Abstract
The retinal pigment epithelium (RPE) is a highly specialized, unique epithelial cell that interacts with photoreceptors on its apical side and with Bruch’s membrane and the choriocapillaris on its basal side. Due to vital functions that keep photoreceptors healthy, the RPE is essential for maintaining vision. With aging and the accumulated effects of environmental stresses, the RPE can become dysfunctional and die. This degeneration plays a central role in age-related macular degeneration (AMD) pathobiology, the leading cause of blindness among the elderly in western societies. Oxidative stress and inflammation have both physiological and potentially pathological roles in RPE degeneration. Given the central role of the RPE, this review will focus on the impact of oxidative stress and inflammation on the RPE with AMD pathobiology. Physiological sources of oxidative stress as well as unique sources from photo-oxidative stress, the phagocytosis of photoreceptor outer segments, and modifiable factors such as cigarette smoking and high fat diet ingestion that can convert oxidative stress into a pathological role, and the negative impact of impairing the cytoprotective roles of mitochondrial dynamics and the Nrf2 signaling system on RPE health in AMD will be discussed. Likewise, the response by the innate immune system to an inciting trigger, and the potential role of local RPE production of inflammation, as well as a potential role for damage by inflammation with chronicity if the inciting trigger is not neutralized, will be debated.
Keywords: Age-related macular degeneration, complement, inflammation, mitochondrial dynamics, Nrf2, oxidative stress, retinal pigment epithelium
1. Introduction
While aging is a decline in the ability to respond and adapt to the cumulative impact of different exposures (Jones, 2015), age-related disease develops when cellular dysfunction from impaired cytoprotective pathways is severe enough to cause tissue injury. Age-related macular degeneration (AMD) is one such disease. It is the leading cause of vision loss among the elderly in the United States and western societies (Congdon et al., 2004) and is a major public health problem that costs $30 billion annually in the United States (Brown et al., 2005a). Over 1.75 million people in the United States have advanced AMD, and as the “baby boomers” age, it is estimated that 3 million people will be affected by 2020 (Friedman et al., 2004), and 1 in 3 people in their 8th decade of life with early AMD will develop advanced disease over the next decade (Gehrs et al., 2006; Mukesh et al., 2004). Currently, 8.7% of the world-wide population has AMD, and the projected number of people afflicted with AMD by 2020 is 196 million, increasing to 288 million by 2040 (Wong et al., 2014). As a result, the cost of AMD is predicted to increase to $59 billion over the next 20 years (Centre for Eye Research Australia, 2006). The impact of AMD on an individual’s quality of life is high. For example, Brown et al found that the decrease in quality of life from early AMD is similar to a person with symptomatic HIV, and with advanced AMD, to one with metastatic prostate cancer having poorly controlled pain (Brown et al., 2005b). With vision loss, a person with AMD is less active (Wysong et al., 2009), and is at higher risk of depression (Augustin et al., 2007; Brody et al., 2001) or anxiety (Berman and Brodaty, 2006) than unaffected elderly people.
The retinal pigment epithelium (RPE) is a highly specialized, polarized epithelial cell whose apical side is in intimate contact with photoreceptor outer segments, and its basal side is adherent to Bruch’s membrane, a specialized, pentalaminar extracellular matrix that separates the RPE from the choriocapillaris (Hogan, 1972). Some of the RPE’s essential functions that maintain the health and function of both the photoreceptors and choriocapillaris include the daily phagocytosis of photoreceptor outer segments, light absorption to optimize vision, heat exchange, vitamin A metabolism, and secreting vascular endothelial growth factor (VEGF), which works as a trophic factor to sustain the health of the choriocapillaris endothelium (Saint-Geniez et al., 2009). With Bruch’s membrane, it maintains the outer blood retinal barrier to provide selective entry and removal of metabolites and molecules to and from the retina and systemic circulation (Strauss, 2005). Along with its remarkable and diverse functions, its sandwiched location makes it pivotal for maintaining normal vision, and in particular, central visual acuity that is provided by the macula. As a result, RPE impairment contributes significantly to AMD.
Oxidative stress has long been considered a major influence on the RPE in AMD pathophysiology. Both environmental and genetic factors support this theory. The macula lives in a high oxidative stress environment, which can be magnified by those who choose to smoke, making it the strongest non-genetic risk factor for AMD aside from chronological aging (Rahman and MacNee, 1996; Rangasamy et al., 2004; Smith and Hansch, 2000). A high fat diet (HFD) intake, which also induces oxidative stress as reviewed in (Uranga et al., 2010), is another preventable AMD risk factor (Chiu et al., 2014; Mares-Perlman et al., 1995; Seddon et al., 2003; Seddon et al., 2001). Over the past decade, nearly 40 genetic variants have been identified that are associated with AMD risk, and include genes that encode proteins involved in oxidative stress, which strengthens the premise that oxidative stress has an etiologic role. For example, since mitochondria are an abundant source of reactive oxygen species (ROS) formation, the identification of polymorphisms in mitochondrial MTND2*LHON4917G, NADH dehydrogenase subunits, and mitochondrial superoxide dismutase 2 (SOD2), that are associated with AMD risk, suggests a pathogenic role for oxidative stress (Canter et al., 2008; SanGiovanni et al., 2009). Likewise, the LOC387715 locus polymorphism that is associated with AMD risk is higher in smokers than either smoking or the polymorphism alone, is additional evidence of a pathogenic role of oxidative stress (Schmidt et al., 2006).
A decade ago, the discovery of polymorphisms in complement factor H (CFH) with up to a sevenfold increased risk of AMD introduced innate immunity as a pathophysiologic factor (Edwards et al., 2005; Hageman et al., 2005; Haines et al., 2005; Klein et al., 2005). Shortly after, genetic abnormalities in other complement pathway genes that influence the risk for AMD were identified such as C3 (rs2230199), factor I (rs10033900), and the C2/factor B locus (rs9332739, rs547154, rs4151667, and rs641153) (Fagerness et al., 2009; Gold et al., 2006; Spencer et al., 2007; Yates et al., 2007). Since these discoveries, scientists around the world have been working to delineate greater understanding of how innate immunity influences AMD pathogenesis. Despite this intensive research in both oxidative stress and innate immunity, several questions remain unanswered. To what degree does oxidative damage contribute to AMD? How does the macula protect itself from oxidative damage? How does innate immunity protect the macula and to what degree does tissue injury from either exaggerated or chronic innate immune response contribute to AMD? Does oxidative damage trigger innate immunity? Given the central anatomic and functional role of the RPE in the macula, in this review, we will take a critical look at the role of oxidative stress and innate immunity on RPE degeneration in non-neovascular AMD pathobiology.
2. Clinical Definition of AMD
According to the American Academy of Ophthalmology, AMD is defined by the presence of at least intermediate-size drusen (63 μm or larger in diameter), or yellowish accumulations of debris within Bruch’s membrane; RPE abnormalities such as hypopigmentation or hyperpigmentation; reticular pseudodrusen or subretinal deposits; and/or the presence of any of the following features: advanced non-neovascular disease or geographic atrophy (GA), which is a circumscribed area or areas of RPE cell loss, and neovascular disease (exudative, wet) including choroidal neovascularization, polypoidal choroidal vasculopathy, or retinal angiomatous proliferation (https://www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp-2015).
3. Histopathologic Changes in Non-neovascular AMD
The clinician defines the macula as the central 6 mm diameter area located between the major retinal vascular arcades while the anatomist defines it as the retina with more than one layer of ganglion cell nuclei (Orth et al., 1977). The macula contains two distinct sub-regions composed of different photoreceptors. The central region or fovea, is 0.8 mm in diameter (2.75° in visual angle), and is dominated by cones. The parafovea surrounds the fovea and is rod-dominated (Curcio et al., 1990). In the macula, rods actually outnumber cones by 9:1. Thus, while the central macula is cone dominated, the rod-dominated parafovea makes the macula cone enriched and not cone-dominated.
In early AMD, patients complain of poor vision despite 20/20 visual acuity, with impaired macular sensitivity, contrast sensitivity, light exposure recovery, and dark adaptation (Chen et al., 1992; Eisner et al., 1992; Eisner et al., 1991; Midena et al., 1997; Owsley et al., 2015), which are due to diminished parafoveal rod responses (Hood et al., 2002; Owsley et al., 2016; Parisi et al., 2007). In the aging parafovea, the region of high rod density, the RPE are hexagonal, enlarged, and have the highest frequency of multinucleated cells (Starnes et al., 2016). Of the possible causes of multinucleation, such as endoreplication, cell fusion, or incomplete cell division, Chen et al found that oxidative stress generated during the phagocytosis of oxidized outer segments, induces RPE proliferation, but cytokinesis failure, indicating that incomplete cell division is likely the primary etiology for multinucleation (Ach et al., 2014; Chen et al., 2016). In early AMD, the RPE degenerates and assumes a mesenchymal morphology while photoreceptors can appear intact (Ach et al., 2015; Hartmann et al., 2011; Iwama et al., 2010; Sulzbacher et al., 2012; Vogt et al., 2011; Wu et al., 2014). With progressive RPE changes, parafoveal rods die before either the RPE or cones (Starnes et al., 2016), and surviving rods often lack outer segments (Curcio et al., 1996; Curcio et al., 1993). The RPE undergoes progressive degeneration as AMD advances with the most severe changes in GA (Ach et al., 2015; Vogt et al., 2011). In the transitional zone surrounding GA, RPE cells have severely irregular shape and pigmentary changes, can dissociate from Bruch’s membrane to become multilayered or they can migrate into the retina or “subduct” into Bruch’s membrane (Guidry et al., 2002; Sarks et al., 1988; Vogt et al., 2011; Zanzottera et al., 2015). These changes are typical of epithelial mesenchymal transition (EMT) type II, where epithelial cells undergo multiple molecular changes to assume a mesenchymal phenotype in an attempt to regenerate (Kalluri and Neilson, 2003), resulting in loss of polarity, loss of epithelial function, increased mobility, and resistance to cell death (Kalluri and Weinberg, 2009; Zeisberg and Neilson, 2009). Collectively, these RPE changes suggest RPE dysfunction has a central role in rod dysfunction and death in both early and late stage AMD.
Coincident with RPE changes, Bruch’s membrane undergoes a series of histopathologic changes known as basal deposits, or accumulations of heterogeneous debris, as classically categorized by Sarks (Sarks, 1976). The composition and location define whether basal deposits are associated with aging or AMD. Beginning early in life, Bruch’s membrane develops outer collagenous layer deposits, which account for much of Bruch’s membrane’s age-related thickening (van der Schaft et al., 1991). Basal laminar deposits (BlamD), which accumulate between the RPE cell and its basement membrane, are associated with aging when they are thin and similar in composition to the RPE basement membrane, including collagen IV, laminin, and heparan sulfate proteoglycans (van der Schaft et al., 1994). When BlamD become thickened and contain heterogeneous debris such as long spacing collagen, inflammatory molecules, and lipids, they are associated with AMD. Basal linear deposits (BlinD), which form within the inner collagenous layer of Bruch’s membrane, are associated with AMD. Histopathologically, BlinD and large drusen (>125microns in diameter) are different morphologic forms of the same lesion (Abdelsalam et al., 1999; Bressler et al., 1994; Curcio and Millican, 1999; Green and Enger, 1993; Spraul et al., 1996).
The RPE appears to be centrally involved in basal deposit and drusen formation. The accumulation of apolipoprotein B100 (apoB100) containing lipoproteins, which are reminiscent of very low density lipoproteins (VLDL) and low density lipoproteins (LDL), is an important antecedent event to basal deposit and drusen formation (Curcio et al., 2005). As the Curcio and Handa labs have demonstrated, these lipoproteins likely originate primarily from the RPE rather than the systemic circulation, and may be secreted in response to lipid overload by the RPE (Curcio et al., 2010; Fujihara et al., 2014; Wu et al., 2010). These lipoproteins transit from the RPE through Bruch’s membrane in linear tracks (Pikuleva and Curcio, 2014), and due to aging related structural changes to Bruch’s membrane, first accumulate in the inner collagenous layer adjacent to the elastic layer, and then toward the RPE, eventually forming a “lipid wall”. Because the lipid wall prevents the passage of material, inflammatory and cellular debris accumulates, forming basal deposits and drusen. These observations correlate with the hypothesis made by Hageman et al, in 2001, that drusen are biomarkers of immune-mediated processes at the RPE-Bruch’s membrane triggered by cellular debris and lipids that accumulate in Bruch’s membrane (Hageman et al., 2001).
Besides drusen, deposits also form in the subretinal space between the photoreceptors and RPE. Originally identified by Mimoun et al. in 1990 (Mimoun et al., 1990), reticular pseudodrusen were first clinically observed in AMD. Zweifel et al., using optical coherence tomography (OCT), showed that these deposits were located in the subretinal space (Zweifel et al., 2010) which have been confirmed by histopathological analysis (Rudolf et al., 2008; Sarks et al., 2011). While long ignored in part, because they can be challenging to visualize on clinical exam, reticular pseudodrusen are now a fundamental criteria for diagnosing and monitoring AMD progression due to improvements in imaging modalities such as OCT and fundus autofluorescence, and recognition that these lesions are a marker for disease advancement (Arnold et al., 1995; Cohen et al., 2007; Hamel et al., 2009; Lee et al., 2012; Zweifel et al., 2010). While the pathogenesis of these lesions is unknown, unesterified cholesterol, apolipoprotein E, CFH, and vitronectin have been found in reticular pseudodrusen (Rudolf et al., 2008). As will be discussed below, our lab found that the C5b-9 complex regulator CD59, is also found in reticular pseudodrusen, and CD59 is secreted in a combination of exosomes and apoptotic blebs by the RPE (Ebrahimi et al., 2012). These results raise the possibility that complement activation by the RPE could contribute to reticular pseudodrusen formation.
The choroid and RPE have a symbiotic interaction whereby the RPE is dependent upon the choriocapillaris for survival. In early AMD, Seddon et al recently found that choriocapillaris loss, using EC-binding lectin from Ulex europaeus (UEA-I) to distinguish live from ghost vessels, preceded RPE cell atrophy (Seddon et al., 2016). The Mullins laboratory, also using UEA-I, found that loss of choriocapillaris area and an increase in ghost vessels, correlated with increased drusen size and density, implicating a role for choriocapillaris dysfunction in drusenogenesis (Mullins et al., 2011). These changes correspond to functional changes such as prolonged filling of the choroid in early AMD with indocyanine green or fluorescein angiography (Pauleikhoff et al., 1999) and decreased choroidal blood volume and flow by laser Doppler flow studies (Berenberg et al., 2012).
