Abstract
The attention system is shaped by reward history, such that learned reward cues involuntarily draw attention. Recent research has begun to uncover the neural mechanisms by which learned reward cues compete for attention, implicating dopamine (DA) signaling within the dorsal striatum. How these elevated priority signals develop in the brain during the course of learning is less well understood, as is the relationship between value-based attention and the experience of reward during learning. We hypothesized that the magnitude of the striatal DA response to reward during learning contributes to the development of a learned attentional bias towards the cue that predicted it, and examined this hypothesis using positron emission tomography with [11C]raclopride. We measured changes in dopamine release for rewarded versus unrewarded visual search for color-defined targets as indicated by the density and distribution of the available D2/D3 receptors. We then tested for correlations of individual differences in this measure of reward-related DA release to individual differences in the degree to which previously reward-associated but currently task-irrelevant stimuli impair performance in an attention task (i.e., value-driven attentional bias), revealing a significant relationship in the right anterior caudate. The degree to which reward-related DA release was right hemisphere lateralized was also predictive of later attentional bias. Our findings provide support for the hypothesis that value-driven attentional bias can be predicted from reward-related DA release during learning.
Keywords: selective attention, reward learning, dopamine, dopamine release positron emission tomography, incentive salience
1. INTRODUCTION
Attention is directed towards stimuli that are physically salient (e.g., bright, high contrast; Theeuwes, 2010) or possess a currently prioritized task-relevant feature (e.g., red stimuli when searching for a red target; Folk et al., 1992). The neural mechanisms of stimulus-driven and goal-directed attention have been extensively studied (e.g., Balan and Gottlieb, 2006; Corbetta and Shulman, 2002). More recently, research has demonstrated that previously reward-associated stimuli automatically capture attention even when explicitly task-irrelevant and physically non-salient (Anderson et al., 2011; see Anderson, 2016a, for a review). Participants first completed a training phase comprising a visual search task in which color-defined targets predicted monetary reward outcomes. Then, in a subsequent test phase, participants performed a different visual search task in which the colors of the stimuli were completely irrelevant to the task. On a subset of trials, one of the non-targets was rendered in a previously reward-associated color, and apart from its reward history this stimulus did not stand out in any salient way. Performance was found to be impaired by the presence of the previously reward-associated stimulus (Anderson et al., 2011), which frequently drew eye movements (Anderson and Yantis, 2012), suggesting automatic attentional processing.
The neural correlates of the attentional processing of previously reward-associated stimuli have been assessed using functional magnetic resonance imaging (fMRI; Anderson, 2017; Anderson et al., 2014; Hickey and Peelen, 2015; Krebs et al., 2011), electroencephalography (MacLean and Giesbrecht, 2015; Qi et al., 2013), magnetoencephalography (Donohue et al., 2016; Hopf et al., 2015), and single unit recording in non-human primates (Hikosaka et al., 2014), consistently implicating elevated neural activity within the posterior parietal cortex and visual corticostriatal loop (see Seger, 2013). These brain areas have been collectively referred to as the value-driven attention network (Anderson, 2017). A recent study utilizing positron emission tomography (PET) further revealed that value-driven attentional bias was strongly related to the release of dopamine (DA) within the dorsal striatum (Anderson et al., 2016b), suggesting a relationship between DA signals within the striatum and the control of visual attention.
These value-based attentional priority signals were measured in extinction, after learning had already occurred. The neural processes by which such elevated cue reactivity develops remain largely unexplored. One recent study demonstrated that the value-driven attention network responds to the receipt of reward, and does so differently based on the preceding reward cue (in this case, target color), reflecting a reward signal that contains information concerning the preceding visual signal (Anderson, 2017). Thus, reward signals may serve as teaching signals to the visual system during the development of value-based attention, mirroring hypothesized mechanisms of perceptual learning (Roelfsema and van Ooyen, 2005; Seitz and Watanabe, 2005). However, evidence directly linking reward signals to variation in attentional performance is lacking, as is the specific neurotransmitter system involved in shaping the attention system during the learning process.
In the present study, we tested the hypothesis that the DA response to reward in the human striatum serves as a teaching signal to the attention system. Regional concentrations of [11C]raclopride, a radiolabelled D2/D3 receptor antagonist, provide a measure of available D2/D3 receptors. By comparing the binding potential of [11C]raclopride across rewarded and unrewarded versions of the same task, relative increases or decreases in the release of endogenous DA due to the experience of extrinsic reward were determined (Martin-Soelch et al., 2011; Wong et al., 2006; Volkow et al., 2006). We predicted that greater reward-related DA release during learning would be associated with greater value-driven attentional bias as measured during a subsequent extinction phase (slowing of response time associated with the presence of a previously high-value distractor).
