Abstract
Importance
Traumatic intracranial bleeding, which is most commonly attributable to falls, is a common concern among clinicians who are hesitant to prescribe oral anticoagulants to older adults with atrial fibrillation.
Objective
To describe the incidence and risk factors for traumatic intracranial bleeding in a large cohort of older adults who are newly prescribed warfarin.
Design
Retrospective cohort study.
Setting
Department of Veterans Affairs (VA).
Participants
31,951 Veterans with atrial fibrillation aged ≥75 years who were new referrals to VA anticoagulation clinics (warfarin therapy) between 1/1/2001–12/31/2012. Patients with comorbid conditions requiring warfarin were excluded.
Main Outcomes and Measures
The primary outcome was traumatic intracranial bleeding. Secondary outcomes included any intracranial bleeding and stroke. We used ICD-9-CM codes to identify incidence rates of these outcomes following warfarin initiation, using VA administrative data (in-system hospitalizations) and Medicare fee-for-service claims data (out-of-system hospitalizations). Clinical characteristics, laboratory, and pharmacy data were extracted from the VA electronic medical record. For traumatic intracranial bleeding, Cox proportional hazards regression was used to determine predictors of interest selected a priori based on prior known associations.
Results
Mean patient age was 81.1 ± 4.1 years, and comorbidities were common (hypertension 82.5%, coronary artery disease 42.6%, diabetes 33.8%). Over the study period, the incidence rate of traumatic intracranial bleeding was 4.8 per 1000 person-years. In unadjusted models, significant predictors for traumatic intracranial bleeding included dementia, fall within past year, anemia, depression, abnormal renal/liver function, anticonvulsant use, labile INR, and antihypertensive medication. After adjusting for potential confounders, the remaining significant predictors were dementia (HR 1.76, 95% CI 1.26–2.46), anemia (HR 1.23, 95% CI 1.00–1.52), depression (HR 1.30, 95% CI 1.05–1.61), anticonvulsant use (HR 1.35, 95% CI 1.04–1.75), and labile INR (HR 1.34, 95% CI 1.04–1.72). The incidence rates of any intracranial bleeding and stroke, respectively, were 14.58 and 13.44 per 1000 person-years.
Conclusions and Relevance
Among patients age ≥75 with atrial fibrillation initiating warfarin, traumatic intracranial bleeding has unique risk factors from those for ischemic stroke. The high overall rate of intracranial bleeding in our sample supports the need to more systematically evaluate the benefits and harms of warfarin in older adults.
Keywords: Atrial fibrillation, bleeding, falls, elderly
BACKGROUND
Advanced age is a powerful risk factor for thromboembolic stroke in patients with atrial fibrillation (AF), and oral anticoagulation reduces this risk by nearly two-thirds in patients at-risk.1,2 However, up to half of eligible older adults with AF are not treated with anticoagulant therapy due to clinicians’ concerns about potential treatment-related harms.3–5 Multiple studies have shown that a primary concern among clinicians is perceived fall risk and the sequelae of traumatic intracranial bleeding, which can have devastating consequences.4–9 However, despite such concerns, the incidence and determinants of this outcome among older adults with AF who are prescribed oral anticoagulants remains largely unknown; most prior studies are relatively small and report that the outcome is rare.10,11
Accordingly, we sought to investigate the long-term incidence and risk factors for traumatic intracranial bleeding among a large cohort of older adults with AF following the initiation of oral anticoagulation with warfarin. To accomplish this, we assembled a retrospective sample of patients from the U.S. Department of Veterans Affairs (VA) Health System, which contains key clinical variables not available in many other large datasets, and then used Medicare fee-for-service (FFS) claims data to capture subsequent out-of-system hospitalizations. We hypothesized that the incidence rate of traumatic intracranial bleeding would be higher than previously reported in clinical trials, and that selected known risk factors for falls and bleeding would independently predict this event. As secondary outcomes we analyzed the incidence of any intracranial bleeding (traumatic and nontraumatic) and stroke.
METHODS
Data sources
The VA is the largest integrated health system in the U.S. and has a well-developed clinical information system that has been used extensively for large-scale epidemiologic investigations.12–16 For our study we obtained VA medical and administrative data through the VA Corporate Data Warehouse, including outpatient encounters and inpatient hospitalizations (dates and ICD-9-CM codes), laboratory results, and pharmacy dispensing. Since we anticipated that nearly our entire cohort would also be covered by Medicare17 and hospitalized at non-VA institutions, we obtained Medicare enrollment information and claims data (Fee-for-Service) for inpatient hospital stays from the Centers for Medicare and Medicaid Services through the VA Information Resource Center (Hines, IL). Veterans’ data were linked to Medicare claims data using unique scrambled social security numbers..
Data were stored on VA Informatics and Computing Infrastructure servers and analyzed through a secure network at the Massachusetts Veterans Epidemiology Research and Information Center (Boston, MA). Study approval and a waiver of informed consent were obtained from Institutional Review Boards at Boston VAHCS and New York Harbor VAHCS.