4. Oxidative stress: when does a friend become a foe?
The generation of ROS has long been considered to have harmful consequences on the RPE. However, ROS have a physiological role that is essential for normal cellular function. Redox signaling can act as an important regulator in both physiological and pathological processes, and can be generated from several sources, as reviewed in (Finkel, 2011), including NADPH-dependent oxidases, mitochondrial electron transport chain (ETC) complexes, and other cellular enzymes including xanthine oxidase, cyclooxygenases, lipoxygenases, and cytochrome p450 enzymes. ROS signal a wide range of cellular responses that protect the RPE, such as feedback regulation after excess metabolism (Brunelle et al., 2005; Guzy et al., 2005), response to hypoxia via HIF-1α (Guzy et al., 2005), regulation of autophagy through ATF4 (Scherz-Shouval et al., 2007), and regulation of inflammatory responses (Bulua et al., 2011; Nakahira et al., 2011; Zhou et al., 2011).
The macula lives in a high oxidative stress environment in part, because it has unique sources of oxidative stress. The RPE has a high metabolic demand that produces high levels of physiologic ROS for signal transduction, but also substantial ROS produced from cellular metabolism to meet its multiple functions. To meet this metabolic demand, the macula receives some of the highest blood flows in the body. As a result, the RPE is exposed to high ambient oxygen partial pressures of 70–90 mm Hg (Winkler et al., 1999).
Due to its high metabolic activity, RPE are enriched with mitochondria, and as a result, is a major source of ROS in the RPE (Jager et al., 2008). Compared with young cells, aging mitochondria generate more ROS, and furthermore, aging cells are progressively susceptible to mitochondria derived ROS. While it has been known since the 1960s that mitochondria produce ROS (Jensen, 1966), the production of ROS by mitochondrial ETC complexes had not been fully elucidated until relatively recently (Yaniv et al., 2013; Zorov et al., 2005). Under physiological conditions during ATP synthesis, the ETC complexes generate ROS that leak out of the mitochondrial membrane (Chen et al., 2012; Liu et al., 2002; Murphy, 2009). Under pathological conditions that compromise the ETC components, the generation of ROS is significantly increased, principally at complexes I (NADH complex) and III (cytochrome complex). Not surprisingly, mutations in the genes that code for these complexes account for more than 50% of all mitochondrial ROS related pathological conditions (Zorov et al., 2005). Additionally, the decrease in mitochondrial membrane potential facilitates the leakage of ROS into the cytoplasm (Korshunov et al., 1997).
The RPE has unique sources of oxidative stress. For example, the RPE has the unusual responsibility of the routine phagocytosis of nearly 30,000 photoreceptor outer segments each day (Ershov and Bazan, 2000). During outer segment phagocytosis, intracellular H2O2 from NADPH oxidase in the phagosome or β-oxidation of outer segment lipids in peroxisomes is generated to the same magnitude that occurs during phagocytosis by macrophages (Miceli et al., 1994; Tate et al., 1995). Photo-oxidative stress from processing light for vision is perhaps the most unique and additional source of oxidative stress. Since the work of Ham et al in 1978 (Ham et al., 1978), photo-oxidative stress has been linked with oxidative damage to the retina, RPE, and choroid, and multiple other works have substantiated this observation, as reviewed in (Beatty et al., 2000). These data correlate with the Chesapeake Bay Watermen studies showing the epidemiologic link of sunlight exposure and AMD risk (Taylor et al., 1990; Taylor et al., 1992).
The oxidative burden contributing to the oxidative stress load on the RPE is enhanced by the western life style. Cigarette smoking clearly adds to the oxidative stress burden because it contains over 4700 chemical components, many of which are strong oxidants (Rangasamy et al., 2004; Smith and Hansch, 2000), and each puff contains 1015 free radicals (Rahman and MacNee, 1996). While the exact role of oxidative damage caused by cigarette smoking in AMD is unknown, chemical oxidants in cigarette smoke deplete tissues of ascorbic acid and protein sulfhydryl groups, causing the oxidation of DNA, lipids and proteins (Cross et al., 1993; Lykkesfeldt et al., 2000; O’Neill et al., 1994). Many of these molecular changes such as malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and advanced glycation endproducts (AGE), have been identified in AMD, and indicate that oxidative damage is an important factor in the mechanism of disease development. These observations correlate well with the multiple studies concluding that smoking is the strongest environmental risk factor for AMD (Rahman and MacNee, 1996; Rangasamy et al., 2004; Smith and Hansch, 2000). To a lesser extent, a high fat diet (HFD) intake also induces oxidative stress, and represents another source of oxidative stress and risk factor for AMD (Chiu et al., 2014; Mares-Perlman et al., 1995; Seddon et al., 2003; Seddon et al., 2001; Uranga et al., 2010). At present, it is unclear when the burden of oxidative stress extends beyond physiological signaling and becomes pathologic, or to what extent the accumulated exogenous and preventable sources of oxidative stress overwhelm the RPE cell’s antioxidant system.
5. How does the RPE protect itself from oxidative stress?
In general, cells have developed several mechanisms to protect themselves from the potentially damaging effects of oxidative stress, yet at the same time, enable vital signaling through physiological ROS. For example, oxidant-producing enzymes are located adjacent to the intended target, or oxidants have a regulated entry, such as H2O2 through aquaporin channels (Bienert et al., 2007). Along with the short half-life of ROS, their transient production, such as during a NOX-dependent oxidant burst, these mechanisms topographically limit ROS to a specific locale where they can be neutralized if excessive. Oxidative stress induces a cellular antioxidant response, including in RPE cells, which has been linked to activation of autophagy through the p62/Keap1/Nrf2 pathway (Azad et al., 2009; Filomeni et al., 2010; Johansson et al., 2015; Wang et al., 2014a). Autophagy is a protective, homeostatic mechanism designed to remove faulty cellular components including those generated by oxidative damage. Importantly, compromised autophagy can result in dysfunctional RPE has been shown to induce an AMD like phenotype in mice (Yao et al., 2015), and is a contributing factor in AMD (Mitter et al., 2014; Viiri et al., 2013; Wang et al., 2009).
5.1 Mitochondrial dynamics maintain a homeostatic level of mitochondrial ROS
As discussed above, a large proportion of ROS is derived from mitochondria. Since RPE cells are predominantly post-mitotic, damaged mitochondria are not removed during cell division (Cai et al., 2000). Instead, mitochondrial health and function rely on a dynamic process of mitochondrial fission and fusion followed by removal of damaged mitochondria (Westermann, 2010). Through fission, damaged mitochondria are sequestered and then degraded through dynamin -1- like protein (Drp1) (Twig et al., 2008). The damaged mitochondrial fragments are selectively removed by mitophagy, a specialized type of autophagy. When the mitochondrial membrane potential (ΔΨm) is decreased, the Pink1-Parkin mediated signaling system is initiated (Lin and Kang, 2008), ultimately leading to ubiquitination and lysosomal degradation of the affected mitochondria. Pink1 is a nuclear encoded mitochondrial serine-threonine kinase. In healthy mitochondria, Pink1 transits through the mitochondrial membranes into the matrix where it is degraded by protease presenilin-associated rhomboid-like protein (PARL), its antagonist (Meissner et al., 2011). With a decrease in ΔΨm, Pink1 remains on the mitochondrial surface, where it phosphorylates a wide range of proteins, most notably Parkin and Ubiquitin. Phosphorylated Parkin in turn ubiquitinates proteins of the outer mitochondrial membrane, eventually resulting in LC3b mediated lysosomal phagophore formation and degradation (Kitagishi et al., 2017; Lazarou et al., 2015; Rub et al., 2017).
Mitophagy protects the mitochondria from environmental insults like chronic oxidative stress, and is highly sensitive to fluctuations in oxidative stress. Under mild oxidative stress that is unable to induce generalized autophagy, mitophagy is induced through mitochondrial fission in a Drp1 dependent manner (Frank et al., 2012). Because any deficiency in mitophagy can result in elevated mitochondrial ROS (Kitagishi et al., 2017; Narendra et al., 2008), impaired mitophagy and mitochondrial dynamics in the RPE have been implicated in early AMD (Feher et al., 2006; Karunadharma et al., 2010). The excessive mitochondrially derived ROS has significant implications that can further injure the mitochondria and threaten the overall health of the RPE (Kinugasa et al., 2015; Zhou et al., 2009) because mitochondrial DNA (mtDNA) are especially susceptible to injury due to the lack of an efficient proof-reading and repair mechanism, and the lack of introns in mitochondrial genes. Thus, any mutation in mtDNA will directly affect the gene product (Blasiak et al., 2013). Since all mitochondria encoded genes are involved in oxidative phosphorylation (OXPHOS), mitochondrial damage leads to defects in OXPHOS, resulting in impaired metabolism and further increases in ROS production, which further damages mitochondria and other cellular elements (Liang and Godley, 2003). In fact, Golestaneh et al recently found that iPS RPE cells derived from AMD patients had mitochondria that were significantly damaged, which reduced ATP production and enhanced glycolysis (Golestaneh et al., 2016). Furthermore, the AMD derived RPE cells were highly susceptible to oxidative stress and produced high levels of ROS under such stress. These changes correlated with decreased Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a regulator of mitochondrial biogenesis and function, and NAD-dependent deacetylase sirtuin1 (SIRT1).
Direct evidence for mitochondrial damage in AMD has been documented in several studies. Feher et al found decreased mitochondrial mass with ultrastructural alterations (Feher et al., 2006) in AMD specimens. The RPE of AMD affected patients have increased mtDNA damage compare to RPE from age matched controls (Blasiak et al., 2013), macular RPE have more mtDNA damage than peripheral RPE (Nordgaard et al., 2008), and mtDNA damage occurs at stages prior to overt visual loss (Karunadharma et al., 2010; Lin et al., 2011). mtDNA damage is correlated with a decrease in 8-oxoguanine glycosylase, a marker for mtDNA repair capacity (Lin et al., 2013) and an increase in disease severity (Blasiak et al., 2013). Importantly, Terluk et al have recently shown that mtDNA damage preferentially develops in the RPE, but not the retina to illustrate the importance of mtDNA damage in the RPE as an early event in AMD progression (Terluk et al., 2015). The mtDNA damage affects regions required for transcription and replication of mitochondrial proteins, and electron transport chain (ETC) complex I and II subunits (Terluk et al., 2015). With damaged mitochondria, the cell has compromised energy production, as well as an imbalance in pro and anti apoptotic signals, leading to RPE dysfunction and death, the two key features of AMD pathogenesis (Kanda et al., 2007; Kinnunen et al., 2012). However, with mitochondrial injury necessitating a severe mitophagic response, EMT or even apoptosis can result (Higgins and Coughlan, 2014; Redza-Dutordoir and Averill-Bates, 2016). Given that the RPE undergoes degeneration reminiscent of EMT type II, and apoptosis is one proposed mechanism of RPE cell death in AMD, future studies should consider the role of mitophagy and mitochondrial dynamics in the pathogenesis of RPE degeneration.
5.2 The Nrf2 cytoprotective signaling system and its decrease in the RPE
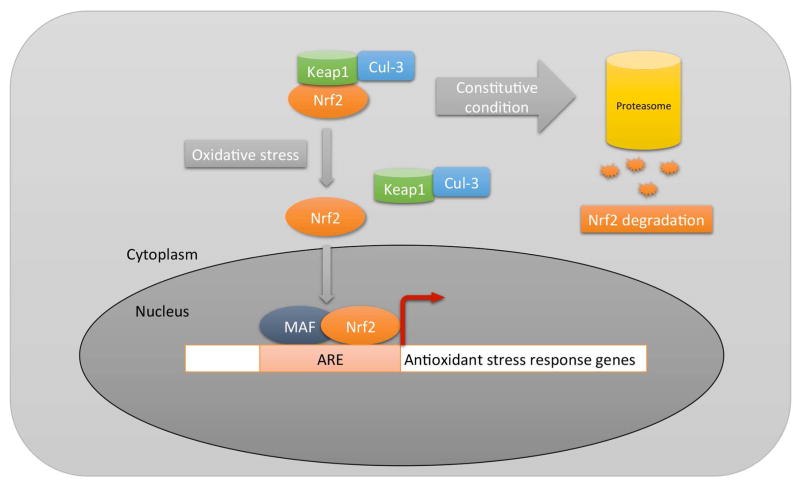
In general, the RPE has a complex series of antioxidant systems that protect cells against oxidative damage. These systems are regulated through various transcription factors including Nf-κB, AP1, the FoxO family, Wnt, or PGC-1α. Perhaps, the most comprehensive transcription system is mediated through Nuclear factor erythroid-2 related factor 2 (Nrf2), a basic leucine zipper transcription factor (Biswas and Rahman, 2008). Nrf2 regulates a coordinated transcriptional program that allows cellular redox homeostasis, yet protects the cell from oxidative injury (Nguyen et al., 2003; Rangasamy et al., 2004; Thimmulappa et al., 2002) (Fig 1). Nrf2 is normally sequestered in the cytosol by interacting with Kelch-like ECH-associated protein 1 (Keap1). Keap1 also functions as a substrate adaptor protein for a Cul3-dependent E3 ubiquitin ligase complex, which also helps to maintain steady-state levels of Nrf2 via proteolysis of Nrf2 by the ubiquitin-proteasome pathway (Adams et al., 2000; Kobayashi et al., 2004; Yu and Kensler, 2005; Zhang et al., 2004). Without oxidative stress, Keap1 constitutively suppresses Nrf2 signaling by degrading Nrf2 to prevent Nrf2 from translocating to the nucleus, which results in a low baseline expression of antioxidant genes that are regulated by other transcription factors (Thimmulappa et al., 2002). With acute oxidative stress, Keap1 undergoes a conformational change after multiple cysteine residues interact with ROS, releasing Nrf2, which inhibits Keap1-mediated proteasomal Nrf2 degradation. The released Nrf2 then translocates to the nucleus, where it dimerizes with Maf proteins, and binds to the antioxidant response element (ARE) in the promoters of its target genes to initiate transcription (Dinkova-Kostova et al., 2005; Kobayashi and Yamamoto, 2005; Wakabayashi et al., 2003). The Nrf2 signaling response regulates both an early acute phase through actions of the “direct” enzymes, such as catalase or SOD, which neutralize H2O2 and superoxide, respectively, and a chronic phase by regulating glutathione and thioredoxin levels, as well as xenobiotic metabolism enzymes that produce reducing equivalents, such as NADPH quinine oxidoreductase (NQO-1) (Osburn et al., 2008). When cellular glutathione is depleted by oxidative stress, cells can die by oxidatively mediated apoptosis (Rahman et al., 2005; Walsh et al., 1995; Will et al., 1999). Several studies have identified Nrf2 as an essential signaling system in the RPE (Gao and Talalay, 2004; Nelson et al., 2002; Nelson et al., 1999; Tanito et al., 2005).
Figure 1.
Diagram of Nrf2 signaling. In the absence of oxidative stress (ROS), Nrf2 is degraded through the proteasome by a Keap1/Cul3 mechanism. With oxidative stress, the ROS induce a conformational change in cytoplasmic Keap1, which releases Nrf2 for transit to the nucleus where it interacts with Maf proteins and binds to antioxidant response elements (ARE), inducing a comprehensive antioxidant response.