2. MATERIALS AND METHODS
2.1. Participants
Eleven (9 female) healthy adult volunteers (19–33 years of age, mean = 26.7, SD = 4.05) who were free of medical or neuropsychiatric disorders participated in the experiment. Screening criteria included a negative drug test and the exclusion of major medical or neuropsychiatric disorders past or present. Axis I diagnoses were ruled out using the Structured Clinical Interview for DSM-IV Axis I Disorders—Clinician Version (SCID-CV) (First et al., 1997), a structured interview to utilize the criteria of the Diagnostic and Statistical Manual of Mental Disorders. All participants received a detailed physical exam including vital signs, 12-lead electrocardiogram, blood for complete blood counts with differential, complete metabolic panel, blood clotting parameters, creatinine (CPK) for muscle toxicity, urine for urinalysis, and toxicology for drugs of abuse and alcohol breathalyzer before the PET scans. Informed consent was obtained from all participants, and all procedures were approved by the Institutional Review Board of the Johns Hopkins University School of Medicine and conformed to the principles outlined in the Declaration of Helsinki.
2.2. Experimental Task
The experiment consisted of a training phase and a test phase. Both phases of the experiment were performed on the same day while the participant lay within the PET scanner, although only during the training phase was PET data acquired – this was done to match the context within which the two phases were completed as much as possible, as value-based attentional biases can be sensitive to contextual information (Anderson, 2015). Participants viewed the stimuli on a LCD monitor using prism mirrors that allow horizontal viewing in the supine position. The experiment was run on a Dell Latitude E6400 computer running Matlab software with Psychophysics Toolbox extensions (Brainard, 1997), and behavioral responses were made using a modified keyboard with all keys except “z” and “m” removed. The test phase was performed immediately after the training phase session that included reward feedback. The test phase took approximately 20 min to complete, leaving at least 55 min of rest between PET scans (see Acquisition of Neuroimaging Data section for additional details on the timing of the PET scans).
2.2.1. Training Phase
During the training phase (see Fig. 1A), each trial consisted of a fixation display, a search array, and, for the rewarded scan, a feedback display. The fixation display was presented for 400, 500, or 600 ms (randomly determined on each trial), the search array for 1000 ms, and the reward feedback display for 1500 ms. A 1000 ms blank screen was inserted between the search and feedback displays and between trials. Participants were instructed to search for a color-defined target circle and report the orientation of a bar within the target as either vertical or horizontal via a button press (“z” and “m”, respectively). Each circle in the search array was approximately 2.3° × 2.3° visual angle in size, placed at equal intervals along an imaginary circle with a radius of 5°.
Figure 1.
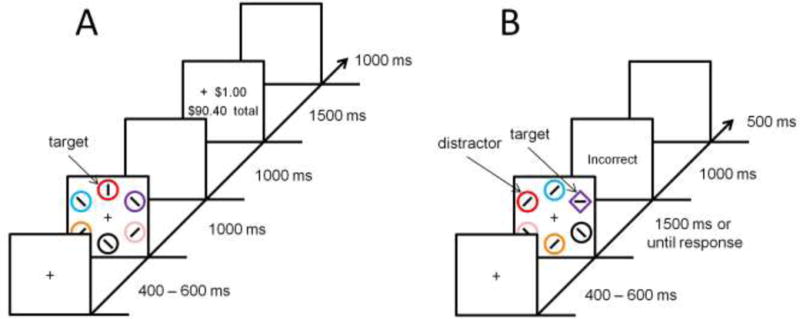
Experimental task. Time course and trial events for the training phase (A) and test phase (B).
The training phase consisted of two 720-trial scans. During one scan, participants searched for red and green targets, and during the other scan, participants searched for blue and yellow targets. The target colors for one scan did not appear as non-targets in the other scan (i.e., the same set of non-targets was used in each scan). Half of the trials in each scan contained one color target and half contained the other color target (only one target was presented on each trial); each target color appeared in each of the six possible stimulus positions equally-often. The order of trials was randomized. Participants were provided monetary rewards for correct responses in one scan (rewarded scan), but not in the other (unrewarded scan). The order of rewarded and unrewarded scans, and the assignment of target colors to rewarded vs. unrewarded scans, alternated across participants. The order in which the scans were completed was not significantly related to our measure of reward-related DA release (p = 0.457 collapsing across regions; p = 0 .036, uncorrected for multiple comparisons, in the left anterior putamen, other ps > 0.58).
For the rewarded scan, following a correct response that fell within a 1000 ms response deadline, money was added to a bank total in a reward-feedback display. If participants responded incorrectly or too slowly, the words “Incorrect” or “Too Slow” appeared in place of the monetary increment. One of the two target colors was followed by a high reward of $1.00 on 80% of the trials on which it was correctly reported, and by a low reward of 20¢ on the remaining 20% of correct trials (high-value color); for the other (low-value) color, these mappings were reversed (as in, e.g., Anderson et al., 2011, 2016b). For the unrewarded scan, the reward feedback display was omitted in the event of a correct response; otherwise, participants were provided “Incorrect” or “Too Slow” feedback. Only incorrect feedback was provided to minimize any reward-related activation associated with the reinforcement of task performance (Seitz and Watanabe, 2005). Participants were provided with a brief rest period every 120 trials.