Study sample
Since the risk of warfarin-related bleeding is highest immediately after initiation of therapy,18 we created an inception cohort of Veterans age ≥75 years who were started on warfarin between 1/1/2002–12/31/2012. Ambulatory patients within the VA system who are prescribed warfarin are followed in dedicated anticoagulation clinics, which can be identified by an administrative “stop code” (code 317). We identified all patients where this code was entered at least once within our study period. Our index date was defined as the date of first warfarin dispensing. Among this population we then identified patients who had a diagnosis of AF (ICD-9-CM code 427.3) within one year prior to index date (one inpatient or two outpatient encounters). To reduce the potential for out-of-system anticoagulation clinic utilization, we also required that patients have an INR available at least every three months between the time of their first and last warfarin prescription. We also excluded patents with no INR values >1.2 since it was unlikely that they actually took warfarin. In order to ensure patients were existing users of VA services, we required at least one outpatient visit or outpatient pharmacy dispensing between six months and two years prior to first warfarin dispensing.
Since risk/benefit considerations with anticoagulation vary by condition, we excluded patients if the following concomitant diagnoses (ICD-9-CM code) were also present: pulmonary embolus (415.1), deep venous thrombosis (453.4), mechanical mitral valve (35.24 or V43.3), or mechanical aortic valve (35.22 or V43.3), within one year prior to first warfarin dispensing. Patients who were prescribed direct oral anticoagulants available during the study period (dabigatran, rivaroxaban, apixaban), were also excluded.
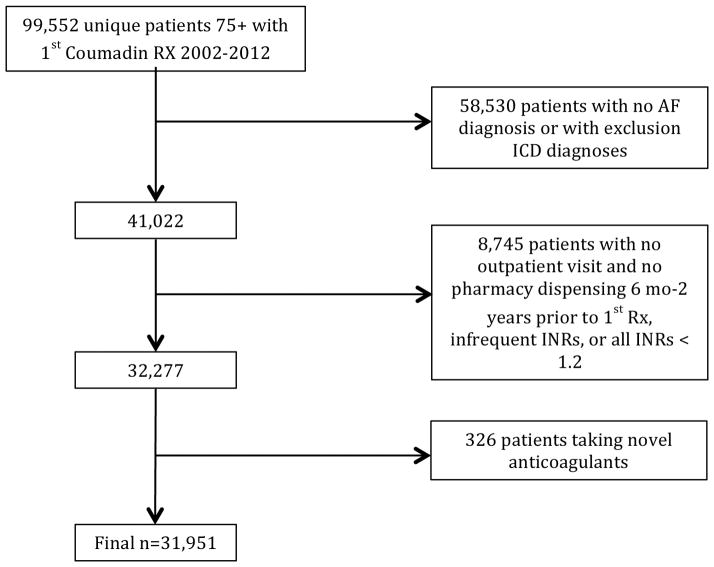
Derivation of the study cohort using the above criteria is shown in Figure 1. Of 99,552 unique patients aged ≥75 with at least warfarin prescription within VA from 2002–2012, 42,838 had AF. Of these patients, after applying our exclusion criteria, our final sample included 31,951 patients.
Figure 1. Derivation of the study cohort.
Of 99,552 patients initially identified through administrative data, 31,951 were included in the final sample.
Outcomes
Our primary outcome was hospitalization for traumatic intracranial bleeding, which was determined by the use of ICD-9-CM codes entered for hospitalizations either within VA hospitals (VA data) or at outside hospitals (Medicare claims data). Use of ICD-9 coding is a previously accepted method of identifying hospitalizations from large datasets.19,20 In claims data, ICD-9 codes for intracranial bleeding are explicitly specified as traumatic or nontraumatic. We selected our ICD-9-CM codes based on prior studies 10,21 and consensus among an expert panel of clinicians (JAD, DRG, MET, HMK, JMG) (eTable 1 in the Supplement). In order to increase the likelihood that these events were attributable to falls, we excluded all patients with a concomitant motor vehicle accident code (E810.0–E819.9).22
Secondary outcomes included (1) hospitalization for any intracranial bleeding (traumatic or nontraumatic) and (2) hospitalization for ischemic stroke. To identify secondary outcomes we followed an algorithm using ICD-9-CM codes entered at discharge (eTable 1 in the Supplement).
Predictors and Covariates
Our predictors of interest were prespecified and based on prior studies showing an association with either falls or bleeding. For falls, we required that risk factors be reported in at least two independent studies22–28 and be available through administrative data; our factors included dementia, arthritis, prior fall, visual impairment, anemia, dizziness, diabetes, depression, low body mass index, anxiolytic use, antipsychotic use, anticonvulsant use, antihypertensive use, and polypharmacy (≥4 chronic medications). Our bleeding risk factors were based on the previously validated HAS-BLED model29(hypertension, abnormal renal or liver function, stroke, prior bleeding, drug or alcohol use, labile INR, age).