In addition to its antioxidant protection, Nrf2 signaling has recently been found to play an unexpected role in maintaining mitochondria and metabolism. Nrf2 controls the expression of several tricarboxylic acid cycle (TCA) enzymes, which increases glucose flux through the TCA cycle to generate NADH, an essential substrate for ETC complex I to promote ATP production (Dinkova-Kostova et al., 2015; Singh et al., 2013). Likewise, Nrf2 also increases the expression of fatty acid oxidation enzymes to provide FADH2 as a substrate for ETC complex II (Dinkova-Kostova et al., 2015). Nrf2 signaling can also increase glycolysis by inducing the expression of several key components (Singh et al., 2013). Finally, Nrf2 promotes the expression of alcohol dehydrogenase, aldehyde dehydrogenase, or NADPH alenol oxidoreductase, which break down 4-HNE modified proteins in ETC complex I and II to rejuvenate mitochondrial function (Miller et al., 2013). Future studies will need to clarify the relative contribution of impaired antioxidant protection to metabolism with Nrf2 failure.
The accumulation of oxidatively damaged molecules in the macula with AMD suggests that the antioxidant response is insufficient. Aging can reduce Nrf2 mRNA or protein, which impairs Nrf2 signaling. Suzuki et al showed an age-dependent decline in Nrf2 from cigarette smoking (Suzuki et al., 2008). These results have also been observed in mice, where aging suppressed the induction of Nrf2 and its target genes in alveolar macrophages in response to cigarette smoking (Suzuki et al., 2008). Our lab has shown that aging impaired the Nrf2 response in the RPE after acute oxidative stress in mice (Sachdeva et al., 2014). While the RPE of young mice elicit a robust induction of Nrf2 mediated antioxidant gene expression after exposure to the chemical oxidant sodium iodate, the RPE of old mice had a blunted antioxidant response that resulted in the accumulation of MDA. MDA damage was reversed by genetic rescue of Nrf2 signaling by conditionally knocking down Keap1.
Nrf2 also declines in several diseases. For example, in emphysema, Nrf2 mRNA and protein, and Nrf2-dependent antioxidants and glutathione levels are reduced, and oxidative stress is increased in diseased compared to normal lungs (Malhotra et al., 2008; Suzuki et al., 2008). These same findings are seen in Nrf2 deficient mice after they develop an emphysema phenotype following chronic cigarette smoke exposure (Rangasamy et al., 2004). We have also provided evidence that Nrf2 is decreased in AMD (Wang et al., 2014b). In maculas of early AMD, Nrf2 immunolabeling was increased with nuclear labeling in morphologically normal RPE compared to the RPE of non-AMD maculas, which is compatible with induced Nrf2 signaling from a high oxidative stress microenvironment of the AMD macula. In dysmorphic RPE cells from AMD maculas, Nrf2 immunolabeling was decreased relative to morphologically normal RPE cells (Fig 2). This finding suggests that Nrf2 signaling has failed in diseased areas, and with this failure, the narrow balance between physiological and pathological oxidative stress is upset, resulting in tissue damage. However, inadequate Nrf2 signaling may not sufficient to induce advanced stage disease. For example, our lab found that C57Bl6J mice treated with chronic cigarette smoking have impaired Nrf2 signaling in the RPE, which was associated with marked RPE degeneration, but without progression to more advanced stages such as neovascular AMD or geographic atrophy (Cano et al., 2010; Fujihara et al., 2008). Similarly, Zhou et al. found that Nrf2−/− mice developed elements of AMD including drusen and RPE abnormalities, but again, no progression to advanced disease (Zhao et al., 2011). It is possible that impairment of more than one cytoprotective pathway in the RPE is necessary to induce further dysfunction and cell death during advanced stages of AMD.
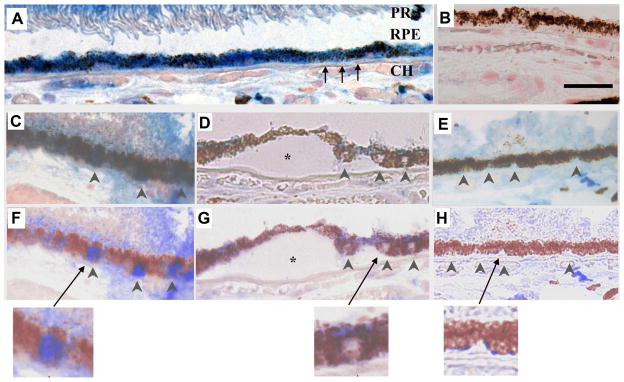
Figure 2.
Nrf2 immunolabeling in a 60-year-old Caucasian female with early AMD. A) Macular RPE with normal, cuboidal morphology have prominent cytoplasmic Nrf2 labeling (blue). B) IgG control. C) Normal macular RPE with both cytoplasmic and nuclear Nrf2 labeling (arrowheads). D) Dysmorphic macular RPE overlying drusen have lighter cytoplasmic labeling than cuboidal RPE from the same section. Nuclei (arrowheads) do not stain for Nrf2. E) Peripheral RPE with minimal Nrf2 labeling in the cytoplasm and no nuclear labeling (arrowheads). F–H) Same images as C–E, respectively, after Nuance software has converted melanin to maroon to improve visualization of Nrf2 labeling in the RPE. The arrow points to the inset of an enlarged image showing nuclear Nrf2 staining in a normal macular RPE cell (F), lack of nuclear Nrf2 staining in a dysmorphic macular RPE cell overlying a druse (*) (G), and lack of Nrf2 staining in a peripheral RPE cell (H). CH, choroid, RPE, retinal pigment epithelium, Black arrows point to Bruch’s membrane. Bar=25μm. Reprinted with permission from Free Radical Biology Medicine.
6. The role of inflammation on the RPE
Inflammation is the body’s response to damage of its cells and tissues in a series of processes designed for the eventual clearance of pathogens and repair of damaged tissue. Acute inflammation is a short-term process that involves leukocyte infiltration, removal of the trigger, and tissue repair. Chronic inflammation is a prolonged response that can result in tissue injury or destruction if the inciting trigger is not neutralized. Persistent inflammation is thought to underlie many diseases including AMD. The inflammatory response includes activation of both innate and adaptive immunity (Patel et al., 2005). At this time, the relative protective or pathologic responses during disease development have not been clearly delineated. In response to oxidatively damaged lipids, macrophages polarized toward an M1 phenotype accumulated in the subretinal space of mice immunized with carboxyethylpyrrole (CEP) prior to lesion development while macrophages and an AMD phenotype were absent in Ccr2-deficient mice, implicating macrophages in tissue injury (Cruz-Guilloty et al., 2013). Furthermore, subretinal mononuclear phagocytes or IL-1β might contribute to cone photoreceptor loss late stage AMD (Eandi et al., 2016) in part, because activated mononuclear phagocytes might resist elimination by the RPE with production of TNF-α, which downregulates OTX2, an important transcription factor that regulates genes that are essential to RPE function (Mathis et al., 2017). In the choroid, macrophages are recruited to basal deposits and drusen (Cherepanoff et al., 2010). Recently, mast cell degranulation has been identified in the choroid of all phases of AMD, which can release proteolytic enzymes that could result in Bruch’s membrane degradation, RPE death, choriocapillaris cell death, and choroidal thinning during AMD development (Bhutto et al., 2016). In the RPE, inflammation involves the innate immunity arms of complement and the inflammasome. The rest of this review will focus on these two arms of immunity.
6.1 Complement: when does a protective response become dysfunctional?
Complement components and its regulatory elements are typically synthesized in large abundance by the liver, and then released into the bloodstream for delivery to tissues in need of a complement defense response. In fact, CFH is one of the most abundant plasma proteins (Zipfel and Skerka, 2009). Indeed, systemically derived complement appears to play a role in AMD. Scholl et al showed that complement activation products C3a, C3d, Ba, C5a, and SC5b-9 were significantly elevated in plasma from AMD patients compared to controls (Scholl et al., 2008). This was especially true for chronic activation markers Ba and C3d (p<0.001) and factor D, which collectively, provided better discriminative accuracy for AMD risk than genetic markers of the complement system. Reynolds et al provided additional evidence of systemic alternative complement pathway component involvement in AMD where increased plasma Bb and C5a were associated with AMD (Reynolds et al., 2009). These studies indicate that systemic complement exerts a role in AMD.
However, a low level of local complement production is necessary for immune surveillance. The RPE is capable of producing complement components and several membrane-bound and soluble regulators to prevent excessive complement activation (Perez and Caspi, 2015). The RPE robustly expresses the classical and alternative pathway components and regulators, but limited production of lectin or terminal pathway components (Anderson et al., 2010). The choroid has a higher expression of classical and alternative complement components and regulators than the RPE (Anderson et al., 2010), and can provide complement components to the RPE in response to an inflammatory trigger, and regulate this response by supplying complement regulators if the RPE is unable to synthesize these components except for C5 and C7, which are not expressed by the RPE or choroid. For C5b-9 formation, the RPE and choroid must rely on systemic components. This interaction between the local microenvironment and the systemic circulation clearly takes place since C5b-9 is found in the choroid as early as 2 years of age (Mullins et al., 2014). C5b-9 localizes to the choriocapillaris rather than the retina or RPE, and increases in the choriocapillaris with aging (Hageman et al., 2005; Seth et al., 2008). In addition, patients homozygous for the 402H CFH AMD risk allele have elevated C5b-9 in the choriocapillaris along with choroidal atrophy compared to low risk eyes. These observations suggest that C5b-9 mediates choroidal atrophy in AMD (Mullins et al., 2014), which can result in hypoxia or reduce the delivery of glucose, a pivotal substrate for the RPE and photoreceptors, to jeopardize the health of both the RPE and photoreceptors due to their extremely high metabolism. Proof of this possibility is that hypoxic RPE cells have increased glucose consumption, which contributes to photoreceptor atrophy (Kurihara et al., 2016).
The alternative complement pathway is distributed differently in AMD than in aging. In normal eyes, C3 immunolocalizes to the choriocapillaris like factors B and D, while in non-neovascular AMD, C3 is additionally found in the RPE and sub-RPE space overlying drusen, which suggests C3 complement activation in the RPE (Anderson et al., 2010). Whether this altered pattern is protective or pathologic is unclear at this time, but studies on CFH immunolabeling indicate that CFH, which is normally confined to the choriocapillaris, intercapillary pillars, and Bruch’s membrane, is decreased in both early AMD and geographic atrophy (Bhutto et al., 2011). CFH is mainly secreted apically by the RPE (Chen et al., 2007; Kim et al., 2009), suggesting a regulatory role in the interphotoreceptor matrix adjacent to photoreceptors, and either systemically or choroidally produced CFH may localize to the basal RPE. Its decrease does not necessarily imply that complement is dysregulated because the C3 regulators CD55 and CD46 may compensate. However, CD55, a 70 kDa a glycophosphatidylinositol (GPI) anchored membrane protein that interferes with the conversion of factor B to Bb by factor D to prevent C3 convertase formation, is minimally expressed by the RPE (Vogt et al., 2006; Yang et al., 2009). CD46, a transmembrane protein, is a cofactor for the serine protease factor I, which cleaves and inactivates surface-bound C3b, to regulate C3. In contrast to CD55, several labs including ours, have found that in normal eyes, CD46 is abundant and localizes to lateral and basal RPE surfaces (Ebrahimi et al., 2012; McLaughlin et al., 2003; Vogt et al., 2006). The basal distribution suggests that CD46 plays a regulatory role of C3 convertase in the basal RPE. Vogt et al found that CD46 is decreased before identifiable AMD changes to the RPE and Bruch’s membrane (Vogt et al., 2011). We found that in intermediate disease, basal CD46 labeling was lost in areas overlying large drusen along with the immunolabeling of extracellular vesicular elements within drusen (Ebrahimi et al., 2012) (Fig 3), observations that were made also by Johnson et al (Johnson et al., 2001). In geographic atrophy, CD46 immunolabeling in the RPE was decreased (Vogt et al., 2011). This work coincides with our discovery that oxidized lipids reduce CD46 by RPE cells due in part, to apoptotic shedding and exosomal release of CD46 into the supernatant (Ebrahimi et al., 2012). Other studies indicate that oxidative stress can reduce complement regulators (Chen et al., 2007; Ebrahimi et al., 2012; Thurman et al., 2009; Wu et al., 2007). Thurman et al found that H2O2 reduced the surfaced accumulation of CFH, CD46, and CD55, which disrupted the barrier function of RPE cells (Thurman et al., 2009) while Wu et al found that CFH expression is decreased by FOXO3 acetylation (Wu et al., 2007). Chen and Forrester found that oxidized lipids reduced CFH production by RPE cells (Chen et al., 2007). Collectively, these studies suggest that the decrease in multiple C3 regulators by oxidative stress could result in dysfunctional C3 activity in AMD. The accumulation of the anaphylatoxins C3a and C5a in drusen of early AMD patients suggests that C3 is abnormally regulated in the basal RPE/Bruch’s membrane (Nozaki et al., 2006).
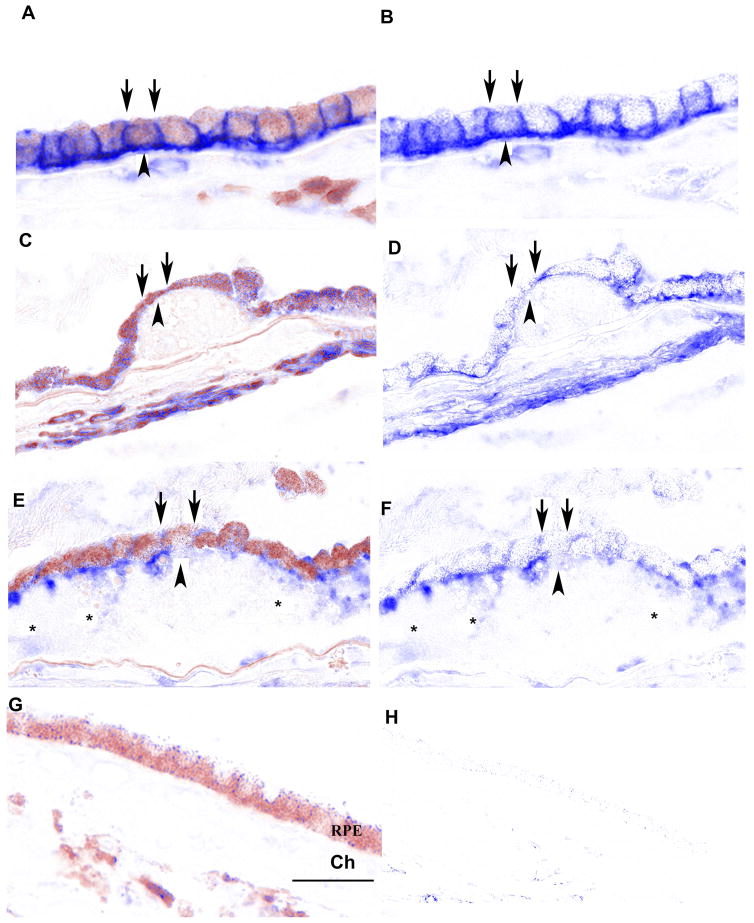
Figure 3.
