of patients with no change or an increased cfDNA VAF (2.5 months; 95% CI, 2.2–2.8 months; P=0.03) (Fig. 3c).
Figure 3. Serial monitoring of variant allele frequency (VAF) of mutant cell-free DNA (cfDNA) during 23 diverse systemic therapies.
A. The median aggregate VAF of mutated cfDNA at baseline (0.82%), during therapy (0.48%), and at disease progression (1.06%) did not differ significantly (P=0.15). B. The best response to therapy (complete [CR] or partial response [PR] vs. stable disease [SD] or progressive disease [PD]) on imaging per Response Evaluation Criteria in Solid Tumors was associated with the best change in the aggregate VAF of mutated cfDNA (median change for CR/PR, −10.51%, vs. median change for SD/PD, 0%; P=0.02). C. The median time to treatment failure of patients with decreased aggregate VAF mutant cfDNA (3.8 months; 95% CI, 1.3–6.3 months) was longer than that of patients with unchanged or increased aggregate VAF mutant cfDNA (blue; 2.5 months; 95% CI, 2.2–2.8 months; P=0.03).
DISCUSSION
We developed a highly sensitive assay that employs protocol changes to commercially available DNA assays and bioinformatics tools widely used for NGS to detect genetic variants in 61 cancer-related genes in plasma cfDNA. Our results demonstrate that our ultra-deep NGS assay confirmed molecular profiles previously reported from clinical testing of archival tumor tissue in a CLIA-certified laboratory in 82% (complete detection) to 87% (complete and partial detection) of patients with advanced cancers. These rates corresponded to variant detection sensitivities of 80% and 86%, respectively.
Our results compare favorably to previously published data. Kim et al. (9) employed a targeted 54-gene digital NGS panel to assess plasma cfDNA samples from 61 patients with advanced cancers and detected molecular variants present in the tumor tissue in 48% (complete detection) to 66% (complete and partial detection) of cases. In another study, using the same technology to assess the plasma cfDNA of NSCLC patients, Thompson et al. found that 58% (complete detection) to 74% (complete and partial detection) of plasma cfDNA samples had molecular profiles that matched those of tumor tissue specimens (10). Wang et al. (11) used the NGS SafeSeqS Pipeline to detect molecular variants in cfDNA isolated from the cerebral spinal fluid of patients with primary brain and spine tumors and demonstrated agreement between plasma and tumor tissue in 74% of cases. Finally, a small study using the NGS CAPP-Seq method to test molecular variants in plasma cfDNA from patients with any stage of NSCLC confirmed 85% of variants previously detected in tumor tissue (12). The CAPP-Seq method was later modified to use integrated digital error suppression (iDES-enhanced CAPP-Seq) and demonstrated a PPV of 72% for plasma cfDNA compared with archival tissue in 24 patients with NSCLC; however, the time between tumor and tissue collection was not clear (13). Jovelet et al. (14) used a standard 50-gene Ion AmpliSeq panel commonly used for archival tumor tissue to test plasma cfDNA from 283 patients with advanced cancer; although the tumor tissue and plasma were obtained around the same time, the sensitivity of the method was 49.9%. The same group later presented an optimized assay with increased sensitivity (15). Compared with NGS, PCR-based methods demonstrate higher concordance rates, which usually range from 85% to 95%; however, these assays have been hindered by their inability to test for multiple aberrations simultaneously (3, 5, 16). A certain level of discordance can be anticipated if the tumor tissue and plasma are not obtained at the same time. Higgins et al. (17) found 100% agreement between PIK3CA mutation testing of simultaneously collected plasma cfDNA (assessed with BEAMing PCR) and tumor tissue in a cohort of patients with advanced breast cancer. However, the concordance between the methods decreased to 79% in a cohort of patients whose tumor and plasma cfDNA samples were obtained at different times. In another study of 100 patients with advanced colorectal cancer, ddPCR detection of RAS mutations in plasma cfDNA was in agreement with RAS mutation status in archival tissue in 97% of cases (18). This compared favorably to most other studies; however, the median time from tissue collection to plasma collection was only 43 days. In the present study, that time was 19.5 months. Indeed, there is increasing evidence that the molecular testing results for cfDNA are highly concordant with those for archival tumor tissue and that concordance decreases with increasing time between tissue and plasma collection (17).
