Abstract
Wound healing is one of the most complex biological processes to occur in life. Repair of tissue following injury involves dynamic interactions between multiple cell types, growth factors, inflammatory mediators and components of the extracellular matrix (ECM). Aberrant and uncontrolled wound healing leads to a non-functional mass of fibrotic tissue. In the eye, fibrotic disease disrupts the normally transparent ocular tissues resulting in irreversible loss of vision. A common feature in fibrotic eye disease is the transdifferentiation of cells into myofibroblasts that can occur through a process known as epithelial-mesenchymal transition (EMT). Myofibroblasts rapidly produce excessive amounts of ECM and exert tractional forces across the ECM, resulting in the distortion of tissue architecture. Transforming growth factor-beta (TGFβ) plays a major role in myofibroblast transdifferentiation and has been implicated in numerous fibrotic eye diseases including corneal opacification, pterygium, anterior subcapsular cataract, posterior capsular opacification, proliferative vitreoretinopathy, fibrovascular membrane formation associated with proliferative diabetic retinopathy, submacular fibrosis, glaucoma and orbital fibrosis. This review serves to introduce the pathological functions of the myofibroblast in fibrotic eye disease. We also highlight recent developments in elucidating the multiple signaling pathways involved in fibrogenesis that may be exploited in the development of novel anti-fibrotic therapies to reduce ocular morbidity due to scarring.
Keywords: transforming growth factor-beta (TGFβ), epithelial-mesenchymal transition (EMT), fibrosis, wound healing, myofibroblast, ocular
1. Introduction
Wound healing is a fundamental biological process that enables the systematic replacement of injured cells; however, prolonged and exaggerated wound healing may result in the pathological condition of fibrosis (Leask & Abraham, 2004). Fibrosis can be defined as the disruption of normal structural components of tissue, with the accumulation of excessive, many times aberrant, forms of extracellular matrix (ECM) proteins, resulting in a distorted and non-functional aggregation of scar tissue (Diegelmann, 1997). This process typically occurs over many months to years in humans and can result in complete organ dysfunction (Leask & Abraham, 2004). To date, there is no effective treatment for fibrotic disease and often, organ transplantation is the only viable option for patients (Leask & Abraham, 2004). In the context of the eye, fibrotic diseases such as corneal opacification and submacular fibrosis render millions of people worldwide visually impaired and blind, and remains one of the major areas of unmet need in clinical ophthalmology (Yu-Wai-Man and Khaw, 2015).
Myofibroblast transdifferentiation is a key feature of pathological tissue repair (Klingberg et al. 2013). Myofibroblasts rapidly synthesize and accumulate excessive amounts of ECM during wound healing and exert synchronized tractional forces across the ECM, resulting in the distortion of tissue architecture and subsequent scarring (Wynn and Ramalingam, 2012). Since their first discovery in healing skin wounds over forty years ago (Gabbiani et al., 1971), our knowledge of the structure and activity of the myofibroblast has progressed profoundly. In addition to skin wound healing, myofibroblasts have been identified in multiple tissues and pathologies including liver cirrhosis, renal fibrosis (Gabbiani, 2003), pulmonary fibrosis (Zhang et al., 1994), epithelial tumours (Radisky et al., 2007) and fibrotic eye diseases (Saika et al., 2008; Yamanaka et al., 2010).
The purpose of this review is to summarize key biological features of the myofibroblast and to discuss the role of the myofibroblast in various fibrotic eye diseases of the cornea, conjunctiva, lens, retina, optic nerve and orbit (Table). We highlight recent developments in elucidating the growth factor signaling pathways, including TGFβ signaling that govern the activation of myofibroblast transdifferentiation in ocular fibrosis; together with the antagonists of this signaling pathway that may hold promise as novel therapeutic agents in the treatment of fibrotic eye disease.
Table.
List of ocular cell types that transdifferentiate to myofibroblasts leading to fibrotic eye disease.
| Ocular Cell Type | Fibrotic Eye Disease | Reference |
|---|---|---|
| Keratocytes | Corneal opacification | Tandon et al., 2010 |
| Corneal endothelial cells | Fuchs endothelial corneal dystrophy | Okumura et al., 2013 |
| Conjunctival epithelial cells | Pterygium | Kato and Shimmura, 2008 |
| Subconjunctival fibrosis post-glaucoma filtration surgery | Cordeiro et al., 1999 | |
| Trabecular meshwork cells | Glaucoma | Takahashi et al., 2014 |
| Lens epithelial cells | Anterior subcapsular cataract | de Iongh et al., 2005 |
| Posterior capsular opacification | Eldred et al., 2011 | |
| Retinal pigment epithelial cells | Proliferative vitreoretinopathy | Tamiya and Kaplan, 2016 |
| Proliferative diabetic retinopathy | Abu El-Asrar et al., 2015 | |
| Subretinal fibrosis | Lopez et al., 1996 | |
| Astrocytes | Glaucoma | Gottanka et al., 2005 |
| Laminar cribrosa cells | Glaucoma | Kirwan et al., 2005a |
| Orbital fibroblasts | Graves Ophthalmopathy | Koumas et al., 2003 |
1.1 The Myofibroblast
Myofibroblasts, sometimes referred to as being ‘activated’ fibroblastic cells, possess similar ultrastructural and physiological characteristics to smooth muscle cells (Darby et al., 2014). The prominent microfilament bundles of myofibroblasts form stress fibers that permit contraction of the cell and hence, remodeling of the adjacent ECM (Darby et al., 2014). One key feature of the myofibroblast is neo-expression of alpha-smooth muscle actin (α-SMA), the actin isoform typically seen in vascular smooth muscle cells (Darby et al., 1990). Incorporation of α-SMA into the cellular stress fibers significantly augments the contractile activity of myofibroblasts and represents a key marker of the myofibroblastic phenotype (Hinz et al., 2001). The actin bundles that comprise the stress fibers terminate at the surface of the myofibroblast and form specialised cell-matrix junctions known as a “fibronexus junctions” in vivo (Dugina et al., 2001), and “large mature focal adhesions” in vitro (Hinz et al., 2003). This creates a mechano-transduction system that enables the force generated by stress fibers to be transmitted to the surrounding ECM. Moreover, this mechano-transduction system also enables extracellular mechanical signals to be transduced into intracellular signaling (Geiger and Bershadsky, 2001).
The expression of α-SMA is precisely regulated by the combined activity of growth factors/cytokines such as TGFβ, specialized ECM proteins such as fibronectin, and the surrounding mechanical microenvironment (Darby et al., 2014). Under normal physiological conditions, the maintenance and turnover of ECM molecules is tightly regulated to maintain a dynamic balance between ECM synthesis and degradation. Following tissue injury, myofibroblasts synthesize and secrete copious amounts of ECM proteins including collagens type I, III, IV and V, fibronectin and tenascin-C, to facilitate tissue remodeling (Zhang et al., 1994). Such extraneous ECM deposits alter the composition, organization and mechanical properties of the existing ECM, hence distorting the normal structure and function of the tissue (Hinz and Gabbiani, 2003). The force generated by the myofibroblast is stabilized by the accumulation of newly synthesized ECM molecules (Tomasek et al., 2002). Active ECM remodeling and deposition of excess ECM results in matrix stiffening, thereby increasing global cellular stress, and facilitating the induction of stress fibers critical for the function of the myofibroblast (Tomasek et al., 2002).
The accumulation of biologically active TGFβ is an important initiation step in myofibroblast transdifferentiation. TGFβ is synthesized and secreted as a biologically inactive precursor protein with a large amino-terminal prodomain, known as the latency-associated peptide (LAP), and a mature TGFβ at the carboxy-terminal region (Massague and Chen, 2000; Weiss and Attisano, 2013). Activation of the TGFβ precursor is essential for the regulation of its functions in vivo and is tightly controlled by multiple mechanisms (Lebrun, 2012). Myofibroblasts themselves can release latent TGFβ complexed with LAP that bind to ECM proteins, forming a sustained reservoir of TGFβ (Darby et al., 2014). Hence, both increased mechanical stress and contraction can further release TGFβ resulting in greater myofibroblastic activity.
Under normal physiological conditions, myofibroblasts disappear by apoptosis with wound healing; however, in pathological wound healing, myofibroblast activity persists, leading to chronic contractile activity and subsequent hypertrophic scarring and tissue contracture (Darby et al., 2014).
1.2 TGFβ signal transduction
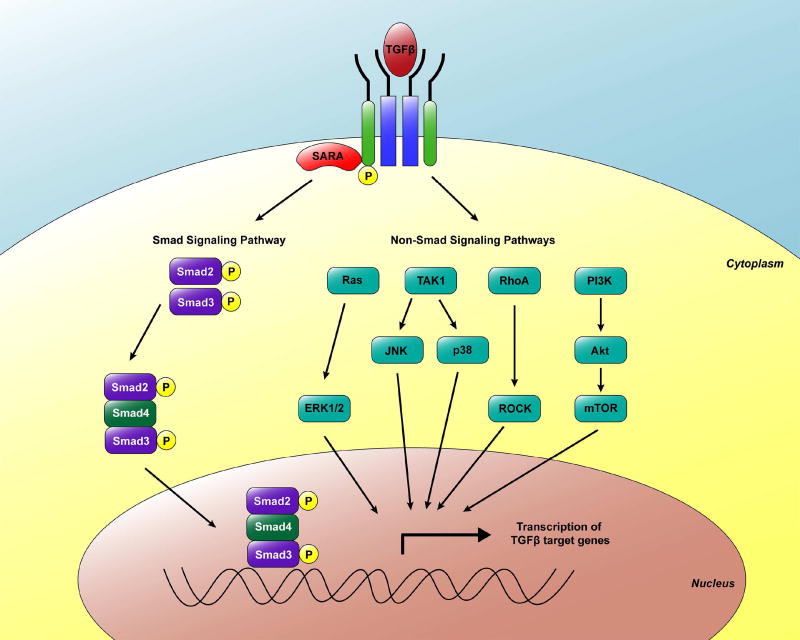
Once activated, the TGFβ ligand binds to the extracellular domain of the type II TGFβ receptor (TβRII), an autophosphorylated serine/threonine kinase receptor (Shi and Massague, 2003). The activated TβRII transphosphorylates and activates the serine and threonine residues in the intracellular glycine-serine rich domain of the type I TGFβ receptor (TβRI) (Lebrun, 2012). The activated TβRI is subsequently able to control several downstream signaling pathways including the Smad-dependent and -independent signaling pathways (Lebrun, 2012) (Fig. 1).
Fig. 1. Smad and Smad-independent signaling pathways downstream of TGFβ.
TGFβ binds to its receptors and the activated receptor complexes relay the signal to the cytoplasm by phosphorylating receptor-regulated Smad proteins (Smad2/3) that hetero-oligomerize with Smad4. This complex then translocates to the nucleus and regulates the transcription of respective target genes. TGFβ can also activate non-Smad signaling pathways including ERK1/2, JNK, p38, Rho/ROCK and PI3K/Akt/mTOR.
1.2.1 Smad-dependent pathways
Activated TβRI phosphorylates the cytoplasmic receptor-regulated small mothers against decapentaplegic (R-Smads), specifically Smad2 and Smad3 (Yi et al., 2005). The amplitude and duration of Smad2/3 signaling is modulated by auxiliary anchoring proteins such as the Smad anchor for receptor activation (SARA) that facilitates access of the R-Smads to the type I receptors (ten Dijke and Hill, 2004). Activated R-Smads associate with the common Smad (Co-Smad, also known as Smad4) to form a heteromeric complex that translocates to the nucleus to regulate transcription of TGFβ-responsive genes (Massague and Chen, 2000; Fleisch et al., 2006; Miyazono, 2009; Wendt et al., 2009). Smad proteins recognize the palindromic DNA sequence (CAGAC), termed the Smad binding element (SBE); however, their affinity for DNA binding is relatively low (Jonk et al., 1998; Massague and Chen, 2000; Denissova and Liu, 2004). Thus, in order for Smads to achieve high-affinity DNA binding, they need to bind synergistically to DNA with cell-specific cofactors (transcriptional co-activators or co-repressors) to ensure the activation of cell type-specific targeted expression of TGFβ-responsive genes (Chen et al., 1997; Miyazono, 2009; Wendt et al., 2009; Lebrun, 2012).
The Smad-dependent pathway is regulated through negative feedback loops (Miyazono, 2009). Smad7 is an inhibitory Smad (I-Smad) that represses TGFβ-signaling through multiple mechanisms (Lebrun, 2012). Smad7 acts as an inert decoy by binding to activated TβRI to limit the phosphorylation of Smad2/3 through competitive inhibition (Shi et al., 1998). Moreover, Smad7 promotes the internalization and degradation of TβRI (Taylor et al., 2010) and competes with R-Smads for receptor binding (Miyazono, 2009).
1.2.2 Smad-independent pathways
While the Smad pathway represents the canonical signaling pathway for TGFβ, several non-Smad intracellular signaling cascades have been implicated in mediating the cellular effects of TGFβ (Yi et al., 2005). The stress-activated kinases p38 and Jun N-terminal Kinase (JNK) have been shown to be induced by TGFβ and synergize with Smad signaling to lead to EMT and apoptosis (Lebrun, 2012). TGFβ can also signal through other mitogen activated protein kinase (MAPK) pathways by activating the extracellular-signal-regulated kinases 1 and 2 (ERK1 and ERK2), leading to the induction of EMT (Zhang, 2009). Rho GTPases have been shown to relay TGFβ signals resulting in cytoskeletal reorganization, cell motility and invasion through activation of small GTPase RhoA, Cdc42 GTPases, Rac1 and the tyrosine kinase Src (Miyazono, 2009; Zhang, 2009; Moustakas and Heldin, 2012). TGFβ can also signal through the mTOR and the phosphoinositide 3-kinase (PI3K)/Akt pathways to regulate cell growth inhibition and induction of EMT (Lebrun, 2012).
1.3 Origin of the myofibroblast: Epithelial-Mesenchymal Transition (EMT)
EMT is the process whereby polarized epithelial cells undergo several morphologic and molecular changes to give rise to motile, extracellular matrix-producing mesenchymal cells (Kalluri and Weinberg, 2009). The process of EMT occurs in three distinct biological settings, namely types 1, 2 and 3, with different functional consequences (Kalluri and Weinberg, 2009). EMT that is activated during implantation, embryogenesis and tissue morphogenesis constitute type 1 EMT, where the generated mesenchymal cells subsequently give rise to secondary epithelia (Kalluri and Weinberg, 2009). Type 2 EMT is normally activated during tissue regeneration, wound healing and repair but can be abnormally activated during organ fibrosis due to dysregulated inflammatory responses (Taylor et al., 2010). In the context of organ fibrosis, type 2 EMT can continue to respond to unabated inflammation, ultimately leading to organ destruction (Kalluri, 2009). Lastly, type 3 EMT is activated by neoplastic cells that have undergone genetic or epigenetic changes to facilitate their acquisition of invasive and metastatic phenotypes, that results in the establishment of secondary sites of tumor growth (Kalluri and Weinberg, 2009). TGFβ-induced EMT has been implicated in certain fibrotic eye diseases and falls under the type 2 EMT category as it involves the transition of adult epithelial cells into fibrogenic myofibroblasts (Zeisberg and Neilson, 2009).
1.3.1 The Role of EMT in Fibrosis
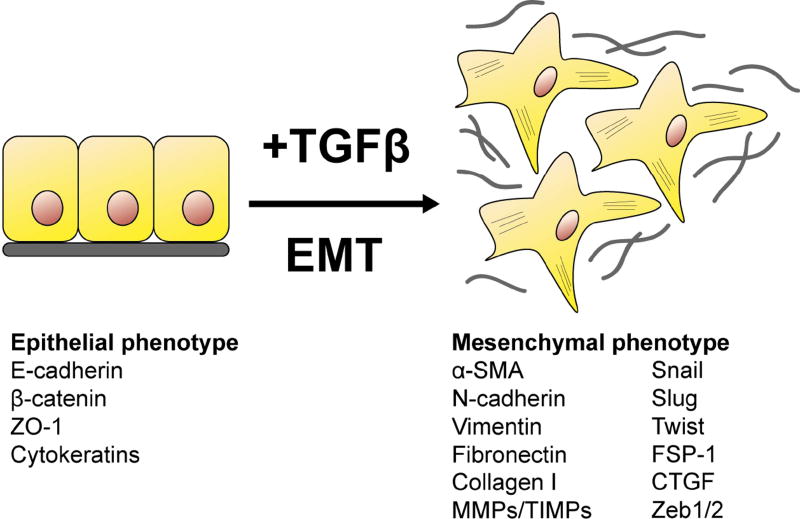
EMT is a complex process that involves the initiation of an intricate cascade of genetic and epigenetic events that culminate in the downregulation of epithelial markers and the upregulation of mesenchymal markers (Taylor et al., 2010). The plasticity of epithelial cells enables them to transdifferentiate during EMT, relinquishing their regular cuboidal morphology and acquiring the characteristic spindle-shape of myofibroblasts (Fig. 2). In undertaking this phenotypic and morphologic transformation, epithelial cells first experience a loss of tight junctional complexes including zonular occludins (ZO)-1 followed by the loss of E-cadherin expression at the cell membrane, resulting also in the dissociation of β-catenin from the membrane that becomes stabilized and translocates to the nucleus (Kalluri and Weinberg, 2009). The loss of E-cadherin facilitates the EMT process and is accompanied by a “cadherin switch” from E-cadherin to N-cadherin, a typical mesenchymal marker (Zeisberg and Neilson, 2009). Epithelial cells undergo a dramatic remodeling of the cytoskeleton with the de novo expression of α-smooth muscle actin (α-SMA) that is incorporated into the newly formed actin stress fibers (Taylor et al., 2010). Other mesenchymal markers include the intermediate filament, vimentin and the fibroblast marker fibroblast-specific protein 1 (FSP-1; Zeisberg and Neilson, 2009). A wide array of transcription factors are involved in controlling the EMT response. Snail transcription factors are zinc finger proteins, of which, Snai1 and Snai2 (formerly known as Slug) play prominent roles in EMT as repressors of E-cadherin expression (Bolos et al., 2003). Twist, a basic helix-loop-helix (bHLH) protein is also upregulated during EMT and can repress E-cadherin independently of Snail (Yang et al., 2007). Another member of the bHLH family, E47 has also been identified as a repressor of E-cadherin and potent inducer of EMT (Cubillo et al., 2013). The zinc finger E-box-binding homeobox (ZEB) 1 and ZEB2 are also transcriptional repressors of E-cadherin and play a key role in fibrotic EMT (Xiong et al., 2012). The completion of EMT is distinguished by the degradation and remodeling of the underlying basement membrane, conferring the newly formed mesenchymal cells with the capability to migrate away from the intact epithelial layer of origin, to facilitate fibrotic tissue contracture (Kalluri and Weinberg, 2009).
Fig. 2. Schematic diagram of epithelial-mesenchymal transition (EMT).
During epithelial-mesenchymal transition, epithelial cells lose their polarity and normal cell-cell and cell-basement membrane adhesions, and transdifferentiate into motile, spindle-shaped mesenchymal cells (myofibroblasts) that secrete extracellular matrix (grey).
2. Corneal fibrotic disease
The cornea is a transparent, avascular tissue composed of three distinct cellular layers: the superficial stratified epithelium, central stroma and endothelium (Fini, 1999). The corneal epithelium provides an ideal barrier from the environment by anchoring itself to its own basement membrane (Chang et al., 1996). The epithelial basement membrane is composed of an organized network of ECM molecules including collagen type IV, thrombospondin, laminins, nidogens, perlecan, matrilins and fibronectin (Kabosova et al. 2007). Adjacent to the epithelial basement membrane is the collagenous Bowman’s layer, composed predominantly of collagen types I, III, V and VI, that protect the underlying corneal stroma (Marshall et al., 1991a; Marshall et al., 1991b). Collagen VII associated with anchoring fibrils of the overlying epithelium is also present within Bowman’s layer (Tisdale et al., 1988). The stroma is composed of keratocytes that maintain the slow turnover of stromal ECM by synthesizing collagen types I and V and keratan sulfate proteoglycans decorated with glucosaminoglycan (GAG) chains (Fini, 1999). The uniquely narrow diameter and uniform, orthogonal arrangement of collagen fibrils and proteoglycans is critical for the maintenance of corneal transparency (Meek and Knupp, 2015). The endothelium also contributes to stromal clarity by controlling ion and fluid movement to maintain stromal hydration through an active pump mechanism (Bourne, 2003).
Due to its anatomical location, the cornea is constantly subject to mechanical trauma and abrasive forces. As a result, it has evolved an efficient system of rapidly resurfacing epithelial defects to avoid microbial infection and further trauma to the underlying stroma (Lee et al., 2013). Migration of the epithelial sheet over the denuded surface and enhanced epithelial cell proliferation, promotes rapid re-epithelialization and the re-establishment of epithelial stratification (Saika et al., 2004a); however, deeper corneal wounds interfere with the proper healing process and result in recurrent corneal erosions, stromal haze, subepithelial fibrosis and epithelial keratinization that may lead to severe visual impairment (Kawashima et al, 2010; Lee et al., 2013). In fact, corneal opacification is the third leading cause of worldwide blindness in the working age population (Foster and Resnikoff, 2005).