2.2.2. Test Phase
For the test phase (see Fig. 1B), each trial consisted of a fixation display (400–600 ms), a search array (1500 ms or until response), and, in the event of an incorrect response, a feedback display (1000 ms). Each trial concluded with an inter-trial-interval during which the fixation cross was visible for 500 ms. Targets were now defined as the unique shape, either a diamond among circles or a circle among diamonds (equally-often), and participants made the same identity judgment concerning the orientation of the bar contained within the target. The colors of the shapes were irrelevant to the task, and participants were instructed to ignore color. Feedback was the same as in the unrewarded version of the training phase.
The test phase was conducted immediately after the rewarded scan of the training phase and consisted of 480 trials. This timing was used instead of administering the test phase at the end of the experiment to avoid potential interference from the performance of an intervening task for some participants (i.e., the unrewarded training phase), as value-based attention can exhibit contextual dependencies (Anderson, 2015). One of the nontarget shapes was rendered in the color of the formerly high-value target (high-value distractor) on 25% of trials, and likewise in the color of the formerly low-value target (low-value distractor) on another 25% of trials. On the remaining 50% of the trials, none of the shapes were rendered in the color of a formerly reward-predictive target (distractor absent trials). Targets and distractors appeared equally-often in each of the six possible stimulus positions. Participants were provided with a brief rest period every 120 trials.
2.3. Acquisition of Neuroimaging Data
2.3.1. MRI
Anatomical MRI scans were obtained for each participant on a day prior to PET scanning using a 3T Siemens Trio MRI. A T1-weighted SPGR (spoiled grass sequence; TR = 2110 ms, TE = 2.7 ms, 0.8 mm cubic voxels) covering the whole brain was used to define volumes of interest (VOIs) for the PET scan analysis; other T1- and T2-weighted images were also acquired as a part of a standardized battery (Brant-Zawadzki et al., 1992; Brašić et al., 2009) and were used to verify that participants met inclusion and exclusion criteria in the present study.
2.3.2. PET
Participants performed the training phase (color search) task over the course of two 60 min PET scans. PET was performed on a high resolution research tomograph (HRRT) in three dimensional mode with a 2.5 mm resolution (Sossi et al., 2005). For each scan, approximately 740 MBq (20 mCi) of [11C]raclopride was administered intravenously as a bolus injection (mean ± SD injected radioactivity: 691.9 ± 51.8 MBq (18.7 ± 1.4 mCi) vs. 666.0 ± 107.3 MBq (18.0 ± 2.9 mCi); mean ± SD injected non-radioactive mass of raclopride: 0.91 ± 0.18 vs. 0.84 ± 0.23 μg, for rewarded and unrewarded scans, respectively; no statistical differences). The two scans were separated by 75 min. The head was stabilized for both PET and MRI by an individualized thermoplastic mask and Velcro straps (Brašić et al., 2012). A laser light in the PET scanner was used to line up an axial line on the mask and the scanner bed and participant head tilt were monitored by the PET technologist for the entire scan.
2.4. Definition of VOIs
VOIs were defined from the MRI data using the 3-D interactive-segmentation mode of a locally developed VOI defining tool (VOILand), as previously reported (Oswald et al., 2005), and using published segmentation guidelines (Diedrichsen et al., 2009; Oswald et al., 2005; Yushkevich et al., 2006). Then, striatal VOIs were subdivided according to the model advanced by Mawlawi et al. (2001) to the ventral striatum, anterior/posterior putamen and anterior/posterior caudate nucleus (5 subdivisions per side) using a semi-automated method that incorporated anatomical guidance based on post-mortem human materials (Baumann et al., 1999; Oswald et al., 2005). VOIs were transferred from MRI to PET space according to MRI-to-PET coregistration parameters obtained with the coregistration module (Ashburner and Friston, 2003) in SPM5 (The Statistical Parametric Mapping 5; The Wellcome Trust Centre for Neuroimaging), and applied to PET frames to obtain regional time (radio-)activity curves (TACs).
2.5. Reconstruction of PET Data
Emission PET scans were reconstructed using the iterative ordered-subset expectation-maximization algorithm correcting for attenuation, scatter, randoms and dead-time (Rahmim et al., 2005) and including inter-frame head motion correction including transmission-emission alignment for the individual frames (Keller et al., 2012). The radioactivity was corrected for physical decay to the injection time. Reconstructions included dynamic PET frames of 256 (left-to-right) by 256 (nasion-to-inion) by 207 (neck-to-cranium) voxels with 1.22mm isotropic dimensions. The frame schedules were four 15-s, four 30-s, three 1-min, two 2-min, five 4-min, and twelve 5-min frames.