Distribution of CD46 in the RPE of early AMD eyes. A. Peripheral RPE/choroid from a 94 yr. old Caucasian female with early AMD. CD46 labeling appears as a prominent line at the basal (arrowhead) and lateral (arrows) surfaces of morphologically normal RPE cells. C. Dysmorphic macular RPE cells, from the same patient, adjacent to small druse with reduced (thinner line) or undetectable CD46 labeling at their lateral surface (arrows). The basal line of CD46 labeling (arrowhead) is preserved, but thinned over the small druse. E. 96 year old Caucasian male with early AMD and a large druse (>500 μm in length). RPE cells overlying the large druse are dysmorphic and the uniform line of lateral (arrows) and basal (arrowhead) CD46 labeling is lost. CD46 labeling is diffusely distributed throughout the druse (*). G. IgG control. B, D, F, H. Same images after RPE melanin pigment is subtracted by Nuance software. Ch, choroid; Dr, druse; RPE, retinal pigmented epithelium. Bar=50μm. Reprinted with permission from Journal of Pathology.
CD59 is a glycoprotein regulator that attaches to cells by a GPI anchor that halts C5b-9 formation by preventing C9 forming with the C5b-8 complex. Our lab found that CD59 immunolabeling in the RPE of normal and early AMD eyes is minimal, but strong in the choroidal vascular endothelium, which suggests an important role in controlling the age-related C5b-9 choroidal deposition (Ebrahimi et al., 2012). In early AMD, CD59 labeling is observed at the apex of morphologically normal RPE and within reticular pseudodrusen, and is decreased in dysmorphic RPE overlying drusen. In the transition zone of geographic atrophy, CD59 immunolabeling is weak in morphologically preserved and dysmorphic RPE cells. Like CD46, we found that oxidized lipids induced the release of CD59 in both apoptotic blebs and exosomes, suggesting a mechanism for its decrease in the RPE and accumulation in reticular pseudodrusen (Ebrahimi et al., 2012). Using polarized primary RPE cells and the ABCA4−/− Stargardt mouse model, Tan et al found two early protective responses of CD59 that preserve RPE cell health (Tan et al., 2016). Accelerated recycling of CD59 to the RPE cell surface inhibited C5b-9 formation and fusion of lysosomes with the RPE plasma membrane limited calcium release and preserved mitochondrial function. This process could involve cholesterol accumulation induced by oxidized lipids or vitamin A dimers to activate acid sphingomyelinase, which increased tubulin acetylation to impede organelle traffic. Thus, defective CD59 recycling and lysosomal exocytosis after sublytic C5b-9 could induce mitochondrial fragmentation and RPE dysfunction. Collectively, with reduced CD46, CFH, and CD59, it is possible that the impairment of multiple regulators and not any single factor is needed for complement dysregulation to contribute to AMD lesion formation.
Compelling mechanistic evidence of a role for complement dysregulation was lacking until the recent work by Toomey et al (Toomey et al., 2015). Using aged CFH+/− mice given a high fat diet, this group found that decreased CFH led to increased basal deposit formation and decreased vision, as quantified by ERG changes. Mechanistically, CFH competes with lipoproteins for binding sites in Bruch’s membrane. With decreased CFH, lipoproteins accumulate in Bruch’s membrane, to trigger basal deposit formation. Importantly, basal deposits did not form in CFH−/− mice where complement activity is minimal due to secondary consumption, indicating that dysregulated complement in heterozygous mice, contributed to lesion formation. Thus, decreased CFH contributed to complement activation, leading to RPE damage and visual impairment, and basal deposit formation. Whether other complement regulators were also impaired is unknown in this model, but is certainly possible given the high oxidative stress environment generated by the combination of aging and HFD.
This work illustrates a non-complement regulatory function of CFH, of competing with lipoproteins for binding to Bruch’s membrane (Clark et al., 2010; Clark et al., 2013; Sarrazin et al., 2011). CFH binds to heparan sulfates in Bruch’s membrane at its short consensus repeats (SCR) 6-8 (Clark et al., 2010; Clark et al., 2013). Because the 402H polymorphism occurs within the SCR6-8 region, heparan binding is reduced with 402H CFH. In addition, Keenan et al have shown that the quantity of heparan sulfate decreases in Bruch’s membrane with aging, resulting in fewer binding sites for CFH, which especially affects the ability of the 402H CFH variant to bind Bruch’s membrane (Keenan et al., 2014). Thus, either an age-related reduction in heparan sulfate or the reduced binding by 402H CFH would encourage lipoprotein accumulation in Bruch’s membrane, a key event prior to basal deposit and drusen formation (Curcio et al., 2011). The ocular “response to retention” hypothesis theorizes that retained lipoproteins become oxidized due to the macula’s high oxidative stress environment, and elicit a pro-inflammatory response. Indeed, we previously showed that oxidized lipoproteins accumulate in Bruch’s membrane including drusen in AMD, and that oxidized lipoproteins induce a pro-inflammatory transcriptional response by RPE cells (Yamada et al., 2008).
Pentraxin-3 (PTX-3) is an additional factor that regulates CFH. The pentraxins are an essential component of humoral innate immunity that function as pattern recognition receptors (PRR) which includes the short pentraxin, C-reactive protein (Garlanda et al., 2005). PTX3, which is typically produced by local tissue following a pro-inflammatory signal (Breviario et al., 1992), serves as a PRR that binds to microbes, P-selectin, and complement components. Our lab recently showed that PTX3 is induced in the RPE by oxidative stress, and that PTX3 preferentially binds to CFH over complement C1q (Fig. 4) (Wang et al., 2016). We found that PTX3 deficiency in vitro and in vivo magnified complement activation induced by oxidative stress with increased C3a, FB, and C3d, but not C5b-9 formation. The increased C3a, by interacting with C3aR, induced IL-1β mRNA expression and secretion of its activated form. In addition, PTX3 deficiency augmented NLRP3 inflammasome activation that enhanced IL-1β, but not IL-18 production by the RPE. With PTX3 deficiency, the complement and inflammasome pathways worked in concert to produce IL-1β in sufficient abundance to recruit macrophages to the choroid. These results demonstrated that PTX3 acts as an essential brake for complement and inflammasome activation by regulating the abundance of CFH in the RPE, and provide critical insight into the complex interplay between oxidative stress and innate immunity in the early stages of AMD. Since PTX3 binds equally to the 402H and 402Y CFH alleles (Deban et al., 2008) and competes with heparan for binding to CFH at SCR19-20 (Blackmore et al., 1998; Blackmore et al., 1996), PTX3 could compensate for impaired 402H CFH binding with heparan, and provide a tissue protecting supply of CFH. Under normal conditions, heparan sulfate proteoglycans likely play the main regulatory role on CFH by insuring an adequate supply. Immunohistochemical studies showed that PTX3 localizes to inner Bruch’s membrane, and thus, acts as a reservoir for functional CFH to modulate complement at the RPE. Since PTX3 production is low without stress, PTX3 is unlikely to have a substantive role during unstressed conditions, but increases with oxidative stress. While an age-related decline or maldistribution of heparan or heparan sulfation could alter CFH distribution and hence, complement regulation, PTX3 could compensate for these changes since CFH and PTX3 co-localized during oxidative stress. However, our lab in immunohistochemical studies, showed that PTX3 is decreased in inner Bruch’s membrane and dysmorphic RPE overlying basal deposits and drusen in AMD (Yamada et al., 2008), which suggests that PTX3 could decline with aging or in AMD. If true, the protective reservoir of CFH would decrease with decreased PTX3 and could result in either lipoprotein accumulation or dysregulated complement activation at C3 during AMD development.
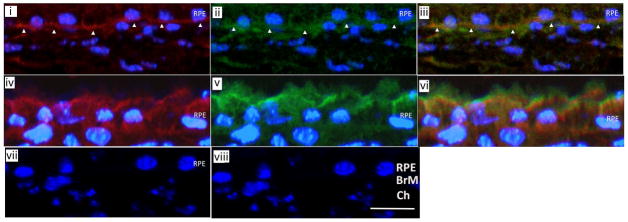
Figure 4.
Immunofluorescence confocal microscopy of a WT mouse given IVit vehicle (n=4) shows linear FH (red) staining (i) and PTX3 (green) staining (ii) in inner BrM (white arrowheads). (iii) Merged image of (i) and (ii) shows FH and PTX3 (yellow) staining overlap. WT mouse given 5 μg 4-HNE IVit (n=4) has diffuse staining in the RPE for FH (red) (iv) and PTX3 (green) (v). (vi) Merged image of (iv) and (v) shows FH and PTX3 (yellow) staining overlap. IgG isotype control for FH (vii) and PTX3 (viii). Reprinted with permission from Journal of Pathology.
CFH has another non-complement function. The Binder lab, with our collaboration, showed that CFH, mediated through SCR7 and SCR20, binds to MDA, which is contained in oxidized LDL (Weismann et al., 2011). In AMD specimens, CFH and MDA co-localized in Bruch’s membrane including drusen, along with C3d, a cleavage product of iC3b, indicating cofactor activity at the same sites. Functionally, CFH binding to MDA prevented a pro-inflammatory response by the RPE that was elicited by the oxidized epitopes. The 402H CFH polymorphism significantly reduced the ability of CFH to bind MDA, thereby suggesting a causal link to AMD etiology. Thus, besides its complement regulatory function, CFH can influence both lipoprotein accumulation and neutralize the deleterious inflammatory effects of MDA by the RPE.
6.2 The inflammasome and its interaction with complement
The inflammasome has emerged as another immunity related factor in non-neovascular AMD pathobiology since the seminal work by Tarallo et al (Tarallo et al., 2012). Inflammasomes are multi-protein complexes that detect cellular danger and implement innate immune defense through activation of IL-1β and IL-18 (Martinon et al., 2002), and recently inactivation of IL-33 (Cayrol and Girard, 2009). The inflammasome can be activated by a number of triggers, notably oxidative stress (Dostert et al., 2008), C5b-9 (Laudisi et al., 2013; Triantafilou et al., 2013), and aluRNAs. Inflammasomes require a priming signal to transcriptionally increase its components (NLRP3, ASC, procaspase-1), and a second activating signal that oligomerizes these components, resulting in caspase-1 activation that cleaves IL-1β and IL-18 for secretion (Schroder and Tschopp, 2010). Tarallo et al demonstrated that alu RNAs, through reduced DICER1, activate the inflammasome in the RPE, with increased IL-18, leading to geographic atrophy (Tarallo et al., 2012). This group followed up with a subsequent study showing that DICER1 deficiency with Alu RNA accumulation resulted in increased IL-18 that led to RPE cell death via caspase-8 activation through a Fas ligand-dependent mechanism (Kim et al., 2014). Doyle et al, on the other hand, reported that inflammasome activation with increased IL-18 protected against choroidal neovascularization in an acute model of AMD (Doyle et al., 2012).
Undoubtedly, inflammasome activation, like most inflammatory responses, is initially protective. What converts its response to tissue injuring? Three factors may be relevant in generating a pathologic response. First, the IL-1β and IL-18 balance is important. IL-1β is a powerful and dominant cytokine that is tightly regulated both at the transcriptional level and by the inflammasome (Dinarello, 1996; Rider et al., 2011). IL-18, which is constitutively expressed and is regulated by the inflammasome (Jordan et al., 2001; Okamura et al., 1998), restrains the pro-inflammatory effects of IL-1β. For example, IL-18 controls the inflammation generated by IL-1β during infection to prevent gastric epithelial cell injury (Hitzler et al., 2012). Secondly, the source (s) of the inflammasome can be relevant. While Inflammasome activation in colon epithelium repairs damaged cells, inflammasome activity by macrophages causes tissue damage (Zaki et al., 2010). This scenario is possible in early AMD where the RPE activates the inflammasome as an initial protective response, and recruits macrophages that accumulate in Bruch’s membrane, potentially adding additional inflammasome activity (Cherepanoff et al., 2010; Kauppinen et al., 2012; Killingsworth et al., 1990; Penfold et al., 1985; Sarks, 1980; Tarallo et al., 2012). Several high quality studies indicate that the NLRP3-inflammasome is activated by the RPE after oxidative stress, such as after exposure to A2E (Anderson et al., 2013) or oxLDLs (Gnanaguru et al., 2016). Tseng et al showed that lysosomal destabilization activated the inflammasome with IL-1β release, which was linked to RPE dysfunction and pyroptotic cell death (Tseng et al., 2013). This finding is significance because autophagy impairment is linked with AMD pathobiology (Kaarniranta et al., 2013; Mitter et al., 2014). Activation of the P2X7 receptor is a widely supported mechanism for inflammasome activation (Rathinam et al., 2012; Schroder and Tschopp, 2010). As in other cell types, Kerur et al showed that P2X7 receptor activation stimulates the inflammasome in the RPE after exposure to Alu RNAs (Kerur et al., 2013). These three triggers, oxidative stress, lysosomal destabilization, and P2X7 receptor activation, like in other cell types, may work in concert to activate the inflammasome in the RPE (Bartlett et al., 2013; Cruz et al., 2007; Rathinam et al., 2012; Schroder and Tschopp, 2010; Wang and Sluyter, 2013). Liu et al recently showed that impaired autophagy, or possibly mitophagy in RPE cells led to recruitment of macrophages with inflammasome activation, which promoted RPE and photoreceptor degeneration (Liu et al., 2016). Thirdly, the duration of inflammasome activity is an important determinant. With inadequate resolution of the inciting insult, chronic inflammasome activity converts the acute protective response into disease. Part of this conversion is mediated through IL-18, which has opposite effects through IL-11 (i.e. pro-proliferative) and IFN-gamma (i.e. anti-proliferative) during acute and chronic inflammation, respectively (Dupaul-Chicoine et al., 2010; Nava et al., 2010; Reuter and Pizarro, 2004; Zaki et al., 2010). The inflammasome, through IL-18, prevented neovascularization using the acute laser model (Doyle et al., 2012), but contributed to RPE cell loss during a chronic model of geographic atrophy (Tarallo et al., 2012). The different outcomes by IL-18 might be explained by the acute and chronic duration of these experiments. Both of these models had low IL-1β since they lacked an obvious IL-1β priming signal. This is in contrast to our work showing increased IL-1β after C3a generation with PTX3 deficiency. Since Cao et al have shown strong IL-1β and IL-18 in the RPE of AMD (Cao et al., 2016), the additional induction of IL-1β could influence the AMD phenotype by upsetting the IL-1β/IL-18 balance to generate a deleterious pro-inflammatory microenvironment.