In the present study, we found that patients with a low aggregate VAF of mutant cfDNA have a significantly longer median OS duration than patients with a high aggregate VAF of mutant cfDNA (10.4 months vs. 5.4 months; P=0.018). We previously used BEAMing PCR to assess plasma cfDNA for 21 common mutations in BRAF, EGFR, KRAS, and PIK3CA in patients with advanced cancers and found that, irrespective of tumor type, patients with a high amount of mutant cfDNA had shorter OS than patients with a low amount of mutant cfDNA did (5.5 months vs. 9.8 months; P=0.001) (3). In another study, using the Idylla system (Biocartis, Mechelen, Belgium) to detect BRAFV600 mutations in plasma-derived cfDNA from patients with diverse advanced cancers, we also found that patients with a high percentage of BRAFV600-mutant cfDNA had shorter OS than patients with a low percentage of BRAFV600-mutant cfDNA did (4.4 months vs. 10.7 months; P=0.005) (4). Similarly, among patients with advanced colorectal cancer who were treated in a phase III randomized trial of regorafenib vs placebo, high baseline levels of KRAS-mutant cfDNA were found to be associated with shorter OS (19). Also, higher amounts of KRAS-mutant cfDNA have been associated with shorter progression-free survival and OS in patients with advanced colorectal cancer treated with irinotecan and cetuximab and in patients with advanced NSCLC treated with carboplatin and vinorelbine (20, 21). Similarly, a BRAFV600E mutation in cfDNA was associated with shorter OS in a combined analysis of clinical trials of BRAF and MEK inhibitors in patients with advanced melanomas (22). To our knowledge, our study is the first report showing the prognostic significance of mutation burden tested by the NGS method.
The serial detection of genomic variants in plasma cfDNA can be used to monitor response to cancer therapy (23–33). In the present study, we assessed serially collected plasma cfDNA from patients treated with systemic therapies and found that a decrease in the aggregate VAF of mutant plasma cfDNA was associated with the best response on radiographic imaging per RECIST (P=0.02) and that the median TTF of patients with a decreased aggregate VAF of mutant cfDNA (3.8 months) was longer than that of patients with an unchanged or increased aggregate VAF (2.5 months; P=0.03). This observation is consistent with previously published data demonstrating that changes in plasma cfDNA can correspond with treatment outcomes (2, 26–34). In a study in which the Idylla PCR system was used to detect BRAFV600 mutations in plasma-derived cfDNA from patients with colorectal and other advanced cancers, therapies associated with a decrease in BRAF-mutant cfDNA produced significantly longer TTF than therapies associated with an increase or no change in BRAF-mutant cfDNA did (10.3 months vs. 7.4 months; P = 0.045) (4). Although several previous studies’ findings support the concept that changes in cfDNA can predict or at least correspond with treatment outcomes, future prospective studies are needed to address the overall evidence.
Our study had several potential limitations. First, our panel assesses only 61 cancer-related genes and is designed to detect mutations, not amplification or fusion events (apart from ALK, RET, and ROS1, for which all exonic and intronic regions were assayed). Second, the assay does not include unique molecular identifiers, which would be useful for removing potentially false positive variant calls due to errors in library preparation or PCR (35). Third, the sample size was limited and included patients with diverse advanced cancers, which might impact the sensitivity of our assay, since the amount of released cfDNA can differ across diverse tumor types (25). Finally, the longitudinal and OS analyses were exploratory, and the associations between mutant VAF in cfDNA and TTF and OS need to be validated in future prospective studies.
In summary, our data highlight the feasibility of performing mutational analysis for 61 cancer-related genes using ultra-deep NGS of plasma cfDNA from patients with advanced cancers. To achieve this, we have employed commercially available consumables and a simplified workflow. Our sequencing strategy demonstrated good sensitivity and relatively simple workflow, which can facilitate adoption of cfDNA sequencing assays in laboratories with NGS expertise. We also demonstrate that our approach allows for robust somatic variant detection, which compares well with standard methods of clinical molecular profiling of archival tumor tissue. Our data suggest that the aggregate VAF of mutant cfDNA is a prognostic biomarker for patient survival and that cfDNA levels are correlated with systemic treatment success, which to our knowledge has not been reported before using the targeted NGS panels. In the future, broad adoption of cfDNA assays could facilitate personalized therapeutic interventions based on patients’ respective cfDNA mutation statuses.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
We demonstrated the feasibility of performing mutational analysis for 61 cancer-related genes using highly sensitive ultra-deep next-generation sequencing of plasma cell-free DNA from patients with advanced cancers using commonly available consumables and a simplified workflow, which can facilitate adoption of the assays in laboratories with next-generation sequencing expertise. We also demonstrate that our approach allows for robust somatic variant detection, which compares well with standard methods of clinical molecular profiling of archival tumor tissue. Our data suggest that the amount of mutated cell-free DNA is a prognostic biomarker for patient survival and that levels of mutated cell-free DNA are correlated with systemic treatment success.