2.6. Data Analysis
2.6.1. Behavior
Mean reaction time (RT) and accuracy were computed for each experimental condition. Only correct RTs were included in the mean, and RTs faster than 200 ms or exceeding 3 SD of the mean were trimmed as in prior studies (e.g., Anderson et al., 2011, 2013, 2016a, 2016b; Anderson and Yantis, 2012).
2.6.2. PET
Nondisplaceable binding potential (BPND; Innis et al., 2007) of [11C]raclopride was obtained by the multilinear reference tissue method with two parameters (MRTM2; Ichise et al., 2003) for striatal subdivisions. Then, DA release (DARel in %; Innis et al., 1992) was obtained using the following formula: (BPND[U] - BPND[R])/BPND[U] × 100, where [U] and [R] stands for BPND of the unrewarded and rewarded scans, respectively.
2.6.3. Brain-Behavior Correlations
Within each of the striatal VOIs, we tested for a correlation (Pearson’s r) across participants of the magnitude of an individual’s value-based attentional bias (slowing of RT on high-value distractor trials compared to distractor absent trials during the test phase) to the magnitude of DA release attributable to reward processing using the calculation described above. Bonferroni correction was used to set the overall type-I error rate at 0.05 (α = 0.005 for each of ten correlations). Significant correlations obtained using Pearson’s r were further scrutinized via a randomization test in which the probability of each correlation was estimated non-parametrically by randomly shuffling the xy pairings (n interations = 10000). The observed correlation between DA release the right anterior caudate and value-based attentional bias was also examined while variance due to DA release in each other VOI was partialled out (i.e., multiple regression using DA release in the right anterior caudate and a different VOI as predictors), in order to examine the unique contribution of DA release in this region to predicting attentional bias.
3. RESULTS
During the rewarded version of the training phase, participants were faster to report the target that was associated with higher reward (559 vs 570 ms), indicating learning of the stimulus-reward associations, t(10) = 2.86, p = 0.017. Similarly, across scans, responses were faster during rewarded compared to unrewarded (618 ms) training, t(10) = 2.73, p = 0.021. Accuracy was similarly high across rewarded (96.7%) and unrewarded (96.4%) training, t(10) = 0.30, p = 0.774, suggesting that participants were similarly motivated to perform the task in each case.
As in prior studies (Anderson et al., 2011, 2013, 2016a, 2016b; Anderson and Yantis, 2012), participants varied in the degree to which performance was affected by the high-value distractor during the test phase. The magnitude of attentional capture by the high-value distractor, measured as the slowing of RT on high-value distractor compared to distractor absent trials (as in, e.g., Anderson et al., 2011, 2013, 2016a, 2016b; Anderson and Yantis, 2012), was marginally correlated with the RT bias observed during reward training (difference in RT to high- and low-value targets), r = .541, p = 0.086, and significantly so with the magnitude of reward-related DA release observed in the right anterior caudate, r = 0.821, p = 0.002 (nonparametric test: p = 0.001; see Fig. 2). Although no significant correlations were observed with reward-related DA release in any other of the other VOIs (ps > 0.049 uncorrected for multiple comparisons, see Fig. 3), the correlations of value-driven attentional capture and reward-related DA release were positive for VOIs in the right hemisphere and negative for VOIs in the left hemisphere.
Figure 2.
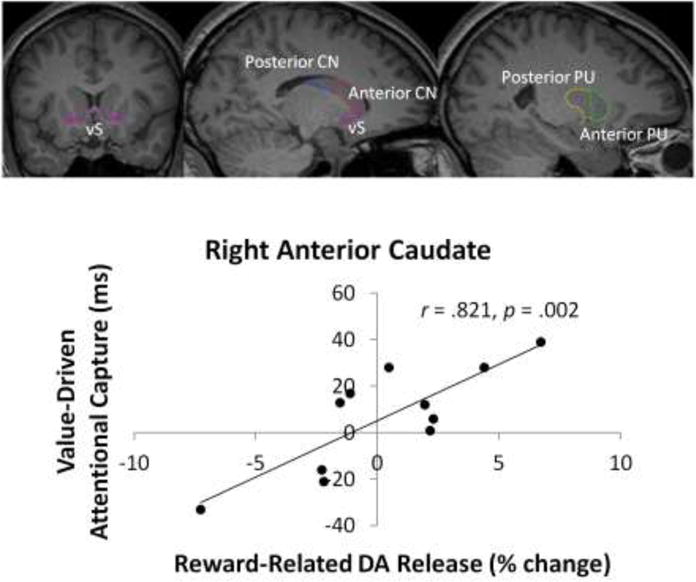
Imaging results. Visual depiction on averaged MRIs of VOIs and observed correlation between value-based distraction post-training and reward-related dopamine release during training in the right anterior caudate across participants. vS = ventral striatum, CN = caudate nucleus, PU = putamen. The left panel presents a coronal slice through the basal ganglia. The middle panel presents a parasagittal slice through the caudate nucleus. The right panel presents a parasagittal slice through the putamen.