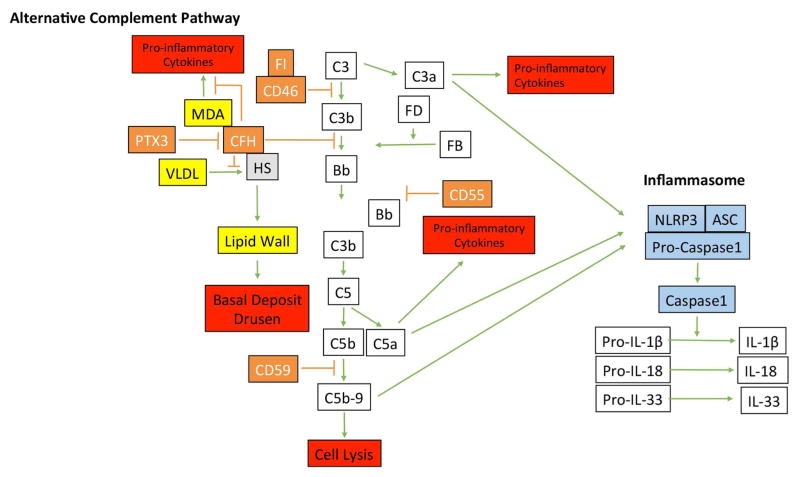
While many studies focus on the impact either of complement or inflammasome activation, several studies indicate that these arms interact with each other. For example, C3a induces ATP release (Asgari et al., 2013) which prompts the P2X7 receptor to associate with NLRP3 and activate the inflammasome (Franceschini et al., 2015). Given the impaired C3 regulators, C3a abundance is a plausible link to inflammasome activation. Likewise, Brandstetter et al have recently shown that C5a acts as a priming signal for RPE cell inflammasome activation with IL-1β secretion after lipofuscin mediated photo-oxidative damage (Brandstetter et al., 2015). This same group also showed that C5a can activate the inflammasome in RPE cells, which increases its susceptibility to photo-oxidative mediated cell death through pyroptosis (Brandstetter et al., 2016). Finally, sublytic C5b-9 is known to activate the inflammasome (Laudisi et al., 2013; Triantafilou et al., 2013), providing an additional possible link between complement and inflammasome activation. Given the accumulation of C5b-9 in the choriocapillaris with aging (Mullins et al., 2014), the potential for sublytic C5b-9 to induce mitochondrial fragmentation and lysosomal exocytosis with RPE dysfunction from defective CD59 recycling (Tan et al., 2016), the documented reduction in CD59 in the RPE in AMD (Ebrahimi et al., 2012), C5b-9 mediated inflammasome activation warrants further investigation. Figure 5 summarizes the elements of the alternative complement pathway, its potential impact on AMD lesion formation, and its potential interaction with the inflammasome.
Figure 5.
Diagram of the alternative complement pathway and the inflammasome. Activation of the alternative complement pathway is regulated by factor H (CFH), CD46, CD55. PTX3, by binding CFH, controls the abundance of CFH. Besides its regulation of complement, CFH binds to malondialdehyde (MDA) to control MDA-mediated pro-inflammatory cytokine production, and heparan sulfates (HS) to compete with very low density lipoproteins (VLDL) that can become deposited in Bruch’s membrane prior to basal deposit or drusen formation. Complement activation can lead to anaphylatoxins generation of C3a and C5a, which can induce pro-inflammatory cytokine production, and C5b-9 complex formation, which is regulated by CD59. C3a, C5a, and C5b-9 complexes are implicated in activating the inflammasome, composed of NLRP3, ASP, and Pro-caspase1, which upon activation, is cleaved to convert pro-IL-1β, pro-IL-18, and pro-IL-33 into their active forms.
7. Does Oxidative stress cause inflammation or does inflammation induce oxidative stress?
While inflammation can produce oxidative stress, compelling evidence indicates that oxidative stress induces inflammation during the development of AMD. Inadequately neutralized oxidative stress can lead to oxidatively damaged proteins, lipids and DNA, which results in structural changes to these molecules. These oxidative modifications can generate novel “oxidation-specific epitopes” (OSE), which can induce pro-inflammatory responses in vitro and in vivo (Berliner et al., 2001). OSEs have been identified in the macula with AMD. In the retina, docosohexanoic acid, the most abundant fatty acid in photoreceptor tips, is specifically oxidized to carboxyethylpyrrole (CEP), which “tag” oxidatively damaged photoreceptors in AMD (Gu et al., 2003; Sun et al., 2005). The RPE contains multiple OSEs including MDA and 4-HNE adducts, as well as AGEs (Schutt et al., 2003; Schutt et al., 2002), while Bruch’s membrane, including drusen, contains AGEs, MDA, CEP, and oxidized phospholipids (OxPL) (Crabb et al., 2002; Farboud et al., 1999; Sun et al., 2005; Suzuki et al., 2007).
The identification of these OSEs is significant because OSEs are recognized as “danger signals” by PRRs (Chou et al., 2008). Indeed, OSEs can activate both innate and adaptive immunity (Binder et al., 2004; Chou et al., 2008). This initial response is intended to neutralize the OSE in order to prevent tissue injury, and is thus, protective. However, inadequate neutralization of the OSE can convert the protective immune reaction into a pathologic response. For example, as mentioned above, by binding MDA, CFH neutralizes the pro-inflammatory response generated by MDA. However, the 402H CFH polymorphism, which confers increased risk for AMD, has reduced binding to MDA, potentially inducing a chronic pro-inflammatory response that can be tissue injuring (Weismann et al., 2011).
Our lab has been interested in the impact of oxidized lipoproteins that accumulate in Bruch’s membrane in early AMD (Yamada et al., 2008). We showed that AGEs induced RPE cell lipoprotein lipase production, which after being secreted into Bruch’s membrane, retains lipoproteins (Cano et al., 2011) which encourages their oxidation. Oxidized phospholipids (OxPL), a component of oxidized lipoproteins, are a prominent consequence of lipid peroxidation of the highly polyunsaturated fatty acids found in the photoreceptors, and are formed in cells undergoing apoptosis, a known mechanism of cell death in AMD (Chang et al., 2004). One OxPL species is the oxidation of the phosphocholine (PC) moiety of phospholipids, which are detected and bound by the IgM natural antibody E06, but not to the PC moiety of unoxidized phospholipids (Friedman et al., 2002; Shaw et al., 2000). We found that this particular OxPL, which is highly abundant in oxidized lipoproteins, accumulates in Bruch’s membrane in early AMD (Handa et al., 2015). One possible PRR that can neutralize this OxPL in Bruch’s membrane is lipoprotein (a), or Lp(a), which is composed of apolipoprotein (a) [apo(a)] covalently bound to apolipoprotein B-100 (apoB) (Dube et al., 2012; Hoover-Plow and Huang, 2013; Kronenberg and Utermann, 2013). Using “mutant Lp(a)” mice with a defective apo(a) lysine binding site for oxidized phospholipids, we found that these mice, when fed a high fat diet for 12 months, developed RPE cell degeneration, basal deposits, and drusen. These changes were associated with increased OxPL, decreased antioxidant genes, increased complement, and decreased complement regulators in the RPE compared to wild-type Lp(a) mice, which developed age-related changes when given a high fat diet. Importantly, Lp(a) appears to localize in Bruch’s membrane including drusen, with OxPL in AMD specimens.
8. Therapeutic Considerations: Current Treatment Recommendations and Future Directions
In the United States, the current recommended treatment for dry AMD is based on the two Age-related Eye Disease Study (AREDS) trials. For patients with early AMD, no treatment is recommended because the AREDS found no benefit with high dose antioxidant micronutrients (Age-Related Eye Disease Study Research, 2001). For patients with intermediate AMD, the AREDS found that daily doses of vitamin C (500 mg), vitamin E (400 IU), β-carotene (15 mg), zinc (80 mg as zinc oxide), and copper (2 mg as cupric oxide) reduced the progression rate to advanced AMD by 25% at 5 years, and the risk of losing vision of three or more lines (doubling of the visual angle) by 19% (Age-Related Eye Disease Study Research, 2001). The AREDS was followed up with AREDS2, where patients were randomized to receive the AREDS formulation with supplemental lutein and zeaxanthin, supplemental omega-3, or the original formulation alone (2013). A secondary randomization to four variations included elimination of β-carotene, lower zinc levels (25 mg), or both. The AREDS2 found no treatment benefit between the original formulation and the AREDS2 modifications. Because the simultaneous use of β-carotene with lutein and zeaxanthin decreases the absorption of the nutrients, plus the higher incidence of lung cancer seen in the β-carotene group that was not observed with lutein and zeaxanthin supplementation, the study concluded that lutein and zeaxanthin represent an appropriate substitute for β-carotene in the supplement, and that there was no detrimental effect of lowering the zinc levels (25 mg). The current recommendation for patients with intermediate AMD is to use the AREDS2 formulation.
The use of antioxidant micronutrients is not uniformly endorsed. A meta-analysis of clinical trials from three large, high quality studies that involved over 23,000 patients with AMD found that antioxidants did not prevent AMD (Evans, 2008). Several components of the AREDS formulation can have unanticipated health risks. For example, vitamin E was associated with heart failure in patients with vascular disease or diabetes mellitus in the Heart Outcomes Prevention Evaluation Study (Lonn et al., 2005) and increased prostate cancer after 7 years in the Selenium and Vitamin E Cancer Prevention Trial (Klein et al., 2011). Elevated zinc has been associated with neurosensory retinal cell death after light exposure (Sheline et al., 2010), and in proteins recovered from drusen (Lengyel et al., 2007) and amyloid plaques of Alzheimer’s patients (Bucciarelli et al., 2002; Lee et al., 1999). In Alzheimer’s disease, zinc chelation has been proposed as a therapy (Lee et al., 2004) in contrast to zinc supplement in the AREDS formulation, which was chosen for its potential antioxidant properties. Given the presence of zinc in drusen and its role in retinal neurotoxicity, some researchers have questioned the use of zinc supplementation for AMD treatment. While considered the best formulation among experts at the time, the AREDS composition was empiric and was not derived from pre-clinical investigation.
Preclinical investigation of inhibiting complement activation has translated into clinical trials. Currently, several trials at different phases are testing the benefit of complement inhibition for non-neovascular AMD. Since the FDA now allows geographic atrophy growth as a surrogate to visual acuity as a primary endpoint, most studies are evaluating geographic atrophy rather than early AMD. At present, the most promising drug is lampalizumab (Roche, Inc.), a humanized anti-factor D monoclonal antibody. In the phase II trial, which was presented at the 31st Annual Meeting of the American Society of Retina Specialists, there was a 20.4% reduction (unconventional p=0.2 significance) in the rate of geographic atrophy growth after 18 months of monthly intravitreal injections of lampalizumab without unexpected or unmanageable serious adverse events. In patients with the factor I AMD risk allele, the GA progression rate was decreased by 44 percent (p<0.005) at 18 months. These data prompted progression to the phase III trial, which is ongoing. APL-2 (Apellis Pharmaceuticals) inhibits C3 activation and thus, blocks all three arms of the complement pathway. APL-2 is a reformulated version of POT-4 (Potentia), which was found to be safe and well tolerated after intravitreal injection in phase I testing. A randomized phase II trial testing APL-2 by monthly or every other month injections for 1 year is now being investigated for treatment of geographic atrophy. LFG316 (Alcon), an anti-C5 monoclonal antibody, is being currently being investigated as either monotherapy, or in combination with CLG561, a properdin inhibitor for the treatment of geographic atrophy. Of note, CLG561 alone, did not slow geographic atrophy enlargement in a phase II study, which was presented at the 2016 Angiogenesis, Exudation, and Degeneration meeting.
What does the future hold? Will targeting elements of oxidative stress replace the antioxidant micronutrients of the AREDS? Oxidative stress targets that rejuvenate endogenous intracellular antioxidant systems have conceptual merit because the oxidative stress can be neutralized within cells where ROS are generated. Nr2 as a target has enjoyed some success with dimethyl fumarate, an Nrf2 activator, in the DEFINE study, which reduced the proportion of patients who had a relapse, the annualized relapse rate, the rate of disability progression, and the number of lesions on MRI for the treatment of relapsing multiple sclerosis (Gold et al., 2012). On the other hand, Bardoloxone, a triterpenoid that also activates Nrf2, was halted in a phase III trial that tested whether it reduced the risk of end-stage renal disease (ESRD) or death from cardiovascular causes among patients with type 2 diabetes mellitus and stage 4 chronic kidney disease because of unacceptable adverse effects (de Zeeuw et al., 2013). These systemic complications could potentially be minimized with intravitreal injections for treating AMD. With the development of nanoparticles, the possibility of more targeted treatment with sustained release is possible. For example, dendrimers can be formulated to specifically target either RPE cells or macrophages/microglia (Kambhampati et al., 2015). When Triamcinolone acetonide (TA) is incorporated into dendrimers, the anti-inflammatory activity is improved 100-fold more than free TA in macrophages. Will other anti-inflammatory targets of complement, PTX3, or the inflammasome emerge? Will future treatment target both oxidative stress and inflammation at the same time? These questions will emerge as we develop a deeper understanding of the role of oxidative stress and inflammation in the RPE.
Acknowledgments
The authors acknowledge the funding support from the National Institutes of Health (EY14005 (JTH), EY019044 (JTH)), RPB Senior Scientist Award (JTH), Wilmer Core Grant EY001765, Unrestricted Grant from RPB (Wilmer Eye Institute), and by the Merlau family and Aleda Wright. Dr. Handa is the Robert Bond Welch Professor.