For medical conditions in our model we used previously validated ICD-9-CM coding algorithms12–14,30–32 when available (eTable 2 in the Supplement). For pharmacy variables, we used categories within the VA pharmacy system.33 Patients were classified as being on a medication if they received a prescription within 6 months prior to cohort entry. Medication status was updated each month thereafter, with individuals counted as discontinuing medication if there was no refill for six months after the supply of their prior fill ran out. For the laboratory variable “abnormal renal or liver function” (including AST, ALT, bilirubin, creatinine), values were based on the most recent available result within six months prior to cohort entry. For the INR variable, we used the Rosendaal linear interpolation method34 which assumes a linear relationship between two INR values and assigns a specific daily INR value for each patient. The average time in therapeutic range (INR 2.0–3.0) is then determined. We initially divided time in therapeutic range into 3 categories based on the classification scheme published by White et al.: poor control (<60%), moderate control (60–75%), and good control (>75%).35 We termed patients with poor control were as having “labile INR”.36
Statistical Analysis
We reported the incidence rate of our primary outcome (traumatic intracranial bleeding) as the number of events per 1000 person-years. Patients were censored at the time of hospitalization for first event, or if they did not experience an event they were censored at death or at end of study period (12/31/2012). We then examined bivariate associations between our predictors of interest and covariates (described above) with the outcome. Subsequently, we used Cox proportional hazards regression to evaluate the independent association of our predictors of interest with the outcome. In addition to adjusting for all prespecified predictors in this model, we adjusted for age, sex, self-identified race, and common comorbidities (peripheral vascular disease, coronary artery disease, hyperlipidemia, chronic pulmonary disease, heart failure). These were identified from ICD-9-CM codes entered within one year prior to patients’ entry into the cohort (eTable 2 in the Supplement). Within our Cox model, medications were updated monthly, and time in therapeutic range was updated every three months. These factors were treated as time-varying covariates. Otherwise baseline variables were used. We reported the strength of associations using hazard ratios (HRs) for point estimates with 95% confidence intervals (CIs). The proportional hazards assumption was tested for each predictor in our multivariable model and was found to be valid. For purposes of clinical applicability, we then ran two separate logistic regression models for the outcomes of 1-year and 3-year traumatic intracranial bleeding, including any variable with a P value of <0.2 in unadjusted analyses. We reported C statistics for these outcomes to evaluate model discrimination for the prediction of traumatic intracranial bleeding at 1 year and 3 years.
For descriptive purposes, we also reported our primary outcome without truncating patient follow-up at the time of first event, in order to account for repeated events in a single patient. Similarly, for our secondary outcomes (any intracranial bleeding, ischemic stroke), in order to capture multiple events, we reported incidence rates without censoring patients after their first event. For stroke, we also classified the observed rates of stroke and traumatic intracranial bleeding based on predicted stroke risk using the CHA2DS2-VASc score.
RESULTS
Study cohort
Of the 31,951 patients in our final cohort, the mean age was 81.1 years, 98.1% were male, and 8.4% were nonwhite. Frequent comorbidities included hypertension (82.5%), coronary artery disease (42.6%), and chronic obstructive pulmonary disease (25.5%). Polypharmacy was common: 87.0% of patients were on ≥4 chronic medications. Mean time in therapeutic range for the entire study sample was 55.5% (standard deviation 22.7%). The majority of patients (55.9%) were classified as having labile INR. On average, among patients with labile INR, 42.7% of INR values were less than 2, 39.4% were 2–3, and 17.9% were greater than 3. Other study sample characteristics are shown in Table 1.
Table 1.
Study Sample Characteristics (N=31,951)
| Age (mean ± SD) | 81.1 ± 4.1 |
| Male, N (%) | 31,332 (98.1%) |
| Race, N (%) | |
| White | 29,255 (91.6%) |
| Black | 2166 (6.8%) |
| Other | 530 (1.5%) |
| Body mass index (mean ± SD) | 28.1 ± 5.1 |
| Comorbid diseases, N (%) | |
| Hypertension | 26,362 (82.5%) |
| Diabetes | 10,806 (33.8%) |
| Prior Stroke | 3512 (11.0%) |
| Hyperlipidemia | 20,213 (63.3%) |
| Coronary artery disease | 13,604 (42.6%) |
| Peripheral vascular disease | 3538 (11.1%) |
| Myocardial infarction | 1329 (4.2%) |
| Chronic obstructive pulmonary disease | 8149 (25.5%) |
| Chronic kidney disease | 5245 (16.4%) |
| Heart failure | 9803 (30.7%) |
| Anemia | 6514 (20.4%) |
| Problem drug/alcohol use | 528 (1.7%) |
| Abnormal renal or liver function a | 1862 (5.8%) |
| Geriatric impairments, N (%) | |
| Dementia | 1498 (4.7%) |
| Depression | 5384 (16.9%) |
| Arthritis | 8878 (27.8%) |
| Prior fall | 1614 (5.1%) |
| Dizziness | 1037 (3.3%) |
| Visual impairment | 10,586 (33.1%) |
| Medications, N (%) | |
| Anxiolytic use | 4021 (12.6%) |
| Antipsychotic use | 919 (2.9%) |
| Anticonvulsant use | 2469 (7.7%) |
| Antihypertensive use | 29,713 (93.0%) |
| Polypharmacy (≥4 medications) | 27,784 (87.0%) |
| INR control | |
| Good (>75% TTR) b | 4864 (15.2%) |
| Moderate (60–75% TTR) | 9141 (28.6%) |
| Labile (<60% TTR) | 17,862 (55.9%) |
| Missing | 84 (0.3%) |
Chronic dialysis, renal transplant, cirrhosis, creatinine ≥2.3 mg/dL, total bilirubin ≥2x upper limit of normal (ULN), or AST/ALT ≥3x ULN
TTR = time in therapeutic range
Primary outcome
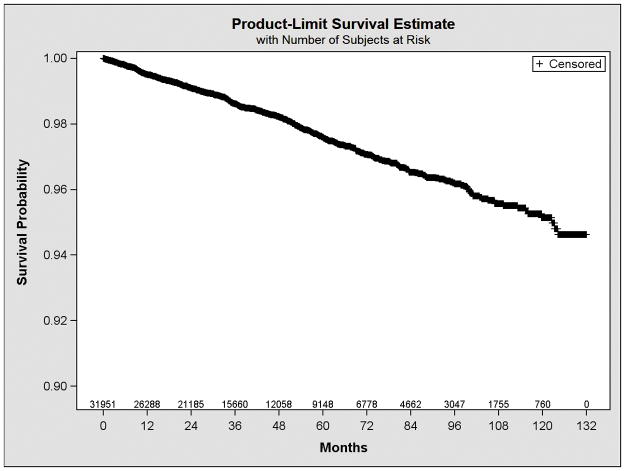
Over a median follow-up period of 2.97 years, the incidence rate of traumatic intracranial bleeding was 4.80 per 1000 person-years. Over three-quarters of events (76%) were identified from Medicare claims data; the remainder occurred within the VA system. The Kaplan-Meier curve illustrating traumatic intracranial bleeding events over time is shown in Figure 2. Based on the Weibull distribution (Weibull shape = 1.28, 95% CI 1.27–1.29), there was a modest increase in the hazard of this outcome over time. The absolute event rate for traumatic intracranial bleeding was 0.54% at 1 year and 2.10% at 3 years. When we included multiple events per patient (without censoring), the incidence rate of traumatic intracranial bleeding was 6.16 per 1000 person-years.