Figure 3.
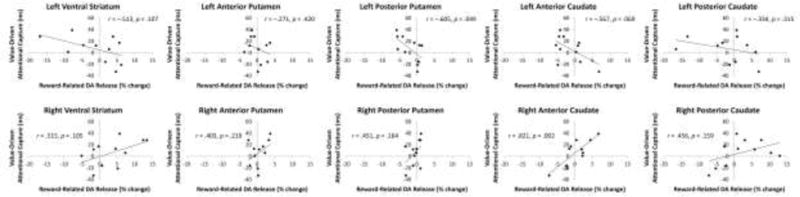
Observed correlation between value-based distraction post-training and reward-related dopamine release during training across all VOIs.
The observed correlation with DA release in the right anterior caudate remains robust when attentional capture is measured as the difference in RT between high-value distractor and low-value distractor trials, r = 0.627, p = 0.039. The difference in RT between low-value distractor trials and distractor absent trials was unrelated to DA release in any of the 10 VOIs (ps > 0.21 uncorrected for multiple comparisons). Nor did baseline RT (mean RT on distractor absent trials) correlate with reward-related DA release in any of the VOIs (ps > 0.20 uncorrected for multiple comparisons), and the reported correlation with DA release in the right anterior caudate remains significant when baseline RT is included as a covariate, β = 0.830, p = 0.004. Similarly, the RT difference between rewarded and unrewarded training was not significantly correlated with reward-related DA release in any of the VOIs (ps > 0.28, uncorrected for multiple comparisons), and the reported correlation with DA release in the right anterior caudate remains significant when this RT difference is included as a covariate, β = 0.839, p = 0.002). Thus, our findings are not confounded by individual differences in overall processing speed.
DA release within the right anterior caudate uniquely predicted value-driven attentional capture. The relationship between DA release in the right anterior caudate and attentional capture remained significant when partialling out variance in DA release in each of the other VOIs, βs > 0.604, ps < 0.026. Likewise, when partialling out DA release in the right anterior caudate, DA release did not significantly predict attentional capture in any other VOI, βs > −0.305, ps > 0.131. Value-driven attentional capture was not predicted by baseline levels of BPND in any region (ps > 0.24, uncorrected for multiple comparisons; p = 0.624 in the right anterior caudate), suggesting that our measure of reward-related DA release was not confounded by variation in baseline receptor D2/D3 availability, consistent with our prior study (Anderson et al., 2016b).
Given the trend towards positive correlations between reward-related DA release and value-driven attentional capture in the right hemisphere, and negative correlations between these same two variables in the left hemisphere, which was not predicted, we further probed this interesting pattern in the data. During the rewarded scan, a pronounced laterality bias was evident such that BPND was greater in the left hemisphere across VOIs, suggesting greater task-related DA release in the right hemisphere, t(10) = 4.57, p = 0.001. This was not the case for the unrewarded scan, however, t(10) = 0.71, p = 0.494. Importantly, the degree to which reward-related DA release was right hemisphere lateralized was strongly predictive of value-driven attentional capture, r = 0.762, p = 0.006 (see Fig. 4). That is, the greater reward-related DA release was in the right compared to the left hemisphere, the greater the consequences of reward learning on subsequently measured attentional bias were.
Figure 4.
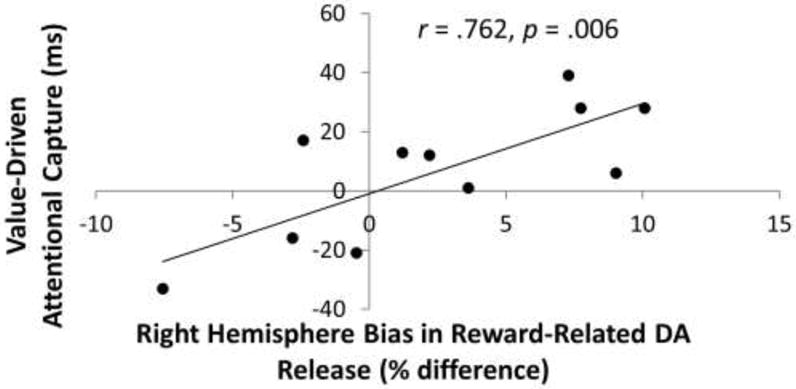
Observed correlation between value-based distraction post-training and the degree to which reward-related striatal DA release was more right hemisphere lateralized (computed as the difference in reward-related DA release in the right minus the left hemisphere).
