Abstract
Studies consistently implicate aberrance of the brain’s reward-processing and decision-making networks in disorders featuring high levels of impulsivity, such as attention-deficit hyperactivity disorder, substance use disorder, and psychopathy. However, less is known about the neurobiological determinants of individual differences in impulsivity in the general population. In this study of 105 healthy adults, we examined relationships between impulsivity and three neurobiological metrics – gray matter volume, resting-state functional connectivity, and spontaneous eye-blink rate, a physiological indicator of central dopaminergic activity. Impulsivity was measured both by performance on a task of behavioral inhibition (go/no-go task) and by self-ratings of attentional, motor, and non-planning impulsivity using the Barratt Impulsiveness Scale (BIS-11). Overall, we found that less gray matter in medial orbitofrontal cortex and paracingulate gyrus, greater resting-state functional connectivity between nodes of the basal ganglia-thalamo-cortical network, and lower spontaneous eye-blink rate were associated with greater impulsivity. Specifically, less prefrontal gray matter was associated with higher BIS-11 motor and non-planning impulsivity scores, but was not related to task performance; greater correlated resting-state functional connectivity between the basal ganglia and thalamus, motor cortices, and prefrontal cortex was associated with worse no-go trial accuracy on the task and with higher BIS-11 motor impulsivity scores; lower spontaneous eye-blink rate was associated with worse no-go trial accuracy and with higher BIS-11 motor impulsivity scores. These data provide evidence that individual differences in impulsivity in the general population are related to variability in multiple neurobiological metrics in the brain’s reward-processing and decision-making networks.
Keywords: impulsivity, gray matter volume, resting-state functional connectivity, spontaneous eye-blink rate, prefrontal cortex, basal ganglia, healthy adults
INTRODUCTION
Impulsivity is a multidimensional trait defined most generally by a propensity for maladaptive decision-making, and is comprised of several related but distinct neurocognitive deficits including a lack of inhibitory control, an inability to wait and adequately plan behavior, and outsized preference for smaller, immediate rewards over larger, delayed rewards (De Wit, 2009). Excessive levels of impulsivity are a prominent feature of numerous clinical disorders, including attention-deficit/hyperactivity disorder (ADHD), substance abuse disorder (SUD), and psychopathy. In the general population, high levels of impulsivity have been linked to poorer life outcomes such as lower levels of academic success (Duckworth and Seligman, 2005) and increased propensity for substance abuse (Kreek et al., 2005). Given the important impact that impulsivity has on both personal and societal well-being, it is of interest to elucidate the neurobiological substrates that contribute to individual differences in impulsivity.
Studies consistently implicate aberrance of the brain’s reward-processing and decision-making networks in disorders featuring excessive impulsivity. These networks consist of gray matter nodes in the ventral midbrain, basal ganglia, thalamus, motor cortices and prefrontal cortex, structural and functional connections between these nodes via basal ganglia-thalamo-cortical circuitry, and the neurotransmitter systems that innervate this circuitry. An increasing number of studies that have examined healthy samples have also linked individual differences in impulsivity to variation in these neurobiological metrics. Several studies have found that self-reported trait measures of impulsivity, such as score on the Barratt Impulsiveness Scale (BIS-11), are negatively correlated with prefrontal gray matter (Matsuo et al., 2009; Schilling et al., 2012; Holmes et al., 2016) (but see (Bjork et al., 2009; Cho et al., 2013). A few recent studies in healthy adults have also found evidence for relationships between impulsivity and resting-state functional connectivity (RSFC), which measures the degree of correlated neural activity between different brain regions while the brain is at rest and is thought to reflect the strength of functional connections. These studies have examined associations between RSFC and self-reported impulsivity (Angelides et al., 2017), delay discounting (Li et al., 2013), and whether changes in RSFC and impulsivity co-occur in response to pharmacological dopamine challenges (Kayser et al., 2012; Cole et al., 2013). In one such study, up-regulation of dopamine levels via tolcapone resulted in a co-occurrence of decreased RSFC between the ventral putamen and pregenual cingulate cortex and decreased delay-discounting (Kayser et al., 2012). In addition, a sizeable literature has found associations in healthy adults between impulsivity and the activity of neurotransmitters such as dopamine. Administration of stimulant drugs such as d-amphetamine, which increase extracellular levels of striatal dopamine (Daberkow et al., 2013), has been associated with improved motor inhibition on the stop-signal and go/no-go tasks and with decreased delay discounting in healthy adults (de Wit et al., 2002). The availability of striatal D2/D3 dopamine receptors in positron emission tomography (PET) studies has also been associated with impulsivity in healthy adults (Lee et al., 2009; Ghahremani et al., 2012; Robertson et al., 2015). Several studies have also examined dopaminergic functioning in relation to impulsivity via spontaneous eye-blink rate (sEBR), which convergent evidence from several lines of research suggests may provide a physiological indicator of dopaminergic functioning (Karson et al., 1982; Karson et al., 1984; Karson, 1988; Elsworth et al., 1991; Lawrence and Redmond, 1991; Kleven and Koek, 1996; Taylor et al., 1999; Jutkiewicz and Bergman, 2004; Kaminer et al., 2011; Cavanagh et al., 2014; Groman et al., 2014a). These studies suggest a positive relationship between sEBR and dopaminergic functioning; for instance, pharmacological studies in both animals and healthy humans show that dopamine agonists increase sEBR while dopamine agonists decrease sEBR (Elsworth et al., 1991; Taylor et al., 1999; Jutkiewicz and Bergman, 2004; Kaminer et al., 2011; Cavanagh et al., 2014), and sEBR has been found to be lower in patients with Parkinson’s disease, which is characterized by low dopamine levels (Karson et al., 1984). However, despite generally consistent relationships with dopaminergic functioning, the relationship of sEBR to impulsivity has been mixed. One study found that higher sEBR was associated with poorer inhibitory control on a stop-signal task (Colzato et al., 2009), while another found that individuals with high self-ratings of disinhibition and low sEBR showed greater delay discounting; this study found no relationships between sEBR and the BIS subscales (Byrne et al., 2016).
The current study aims to advance this literature by providing a holistic account of impulsivity’s relationship to multiple neurobiological metrics in the brain’s reward-processing and decision-making networks in healthy adults. We present the first multi-modal dataset to simultaneously examine gray matter volume and resting-state functional connectivity in relation to impulsivity in a healthy sample. We also examine the relationship between impulsivity and spontaneous eye-blink rate to glean possible relationships with dopaminergic activity. Furthermore, we examine each of these neurobiological metrics with respect to both trait-based and task-based measures of impulsivity. The prior literature in healthy adults led us to hypothesize a negative relationship between impulsivity and prefrontal gray matter, though hypotheses for the RSFC and sEBR relationships were non-directional due to a lack of consistent findings on these metrics in prior literature.