2.1 Myofibroblast transdifferentiation in stromal wound healing
Wound healing in the corneal stroma is a complex and regulated sequence of events directed towards wound closure (Ljubimov and Saghizadeh, 2015; Torricelli et al., 2016). The normally quiescent stromal keratocytes transdifferentiate into myofibroblasts and proliferate and migrate towards the site of injury to completely fill the wound, depositing and cross-linking excessive amounts of ECM proteins including collagen and fibronectin resulting in disruption of the normal organization of the corneal ECM (Tandon et al., 2010; Chaurasia et al., 2015). It should be noted that this transdifferentiation process does not constitute an epithelial-mesenchymal transition in the strictest sense, but more a “keratocyte-myofibroblast transformation.” These myofibroblasts contain intracellular α-SMA-labeled filaments, become highly motile and exert strong contractile forces on the ECM, enabling wound closure and restoration of corneal integrity (Wilson, 2012). The expression of α-SMA is directly proportional to the contractile force generated by the myofibroblasts (Jester et al., 1995). Corneal myofibroblasts also express receptors for fibronectin, α5β1 and αvβ3 integrins, that link the actin cytoskeleton to the ECM, enabling the cells to exert the contractile forces that participate in wound matrix reorganization and contraction (Jester et al., 1999a). This contraction by the myofibroblasts disrupts the normal corneal curvature, inducing refractive error and impairing normal corneal function (Fini and Stramer, 2005).
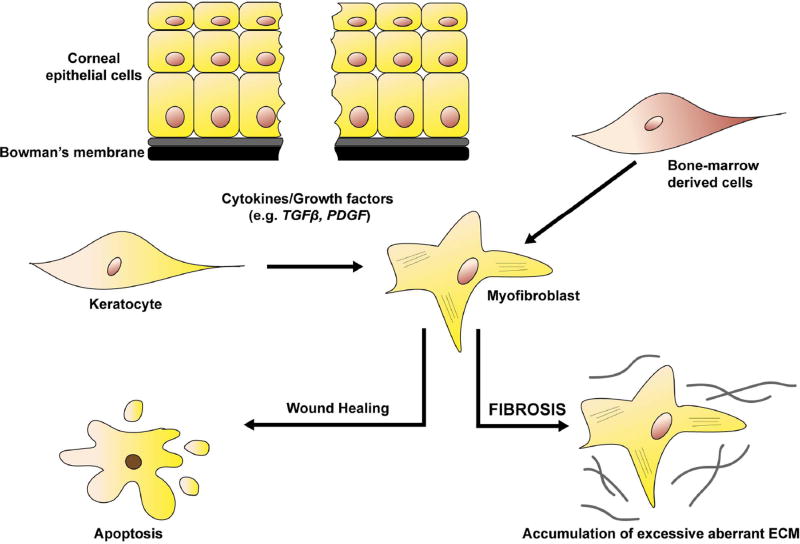
The activated stromal keratocytes (also known as stromal fibroblasts) downregulate the expression of proteins such as corneal crystallins resulting in persistent stromal haze (Jester et al., 1999b; Stramer and Fini, 2004) and start producing matrix metalloproteinases (MMPs) to enable ECM-remodeling at the site of injury (West-Mays and Dwivedi, 2006). Upon completion of wound healing, upregulation of IL-1 produced by stromal cells triggers apoptosis of the myofibroblasts, depriving them of TGFβ (Kaur et al., 2009a; Barbosa et al., 2010a). Keratocytes go on to then reoccupy the anterior stroma, resorb the abnormal ECM proteins, restoring corneal integrity and transparency (Torricelli et al., 2013a). However, any persistence of TGFβ leads to the maintenance of the myofibroblasts that continue to secrete and deposit anomalous ECM leading to corneal opacification (see Fig. 3). This opacification will persist long after the myofibroblasts have disappeared from the site of injury (Wilson, 2012).
Fig. 3. Schematic diagram of the corneal wound healing process.
Corneal injury involving disruption to Bowman’s membrane results in the increased exposure of cytokines and growth factors including TGFβ and PDGF into the anterior stroma. TGFβ induces the normally quiescent keratocytes and/or bone-marrow derived cells to transdifferentiate into myofibroblasts to facilitate corneal repair. During physiological wound healing, myofibroblasts disappear by apoptosis and tissue transparency is maintained. However, in pathological conditions, myofibroblasts persist and secrete excessive amounts of aberrant extracellular matrix proteins (fibrosis) resulting in corneal scarring.
Corneal cells are known to express many different growth factors and cytokines, including members of the fibroblast growth factor (FGF; including keratinocyte growth factor, KGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), TGFβ, insulin-like growth factor (IGF), hepatocyte growth factor (HGF), tumor necrosis factor (TNF), and interleukin (IL) families (for review see Ljubimov and Saghizadeh, 2015). The corneal wound healing process is orchestrated by the coordinated activity of many of these growth factors, in particular TGFβ and PDGF (Wilson, 2012; Singh et al., 2014; see Fig. 3). All three TGFβ isoforms and their receptors are constitutively expressed by the corneal epithelium (Nishida et al., 1994). High levels of TGFβ1 protein have been identified in basal epithelial cells during the corneal wound healing response in rats following excimer laser ablation injury (Tuli et al., 2006). TGFβ has been found to play a key role in the generation of highly metabolically active corneal myofibroblasts (Fini and Stramer, 2005). Myofibroblasts themselves are able to secrete TGFβ and hence, sustain their own viability through autocrine mechanisms once generated (Kaur et al., 2009b).
In vitro studies report that keratocytes can transdifferentiate into myofibroblasts upon treatment with TGFβ (Masur et al., 1996; Petridou et al., 2000; Jester et al., 1999a; Kaur et al., 2009a). Interestingly, these keratocytes displayed a cell density-dependent model of myofibroblast transdifferentiation where low-density cultures expressed increased levels of α-SMA mRNA and protein compared to high-density cultures (Petridou et al., 2000). Moreover, low-density cultures displayed increased functional expression of TGFβ receptors and greater Smad2 nuclear translocation compared to high-density cells (Petridou et al., 2000), indicating that decreased cell density and loss of cell contact during injury may sensitize the keratocytes to TGFβ-driven myofibroblast transdifferentiation.
Following corneal trauma, an influx of bone marrow-derived cells migrate into the corneal stroma from the limbal region (Barbosa et al., 2010b). In an elegant in vivo study, Barbosa et al., showed that green fluorescent protein (GFP)-tagged bone marrow-derived cells co-localized with α-SMA-positive corneal myofibroblasts following corneal injury in mice, indicating that a significant proportion of myofibroblasts in the cornea originated from these bone marrow-derived cells (Barbosa et al., 2010b). Santhiago et al. (2011) corroborated these findings using a rabbit model whereby subconjunctival injection of the anti-fibrotic agent, PRM-151, a recombinant form of human pentraxin-2, significantly reduced myofibroblast generation in rabbit corneas following haze-producing photorefractive keratectomy (PRK; Santhiago et al., 2011). Using an in vitro model, mouse keratocytes and bone marrow-derived cells were both capable of transdifferentiating into myofibroblasts and the interactions between these two cell types potentiated myofibroblast transdifferentiation from either precursor cell type (Singh et al., 2012). The addition of TGFβ inhibitors, TGFβ type I receptor kinase inhibitor (LY-364947) and/or anti-LAP antibody significantly reduced the proportion of α-SMA-positive myofibroblasts indicating that TGFβ was a key factor in promoting myofibroblast transdifferentiation from either cell type (Singh et al., 2012). Moreover, further studies by Singh et al. reported a role for an additional growth factor, PDGF-B to work in concert with TGFβ in this process (see Fig. 3; Singh et al., 2014). Presently, it is unknown whether myofibroblasts generated from corneal fibroblasts have identical properties to myofibroblasts generated from bone marrow-derived cells, nor the conditions under which either differentiation pathway is favored, posing interesting areas for future investigation.
Although myofibroblast transdifferentiation is typically associated with TGFβ-mediated EMT, basal limbal epithelial progenitor cells invading the corneal stroma have been shown to undergo EMT into keratocytes in a rabbit limbal explant model (Kawakita et al., 2005). It should be noted, however, that the authors defined EMT solely by the loss of epithelial markers, and did not investigate any associated mesenchymal markers. Based on this, it is therefore difficult to discern whether cells actually underwent an EMT as no further studies have since been conducted to corroborate this. The process of EMT has also been implicated in the development of corneal subepithelial fibrosis (also known as pannus) secondary to limbal stem cell deficiency (Kawashima et al., 2010). Elevations of α-SMA-positive myofibroblasts and downregulation of the epithelial phenotype marker, E-cadherin were also observed in subepithelial locations in donor human pannus tissue (Kawashima et al., 2010). Other genes, such as Snail1, MMP-2 and MMP-9 were also upregulated in pannus tissue, together with the translocation of β-catenin from intercellular junctions to the cytoplasm, indicating activation of the Wnt signaling pathway (Kawashima et al., 2010). As this study was conducted by comparing pannus specimens with normal human corneas, the EMT process can only be inferred rather than observed in real-time. Hence, it is unclear whether the myofibroblasts were epithelial in origin, with further research required to better characterize the involvement of EMT in the pathogenesis of corneal opacification. Interestingly, human limbal keratocytes have been reported to undergo a mesenchymal-epithelial transition (MET) in cell culture, with acquisition of the epithelial-specific marker, cytokeratin 3 (CK3) (Perrella et al., 2012). It is hypothesized in this study that at the limbus, CK3-positive keratocytes migrate to the site of injury to maintain the epithelial population. Interestingly, as only suprabasal corneal epithelial cells were positive for CK3 (Perrella et al., 2012), this would imply that these cells underwent terminal differentiation independent of their progenitor stage. Another possibility is that the CK3-positive epithelial cells may have been derived from a population of corneal stromal cells co-expressing CD34 and CD105 that exhibit stem-like properties (Hashmani et al., 2013). Further in vivo studies are required to ensure that these findings are not limited to cell culture (Perrella et al., 2012).
The role of Bowman’s layer in regulating the release of cytokines into the corneal stroma is poorly understood. It has been proposed that Bowman’s layer may act as an integral corneal regulatory structure that limits the fibrotic response by modulating the entry of epithelial derived-TGFβ into the stroma (Torricelli et al., 2013b). In PRK, the epithelium is debrided and Bowman’s layer is ablated together with part of the anterior stroma (Moller-Pedersen et al., 1998). It is hypothesized that penetration of Bowman’s layer results in the expression of fibrotic markers and subsequent corneal haze. Laser-assisted in situ keratomileusis (LASIK) is advantageous as Bowman’s layer is only pierced at the borders of the corneal flap allowing for regeneration of the stroma without stimulating a fibrotic response, thus maintaining corneal transparency (Nakamura, 2001). Moreover, epithelial-debridement results in the upregulation of type I and II TGFβ receptor expression by migrating corneal epithelial cells, thereby augmenting the TGFβ signaling response in PRK (Zieske et al., 2001). Despite the presence of structural abnormalities and defects in Bowman’s layer in keratoconic corneas (Sawaguchi et al., 1998), myofibroblasts are not commonly observed, and have only been reported in more severe cases presenting with subepithelial fibrosis (Maatta et al., 2006). Moreover, the fibrotic areas in keratoconus are typically focal rather than widespread (Maatta et al., 2006). Hence, it still remains unclear whether the integrity of Bowman’s layer acts as a physical barrier to cytokines into the stroma to facilitate this keratocyte-myofibroblast transdifferentiation.
BMP-7 and its downstream signaling molecules have shown promise in antagonizing TGFβ-induced corneal stromal injury. Gene transfer of an endogenous inhibitor of TGFβ, BMP-7, into the corneal stroma using gold nanoparticles effectively reduced haze in an in vivo rabbit PRK model (Tandon et al., 2013). BMP-7 target genes, inhibitors of differentiation (Id) 2 and 3 have been found to be constitutively expressed in corneal epithelial cells and stromal keratocytes (Mohan et al., 2016). Treatment of human corneal fibroblasts with TGFβ1 resulted in an acute increase in Id1/2/4 which progressively decreased within 48 hours (Mohan et al., 2016). In contrast, a time-dependent increase in the expression of Id1/2/3 was evident when treated with BMP-7 alone (Mohan et al., 2016). Treatment with a combination of TGFβ1 and BMP-7 revealed a significant increase in Id1 with reduced TGFβ-induced α-SMA expression highlighting the role of Id genes in regulating the anti-fibrotic effects of BMP-7 (Mohan et al., 2016). More recently, treatment of primary human corneal stromal fibroblasts with the histone deacetylase inhibitor (HDACi), ITF2357, reduced myofibroblast transdifferentiation and inhibited ECM components including collagen type I, collagen type IV, fibronectin and integrin αvβ3 expression (Lim et al., 2016). The inhibitory effect of ITF2357 involved the activation of Id3 and suppression of phosphorylation of TGFβ-dependent Smad pathways (Lim et al., 2016). Furthermore, endogenous BMP-7 mRNA and protein is downregulated following alkali burn to the mouse cornea (Saika et al., 2005). Resurfacing of the burned cornea was accelerated by adenoviral gene transfer of BMP-7 that resulted in suppression of myofibroblast generation and reduced expression of TGFβ2 and collagen Iα2 in the affected stroma (Saika et al., 2005). The authors also showed that BMP-7 induced the activation of Smad1/5/8 signaling and partially suppressed phosphorylation of TGFβ-dependent Smad2 (Saika et al., 2005). Targeting the BMP-7 pathway shows promise in abrogating TGFβ-driven corneal stromal fibrosis, and continued research in elucidating the signaling pathways and downstream target genes of BMP-7 is required to better understand its antagonistic effects on TGFβ.
Matricellular proteins have been shown to play a significant role in modulating TGFβ signaling during corneal wound healing (Saika et al., 2002; Saika et al., 2016). Thrombospondin-1 (TSP-1) is a matricellular protein known to activate LAP, the latent complex of TGFβ, enabling TGFβ to interact with its receptors (Schultz-Cherry et al., 1995). In the uninjured cornea, TSP-1 is only present in the endothelial cell layer. However, following corneal abrasion, TSP-1 is elevated in the corneal stroma of the wounded area and disappears from the stroma following re-epithelization in a mouse model (Uno et al., 2004). Using a rat corneal wound healing model, Matsuba et al., found that α-SMA-positive cells co-localized with the stromal zone expressing TSP-1, highlighting the involvement of TSP-1 in keratocyte-myofibroblast transformation (Matsuba et al., 2011). Another matricellular protein, SPARC, has been found to be upregulated during keratocyte-myofibroblast transformation in an in vitro bovine corneal stromal repair model system following treatment with TGFβ (Berryhill et al., 2003). Osteopontin (OPN), a matrix structural glycophosphoprotein, has also been shown to be upregulated during normal corneal stromal wound repair (Miyazaki et al., 2008). OPN-null mice exhibited delayed wound healing following incision injury to the corneal stroma and showed a higher incidence of corneal ulceration and perforation following alkali burn (Miyazaki et al., 2008). The absence of OPN also reduced the appearance of α-SMA-positive myofibroblasts and delayed the expression of collagen Iα2 and TGFβ1 deposition in healing corneas compared to wild type mice (Miyazaki et al., 2008). Similarly, another matricellular protein, tenascin-C was also found to be markedly upregulated following corneal incisional injury in mice (Sumioka et al., 2013). Tenascin C-null mice showed delayed corneal stromal wound healing with reduced appearance of myofibroblasts and decreased expression of collagen Iα1, fibronectin and TGFβ1 compared to wild type mice (Sumioka et al., 2013). In vitro experiments further corroborated these findings showing that the loss of tenascin C abrogated TGFβ1-induced keratocyte to myofibroblast conversion with associated reduction in mRNA expression of collagen Iα1, α-SMA and TGFβ1 (Sumioka et al., 2013). The upregulation of tenascin C has also been well documented following photorefractive keratectomy in corneas of both human (Vesalouma et al., 1995; Vesalouma et al., 1998) and rabbit (Latvala et al., 1995; Wachtlin et al., 1999). Clearly, matricellular proteins play an important role in corneal stromal wound healing and further research into how these ECM components individually and synergistically mediate TGFβ signaling is required.
Several other emerging strategies for targeting TGFβ signaling in corneal fibrosis have been identified. Gene transfer of decorin, an endogenous inhibitor of TGFβ, suppressed the TGFβ-induced upregulation of α-SMA expression and levels of profibrogenic genes including fibronectin, collagen types I, III and IV in cultured human corneal stromal fibroblasts treated with TGFβ (Mohan et al., 2010). Similarly, in vivo, decorin gene therapy effectively reduced corneal scarring following PRK-induced laser injury to the rabbit cornea with no adverse events observed up to 4 months (Mohan et al., 2011; Mohan et al., 2012). Overexpression of a specific fibril collagenase, MMP14, by a single intrastromal injection of MMP14 viral vector also prevented collagen deposition and subsequent scarring with reduced mRNA expression of two key gene markers of corneal fibrosis, α-SMA and type III collagen (Galiacy et al., 2011). In a rabbit corneal injury model, adenoviral-mediated targeted delivery of Smad7 to the corneal stroma also inhibited TGFβ1-induced myofibroblastic transdifferentiation (significant reduction in α-SMA levels), as well as corneal haze and fibrosis post-PRK (Gupta et al., 2017). More recently, treatment with cationic nanoparticles encapsulating a combination of silencing RNAs (siRNAs) targeting TGFβ1, type II TGFβ receptor and connective tissue growth factor (CTGF) significantly reduced the levels of α-SMA expression and scar formation in an organ culture model of excimer ablated rabbit corneas (Sriram et al., 2014). Similarly, pirfenidone-loaded nanoparticles also inhibited TGFβ-induced upregulation of α-SMA, collagen type I expression and subsequent corneal haze using an in vivo rat cornea alkali burn model (Chowdhury et al., 2013). Further research is required to better understand the role of TGFβ in corneal fibrosis to enable promising new translational areas in the development of anti-fibrotic and accelerated healing agents as well as combination gene therapy approaches.
Although blocking TGFβ signaling shows promise in the treatment of corneal fibrosis, it should be noted that TGFβ signaling also plays a key role in the normal corneal wound healing process. Epithelial debridement results in an upregulation of TGFβ receptor expression in migrating corneal epithelial cells indicating the importance of TGFβ signaling in modulating corneal epithelial cell migration during wound repair (Zieske et al., 2001). Corneal epithelial wound healing was significantly delayed in transgenic mice with conditional knockout of the type II TGFβ receptor from the corneal epithelium (Terai et al., 2011). In the absence of TGFβ signaling, in corneal wound healing, expansion of actin stress fibers at the migrating edge was also inhibited (Terai et al., 2011). This wound healing defect was accompanied by a suppression of TGFβ/Smad signaling and a delayed activation of p38/MAPK signaling. This suggests that while canonical Smad signaling is essential for the initial activation of p38/MAPK, there is a possibility that the p38/MAPK cascade is activated later through an alternate signaling cascade that bypasses TGFβ receptor signaling (Terai et al., 2011). It is also important to note that TGFβ isoforms exhibit differential activity in the context of corneal wound healing. Using a 3D culture model of human corneal fibroblasts, treatment with TGFβ1 induced fibrosis and upregulation of α-SMA, while TGFβ3 did not (Guo et al., 2016). The opposing roles of these TGFβ isoforms were associated with a differential regulation of Smad7 protein, whereby TGFβ1 reduced Smad7, while TGFβ3 stimulated Smad7 upregulation (Guo et al., 2016). Moreover, TGFβ3 was found to reduce the expression of α-SMA in both a 3D model of rabbit corneal fibroblasts and ex vivo wounded rabbit cornea model, thereby improving corneal transparency (Sriram et al., 2017). The anti-fibrotic effect of TGFβ3 was found to be associated with an inhibition of TGFβ1-induced expression of PDGF (Sriram et al., 2017). Hence, when designing new therapeutic approaches to corneal fibrosis, targeting different specific downstream targets of TGFβ isoforms, whilst preserving TGFβ signaling pathways required for normal corneal wound healing, may be more efficacious.
2.2 Myofibroblast transdifferentiation in endothelial wound healing
Human corneal endothelial cells (CECs) show marked differences in behavior to epithelial cells and keratocytes in their approach to wound healing, as they are mitotically arrested in the G1 phase of the cell cycle and remain a non-proliferative monolayer throughout life (Roy et al., 2015). CECs heal wounds mainly by their migration and spreading and thus, when the endothelial density diminishes, the normal barrier and pump functions of the endothelial layer no longer suffice to maintain corneal transparency, resulting in corneal edema and eventual vision loss and ocular pain (Roy et al., 2015). Fuchs endothelial corneal dystrophy (FECD), a degenerative disease of the corneal endothelium, is characterized by the chronic loss of morphologically and physiologically altered endothelial cells, necessitating corneal transplantation in severe cases (Okumura et al., 2013).