4. DISCUSSION
Our results provide support for the hypothesis that dopaminergic reward signals in the human striatum serve as teaching signals to the attention system. Reward-related DA release in the right anterior caudate, as measured using PET, was predictive of the magnitude of subsequent attentional capture by reward-predictive cues. The more that extrinsic rewards engaged the striatal DA system, the more strongly the stimuli that previously predicted these rewards automatically captured attention.
DA release within the caudate plays an important role in habit learning and the expression of habitual behaviors (Graybiel, 2008). DA release within the caudate is also associated with craving elicited by drug cues (Wong et al., 2006; Volkow et al., 2006), as well as the signaling of value-based attentional priority following reward learning (Anderson et al., 2016b). Our findings suggest that DA signals within the right anterior caudate are closely associated with reward-driven plasticity within the attention system during associative learning, strongly predicting subsequent distraction by reward cues.
More broadly, our findings contribute to a growing literature linking DA levels as measured via PET to individual differences in cognitive and behavioral processes (Buckholtz et al., 2010; Kohno et al., 2013; Robertson et al., 2015; Treadway et al., 2012; Wong et al., 2006; Volkow et al., 2006). Our findings complement our earlier demonstration of the feasibility of using PET to measure learning-related changes in DA signaling (Anderson et al., 2016b), providing a powerful tool for examining the neurochemical basis of such learning in vivo in humans. By implicating striatal DA in the development of value-driven attentional bias, we identify a potential target for pharmacological manipulation of value-based attention. Value-driven attention plays a role in addiction (see Anderson, 2016b, for a review) and impulsive non-planning behaviors (Anderson et al., 2016a), and the successful pharmacological manipulation of value-driven attention could have benefits for these and other problematic behaviors to which value-driven attention contributes.
Although common in the PET literature given practical considerations, the sample size of the present study was small. Correlations between reward-related DA release and subsequent attentional capture may be present in other regions of the striatum, but were not sufficiently robust to pass corrections for multiple comparisons given the current sample size. Care should be taken, therefore, in drawing conclusions about which specific brain regions are involved in the shaping of value-driven attention. The detection of a robustly significant correlation in the right anterior caudate supports the role of striatal DA in value-driven attention more broadly, but the contribution of other striatal subdivisions should not be ruled out on the basis of the results of the present study.
Our data additionally support the idea that DA release in the right anterior caudate uniquely contributes to the learning of value-driven attentional bias. Even when accounting for DA release in other striatal regions, DA release in the right anterior caudate continued to predict attentional capture. Based on the findings of the present study, we conclude that DA signaling in the right anterior caudate plays a specific role in the shaping of attentional priority, one that is not reducible to DA signaling within the striatum more broadly.
Our data also suggested that the degree to which reward-related DA release was right hemisphere lateralized was also predictive of subsequent attentional capture by reward-related stimuli. This finding fits with several studies demonstrating right hemisphere dominance in DA signaling related to difficulty ignoring reward-related stimuli (Anderson et al., 2016b), craving elicited by drug cues (Wong et al., 2006), the processing of unexpected reward (particularly in males; Martin-Soelch et al., 2011), impulsiveness (Oswald et al., 2015), and incentive motivation (Tomer et al., 2008; see also Tomer et al., 2014). Attention-related processes also exhibit pronounced right hemisphere dominance, as is evident, for example, in unilateral spatial neglect (e.g., Mesulam, 1981). Thus, our findings support a distinction between the right and left striatum in the learning that underlies value-driven attention.
The sample of participants recruited for the present study were primarily female. If anything, value-driven attentional biases tend to be more pronounced in males, which may be related to impulsiveness and reward sensitivity (Anderson et al., 2013; Della Libera et al., 2017). Right-hemisphere lateralization of DA release attributable to unexpected reward was also shown to be more pronounced in males (Martin-Soelch et al., 2011), and our prior study relating striatal DA release measured during the test phase to value-driven attentional capture included a sex-balanced sample. Therefore, we do not think that our findings are specific to females, although our data cannot speak to this issue directly.
As in our prior study using PET imaging, our measure of DA release included both positive and negative values. Some participants had lesser availability of dopamine receptors during the unrewarded scan, indicating greater dopamine release in the absence of extrinsic rewards. The specific reasons for such negative values of reward-related DA release are unclear. One possibility is that these participants found the intrinsic reward of correct performance (see Seitz and Watanabe, 2005) more salient than extrinsic rewards, or tended to focus on low and missed rewards, exhibiting large reward prediction errors during the rewarded scan (which suppress striatal DA; O’Doherty, 2004; Shultz et al., 1997). Relatedly, given the coarse temporal resolution of PET, it is unclear to what degree the observed reward-related DA release was mediated by the processing of reward feedback vs. anticipated reward at the time of the stimulus array; nor can the different contributions of reward predictions and reward prediction errors be determined. Rather, our measure of reward-related DA release reflects a sum total over the course of learning. Decomposition of the different components of DA signaling within the striatum requires combining PET with other neuroimaging techniques with greater temporal resolution, such as fMRI.