MATERIALS AND METHODS
Overview
This study of n=105 healthy adults examines relationships between impulsivity and gray matter volume, resting-state functional connectivity, and spontaneous eye-blink rate (sEBR), a peripheral measure of central dopaminergic activity. Impulsivity was measured both by self-rating of impulsivity using the Barratt Impulsiveness Scale (BIS-11) and by performance on a go/no-task. Since impulsivity is a multi-dimensional construct encapsulating a number of distinct cognitive processes, we evaluated the neurobiological metrics with respect to multiple metrics from the BIS-11 and go/no-go task that captured this range of impulsivity subdomains. On the BIS-11, we measured attentional, motor and non-planning impulsivity subscale scores in addition to total score; on the task, we measured accuracy on no-go trials to gauge the capacity to withhold prepotent responses, and post-error slowdown to measure the tendency to proceed more deliberately after negative feedback.
First, whole-brain voxel-wise analyses were used to examine relationships between GMV and the impulsivity metrics. Next, we examined the relationship between RSFC and the impulsivity metrics using seed-to-whole-brain analyses; in order to assess RSFC in different networks in the basal-ganglia-thalamo-cortical circuitry, we used six a priori basal ganglia seeds (Di Martino et al., 2008) that have been shown to participate in functionally distinct networks, in addition to a substantia nigra seed (Tomasi and Volkow, 2012a) and ventral tegmental area seed (Tomasi and Volkow, 2012a) to assess RSFC in distinct dopaminergic pathways. Lastly, we examined correlations between sEBR and the impulsivity metrics. Age and sex were used as covariates in all analyses. Additionally, all volumetric analyses also included intracranial volume as a covariate.
Participants
127 healthy adults were recruited from the community for a study on health and well-being through internet and local newspaper advertisements. Participants provided written informed consent for study procedures that were approved by the UW-Madison Health Sciences Internal Review Board. Criteria for exclusion included use of psychotropic or steroid drugs, night-shift work, diabetes, peripheral vascular disease or other diseases affecting circulation, pregnancy, and current smoking habit or alcohol or drug dependency; exclusion criteria were assessed via self-report. Structural magnetic resonance imaging (MRI) scans were obtained for 106 subjects; one subject’s scan was excluded due to poor image registration. Thus, data from a total of 105 subjects [age, 48.6 ± 10.9 years (mean ± SD); 65 women, 40 men] were included in the gray matter volume analyses. 27 of these 105 subjects were excluded from RSFC analyses due to excessive motion during the functional MRI scan, and so data from 78 subjects were used in the RSFC analyses [age, 48.9 ± 11.0 (mean ± SD); 50 women, 28 men]. Spontaneous eye blink rate (sEBR) data was obtained for 98 of these 105 subjects, and so analyses involving sEBR included data from 98 subjects [age, 48.9 ± 10.8 years (mean ± SD); 58 women, 40 men].
Barratt Impulsiveness Scale (BIS-11)
The BIS-11(Patton and Stanford, 1995) is a self-report questionnaire containing 30 questions, each of which requires the subject to choose between ‘Rarely/Never’, ‘Occasionally’, ‘Often’ and ‘Almost Always’. Items are scored from 1 to 4. Scoring yields a total score and three subscale scores derived by factor analysis: attentional impulsivity (e.g. “I am restless at the theatre or lectures”), motor impulsivity (e.g. “I do things without thinking”), and non-planning impulsivity (e.g. “I am more interested in the present than the future”) (Patton et al., 1995). Higher scores indicate higher levels of impulsivity. The BIS-11 has good internal consistency (Cronbach’s α= 0.83) and test–retest reliability (Spearman’s rho= 0.83) (Stanford et al., 2009).
Go/No-Go Task
Subjects completed an auditory go/no-go task based on the paradigm described in Shalgi et al (2009) (Shalgi et al., 2009). Subjects were instructed to push the spacebar on a keyboard upon the presentation of an auditory syllable stimulus, except when the same syllable was repeated (no-go repeat trials) or when the syllable was “ke/” or “pa/” (no-go syllable trials). Subjects completed four blocks of 252 trials, of which 196 trials were go trials, 16 were no-go repeat trials, and 40 were no-go syllable trials. Accuracy was calculated as the percentage of correct button-pushes for go-trials and the percentage of correct withholds for no-go trials. Post-error slowdown was calculated as the difference between average reaction time on go-trials following incorrect no-go trials and average reaction time on go-trials following correct no-go trials.
Previous studies have shown that inhibitory capacity on no-go trials is sensitive to task demands, and that different task demands recruit distinct sets of brain regions and cognitive functions. For instance, Shalgi and colleagues find that subjects perform better on no-go syllable trials than no-go repeat trials (Shalgi et al., 2009). This is consistent with a meta-analysis that classifies go/no-go tasks in which the no-go stimuli are constant (as in the no-go syllable trials) as “simple”, and classifies go/no-go tasks in which the no-go stimuli change depending on context (as in the no-go repeat trials) as “complex” (Simmonds et al., 2008); this study also found that complex no-go trials recruit prefrontal regions to a greater extent than simple no-go trials, likely due to the increased attentional and working memory loads required for these trials. Given these performance and neurobiological differences, we analyzed accuracy on each type of no-go trial separately.
Image Acquisition
Images were acquired on a GE X750 3.0 Tesla MRI scanner device with an eight-channel head coil. Anatomical scans consisted of a high-resolution 3D T1-weighted inversion recovery fast gradient echo image (inversion time = 450 ms, 256 × 256 in-plane resolution, 256 mm FOV, 124 × 1.0 mm axial slices). Resting-state functional images were acquired in a single scan run using a gradient echo EPI sequence (64×64 in-plane resolution, 240mm FOV, TR/TE/Flip = 2000ms/25ms/60°, 40×4mm interleaved sagittal slices, and 210 3D volumes).
Structural MRI Preprocessing and Analysis
Preprocessing and analyses of structural MRI data were conducted in Statistical Parametric Mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm). For preprocessing, T1 images were manually realigned; segmented into gray matter, white matter, and cerebrospinal fluid; normalized to Montreal Neurological Institute (MNI)-152 space; modulated after normalization to preserve volume; and smoothed with an 8mm full-width at half-maximum (FWHM) Gaussian kernel(Ashburner and Friston, 2000). Individual Brain Atlases using Statistical Parametric Mapping (IBASPM) (http://www.thomaskoenig.ch/Lester/ibaspm.htm) in the Wake Forest University (WFU) Pick Atlas Toolbox was used to create any region of interest masks for small volume correction (SVC) analyses.