Endothelial-mesenchymal transition (EndMT), a variant of EMT, has been postulated to play a role in the pathogenesis of FECD. The addition of TGFβ induces CECs to migrate and undergo EndMT, accompanied by loss of ZO-1, upregulation of α-SMA (Zhu et al., 2012) and abnormal ECM secretion, corroborating the clinical features of Descemet’s membrane thickening in FECD (Okumura et al., 2013). Okumura et al. showed that simian CECs underwent TGFβ-induced EndMT through phosphorylation of Smad2, ERK and p38 MAPK signaling (Okumura et al., 2013). Inhibition of TGFβ signaling using SB431542, a TβRI inhibitor, abrogated the EndMT process and allowed expression of the zonular occludens scaffolding protein (ZO-1), a tight junction marker, to be retained in CECs (Okumura et al., 2013). In vitro and in vivo models of corneal endothelial wound healing have shown that addition of Smad7, an inhibitor of canonical TGFβ signaling, suppressed TGFβ-induced fibrosis in CECs, emphasizing the importance of Smad2/3 activation in EndMT (Sumioka et al., 2008).
The capacity to induce EndMT is not exclusive to TGFβ. A pro-mitogenic factor, fibroblast growth factor (FGF2), has also demonstrated the capacity to induce EndMT in CECs and is believed to play a role in the pathogenesis of retrocorneal fibrous membrane (RCFM) (Lee and Kay, 2006a). In RCFM, corneal endothelial cells regain their proliferative ability, lose their contact inhibition and transform into multilayered fibroblast-like cells underneath Descemet’s membrane (Lee and Kay, 2003a). These fibroblast-like cells in turn produce and deposit fibrillary ECM molecules (Lee and Kay, 2003a). FGF2 directly stimulates cell proliferation in CECs by degrading p27 and regulating the translocation of Cdk4 through the PI3K pathway (Lee and Kay, 2003b). The PI3K pathway is also involved in FGF2-induced EndMT of CECs, including synthesis and secretion of collagen type I (Ko and Kay, 2005). The elongated cell morphology and actin cytoskeletal rearrangement induced by FGF-2 in CECs have been associated with the sequential activation of Rho GTPases through PI3K (Lee and Kay, 2006b). FGF2 initially induces the formation of cortical actin by inhibiting Rho activity and activating Rac through the PI3K pathway. This is followed by the formation of protrusive processes by activation of Cdc42 via PI3K, thus allowing cells to migrate to the injury site and produce fibrillary ECM proteins to facilitate repair. Inhibition of RhoA kinase (ROCK) expression has shown promise in enhancing endothelial wound healing by promoting cell adhesion and migration in monkey and human models (Pipparelli et al., 2013). Additionally, ROCK inhibitor eye drops have also been found to accelerate endothelial healing in a rabbit model of FECD with reduction in central corneal edema and improvement in corneal clarity (Okumura et al., 2011; Koizumi et al., 2014). Future studies should be aimed at studying potential inhibitors of EndMT in CECs to further explore novel ways of engineering functional corneal endothelium in vitro by potentially unlocking the mitotic arrest in CECs and permitting endothelial cell regeneration.
3. Pterygium
Pterygium is a wing-shaped fibrovascular outgrowth of the bulbar conjunctiva onto the cornea (Di Girolamo et al., 2004). The corneal epithelium is invaded by the apex of the pterygium, displaying irregularities in epithelial thickness with areas of thinning and fibrous proliferation (Kato and Shimmura, 2008). Other pertinent histological features include squamous metaplasia, goblet cell hyperplasia and an underlying breakdown of Bowman’s layer (Li et al., 2001). It is hypothesized that the epithelial cells present in pterygia acquire an altered balance between proliferation and apoptosis, resulting in a stromal overgrowth of activated fibroblasts, neovascularization, inflammatory cell infiltrate and aberrant ECM accumulation of elastin and collagen (Kato and Shimmura, 2008).
The current understanding of the pathogenesis of pterygium implicates multifactorial mechanisms including ultraviolet (UV) exposure (Threlfall and English, 1999), oxidative stress (Kau et al., 2006; Balci et al., 2011), anti-apoptotic factors (Tan et al., 2000; Liang et al., 2011), inflammatory mediators (Baser et al., 2016), immunologic mediators (Di Girolamo et al., 2002), viral infections (Hsiao et al., 2010), genetic factors (Weinstein et al., 2002), extracellular matrix modulators (Tsai et al., 2010) and growth factors (Kria et al., 1996; Kria et al., 1998; Jin et al., 2003; Nolan et al., 2003). A detailed discussion of these pathogenic factors is beyond the scope of this review and is proficiently covered in other comprehensive reviews (see Di Girolamo et al., 2004; Chui et al., 2008; Liu et al., 2013; Cardenas-Cantu et al., 2014).
Several growth factors have been investigated in the development of pterygium including heparin-binding-epidermal growth factor (HB-EGF) (Nolan et al., 2003), vascular endothelial growth factor (VEGF) (Jin et al., 2003), FGF, PDGF and TGFβ (Kria et al., 1996). TGFβ has been identified as an inducer of myofibroblast transdifferentiation in pterygia (Di Girolamo et al., 2004). Relative to normal conjunctiva, elevated expression levels of TGFβ have been reported in pterygium tissue (Kria et al., 1996) and in pterygium fibroblast cultures (Kria et al., 1998). While all three TGFβ isoforms and their receptors are expressed in cultured pterygium fibroblasts, their expression patterns and modes of action have not been fully characterized (Kria et al., 1998; Lee et al., 2000). Inhibition of TGFβ expression with the anti-fibrotic agent pirfenidone in cultured human pterygium fibroblasts (obtained during pterygium surgery), effectively abrogated cellular proliferation, migration, collagen synthesis and subsequent contraction compared to control cells (Lee et al., 2014). Additionally, application of human amniotic membranes suppressed gene expression of TGFβ2, TGFβ3 and all three TGFβ receptors in cultured pterygium fibroblasts with subsequent inhibition of contractility (Lee et al., 2000). These amniotic membranes have also been shown to reduce α-SMA expression in cultured human pterygial fibroblasts (Sha et al., 2014) and enhance healing of corneal scarring secondary to recurrent pterygium ingrowth in a clinical setting (Gabric et al., 1999).
Pterygial epithelial cells display characteristic features of EMT, including repression of E-cadherin and nuclear accumulation of β-catenin and lymphoid-enhancer-factor-1 (LEF1) (Kato et al., 2007). Apical pterygial epithelial cells invading the corneal stroma express both epithelial (cytokeratin 14) and mesenchymal markers (α-SMA, vimentin, snail, slug), characteristic of EMT (Touhami et al., 2005; Kato et al., 2007). Transmission electron microscopy of human pterygium specimens revealed a population of dissociated epithelial cells surrounded by activated myofibroblastic cells, with processes extending into the corneal stroma (Kato et al., 2007). It is hypothesized that pterygium myofibroblasts may originate from limbal epithelial stem cells, periorbital fibro-adipose tissue posterior to Tenon’s capsule (Kato and Shimmura, 2008), or circulating chemokine receptor (CXCR) 4-positive bone marrow-derived cells, as part of an exaggerated repair process following trauma to the ocular surface (Kim et al. 2013). Moreover, it is still unclear whether TGFβ plays a role in inducing this EMT process and whether the precursor cells of myofibroblasts are locally or systemically derived in pterygium, opening up new avenues for research in understanding the pathogenesis of pterygium (Kim et al. 2016).
Given the complex multifactorial pathogenesis of pterygium, it is important to understand the relationship between all the pathogenic factors and their respective roles in initiating the development of pterygium and/or progression of the disease. Nolan et al. (2003) showed a link between UV irradiation and TGFβ overexpression. Microarray data in UVB irradiated-pterygial epithelial cells showed the upregulation of TGFβ3 and TGFβRIII transcripts; however, changes in protein levels and the activation level of these proteins were not investigated (Nolan et al., 2003). Previous studies have shown that UV irradiation also upregulates inflammatory mediators including IL-6 and IL-8 in pterygium and cultured pterygium epithelial cells (Di Girolamo et al., 2002). The precise mechanisms by which UV-irradiation induces pterygium is yet to be fully elucidated and future research directed at understanding the complex interactions between growth factors, inflammatory mediators and UV irradiation is warranted.
4. Anterior subcapsular cataract and posterior capsular opacification
The crystalline lens has a simple yet highly ordered architecture characterized by an anterior monolayer of cuboidal lens epithelial cells (LECs) overlying a mass of elongated and differentiated fiber cells, all encased within a thick basement membrane, the lens capsule (Lovicu and McAvoy, 2005). Cataract, the loss of lens transparency, is a leading cause of blindness worldwide (Foster and Resnikoff, 2005). The only means of treatment is surgical intervention; however, despite restoring vision initially, 35% of patients develop posterior capsular opacification (PCO), necessitating further surgery (Wormstone et al., 2002).
Both TGFβ1 and TGFβ2 isoforms are constitutively expressed by LECs and exist in the aqueous humour in their latent, inactive forms (Gordon-Thomson et al., 1998). Following ocular trauma e.g. surgery, active levels of TGFβ isoforms become dramatically elevated (Eldred et al., 2011). TGFβ has been found to induce LECs to undergo EMT and transdifferentiate into myofibroblastic cells bearing morphological and biochemical resemblance to human anterior subcapsular cataract (ASC) and PCO (de Iongh et al., 2005). Treatment of LECs with TGFβ induces myofibroblast transdifferentiation and production of aberrant ECM in both rat lens epithelial explants (Liu et al., 1994) and human capsular bag models (Wormstone et al., 2002). In vivo models including intravitreal injection of active TGFβ into rodent eyes (Hales et al., 1999), ectopic overexpression of mature TGFβ in transgenic mouse lenses (Srinivasan et al., 1998; Lovicu et al., 2002) and adenoviral gene delivery of TGFβ into the mouse anterior chamber (Robertson et al., 2007), all induce ASC-like plaques with characteristic EMT features.
ASC has been associated with several conditions involving altered levels of TGFβ expression and/or activation, including trauma (Tang et al., 2003), ocular inflammation, such as atopic dermatitis (Sasaki et al., 1998; Brandonisio et al., 2001; Shu et al., 2016) and iritis (Marcantonio et al., 2003). Biopsy specimens of human ASC have been found to display α-SMA-reactive myofibroblastic cells (Shu et al., 2016), consistent with TGFβ-overexpressing transgenic mice models (Lovicu et al., 2004). Human ASC plaques have been shown to consist of myofibroblastic cells surrounded by accumulations of ECM proteins including fibronectin, laminin, collagen type I and type IV directly beneath the anterior capsule (Joo et al., 1999).
Several features of TGFβ-induced EMT have also been identified in PCO. Residual LECs, that remain on the anterior capsule following cataract surgery, have been found to proliferate, migrate and transdifferentiate to form fibrotic plaques (Wormstone et al, 2002). An in vitro human capsular bag model that involves performing sham cataract operations on human donor lenses, has been used to investigate the pathogenesis of PCO (Liu et al., 1996; Wormstone et al., 1997; Saxby et al., 1998). The transdifferentiated cells in this model have been found to express α-SMA as well as aberrant ECM similar to that of human ASC (Marcantonio et al., 2003).
In a mouse lens injury model of ASC, capsular puncture has been found to upregulate the TGFβ/Smad signaling pathway and induce myofibroblastic transdifferentiation of LECs (Saika et al., 2004b). Pre-treatment with TGFβ-neutralising antibodies prior to capsular puncture injury inhibited the nuclear translocation of Smad3/4 and subsequent lens fibrosis (Saika et al., 2004b). Moreover, injury-induced EMT was inhibited in Smad3 knockout transgenic mice, supporting the role of Smad signaling in lens fibrosis (Saika et al., 2004b). In addition, knockdown of Smad3 signaling using siRNA inhibited the effect of TGFβ2 on cell proliferation and the production of ECM proteins such as fibronectin and collagen type I, whereas siRNA against Smad2 blocked cell migration and the production of α-SMA (Li and Chen, 2011). Silencing both Smad2 and Smad3 effectively blocked the effect of TGFβ2-mediated cell proliferation, migration and ECM production, emphasizing the importance of TGFβ/Smad signaling in mediating EMT in the lens (Li and Chen, 2011). Moreover, adenoviral gene transfer of the inhibitory Smad, Smad7, prevented injury-induced EMT of LECs in mice with reduction in TGFβ1 and EMT markers including α-SMA, lumican and collagen VI (Saika et al., 2004c).
While the Smad signaling pathway represents the canonical signaling pathway for TGFβ, several Smad-independent intracellular signaling cascades have been implicated in regulating EMT in the lens (de Iongh et al., 2005). Chen et al. showed that MAPK/ERK1/2 was activated in TGFβ2-induced EMT, and inhibition of ERK1/2 signaling with U0126, completely prevented TGFβ2-induced expression of α-SMA, fibronectin, collagen types I, IV and suppressed the myofibroblastic phenotype (Chen et al., 2014a). More recently, work in our laboratory have extended these findings to show that ERK1/2 is required for the initiation but not the progression of TGFβ2-induced EMT in rat lens epithelial explants (Wojciechowski et al., 2017). We have also reported that Sprouty (Spry), an antagonist of receptor tyrosine kinases and ERK1/2 signaling, is a negative regulator of TGFβ-signaling in the lens (Shin et al 2012; Lovicu et al., 2016). Conditional knockout of Spry from the lenses of mice induced ASC, with subpopulations of cells expressing α-SMA and a concomitant loss of E-cadherin, highlighting the loss of the epithelial phenotype through an EMT (Shin et al., 2012). Overexpression of Spry in LECs in vivo and in vitro rendered the cells less responsive to TGFβ signaling and maintained lens cell integrity and transparency (Shin et al., 2012; Zhao et al., 2015). Work in our laboratory has also identified a role for the reactive oxygen species (ROS)-producing enzyme, Nox4 in mediating TGFβ2-induced EMT in the lens (Das et al., 2016). Inhibition of Nox4 expression and its production of ROS using a pan-NADPH oxidase inhibitor, VAS2870, suppressed the progression of EMT (Das et al., 2016). Recent work in our laboratory has shown that BMP-7 can also suppress TGFβ2-induced EMT in the lens by upregulating the phosphorylation of BMP-7-responsive Smad1/5 signaling and concurrently downregulating phosphorylation of TGFβ-responsive Smad2/3 nuclear translocation (Shu et al., 2017). The inhibitory activity of BMP-7 on TGFβ2-induced lens EMT is believed to be mediated by BMP-7 target genes, Id2 and Id3 (Kowanetz et al., 2004; Saika et al., 2006; Shu et al., 2017). Furthermore, the Rho/ROCK pathway has been found to promote TGFβ activity leading to cytoskeletal reorganization, cell motility and invasion in LECs (Maddala et al., 2003). Pre-treatment of cells with C3-exoenzyme (an irreversible inhibitor of Rho-GTPase) or the ROCK inhibitor, Y-27632, inhibited TGFβ-induced EMT by abolishing the formation of actin stress fibers and focal adhesions (Maddala et al., 2003). Interestingly, although Smad3 and Rho/ROCK signal activation were both required for TGFβ-induced EMT, their activation was found to be independent of each other, highlighting the complexity of the network of signaling pathways involved in myofibroblastic transdifferentiation in the lens (Cho and Yoo, 2007).
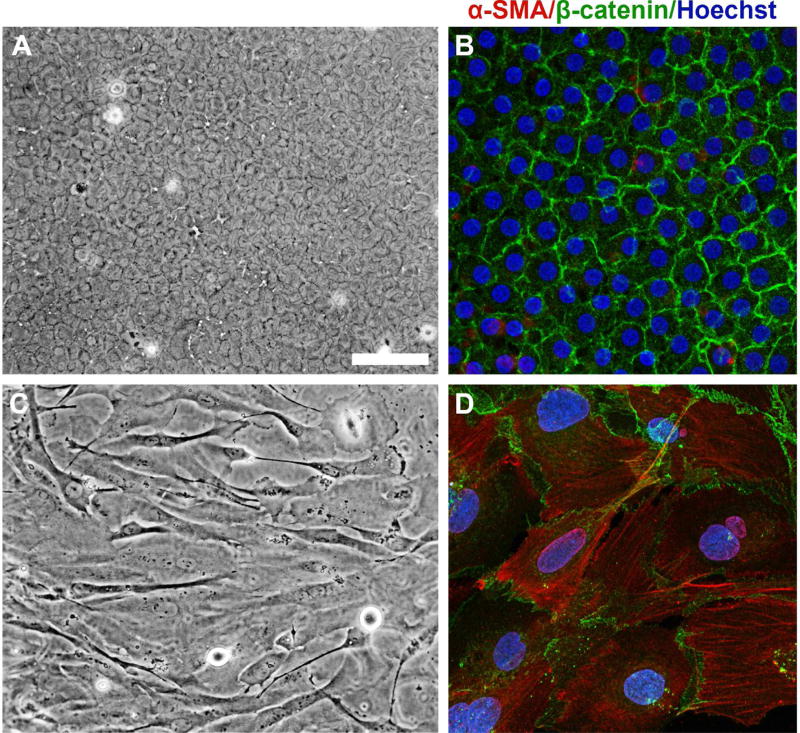
Although animal models have played an essential role in elucidating the growth factor signaling pathways regulating cataractogenesis, human models need to be developed further in order to translate these findings into human applications. Based on our established rat lens epithelial explant model, we have developed a human lens explant system by culturing anterior capsulorhexis flaps obtained during cataract surgery. Similar to the rat lens epithelial explant model, the anterior monolayer of LECs remain adherent to the lens capsule of the capsulorhexis, thus enabling the observation of changes in morphology of the LECs whilst on their native basement membrane. In our rat explant system, treatment of LECs obtained from 21-day-old Wistar rats with 200 pg/ml TGFβ2 induced myofibroblastic transdifferentiation (Das et al., 2016; Shu et al., 2017). Anterior capsulorhexis specimens obtained from patients aged 59 to 76 years undergoing routine surgery for age-related nuclear and/or cortical cataract were subsequently pinned down onto a culture dish in a similar manner to rat lens epithelial explants. Treatment with a higher dose of TGFβ2 (10 ng/ml) was required to induce transdifferentiation of the normal human LECs into spindle-shaped myofibroblastic cells, with loss of membranous β-catenin labeling and acquisition of α-SMA stress fibers (Fig. 4), similar to the rat lens epithelial explant system (Das et al., 2016; Shu et al., 2017). This in vitro human model will enable the translation of animal-based EMT research to the human lens and may contribute to the development of novel pharmaceutical alternatives for the treatment of ASC and PCO.
Fig. 4. A novel human lens epithelial explant model for studying cataractogenesis.
Following 5 days of culture, phase contrast microscopy shows that untreated human lens epithelial explants (A, anterior capsulorhexis tissue obtained in surgery) remain as a normal cuboidal layer of epithelial cells, with membranous localization of β-catenin and minimal α-SMA (B) viewed with confocal microscopy. Immunofluorescence labeling was performed as described previously (Shu et al., 2016). Treatment with 10 ng/ml TGFβ2 displayed an elongate, spindle-shaped morphology (C). Although β-catenin remained localized to the membrane in TGFβ2-treated explants, the label no longer highlighted the normal cobblestone-packed arrangement as in control explants (D). TGFβ-treated explants exhibited strong immunoreactivity for α-SMA, co-labeling to stress fibers (D). Scale bar: 100 µm (A, C), 25 µm (B, D).
5. Glaucoma
Glaucoma is a progressive optic neuropathy characterized by loss of retinal ganglion cells (RGCs) resulting in morphological changes of the optic nerve head (ONH) and associated visual field defects. Despite the presence of normotensive glaucoma (NTG) where IOP is not elevated (CNTGS 1998), intraocular pressure (IOP) is the only modifiable risk factor, and reducing IOP is the mainstay of treatment for glaucoma (Bagnis et al., 2011). The first report of myofibroblast transdifferentiation was observed in trabecular meshwork (TM) cells of patients with corticosteroid-induced ocular hypertension (Johnson et al., 1997) and has since been reported in TM cells, astrocytes and laminar cribrosa cells in primary open angle glaucoma (POAG) (Prendes et al., 2013). Studies regarding myofibroblast transdifferentiation in glaucoma have been limited to those with elevated IOP, and to the best of our knowledge no information regarding its role in NTG has been reported.
Elevated levels of TGFβ have been detected in both aqueous humor (Tripathi et al. 1994) and ONHs (Pena et al., 1999) of patients with POAG. It is postulated that TGFβ contributes to ECM changes evident in the TM, leading to increased aqueous humor outflow resistance and elevated IOP (Fuchshofer and Tamm, 2012; Prendes et al., 2013). In the ONH, TGFβ induces fibrotic changes at the lamina cribrosa (LC) resulting in mechanical deformation of RGC axons, impairing axonal transport and neurotrophic supply (Fuchshofer and Tamm, 2012). Additionally, TGFβ also plays a key role in the aberrant wound healing response of conjunctival fibroblasts following filtration glaucoma surgery (Schlunck et al., 2016). In the following sections, we will elaborate on the molecular mechanisms that control TGFβ signaling in these areas of glaucomatous damage.