Figure 2. Kaplan-Meier plot for traumatic intracranial bleeding events over time.
Based on the Weibull distribution (Weibull shape = 1.28, 95% CI 1.27–1.29), there was a modest increase in the hazard of traumatic intracranial bleeding over time.
In our unadjusted model, antihypertensive use was the strongest risk factor for traumatic intracranial bleeding (hazard ratio [HR] 2.63, 95% confidence interval [CI] 2.06–3.35), although this effect was no longer significant after multivariable adjustment (HR 1.15, 95% CI 0.89–1.49). Other significant risk factors included dementia (HR 2.11, 95% CI 1.53–2.92), fall within the past year (HR 1.72, 95% CI 1.21–2.44), anemia (HR 1.35, 95% CI 1.10–1.65), depression (HR 1.49, 95% CI 1.22–1.82), abnormal renal/liver function (HR 1.42, 95% CI 1.00–2.01), anticonvulsant use (HR 1.30, 95% CI 1.01–1.69), and labile INR (HR 1.40, 95% CI 1.10–1.80). After adjusting for potential confounders, dementia, anemia, depression, anticonvulsant use, and labile INR remained significant predictors (Table 2), with the strongest association seen with dementia (HR 1.76, 95% CI 1.26–2.46). The incidence rates for traumatic intracranial bleeding were 3.36 per 1000 person-years in patients with none of these risk factors (N=9422) and 6.38 per 1000 person-years in those with ≥2 risk factors (N=9271). Using logistic regression models to evaluate traumatic intracranial bleeding at 1 year and 3 years, and retaining risk factors with a P value of <0.2 in adjusted analyses, we found that model discrimination was poor (C statistic = 0.55 at both 1 year and 3 years).
Table 2.
Unadjusted and adjusted models
| Hazard Ratio (95% Confidence Interval) | ||
|---|---|---|
| Unadjusted | Adjusted a | |
| Dementia | 2.11 (1.53–2.92) ‡ | 1.76 (1.26–2.46) ‡ |
| Arthritis | 0.98 (0.81–1.18) | 0.91 (0.75–1.10) |
| Prior fall | 1.72 (1.21–2.44) ‡ | 1.32 (0.88–1.98) |
| Visual impairment | 1.09 (0.92–1.30) | 1.03 (0.86–1.23) |
| Anemia | 1.35 (1.10–1.65) ‡ | 1.23 (1.00–1.52) † |
| Dizziness | 1.43 (0.93–2.19) | 1.22 (0.79–1.87) |
| Diabetes | 1.04 (0.87–1.24) | 0.98 (0.81–1.18) |
| Depression | 1.49 (1.22–1.82) ‡ | 1.30 (1.05–1.61) † |
| Body mass index <20 | 1.07 (0.63–1.83) | 1.12 (0.67–1.88) |
| Hypertension | 1.17 (0.94–1.46) | 1.16 (0.92–1.46) |
| Abnormal renal/liver function | 1.42 (1.00–2.01) | 1.32 (0.93–1.89) |
| Prior stroke | 1.24 (0.96–1.60) | 1.11 (0.86–1.44) |
| Prior bleed | 0.93 (0.54–1.61) | 0.80 (0.46–1.39) |
| Drug/alcohol use | 0.48 (0.18–1.29) | 0.45 (0.17–1.20) |
| Polypharmacy (≥4 medications) | 1.15 (0.90–1.47) | 0.98 (0.75–1.29) |
| Anxiolytic use | 1.33 (1.04–1.70) † | 1.24 (0.96–1.60) |
| Antipsychotic use | 1.02 (0.64–1.61) | 1.02 (0.64–1.61) |
| Anticonvulsant use | 1.30 (1.01–1.69) † | 1.35 (1.04–1.75) † |
| Antihypertensive use | 2.63 (2.06–3.35) ‡ | 1.15 (0.88–1.49) |
| INR control | ||
| Moderate | 1.02 (0.78–1.34) | 1.00 (0.77–1.32) |
| Poor | 1.43 (1.10–1.80) ‡ | 1.34 (1.04–1.72) † |
Adjusted for other covariates listed plus age, sex, race, coronary artery disease, chronic obstructive pulmonary disease, peripheral vascular disease, heart failure
P<0.05;
P<0.01
Secondary outcomes
The incidence rate of any intracranial bleeding (traumatic or nontraumatic) was 14.58 per 1000 person-years. More than half of intracranial bleeding events (57.2%) were nontraumatic. Among the 1317 patients who experienced any intracranial bleeding event, 407 (30.1%) experienced more than one episode. Over the same period of observation, the rate of ischemic stroke was 13.44 per 1000 person-years.