Footnotes
Conflict of Interest: JTH and MC have received funding from Bayer Pharmaceuticals, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 2.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999;44:1–29. doi: 10.1016/s0039-6257(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 3.Ach T, Huisingh C, McGwin G, Jr, Messinger JD, Zhang T, Bentley MJ, Gutierrez DB, Ablonczy Z, Smith RT, Sloan KR, Curcio CA. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014;55:4832–4841. doi: 10.1167/iovs.14-14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ach T, Tolstik E, Messinger JD, Zarubina AV, Heintzmann R, Curcio CA. Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:3242–3252. doi: 10.1167/iovs.14-16274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends in cell biology. 2000;10:17–24. doi: 10.1016/s0962-8924(99)01673-6. [DOI] [PubMed] [Google Scholar]
- 6.Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson OA, Finkelstein A, Shima DT. A2E induces IL-1ss production in retinal pigment epithelial cells via the NLRP3 inflammasome. PLoS One. 2013;8:e67263. doi: 10.1371/journal.pone.0067263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995;15:183–191. [PubMed] [Google Scholar]
- 10.Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Kohl J, Cook HT, Kemper C. C3a modulates IL-1beta secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122:3473–3481. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- 11.Augustin A, Sahel JA, Bandello F, Dardennes R, Maurel F, Negrini C, Hieke K, Berdeaux G. Anxiety and depression prevalence rates in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:1498–1503. doi: 10.1167/iovs.06-0761. [DOI] [PubMed] [Google Scholar]
- 12.Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 13.Bartlett R, Yerbury JJ, Sluyter R. P2X7 receptor activation induces reactive oxygen species formation and cell death in murine EOC13 microglia. Mediators of inflammation. 2013;2013:271813. doi: 10.1155/2013/271813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 15.Berenberg TL, Metelitsina TI, Madow B, Dai Y, Ying GS, Dupont JC, Grunwald L, Brucker AJ, Grunwald JE. The association between drusen extent and foveolar choroidal blood flow in age-related macular degeneration. Retina. 2012;32:25–31. doi: 10.1097/IAE.0b013e3182150483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berliner JA, Subbanagounder G, Leitinger N, Watson AD, Vora D. Evidence for a role of phospholipid oxidation products in atherogenesis. Trends Cardiovasc Med. 2001;11:142–147. doi: 10.1016/s1050-1738(01)00098-6. [DOI] [PubMed] [Google Scholar]
- 17.Berman K, Brodaty H. Psychosocial effects of age-related macular degeneration. Int Psychogeriatr. 2006;18:415–428. doi: 10.1017/S1041610205002905. [DOI] [PubMed] [Google Scholar]
- 18.Bhutto IA, Baba T, Merges C, Juriasinghani V, McLeod DS, Lutty GA. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br J Ophthalmol. 2011;95:1323–1330. doi: 10.1136/bjo.2010.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhutto IA, McLeod DS, Jing T, Sunness JS, Seddon JM, Lutty GA. Increased choroidal mast cells and their degranulation in age-related macular degeneration. Br J Ophthalmol. 2016;100:720–726. doi: 10.1136/bjophthalmol-2015-308290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 21.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biswas SK, Rahman I. Molecular aspects of medicine. 2008. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackmore TK, Hellwage J, Sadlon TA, Higgs N, Zipfel PF, Ward HM, Gordon DL. Identification of the second heparin-binding domain in human complement factor H. J Immunol. 1998;160:3342–3348. [PubMed] [Google Scholar]
- 24.Blackmore TK, Sadlon TA, Ward HM, Lublin DM, Gordon DL. Identification of a heparin binding domain in the seventh short consensus repeat of complement factor H. J Immunol. 1996;157:5422–5427. [PubMed] [Google Scholar]
- 25.Blasiak J, Glowacki S, Kauppinen A, Kaarniranta K. Mitochondrial and nuclear DNA damage and repair in age-related macular degeneration. Int J Mol Sci. 2013;14:2996–3010. doi: 10.3390/ijms14022996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandstetter C, Holz FG, Krohne TU. Complement Component C5a Primes Retinal Pigment Epithelial Cells for Inflammasome Activation by Lipofuscin-mediated Photooxidative Damage. J Biol Chem. 2015;290:31189–31198. doi: 10.1074/jbc.M115.671180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandstetter C, Patt J, Holz FG, Krohne TU. Inflammasome priming increases retinal pigment epithelial cell susceptibility to lipofuscin phototoxicity by changing the cell death mechanism from apoptosis to pyroptosis. J Photochem Photobiol B. 2016;161:177–183. doi: 10.1016/j.jphotobiol.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994;14:130–142. [PubMed] [Google Scholar]
- 29.Breviario F, d’Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- 30.Brody BL, Gamst AC, Williams RA, Smith AR, Lau PW, Dolnak D, Rapaport MH, Kaplan RM, Brown SI. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108:1893–1900. doi: 10.1016/s0161-6420(01)00754-0. discussion 1900–1891. [DOI] [PubMed] [Google Scholar]
- 31.Brown GC, Brown MM, Sharma S, Stein JD, Roth Z, Campanella J, Beauchamp GR. The burden of age-related macular degeneration: a value-based medicine analysis. Trans Am Ophthalmol Soc. 2005a;103:173–184. discussion 184–176. [PMC free article] [PubMed] [Google Scholar]
- 32.Brown MM, Brown GC, Stein JD, Roth Z, Campanella J, Beauchamp GR. Age-related macular degeneration: economic burden and value-based medicine analysis. Can J Ophthalmol. 2005b;40:277–287. doi: 10.1016/S0008-4182(05)80070-5. [DOI] [PubMed] [Google Scholar]
- 33.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 35.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 37.Cano M, Fijalkowski N, Kondo N, Dike S, Handa J. Advanced Glycation Endproduct Changes to Bruch’s Membrane Promotes Lipoprotein Retention by Lipoprotein Lipase. Am J Pathol. 2011;179:850–859. doi: 10.1016/j.ajpath.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cano M, Thimmalappula R, Fujihara M, Nagai N, Sporn M, Wang AL, Neufeld AH, Biswal S, Handa JT. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision Res. 2010;50:652–664. doi: 10.1016/j.visres.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canter JA, Olson LM, Spencer K, Schnetz-Boutaud N, Anderson B, Hauser MA, Schmidt S, Postel EA, Agarwal A, Pericak-Vance MA, Sternberg P, Jr, Haines JL. Mitochondrial DNA polymorphism A4917G is independently associated with age-related macular degeneration. PLoS ONE. 2008;3:e2091. doi: 10.1371/journal.pone.0002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao S, Wang JC, Gao J, Wong M, To E, White VA, Cui JZ, Matsubara JA. CFH Y402H polymorphism and the complement activation product C5a: effects on NF-kappaB activation and inflammasome gene regulation. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2015-307213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centre for Eye Research Australia. Access Australia. 2006. Centrally Focused: The Impact of Age-Related Macular Degeneration. [Google Scholar]
- 43.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen A, Raule N, Chomyn A, Attardi G. Decreased reactive oxygen species production in cells with mitochondrial haplogroups associated with longevity. PLoS One. 2012;7:e46473. doi: 10.1371/journal.pone.0046473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen JC, Fitzke FW, Pauleikhoff D, Bird AC. Functional loss in age-related Bruch’s membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992;33:334–340. [PubMed] [Google Scholar]
- 46.Chen M, Forrester JV, Xu H. Synthesis of complement factor H by retinal pigment epithelial cells is down-regulated by oxidized photoreceptor outer segments. Exp Eye Res. 2007;84:635–645. doi: 10.1016/j.exer.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Chen M, Rajapakse D, Fraczek M, Luo C, Forrester JV, Xu H. Retinal pigment epithelial cell multinucleation in the aging eye - a mechanism to repair damage and maintain homoeostasis. Aging Cell. 2016;15:436–445. doi: 10.1111/acel.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010;94:918–925. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- 49.Chiu CJ, Chang ML, Zhang FF, Li T, Gensler G, Schleicher M, Taylor A. The relationship of major American dietary patterns to age-related macular degeneration. Am J Ophthalmol. 2014;158:118–127. e111. doi: 10.1016/j.ajo.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chou MY, Hartvigsen K, Hansen LF, Fogelstrand L, Shaw PX, Boullier A, Binder CJ, Witztum JL. Oxidation-specific epitopes are important targets of innate immunity. J Intern Med. 2008;263:479–488. doi: 10.1111/j.1365-2796.2008.01968.x. [DOI] [PubMed] [Google Scholar]
- 51.Clark SJ, Perveen R, Hakobyan S, Morgan BP, Sim RB, Bishop PN, Day AJ. Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch’s membrane in human retina. J Biol Chem. 2010;285:30192–30202. doi: 10.1074/jbc.M110.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark SJ, Ridge LA, Herbert AP, Hakobyan S, Mulloy B, Lennon R, Wurzner R, Morgan BP, Uhrin D, Bishop PN, Day AJ. Tissue-specific host recognition by complement factor H is mediated by differential activities of its glycosaminoglycan-binding regions. J Immunol. 2013;190:2049–2057. doi: 10.4049/jimmunol.1201751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol. 2007;91:354–359. doi: 10.1136/bjo.2006.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 55.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cross CE, O’Neill CA, Reznick AZ, Hu ML, Marcocci L, Packer L, Frei B. Cigarette smoke oxidation of human plasma constituents. Ann N Y Acad Sci. 1993;686:72–89. doi: 10.1111/j.1749-6632.1993.tb39157.x. discussion 89–90. [DOI] [PubMed] [Google Scholar]
- 57.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cruz-Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, Betancourt M, Viteri E, Ramkhellawan GC, Ewald E, Feuer W, Huang D, Wen R, Hong L, Wang H, Laird JM, Sene A, Apte RS, Salomon RG, Hollyfield JG, Perez VL. Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. Int J Inflam. 2013;2013:503725. doi: 10.1155/2013/503725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Curcio CA, Johnson M, Huang JD, Rudolf M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J Lipid Res. 2010;51:451–467. doi: 10.1194/jlr.R002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–1249. [PubMed] [Google Scholar]
- 62.Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- 63.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3278–3296. [PubMed] [Google Scholar]
- 64.Curcio CA, Presley JB, Malek G, Medeiros NE, Avery DV, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res. 2005;81:731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. The Journal of comparative neurology. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 66.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM Investigators BT. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deban L, Jarva H, Lehtinen MJ, Bottazzi B, Bastone A, Doni A, Jokiranta TS, Mantovani A, Meri S. Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J Immunol. 2008;181:8433–8440. doi: 10.4049/jimmunol.181.12.8433. [DOI] [PubMed] [Google Scholar]
- 68.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 69.Dinkova-Kostova AT, Baird L, Holmstrom KM, Meyer CJ, Abramov AY. The spatiotemporal regulation of the Keap1-Nrf2 pathway and its importance in cellular bioenergetics. Biochem Soc Trans. 2015;43:602–610. doi: 10.1042/BST20150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 71.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC, O’Neill LA, Hollyfield JG, Humphries P. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012 doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dube JB, Boffa MB, Hegele RA, Koschinsky ML. Lipoprotein(a): more interesting than ever after 50 years. Current opinion in lipidology. 2012;23:133–140. doi: 10.1097/MOL.0b013e32835111d8. [DOI] [PubMed] [Google Scholar]
- 74.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, Vallance BA, Saleh M. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 75.Eandi CM, Charles Messance H, Augustin S, Dominguez E, Lavalette S, Forster V, Hu SJ, Siquieros L, Craft CM, Sahel JA, Tadayoni R, Paques M, Guillonneau X, Sennlaub F. Subretinal mononuclear phagocytes induce cone segment loss via IL-1beta. Elife. 2016:5. doi: 10.7554/eLife.16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased Membrane Complement Regulators in the Retinal Pigmented Epithelium Contributes to Age-Related Macular Degeneration. The Journal of pathology. 2012 doi: 10.1002/path.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Edwards AO, Ritter R, Iii, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement Factor H Polymorphism and Age-Related Macular Degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 78.Eisner A, Klein ML, Zilis JD, Watkins MD. Visual function and the subsequent development of exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 1992;33:3091–3102. [PubMed] [Google Scholar]
- 79.Eisner A, Stoumbos VD, Klein ML, Fleming SA. Relations between fundus appearance and function. Eyes whose fellow eye has exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 1991;32:8–20. [PubMed] [Google Scholar]
- 80.Ershov AV, Bazan NG. Photoreceptor phagocytosis selectively activates PPARgamma expression in retinal pigment epithelial cells. J Neurosci Res. 2000;60:328–337. doi: 10.1002/(SICI)1097-4547(20000501)60:3<328::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 81.Evans J. Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis. Eye (Lond) 2008;22:751–760. doi: 10.1038/eye.2008.100. [DOI] [PubMed] [Google Scholar]
- 82.Fagerness JA, Maller JB, Neale BM, Reynolds RC, Daly MJ, Seddon JM. Variation near complement factor I is associated with risk of advanced AMD. Eur J Hum Genet. 2009;17:100–104. doi: 10.1038/ejhg.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farboud B, Aotaki-Keen A, Miyata T, Hjelmeland LM, Handa JT. Development of a polyclonal antibody with broad epitope specificity for advanced glycation endproducts and localization of these epitopes in Bruch’s membrane of the aging eye. Mol Vis. 1999;5:11. [PubMed] [Google Scholar]
- 84.Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Balacco Gabrieli C. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006;27:983–993. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 85.Filomeni G, Desideri E, Cardaci S, Rotilio G, Ciriolo MR. Under the ROS…thiol network is the principal suspect for autophagy commitment. Autophagy. 2010;6:999–1005. doi: 10.4161/auto.6.7.12754. [DOI] [PubMed] [Google Scholar]
- 86.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Franceschini A, Capece M, Chiozzi P, Falzoni S, Sanz JM, Sarti AC, Bonora M, Pinton P, Di Virgilio F. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J. 2015 doi: 10.1096/fj.14-268714. [DOI] [PubMed] [Google Scholar]
- 88.Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta. 2012;1823:2297–2310. doi: 10.1016/j.bbamcr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 89.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 90.Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J Biol Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 91.Fujihara M, Cano M, Handa JT. Mice that produce ApoB100 lipoproteins in the RPE do not develop drusen yet are still a valuable experimental system. Invest Ophthalmol Vis Sci. 2014;55:7285–7295. doi: 10.1167/iovs.14-15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fujihara M, Nagai N, Sussan TE, Biswal S, Handa JT. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS ONE. 2008;3:e3119. doi: 10.1371/journal.pone.0003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci U S A. 2004;101:10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annual review of immunology. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 95.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gnanaguru G, Choi AR, Amarnani D, D’Amore PA. Oxidized Lipoprotein Uptake Through the CD36 Receptor Activates the NLRP3 Inflammasome in Human Retinal Pigment Epithelial Cells. Invest Ophthalmol Vis Sci. 2016;57:4704–4712. doi: 10.1167/iovs.15-18663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT Investigators DS. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 99.Golestaneh N, Chu Y, Cheng SK, Cao H, Poliakov E, Berinstein DM. Repressed SIRT1/PGC-1alpha pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration. J Transl Med. 2016;14:344. doi: 10.1186/s12967-016-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- 101.Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 102.Guidry C, Medeiros NE, Curcio CA. Phenotypic variation of retinal pigment epithelium in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:267–273. [PubMed] [Google Scholar]