Acknowledgments
Financial support: This study was supported by the Sidney Kimmel Foundation for Cancer Research (Filip Janku), the Sheikh Khalifa Al Nahyan Ben Zayed Institute for Personalized Cancer Therapy (Filip Janku), and the National Center for Advancing Translational Sciences (grant no. UL1 TR000371); by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (P30 CA016672).
We thank Karen Gutekunst, Paul Van Hummelen, and Matt Friedenberg for intellectual contribution and the sequencing core team at Illumina for sequencing support.
FUNDING
This study was supported by the Sidney Kimmel Foundation for Cancer Research, the Sheikh Khalifa Al Nahyan Ben Zayed Institute for Personalized Cancer Therapy, and the National Center for Advancing Translational Sciences (grant no. UL1 TR000371). This study was also supported by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (P30 CA016672).
Footnotes
Conflict of interest: Filip Janku has research support from Novartis, Agios, Astellas, Deciphera, Symphogen, Piqur, Roche, BioMed Valley Discoveries, and Upsher-Smith Laboratories, is on the Scientific Advisory Boards of Deciphera, Illumina and Guardant Health, provides paid consulting for Trovagene and has ownership interest in Trovagene. Gordon B. Mills has research support from AstraZeneca, Critical Outcomes Technology, and GSK; is on the Scientific Advisory Board of AstraZeneca, Blend, Critical Outcome Technologies, HanAl Bio Korea, Nuevolution, Provista Diagnostics, Signalchem Lifesciences, and Symphogen; and has stock options or financial considerations with Catena Pharmaceuticals, PTV Ventures, and Spindle Top Ventures. Shile Zhang, Jill Waters, Li Liu, Han-Yu Chuang, Kristina M. Kruglyak, Chen Zhao, Neeraj S. Salathia are current employees and shareholders of Illumina, Inc. Yue Zhao, Hyunsung J. Kim, Sante Gnerre, Ravi Vijaya Satya, Richard Shen, Jian-Bing Fan are shareholders of Illumina, Inc.
References
- 1.Polivka J, Jr, Pesta M, Janku F. Testing for oncogenic molecular aberrations in cell-free DNA-based liquid biopsies in the clinic: are we there yet? Expert review of molecular diagnostics. 2015;15:1631–44. doi: 10.1586/14737159.2015.1110021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O’Connell A, Feeney N, et al. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janku F, Angenendt P, Tsimberidou AM, Fu S, Naing A, Falchook GS, et al. Actionable mutations in plasma cell-free DNA in patients with advanced cancers referred for experimental targeted therapies. Oncotarget. 2015;6:12809–21. doi: 10.18632/oncotarget.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janku F, Huang HJ, Claes B, Falchook GS, Fu S, Hong D, et al. BRAF Mutation Testing in Cell-Free DNA from the Plasma of Patients with Advanced Cancers Using a Rapid, Automated Molecular Diagnostics System. Mol Cancer Ther. 2016 doi: 10.1158/1535-7163.MCT-15-0712. [DOI] [PubMed] [Google Scholar]
- 5.Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez-Crapez E, Rolet F, et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med. 2014;20:430–5. doi: 10.1038/nm.3511. [DOI] [PubMed] [Google Scholar]
- 6.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–11. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arkenau HT, Barriuso J, Olmos D, Ang JE, de Bono J, Judson I, et al. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol. 2009;27:2692–6. doi: 10.1200/JCO.2008.19.5081. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Kim ST, Lee WS, Lanman RB, Mortimer S, Zill OA, Kim KM, et al. Prospective blinded study of somatic mutation detection in cell-free DNA utilizing a targeted 54-gene next generation sequencing panel in metastatic solid tumor patients. Oncotarget. 2015;6:40360–9. doi: 10.18632/oncotarget.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson JC, Yee SS, Troxel AB, Savitch SL, Fan R, Balli D, et al. Detection of therapeutically targetable driver and resistance mutations in lung cancer patients by next generation sequencing of cell-free circulating tumor DNA. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Springer S, Zhang M, McMahon KW, Kinde I, Dobbyn L, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci U S A. 2015;112:9704–9. doi: 10.1073/pnas.1511694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–54. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman AM, Lovejoy AF, Klass DM, Kurtz DM, Chabon JJ, Scherer F, et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol. 2016;34:547–55. doi: 10.1038/nbt.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jovelet C, Ileana E, Le Deley MC, Motte N, Rosellini S, Romero A, et al. Circulating Cell-Free Tumor DNA Analysis of 50 Genes by Next-Generation Sequencing in the Prospective MOSCATO Trial. Clin Cancer Res. 2016;22:2960–8. doi: 10.1158/1078-0432.CCR-15-2470. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y, Jovelet C, Filleron T, Pedrero M, Motte N, Boursin Y, et al. Improving the Performance of Somatic Mutation Identification by Recovering Circulating Tumor DNA Mutations. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-3457. [DOI] [PubMed] [Google Scholar]