5.1 Myofibroblast transdifferentiation of trabecular meshwork cells
The TM forms an integral part of the conventional outflow pathway through which the aqueous humor is drained (Takahashi et al., 2014). Since the rate of aqueous humor production is unchanged in POAG (Brubaker, 1991), the primary cause of elevated IOP is due to the increased outflow resistance in the TM (Johnson, 2006). TM cells are continuously exposed to growth factors and cytokines present in the aqueous humor that trigger microenvironmental changes in the trabeculae (Takahashi et al., 2014). Cultured human TM cells and ex vivo human TM tissue from normal and glaucomatous eyes express transcripts for all three TGFβ receptor isoforms (Wordinger et al., 1998). The levels of TGFβ2 are significantly higher in TM cells (Tovar-Vidales et al., 2011) and in the surrounding aqueous humor of patients with POAG (Picht et al., 2001; Ochiai and Ochiai, 2002; Ozcan et al., 2004; Yamamoto et al., 2005; Trivedi et al., 2011). Importantly, the level of biologically active TGFβ2 was found to be significantly elevated in the aqueous humor of patients with POAG compared to those without glaucoma (Min et al., 2006; Agarwal et al., 2015). Interestingly, POAG patients have been found to have higher levels of active TGFβ2 compared to those with other forms of glaucoma, specifically primary angle closure glaucoma, uveitic glaucoma and pseudoexfoliative glaucoma (Inatani et al., 2001).
The association between elevated levels of both TGFβ2 levels and IOP in POAG, raises the cause-and-effect question: does TGFβ induce elevated IOP or vice versa? Using in vitro monkey and pig anterior ocular segment culture models, infusion with TGFβ2 induced elevated IOP and reduced aqueous humor outflow facility (Bhattacharya et al., 2009). Similarly, adenoviral gene transfer of active human TGFβ2 (Shepard et al., 2010) and TGFβ1 (Robertson et al., 2010) to rodent eyes also resulted in elevated IOP. Thus, it appears that elevated levels of aqueous humor-derived TGFβ may induce increased IOP leading to subsequent glaucomatous damage.
Exposure to TGFβ induces TM cells to undergo myofibroblastic transdifferentiation with concomitant upregulation of mesenchymal markers including α-SMA in primary human TM cells (Tamm et al., 1996; Pattabiraman and Rao, 2010; Pattabiraman et al., 2014) and primary monkey TM cell lines (Takahashi et al., 2014). The myofibroblastic TM cells display a spindle-shaped phenotype with distinct α-SMA-labeled stress fibers (Tamm et al., 1996). Contrary to these findings, adenoviral transfer of the active TGFβ1 isoform in the rodent eye resulted in reduced α-SMA expression despite a substantial increase in ECM proteins (Robertson et al., 2007). However, it should be noted that the morphologic changes observed in this rodent model were more consistent with primary angle closure glaucoma compared to POAG, and that the TGFβ2 isoform is reported to be more elevated compared to TGFβ1 in POAG (Inatani et al., 2001). Since TM cells are not epithelial in nature, the myofibroblastic transdifferentiation of these cells cannot be categorized as an EMT response in the strictest sense, although it has been described to display “EMT-like” features including loss of cell-cell contact, acquisition of the mesenchymal phenotype, increase in cell motility, upregulation of ECM ligands and phosphorylation of downstream TGFβ signaling molecules such as Smad2 (Takahashi et al., 2014).
Biomechanical alterations in the TM play a key role in the increased outflow resistance observed in glaucoma (Overby et al., 2014). Outflow resistance is regulated through a homeostatic mechanism (Keller et al., 2009). TM cells are sensitive to changes in pressure or mechanical stretching of the cribriform and juxtacanalicular TM regions, and adjust their ECM turnover accordingly through selective proteinase secretion and activation, targeted ECM degradation and synthesis of replacement ECM components (Acott and Kelley, 2008). MMPs-1 and -3 are significantly elevated in primary human TM cells isolated from POAG patients compared to healthy donor eyes (Ronkko et al., 2007). It is postulated that the persistent elevation of these MMPs may result in the continual cleavage of ECM components and subsequent overproduction of replacement ECM (Keller et al., 2009). In contrast, TGFβ has been found to upregulate plasminogen activator inhibitor- (PAI)-1, which is a known inhibitor of ECM degradation (Zhao et al., 2004; Fleenor et al., 2006). Interestingly, TGFβ2-induced upregulation of PAI-1 and reduced MMP-2 activity leading to the accumulation of ECM proteins in the TM of glaucomatous eyes (Fuchshofer et al., 2003). Indeed, accumulation of fibronectin in trabeculae by mesenchymal TM cells contributes to the increased outflow resistance (Takahashi et al., 2014). Taken together, it is likely that a dynamic balance between ECM degradation and synthesis exists in the TM, and disruption of normal ECM turnover profoundly affects outflow facility.
Changes in the cytoskeletal architecture of the TM have been observed in glaucomatous patients (WuDunn, 2009). TGFβ enables TM cells to acquire a high level of motility as it disrupts the normal cell-cell interactions, as well as interactions between the cells and the dense collagen- and elastic fiber-rich TM lamellae (TM beams) (Takahashi et al., 2014). TGFβ also increases the expression and activity of tissue transglutaminase, an enzyme that catalyzes irreversible cross-linking of TM fibronectin (Welge-Lussen et al., 2000). Tissue transglutaminase has been found in higher amounts in patients with POAG, and tissue transglutaminase from TM cells of POAG patients exhibit higher enzymatic activity (Tovar-Vidales et al., 2011). Increased cross-linked fibronectin may contribute to the elevated TM stiffness and subsequent obstruction of aqueous outflow observed in patients with POAG (Last et al., 2011). Additionally, cross-linked actin network (CLAN) formation has been observed following exposure to dexamethasone in cultured human (Clark et al., 1994) and bovine (Wade et al., 2009) TM cells. CLAN formation has also been identified following treatment with TGFβ2 despite the absence of dexamethasone in bovine TM cells (O’Reilley et al., 2011). Blockade of the TGFβ2 receptor, treatment with a TGFβ2-neutralising antibody or inhibition of TGFβ2/Smad3 signaling effectively blocked this CLAN formation (O’Reilley et al., 2011). More recently, TGFβ2 has been shown to induce CLAN formation in cultured nonglaucomatous human TM cells through both Smad-dependent and Smad-independent pathways (Montecchi-Palmer et al., 2017). The authors showed that TGFβ2-induced CLAN formation was completely blocked using inhibitors of the TGFβ receptor, Smad3 and ERK signaling and was partially blocked by inhibitors of JNK, p38 and ROCK, indicating varying levels of involvement of each of these signaling pathways (Montecchi-Palmer et al., 2017). Given that CLANs are polygonal networks of actin filaments, they have the potential to alter the biochemical behavior of TM cells including stiffness, contractility, phagocytic activity and ECM turnover, that all ultimately influence outflow facility (Overby and Clark, 2015).
Multiple signaling pathways and transcription factors have been implicated in the myofibroblast transdifferentiation of TM cells. Pattabiraman and Rao found that TGFβ-activated Rho/ROCK signaling is involved in enhancing actomyosin assembly in TM cells (Pattabiraman and Rao, 2010). The resultant increased contractile force generated by the transformed TM cells is presumed to play a key role in altering the geometry and stiffness of the pressure-sensitive trabecular outflow pathway, thus reducing aqueous humor outflow facility. Additionally, CTGF is markedly upregulated in cultured TM cells treated with TGFβ, and is involved in remodeling cell-matrix adhesions by enhancing the expression of ECM molecules and collagen type IV binding integrins (Fuchshofer et al., 2007; Fuchshofer and Tamm, 2009). Knockdown of CTGF expression by RNA interference prevented TGFβ-induced upregulation of fibronectin in TM cells (Junglas et al., 2012).
The actions of both TGFβ and CTGF on ECM remodeling in the TM can also be abrogated by BMPs, namely BMP-7 and BMP-4. BMP-7 suppressed TGFβ-induced expression of CTGF, fibronectin and collagen types IV and VI (Fuchshofer et al., 2007). The antagonistic effect of BMP-7 on TGFβ signaling is dependent on Smad7 which is known to play a critical role in the auto-inhibitory negative feedback regulation of TGFβ signaling (Fuchshofer et al., 2009). Both BMP-7 and TGFβ increase Smad7 expression in TM cells; however, their combined activity induces sufficient Smad7 to block TGFβ-induced effects on ECM synthesis (Fuchshofer et al., 2009). Depletion of Smad7 by RNA interference completely inhibited the antagonizing effect of BMP-7 on TGFβ signaling (Fuchshofer et al., 2009). Wordinger et al. showed that BMP-4 exhibited similar properties to BMP-7 in counteracting the TGFβ activity in TM cells and significantly reduced the induction of fibronectin (Wordinger et al., 2007). Gremlin, a BMP antagonist, blocked the negative effect of BMP-4 on TGFβ resulting in induction of fibronectin and elevated IOP when added to the medium of ex vivo cultured human anterior segments (Wordinger and Clark, 2007). More recently, Mody et al. showed that BMP-4 attenuated the pro-fibrotic effects of TGFβ2 in human TM cells by upregulating its downstream target genes, Id1 and Id3 (Mody et al., 2017). On the other hand, adenoviral gene transfer of BMP-2 in rat eyes induced calcification in TM cells with resultant IOP elevation (Buie et al., 2013). Hence, further research is warranted in clarifying the contrasting roles of various BMP isoforms in the pathogenesis of glaucoma.
5.2 Myofibroblast transdifferentiation of astrocytes and laminar cribrosa cells in optic nerve head fibrosis
In glaucoma, elevated IOP leads to damage of RGC axons and ECM remodeling at the LC region of the ONH (Wallace and O’Brien, 2016). The LC is a sieve-like structure consisting of perforated fibroelastic plates through which RGC axons pass through before converging as the optic nerve (Schneider and Fuchshofer, 2016). The laminar plates contain ECM macromolecules including elastin and collagen types I, III, V and VI (Schneider and Fuchshofer, 2016). ECM remodeling in this region results in fibrosis and biomechanical stress to RGC axons (Schneider and Fuchshofer, 2016). Moreover, ECM changes may also contribute to impaired antero- and retrograde axonal transport of neurotrophic factors leading to further RGC degeneration (Anderson and Hendrickson, 1974). ECM remodeling of the LC in glaucoma is due to altered expression patterns of the two main cell types found at the LC, namely optic nerve astrocytes and LC cells (Hernandez, 2000).
Optic nerve astrocytes are essential for maintaining RGC homeostasis, providing cellular support to RGC axons at the laminar cribrosa and prelaminar regions of the ONH (Hernandez, 2000). Raised IOP or optic disc hypo-perfusion stimulate the normally quiescent astrocytes to transdifferentiate into reactive astrocytes with myofibroblastic properties (Pena et al., 1999). TGFβ2 has been localized to reactive astrocytes in glaucomatous ONHs, and levels of TGFβ2 have been found to be 70–100 times higher in glaucomatous ONHs compared to age-matched controls (Pena et al., 1999). It is hypothesized that reactive astrocytes secrete TGFβ2 once activated, facilitating ECM remodeling (Zode et al., 2011). Reactive astrocytes migrate into nerve fiber layer bundles and synthesize new ECM macromolecules including collagen types IV and VI (Gottanka et al., 2005), elastin (Pena et al., 1998), laminin and tenascin (Qu and Jakobs, 2013) in the laminar region; characteristic signs of glaucomatous discs.
Laminar cribrosa cells, localized within or between the cribriform plates of the LC region also play a crucial role in ECM remodeling by producing ECM when exposed to TGFβ (Kirwan et al., 2005b), or exposed to mechanical stretch (Kirwan et al., 2005a). Treatment with TGFβ induces pro-fibrotic ECM gene expression in the LC region, and interestingly, CTGF has also been reported as one of the highly expressed genes in the glaucomatous TM and is similarly upregulated in glaucomatous ONHs (Kirwan et al., 2005b). Pretreatment of LC cells with an anti-CTGF antibody suppressed the expression of pro-fibrotic genes including fibronectin, α-SMA and collagen type I, induced by incubation with aqueous humor from glaucoma patients (Wallace et al., 2013). In the early stages of glaucoma, the LC undergoes thickening and posterior migration, and later, shearing and collapse of the LC plates leads to a thin fibrotic connective tissue scar with increased deposition of collagens and elastin (Wallace and O’Brien, 2016). The aberrant accumulation of connective tissue disrupts the biomechanical properties of the lamina cribrosa, and connective tissue septae surrounding RGC axons leading to the characteristic clinical signs of glaucomatous cupping and excavation of the optic disc (Fuchshofer and Tamm, 2012).
The regulation of ECM deposition is dependent on a homeostatic balance between ECM synthesis and degradation. In glaucoma, the increased synthesis of ECM proteins is also accompanied by an impaired degradation system leading to aberrant ECM accumulation in the LC region of the ONH (Schneider and Fuchshofer, 2016). The major enzymes involved in ECM degradation are the matrix metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of MMPs (TIMPs) (Kompass et al., 2008). Microarray analyses of glaucomatous primate ONHs exhibited an upregulation of MMP-1 and TIMP-1 compared to healthy ONHs (Kompass et al., 2008). Treatment of ONH astrocytes with TGFβ increased expression of TIMPs, further amplifying ECM accumulation and elastosis (Neumann et al., 2008). In contrast, treatment of LC cells induced elevated levels of MMP-2 (Kirwan et al., 2005a). Hence, an imbalance of ECM production and degradation at the LC region also plays a key role in ONH fibrosis.
Although CLANs were previously believed to be exclusively found in TM cells, recent evidence shows that CLANs can be induced in LC cells in both glaucomatous human eye specimens and cultured bovine LC cells (Job et al., 2010). Similar to TM cells, CLANs in LC cells were significantly upregulated with dexamethasone treatment and such CLANs were larger and more abundant in LC cells cultured from glaucoma donors (Job et al., 2010). Intriguingly, CLANs were not associated with ONH astrocytes (Job et al., 2010). It is interesting that these unique cytoskeletal structures are found in two of the major tissues associated with glaucomatous damage. The biomechanical changes in cytoskeletal actin observed in CLANs may play a significant role in compromising cellular behavior at the LC and TM. The precise biological and pathogenic functions of CLAN formation in glaucoma continues to be an active area of research.
It is clear that much progress has been made in characterizing the pathological mechanisms underlying degeneration of the trabecular meshwork and RGCs in glaucoma. While current therapies for glaucoma aimed at lowering IOP are effective in slowing disease progression, they do not specifically target the underlying pathological mechanisms. Hence, continued research into this area will enable the development of new therapeutic strategies that impart TM-protective and neuro-protective capabilities to halt progression, or possibly even reverse glaucomatous damage.
5.3 Myofibroblast transdifferentiation of conjunctival fibrosis following glaucoma filtration surgery
Glaucoma filtration surgery, in particular trabeculectomy, involves creating a new transscleral route for aqueous humor to exit into the subconjunctival space, thereby lowering IOP (Schlunck et al., 2016). A major post-operative complication is conjunctival fibrosis at the wound site that may seal off this new route, leading to inadequate IOP reduction. Currently, the antimetabolite mitomyocin C (MMC) is the mainstay anti-fibrotic treatment for glaucoma filtration surgery (Amoozgar et al., 2016). A single treatment effectively inhibits cell proliferation by intercalating DNA (Schlunck et al., 2016). Due to the direct DNA damage and excess inhibition of cell proliferation, adverse events such as bleb leakage, blebitis, hypotony, endophthalmitis and conjunctival erosion over the tube have been reported (Yamanaka et al., 2015; Amoozgar et al., 2016). Hence, there is interest in developing alternative drugs to maximize bleb survival and minimize adverse events.
Glaucoma filtration surgery induces tissue trauma, triggering a localized inflammatory response involving platelet activation, recruitment of macrophages and polymorphonuclear cells, and release of cytokines including TGFβ, PDGF and VEGF-A (Schlunck et al., 2016). PDGF drives the proliferation and propagation of fibroblasts (Pierce et al., 1989) while TGFβ induces the transdifferentiation of fibroblasts into contractile, ECM-producing myofibroblasts (Desmouliere et al., 1993). Using a rabbit model of glaucoma filtration surgery, subconjunctival injections of an anti-TGFβ2 antibody effectively reduced conjunctival scarring both in vitro and in vivo, with minimal damage to local tissue (Cordeiro et al., 1999). Despite this promising finding, the antibody showed no superiority to placebo in two phase III clinical trials (CAT-152 Trabeculectomy Study Group, 2007). Fibroblasts, platelets and inflammatory cells have been found to release the TGFβ1 isoform which is not targeted by this antibody (Schlunck et al., 2016). It is possible that the TGFβ2 isoform may play a less significant role in a clinical setting, suggesting that more generalized and isoform-independent inhibition of TGFβ signaling may be warranted. Instead of targeting the TGFβ ligand, Nakamura et al. inhibited activation of the TGFβ II receptor using siRNA in an in vivo mouse model, which blocked TGFβ-induced matrix deposition and inflammation (Nakamura et al., 2004). Moreover, treatment with TGFβ II receptor-specific siRNA significantly impeded cell migration in an in vitro culture of human corneal fibroblasts (Nakamura et al., 2004). Although effective, it is possible that blocking TGFβ signaling on a receptor level may also compromise the normal ocular healing process that is dependent on epithelial migration (Yamanaka et al., 2010). It may be more advantageous to block downstream TGFβ signaling pathways specifically involved in myofibroblast transdifferentiation, whilst maintaining the TGFβ signaling pathways required for epithelial homeostasis and normal healing.
Similar to fibrotic changes at the TM and laminar region of the ONH, increased expression levels of TGFβ and CTGF have been reported in conjunctival bleb tissue following glaucoma filtration surgery in a rabbit model, reaching peak concentrations at 5-days post-surgery (Esson et al., 2004). Exogenous addition of CTGF increased the rate of conjunctival bleb fibrosis, highlighting its role in the scarring process (Esson et al., 2004). Hence, it may be more efficacious to inhibit both TGFβ and CTGF signaling to enhance the success rate of glaucoma filtration surgery.
A promising approach to suppression of injury-induced conjunctival scarring is through activation of peroxisome proliferator-activated receptor (PPAR)-γ signaling. Yamanaka et al. showed that overexpression of PPAR-γ using an adenoviral vector effectively inhibited TGFβ1-induced upregulation of collagen type I, fibronectin and CTGF in cultured human subconjunctival fibroblasts (Yamanaka et al., 2009). These findings were further supported by in vivo experiments using an injury-induced conjunctival scarring mouse model where topical application of the PPAR-γ adenoviral vector inhibited macrophage invasion, myofibroblast transdifferentiation and upregulation of collagen type 1A2, CTGF and monocyte chemotactic protein (MCP)-1 (Yamanaka et al., 2009). Another promising strategy for reducing post-operative scarring is through topical application of the anti-allergy drug, tranilast (Chihara et al., 2002). Following trabeculectomy in human patients, 0.5% tranilast used thrice daily for 3 months induced enlargement of the filtering bleb and reduced postoperative IOP (Chihara et al., 2002). It is believed that tranilast suppresses post-operative scarring and inflammation by inhibiting the actions of various cytokines including interleukins and TGFβ (Ikeda et al., 1996). Although PPAR-γ and tranilast show therapeutic potential, further characterization of the mechanisms underlying their roles in modulating TGFβ signaling is required.
Biomechanical cues play a significant role in scar formation. Increased tissue stiffness facilitate myofibroblast transdifferentiation and subsequent fibrosis. The Rho subfamily of small GTPases have important functions in regulating actin cytoskeletal organization, cell adhesion and motility (Fukata et al., 2001). Rho kinases (ROCKs) are critical for regulating focal adhesions and stress fiber formation (Riento and Ridley, 2003). Targeted inhibition of Rho kinase (ROCK) I using Y-27632 abrogated cell contractility and α-SMA expression in human Tenon fibroblast cells and prevented post-operative scar formation in a rabbit model of glaucoma filtration surgery (Honjo et al., 2007). Hence, further investigation into the role of biomechanical signaling in conjunctival scarring is warranted, and ultimately, integrating our knowledge on biochemical and mechanical signaling cascades will enable the development of novel combination approaches to modulate conjunctival scarring.
6. Proliferative retinal disease
In the retina, myofibroblast transdifferentiation manifests in diseases such as proliferative vitreoretinopathy (PVR), subretinal fibrosis in neovascular age-related macular degeneration (AMD), and fibrovascular membrane formation associated with proliferative diabetic retinopathy (PDR) (Friedlander, 2007). Neovascularization is initiated by rupture of the blood-retinal barrier, through which serum growth factors, cytokines and inflammatory cells penetrate into the vitreous cavity and/or sub-retinal space (Xiao et al., 2014). These new vessels are abnormal and leak fluid, resulting in retinal edema and hemorrhaging. Vision loss is due to fibrovascular scar proliferation and secondary tractional retinal detachment.