A separate whole-brain voxel-wise regression was conducted for each impulsivity metric (i.e. each BIS-11 score and each go/no-go performance metric). Significance for these regressions was evaluated using family wise error (FWE) cluster correction. The cluster extent threshold corresponded to the statistical probability (α=0.05, or 5% chance) of identifying a random noise cluster at a predefined voxel-wise (i.e., whole-brain) threshold of p<0.001 (uncorrected). We used 3dClustSim (updated December 2015) to determine that a cluster-corrected size of ≥236 voxels was significant at pFWE<0.05.
Resting-State fMRI Preprocessing and Analysis
Resting-state data were processed using a combination of FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl) and AFNI (Cox, 1996). We removed the first four volumes from each subject’s data, then used FSL’s FEAT tool for motion correction with MCFLIRT (Jenkinson et al., 2002), and non-brain removal using BET (Smith, 2002). Transformation matrices for registration were computed and applied using FSL to register the subject’s time series data to their anatomical template, and a 12DOF affine transformation was used to register the subject’s anatomical to Montreal Neurological Institute (MNI) space using FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002). Registration from high resolution structural to standard space was then further refined using FNIRT nonlinear registration (Andersson et al., 2007a, b). MNI-normalized structural images were segmented with FAST and these segmentations were used to create voxel-wise average signal and derivates from eroded CSF and 2X eroded white-matter masks. These and 6 motion regressors of no interest were included in a nuisance regression using AFNI’s 3dDeconvolve. The results from this regression were smoothed using a 5mm FWHM Gaussian kernel.
Six functionally distinct basal ganglia seeds, each with a radius of 3.5 mm, were created in each hemisphere based on coordinates reported by Di Martino and colleagues (2008) (Di Martino et al., 2008). The origin coordinates of these seeds were in the inferior ventral striatum (±9, 9, −8), superior ventral striatum (±10, 15, 0), dorsal caudate (±13, 15, 9), dorsal caudal putamen (±28, 1, 3), dorsal rostral putamen (±25, 8, 6), and ventral rostral putamen (±20, 12, −3). Furthermore, a substantia nigra seed (±12, −12, −12) and midline ventral tegmental area seed (0, −15, −12), each with a radius of 2 mm, were created based on coordinates reported by Tomasi and Volkow (2012) (Tomasi and Volkow, 2012a).
Average time-series were extracted for each seed from each participant’s preprocessed data. These timeseries were regressed back onto each participant’s data using 3dDeconvolve. To further address motion, high motion time points (a frame-wise displacement (FD) measure larger than 0.2 mm) were modeled out of the data with an individual regressor. Participants with more than 25% (52 TRs) of the data censored were omitted from analysis, leading to a total of 27 excluded participants. The resultant maps were Fisher-Z transformed to stabilize correlation variance. Average Fisher-Z correlations were extracted from the target ROIs. Whole-brain, voxel-wise regressions were conducted using FSL’s Randomise (Winkler et al., 2014) thresholded at p<0.05 with 5000 permutations. A separate regression analysis was conducted for each impulsivity metric (i.e. each BIS-11 score and each go/no-go performance metric) for each seed.
Spontaneous Eye-blink Recording
Spontaneous eye-blink rates are affected by the time of day (Barbato et al., 2000), and so data were collected around 7 pm for all participants to ensure that differences in the time of data collection could not contribute to any observed difference in eye-blink activity. Baseline sEBR was extracted from high- density, 256-channel EEG data that were collected during a 10-min baseline EEG recording. Participants were seated in front of a computer screen. During the first 2 min and last 2 min of the baseline recording, participants were instructed to keep their eyes closed. During the 6 min in between these periods, participants were instructed to keep their eyes open while looking at a cross in the middle of a fixation screen. No explicit instruction was given about blinking behavior to insure its spontaneity. Eye-blink data were extracted from the 6-min baseline recording with eyes open. Artifacts and bad channels (i.e., channels with high impedance/poor contact with the scalp) were removed from the raw EEG data using EEGLAB, and a low-pass filter of 100 Hz was applied before data analysis. After performing an independent component analysis (ICA) in MATLAB, maximally independent components were selected based on the presence of eye-blink activity, its temporal activity, and its frontal distribution. Based on the time points of the individual eye-blinks, sEBR per minute was computed. The vertical eye-blink power spectrum is concentrated in the range 0.5 to 3 Hz. There, the power of blinks is in the order of 10 times larger in amplitude than the average cortical signals, and lasts for approximately 300 ms (Nazarpour et al., 2008). These particular characteristics enable reliable statistical separation of eye-blink-related signals from brain-related or EMG-related signal from the EEG signals. The amplitude threshold for peak detection was verified manually for every participant and manually adapted if needed to assure correct quantification of eye-blink rates.
RESULTS
Self-report and Task-based Impulsivity Measures
Participant data for key self-report and task-based measures of impulsivity are summarized in Table 1.
Table 1.
Self-report and task-based impulsivity measures
| Variable | Mean ± S.D. (min-max) | Correlation with Age (p-value) | Males – Females (p-value) |
|---|---|---|---|
| BIS-11 Total | 48.2 ± 8.1 (32–70) | .01 (0.962) | 1.73 (0.291) |
| BIS-11 Attentional Impulsivity | 13.7 ± 3.1 (6–22) | −.01 (0.932) | 0.79 (0.208) |
| BIS-11 Motor Impulsivity | 20.3 ± 3.0 (13–27) | −.16 (0.115) | −0.20 (0.741) |
| BIS-11 Non-planning Impulsivity | 14.1 ± 4.1 (5–23) | .13 (0.189) | 1.14 (0.165) |
| Go Trial Accuracy (%) | 91.6 ± 7.0 (67.7–98.8) | −.20 (0.045) | 0.20 (0.887) |
| No-Go (repeat) Trial Accuracy (%) | 68.6 ± 18.1 (15.9–96.2) | .01 (0.960) | −5.88 (0.107) |
| No-Go (syllable) Trial Accuracy (%) | 73.7 ± 15.3 (12.6–92.9) | .16 (0.110) | −8.86 (0.004) |
| Post-error Slowdown (ms) | 84.8 ± 64.3 (−57.4–277.9) | .24 (0.014) | 1.21 (0.931) |
Relationships with p<0.10 are italicized. Relationships with p<0.05 are bolded.
Bivariate Pearson correlations between and within BIS-11 scores and go/no-go task performance metrics are summarized in Table 2.
Table 2.