6. CONCLUSIONS
The findings from the present study shed light on the mechanism by which the experience of reward shapes the human attention system. Stronger reward responsiveness, as reflected in the release of DA in the right anterior caudate, is associated with elevated attentional biases towards the cues that predicted the reward. The effects of reward on DA release, as they relate to subsequent attentional capture, also appear to exhibit right hemisphere dominance. Our study provides neural evidence linking reward processing to attentional learning, and speaks to the neurochemical basis of the development of value-driven attention.
Highlights.
We related DA-release attributable to reward processing to distraction by reward cues
Reward-related DA-release in the right anterior caudate predicted later distraction
The right anterior caudate uniquely contributed to attentional capture by reward cues
The attention-related effects of reward on DA-release exhibited right hemisphere dominance
Our findings provide neural evidence linking reward processing to attentional learning
Acknowledgments
Special thanks to Andrew Crabb, MS, and Ayon Nandi, MS for technical and/or editorial assistance.
FUNDING
The reported research was supported by NIH grants R01-DA013165 to S.M.C., S10-RR017219 to D.F.W., S10-RR023623 to D.F.W. The funding sources played no role in the study beyond financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
B.A.A. & D.F.W. developed the study concept; B.A.A., H.K., D.F.W., & S.M.C. designed the experiment; D.F.W., J.R., & J.R.B. conducted the experiment; B.A.A., H.K., D.F.W., & A.R. analyzed and chose the analytic tools for the data; A.R. contributed custom software used in data analysis; all authors contributed to the interpretation of the data and the writing and editing of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
References
- Anderson BA. Value-driven attentional priority is context specific. Psychonomic Bulletin and Review. 2015;22:750–756. doi: 10.3758/s13423-014-0724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA. The attention habit: How reward learning shapes attentional selection. Annals of the New York Academy of Sciences. 2016a;1369:24–39. doi: 10.1111/nyas.12957. [DOI] [PubMed] [Google Scholar]; Anderson BA. What is abnormal about addiction-related attentional biases? Drug and Alcohol Dependence. 2016b;167:8–14. doi: 10.1016/j.drugalcdep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA. Reward processing in the value-driven attention network: Reward signals tracking cue identity and location. Social, Cognitive, and Affective Neuroscience. 2017;12:461–467. doi: 10.1093/scan/nsw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Faulkner ML, Rilee JJ, Yantis S, Marvel CL. Attentional bias for non-drug reward is magnified in addiction. Experimental and Clinical Psychopharmacology. 2013;21:499–506. doi: 10.1037/a0034575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Kronemer SI, Rilee JJ, Sacktor N, Marvel CL. Reward, attention, and HIV-related risk in HIV+ individuals. Neurobiology of Disease. 2016a;92:157–165. doi: 10.1016/j.nbd.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Kuwabara H, Wong DF, Gean EG, Rahmim A, Brašić JR, et al. The role of dopamine in value-based attentional orienting. Current Biology. 2016b;26:550–555. doi: 10.1016/j.cub.2015.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proceedings of the National Academy of Science, USA. 2011;108:10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional priority signals in human basal ganglia and visual cortex. Brain Research. 2014;1587:88–96. doi: 10.1016/j.brainres.2014.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Yantis S. Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Attention, Perception, and Psychophysics. 2012;74:1644–1653. doi: 10.3758/s13414-012-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Rigid body registration. In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD, editors. Human Brain Function. 2nd Academic Press; 2003. [Google Scholar]
- Brant-Zawadzki M, Gillan GD, Nitz WR. MP RAGE: a three-dimensional, T1-weighted, gradient-echo sequence–initial experience in the brain. Radiology. 1992;182:769–775. doi: 10.1148/radiology.182.3.1535892. [DOI] [PubMed] [Google Scholar]
- Brašić JR, Bibat G, Kumar A, Zhou Y, Hilton J, Yablonski ME, et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with Rett syndrome. Synapse. 2012;66:471–482. doi: 10.1002/syn.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brašić JR, Zhou Y, Musachio JL, Hilton J, Fan H, Crabb A, et al. Single photon emission computed tomography experience with (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine in the living human brain of smokers and nonsmokers. Synapse. 2009;63:339–358. doi: 10.1002/syn.20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan PF, Gottlieb J. Integration of exogenous input into a dynamic salience map revealed by perturbing attention. Journal of Neuroscience. 2006;26:9239–9249. doi: 10.1523/JNEUROSCI.1898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, et al. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. Journal of Neuropsychiatry and Clinical Neurosciences. 1999;11:71–78. doi: 10.1176/jnp.11.1.71. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Della Libera C, Calletti R, Eštočinová J, Chelazzi L, Santandrea E. Reward-based plasticity of spatial priority maps: exploiting inter-subject variability to probe the underlying neurobiology. Cognitive Neuroscience. 