Traumatic intracranial bleeding based on stroke risk
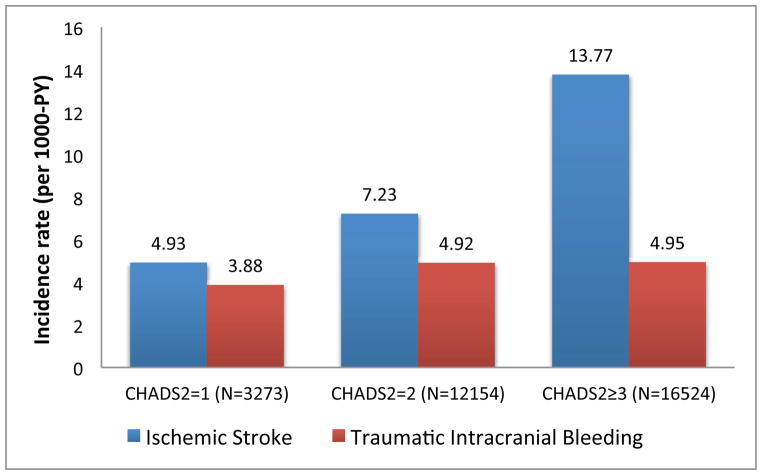
After dividing our sample into three categories of stroke risk based on CHA2DS2-VASc scores (CHA2DS2-VASc=2, CHA2DS2-VASc=3–4, CHA2DS2-VASc≥5), over one-third of patients (40.3%) fell into the category of very high stroke risk (CHA2DS2-VASc≥5). The observed rate of ischemic stroke increased as CHA2DS2-VASc score increased (stroke per 1000 person years: CHA2DS2-VASc=2: 4.59; CHA2DS2-VASc=3–4: 7.63; CHA2DS2-VASc≥5: 14.90) (Figure 3). Conversely, across CHA2DS2-VASc scores the observed rate of traumatic intracranial bleeding was relatively constant (traumatic intracranial bleeding per 1000 person years: CHA2DS2-VASc=2: 3.85; CHA2DS2-VASc=3–4: 4.81; CHA2DS2-VASc≥5: 5.00).
Figure 3. Rate of traumatic intracranial bleeding and stroke, stratified by CHA2DS2-VASc score.
The observed rate of stroke increased as CHA2DS2-VASc score increased, while the rate of traumatic intracranial bleeding remained relatively constant across CHA2DS2-VASc categories. PY = person-years.
DISCUSSION
To our knowledge, our study represents the largest investigation to date focused on traumatic intracranial bleeding among older adults (patients age ≥75) initiating warfarin for AF. Among the 31,951 patients in our sample, we found that the rate of traumatic intracranial bleeding was 4.80 per 1000 person years. As expected, this rate was considerably higher than in previous clinical trials,37,38 but relatively in line with a smaller population estimate from Medicare beneficiaries previously described by Gage et al.10 While we found that traumatic intracranial bleeding was a less common event than ischemic stroke, when we analyzed all intracranial bleeding events (traumatic and nontraumatic) the rates were similar (ischemic stroke: 13.44 per 1000 person years; any intracranial bleeding: 14.58 per 1000 person years).
Identified risk factors for traumatic intracranial bleeding
Several variables among those we analyzed placed patients at increased risk for traumatic intracranial bleeding. Most notably, the presence of dementia was associated with a doubling of risk, which was only mildly attenuated after adjusting for other factors. Prior studies have shown that patients with dementia are prescribed oral anticoagulants less frequently than patients without dementia, presumably due to concerns over harm or futility.39,40 Dementia is known from prior studies to increase fall risk considerably; in one cohort of 2,015 older adult nursing home residents, the relative risk for dementia and falls was 1.93 after adjusting for other characteristics.28 While not all falls lead to traumatic intracranial bleeding, the presence of cerebral amyloid angiopathy in dementia subtypes (e.g. Alzheimer’s disease) is known to increase bleeding risk41 and this may act synergistically with falls to predispose patients to traumatic intracranial bleeding. Other factors such as difficulty following medication instructions or dietary noncompliance may also play a role through excessive anticoagulation or increased INR variability. In this context, we believe our findings further support the perceived increased risk of warfarin in patients with dementia, and can potentially be used in individualized discussions with patients and caregivers when considering the risk-benefit tradeoff.