6.1 Proliferative vitreoretinopathy
PVR is a severe complication of surgical repair for rhegmatogenous retinal detachment (RD), characterized by the formation of fibrous membranes on the epiretinal surface (Charteris, 1995). The contraction of these epiretinal membranes (ERMs) results in distortion of the retinal architecture due to retinal wrinkling, retinal folds and ultimately, tractional retinal detachment (RD) (Tamiya and Kaplan, 2016). Ultrastructural studies show that PVR membranes are composed of a heterogenous population of cells including retinal pigment epithelial (RPE) cells, fibroblasts, myofibroblasts, glial cells and macrophages (Oberstein et al., 2011). Of these cell types, fibroblasts and myofibroblasts are considered the primary contractile cellular phenotypes within the ERM and play a crucial role during the contractile phase of PVR (Tamiya and Kaplan, 2016).
Emerging evidence indicates that myofibroblast transdifferentiation of RPE cells plays a key role in the pathogenesis of PVR (Wiedemann, 1992; Bochaton-Piallat et al., 2000; Xiao et al., 2014). In the normal eye, RPE cells form a cobblestone-packed monolayer of immotile, polarized and non-proliferative cells connected by apical tight and adherens junctions (Tosi et al., 2014). During this EMT, RPE cells lose their specialized cell-to-cell adhesions and acquire migratory mesenchymal characteristics (Tamiya and Kaplan, 2016). Transdifferentiated RPE cells express α-SMA in stress fibers, increasing their contractility (Tamiya and Kaplan, 2016). A recent analysis of human PVR membranes confirmed that the majority of α-SMA-expressing myofibroblasts were derived from cytokeratin-positive RPE cells (Feist et al., 2014).
In PVR, the compromised retinal architecture and breakdown of the blood-retinal-barrier exposes RPE cells to growth factors and inflammatory cells resident in the vitreous and systemic circulation (Pastor et al., 2002). Levels of TGFβ are upregulated in both the vitreous body and ERMs in human PVR patients (Connor et al., 1989; Baudouin et al., 1993; Bochaton-Piallat et al., 2000; Kita et al, 2008; Winkler and Hoerauf, 2011), and expression levels of TGFβ2 are positively correlated with disease severity (Connor et al., 1989). Moreover, the TGFβ2 isoform and its receptor (TGFβ-RII) have been identified within excised human PVR membranes (Cui et al., 2007). Numerous studies have shown that TGFβ induces myofibroblast transdifferentiation in RPE cells in human (Stocks et al., 2001; Li et al., 2011; Xiao et al., 2014; Dvashi et al., 2015) and murine models (Saika et al., 2004b; Saika et al., 2007) of PVR.
Several in vivo and in vitro studies demonstrate the importance of Smad signaling in myofibroblast transdifferentiation of RPE cells. In Smad3-deficient mice, suppression of the EMT process in both TGFβ2-treated RPE cells and in an experimental retinal detachment model was reported (Saika et al., 2004d). Inhibition of the nuclear translocation of Smad2/3 using the anti-fibrotic drug pirfenidone also prevented TGFβ1-induced EMT in a human RPE cell line (Choi et al., 2012). Overexpression of Smad7 attenuated phosphorylation of Smad2/3 in RPE cells treated with TGFβ2 and concurrently inhibited collagen type I and fibronectin, highlighting the key role of Smad signaling in the progression of EMT in RPE cells (Saika et al., 2007).
Smad-independent signaling pathways also play a significant role in regulating the EMT process in RPE cells. TGFβ2 activates the p38 MAPK pathway and blockade of this pathway inhibited the expression and synthesis of collagen type I in human RPE cells (Kimoto et al., 2004). Chen et al. showed that TGFβ2 induced the activation of ERK1/2 and Jagged/Notch signaling in human RPE cells (Chen et al., 2014b). Inhibition of ERK1/2 signaling using U0126 prevented the progression of EMT in RPE cells by abrogating both the Smad and Jagged/Notch signaling pathways (Chen et al., 2014b). Interestingly, blockade of Jagged/Notch signaling using DAPT also inhibited TGFβ2-induced activation of ERK1/2 signaling suggesting cross-interaction amongst the ERK1/2, Jagged/Notch and Smad signaling pathways (Chen et al., 2014b). The synthesis of collagen type I in RPE cells is also dependent on the TGFβ2-induced activation of the PI3K/Akt signaling pathway (Yokoyama et al., 2012). MicroRNAs, in particular miR-29b, play a role in the pathogenesis of PVR by negatively regulating Akt2 signaling (Li et al., 2016). Trichostatin A (TSA), a histone deacetylase inhibitor (HDACi) has been shown to inhibit both the canonical Smad signaling pathway and non-canonical pathways including Jagged/Notch, PI3K/Akt, and MAPK/ERK1/2 pathways, and in doing so, suppresses TGFβ-induced morphological changes in RPE cells, preventing the up-regulation of α-SMA, fibronectin, collagen types I and IV, Snail and Slug (Xiao et al., 2014). Clearly, much progress has been made in elucidating the multiple signaling pathways activated during TGFβ-induced EMT of RPE cells and targeting these intracellular mediators holds promise as potential therapy in inhibiting contracture of retinal tissue in PVR (Yang et al., 2015).
6.2 Proliferative diabetic retinopathy
One of the leading causes of visual loss in the working-age population is diabetic retinopathy (Yau et al., 2012). Diabetes induces retinal ischemia and hypoxia leading to the proliferation of new blood vessels across the retinal surface. The outgrowth of new blood vessels is accompanied by an accumulation of ECM resulting in the formation of fibrovascular membranes that extend into the vitreous (Abu El-Asrar et al., 2015). Although these membranes are only present in approximately 8% of PDR patients (Smiddy et al., 1995), they can lead to devastating visual consequences due to tractional RD following hemorrhage and contracture of the membranes (Friedlander, 2007).
Fibrovascular ERMs that form in some patients with proliferative diabetic retinopathy (PDR) have been observed to contain aggregations of myofibroblasts (Abu El-Asrar et al., 2006). The origin of these myofibroblasts is yet to be fully elucidated; however, recent evidence suggests that they may be endothelial in origin (Abu El-Asrar et al., 2015). EndMT has been identified as an important mechanism in the pathogenesis of cardiac, kidney and pulmonary fibrosis, as endothelial cells lose their apical-basal polarity and form migratory, spindle-shaped mesenchymal cells (Piera-Velazquez et al., 2011). The cytochemical changes accompanying the morphologic changes include decreased expression of endothelial markers (e.g. CD31, vascular endothelial (VE)-cadherin) and acquisition of mesenchymal markers (e.g. α-SMA, fibroblast-specific protein-1; Piera-Velazquez et al., 2011).
Human PDR fibrovascular membranes were found to contain a heterogeneous population of myofibroblasts derived from both endothelial cells via EndMT and from circulating bone marrow-derived fibroblasts that traffic to sites of injury (Abu El-Asrar et al., 2015). Retinal endothelial cells treated with TGFβ1 and CTGF, as well as pro-inflammatory cytokines, interleukin (IL)-1β and tumor necrosis factor-α were found to lose endothelial cell markers and acquire mesenchymal markers (Abu El-Asrar et al., 2015). Co-stimulation with IL-1β and TGFβ2 resulted in the synergistic induction of EndMT (Abu El-Asrar et al., 2015). Myofibroblasts were found to secrete both MMP-2 (Noda et al., 2003) and MMP-9 (Abu El-Asrar et al., 2007) which facilitate endothelial cell migration and liberate matrix bound growth factors such as VEGF that play a significant role in neovascularization. Further research is required to elucidate the pro-inflammatory and pro-fibrotic pathways contributing to the progression of PDR.
6.3 Subretinal fibrosis in neovascular age-related macular degeneration
AMD is the leading cause of blindness over the age of 65 in developed countries (Lim et al., 2012). Loss of central vision occurs in 10–15% of cases as a consequence of choroidal neovascularization (CNV) (Ferris et al., 1984). AMD is characterized by the accumulation of angiogenic drusen and atrophic RPE changes that impede the diffusion of oxygen from the choriocapillaris to the photoreceptors. This results in ischaemia and hypoxia that stimulates the growth of new blood vessels that extend into the subretinal space (Lim et al., 2012). These new vessels are leaky and hemorrhage, leading to the formation of end- stage subretinal fibrotic plaques or disciform scars (Ryan, 1979).
Intravitreal anti-VEGF injections are the mainstay of treatment for CNV; however, inhibiting angiogenic cytokines does not address the underlying ischaemic and inflammatory pathophysiology of neovascular AMD (Ishikawa et al., 2016). Although anti-VEGF therapy improves visual function in the majority of patients, unsuccessful treatment outcomes can be attributed, in part, to the progression of subretinal fibrosis. Subretinal fibrosis is a wound healing response that follows CNV that is characterized by proliferation and infiltration of various cell types including glial cells, RPE, macrophages, fibroblasts and myofibroblastic cells, that interact with growth factors and inflammatory cytokines resulting in substantial remodeling of the ECM (Kent and Sheridan, 2003).
The presence of inflammatory cells and α-SMA-positive transdifferentiated RPE cells have been identified in surgically excised human CNV fibrous membranes (Lopez et al., 1996). A gradient change was observed in the CNV membranes from cytokeratin-positive, mildly α-SMA-positive RPE adjacent to the normal RPE monolayer, to cytokeratin-negative and strongly α-SMA-positive cells in the stroma (Lopez et al., 1996). In vitro, low-density culture of RPE cells promotes more rapid transdifferentiation compared to tightly interconnected cultures due to the diminished support by cell-cell contacts. Furthermore, TGFβ does not initiate EMT in RPE with well-established cell-cell contacts suggesting that disruption of these contacts plays a significant role in the initiation of EMT in RPE cells (Tamiya et al., 2010). This is corroborated by the clinical progression of neovascular AMD where RPE detachment and dissociation has been found to precede the development of CNV (Ambati and Fowler, 2012).
The complex interactions of growth factors including TNF-α, TGFβ, FGF-2, EGF and PDGF direct RPE cell behavior, promoting pro-fibrotic activities such as cell proliferation, transdifferentiation, migration and ECM remodeling through a coordinated interplay of several signaling pathways (Ishikawa et al., 2016). There, under the guidance of TGFβ in particular, secretion of ECM and remodeling begin (Ishikawa et al., 2016). Concomitantly, VEGF facilitates angiogenesis and initiates remodeling and maturation through pericyte recruitment, resulting in the formation of a local mature arterial and venous supply within the area (Ishikawa et al., 2016). Angiogenesis continues until a state of normoxia or hyperoxia exists, thereby switching off VEGF synthesis (Ishikawa et al., 2016). Finally, after many months, wound quiescence gives rise to a cicatricial membrane causing local destruction of photoreceptors, RPE and choroidal vessels, resulting in irreversible damage to the macula (Ishikawa et al., 2016).
Due to the complexity of cellular interactions and the numerous mediators involved, there is a paucity of research in effective therapeutic interventions for fibrosis. One molecular target showing promise in the treatment of subretinal fibrosis is sphingosine-1-phosphate (S1P), a pro-angiogenic growth factor that interacts with pro-fibrotic mediators including TGFβ, CTGF and PDGF (Caballero et al., 2009). Intravitreal injection of anti-S1P monoclonal antibodies potently suppressed CNV lesion volume, angiogenesis and markedly attenuated collagen deposition in a murine model of laser-induced CNV (Caballero et al., 2009). Neutralization of S1P activity using monoclonal antibodies clearly has potential as a novel therapy for AMD and human clinical trials are currently underway (Caballero et al., 2009).
7. Orbital fibrosis in Graves’ ophthalmopathy
Graves’ ophthalmopathy (GO), also known as thyroid eye disease, is an autoimmune disease associated with dysregulated thyroid gland activity (Wang and Smith, 2014). It is characterized by extensive inflammation, expansion, remodeling and fibrosis of the soft tissues surrounding the eyes, resulting in enlargement of the extraocular muscles and accumulation of retrobulbar fat and connective tissue (Bahn, 2010). Due to the rigidity of the bony orbit, this increased orbital tissue volume displaces the globe forward resulting in clinical signs of proptosis, swollen eyelids, keratitis, conjunctivitis and optic neuropathy from optic nerve compression (Dik et al., 2016).
Orbital fibroblasts have been implicated as the primary target cells of the autoimmune process in GO (Dik et al., 2016). Once activated, orbital fibroblasts fulfil their key roles in orbital inflammation and tissue remodeling in GO by increasing their proliferative activity, producing inflammatory cytokines and differentiating into adipocytes or myofibroblasts (Dik et al., 2016). The adipogenic and myofibroblastic differentiation potential of orbital fibroblasts is determined by the expression of the cell-surface marker, Thy-1 (CD90) (Smith et al., 2002; Koumas et al., 2003). When treated with TGFβ, Thy-1-positive orbital fibroblasts differentiate into myofibroblasts, displaying strong α-SMA immunoreactivity and produce excessive amounts of ECM components (Koumas et al., 2003). Thy-1 negative fibroblasts, however, exhibit a high capacity to differentiate into adipocytes (Koumas et al., 2003). The degree of Thy-1 expression determines disease progression in GO, including the extent of fibrosis and tissue expansion/remodeling (Dik et al., 2016). Agonists of the nuclear receptor peroxisome proliferator-activated receptor-gamma (PPARγ) have been found to suppress TGFβ-induced activation of orbital fibroblasts and associated orbital inflammation by regulating energy homeostasis and metabolic function (Koumas et al., 2003). Hence, further exploration of the potential molecular mechanisms underlying PPARγ agonists is warranted in the development of novel treatment strategies for GO.
8. Conclusions, challenges and future directions
Significant progress has been made over the past few years in understanding the mechanisms underlying fibrogenesis, particularly in the context of the eye. A growing number of novel mediators and biochemical signaling pathways have been implicated in myofibroblastic transdifferentiation that can be exploited in developing anti-fibrotic therapies. It is clear that TGFβ signaling plays a key role in the pathogenesis of ocular fibrosis; however, given the diversity of known regulatory factors of TGFβ signaling and the complexity of the underlying signaling pathways, crosstalk and feedback is highly likely and the interactions and integration of downstream TGFβ signaling molecules in fibrogenesis remain elusive. Moreover, an inherent functional redundancy in the activity of these signaling molecules may also exist, resulting in a highly robust signaling network that is resistant to treatment. Further research is therefore required to identify key elements in the system that may lack redundancy and are susceptible to targeted treatment. The current challenge is to develop a comprehensive connectivity model of the molecular mechanisms regulating TGFβ signaling using high-throughput proteomic mapping of molecular targets and signaling networks with time-resolved analysis. In doing so, it may be possible to identify a “master switch” that integrates these dynamic inputs and controls myofibroblast transdifferentiation.
It is clear that multiple signaling pathways, transcription factors and inhibitory agents are common across the various fibrotic eye diseases, highlighting similarities in the underlying pathophysiology of ocular fibrosis. In particular, Smad7 gene therapy has been found to be efficacious in corneal stromal fibrosis (Gupta et al., 2017), endothelial wound healing (Sumioka et al., 2008), injury-induced lens fibrosis (Saika et al., 2004c) and EMT of RPE cells (Saika et al., 2007). BMPs and their downstream target genes, Id, have shown promise in antagonizing TGFβ-induced corneal stromal injury (Saika et al., 2005; Tandon et al., 2013; Mohan et al., 2016), EMT of lens epithelial cells (Kowanetz et al., 2004; Saika et al., 2006; Shu et al., 2017) and myofibroblast transdifferentiation of TM cells (Wordinger et al., 2007; Fuchshofer et al., 2009; Mody et al., 2017). Interestingly, the inhibitory effect of BMP-7 on TGFβ signaling has been shown to be dependent on upregulation of Smad7 in myofibroblast transdifferentiation of TM cells (Fuchshofer et al., 2009), indicating a link between BMPs and Smad7. Since these potential drug targets are effective across multiple ocular structures, further research is required to develop tissue-specific drugs. Concerted efforts in mechanistic biomedical research is critical in identifying key elements of the pathophysiological networks underlying ocular fibrosis to enable their selective targeting. Given the complexity of TGFβ-mediated fibrosis, it is likely that a combined targeting of several factors may be necessary.
Although TGFβ plays a key role in ocular scarring, it is also required for normal physiological wound healing. Hence, when designing new therapeutic approaches to corneal fibrosis, this dual role of TGFβ needs to be taken into consideration. It may be more advantageous to block downstream TGFβ signaling pathways specifically involved in myofibroblast transdifferentiation whilst preserving and harnessing the TGFβ signaling pathways required for homeostasis and normal healing. Whether it is an excess of TGFβ that induces ocular scarring or perhaps the timing of its upregulation that dictates how TGFβ switches between its physiological and pathological functions would be a pertinent area of research to pursue.
Improved culture models are also required to study myofibroblast transdifferentiation. Conventional 2D-culture systems allow the basic identification of pathways involved in the morphological transformation of epithelial cells; however, may not be reflective of the in vivo situation. Whilst in vivo studies are invaluable, they are more challenging and expensive. Instead, 3D-cultures may hold promise in preserving the polarity of epithelial cells and more accurately depict the mechanical properties of fibrotic tissue, in particular, ECM aggregation and subsequent matrix contraction. Understanding the local and global matrix properties within tissues is crucial in understanding how cells communicate, coordinate and adapt to their dynamic environment, and how mechanical signals influence cell signaling networks.
Beyond the eye, significant progress has been made in understanding fibrosis in various parts of the body with progression into the development of anti-fibrotic clinical drugs. Similar to many fibrotic eye diseases, TGFβ-driven myofibroblast transdifferentiation is evident in various non-ocular fibrotic diseases (Wynn and Ramalingam, 2012). Drugs designed to inhibit TGFβ-signaling such as Freolimumab (Trachtman et al., 2011) and Bortezomib (Fineschi et al., 2008) have shown promise in renal and pulmonary fibrosis. Although these therapeutic agents are designed for systemic fibrotic diseases, the novel pathogenic regulators targeted by these agents can be investigated further in the context of fibrotic eye disease.
Ultimately, the challenge ahead is the development of a cogent plan to translate experimental innovations into clinical practice. A limitation with most cell culture models is that morphological changes associated with myofibroblast transdifferentiation are observed within a few days or weeks while most human fibrotic eye diseases progress slowly over the course of months or years. A major obstacle will be in designing and administering effective clinical trials that may be protracted and costly, as fibrotic eye diseases generally progress slowly.
Future studies should be aimed at identifying early biomarkers associated with pathological wound healing responses to enhance clinical decision making and predictions of patient prognosis. The overarching goal is to inhibit the pathological aspects of fibrosis whilst promoting the beneficial aspects of our vital normal wound healing response. As we continue to learn new information about the pathogenesis of fibrotic eye disease, we will be able to develop new and effective anti-fibrotic therapies to combat these visually impairing and costly clinical problems.
Highlights.
TGFβ plays a major role in EMT leading to different forms of eye fibrotic disease
The myofibroblast results from this EMT contributing to the fibrotic pathology
Multiple and overlapping signaling pathways are involved in ocular fibrogenesis
Acknowledgments
Supported by the NIH (R01 EY0-3177) and the Rebecca L. Cooper Foundation, Australia. DYS was supported through an Australian Postgraduate Award PhD scholarship and a Sydney Eye Hospital Foundation Postgraduate scholarship. The authors thank Keith Ong for providing the human anterior capsulorhexis samples. Human ethics was approved by The University of Sydney Human Research Ethics Committee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial disclosures or conflicts of interest.