Bivariate correlations between self-report and task-based impulsivity measures
| 1. BIS-11 Total | 2. BIS-11 Attentional Impulsivity | 3. BIS-11 Motor Impulsivity | 4. BIS-11 Non-planning Impulsivity | 5. Go Trial Accuracy | 6. No-Go (repeat) Trial Accuracy | 7. No-Go (syllable) Trial Accuracy | 8. Post-error Slowdown | |
|---|---|---|---|---|---|---|---|---|
| 1. | – | .779 (<.001†) |
.728 (<.001†) |
.859 (<.001†) |
−.077 (.436) |
−.162 (.099) |
−.003 (.979) |
−.043 (.665) |
| 2. | – | .374 (<.001†) |
.513 (<.001†) |
−.029 (.770) |
−.166 (.090) |
−.039 (.694) |
−.172 (.078) |
|
| 3. | – | .428 (<.001†) |
−.042 (.669) |
−.166 (.091) |
−.029 (.771) |
−.004 (.968) |
||
| 4. | – | −.099 (.313) |
−.073 (.459) |
.045 (.647) |
.049 (.621) |
|||
| 5. | – | .076 (.444) |
.160 (.102) |
−.420 (<.001†) |
||||
| 6. | – | .493 (.001†) |
.178 (.069) |
|||||
| 7. | – | .195 (.046) |
||||||
| 8. | – |
Relationships with p<0.10 are italicized. Relationships with p<0.05 are bolded.
Relationship remained significant after controlling for age and gender.
Weak trending (p<0.1) negative relationships were observed between accuracy on no-go repeat syllable trials and BIS-11 total, BIS-11 attentional impulsivity, and BIS-11 motor impulsivity scores; BIS-11 attentional impulsivity score also had a trending negative relationship with post-error slowdown. Post-error slowdown was significantly (p<0.05) negatively correlated with accuracy on go trials, significantly positively correlated with accuracy on no-go syllable trials, and had a positive trending relationship with accuracy on no-go repeat syllable trials. Accuracy on both types of no-go trials was significantly positively correlated.
Gray Matter Volume (GMV) and Impulsivity Measures
BIS-11
Whole-brain analyses revealed that, at the corrected level, GMV in the right medial orbitofrontal cortex was negatively correlated with BIS-11 total, BIS-11 motor impulsivity, and BIS-11 non-planning impulsivity scores; GMV in the right and left paracingulate gyrus was also negatively correlated with BIS-11 non-planning impulsivity score (Table 3; Figure 1).
Table 3.
Relationships between gray matter volume and BIS-11 scores
| Region | Cluster Size | MNI Peak Coordinates | Relationship | |
|---|---|---|---|---|
| BIS-11 Total | Medial Orbitofrontal Cortex (R) | 487 | (6, 34, −20) | Negative |
| BIS-11 Motor Impulsivity | Medial Orbitofrontal Cortex (R) | 336 | (4, 30, −21) | Negative |
| BIS-11 Non-planning Impulsivity | Paracingulate Gyrus (R) | 427 | (10, 28, 32) | Negative |
| Paracingulate Gyrus (L) | 289 | (−4, 52, 9) | Negative | |
| Medial Orbitofrontal Cortex (R) | 411 | (6, 33, −21) | Negative |
A cluster size of ≥236 voxels was significant at pFWE<0.05
Figure 1.
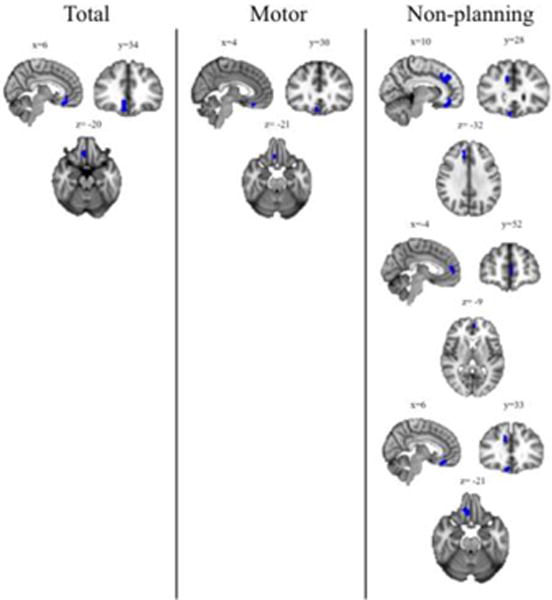
Gray Matter Volume and Impulsivity Results of voxel-wise whole-brain analyses examining the relationship between gray matter volume (controlling for age, gender, and intracranial volume) and BIS-11 total and subscale scores. Blue clusters indicate areas where volume is significantly (k>236; p<0.05 corrected) negatively correlated with score. “Total” = BIS-11 total score; “Motor” = BIS-11 motor impulsivity score; “Non-planning” = BIS-11 non-planning impulsivity score. There were no significant clusters related to BIS-11 attentional impulsivity score.
Go/No-Go
Whole-brain analyses did not reveal any significant relationships between GMV and accuracy on either go or no-go trials or post-error slowdown at the corrected level.
Resting-State Functional Connectivity (RSFC) and Impulsivity Measures
BIS-11
Seed-to-whole-brain analyses revealed a significant relationship between BIS-11 motor impulsivity score and RSFC between the right dorsal caudate and clusters in the parietal and occipital lobes that included the precentral gyrus and precuneus (Table 4).
Table 4.
Relationships between RSFC and BIS-11motor impulsivity score
| Focal Seed | RSFC Relationship with: | MNI Peak Coordinates | Cluster Size | t-value |
|---|---|---|---|---|
| R Dorsal Caudate | L Superior Lateral Occipital Cortex | (−16, −62, 66) | 2153 | 4.80 |
| L Cuneal Cortex | (−18, −74, 26) | 573 | 4.15 | |
| R Superior Parietal Lobule | (34, −44, 58) | 292 | 4.45 |
Increased correlated coupling between these regions was associated with higher BIS-11 motor impulsivity score. In addition, a significant relationship was observed between BIS-11 non-planning impulsivity score and RSFC between the left substantia nigra and bilateral thalamus (Table 5).
Table 5.
Relationships between RSFC and BIS-11 non-planning impulsivity score
| Focal Seed | RSFC Relationship with: | MNI Peak Coordinates | Cluster Size | t-value |
|---|---|---|---|---|
| L Substantia Nigra | R Thalamus | (0, −18, 10) | 179 | 4.52 |
Decreased correlated coupling between these regions was associated with increased BIS-11 non-planning impulsivity score. There were no relationships observed between RSFC and BIS-11 total score or BIS-11 attentional impulsivity score.
Go/No-Go
Seed-to-whole-brain analyses revealed significant relationships between no-go repeat trial accuracy and RSFC strength between a number of basal ganglia and ventral midbrain seeds and other nodes of the basal ganglia-thalamo-cortical network including the thalamus, motor cortex, temporal lobe, prefrontal cortex and neighboring areas of the basal ganglia (Table 6; Figure 2).
Table 6.