- 103.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 104.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 106.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement Factor H Variant Increases the Risk of Age-Related Macular Degeneration. Science. 2005 doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 107.Ham WT, Jr, Ruffolo JJ, Jr, Mueller HA, Clarke AM, Moon ME. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Invest Ophthalmol Vis Sci. 1978;17:1029–1035. [PubMed] [Google Scholar]
- 108.Hamel CP, Meunier I, Arndt C, Ben Salah S, Lopez S, Bazalgette C, Bazalgette C, Zanlonghi X, Arnaud B, Defoort-Dellhemmes S, Puech B. Extensive macular atrophy with pseudodrusen-like appearance: a new clinical entity. Am J Ophthalmol. 2009;147:609–620. doi: 10.1016/j.ajo.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 109.Handa JT, Tagami M, Ebrahimi K, Leibundgut G, Janiak A, Witztum JL, Tsimikas S. Lipoprotein(A) with An Intact Lysine Binding Site Protects the Retina From an Age-Related Macular Degeneration Phenotype in Mice (An American Ophthalmological Society Thesis) Trans Am Ophthalmol Soc. 2015;113:T51–T522. [PMC free article] [PubMed] [Google Scholar]
- 110.Hartmann KI, Bartsch DU, Cheng L, Kim JS, Gomez ML, Klein H, Freeman WR. Scanning laser ophthalmoscope imaging stabilized microperimetry in dry age-related macular degeneration. Retina. 2011;31:1323–1331. doi: 10.1097/IAE.0b013e31820a6850. [DOI] [PubMed] [Google Scholar]
- 111.Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol. 2014;171:1917–1942. doi: 10.1111/bph.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, Muller A. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1beta and IL-18. J Immunol. 2012;188:3594–3602. doi: 10.4049/jimmunol.1103212. [DOI] [PubMed] [Google Scholar]
- 113.Hogan MJ. Role of the retinal pigment epithelium in macular disease. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:64–80. [PubMed] [Google Scholar]
- 114.Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002;43:1673–1685. [PubMed] [Google Scholar]
- 115.Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 2013;62:479–491. doi: 10.1016/j.metabol.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iwama D, Tsujikawa A, Ojima Y, Nakanishi H, Yamashiro K, Tamura H, Ooto S, Yoshimura N. Relationship between retinal sensitivity and morphologic changes in eyes with confluent soft drusen. Clin Experiment Ophthalmol. 2010;38:483–488. doi: 10.1111/j.1442-9071.2010.02294.x. [DOI] [PubMed] [Google Scholar]
- 117.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 118.Jensen PK. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. II. Steroid effects. Biochim Biophys Acta. 1966;122:167–174. doi: 10.1016/0926-6593(66)90058-0. [DOI] [PubMed] [Google Scholar]
- 119.Johansson I, Monsen VT, Pettersen K, Mildenberger J, Misund K, Kaarniranta K, Schonberg S, Bjorkoy G. The marine n-3 PUFA DHA evokes cytoprotection against oxidative stress and protein misfolding by inducing autophagy and NFE2L2 in human retinal pigment epithelial cells. Autophagy. 2015;11:1636–1651. doi: 10.1080/15548627.2015.1061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 121.Jones DP. Redox theory of aging. Redox Biol. 2015;5:71–79. doi: 10.1016/j.redox.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jordan JA, Guo RF, Yun EC, Sarma V, Warner RL, Crouch LD, Senaldi G, Ulich TR, Ward PA. Role of IL-18 in acute lung inflammation. J Immunol. 2001;167:7060–7068. doi: 10.4049/jimmunol.167.12.7060. [DOI] [PubMed] [Google Scholar]
- 123.Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Vereb Z, Salminen A, Boulton ME, Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kambhampati SP, Mishra MK, Mastorakos P, Oh Y, Lutty GA, Kannan RM. Intracellular delivery of dendrimer triamcinolone acetonide conjugates into microglial and human retinal pigment epithelial cells. Eur J Pharm Biopharm. 2015;95:239–249. doi: 10.1016/j.ejpb.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2007;104:16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karunadharma PP, Nordgaard CL, Olsen TW, Ferrington DA. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:5470–5479. doi: 10.1167/iovs.10-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kauppinen A, Niskanen H, Suuronen T, Kinnunen K, Salminen A, Kaarniranta K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells--implications for age-related macular degeneration (AMD) Immunol Lett. 2012;147:29–33. doi: 10.1016/j.imlet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 130.Keenan TD, Pickford CE, Holley RJ, Clark SJ, Lin W, Dowsey AW, Merry CL, Day AJ, Bishop PN. Age-dependent changes in heparan sulfate in human Bruch’s membrane: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:5370–5379. doi: 10.1167/iovs.14-14126. [DOI] [PubMed] [Google Scholar]
- 131.Kerur N, Hirano Y, Tarallo V, Fowler BJ, Bastos-Carvalho A, Yasuma T, Yasuma R, Kim Y, Hinton DR, Kirschning CJ, Gelfand BD, Ambati J. TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest Ophthalmol Vis Sci. 2013;54:7395–7401. doi: 10.1167/iovs.13-12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye. 1990;4(Pt 4):613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 133.Kim Y, Tarallo V, Kerur N, Yasuma T, Gelfand BD, Bastos-Carvalho A, Hirano Y, Yasuma R, Mizutani T, Fowler BJ, Li S, Kaneko H, Bogdanovich S, Ambati BK, Hinton DR, Hauswirth WW, Hakem R, Wright C, Ambati J. DICER1/Alu RNA dysmetabolism induces Caspase-8-mediated cell death in age-related macular degeneration. Proc Natl Acad Sci U S A. 2014;111:16082–16087. doi: 10.1073/pnas.1403814111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim YH, He S, Kase S, Kitamura M, Ryan SJ, Hinton DR. Regulated secretion of complement factor H by RPE and its role in RPE migration. Graefes Arch Clin Exp Ophthalmol. 2009;247:651–659. doi: 10.1007/s00417-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kinnunen K, Petrovski G, Moe MC, Berta A, Kaarniranta K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012;90:299–309. doi: 10.1111/j.1755-3768.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 136.Kinugasa H, Whelan KA, Tanaka K, Natsuizaka M, Long A, Guo A, Chang S, Kagawa S, Srinivasan S, Guha M, Yamamoto K, St Clair DK, Avadhani NG, Diehl JA, Nakagawa H. Mitochondrial SOD2 regulates epithelial-mesenchymal transition and cell populations defined by differential CD44 expression. Oncogene. 2015;34:5229–5239. doi: 10.1038/onc.2014.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kitagishi Y, Nakano N, Ogino M, Ichimura M, Minami A, Matsuda S. PINK1 signaling in mitochondrial homeostasis and in aging (Review) Int J Mol Med. 2017;39:3–8. doi: 10.3892/ijmm.2016.2827. [DOI] [PubMed] [Google Scholar]
- 138.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, Sangiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science. 2005 doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Molecular and cellular biology. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 142.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS letters. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 143.Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med. 2013;273:6–30. doi: 10.1111/j.1365-2796.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 144.Kurihara T, Westenskow PD, Gantner ML, Usui Y, Schultz A, Bravo S, Aguilar E, Wittgrove C, Friedlander M, Paris LP, Chew E, Siuzdak G, Friedlander M. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife. 2016:5. doi: 10.7554/eLife.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Laudisi F, Spreafico R, Evrard M, Hughes TR, Mandriani B, Kandasamy M, Morgan BP, Sivasankar B, Mortellaro A. Cutting edge: The NLRP3 inflammasome links complementmediated inflammation and IL-1beta release. J Immunol. 2013;191:1006–1010. doi: 10.4049/jimmunol.1300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 148.Lee JY, Friedman JE, Angel I, Kozak A, Koh JY. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol Aging. 2004;25:1315–1321. doi: 10.1016/j.neurobiolaging.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 149.Lee MY, Yoon J, Ham DI. Clinical characteristics of reticular pseudodrusen in Korean patients. Am J Ophthalmol. 2012;153:530–535. doi: 10.1016/j.ajo.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 150.Lengyel I, Flinn JM, Peto T, Linkous DH, Cano K, Bird AC, Lanzirotti A, Frederickson CJ, van Kuijk FJ. High concentration of zinc in sub-retinal pigment epithelial deposits. Exp Eye Res. 2007;84:772–780. doi: 10.1016/j.exer.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 151.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 152.Lin H, Xu H, Liang FQ, Liang H, Gupta P, Havey AN, Boulton ME, Godley BF. Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:3521–3529. doi: 10.1167/iovs.10-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin T, Walker GB, Kurji K, Fang E, Law G, Prasad SS, Kojic L, Cao S, White V, Cui JZ, Matsubara JA. Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye. Cytokine. 2013;62:369–381. doi: 10.1016/j.cyto.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. Journal of neurochemistry. 2008;106:464–474. doi: 10.1111/j.1471-4159.2008.05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu J, Copland DA, Theodoropoulou S, Chiu HA, Barba MD, Mak KW, Mack M, Nicholson LB, Dick AD. Impairing autophagy in retinal pigment epithelium leads to inflammasome activation and enhanced macrophage-mediated angiogenesis. Sci Rep. 2016;6:20639. doi: 10.1038/srep20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. Journal of neurochemistry. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 157.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 158.Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, Ames BN. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr. 2000;71:530–536. doi: 10.1093/ajcn/71.2.530. [DOI] [PubMed] [Google Scholar]
- 159.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. American journal of respiratory and critical care medicine. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 160.Mares-Perlman JA, Brady WE, Klein R, VandenLangenberg GM, Klein BE, Palta M. Dietary fat and age-related maculopathy. Arch Ophthalmol. 1995;113:743–748. doi: 10.1001/archopht.1995.01100060069034. [DOI] [PubMed] [Google Scholar]
- 161.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 162.Mathis T, Housset M, Eandi C, Beguier F, Touhami S, Reichman S, Augustin S, Gondouin P, Sahel JA, Kodjikian L, Goureau O, Guillonneau X, Sennlaub F. Activated monocytes resist elimination by retinal pigment epithelium and downregulate their OTX2 expression via TNF-alpha. Aging Cell. 2017;16:173–182. doi: 10.1111/acel.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McLaughlin BJ, Fan W, Zheng JJ, Cai H, Del Priore LV, Bora NS, Kaplan HJ. Novel role for a complement regulatory protein (CD46) in retinal pigment epithelial adhesion. Invest Ophthalmol Vis Sci. 2003;44:3669–3674. doi: 10.1167/iovs.02-0813. [DOI] [PubMed] [Google Scholar]
- 164.Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK. The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. Journal of neurochemistry. 2011;117:856–867. doi: 10.1111/j.1471-4159.2011.07253.x. [DOI] [PubMed] [Google Scholar]
- 165.Miceli MV, Liles MR, Newsome DA. Evaluation of oxidative processes in human pigment epithelial cells associated with retinal outer segment phagocytosis. Exp Cell Res. 1994;214:242–249. doi: 10.1006/excr.1994.1254. [DOI] [PubMed] [Google Scholar]
- 166.Midena E, Degli Angeli C, Blarzino MC, Valenti M, Segato T. Macular function impairment in eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997;38:469–477. [PubMed] [Google Scholar]
- 167.Miller DM, Singh IN, Wang JA, Hall ED. Administration of the Nrf2-ARE activators sulforaphane and carnosic acid attenuates 4-hydroxy-2-nonenal-induced mitochondrial dysfunction ex vivo. Free Radic Biol Med. 2013;57:1–9. doi: 10.1016/j.freeradbiomed.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mimoun G, Soubrane G, Coscas G. Macular drusen. J Fr Ophtalmol. 1990;13:511–530. [PubMed] [Google Scholar]
- 169.Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W, Jr, Ding J, Bowes Rickman C, Boulton M. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10:1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mukesh BN, Dimitrov PN, Leikin S, Wang JJ, Mitchell P, McCarty CA, Taylor HR. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology. 2004;111:1176–1182. doi: 10.1016/j.ophtha.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 171.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mullins RF, Schoo DP, Sohn EH, Flamme-Wiese MJ, Workamelahu G, Johnston RM, Wang K, Tucker BA, Stone EM. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol. 2014;184:3142–3153. doi: 10.1016/j.ajpath.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner-Smidt K, Neumaier I, Skerra A, Li L, Parkos CA, Nusrat A. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nelson KC, Armstrong JS, Moriarty S, Cai J, Wu MW, Sternberg P, Jr, Jones DP. Protection of retinal pigment epithelial cells from oxidative damage by oltipraz, a cancer chemopreventive agent. Invest Ophthalmol Vis Sci. 2002;43:3550–3554. [PubMed] [Google Scholar]
- 178.Nelson KC, Carlson JL, Newman ML, Sternberg P, Jr, Jones DP, Kavanagh TJ, Diaz D, Cai J, Wu M. Effect of dietary inducer dimethylfumarate on glutathione in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1999;40:1927–1935. [PubMed] [Google Scholar]
- 179.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 180.Nordgaard CL, Karunadharma PP, Feng X, Olsen TW, Ferrington DA. Mitochondrial proteomics of the retinal pigment epithelium at progressive stages of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:2848–2855. doi: 10.1167/iovs.07-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.O’Neill CA, Halliwell B, van der Vliet A, Davis PA, Packer L, Tritschler H, Strohman WJ, Rieland T, Cross CE, Reznick AZ. Aldehyde-induced protein modifications in human plasma: protection by glutathione and dihydrolipoic acid. J Lab Clin Med. 1994;124:359–370. [PubMed] [Google Scholar]
- 183.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Current opinion in immunology. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 184.Orth DH, Fine BS, Fagman W, Quirk TC. Clarification of foveomacular nomenclature and grid for quantitation of macular disorders. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83:OP506–514. [PubMed] [Google Scholar]
- 185.Osburn WO, Yates MS, Dolan PD, Chen S, Liby KT, Sporn MB, Taguchi K, Yamamoto M, Kensler TW. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicological sciences: an official journal of the Society of Toxicology. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Owsley C, Huisingh C, Clark ME, Jackson GR, McGwin G., Jr Comparison of Visual Function in Older Eyes in the Earliest Stages of Age-related Macular Degeneration to Those in Normal Macular Health. Curr Eye Res. 2015:1–7. doi: 10.3109/02713683.2015.1011282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Owsley C, McGwin G, Jr, Clark ME, Jackson GR, Callahan MA, Kline LB, Witherspoon CD, Curcio CA. Delayed Rod-Mediated Dark Adaptation Is a Functional Biomarker for Incident Early Age-Related Macular Degeneration. Ophthalmology. 2016;123:344–351. doi: 10.1016/j.ophtha.2015.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Parisi V, Perillo L, Tedeschi M, Scassa C, Gallinaro G, Capaldo N, Varano M. Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina. 2007;27:879–890. doi: 10.1097/IAE.0b013e318042d6aa. [DOI] [PubMed] [Google Scholar]
- 189.Patel N, Ohbayashi M, Nugent AK, Ramchand K, Toda M, Chau KY, Bunce C, Webster A, Bird AC, Ono SJ, Chong V. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunology. 2005;115:422–430. doi: 10.1111/j.1365-2567.2005.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pauleikhoff D, Spital G, Radermacher M, Brumm GA, Lommatzsch A, Bird AC. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Arch Ophthalmol. 1999;117:1353–1358. doi: 10.1001/archopht.117.10.1353. [DOI] [PubMed] [Google Scholar]
- 191.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 192.Perez VL, Caspi RR. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015;36:354–363. doi: 10.1016/j.it.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pikuleva IA, Curcio CA. Cholesterol in the retina: the best is yet to come. Prog Retin Eye Res. 2014;41:64–89. doi: 10.1016/j.preteyeres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal. 2005;7:42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- 195.Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med. 1996;21:669–681. doi: 10.1016/0891-5849(96)00155-4. [DOI] [PubMed] [Google Scholar]