References
- Abu El-Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur. Cytokine. Netw. 2006;17:155–165. [PubMed] [Google Scholar]
- Abu El-Asrar AM, van den Steen PE, Al-Amro SA, Missotten L, Opdenakker G, Geboes K. Expression of angiogenic and fibrogenic factors in proliferative vitreoretinal disorders. Int. Ophthalmol. 2007;27:11–22. doi: 10.1007/s10792-007-9053-x. [DOI] [PubMed] [Google Scholar]
- Abu El-Asrar AM, De Hertogh G, van den Eynde K, Alam K, van Raemdonck K, Opdenakker G, van Damme J, Geboes K, Struyf S. Myofibroblasts in proliferative diabetic retinopathy can originate from infiltrating fibrocytes and through endothelial-to-mesenchymal transition (EndoMT) Exp. Eye. Res. 2015;132:179–189. doi: 10.1016/j.exer.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp. Eye. Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoozgar B, Lin SC, Han Y, Kuo J. A role for antimetabolites in glaucoma tube surgery. Curr Opin Ophthalmol. 2016;27:164–169. doi: 10.1097/ICU.0000000000000244. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest. Ophthalmol. 1974;13:771–783. [PubMed] [Google Scholar]
- Agarwal P, Daher AM, Agarwal R. Aqueous humor TGF-β2 levels in patients with open-angle glaucoma: a meta-analysis. Mol. Vis. 2015;21:612–620. [PMC free article] [PubMed] [Google Scholar]
- Bagnis A, Papdia M, Scotto R, Traverso CE. Current and emerging medical therapies in the treatment of glaucoma. Expert Opin Emerg Drugs. 2011;16:293–307. doi: 10.1517/14728214.2011.563733. [DOI] [PubMed] [Google Scholar]
- Bahn BS. Graves’ ophthalmolopathy. N Engl J Med. 2010;25:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balci M, Sahin S, Mutlu FM, Yagci R, Karanci P, Yildiz M. Investigation of oxidative stress in pterygium tissue. Mol. Vis. 2011;17:443–447. [PMC free article] [PubMed] [Google Scholar]
- Barbosa FL, Chaurasia S, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Stromal interleukin-1 expression in the cornea after haze-associated injury. Exp. Eye. Res. 2010a;91:456–461. doi: 10.1016/j.exer.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FL, Chaurasia SS, Culter A, Asosingh K, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye. Res. 2010b;91:92–96. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baser G, Sivrikoz ON, Karahan E, Un ES, Yildirim H. The influence of chemokine CXCR4 and cyclooxygenase-2 in the recurrence of pterygium. Ocul. Immunol. Inflamm. 2016;22:1–5. doi: 10.3109/09273948.2015.1116588. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Fredj-Reygrobellet D, Brignole F, Negre F, Lapalus P, Gastaud P. Growth factors in vitreous and subretinal fluid cells from patients with proliferative vitreoretinopathy. Ophthalmic. Res. 1993;25:52–59. doi: 10.1159/000267221. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Gabelt BT, Ruiz J, Picciani R, Kaufman PL. Cochlin expression in anterior segment organ culture models after TGFbeta2 treatment. Invest. Ophthalmol. Vis. Sci. 2009;50:551–559. doi: 10.1167/iovs.08-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill BL, Kane B, Stramer B, Fini EM, Hassell JR. Increased SPARC accumulation during corneal repair. Exp. Eye. Res. 2002;77:85–92. doi: 10.1016/s0014-4835(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Bochaton-Piallat ML, Kapetanios AD, Donati G, Redard M, Gabbiani G, Pournaras CJ. TGF-beta1, TGF-beta receptor II and ED-A fibronectin expression in myofibroblast of vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 2000;41:2336–2342. [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell. Sci. 2003;116:449–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Bourne WM. Biology of the corneal endothelium in health and disease. Eye (Lond) 2003;17:912–918. doi: 10.1038/sj.eye.6700559. [DOI] [PubMed] [Google Scholar]
- Brandonisio TM, Bachman JA, Sears JM. Atopic dermatitis: a case report and current clinical review of systemic and ocular manifestations. Optometry. 2001;72:94–102. [PubMed] [Google Scholar]
- Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture] Invest. Ophthalmol. Vis. Sci. 1991;32:3145–3166. [PubMed] [Google Scholar]
- Buie LK, Karim MZ, Smith MH, Borras T. Development of a model of elevated intraocular pressure in rats by gene transfer of bone morphogenetic protein 2. Invest. Ophthalmol. Vis. Sci. 2013;54:5441–5455. doi: 10.1167/iovs.13-11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero S, Swaney J, Moreno K, Afzal A, Kielczewski J, Stoller G, Cavalli A, Garland W, Hansen G, Sabbadini R, Grant MB. Exp. Eye. Res. 2009;88:367–377. doi: 10.1016/j.exer.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas-Cantu E, Zavala J, Valenzuela J, Valdez-Garcia JE. Molecular basis of pterygium development. Semin. Ophthalmol. 2014;31:1–17. doi: 10.3109/08820538.2014.971822. [DOI] [PubMed] [Google Scholar]
- CAT-152 0201 Trabeculectomy Study Group. Grehn F, Hollo G, Khaw P, Overton B, Wilson R, Vogel R, Smith Z. Factors affecting the outcome of trabeculectomy: an analysis based on combined data from two phase III studies of an antibody to transforming growth factor beta2, CAT-152. Ophthalmology. 2007;114:1831–1838. doi: 10.1016/j.ophtha.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Chang SW, Hu FR, Hou PK. Corneal epithelial recovery following photorefractive keratectomy. Br. J. Ophthalmol. 1996;80:663–668. doi: 10.1136/bjo.80.7.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charteris DG. Proliferative vitreoretinopathy: pathobiology, surgical management, and adjunctive treatment. Br. J. Ophthalmol. 1995;79:953–960. doi: 10.1136/bjo.79.10.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Lim RR, Lakshminarayanan R, Mohan RR. Nanomedicine approaches for corneal diseases. J. Funct. Biomater. 2015;6:277–298. doi: 10.3390/jfb6020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Smad4 and FAST-1 in the assembly of activing-responsive factor. Nature. 1997;389:85–89. doi: 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye S, Xiao W, Wang W, Luo L, Liu Y. ERK1/2 pathway mediates epithelial-mesenchymal transition by cross-interacting with TGFβ/Smad and Jagged/Notch signaling pathways in lens epithelial cells. Int. J. Mol. Med. 2014a;33:1664–1670. doi: 10.3892/ijmm.2014.1723. [DOI] [PubMed] [Google Scholar]
- Chen X, Xiao W, Wang W, Luo L, Ye S, Liu Y. The complex interplay between ERK1/2, TGFβ/Smad, and Jagged/Notch signaling pathways in the regulation of epithelial-mesenchymal transition of retinal pigment epithelial cells. PLos. One. 2014b;9:e96365. doi: 10.1371/journal.pone.0096365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara E, Dong J, Ochiai H, Hamada S. Effects of tranilast on filtering blebs: a pilot study. J. Glaucoma. 2002;11:127–133. doi: 10.1097/00061198-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Yoo J. Rho activation is required for transforming growth factor-beta-induced epithelial-mesenchymal transition of lens epithelial cells. Cell. Biol. Int. 2007;31:1225–1230. doi: 10.1016/j.cellbi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Choi K, Lee K, Ryu SW, Im M, Kook KH, Choi C. Pirfenidone inhibits transforming growth factor-β1-induced fibrogenesis by blocking nuclear translocation of Smads in human retinal pigment epithelial cell line ARPE-19. Mol. Vis. 2012;18:1010–1020. [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Guha R, Trivedi R, Kompella UB, Konar A, Hazra S. Pirfenidone nanoparticles improve corneal wound healing and prevent scarring following alkali burn. PLoS. One. 2013;8:e70528. doi: 10.1371/journal.pone.0070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui J, Di Girolamo N, Wakefield D, Coroneo MT. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul. Surf. 2008;6:24–43. doi: 10.1016/s1542-0124(12)70103-9. [DOI] [PubMed] [Google Scholar]
- Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 1994;35:281–294. [PubMed] [Google Scholar]
- Connor TB, Jr, Roberts AB, Sporn MB, Danielpour D, Dart LL, Michels RG, de Bustros S, Enger C, Kato H, Lansing M. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J. Clin. Invest. 1989;83:1661–1666. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro MF, Gay JA, Khaw PT. Human anti-transforming growth factor-beta2 antibody: a new glaucoma anti-scarring agent. Invest. Ophthalmol. Vis. Sci. 1999;40:2225–2234. [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- Cubillo E, Diaz-Lopez A, Cuevas EP, Moreno-Bueno G, Peinado H, Montes A, Santos V, Portillo F, Cano A. E47 and Id1 interplay in epithelial-mesenchymal transition. PLoS. One. 2013;8:e59948. doi: 10.1371/journal.pone.0059948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JZ, Chiu A, Maberley D, Ma P, Samad A, Matsubara JA. Stage specificity of novel growth factor expression during development of proliferative vitreoretinopathy. Eye (Lond) 2007;21:200–208. doi: 10.1038/sj.eye.6702169. [DOI] [PubMed] [Google Scholar]
- Darby I, Skalli O, Gabbiani G. α-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab. Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- Darby IA, Laverdet B, Bonte F, Desmouliere A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SJ, Lovicu FJ, Collinson EJ. Nox4 plays a role in TGF-β-dependent lens epithelial to mesenchymal transition. Invest. Ophthalmol. Vis. Sci. 2016;57:3665–3673. doi: 10.1167/iovs.16-19114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells. Tissues. Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- Denissova NG, Liu F. Repression of endogenous Smad7 by Ski. J. Biol. Chem. 2004;279:28143–28148. doi: 10.1074/jbc.M404961200. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell. Biol. 1993;122:103–1111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. UVB-mediated induction of interleukin-6 and-8 in pterygia and cultured human pterygium epithelial cells. Invest. Ophthalomol. Vis. Sci. 2002;43:3430–3437. [PubMed] [Google Scholar]
- Di Girolama N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog. Retin. Eye. Res. 2004;23:195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Diegelmann RF. Cellular and biochemical aspects of normal and abnormal wound healing: an overview. J. Urol. 1997;157:298–302. [PubMed] [Google Scholar]
- Dik WA, Virakul S, van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp. Eye. Res. 2016;142:83–91. doi: 10.1016/j.exer.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Dugina V, Fontao L, Chaponnier C, Vsiliev J, Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J. Cell. Sci. 2001;114:3285–3296. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- Dvashi Z, Goldberg M, Adir O, Shapira M, Pollack A. TGF-β1 induced transdifferentiation of RPE cells is mediated by TAK1. PLoS. One. 2015;10:e0122229. doi: 10.1371/journal.pone.0122229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldred JA, Dawes LJ, Wormstone IM. The lens as a model for fibrotic disease. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011;366:1301–1319. doi: 10.1098/rstb.2010.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esson DW, Neelakantan A, Iyer SA, Blalock TD, Balasubramanian L, Grotendorst GR, Schulz GS, Sherwood MB. Expression of connective tissue growth factor after glaucoma filtration surgery in a rabbit model. Invest. Ophthalomol. Vis. Sci. 2004;45:485–491. doi: 10.1167/iovs.03-0485. [DOI] [PubMed] [Google Scholar]
- Feist RM, Jr, King JL, Morris R, Witherspoon CD, Guidry C. Myofibroblast and extracellular matrix origins in proliferative vitreoretinopathy. Graefes. Arch. Clin. Exp. Ophthalmol. 2014;252:347–357. doi: 10.1007/s00417-013-2531-0. [DOI] [PubMed] [Google Scholar]
- Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch. Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- Fineschi S, Bongiovanni M, Donati Y, Djaafar S, Naso F, Goffin L, Argiroffo CB, Pache JM, Ferrari-Lacraz S, Chizzolini C. In vivo investigations on anti-fibrotic potential of proteasome inhibition in lung and skin fibrosis. Am. J. Respir. Cell. Mol. Biol. 2008;39:458–465. doi: 10.1165/rcmb.2007-0320OC. [DOI] [PubMed] [Google Scholar]
- Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye. Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24:S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFβ2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest. Ophthalmol. Vis. Sci. 2006;47:226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- Fleisch MC, Maxwell CA, Barcellos-Hoff MH. The pleiotropic roles of transforming growth factor beta in homeostasis and carcinogenesis of endocrine organs. Endocr. Relat. Cancer. 2006;13:379–400. doi: 10.1677/erc.1.01112. [DOI] [PubMed] [Google Scholar]
- Foster A, Resnikoff S. The impact of Vision 2020 on global blindness. Eye (Lond) 2005;19:1133–1135. doi: 10.1038/sj.eye.6701973. [DOI] [PubMed] [Google Scholar]
- Friedlander M. Fibrosis and diseases of the eye. J. Clin. Invest. 2007;117:576–586. doi: 10.1172/JCI31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp. Eye. Res. 2003;77:757–765. doi: 10.1016/s0014-4835(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Yu AH, Welge-Lussen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2007;48:715–726. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Stephan DA, Russell P, Tamm ER. Gene expression profiling of TGFβ2- and/or BMP-7-treated trabecular meshwork cells: identification of Smad7 as a critical inhibitor of TGF-β2 signaling. Exp. Eye. Res. 2009;88:1020–1032. doi: 10.1016/j.exer.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER. Modulation of extracellular matrix turnover in the trabecular meshwork. 2009;88:683–688. doi: 10.1016/j.exer.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell. Tissues. Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Kaibuchi K, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends. Pharmacol. Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- Gabric N, Mravicic I, Dekaris I, Karaman Z, Mitrovic S. Human amniotic membrane in the reconstruction of the ocular surface. Doc. Ophthalmol. 1999;98:273–283. doi: 10.1023/a:1002423621010. [DOI] [PubMed] [Google Scholar]
- Galiacy SD, Fournie P, Massoudi D, Ancele E, Quintyn JC, Erraud A, Raymond-Letron I, Rolling F, Malecaze F. Matrix metalloproteinase 14 overexpression reduces corneal scarring. Gene. Ther. 2011;18:462–468. doi: 10.1038/gt.2010.159. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr. Opin. Cell. Biol. 2001;13:584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- Gordon-Thomson C, de Iongh RU, Hales AM, Chamberlain CG, McAvoy JW. Differential cataractogenic potency of TGF-beta1, -beta2, and –beta3 and their expression in the postnatal rat eye. Invest. Ophthalmol. Vis. Sci. 1998;39:1399–1409. [PubMed] [Google Scholar]
- Gottanka J, Kuhlmann A, Scholz M, Johnson DH, Lutjen-Drecoll E. Pathophysiologic changes in the optic nerves of eyes with primary open angle and pseudoexfoliation glaucoma. Invest. Ophthalmol. Vis. Sci. 2005;46:4170–4181. doi: 10.1167/iovs.05-0289. [DOI] [PubMed] [Google Scholar]
- Guo X, Hutcheon AEK, Zieske JD. Molecular insights on the effect of TGF-β1/-β3 in human corneal fibroblasts. Exp. Eye. Res. 2016;146:233–241. doi: 10.1016/j.exer.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Rodier JT, Sharma A, Giuliano EA, Sinha PR, Hesemann NP, Ghosh A, Mohan RR. Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo. PLoS ONE. 2017;12:e0172928. doi: 10.1371/journal.pone.0172928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales AM, Chamberlain CG, Dreher B, McAvoy JW. Intravitreal injection of TGFbeta induces cataract in rats. Invest. Ophthalmol. Vis. Sci. 1999;40:3231–3236. [PubMed] [Google Scholar]
- Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Wang LC, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF, Sheppard D, Gardner HA, Violeette SH. αvβ6 Integrin Regulates Renal Fibrosis and Inflammation in Alport Mouse. Am. J. Pathol. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmani K, Branch MJ, Sidney LE, Dhillon PS, Verma M, McIntosh OD, Hopkinson A, Dua HS. Characterization of corneal stromal stem cells with the potential for epithelial transdifferentiation. Stem Cell Res. Ther. 2013;4:75. doi: 10.1186/scrt226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog. Retin. Eye. Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotech. 2003;14:538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. α-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol. Biol. Cell. 2003;14:2508–2519. doi: 10.1091/mbc.E02-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Kameda T, Kawaji T, Yoshimura N, Araie M. Potential role of Rho-associated protein kinase inhibitor Y-27632 in glaucoma filtration surgery. Invest. Ophthalmol. Vis. Sci. 2007;122:103–111. doi: 10.1167/iovs.07-0878. [DOI] [PubMed] [Google Scholar]
- Hsiao CH, Lee BH, Ngan KW, Chuang WY, Yeung L, Yeh LK, Tan HY, Hui-Kang D, Lin KK. Presence of human papillomavirus in pterygium in Taiwan. Cornea. 2010;43:3430–3437. doi: 10.1097/ICO.0b013e3181afdb06. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Inao M, Fujiwara K. Inhibitory effect of tranilast on activation and transforming growth factor β1 expression in cultured rat stellate cells. Biochem. Biophys. Res. Commun. 1996;227:322–327. doi: 10.1006/bbrc.1996.1508. [DOI] [PubMed] [Google Scholar]
- Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Kannan R, Hinton DR. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp. Eye. Res. 2016;142:19–25. doi: 10.1016/j.exer.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest. Ophthalmol. Vis. Sci. 1995;36:809–819. [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest. Ophthalmol. Vis. Sci. 1999a;40:1959–1967. [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavangh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog. Retin. Eye. Res. 1999b;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jin J, Guan M, Sima J, Gao G, Zhang M, Liu Z, Fant J, Ma JX. Decreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea. 2003;22:473–477. doi: 10.1097/00003226-200307000-00015. [DOI] [PubMed] [Google Scholar]
- Job R, Raja V, Grierson I, Currie L, O’Reilly S, Pollock N, Knight E, Clark AF. Cross-linked actin networks (CLANs) are present in lamina cribrosa cells. Br. J. Ophthalmol. 2010;94:1388–1392. doi: 10.1136/bjo.2009.176032. [DOI] [PubMed] [Google Scholar]
- Johnson D, Gottanka J, Flugel C, Hoffmann F, Futa R, Lutjen-Drecoll E. Ultrastructural changes in the trabecular meshwork of human eyes treated with corticosteroids. Arch. Ophthalmol. 1997;115:375–383. doi: 10.1001/archopht.1997.01100150377011. [DOI] [PubMed] [Google Scholar]
- Johnson M. ‘What controls aqueous humour outflow resistance?’. Exp. Eye. Res. 2006;82:545–557. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonk LJ, Itok S, Heldin CH, ten Dijke P, Kruijer W. Identification and functional characterization of a Smad binding element (SBE) in the JunB promoter that acts as a transforming growth factor-beta, activing, and bone morphogenetic protein-inducible enhancer. J. Biol. Chem. 1998;14:21145–21152. doi: 10.1074/jbc.273.33.21145. [DOI] [PubMed] [Google Scholar]
- Joo CK, Lee EH, Kim JC, Kim YH, Lee JH, Kim JT, Chung KH, Kim J. Degeneration and transdifferentiation of human lens epithelial cells in nuclear and anterior polar cataracts. J. Cataract. Refract. Surg. 1999;25:652–658. doi: 10.1016/s0886-3350(99)00009-7. [DOI] [PubMed] [Google Scholar]
- Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bosl M, Bosserhoff A, Kostler J, Wagner R, Tamm ER, Fuchshofer R. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathology. 2012;180:2386–2403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV. Compositional differences between infant and adult human corneal basement membranes. Invest. Ophthalmol. Vis. Sci. 2007;48:4989–4999. doi: 10.1167/iovs.07-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Shimmura S, Kawakita T, Miyashita H, Ogawa Y, Yoshida S, Higa K, Okano H, Tsubota K. Beta-catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. 2007;48:1511–1517. doi: 10.1167/iovs.06-1060. [DOI] [PubMed] [Google Scholar]
- Kato N, Shimmura S. Epithelial-mesenchymal transition in the pathogenesis of pterygium. Inflamm. Regen. 2008;28:434–439. [Google Scholar]
- Kau HC, Tsai CC, Lee CF, Kao SC, Hsu WM, Liu JH, Wei YH. Increased oxidative DNA damage, 8-hydroxydeoxyguanosine, in human pterygium. Eye. 2006;20:826–831. doi: 10.1038/sj.eye.6702064. [DOI] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Suto C, Wilson SE. Corneal myofibroblast viability: opposing effects of IL-1 and TGFβ1. Exp. Eye. Res. 2009a;89:152–158. doi: 10.1016/j.exer.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, de Medeiros FW, Agrawal V, Salomao MQ, Singh N, Ambati BK, Wilson SW. Corneal stroma PDGF blockade and myofibroblast development. Exp. Eye. Res. 2009b;88:960–965. doi: 10.1016/j.exer.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC. Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am. J. Pathol. 2005;167:381–393. doi: 10.1016/S0002-9440(10)62983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima M, Kawakita T, Higa K, Satake Y, Omoto M, Tsubota K, Shimmura S, Shimazaki J. Subepithelial corneal fibrosis partially due to epithelial-mesenchymal transition of ocular surface epithelium. Mol. Vis. 2010;16:2727–2732. [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp. Eye. Res. 2009;88:676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent D, Sheridan C. Choroidal neovascularization: a wound healing perspective. Mol. Vis. 2003;22:747–755. [PubMed] [Google Scholar]