Relationships between RSFC and no-go (repeat) trial accuracy
| Focal Seed | RSFC Relationship with: | MNI Peak Coordinates | Cluster Size | t-value |
|---|---|---|---|---|
| R Inferior Ventral Striatum | R Thalamus | (6, −24, 4) | 1106 | 4.76 |
| L Precentral Gyrus | (−26, −18, 68) | 68 | 3.74 | |
| L Postcenttral Gyrus | (−46, −18, 48) | 61 | 3.78 | |
| L Orbitofrontal Cortex | (−28, 8, −14) | 56 | 3.56 | |
| L Inferior Ventral Striatum | L Amygdala | (−24, −2, −14) | 15954 | 4.56 |
| L Precuneus | (−12, −46, 54) | 1139 | 4.65 | |
| L Superior Parietal Lobule | (−42, −50, 62) | 178 | 3.37 | |
| L Temporal Pole | (−48, 12, −8) | 4068 | 5.02 | |
| R Postcentral Gyrus | (58, −14, 42) | 749 | 3.97 | |
| R Superior Ventral Striatum | L Precuneus | (−6, −56, 62) | 648 | 3.76 |
| L Anterior Cingulate Cortex | (−8, 22, 24) | 399 | 4.03 | |
| L Supplementary Motor Cortex | (−4, −10, 52) | 267 | 3.57 | |
| R Thalamus | (6, −20, 2) | 134 | 3.47 | |
| L Planum Polare | (−50, −6, −6) | 4441 | 5.38 | |
| L Superior Ventral Striatum | R Thalamus | (14, −16, −2) | 771 | 4.75 |
| R Paracingulate Gyrus | (8, 24, 36) | 308 | 4.97 | |
| R Dorsal Rostral Putamen | L Thalamus | (−12, −36, 6) | 1264 | 5.23 |
| R Dorsal Caudate | L Precentral Gyrus | (−28, −18, 70) | 497 | 4.48 |
| L Temporal Pole | (−48, 12, −8) | 3793 | 5.37 | |
| L Dorsal Caudate | R Thalamus | (14, −16, −2) | 512 | 3.94 |
| L Anterior Cingulate Cortex | (−8, 26, 24) | 318 | 4.78 | |
| Ventral Tegmental Area | R Middle Temporal Gyrus | (62, −8, −18) | 1268 | 4.54 |
| L Medial Orbitofrontal Cortex | (−4, 42, −18) | 839 | 5.00 | |
| L Precuneus Cortex | (−6, −64, 46) | 28178 | 4.57 | |
| L Dorsal Caudal Putamen | R Postcentral Gyrus | (42, −24, 54) | 1579 | 3.89 |
| L Superior Frontal Gyrus | (−14, 24, 48) | 251 | 3.63 |
Figure 2.
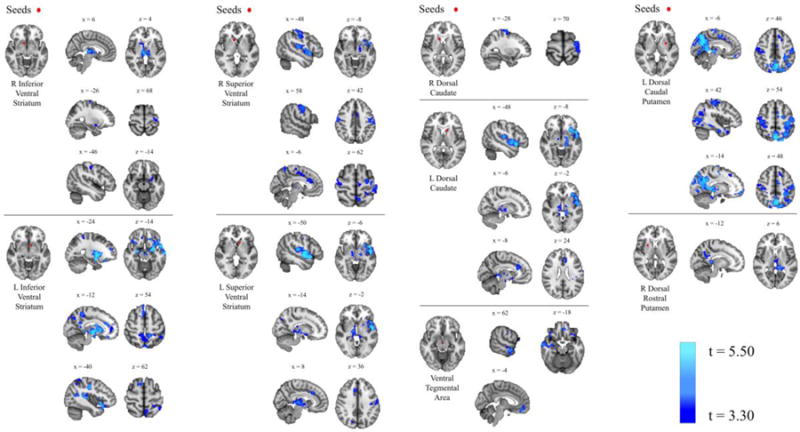
Resting-State Functional Connectivity and Impulsivity Results of seed-to-whole-brain analyses examining the relationship between resting-state functional connectivity and no-go (repeat trial) accuracy, controlling for age and gender. Blue clusters indicate areas where correlated coupling with the seed region is significantly inversely associated with no-go (repeat trial) accuracy.
Stronger correlated coupling in this circuitry was associated with worse accuracy, while decreased correlated coupling was associated with better accuracy. No relationships were found between RSFC and go trial accuracy, no-go syllable trial accuracy, or post-error slowdown.
Spontaneous Eye Blink Rate (sEBR) and Impulsivity Measures
Subjects had sEBRs ranging from 1.69 to 39.94 per minute (mean = 16.84, SD = 9.04). Relationships between sEBR and self-report and task-based impulsivity measures are summarized in Table 7.
Table 7.
Relationships between sEBR and self-report and task based impulsivity measures
| Partial Correlation with sEBR (p-value) | |
|---|---|
| BIS-11 Total | −.034 (.746) |
| BIS-11 Attentional Impulsivity | .035 (.738) |
| BIS-11 Motor Impulsivity | −.202 (.048) |
| BIS-11 Non-planning Impulsivity | .056 (.587) |
| Go Trial Accuracy (%) | .204 (.047) |
| No-Go (repeat syllable) Trial Accuracy (%) | .239 (.019) |
| No-Go (no-go syllable) Trial Accuracy (%) | .268 (.008) |
| Post-error Slowdown (ms) | −.122 (.238) |
Relationships with p<0.05 are bolded.
As sEBR can be affected by age(Chen et al., 2003; Sforza et al., 2008) and gender(Bentivoglio et al., 1997; Chen et al., 2003; Sforza et al., 2008), these variables were regressed out of the analyses. sEBR was significantly positively correlated with accuracy on go trials and both types of no-go trials, and was significantly negatively correlated with BIS-11 motor impulsivity score (Figure 3).
Figure 3.
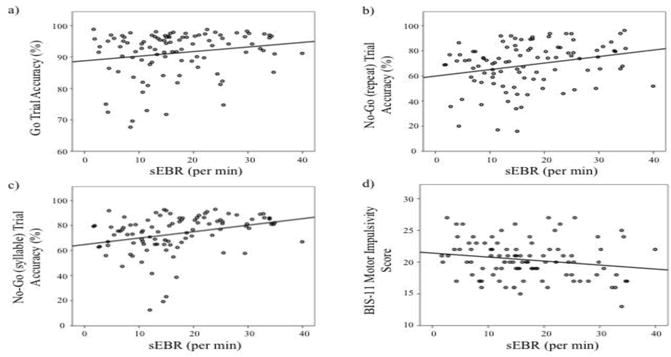
Spontaneous Eye Blink Rate and Impulsivity Plots of relationships between sEBR and go/no-go trial accuracy (a-c) and BIS-11 motor impulsivity score (d).