2017;8:85–101. doi: 10.1080/17588928.2016.1213226. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Donohue SE, Hopf JM, Bartsch MV, Schoenfeld MA, Heinze HJ, Woldorff MG. The rapid capture of attention by rewarded objects. Journal of Cognitive Neuroscience. 2016;28:529–541. doi: 10.1162/jocn_a_00917. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annual Review of Neuroscience. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hickey C, Peelen MV. Neural mechanisms of incentive salience in naturalistic human vision. Neuron. 2014;85:512–518. doi: 10.1016/j.neuron.2014.12.049. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Kim HF, Yasuda M, Yamamoto S. Basal ganglia circuits for reward value guided behavior. Annual Review of Neuroscience. 2014;37:289–306. doi: 10.1146/annurev-neuro-071013-013924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf JM, Schoenfeld MA, Buschschulte A, Rautzenberg A, Krebs RM, Boehler CN. The modulatory impact of reward and attention on global feature selection in human visual cortex. Visual Cognition. 2015;23:229–248. [Google Scholar]
- Ichise M, Liow J-S, Lu J-Q, Tanako A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. Journal of Cerebral Blood Flow and Metabolism. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Innis RB, Malison RT, al-Tikriti M, Hoffer PB, Sybirska EH, Seibyl JP, et al. Amphetamine-stimulated dopamine release competes in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse. 1992;10:177–184. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
- Keller SH, Sibomana M, Olesen OV, Svarer C, Holm S, Andersen FL, Højgaard L. Methods for motion correction evaluation using 18F-FDG human brain scans on a high-resolution PET scanner. Journal of Nuclear Medicine. 2012;53:495–504. doi: 10.2967/jnumed.111.095240. [DOI] [PubMed] [Google Scholar]
- Kohno M, Ghahremani DG, Morales AM, Robertson CL, Ishibashi K, Morgan AT, et al. Risk-taking behavior: dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cerebral Cortex. 2013;25:236–245. doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Egner T, Woldorff MG. The neural underpinnings of how reward associations can both guide and misguide attention. Journal of Neuroscience. 2011;31:9752–9759. doi: 10.1523/JNEUROSCI.0732-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean MH, Giesbrecht B. Neural evidence reveals the rapid effects of reward history on selective attention. Brain Research. 2015;1606:86–94. doi: 10.1016/j.brainres.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A, Barhaghi K, Rallis D, Herscovitch P, et al. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. European Journal of Neuroscience. 2011;33:1706–1715. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow and Metabolism. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS, Wong DF, Brown CH, Kuwabara H, Brašić JR. Risky decision-making and ventral striatal dopamine responses to amphetamine: a positron emission tomography [11C]raclopride study in healthy adults. NeuroImage. 2015;113:26–36. doi: 10.1016/j.neuroimage.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Qi S, Zeng Q, Ding C, Li H. Neural correlates of reward-driven attentional capture in visual search. Brain Research. 2013;1532:32–43. doi: 10.1016/j.brainres.2013.07.044. [DOI] [PubMed] [Google Scholar]
- Rahmim A, Cheng JC, Blinder S, Camborde ML, Sossi V. Statistical dynamic image reconstruction in state-of-the-art high-resolution PET. Physics in Medicine and Biology. 2005;50:4887–4912. doi: 10.1088/0031-9155/50/20/010. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Mandelkern MA, Brown AK, Ghahremani DG, Sabb F, et al. Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. Journal of Neuroscience. 2015;35:5990–5997. doi: 10.1523/JNEUROSCI.4850-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema PR, van Ooyen A. Attention-gated reinforcement learning of internal representations for classification. Neural Computation. 2005;17:2176–2214. doi: 10.1162/0899766054615699. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seger CA. The visual corticostriatal loop through the tail of the caudate: circuitry and function. Frontiers in Systems Neuroscience. 2013;7 doi: 10.3389/fnsys.2013.00104. article no. 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz A, Watanabe T. A unified model for perceptual learning. Trends in Cognitive Sciences. 2005;9:329–334. doi: 10.1016/j.tics.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Sossi V, de Jong HWAM, Barker WC, Bloomfield P, Burbar Z, Camborde M-L, et al. The second generation HRRT: a multi-centre scanner performance investigation. IEEE Nuclear Science Symposium Conference Record (2005) 2005:2195–2199. [Google Scholar]
- Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychologica. 2010;135:77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biological Psychology. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Slagter HE, Christian BT, Fox AS, King RC, Murali D, et al. Love to win or hate to lose? asymmetry of dopamine D2 receptor binding predicts sensitivity to reward versus punishment. Journal of Cognitive Neuroscience. 2014;26:1039–1048. doi: 10.1162/jocn_a_00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. Journal of Neuroscience. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]