- Kim KW, Park SH, Lee SH, Kim JC. Upregulated stromal cell-derived factor 1 (SDF-1) expression and its interaction with CXCR4 contribute to the pathogenesis of severe pterygia. Invest. Ophthalmol. Vis. Sci. 2013;54:7198–7206. doi: 10.1167/iovs.13-13044. [DOI] [PubMed] [Google Scholar]
- Kim KW, Park SH, Kim JC. Fibroblast biology in pterygia. Exp. Eye. Res. 2016;142:32–39. doi: 10.1016/j.exer.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Kimoto K, Nakatsuka K, Matsuo N, Yoshioka H. p38 MAPK mediates the expression of type I collagen induced by TGF-beta 2 in human retinal pigmented epithelial cells ARPE-19. Invest. Ophthalmol. Vis. Sci. 2004;45:2431–2437. doi: 10.1167/iovs.03-1276. [DOI] [PubMed] [Google Scholar]
- Kirwan RP, Leonard MO, Murphy M, Clark AF, O’Brien CJ. Transforming growth factor-beta-regulated gene transcription and protein expression in human GFAP-negative lamina cribrosa cells. Glia. 2005a;52:309–324. doi: 10.1002/glia.20247. [DOI] [PubMed] [Google Scholar]
- Kirwan RP, Fenerty CH, Crean J, Wordinger RJ, Clark AF, O’Brien CJ. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Mol. Vis. 2005b;11:798–810. [PubMed] [Google Scholar]
- Kita T, Hata Y, Arita R, Kawahara S, Miura M, Nakao S, Mochizuki Y, Enaida H, Goto Y, Shimokawa H, Hafezi-Moghadam A, Ishibashi R. Role of TGF-beta in proliferative vitreoretinal diseases and ROCK as a therapeutic target. Proc. Natl. Acad. Sci. USA. 2008;105:17504–17509. doi: 10.1073/pnas.0804054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J. Pathol. 2013;229:298–309. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N, Okumura N, Ueno M, Kinoshita S. New therapeutic modality for corneal endothelial disease using Rho-associated kinase inhibitor eye drops. Cornea. 2014;33:S25–S31. doi: 10.1097/ICO.0000000000000240. [DOI] [PubMed] [Google Scholar]
- Kompass KS, Agapova OA, Li W, Kaufman PL, Rasmussen CA, Hernandez MR. Bioinformatic and statistical analysis of the optic nerve head in a primate model of ocular hypertension. BMC. Neurosci. 2008;26:93. doi: 10.1186/1471-2202-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am. J. Pathol. 2003;163:1291–1300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz M, Valcourt U, Bergstrom R, Heldin CH, Moustakas A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol. Cell. Biol. 2004;24:4241–4254. doi: 10.1128/MCB.24.10.4241-4254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta. Histochem. 1996;98:195–201. doi: 10.1016/s0065-1281(96)80038-9. [DOI] [PubMed] [Google Scholar]
- Kria L, Ohira A, Amemiya T. Growth factors in cultured pterygium fibroblasts: immunohistochemical and ELISA analysis. Graefes. Arch. Clin. Exp. Ophthalmol. 1998;236:702–708. doi: 10.1007/s004170050144. [DOI] [PubMed] [Google Scholar]
- Ko MK, Kay EP. Regulatory role of FGF-2 on type I collagen expression during endothelial mesenchymal transformation. Invest. Ophthalmol. Vis. Sci. 2005;46:4495–4503. doi: 10.1167/iovs.05-0818. [DOI] [PubMed] [Google Scholar]
- Last JA, Pan T, Ding Y, Reilly CM, Keller K, Acott TS, Fautsch MP, Murphy CJ, Russell P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. 2011. Invest. Ophthalmol. Vis. Sci. 2011;52:2147–2152. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latvala T, Tervo K, Mustonen R, Tervo T. Expression of cellular fibronectin and tenascin in the rabbit cornea after excimer laser photorefractive keratectomy: a 12 month study. Br. J. Ophthalmol. 1995;79:65–69. doi: 10.1136/bjo.79.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB. J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Lebrun JJ. The dual role of TGFβ in human cancer: from tumor suppression to cancer metastasis. ISRN. Mol. Biol. 2012:381428. doi: 10.5402/2012/381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HT, Kay EP. FGF-2 induced reorganization and disruption of actin cytoskeleton through PI 3-kinase, Rho, and Cdc42 in corneal endothelial cells. Mol. Vis. 2003a;8:624–634. [PubMed] [Google Scholar]
- Lee HT, Kay EP. Regulatory role of PI 3-kinase on expression of Cdk4 and p27, nuclear localization of Cdk4, and phosphorylation of p27 in corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2003b;44:1521–1528. doi: 10.1167/iovs.02-0637. [DOI] [PubMed] [Google Scholar]
- Lee HT, Kay EP. FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Exp. Eye. Res. 2006a;83:1309–1316. doi: 10.1016/j.exer.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Lee HT, Kay EP. Cross-talk among Rho GTPases acting downstream of PI 3-kinase induces mesenchymal transformation of corneal endothelial cells mediated by FGF-2. Invest. Ophthalmol. Vis. Sci. 2006b;47:2358–2368. doi: 10.1167/iovs.05-1490. [DOI] [PubMed] [Google Scholar]
- Lee K, Young LS, Park SY, Yang H. Antifibrotic effect of pirfenidone on human pterygium fibroblasts. 2014;39:680–685. doi: 10.3109/02713683.2013.867063. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Lee JY, Lee SH, Choi TH. Accelerating repaired basement membrane after bevacizumab treatment on alkali-burned mouse cornea. BMB. Rep. 2013;46:195–200. doi: 10.5483/BMBRep.2013.46.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. 2000;20:325–334. [PubMed] [Google Scholar]
- Liang K, Jiang Z, Ding BQ, Cheng P, Huang DK, Tao L. Expression of cell proliferation and apoptosis biomarkers in pterygia and normal conjunctiva. Mol. Vis. 2011;17:1687–1693. [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Lee SB, Gunja-Smith Z, Liu Y, Solomon A, Meller D, Tseng SC. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by pterygium head fibroblasts. Arch. Ophthalmol. 2001;119:71–80. [PubMed] [Google Scholar]
- Li H, Wang H, Wang F, Gu Q, Xu X. Snail involves in the transforming growth factor β1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS. One. 2011;6:e23322. doi: 10.1371/journal.pone.0023322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tang X, Chen X. Comparative effects of TGF-β2/Smad2 and TGFβ2/Smad3 signaling pathways on proliferation, migration, and extracellular matrix production in a human lens cell line. Exp. Eye. Res. 2011;92:173–179. doi: 10.1016/j.exer.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Li M, Li H, Liu X, Xu D, Wang F. MicroRNA-29b regulates TGF-β1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells by targeting AKT2. Exp. Cell. Res. 2016;345:115–124. doi: 10.1016/j.yexcr.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- Lim RR, Tan A, Liu YC, Barathi VA, Mohan RR, Mehta JS, Chaurasia SS. ITF2357 transactivates Id3 and regulate TGFβ/BMP7 signaling pathways to attenuate corneal fibrosis. Sci. Rep. 2016;11:20841. doi: 10.1038/srep20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CS, Wormstone IM, Duncan G, Marcantonio JM, Webb SF, Davies PD. A study of human lens cell growth in vitro. A model for posterior capsule opacification. Invest. Ophthalmol. Vis. Sci. 1996;37:906–914. [PubMed] [Google Scholar]
- Liu J, Hales AM, Chamberlain CG, McAvoy JW. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Invest. Ophthalmol. Vis. Sci. 1994;35:388–401. [PubMed] [Google Scholar]
- Liu T, Liu Y, Xie L, He X, Bai J. Progress in the pathogenesis of pterygium. Curr. Eye. Res. 2013;38:1191–1197. doi: 10.3109/02713683.2013.823212. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog. Retin. Eye. Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest. Ophthalmol. Vis. Sci. 1996;37:855–868. [PubMed] [Google Scholar]
- Lovicu FJ, Steven P, Saika S, McAvoy JW. Aberrant lens fibre differentiation in anterior subcapsular cataract formation: a process dependent on reduced levels of Pax6. Invest. Ophthalmol. Vis. Sci. 2004;45:1946–1953. doi: 10.1167/iovs.03-1206. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, McAvoy JW. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br. J. Ophthalmol. 2002;86:220–226. doi: 10.1136/bjo.86.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev. Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Shin EH, McAvoy JW. Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract. Exp. Eye. Res. 2016;142:92–101. doi: 10.1016/j.exer.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddala R, Reddy VN, Epstein DL, Rao V. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol. Vis. 2003;17:329–336. [PubMed] [Google Scholar]
- Marcantonio JM, Syam PP, Liu CS, Duncan G. Epithelial transdifferentiation and cataract in the human lens. Exp. Eye. Res. 2003;77:339–346. doi: 10.1016/s0014-4835(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Marshall GE, Konstas AG, Lee WR. Immunogold fine structural localization of extracellular matrix components in aged human cornea. I. Collagen types I–IV and laminin. Graefes Arch. Clin. Exp. Ophthalmol. 1991a;229:157–163. doi: 10.1007/BF00170550. [DOI] [PubMed] [Google Scholar]
- Marshall GE, Konstas AG, Lee WR. Immunogold fine structural localization of extracellular matrix components in aged human cornea. II. Collagen types V and VI. Graefes Arch. Clin. Exp. Ophthalmol. 1991b;229:164–171. doi: 10.1007/BF00170551. [DOI] [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc. Natl. Acad. Sci. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Chen YG. Controlling TGF-beta signaling. Genes. Dev. 2000;14:627–244. [PubMed] [Google Scholar]
- Matsuba M, Hutcheon AEK, Zieske JD. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp. Eye. Res. 2011;93:534–540. doi: 10.1016/j.exer.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatta M, Vaisanen T, Vaisanen MR, Pihlajaniemi T, Tervo T. Altered expression of type XIII collagen in keratoconus and scarred human cornea: increased expression in scarred cornea is associated with myofibroblast transformation. Cornea. 2006;4:448–453. doi: 10.1097/01.ico.0000183537.45393.1f. [DOI] [PubMed] [Google Scholar]
- Meek KM, Knupp C. Corneal structure and transparency. Prog. Retin. Eye. Res. 2015;46:1–16. doi: 10.1016/j.preteyeres.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SH, Lee TI, Chung YS, Kim HK. Transforming growth factor-β levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean. J. Ophthalmol. 2006;20:162–165. doi: 10.3341/kjo.2006.20.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Okada Y, Yamanaka O, Kitano A, Ikeda K, Kon S, Uede T, Rittling SR, Denhardt DT, Kao WWY, Saika W. Corneal wound healing in an osteopontin-deficient mouse. Invest. Ophthalmol. Vis. Sci. 2008;49:1367–1375. doi: 10.1167/iovs.07-1007. [DOI] [PubMed] [Google Scholar]
- Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 2009;85:314–323. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody AA, Wordinger RJ, Clark AF. Role of ID proteins in BMP4 inhibition of profibrotic effects of TGF-β2 in human TM cells. Invest. Ophthalmol. Vis. Sci. 2017;58:849–859. doi: 10.1167/iovs.16-20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Gupta R, Mehan MK, Cowden JW, Sinha S. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp. Eye Res. 2010;91:238–245. doi: 10.1016/j.exer.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JCK, Sharma A, Schultz GS, Cowden JW, Tandon A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS. One. 2011;6:e26432. doi: 10.1371/journal.pone.0026432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Sharma A, Tandon A. Gene therapy in the cornea: 2005—present. Prog. Retin. Eye. Res. 2012;31:43–64. doi: 10.1016/j.preteyeres.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Morgan BR, Anumanthan G, Sharma A, Chaurasia SS, Rieger FG. Characterization of Inhibitor of differentiation (Id) proteins in human cornea. Exp. Eye. Res. 2016;146:145–153. doi: 10.1016/j.exer.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester J. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998;17:627–639. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Montecchi-Palmer M, Bermudez JY, Webber H, Clark AF, Mao W. TGFβ2 induces the formation of cross-linked actin networks (CLANs) in human trabecular meshwork cells through Smad and non-Smad pathways. Invest. Ophthalmol. Vis. Sci. 2017;58:1288–1295. doi: 10.1167/iovs.16-19672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin C. Induction of epithelial-mesenchymal transition by transforming growth factor β. Semin. Cancer. Biol. 2012;22:446–454. doi: 10.1016/j.semcancer.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kurosaka D, Bissen-Miyajima H, Tsubota K. Intact corneal epithelium is essential for the prevention of stromal haze after laser assisted in situ keratomileusis. Br. J. Ophthalmol. 2001;85:209–213. doi: 10.1136/bjo.85.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Siddiqui SS, Shen X, Malik AB, Pulido JS, Kumar NM, Yue BYJT. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol. Vis. 2004;10:703–711. [PubMed] [Google Scholar]
- Neumann C, Yu A, Welge-Lussen U, Lutjen-Drecoll E, Birke M. The effect of TGF-beta2 on elastin, type VI collagen, and components of the proteolytic degradation system in human optic nerve astrocytes. Invest. Ophthalmol. Vis. Sci. 2008;48:1464–1472. doi: 10.1167/iovs.07-1053. [DOI] [PubMed] [Google Scholar]
- Nishida K, Kinoshita S, Yokoi N, Kaneda M, Hashimoto K, Yamamoto S. Immunohistochemical localization of transforming growth factor-beta 1, -beta 2, and –beta3 latency-associated peptide in human cornea. Invest. Ophthalmol. Vis. Sci. 1994;35:3289–3294. [PubMed] [Google Scholar]
- Noda K, Ishida S, Inoue M, Obata K, Oguchi Y, Ikeda E. Production and activation of matrix metalloproteinase-2 in proliferative diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2003;44:3263–2170. doi: 10.1167/iovs.02-0662. [DOI] [PubMed] [Google Scholar]
- Nolan TM, Di Girolamo N, Sachdev NH, Hampartzoumian T, Coroneo MT, Wakefield D. The role of ultraviolet irradiation and heparin-binding epidermal growth factor-like growth factor in the pathogenesis of pterygium. Am. J. Pathol. 2003;162:567–574. doi: 10.1016/S0002-9440(10)63850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstein SY, Byun J, Herrera D, Chapin EA, Fisher SK, Lewis GP. Cell proliferation in human epiretinal membranes: characterization of cell types and correlation with disease condition and duration. Mol. Vis. 2011;17:1794–1805. [PMC free article] [PubMed] [Google Scholar]
- Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn. J. Ophthalmol. 2002;46:249–253. doi: 10.1016/s0021-5155(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Okumura N, Koizumi N, Ueno M, Sakamoto Y, Takahashi H, Hirata K, Torii R, Hamuro J, Kinoshita S. Enhancement of corneal endothelium wound healing by Rho-associated kinase (ROCK) inhibitor eye drops. Br. J. Ophthalmol. 2011;95:1006–1009. doi: 10.1136/bjo.2010.194571. [DOI] [PubMed] [Google Scholar]
- Okumura N, Kay EP, Nakahara M, Hamuro J, Kinoshita S, Koizumi N. Inhibition of TGF-β signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS One. 2013;8:e58000. doi: 10.1371/journal.pone.0058000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly S, Pollock N, Currie L, Paraoan L, Clark AF, Grierson I. Inducers of cross-linked actin networks in trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2011;52:7316–7324. doi: 10.1167/iovs.10-6692. [DOI] [PubMed] [Google Scholar]
- Overby DR, Clark AF. Animal models of glucocorticoid-induced glaucoma. Exp. Eye. Res. 2015;141:15–22. doi: 10.1016/j.exer.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby DR, Zhou EH, Vargas-Pinto R, Pedrigi RM, Fuchshofer R, Braakman ST, Gupta R, Perkumas KM, Sherwood JM, Vahabikashi A, Dang Q, Kim JH, Ethier CR, Stamer WD, Fredberg JJ, Johnson M. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc. Natl. Acad. Sci. USA. 2014;111:13876–13881. doi: 10.1073/pnas.1410602111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan AA, Ozdemir N, Canataroglu A. The aqueous levels of TGF-beta2 in patients with glaucoma. Int. Ophthalmol. 2004;25:19–22. doi: 10.1023/b:inte.0000018524.48581.79. [DOI] [PubMed] [Google Scholar]
- Pastor JC, de la Rua ER, Martin F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog. Retin. Eye. Res. 2002;21:127–144. doi: 10.1016/s1350-9462(01)00023-4. [DOI] [PubMed] [Google Scholar]
- Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am. J. Physiol. Cell. Physiol. 2010;298:C749–C763. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman PP, Maddala R, Rao PV. Regulation of plasticity and fibrogenic activity of trabecular meshwork cells by Rho GTPase signaling. J. Cell. Physio. 2014;229:927–942. doi: 10.1002/jcp.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JD, Netland PA, Vidal I, Dorr DA, Rasky A, Hernandez MR. Elastosis of the lamina cribrosa in glaucomatous optic neuropathy. Exp. Eye. Res. 1998;67:517–524. doi: 10.1006/exer.1998.0539. [DOI] [PubMed] [Google Scholar]
- Pena JD, Taylor AW, Ricard CS, Vidal I, Hernandez MR. Transforming growth factor beta isoforms in human optic nerve heads. Br. J. Ophthalmol. 1999;83:209–218. doi: 10.1136/bjo.83.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrella G, Scott CA, Spelat R, Brusini P, D’Aurizio F, De Pol I, Dua HS. Cultured human keratocytes from the limbus and cornea both express epithelial cytokeratin 3: possible mesenchymal-epithelial transition. Int. J. Ophthalmol. Pathol. 2012;1:1–7. [Google Scholar]
- Petridou S, Maltseva O, Spanakis S, Masur SK. TGF-beta receptor expression and smad2 localisation are cell density dependent in fibroblasts. Invest. Ophthalmol. Vis. Sci. 2000;41:89–95. [PubMed] [Google Scholar]
- Picht G, Welge-Luessen U, Grehn F, Lutjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes. Arch. Clin. Exp. Ophthalmol. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- Piera-Velaquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am. J. Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, Deuel TF. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J. Cell. Bio. 1989;109:429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipparelli A, Arsenijevic Y, Thuret G, Gain P, Nicolas M, Majo F. ROCK inhibitor enhances adhesion and wound healing of human corneal endothelial cells. PLoS One. 2013;23:e62095. doi: 10.1371/journal.pone.0062095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendes MA, Harris A, Wirostko BM, Gerber AL, Siesky B. The role of transforming growth factor β in glaucoma and the therapeutic implications. Br. J. Ophthalmol. 2013;97:680–686. doi: 10.1136/bjophthalmol-2011-301132. [DOI] [PubMed] [Google Scholar]
- Qu J, Jakobs TC. The time course of gene expression during reactive gliosis in the optic nerve. PLoS. One. 2013;8:e67094. doi: 10.1371/journal.pone.0067094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J. Cell. Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behavior. Nat. Rev. Mol. Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Robertson JV, Nathu Z, Najjar A, Dwivedi D, Gauldi J, West-Mays JA. Adenoviral gene transfer of bioactive TGFbeta1 to the rodent eye as a novel model for anterior subcapsular cataract. Mol. Vis. 2007;13:457–469. [PMC free article] [PubMed] [Google Scholar]
- Robertson JV, Golesic E, Gauldie J, West-Mays JA. Ocular gene transfer of active TGF-beta induces changes in anterior segment morphology and elevated IOP in rats. Invest. Ophthalmol. Vis. Sci. 2010;51:308–318. doi: 10.1167/iovs.09-3380. [DOI] [PubMed] [Google Scholar]
- Ronkko S, Rekonen P, Kaarniranta K, Puustjarvi T, Teravirta M, Uusitalo H. Matrix metalloproteinases and their inhibitors in the chamber angle of normal eyes and patients with primary open-angle glaucoma and exfoliation glaucoma. Graefes. Arch. Clin. Exp. Ophthalmol. 2007;245:697–704. doi: 10.1007/s00417-006-0440-1. [DOI] [PubMed] [Google Scholar]
- Roy O, Leclerc VB, Bourget JM, Theriault M, Proulx S. Understanding the process of corneal endothelial morphological change in vitro. Invest. Ophthalmol. Vis. Sci. 2015;56:1228–1237. doi: 10.1167/iovs.14-16166. [DOI] [PubMed] [Google Scholar]
- Ryan SJ. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans. Am. Ophthalmol. Soc. 1979;77:707–745. [PMC free article] [PubMed] [Google Scholar]
- Saika S, Ohnishi Y, Ooshima A, Liu CY, Kao WWY. Epithelial repair: roles of extracellular matrix. Cornea. 2002;21:S23–S29. doi: 10.1097/00003226-200203001-00006. [DOI] [PubMed] [Google Scholar]