Relation of sEBR to GMV and RSFC
A whole-brain analysis identified a cluster of size k=42 with peak coordinates at (−21, 8, −15) in the left putamen and extending into the left medial orbitofrontal cortex where volume was significantly positively correlated with sEBR at the uncorrected (p<0.001) level. This finding did not survive FWE cluster correction. Regression analyses did not find significant relationships between sEBR and RSFC between the seed regions and the rest of the brain.
DISCUSSION
This study aimed to characterize how multiple neurobiological metrics relate to individual differences in impulsivity in the general population. Overall, we found that levels of gray matter volume, resting-state functional connectivity, and spontaneous eye-blink rate all predicted variability in self-reported and/or task-based measures of impulsivity. Specifically, greater levels of impulsivity were associated with lower gray matter volume in the orbital and medial prefrontal cortex and paracingulate gyrus, greater correlated functional connectivity throughout basal ganglia-thalamo-cortical circuitry, and lower spontaneous eye-blink rate, potentially indicative of reduced dopaminergic activity.
In terms of GMV, the present results align with prior studies finding a negative relationship between prefrontal gray matter and BIS-11 scores (Matsuo et al., 2009; Schilling et al., 2012; Holmes et al., 2016). We found prefrontal GMV to be negatively correlated with the BIS-11 motor impulsivity subscale as well as the non-planning impulsivity subscale. The association of prefrontal regions with aspects of both rapid motor inhibitory control (motor impulsivity subscale) and deliberative decision-making (non-planning impulsivity subscale) is consistent with the fMRI literature, which shows prefrontal activation during response inhibition on both go/no-go (Garavan et al., 1999; Liddle et al., 2001; Horn et al., 2003; Brown et al., 2006) and stop-signal tasks (Ghahremani et al., 2012) as well as during delay discounting tasks when long-term options are chosen (McClure et al., 2004; McClure et al., 2007). However, whereas the fMRI literature has generally implicated lateral prefrontal regions in these processes, our findings are in medial prefrontal regions. At the same time, we did not find GMV to be associated with any of the task-based measures of motor inhibition. This is at least the third study to report the absence of a relationship between GMV and performance on a rapid motor inhibition task in healthy subjects (Casey et al., 1997; McAlonan et al., 2009). This suggests that the relationship between BOLD activation and gray matter volume in these regions may not be related in a linear or otherwise straightforward fashion. It is also worth noting here that, although worse performance on the go/no-go task was associated with higher BIS-11 scores, these correlations were not statistically significant, consistent with other studies that have found a lack of significant correlation between self-report and task-based measures of impulsivity (Reynolds et al., 2006).
Regarding RSFC, we found that subjects with the greatest correlated coupling at rest between the basal ganglia and the thalamus, motor cortex, temporal lobe and prefrontal cortex had the poorest inhibitory capacity on the go/no-go task. One interpretation of these findings is that more impulsive individuals have a basal ganglia-thalamo-cortical system that is more primed for action at baseline; these individuals may thus have difficulty inhibiting this prepotent urge to act. These findings may be consistent with a recent study finding that self-reported “fun-seeking” as measured by the Behavioral Inhibition and Activation Systems (BIS/BAS) Questionnaire was positively correlated with RSFC between the putamen and middle orbitofrontal cortex (Angelides et al., 2017), though this study also found a negative correlation between self-reported “drive” and RSFC between the caudate and mid-cingulate cortex. The present findings also appear to be consistent with a pharmacological challenge study that found that a decrease in RSFC between the ventral putamen and pregenual cingulate cortex was associated with a decrease in impulsivity on a delay-discounting task (Kayser et al., 2012). It is interesting to note that this study found that the decrease in RSFC and impulsivity was induced by the up-regulation of dopamine levels via administration of tolcapone, which may be consistent with the present sEBR findings as both lower RSFC and higher sEBR (potentially indicative of elevated dopamine levels) were associated with less impulsivity.
The association of lower sEBR with greater self-reported motor impulsivity and worse motor inhibition on the go/no-go task found here is consistent with some lines of research on dopaminergic functioning, but at odds with others. First, these relationships align with findings from pharmacological studies showing that administration of drugs that increase extracellular levels of dopamine, such as methylphenidate and d-amphetamine, improves response inhibition (i.e. reduces motor impulsivity) in healthy subjects (de Wit et al., 2002). They also appear to be consistent with fMRI literature showing that striatal BOLD activation, which has been positively correlated with dopamine release (Schott et al., 2008; Buckholtz et al., 2010b), is present during motor inhibitory control (Kelly et al., 2004; Vink et al., 2005; Brown et al., 2006; Li et al., 2008; Simmonds et al., 2008; Zandbelt and Vink, 2010; Ghahremani et al., 2012). However, the present results conflict with a prior study examining the relationship between sEBR and stop-signal task performance in healthy adults, which found that higher sEBR was associated with diminished inhibitory capacity (Colzato et al., 2009). Consistency with PET studies is more challenging to ascertain. PET imaging quantifies the availability of receptors for radiotracer binding, and this metric is difficult to interpret because it likely reflects the confluence of a variety of factors, including levels of the endogenous ligand that competes for receptor binding with the radiotracer and the density of receptors themselves (Groman et al., 2014b). In healthy adults, several studies have found that decreased dopamine receptor availability in the dorsal striatum is associated with better rapid motor response inhibition (Ghahremani et al., 2012; Robertson et al., 2015). These findings are consistent with the interpretation that the reduced receptor availability is in part due to increased competition from elevated levels of endogenous dopamine in individuals with better response inhibition capacities. However, in apparent contrast, another PET study found that decreased striatal dopamine receptor availability was associated with increased levels of trait impulsivity as measured by the BIS-11 (Lee et al., 2009). Other PET studies are more difficult to interpret within this framework: a study in healthy adults found that trait impulsivity measured with the BIS-11 was negatively correlated with availability of presynaptic D2 autoreceptors in the substantia nigra/ventral tegmental area, which have an inhibitory drive on striatal dopamine release (Buckholtz et al., 2010a). Further research is needed in this area to elucidate the mechanisms determining how exactly dopamine affects motor response inhibition.