- 16.Janku F, Huang HJ, Claes B, Falchook GS, Fu S, Hong D, et al. BRAF Mutation Testing in Cell-Free DNA from the Plasma of Patients with Advanced Cancers Using a Rapid, Automated Molecular Diagnostics System. Mol Cancer Ther. 2016;15:1397–404. doi: 10.1158/1535-7163.MCT-15-0712. [DOI] [PubMed] [Google Scholar]
- 17.Higgins MJ, Jelovac D, Barnathan E, Blair B, Slater S, Powers P, et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin Cancer Res. 2012;18:3462–9. doi: 10.1158/1078-0432.CCR-11-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:795–801. doi: 10.1038/nm.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabernero J, Lenz HJ, Siena S, Sobrero A, Falcone A, Ychou M, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015;16:937–48. doi: 10.1016/S1470-2045(15)00138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nygaard AD, Garm Spindler KL, Pallisgaard N, Andersen RF, Jakobsen A. The prognostic value of KRAS mutated plasma DNA in advanced non-small cell lung cancer. Lung Cancer. 2013;79:312–7. doi: 10.1016/j.lungcan.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Spindler KL, Pallisgaard N, Vogelius I, Jakobsen A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin Cancer Res. 2012;18:1177–85. doi: 10.1158/1078-0432.CCR-11-0564. [DOI] [PubMed] [Google Scholar]
- 22.Santiago-Walker A, Gagnon R, Mazumdar J, Casey M, Long GV, Schadendorf D, et al. Correlation of BRAF Mutation Status in Circulating-Free DNA and Tumor and Association with Clinical Outcome across Four BRAFi and MEKi Clinical Trials. Clin Cancer Res. 2016;22:567–74. doi: 10.1158/1078-0432.CCR-15-0321. [DOI] [PubMed] [Google Scholar]
- 23.Hyman DM, Diamond EL, Vibat CR, Hassaine L, Poole JC, Patel M, et al. Prospective blinded study of BRAFV600E mutation detection in cell-free DNA of patients with systemic histiocytic disorders. Cancer Discov. 2015;5:64–71. doi: 10.1158/2159-8290.CD-14-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–6. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 27.Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Science translational medicine. 2012;4:136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 28.Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–12. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 29.Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O’Connell A, Messineo MM, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20:1698–705. doi: 10.1158/1078-0432.CCR-13-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mok T, Wu YL, Lee JS, Yu CJ, Sriuranpong V, Sandoval-Tan J, et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin Cancer Res. 2015;21:3196–203. doi: 10.1158/1078-0432.CCR-14-2594. [DOI] [PubMed] [Google Scholar]
- 31.Karlovich C, Goldman JW, Sun JM, Mann E, Sequist LV, Konopa K, et al. Assessment of EGFR Mutation Status in Matched Plasma and Tumor Tissue of NSCLC Patients from a Phase I Study of Rociletinib (CO-1686) Clin Cancer Res. 2016;22:2386–95. doi: 10.1158/1078-0432.CCR-15-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchetti A, Palma JF, Felicioni L, De Pas TM, Chiari R, Del Grammastro M, et al. Early Prediction of Response to Tyrosine Kinase Inhibitors by Quantification of EGFR Mutations in Plasma of NSCLC Patients. J Thorac Oncol. 2015;10:1437–43. doi: 10.1097/JTO.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 33.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frenel JS, Carreira S, Goodall J, Roda D, Perez-Lopez R, Tunariu N, et al. Serial Next-Generation Sequencing of Circulating Cell-Free DNA Evaluating Tumor Clone Response To Molecularly Targeted Drug Administration. Clin Cancer Res. 2015;21:4586–96. doi: 10.1158/1078-0432.CCR-15-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108:9530–5. doi: 10.1073/pnas.1105422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.