Patients with labile INR in our cohort were also more likely to experience traumatic intracranial bleeding after multivariable adjustment. This finding mirrors prior investigations linking labile INR with all-cause bleeding.29,35 For example, a study of patients enrolled in the SPORTIF III and V trials found that patients with labile INR were over twice as likely to experience major bleeding as patients with >75% of values within therapeutic range.35 The high prevalence of labile INR in our population (56%) may be due in part to advanced age. Studies have shown that older patients have a greater anticoagulant response to warfarin despite administration of a lower weight-related dose,42 and require a longer period to return to therapeutic range when their INR is supratherapeutic.43 In practice, there is considerable variability in the effectiveness of warfarin management between health systems, and labile INR therefore represents a potentially modifiable risk factor. The degree of therapeutic warfarin values in our sample was similar to a report by Rose et al. who studied 124,551 Veterans receiving warfarin over a 2-year time period and found a mean time in therapeutic range of 58%, with values ranging from 38% to 69% at different sites.44 Other reports have shown considerably better control of INR; for example, in the Swedish AuriculA registry, mean time in therapeutic range among 18,391 patients was 76%.45
While the strength of association was lower for other independent risk factors, all have been shown in prior studies to be associated with either falls or bleeding. Depression, for example, may predispose patients to falling through daytime sleepiness, impaired attention, or psychomotor slowing.46 Furthermore, SSRIs, the most commonly used antidepressants, have been shown to increase bleeding risk.47 Anemia in older adults has been associated with a doubling of fall risk,26 which may be due to its association with fatigue and poor muscle strength, and as also been implicated as an independent risk factor for bleeding.48 Anticonvulsant medications have been associated with fall-related fractures, possibly due to adverse effects of dizziness or unsteady gait,49 as well as resistance to warfarin which may make it challenging to maintain INR in therapeutic range and place patients at risk for a rapid increase in INR if the anticonvulsant is discontinued for any reason.50
Comparative risk of ischemic stroke
The rate of ischemic stroke in our population (13.44 per 1000 person-years), despite anticoagulation with warfarin, was over twice as high as the rate of traumatic intracranial bleeding. While this underscores the burden of stroke in patients age ≥75 with AF, the difference between our observed rates of stroke and traumatic intracranial bleeding was considerably less than those reported in prior studies.10,11,51 For example, in the previously noted study of Medicare beneficiaries by Gage et al., the incidence of ischemic stroke was nearly seven times as common as traumatic intracranial bleeding.10 We also found that, as expected, the rate of ischemic stroke increased with higher CHA2DS2-VASc score. However, there was no concomitant increase in the rate of traumatic intracranial bleeding, which underscores that the risk factors for this outcome are unique. In addition, as previously noted, the rate of any intracranial bleeding (traumatic or nontraumatic) was similar to the rate of ischemic stroke. This observation highlights the difficult risk-benefit tradeoff that clinicians and older patients face when considering warfarin therapy.
Strength and limitations
There are several strengths of our study, including our linkage of VA data with Medicare claims data to capture out-of-system hospitalizations, as well as our ability to analyze variables that are unavailable in many other administrative datasets. However, our findings must also be interpreted within the limitations of our study design. First, we were unable to analyze fall risk factors that are generally not coded during routine clinic visits such as slow gait or balance impairment.24,25 In addition, we could not precisely characterize the severity of each risk factor, and certain risk factors of interest (such as problem drug or alcohol use) may have been under-identified given our requirement that they were explicitly coded during inpatient or outpatient visits. Second, our study represents a single healthcare system with a nearly entirely male population; our findings will therefore need to be evaluated in other groups including samples with a larger proportion of women (where risk factors may differ). Third, as we excluded patients who had infrequent INRs due to the potential for incomplete data, we may have excluded noncompliant patients with higher bleeding risk. Similarly, we excluded AF patients who never received warfarin, and we cannot comment on bleeding rates within this subset. Fourth, we were unable to capture information on hospitalizations for patients enrolled in Medicare managed care; while only 23% of patients were enrolled in one of these plans for more than one year during the study period, we may still have underestimated the incidence rates of our outcomes. Finally, our study period predated the common use of direct oral anticoagulants. These medications are increasingly used for stroke prevention in AF, and may have a different risk/benefit profile than warfarin in older adults. For example, the reliable effect of these medications, taken regularly, would obviate the “labile INR” variable which we found to be significantly associated with bleeding risk. We therefore believe that the comparative risk of direct oral anticoagulants versus warfarin, in the context of traumatic intracranial bleeding among older adults in clinical practice, warrants further investigation in future observational studies.
Conclusions
In summary, we found that the rate of traumatic intracranial bleeding among older adults with AF initiating warfarin was higher than previously reported in clinical trials. After multivariable adjustment, several factors placed patients at increased risk for traumatic intracranial bleeding including dementia, anemia, depression, anticonvulsant use, and labile INR. While we were unable to generate a clinical prediction tool to evaluate risk, given poor model discrimination, we still believe that the individual factors we identified may potentially be used in patient-centered discussions about the benefits and harms of anticoagulant therapy. Our findings should be validated in other datasets, particularly given the underrepresentation of women in our sample.
Supplementary Material
Acknowledgments
Author Contributions:
Dr. Dodson and Dr. Gaziano had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dodson, Gagnon, Tinetti, Krumholz, Gaziano.
Acquisition, analysis, or interpretation of data: Dodson, Petrone, Gagnon, Gaziano.
Drafting of the manuscript: Dodson, Tinetti, Gaziano.
Critical revision of the manuscript for important intellectual content: Petrone, Gagnon, Krumholz.
Statistical analysis: Gagnon, Petrone.
Obtained funding: Dodson, Gaziano.
Administrative, technical, or material support: Gagnon, Gaziano.
Study supervision: Dodson, Gagnon, Tinetti, Krumholz, Gaziano.
Conflict of Interest Disclosures: Dr. Krumholz is a recipient of research agreements from Medtronic and from Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing, and is chair of a cardiac scientific advisory board for UnitedHealth. No other authors report disclosures.