In addition to contextualizing these results within prior findings in healthy samples, it is also interesting to view them in the context of relationships found in clinical populations with very high levels of impulsivity. Regarding GMV, the inverse relationship found here between prefrontal GMV and impulsivity resembles findings from the ADHD literature, in that adults with ADHD have been found to have lower prefrontal GMV compared to healthy adults (Hesslinger et al., 2002; Seidman et al., 2006). On the other hand, a number of studies have found that impulsive-antisocial traits in psychopathy are associated with greater prefrontal GMV (Cope et al., 2012; Contreras-Rodriguez et al., 2015; Korponay et al., in press) (but see (Cope et al., 2014)). Contextualizing the RSFC findings is more difficult, as RSFC studies in ADHD patients have found both greater and lower RSFC across different networks (Konrad and Eickhoff, 2010; Tomasi and Volkow, 2012b); impulsive-antisocial traits in psychopathy have typically been associated with greater strength of RSFC in this circuitry (Korponay et al., 2017). Finally, the inverse relation found here between impulsivity and sEBR is consistent with findings pointing to hypoactivity of the dopamine system in individuals with ADHD and studies showing that pharmacological up-regulators of dopamine improve response inhibition and decrease impulsivity in subjects with ADHD (Tannock et al., 1989; Aron et al., 2003); evidence points to hyperactivity of the dopamine system in psychopathy (Buckholtz et al., 2010b). Collectively, these observations suggest that the neurobiological underpinnings of impulsivity in the general population may differ in degree, rather than in kind, from those responsible for excessive impulsivity in certain disorders such as ADHD; other disorders, such as psychopathy, may represent an opposite extreme on this continuum.
SIGNIFICANCE STATEMENT.
Trait impulsivity and deficient self-control are predictors of numerous poor life outcomes in otherwise healthy individuals, including lower levels of academic success and increased propensity for drug abuse. This study is the first to simultaneously characterize how gray matter volume, resting-state functional connectivity, and spontaneous eye-blink rate – a physiological indicator of dopaminergic functioning – relate to individual differences in impulsivity in healthy adults (n=105). Our results indicate that variation in all three of these metrics in key areas of the brain’s self-control circuitry is associated individual differences in impulsivity in the general population.
HIGHLIGHTS.
Differences in impulsivity are linked to variability in multiple metrics
Greater impulsivity is associated with less prefrontal gray matter volume
Greater impulsivity is associated with increased functional connectivity
Greater impulsivity is associated with lower spontaneous eye-blink rate
Acknowledgments
This work was supported by the National Center for Complementary and Alternative Medicine (NCCAM) P01AT004952 to RJD, grants from the National Institute of Mental Health (NIMH) R01MH43454, P50-MH084051 to RJD, grants from the Fetzer Institute and the John Templeton Foundation to RJD, and a core grant to the Waisman Center from the National Institute of Child Health and Human Development (NICHD) P30 HD003352 to Albee Messing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Dr. Richard J. Davidson is the founder, president, and serves on the board of directors for the non-profit organization, Healthy Minds Innovations, Inc. In addition, Dr. Davidson serves on the board of directors for the Mind and Life Institute.
References
- Andersson JLR, Jenkinson M, Smith SM. Non-linear optimisation. FMRIB technical report TR07JA1 2007a [Google Scholar]
- Andersson JLR, Jenkinson M, Smith SM. Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2 2007b [Google Scholar]
- Angelides NH, Gupta J, Vickery TJ. Associating resting-state connectivity with trait impulsivity. Soc Cogn Affect Neurosci. 2017 doi: 10.1093/scan/nsx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barbato G, Ficca G, Muscettola G, Fichele M, Beatrice M, Rinaldi F. Diurnal variation in spontaneous eye-blink rate. Psychiatry Res. 2000;93:145–151. doi: 10.1016/s0165-1781(00)00108-6. [DOI] [PubMed] [Google Scholar]
- Bentivoglio AR, Bressman SB, Cassetta E, Carretta D, Tonali P, Albanese A. Analysis of blink rate patterns in normal subjects. Mov Disord. 1997;12:1028–1034. doi: 10.1002/mds.870120629. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW. Delay discounting correlates with proportional lateral frontal cortex volumes. Biol Psychiatry. 2009;65:710–713. doi: 10.1016/j.biopsych.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010a;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Cole D, Kessler RM, Zald DH. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010b;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne KA, Patrick CJ, Worthy DA. Striatal Dopamine, Externalizing Proneness, and Substance Abuse: Effects on Wanting and Learning during Reward-Based Decision Making. Clin Psychol Sci. 2016;4:760–774. doi: 10.1177/2167702615618163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Masters SE, Bath K, Frank MJ. Conflict acts as an implicit cost in reinforcement learning. Nat Commun. 2014;5 doi: 10.1038/ncomms6394. [DOI] [PubMed] [Google Scholar]
- Chen WH, Chiang TJ, Hsu MC, Liu JS. The validity of eye blink rate in Chinese adults for the diagnosis of Parkinson’s disease. Clin Neurol Neurosurg. 2003;105:90–92. doi: 10.1016/s0303-8467(02)00107-5. [DOI] [PubMed] [Google Scholar]
- Cho SS, Pellecchia G, Aminian K, Ray N, Segura B, Obeso I, Strafella AP. Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topogr. 2013;26:479–487. doi: 10.1007/s10548-012-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Oei NY, Soeter RP, Both S, van Gerven JM, Rombouts SA, Beckmann CF. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex. 2013;23:1509–1516. doi: 10.1093/cercor/bhs136. [DOI] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WP, van Wouwe NC, Pannebakker MM, Hommel B. Dopamine and inhibitory action control: evidence from spontaneous eye blink rates. Exp Brain Res. 2009;196:467–474. doi: 10.1007/s00221-009-1862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Rodriguez O, Pujol J, Batalla I, Harrison BJ, Soriano-Mas C, Deus J, Lopez-Sola M, Macia D, Pera V, Hernandez-Ribas R, Pifarre J, Menchon JM, Cardoner N. Functional Connectivity Bias in the Prefrontal Cortex of Psychopaths. Biol Psychiatry. 2015;78:647–655. doi: 10.1016/j.biopsych.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Cope LM, Ermer E, Nyalakanti PK, Calhoun VD, Kiehl KA. Paralimbic gray matter reductions in incarcerated adolescent females with psychopathic traits. J Abnorm Child Psychol. 2014;42:659–668. doi: 10.1007/s10802-013-9810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope LM, Shane MS, Segall JM, Nyalakanti PK, Stevens MC, Pearlson GD, Calhoun VD, Kiehl KA. Examining the effect of psychopathic traits on gray matter volume in a community substance abuse sample. Psychiatry Res. 2012;204:91–100. doi: 10.1016/j.pscychresns.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME, Garris PA, Roitman MF. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci. 2013;33:452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Seligman ME. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol Sci. 2005;16:939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Lawrence MS, Roth RH, Taylor JR, Mailman RB, Nichols DE, Lewis MH, Redmond DE. D(1) and D(2) Dopamine-Receptors Independently Regulate Spontaneous Blink Rate in the Vervet Monkey. J Pharmacol Exp Ther. 1991;259:595–606. [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED. Striatal Dopamine D-2/D-3 Receptors Mediate Response Inhibition and Related Activity in Frontostriatal Neural Circuitry in Humans. Journal of Neuroscience. 2012;32:7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, Seu E, Tran S, Clark TA, Harpster SN, Crawford M, Burtner JL, Feiler K, Roth RH, Elsworth JD, London ED, Jentsch JD. In the Blink of an Eye: Relating Positive-Feedback Sensitivity to Striatal Dopamine D-2-Like Receptors through Blink Rate. Journal of Neuroscience. 2014a;34:14443–14454. doi: 10.1523/JNEUROSCI.3037-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, Seu E, Tran S, Clark TA, Harpster SN, Crawford M, Burtner JL, Feiler K, Roth RH, Elsworth JD, London ED, Jentsch JD. In the blink of an eye: relating positive-feedback sensitivity to striatal dopamine D2-like receptors through blink rate. J Neurosci. 2014b;34:14443–14454. doi: 10.1523/JNEUROSCI.3037-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslinger B, Tebartz van Elst L, Thiel T, Haegele K, Hennig J, Ebert D. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neurosci Lett. 2002;328:319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Hollinshead MO, Roffman JL, Smoller JW, Buckner RL. Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. Journal of Neuroscience. 2016;36:4038–4049. doi: 10.1523/JNEUROSCI.3206-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Bergman J. Effects of dopamine D1 ligands on eye blinking in monkeys: Efficacy, antagonism, and D1/D2 interactions. J Pharmacol Exp Ther. 2004;311:1008–1015. doi: 10.1124/jpet.104.071092. [DOI] [PubMed] [Google Scholar]
- Kaminer J, Powers AS, Horn KG, Hui CN, Evinger C. Characterizing the Spontaneous Blink Generator: An Animal Model. Journal of Neuroscience. 2011;31:11256–11267. doi: 10.1523/JNEUROSCI.6218-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson CN. Physiology of normal and abnormal blinking. Adv Neurol. 1988;49:25–37. [PubMed] [Google Scholar]
- Karson CN, LeWitt PA, Calne DB, Wyatt RJ. Blink rates in parkinsonism. Ann Neurol. 1982;12:580–583. doi: 10.1002/ana.410120614. [DOI] [PubMed] [Google Scholar]
- Karson CN, Burns RS, LeWitt PA, Foster NL, Newman RP. Blink rates and disorders of movement. Neurology. 1984;34:677–678. doi: 10.1212/wnl.34.5.677. [DOI] [PubMed] [Google Scholar]
- Kayser AS, Allen DC, Navarro-Cebrian A, Mitchell JM, Fields HL. Dopamine, corticostriatal connectivity, and intertemporal choice. J Neurosci. 2012;32:9402–9409. doi: 10.1523/JNEUROSCI.1180-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. Eur J Neurosci. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Differential effects of direct and indirect dopamine agonists on eye blink rate in cynomolgus monkeys. J Pharmacol Exp Ther. 1996;279:1211–1219. [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31:904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay C, Pujara M, Deming P, Philippi C, Decety J, Kosson DS, Kiehl KA, Koenigs M. Impulsive-antisocial dimension of psychopathy linked to enlargement and abnormal functional connectivity of the striatum. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:149–157. doi: 10.1016/j.bpsc.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay C, Pujara M, Deming P, Philippi C, Decety J, Kosson DS, Kiehl KA, Koenigs M. Impulsive-antisocial psychopathic traits linked to increased volume and functional connectivity within prefrontal cortex. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsx042. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Redmond DE. Mptp Lesions and Dopaminergic Drugs Alter Eye Blink Rate in African-Green Monkeys. Pharmacol Biochem Be. 1991;38:869–874. doi: 10.1016/0091-3057(91)90255-z. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Yan P, Sinha R, Lee TW. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Ma N, Liu Y, He XS, Sun DL, Fu XM, Zhang X, Han S, Zhang DR. Resting-state functional connectivity predicts impulsivity in economic decision-making. J Neurosci. 2013;33:4886–4895. doi: 10.1523/JNEUROSCI.1342-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30:1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Chua SE, Oosterlaan J, Hung SF, Tang CP, Lee CC, Kwong SL, Ho TP, Cheung C, Suckling J, Leung PW. Age-related grey matter volume correlates of response inhibition and shifting in attention-deficit hyperactivity disorder. Br J Psychiatry. 2009;194:123–129. doi: 10.1192/bjp.bp.108.051359. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarpour K, Wongsawat Y, Sanei S, Chambers JA, Oraintara S. Removal of the eye-blink artifacts from EEGs via STF-TS modeling and robust minimum variance beamforming. IEEE Trans Biomed Eng. 2008;55:2221–2231. doi: 10.1109/TBME.2008.919847. [DOI] [PubMed] [Google Scholar]
- Patton J, Stanford M. Barratt impulsiveness scale, version 11 [BIS 11] Sourcebook of adult assessment. 1995:361–364. [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit D. Dimensions of impulsive behavior: Personality and behavioral measures. Pers Indiv Differ. 2006;40:305–315. [Google Scholar]
- Robertson CL, Ishibashi K, Mandelkern MA, Brown AK, Ghahremani DG, Sabb F, Bilder R, Cannon T, Borg J, London ED. Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. J Neurosci. 2015;35:5990–5997. doi: 10.1523/JNEUROSCI.4850-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling C, Kuhn S, Romanowski A, Schubert F, Kathmann N, Gallinat J. Cortical thickness correlates with impulsiveness in healthy adults. Neuroimage. 2012;59:824–830. doi: 10.1016/j.neuroimage.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Duzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Sforza C, Rango M, Galante D, Bresolin N, Ferrario VF. Spontaneous blinking in healthy persons: an optoelectronic study of eyelid motion. Ophthalmic Physiol Opt. 2008;28:345–353. doi: 10.1111/j.1475-1313.2008.00577.x. [DOI] [PubMed] [Google Scholar]
- Shalgi S, Barkan I, Deouell LY. On the positive side of error processing: error-awareness positivity revisited. Eur J Neurosci. 2009;29:1522–1532. doi: 10.1111/j.1460-9568.2009.06690.x. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks, demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers Indiv Differ. 2009;47:385–395. [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Lawrence MS, Sladek JR, Roth RH, Redmond DE. Spontaneous blink rates correlate with dopamine levels in the caudate nucleus of MPTP-treated monkeys. Exp Neurol. 1999;158:214–220. doi: 10.1006/exnr.1999.7093. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb Cortex. 2012a;24:935–944. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012b;71:443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp. 2005;25:336–344. doi: 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M. On the Role of the Striatum in Response Inhibition. Plos One. 2010;5 doi: 10.1371/journal.pone.0013848. [DOI] [PMC free article] [PubMed] [Google Scholar]