- Saika S, Okada Y, Miyamoto T, Yamanaka O, Ohnishi Y, Ooshima A, Liu CY, Weng D, Kao WW. Role of p38 MAP kinase in regulation of cell migration and proliferation in healing corneal epithelium. Invest. Ophthalmol. Vis. Sci. 2004a;45:100–109. doi: 10.1167/iovs.03-0700. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, Kao WW, Roberts AB. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am. J. Pathol. 2004b;164:651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Sato M, Muragaki Y, Ohnishi Y, Ooshima A, Nakajima Y, Namikawa K, Kiyama H, Flanders KC. Transient adenoviral gene transfer of Smad7 prevents injury-induced epithelial-mesenchymal transition of lens epithelium in mice. Lab. Invest. 2004c;84:1259–1270. doi: 10.1038/labinvest.3700151. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Tanaka T, Yamanaka O, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Yoo J, Flanders KC, Roberts AB. Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab. Invest. 2004d;84:1245–1258. doi: 10.1038/labinvest.3700156. [DOI] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Flanders KC, Nakajima Y, Miyamoto T, Ohnishi Y, Kao WW, Muragaki Y, Ooshima A. Therapeutic effects of adenoviral gene transfer of bone morphogenetic protein-7 on a corneal alkali injury model in mice. Lab. Invest. 2005;85:474–486. doi: 10.1038/labinvest.3700247. [DOI] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Flanders KC, Ohnishi Y, Nakajima Y, Muragaki Y, Ooshima A. Adenoviral gene transfer of BMP-7, Id2, or Id3 suppresses injury-induced epithelial-to-mesenchymal transition of lens epithelium in mice. Am. J. Physiol. Cell. Physiol. 2006;290:C282–289. doi: 10.1152/ajpcell.00306.2005. [DOI] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Nishikawa-Ishida I, Kitano A, Flanders KC, Okada Y, Ohnishi Y, Nakajima Y, Ikeda K. Effect of Smad7 gene overexpression on transforming growth factor beta-induced retinal pigment fibrosis in a proliferative vitreoretinopathy mouse model. Arch. Ophthalmol. 2007;125:647–654. doi: 10.1001/archopht.125.5.647. [DOI] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Sumioka T, Miyamoto T, Miyazaki K, Okada Y, Kitano A, Shirai K, Tanaka S, Ikeda K. Prog. Retin. Eye. Res. 2008;27:177–196. doi: 10.1016/j.preteyeres.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Okada Y, Sumioka T. Modulation of Smad signaling by non-TGFβ components in myofibroblast generation during wound healing in corneal stroma. Exp. Eye. Res. 2016;142:40–48. doi: 10.1016/j.exer.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Santhiago MR, Singh V, Barbosa FL, Agrawal V, Wilson SE. Monocyte development inhibitor PRM-151 decreases corneal myofibroblast generation in rabbits. Exp. Eye. Res. 2011;93:786–789. doi: 10.1016/j.exer.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Kojima M, Nakaizumi H, Kitagawa K, Yamada Y, Ishizaki H. Early lens changes seen in patients with atopic dermatitis applying image analysis processing of Scheimpflug and specular microscopic images. 1998;212:88–94. doi: 10.1159/000027285. [DOI] [PubMed] [Google Scholar]
- Saxby L, Rosen E, Boulton M. Lens epithelial cell proliferation, migration, and metaplasia following capsulorhexis. Br. J. Ophthalmol. 1998;82:945–952. doi: 10.1136/bjo.82.8.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi S, Fukuchi T, Abe H, Kaiya T, Sugar J, Yue BTYT. Three dimensional scanning electron microscopic study of keratoconus corneas. Arch. Ophthalmol. 1998;116:62–68. doi: 10.1001/archopht.116.1.62. [DOI] [PubMed] [Google Scholar]
- Schlunck G, Meyer-ter-Vehn T, Klink T, Grehn F. Conjunctival fibrosis following filtering glaucoma surgery. Exp. Eye. Res. 2016;142:76–82. doi: 10.1016/j.exer.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Schneider M, Fuchshofer R. The role of astrocytes in optic nerve head fibrosis in glaucoma. Exp. Eye. Res. 2016;142:49–55. doi: 10.1016/j.exer.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin 1. J. Biol. Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- Singh V, Agrawal V, Santhiago MR, Wilson SE. Stromal fibroblast-bone marrow-derived cell interactions: implications for myofibroblast development in the cornea. Exp. Eye. Res. 2012;98:1–8. doi: 10.1016/j.exer.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Barbosa FL, Torricelli AA, Santhiago MR, Wilson SE. Transforming growth factor β and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Exp. Eye. Res. 2014;120:152–160. doi: 10.1016/j.exer.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha X, Wen Y, Liu Z, Song L, Peng J, Xie L. Inhibition of α-smooth muscle actin expression and migration of pterygium fibroblasts by coculture with amniotic mesenchymal stem cells. Curr. Eye. Res. 2014;39:1081–1089. doi: 10.3109/02713683.2014.900806. [DOI] [PubMed] [Google Scholar]
- Shepard A, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-β2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest. Ophthalmol. Vis. Sci. 2010;51:2067–2076. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wang YF, Jayaraman L, Yang H, Massague J, Pavletich NP. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell. 1998;94:585–594. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shin EH, Basson MA, Robinson ML, McAvoy JW, Lovicu FJ. Sprouty is a negative regulator of transforming growth factor β-induced epithelial-to-mesenchymal transition and cataract. Mol. Med. 2012;18:861–873. doi: 10.2119/molmed.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu DY, Ong K, Lovicu FJ. Histopathology of subcapsular cataract in a patient with atopic dermatitis. Optom. Vis. Sci. 2016;94:1–16. doi: 10.1097/OPX.0000000000001011. [DOI] [PubMed] [Google Scholar]
- Shu DY, Wojciechowski MC, Lovicu FJ. Bone morphogenetic protein-7 suppresses TGFβ2-induced epithelial-mesenchymal transition in the lens: implications for cataract prevention. Invest. Ophthalmol. Vis. Sci. 2017;58:781–796. doi: 10.1167/iovs.16-20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiddy WE, Feuer W, Irvine D, Flynn HW, Blankenship GW. Vitrectomy for complications of proliferative diabetic retinopathy. Ophthalmology. 1995;102:1688–1695. doi: 10.1016/s0161-6420(95)30808-1. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP, Sorisky A. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 2002;87:385–392. doi: 10.1210/jcem.87.1.8164. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataract. J. Clin. Invest. 1998;101:625–634. doi: 10.1172/JCI1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S, Gibson DJ, Robinson P, Pi L, Tuli S, Lewin AS, Schultz G. Assessment of anti-scarring therapies in ex vivo organ cultured rabbit corneas. Exp. Eye. Res. 2014;125:173–182. doi: 10.1016/j.exer.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S, Tran JA, Guo X, Hutcheon AEK, Kazlauskas A, Zieske JD. Development of wound healing models to study TGFβ3’s effect on SMA. Exp. Eye. Res. 2017;161:52–60. doi: 10.1016/j.exer.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocks SZ, Taylor SM, Shiels IA. Transforming growth factor-beta1 induces alpha-smooth muscle actin expression and fibronectin synthesis in cultured human retinal pigment epithelial cells. Clin. Exp. Ophthalmol. 2001;29:33–37. doi: 10.1046/j.1442-9071.2001.00368.x. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Fini ME. Uncoupling keratocyte loss of corneal crystallin from markers of fibrotic repair. Invest. Ophthalmol. Vis. Sci. 2004;45:4010–4015. doi: 10.1167/iovs.03-1057. [DOI] [PubMed] [Google Scholar]
- Sumioka T, Ikeda K, Okada Y, Yamanaka O, Kitano A, Saika S. Inhibitory effect of blocking TGF-beta/Smad signal on injury-induced fibrosis of corneal endothelium. Mol. Vis. 2008;14:2272–2281. [PMC free article] [PubMed] [Google Scholar]
- Sumioka T, Kitano A, Flanders KC, Okada Y, Yamanaka O, Fujita N, Iwanishi H, Kao WWY, Saika S. Impaired cornea wound healing in a tenascin C-deficient mouse model. Lab. Invest. 2013;93:207–217. doi: 10.1038/labinvest.2012.157. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Inoue T, Fujimoto T, Kojima S, Tanihara H. Epithelial mesenchymal transition-like phenomenon in trabecular meshwork cells. Exp. Eye. Res. 2014;118:72–79. doi: 10.1016/j.exer.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Tamiya S, Kaplan HJ. Role of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Exp. Eye. Res. 2016;142:26–31. doi: 10.1016/j.exer.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Tamiya S, Liu L, Kaplan HJ. Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Invest. Ophthalmol. Vis. Sci. 2010;51:2755–2763. doi: 10.1167/iovs.09-4725. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Siegner A, Baur A, Lutjen-Drecoll E. Transforming growth factor-β1 induces α-smooth muscle actin expression in cultured human and monkey trabecular meshwork. Exp. Eye. Res. 1996;62:389–398. doi: 10.1006/exer.1996.0044. [DOI] [PubMed] [Google Scholar]
- Tan DT, Tang WY, Liu YP, Goh HS, Smith DR. Apoptosis and apoptosis relative gene expression in normal conjunctiva and pterygium. Br. J. Opthhalmol. 2000;84:212–216. doi: 10.1136/bjo.84.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon A, Sharma A, Rodier JT, Klibanov AM, Rieger FG, Mohan RR. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PLoS. One. 2013;8:e66434. doi: 10.1371/journal.pone.0066434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon A, Tovey JC, Sharma A, Gupta R, Mohan RR. Role of transforming growth factor beta in corneal function, biology and pathology. Curr. Mol. Med. 2010;10:565–578. doi: 10.2174/1566524011009060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Salzman IJ, Sable MD. Traumatic cataract formation after vigorous ocular massage. J. Cataract. Refract. Surg. 2003;29:1641–1642. doi: 10.1016/s0886-3350(03)00120-2. [DOI] [PubMed] [Google Scholar]
- Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J. Mammary. Gland. Biol. Neoplasia. 2010;15:169–190. doi: 10.1007/s10911-010-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends. Biochem. Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Terai K, Call M, Liu H, Saika S, Liu C, Hayashi Y, Chikama TI, Zhang J, Terai N, Kao CWC, Kao WWY. Crosstalk between TGF-β and MAPK signaling during corneal wound healing. Invest. Ophthalmol. Vis. Sci. 2011;52:8208–8215. doi: 10.1167/iovs.11-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall TJ, English DR. Sun exposure and pterygium of the eye: a dose-response curve. Am. J. Ophthalmol. 1999;128:280–287. doi: 10.1016/s0002-9394(99)00161-0. [DOI] [PubMed] [Google Scholar]
- Tisdale AS, Spurr-Michaud SJ, Rodrigues M, Hackett J, Krachmer J, Gipson IK. Development of anchoring structures of the epithelium in rabbit and human fetal corneas. Invest. Ophthalmol. Vis. Sci. 1988;29:727–736. [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat. Rev. Mol. Cell. Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Invest. Ophthalmol. Vis. Sci. 2013a;54:6390–6400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Agrawal V, Santhiago MR, Wilson SE. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest. Ophthalmol. Vis. Sci. 2013b;54:4026–4033. doi: 10.1167/iovs.13-12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye. Res. 2016;142:110–118. doi: 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi GM, Marigliani D, Romeo N, Toti P. Disease pathways in proliferative vitreoretinopathy: an ongoing challenge. J. Cell. Physiol. 2014;229:1577–1583. doi: 10.1002/jcp.24606. [DOI] [PubMed] [Google Scholar]
- Touhami A, Di Pascuale MA, Kawatika T, Del Valle M, Rosa RH, Jr, Dubovy S, Tseng SC. Characterisation of myofibroblasts in fibrovascular tissues of primary and recurrent pterygia. Br. J. Ophthalmol. 2005;89:269–274. doi: 10.1136/bjo.2004.050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Vidales T, Clark AF, Wordinger RJ. Transforming growth factor-beta2 utilizes the canonical Smad-signaling pathway to regulate tissue transglutaminase expression in human trabecular meshwork cells. Exp. Eye. Res. 2011;93:442–451. doi: 10.1016/j.exer.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DRW, Peters H, Rota S, Remuzzi G, Rump LC, Sellin LK, Heaton JPW, Streisand JB, Hard ML, Ledbetter SR, Vincenti F. A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney. Int. 2011;79:1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp. Eye. Res. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- Trivedi RH, Nutaitis M, Vroman D, Crosson CE. Influence of race and age on aqueous humor levels of transforming growth factor-beta 2 in glaucomatous and nonglaucomatous eyes. J. Occul. Pharmacol. Ther. 2011;27:477–480. doi: 10.1089/jop.2010.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TT, Chiang CC, Yeh KT, Lee H, Cheng YW. Effect of TIMP-1 and MMP in pterygium invasion. Invest. Ophthalmol. Vis. Sci. 2010;51:3462–3467. doi: 10.1167/iovs.09-4921. [DOI] [PubMed] [Google Scholar]
- Tuli SS, Liu R, Chen C, Blalock TD, Goldstein M, Schultz GS. Immunohistochemical localization of EGF, TGF-alpha, TGF-beta, and their receptors in rat corneas during healing of excimer laser ablation. Curr. Eye. Res. 2006;31:709–719. doi: 10.1080/02713680600837390. [DOI] [PubMed] [Google Scholar]
- Uno K, Hayashi H, Kuroki M, Uchida H, Yamauchi Y, Kuroki M, Oshima K. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem. Biophys. Res. Comm. 2004;315:928–934. doi: 10.1016/j.bbrc.2004.01.146. [DOI] [PubMed] [Google Scholar]
- Vesalouma M, Ylatupa S, Mertaniemi P, Tervo K, Partanen P, Tervo T. Increased release of tenascin in tear fluid after photorefractive keratectomy. Graefes Arch. Clin. Exp. Ophthalmol. 1995;233:479–483. doi: 10.1007/BF00183428. [DOI] [PubMed] [Google Scholar]
- Vesalouma MH, Tervo TT. Tenascin and cytokines in tear fluid after photorefractive keratectomy. J. Refract. Surg. 1998;14:447–454. doi: 10.3928/1081-597X-19980701-11. [DOI] [PubMed] [Google Scholar]
- Wade NC, Grierson I, O’Reilly S, Hoare MJ, Cracknell KPB, Paraoan LI, Brotchie D, Clark AF. Cross-linked actin networks (CLANs) in bovine trabecular meshwork cells. Exp. Eye. Res. 2009;89:648–659. doi: 10.1016/j.exer.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Clark AF, Lipson KE, Andrew D, Crean JK, O’Brien CJ. Anti-connective tissue growth factor antibody treatment reduces extracellular matrix production in trabecular meshwork and lamina cribrosa cells. Invest. Ophthalmol. Vis. Sci. 2013;54:7836–7848. doi: 10.1167/iovs.13-12494. [DOI] [PubMed] [Google Scholar]
- Wallace DM, O’Brien CJ. The role of lamina cribrosa cells in optic nerve head fibrosis in glaucoma. Exp. Eye. Res. 2016;142:102–109. doi: 10.1016/j.exer.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest. Ophthalmol. Vis. Sci. 2014;55:1735–1748. doi: 10.1167/iovs.14-14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley. Interdiscip. Rev. Dev. Biol. 2013;2:47–63. doi: 10.1002/wdev.86. [DOI] [PubMed] [Google Scholar]
- Welge-Lussen U, May CA, Lutjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-beta1 and TGF-beta2. Invest. Ophthalmol. Vis. Sci. 2000;41:2229–2238. [PubMed] [Google Scholar]
- Weinstein O, Rosenthal G, Zirkin H, Monos T, Lifshitz T, Argov S. Overexpression of p53 tumor suppressor gene in pterygia. Eye. 2002;16:619–621. doi: 10.1038/sj.eye.6700150. [DOI] [PubMed] [Google Scholar]
- Wendt MK, Allington TM, Schiemann WP. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future. Oncol. 2009;5:1145–1168. doi: 10.2217/fon.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell. Biol. 2006;38:1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtlin J, Langenbeck K, Schrunder S, Zhang EP, Hoffmann F. Immunohistology of corneal wound healing after photorefractive keratectomy and laser in situ keratomileusis. J. Refract. Surg. 1999;15:451–458. doi: 10.3928/1081-597X-19990701-11. [DOI] [PubMed] [Google Scholar]
- Wiedemann P. Growth factors in retinal disease: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv. Ophthalmol. 1992;36:373–384. doi: 10.1016/0039-6257(92)90115-a. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp. Eye. Res. 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J, Hoerauf H. TGF-β and RPE-derived cells in taut subretinal strands from patients with proliferative vitreoretinopathy. Eur. J. Ophthalmol. 2011;21:422–426. doi: 10.5301/EJO.2010.6067. [DOI] [PubMed] [Google Scholar]
- Wojciechowski MC, Mahmutovic L, Shu DY, Lovicu FJ. ERK1/2 signaling is required for the initiation but not progression of TGFβ-induced lens epithelial to mesenchymal transition (EMT) Exp. Eye. Res. 2017;159:98–113. doi: 10.1016/j.exer.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordinger RJ, Clark AF, Agarwal R, Lambert W, McNatt L, Wilson SE, Qu Z, Fung BK. Cultured human trabecular meshwork cells express functional growth factor receptors. Invest. Ophthalmol. Vis. Sci. 1998;39:1575–1589. [PubMed] [Google Scholar]
- Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, Zode GS, Fuller JA, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest. Ophthalmol. Vis. Sci. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Clark AF. Bone morphogenetic proteins and their receptors in the eye. Exp. Bio. Med (Maywood) 2007;232:979–992. doi: 10.3181/0510-MR-345. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Liu CS, Rakic JM, Marcantonio JM, Vrensen GF, Duncan G. Human lens epithelial cell proliferation in a protein-free medium. Invest. Ophthalmol. Vis. Sci. 1997;38:396–404. [PubMed] [Google Scholar]
- Wormstone IM, Tamiya S, Anderson I, Duncan G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest. Ophthalmol. Vis. Sci. 2002;43:2301–2308. [PubMed] [Google Scholar]
- WuDunn D. Mechanobiology of trabecular meshwork cells. Exp. Eye. Res. 2009;88:718–723. doi: 10.1016/j.exer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Chen X, Liu X, Luo L, Ye S, Liu Y. Trichostatin A, a histone deacetylase inhibitor, suppresses proliferation and epithelial-mesenchymal transition in retinal pigment epithelium cells. J. Cell. Mol. Med. 2014;18:646–655. doi: 10.1111/jcmm.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, Jiang L, Zhou Y, Qiu Q, Fang L, Tan R, Wen P, Yang J. The miR-200 family regulates TGF-β1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. J. Physiol. Renal. Physiol. 2012;302:F369–F379. doi: 10.1152/ajprenal.00268.2011. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Itonaga K, Marunouchi T, Majima K. Concentration of transforming growth factor beta2 in aqueous humor. Ophthalmic. Res. 2005;37:29–33. doi: 10.1159/000083019. [DOI] [PubMed] [Google Scholar]
- Yamanaka O, Miyazaki K, Kitano A, Saika S, Nakajima Y, Ikeda K. Suppression of injury-induced conjunctiva scarring by peroxisome proliferator-activated receptor γ gene transfer in mice. Invest. Ophthalmol. Vis. Sci. 2009;50:187–193. doi: 10.1167/iovs.08-2282. [DOI] [PubMed] [Google Scholar]
- Yamanaka O, Liu CY, Kao WWY. Fibrosis in the anterior segments of the eye. Endocr. Metab. Immune. Disord. Drug. Targets. 2010;10:331–335. doi: 10.2174/1871530311006040331. [DOI] [PubMed] [Google Scholar]
- Yamanaka O, Kitano-Izutani A, Tomoyose K, Reinach PS, Okada Y, Shirai K. Pathobiology of wound healing after glaucoma filtration surgery. BMC Ophthalmol. 2015;15(S1):157. doi: 10.1186/s12886-015-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2007;129:43–55. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yang S, Li H, Li M, Wang F. Mechanisms of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Discov. Med. 2015;20:207–217. [PubMed] [Google Scholar]
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease (META-EYE) Study Group Global prevalence and major risk factors of diabetic retinopathy. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JY, Shin I, Arteago CL. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J. Biol. Chem. 2005;280:10870–10876. doi: 10.1074/jbc.M413223200. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Kimoto K, Itoh Y, Nakatsuka K, Matsuo N, Yoshioka H, Kubota T. The PI3K/Akt pathway mediates the expression of type I collagen induced by TGF-β2 in human retinal pigment epithelial cells. Graefes. Arch. Clin. Exp. Ophthalmol. 2012;250:15–23. doi: 10.1007/s00417-011-1766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Wai-Man C, Khaw PT. Development novel anti-fibrotic therapeutics to modulate post-surgical wound healing in glaucoma: big potential for small molecules. Expert. Rev. Ophthalmol. 2015;10:65–76. doi: 10.1586/17469899.2015.983475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am. J. Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell. Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Wojciechowski MC, Jee S, Boros J, McAvoy JW, Lovicu FJ. Negative regulation of TGFβ-induced lens epithelial to mesenchymal transition (EMT) by RTK antagonists. Exp. Eye. Res. 2015;132:9–16. doi: 10.1016/j.exer.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ramsey KE, Stephan DA, Russell P. Gene and protein expression changes in human trabecular meshwork cells treated with transforming growth factor-beta. Invest. Ophthalmol. Vis. Sci. 2004;45:4023–4034. doi: 10.1167/iovs.04-0535. [DOI] [PubMed] [Google Scholar]
- Zhu YT, Chen HC, Chen SY, Tseng SC. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. J. Cell. Sci. 2012;125:3636–3648. doi: 10.1242/jcs.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Hutcheon AE, Guo X, Chung EH, Joyce NC. TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Invest. Ophthalmol. Vis. Sci. 2001;42:1465–1471. [PubMed] [Google Scholar]
- Zode GS, Sethi A, Brun-Zinkernagel AM, Chang IF, Clark AF, Wordinger RJ. Transforming growth factor-β2 increases extracellular matrix proteins in optic nerve head cells via activation of the Smad signaling pathway. Mol. Vis. 2011;17:1745–1758. [PMC free article] [PubMed] [Google Scholar]