Funding/Support: This project was supported by National Institutes of Health (NIH)/National Institute on Aging (NIA) grant R03AG045067 and a T. Franklin Williams Scholarship Award (funding provided by: Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Alliance for Academic Internal Medicine-Association of Specialty Professors, and the American College of Cardiology). Dr. Krumholz is supported by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Role of the funder/sponsor: The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 3.Antani MR, Beyth RJ, Covinsky KE, et al. Failure to prescribe warfarin to patients with nonrheumatic atrial fibrillation. J Gen Intern Med. 1996;11(12):713–720. doi: 10.1007/BF02598984. [DOI] [PubMed] [Google Scholar]
- 4.Beyth RJ, Antani MR, Covinsky KE, et al. Why isn’t warfarin prescribed to patients with nonrheumatic atrial fibrillation? J Gen Intern Med. 1996;11(12):721–728. doi: 10.1007/BF02598985. [DOI] [PubMed] [Google Scholar]
- 5.Glader E, Sjölander M, Eriksson M, Lundberg M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke. 2010;41:397–401. doi: 10.1161/STROKEAHA.109.566950. [DOI] [PubMed] [Google Scholar]
- 6.Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J. 2011;161(2):241–246. doi: 10.1016/j.ahj.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Lotze U, Liebetrau J, Malsch I, et al. Medical treatment of patients with atrial fibrillation aged over 80 years in daily clinical practice: Influence of age and CHADS2 score. Arch Gerontol Geriatr. 2010;50(1):36–41. doi: 10.1016/j.archger.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Monette J, Gurwitz JH, Rochon PA, Avorn J. Physician attitudes concerning warfarin for stroke prevention in atrial fibrillation: Results of a survey of long-term care practitioners. J Am Geriatr Soc. 1997;45(9):1060–1065. doi: 10.1111/j.1532-5415.1997.tb05967.x. [DOI] [PubMed] [Google Scholar]
- 9.Dharmarajan TS, Varma S, Akkaladevi S, Lebelt AS, Norkus EP. To anticoagulate or not to anticoagulate? A common dilemma for the provider: Physicians’ opinion poll based on a case study of an older long-term care facility resident with dementia and atrial fibrillation. J Am Med Dir Assoc. 2006;7(1):23–28. doi: 10.1016/j.jamda.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118(6):612–617. doi: 10.1016/j.amjmed.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159(7):677–685. doi: 10.1001/archinte.159.7.677. [DOI] [PubMed] [Google Scholar]
- 12.Butler D, Kowall NW, Lawler E, Michael Gaziano J, Driver JA. Underuse of diagnostic codes for specific dementias in the Veterans Affairs New England healthcare system. J Am Geriatr Soc. 2012;60(5):910–915. doi: 10.1111/j.1532-5415.2012.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choma NN, Griffin MR, Kaltenbach LA, Greevy RA, Roumie CL. Blood pressure control among patients with hypertension and newly diagnosed diabetes. Diabet Med. 2012;29(9):1126–1133. doi: 10.1111/j.1464-5491.2011.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller DR, Safford MM, Pogach LM. Who Has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27:B10–B21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 15.Rosen AK, Rivard P, Zhao S, et al. Evaluating the patient safety indicators: How well do they perform on Veterans Health Administration data? Med Care. 2005;43(9):873–884. doi: 10.1097/01.mlr.0000173561.79742.fb. [DOI] [PubMed] [Google Scholar]
- 16.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007;120(1):26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Shen Y, Hendricks A, Zhang S, Kazis LE. VHA enrollees’ health care coverage and use of care. Med Care Res Rev. 2003;60(2):253–267. doi: 10.1177/1077558703060002007. [DOI] [PubMed] [Google Scholar]
- 18.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115(21):2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 19.Tinetti ME, Baker DI, King M, et al. Effect of dissemination of evidence in reducing injuries from falls. N Engl J Med. 2008;359(3):252–261. doi: 10.1056/NEJMoa0801748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartholt KA, van der Velde N, Looman CW, et al. Trends in fall-related hospital admissions in older persons in the Netherlands. Arch Intern Med. 2010;170(10):905–911. doi: 10.1001/archinternmed.2010.106. [DOI] [PubMed] [Google Scholar]
- 21.Pieracci FM, Eachempati SR, Shou J, Hydo LJ, Barie PS. Use of long-term anticoagulation is associated with traumatic intracranial hemorrhage and subsequent mortality in elderly patients hospitalized after falls: Analysis of the New York State Administrative Database. J Trauma. 2007;63(3):519–524. doi: 10.1097/TA.0b013e31812e519b. [DOI] [PubMed] [Google Scholar]
- 22.Tinetti ME, Han L, Lee DH, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174(4):588–595. doi: 10.1001/jamainternmed.2013.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 24.Graafmans WC, Ooms ME, Hofstee HMA, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: A prospective study of risk factors and risk profiles. Am J Epidemiol. 1996;143(11):1129–1136. doi: 10.1093/oxfordjournals.aje.a008690. [DOI] [PubMed] [Google Scholar]
- 25.Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297(1):77–86. doi: 10.1001/jama.297.1.77. [DOI] [PubMed] [Google Scholar]
- 26.Penninx BWJH, Pluijm SMF, Lips P, et al. Late-life anemia is associated with increased risk of recurrent falls. J Am Geriatr Soc. 2005;53(12):2106–2111. doi: 10.1111/j.1532-5415.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 27.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls: A prospective study. JAMA. 1989;261(18):2663–2668. [PubMed] [Google Scholar]
- 28.Van Doorn C, Gruber-Baldini AL, Zimmerman S, et al. Dementia as a risk factor for falls and fall injuries among nursing home residents. J Am Geriatr Soc. 2003;51(9):1213–1218. doi: 10.1046/j.1532-5415.2003.51404.x. [DOI] [PubMed] [Google Scholar]
- 29.Lip GYH, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: The HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) Score. J Am Coll Cardiol. 2011;57(2):173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, revisions 9 and 10. Stroke. 2005;36(8):1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 31.Buresly K, Eisenberg MJ, Zhang X, Pilote L. Bleeding complications associated with combinations of aspirin, thienopyridine derivatives, and warfarin in elderly patients following acute myocardial infarction. Arch Intern Med. 2005;165(7):784–789. doi: 10.1001/archinte.165.7.784. [DOI] [PubMed] [Google Scholar]
- 32.Jia H, Damush TM, Qin H, et al. The impact of poststroke depression on healthcare use by Veterans with acute stroke. Stroke. 2006;37(11):2796–2801. doi: 10.1161/01.STR.0000244783.53274.a4. [DOI] [PubMed] [Google Scholar]
- 33.VA Pharmacy Benefits Management Services. [Accessed August 14, 2015]; Available at: http://www.pbm.va.gov/nationalformulary.asp.