- 196.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–342. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 199.Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol. 2004;34:2347–2355. doi: 10.1002/eji.200425351. [DOI] [PubMed] [Google Scholar]
- 200.Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818–5827. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 202.Rub C, Wilkening A, Voos W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 2017;367:111–123. doi: 10.1007/s00441-016-2485-8. [DOI] [PubMed] [Google Scholar]
- 203.Rudolf M, Malek G, Messinger JD, Clark ME, Wang L, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008;87:402–408. doi: 10.1016/j.exer.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sachdeva MM, Cano M, Handa JT. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.SanGiovanni JP, Arking DE, Iyengar SK, Elashoff M, Clemons TE, Reed GF, Henning AK, Sivakumaran TA, Xu X, DeWan A, Agron E, Rochtchina E, Sue CM, Wang JJ, Mitchell P, Hoh J, Francis PJ, Klein ML, Chew EY, Chakravarti A. Mitochondrial DNA variants of respiratory complex I that uniquely characterize haplogroup T2 are associated with increased risk of age-related macular degeneration. PLoS One. 2009;4:e5508. doi: 10.1371/journal.pone.0005508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sarks J, Arnold J, Ho IV, Sarks S, Killingsworth M. Evolution of reticular pseudodrusen. Br J Ophthalmol. 2011;95:979–985. doi: 10.1136/bjo.2010.194977. [DOI] [PubMed] [Google Scholar]
- 208.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2(Pt 5):552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 209.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sarks SH. Council Lecture. Drusen and their relationship to senile macular degeneration. Aust J Ophthalmol. 1980;8:117–130. doi: 10.1111/j.1442-9071.1980.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 211.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harbor perspectives in biology. 2011:3. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed]
- 212.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Schmidt S, Hauser MA, Scott WK, Postel EA, Agarwal A, Gallins P, Wong F, Chen YS, Spencer K, Schnetz-Boutaud N, Haines JL, Pericak-Vance MA. Cigarette smoking strongly modifies the association of LOC387715 and age-related macular degeneration. American journal of human genetics. 2006;78:852–864. doi: 10.1086/503822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Scholl HP, Charbel Issa P, Walier M, Janzer S, Pollok-Kopp B, Borncke F, Fritsche LG, Chong NV, Fimmers R, Wienker T, Holz FG, Weber BH, Oppermann M. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3:e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 216.Schutt F, Bergmann M, Holz FG, Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3663–3668. doi: 10.1167/iovs.03-0172. [DOI] [PubMed] [Google Scholar]
- 217.Schutt F, Ueberle B, Schnolzer M, Holz FG, Kopitz J. Proteome analysis of lipofuscin in human retinal pigment epithelial cells. FEBS letters. 2002;528:217–221. doi: 10.1016/s0014-5793(02)03312-4. [DOI] [PubMed] [Google Scholar]
- 218.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121:1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Seddon JM, McLeod DS, Bhutto IA, Villalonga MB, Silver RE, Wenick AS, Edwards MM, Lutty GA. Histopathological Insights Into Choroidal Vascular Loss in Clinically Documented Cases of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016;134:1272–1280. doi: 10.1001/jamaophthalmol.2016.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Seddon JM, Rosner B, Sperduto RD, Yannuzzi L, Haller JA, Blair NP, Willett W. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119:1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 221.Seth A, Cui J, To E, Kwee M, Matsubara J. Complement-associated deposits in the human retina. Invest Ophthalmol Vis Sci. 2008;49:743–750. doi: 10.1167/iovs.07-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sheline CT, Zhou Y, Bai S. Light-induced photoreceptor and RPE degeneration involve zinc toxicity and are attenuated by pyruvate, nicotinamide, or cyclic light. Mol Vis. 2010;16:2639–2652. [PMC free article] [PubMed] [Google Scholar]
- 224.Singh A, Happel C, Manna SK, Acquaah-Mensah G, Carrerero J, Kumar S, Nasipuri P, Krausz KW, Wakabayashi N, Dewi R, Boros LG, Gonzalez FJ, Gabrielson E, Wong KK, Girnun G, Biswal S. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J Clin Invest. 2013;123:2921–2934. doi: 10.1172/JCI66353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Smith CJ, Hansch C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem Toxicol. 2000;38:637–646. doi: 10.1016/s0278-6915(00)00051-x. [DOI] [PubMed] [Google Scholar]
- 226.Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, Agarwal A, Postel EA, Pericak-Vance MA, Haines JL. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum Mol Genet. 2007;16:1986–1992. doi: 10.1093/hmg/ddm146. [DOI] [PubMed] [Google Scholar]
- 227.Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:2724–2735. [PubMed] [Google Scholar]
- 228.Starnes AC, Huisingh C, McGwin G, Jr, Sloan KR, Ablonczy Z, Smith RT, Curcio CA, Ach T. Multi-nucleate retinal pigment epithelium cells of the human macula exhibit a characteristic and highly specific distribution. Vis Neurosci. 2016;33:e001. doi: 10.1017/S0952523815000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Strauss O. The retinal pigment epithelium in visual function. Physiological reviews. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 230.Sulzbacher F, Kiss C, Kaider A, Eisenkoelbl S, Munk M, Roberts P, Sacu S, Schmidt-Erfurth U. Correlation of SD-OCT features and retinal sensitivity in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:6448–6455. doi: 10.1167/iovs.11-9162. [DOI] [PubMed] [Google Scholar]
- 231.Sun M, Finnemann SC, Febbraio M, Shan L, Annangudi SP, Podrez EA, Hoppe G, Darrow R, Organisciak DT, Salomon RG, Silverstein RL, Hazen SL. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: A potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J Biol Chem. 2005 doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. American journal of respiratory cell and molecular biology. 2008;39:673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- 233.Suzuki M, Kamei M, Itabe H, Yoneda K, Bando H, Kume N, Tano Y. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol Vis. 2007;13:772–778. [PMC free article] [PubMed] [Google Scholar]
- 234.Tan LX, Toops KA, Lakkaraju A. Protective responses to sublytic complement in the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2016;113:8789–8794. doi: 10.1073/pnas.1523061113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tanito M, Masutani H, Kim YC, Nishikawa M, Ohira A, Yodoi J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest Ophthalmol Vis Sci. 2005;46:979–987. doi: 10.1167/iovs.04-1120. [DOI] [PubMed] [Google Scholar]
- 236.Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, Albuquerque RJ, Hauswirth WW, Chiodo VA, Kugel JF, Goodrich JA, Ponicsan SL, Chaudhuri G, Murphy MP, Dunaief JL, Ambati BK, Ogura Y, Yoo JW, Lee DK, Provost P, Hinton DR, Nunez G, Baffi JZ, Kleinman ME, Ambati J. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tate DJ, Jr, Miceli MV, Newsome DA. Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995;36:1271–1279. [PubMed] [Google Scholar]
- 238.Taylor HR, Munoz B, West S, Bressler NM, Bressler SB, Rosenthal FS. Visible light and risk of age-related macular degeneration. Trans Am Ophthalmol Soc. 1990;88:163–173. discussion 173–168. [PMC free article] [PubMed] [Google Scholar]
- 239.Taylor HR, West S, Munoz B, Rosenthal FS, Bressler SB, Bressler NM. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110:99–104. doi: 10.1001/archopht.1992.01080130101035. [DOI] [PubMed] [Google Scholar]
- 240.Terluk MR, Kapphahn RJ, Soukup LM, Gong H, Gallardo C, Montezuma SR, Ferrington DA. Investigating mitochondria as a target for treating age-related macular degeneration. J Neurosci. 2015;35:7304–7311. doi: 10.1523/JNEUROSCI.0190-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 242.Thurman JM, Renner B, Kunchithapautham K, Ferreira VP, Pangburn MK, Ablonczy Z, Tomlinson S, Holers VM, Rohrer B. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009;284:16939–16947. doi: 10.1074/jbc.M808166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Toomey CB, Kelly U, Saban DR, Bowes Rickman C. Regulation of age-related macular degeneration-like pathology by complement factor H. Proc Natl Acad Sci U S A. 2015;112:E3040–3049. doi: 10.1073/pnas.1424391112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Triantafilou K, Hughes TR, Triantafilou M, Morgan BP. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126:2903–2913. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- 245.Tseng WA, Thein T, Kinnunen K, Lashkari K, Gregory MS, D’Amore PA, Ksander BR. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:110–120. doi: 10.1167/iovs.12-10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Uranga RM, Bruce-Keller AJ, Morrison CD, Fernandez-Kim SO, Ebenezer PJ, Zhang L, Dasuri K, Keller JN. Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. Journal of neurochemistry. 2010;114:344–361. doi: 10.1111/j.1471-4159.2010.06803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.van der Schaft TL, de Bruijn WC, Mooy CM, Ketelaars DA, de Jong PT. Is basal laminar deposit unique for age-related macular degeneration? Arch Ophthalmol. 1991;109:420–425. doi: 10.1001/archopht.1991.01080030122052. [DOI] [PubMed] [Google Scholar]
- 249.van der Schaft TL, Mooy CM, de Bruijn WC, Bosman FT, de Jong PT. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol. 1994;232:40–46. doi: 10.1007/BF00176436. [DOI] [PubMed] [Google Scholar]
- 250.Viiri J, Amadio M, Marchesi N, Hyttinen JM, Kivinen N, Sironen R, Rilla K, Akhtar S, Provenzani A, D’Agostino VG, Govoni S, Pascale A, Agostini H, Petrovski G, Salminen A, Kaarniranta K. Autophagy activation clears ELAVL1/HuR-mediated accumulation of SQSTM1/p62 during proteasomal inhibition in human retinal pigment epithelial cells. PLoS One. 2013;8:e69563. doi: 10.1371/journal.pone.0069563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Vogt SD, Barnum SR, Curcio CA, Read RW. Distribution of complement anaphylatoxin receptors and membrane-bound regulators in normal human retina. Exp Eye Res. 2006;83:834–840. doi: 10.1016/j.exer.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 252.Vogt SD, Curcio CA, Wang L, Li CM, McGwin G, Jr, Medeiros NE, Philp NJ, Kimble JA, Read RW. Retinal pigment epithelial expression of complement regulator CD46 is altered early in the course of geographic atrophy. Exp Eye Res. 2011 doi: 10.1016/j.exer.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 254.Walsh AC, Michaud SG, Malossi JA, Lawrence DA. Glutathione depletion in human T lymphocytes: analysis of activation-associated gene expression and the stress response. Toxicology and applied pharmacology. 1995;133:249–261. doi: 10.1006/taap.1995.1149. [DOI] [PubMed] [Google Scholar]
- 255.Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy. 2009;5:563–564. doi: 10.4161/auto.5.4.8163. [DOI] [PubMed] [Google Scholar]
- 256.Wang B, Sluyter R. P2X7 receptor activation induces reactive oxygen species formation in erythroid cells. Purinergic Signal. 2013;9:101–112. doi: 10.1007/s11302-012-9335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang L, Cano M, Datta S, Wei H, Ebrahimi KB, Gorashi Y, Garlanda C, Handa JT. PTX3 recruits complement factor H to protect against oxidative stress-induced complement and inflammasome overactivation. The Journal of pathology. 2016 doi: 10.1002/path.4811. [DOI] [PubMed] [Google Scholar]
- 258.Wang L, Cano M, Handa JT. p62 provides dual cytoprotection against oxidative stress in the retinal pigment epithelium. Biochim Biophys Acta. 2014a;1843:1248–1258. doi: 10.1016/j.bbamcr.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang L, Kondo N, Cano M, Ebrahimi K, Yoshida T, Barnett BP, Biswal S, Handa JT. Nrf2 signaling modulates cigarette smoke-induced complement activation in retinal pigmented epithelial cells. Free Radic Biol Med. 2014b doi: 10.1016/j.freeradbiomed.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, Cano M, Brandstatter H, Tsimikas S, Skerka C, Superti-Furga G, Handa JT, Zipfel PF, Witztum JL, Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 262.Will O, Mahler HC, Arrigo AP, Epe B. Influence of glutathione levels and heat-shock on the steady-state levels of oxidative DNA base modifications in mammalian cells. Carcinogenesis. 1999;20:333–337. doi: 10.1093/carcin/20.2.333. [DOI] [PubMed] [Google Scholar]
- 263.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 264.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 265.Wu T, Tian J, Cutler RG, Telljohann RS, Bernlohr DA, Mattson MP, Handa JT. Knockdown of FABP5 mRNA decreases cellular cholesterol levels and results in decreased apoB100 secretion and triglyceride accumulation in ARPE-19 cells. Lab Invest. 2010;90:963–965. doi: 10.1038/labinvest.2010.87. [DOI] [PubMed] [Google Scholar]
- 266.Wu Z, Ayton LN, Luu CD, Guymer RH. Relationship between retinal microstructures on optical coherence tomography and microperimetry in age-related macular degeneration. Ophthalmology. 2014;121:1445–1452. doi: 10.1016/j.ophtha.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 267.Wu Z, Lauer TW, Sick A, Hackett SF, Campochiaro PA. Oxidative Stress Modulates Complement Factor H Expression in Retinal Pigmented Epithelial Cells by Acetylation of FOXO3. J Biol Chem. 2007;282:22414–22425. doi: 10.1074/jbc.M702321200. [DOI] [PubMed] [Google Scholar]
- 268.Wysong A, Lee PP, Sloan FA. Longitudinal incidence of adverse outcomes of age-related macular degeneration. Arch Ophthalmol. 2009;127:320–327. doi: 10.1001/archophthalmol.2008.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yamada Y, Tian J, Yang Y, Cutler RG, Wu T, Telljohann RS, Mattson MP, Handa JT. Oxidized Low Density Lipoproteins Induce a Pathologic Response by Retinal Pigmented Epithelial Cells. Journal of neurochemistry. 2008;105:1187–1197. doi: 10.1111/j.1471-4159.2008.05211.x. [DOI] [PubMed] [Google Scholar]
- 270.Yang P, Tyrrell J, Han I, Jaffe GJ. Expression and modulation of RPE cell membrane complement regulatory proteins. Invest Ophthalmol Vis Sci. 2009;50:3473–3481. doi: 10.1167/iovs.08-3202. [DOI] [PubMed] [Google Scholar]
- 271.Yaniv Y, Juhaszova M, Sollott SJ. Age-related changes of myocardial ATP supply and demand mechanisms. Trends Endocrinol Metab. 2013;24:495–505. doi: 10.1016/j.tem.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yao J, Jia L, Khan N, Lin C, Mitter SK, Boulton ME, Dunaief JL, Klionsky DJ, Guan JL, Thompson DA, Zacks DN. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy. 2015;11:939–953. doi: 10.1080/15548627.2015.1041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT. Complement C3 Variant and the Risk of Age-Related Macular Degeneration. N Engl J Med. 2007 doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 274.Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat Res. 2005;591:93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 275.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zanzottera EC, Messinger JD, Ach T, Smith RT, Curcio CA. Subducted and melanotic cells in advanced age-related macular degeneration are derived from retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2015;56:3269–3278. doi: 10.1167/iovs.15-16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Molecular and cellular biology. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zhao Z, Chen Y, Wang J, Sternberg P, Freeman ML, Grossniklaus HE, Cai J. Age-related retinopathy in NRF2-deficient mice. PLoS One. 2011;6:e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q, Varga J, Sznajder JI. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. American journal of physiology Lung cellular and molecular physiology. 2009;297:L1120–1130. doi: 10.1152/ajplung.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 282.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nature reviews Immunology. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 283.Zorov DB, Bannikova SY, Belousov VV, Vyssokikh MY, Zorova LD, Isaev NK, Krasnikov BF, Plotnikov EY. Reactive oxygen and nitrogen species: friends or foes? Biochemistry (Mosc) 2005;70:215–221. doi: 10.1007/s10541-005-0103-6. [DOI] [PubMed] [Google Scholar]
- 284.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–312. e301. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]