- 34.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. [PubMed] [Google Scholar]
- 35.White HD, Gruber M, Feyzi J, Kaatz S, Husted S, Albers GW. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: Results from SPORTIF III and V. Arch Intern Med. 2007;167(3):239–245. doi: 10.1001/archinte.167.3.239. [DOI] [PubMed] [Google Scholar]
- 36.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 37.Gulløv A, Koefoed B, Petersen P, et al. Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation: Second Copenhagen Atrial Fibrillation, Aspirin, and Anticoagulation Study. Arch Intern Med. 1998;158(14):1513–1521. doi: 10.1001/archinte.158.14.1513. [DOI] [PubMed] [Google Scholar]
- 38.Stroke Prevention in Atrial Fibrillation Investigators. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348(9028):633–638. [PubMed] [Google Scholar]
- 39.Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: The risk–treatment paradox in patients with newly diagnosed non-valvular atrial fibrillation. Heart. 2011;97(24):2046–2050. doi: 10.1136/heartjnl-2011-300901. [DOI] [PubMed] [Google Scholar]
- 40.Abel Latif AK, Peng X, Messinger-Rapport BJ. Predictors of anticoagulation prescription in nursing home residents with atrial fibrillation. J Am Med Dir Assoc. 2005;6(2):128–131. doi: 10.1016/j.jamda.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Yamada M. Cerebral amyloid angiopathy: Emerging concepts. J Stroke. 2015;17(1):17–30. doi: 10.5853/jos.2015.17.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shepherd AM, Hewick DS, Moreland TA, Stevenson IH. Age as a determinant of sensitivity to warfarin. Br J Clin Pharmacol. 1977;4(3):315–320. doi: 10.1111/j.1365-2125.1977.tb00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hylek EM, Regan S, Go AS, Hughes RA, Singer DE, Skates SJ. Clinical predictors of prolonged delay in return of the international normalized ratio to within the therapeutic range after excessive anticoagulation with warfarin. Ann Intern Med. 2001;135(6):393–400. doi: 10.7326/0003-4819-135-6-200109180-00008. [DOI] [PubMed] [Google Scholar]
- 44.Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Risk-Adjusted Percent Time in Therapeutic Range as a Quality Indicator for Outpatient Oral Anticoagulation: Results of the Veterans Affairs Study To Improve Anticoagulation (VARIA) Circ Cardiovasc Qual Outcomes. 2011;4(1):22–29. doi: 10.1161/CIRCOUTCOMES.110.957738. [DOI] [PubMed] [Google Scholar]
- 45.Wieloch M, Sjalander A, Frykman V, Rosenqvist M, Eriksson N, Svensson PJ. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA. Eur Heart J. 2011;32(18):2282–2289. doi: 10.1093/eurheartj/ehr134. [DOI] [PubMed] [Google Scholar]
- 46.Iaboni A, Flint AJ. The complex interplay of depression and falls in older adults: A clinical review. Am J Geriatr Psychiatry. 2013;21(5):484–492. doi: 10.1016/j.jagp.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrade C, Sandarsh S, Chethan KB, Nagesh KS. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: A review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71(12):1565–1575. doi: 10.4088/JCP.09r05786blu. [DOI] [PubMed] [Google Scholar]
- 48.Dauerman HL, Lessard D, Yarzebski J, Gore JM, Goldberg RJ. Bleeding complications in patients with anemia and acute myocardial infarction. Am J Cardiol. 2005;96(10):1379–1383. doi: 10.1016/j.amjcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 49.Carbone LD, Johnson KC, Robbins J, et al. Antiepileptic drug use, falls, fractures, and BMD in postmenopausal women: Findings from the Women’s Health Initiative (WHI) J Bone Miner Res. 2010;25(4):873–881. doi: 10.1359/jbmr.091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61(3):246–255. doi: 10.1111/j.1365-2125.2005.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hart RG, Diener H, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: The RE-LY Trial. Stroke. 2012;43(6):1511–1517. doi: 10.1161/STROKEAHA.112.650614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.