Abstract
Parasitic protozoa cause considerable disease in humans and, due to their intracellular life cycle, induce robust CD8+ T cell responses. A greater understanding of the factors that promote and maintain CD8+ T cell-mediated immunity against these pathogens is likely needed for the development of effective vaccines. Immunization with radiation-attenuated sporozoites, the infectious stage of the malaria parasite transmitted by mosquitoes, is an excellent model to study these questions as CD8+ T cells specific for a single epitope can completely eliminate parasite infection in the liver. Furthermore, live, radiation-attenuated parasites represent the ‘gold standard’ for malaria vaccination. Here, we will highlight recent studies aimed at understanding the factors required for the induction, recruitment, and maintenance of effector and memory CD8+ T cells against malaria liver stages.
Keywords: Plasmodium, CD8+ T cell responses, dendritic cells (DCs), T cell memory, in vivo imaging, vaccines
Introduction
CD8+ T cells are critical for the control of intracellular pathogens, including viral, bacterial, and protozoan parasites. To date, most of our knowledge regarding the antigen presentation requirements, generation, and maintenance of effector and memory CD8+ T cells is based on non-infectious or viral models that fail to account for the complexity and antigenic diversity of protozoan parasites. This knowledge gap is significant considering that parasitic protozoa— Plasmodium spp., Toxoplasma gondii, Leishmania spp., and Trypanosma cruzi— pose major threats to human health. Owing to their ability to invade and replicate within host cells, intracellular pathogens are susceptible to CD8+ T cell-mediated elimination. Thus, CD8+ T cells are important for protective immunity against these pathogens; however, the degree of protection conferred by these cells depends on the species and the developmental stage of the parasite. Importantly, protective immunity against malaria liver stages is mediated, to a significant extent, by CD8+ T cells specific for the Plasmodium circumsporozoite (CS) antigen [1,2]. Here, we review how CD8+ T cell responses against malaria parasites are initiated and sustained following a natural course of infection while drawing parallels to other intracellular pathogens. Furthermore, we will discuss the implications of these findings on the development of whole parasite vaccines.
Early host-pathogen interactions in the skin
Many pathogens enter their mammalian host through the skin, a complex organ that is critical for both physical protection and host defense. In accordance with its key role in immune surveillance, the skin supports a diverse community of resident and migratory immune cells including neutrophils, macrophages, mast cells, dendritic cells (DCs), and lymphocytes [3,4]. Host-pathogen interactions in the skin have a tremendous impact on disease outcome and protective immunity. Consequently, the ‘skin stage of malaria’ has garnered considerable attention within recent years. Malaria infection begins when a female Anopheles mosquito injects Plasmodium sporozoites into the skin of its host during blood feeding. After their deposition in the skin, sporozoites glide rapidly (~1–2 µm/second) before exiting the dermis via blood or lymphatic vessels [5]. The exquisite motility of malaria sporozoites appears to limit degradation by skin-resident phagocytes while promoting progression from the skin site of inoculation to the liver site of infection [6]. Although some sporozoites enter the bloodstream and access the liver within minutes after their inoculation, many take hours to exit the skin [7] and a small proportion (~0.5–5%) remain and develop into exoerythrocytic forms [8,9]. The prolonged residence and development of parasites in the skin likely provides ample chemoattractant signals for innate leukocytes. Neutrophils are rapidly recruited to the skin after needle or mosquito bite inoculation of infectious sporozoites and sustain significantly high numbers in the skin and skin-draining lymph nodes (DLNs) for up to 24 hours post-inoculation [6,10]. Following the first wave of neutrophil recruitment, inflammatory monocytes populate the skin and DLNs [10]. Despite the dramatic neutrophilic response in these organs after skin deposition of sporozoites, neutrophil depletion appears to have no impact on the development of a protective CD8+ T cell response [10]. Interestingly, neutrophils also infiltrate the skin after sham injection, needle inoculation of salivary gland extract from arthropod vectors, and uninfected mosquito and sand fly bites [10,11]. The early neutrophilic response generated under these conditions is likely a byproduct of a host response aimed at wound repair and sterilization as neutrophils were recently shown to be recruited to the skin after sterile laser damage [12].
Although neutrophils and inflammatory monocytes can contribute to adaptive immunity [13,14], DCs are critically involved in both the detection of pathogens in the periphery as well as the activation and differentiation of T cells in lymphoid organs [15]. Skin-derived DCs are a heterogeneous population of cells that differs in their ability to present antigens to CD4+ and CD8+ T cells [15,16] and can be broadly defined into the following three subsets: langerin-positive CD103+ dermal DCs, langerin-negative CD11b+ CD103− dermal DC, and langerin-positive CD103− Langerhans cells (LCs) [15]. After intradermal (ID) injection of sporozoites, ~20% of skin-deposited sporozoites were found to be closely associated with CD11b+ myeloid cells in the skin [10]. However, we recently demonstrated a nonessential role for Langerhans cells and langerin+ dermal DCs in sporozoite antigen presentation to CD8+ T cells using a mouse model system that allowed for the selective depletion of these DC subsets [17]. In addition, we did not observe appreciable migration of skin DCs to the DLNs after sporozoite injection into the dermis by mosquito bite or needle inoculation, nor did we detect a difference in CD8+ T cell priming after chemical inhibition of DC migration to the DLNs [18]. Nevertheless, the immunological significance of malaria parasites that remain and undergo partial development within the skin is largely unknown. It is possible that the inflammatory response induced by parasites in the skin may exert “remote control” over the composition of leukocytes in the DLNs as described following cutaneous inflammation with Complete Freud’s Adjuvant and Keyhole Limpet Hemocyanin [19]. In support of this, a Th1 chemokine/cytokine profile was observed in the DLNs 24 hours after needle inoculation of P. berghei sporozoites [10]. Additionally, parasite development in the skin may provide late liver stage antigens and, thus contribute to a broader anti-malaria CD8+ T cell response [20,21].
Innate and adaptive immunity to malaria parasites in the DLNs
While many pathogens initiate infection in the skin of their mammalian host, several pathogens— Plasmodium spp., Schistosma mansoni, L major, and Toxoplasma gondii—additionally gain access to the DLNs [5,7,22–24], raising key questions about the fate of parasites in these organs as well as their contribution to adaptive immunity. These questions are especially pertinent in the context of a malaria infection as a significant proportion (~15–20%) of skin-deposited sporozoites migrate to the DLNs [5,7,25,26]. Using a model mimicking natural exposure to sporozoite-infected mosquitoes, we previously demonstrated that CD8+ T cells specific for the malaria CS protein, a leading vaccine candidate, were primed by DCs in the DLNs of mice [25]. Remarkably, IFN-γ producing CD8+ T cells were detected in the DLNs within 48 hours after immunization by irradiated P. yoelii-infected mosquito bites, whereas significant responses in the spleen, liver, and liver-DLNs were not observed until 72 hours post-immunization. Furthermore, animals that had their DLNs removed prior to sporozoite immunization generated poor CS-specific CD8+ T cell responses [25]. In agreement with these findings, Obeid and colleagues demonstrated that CD8+ T cell responses generated in the DLNs were sufficient for sterile protection against live sporozoites [27]. The DLNs were also found to be the primary site of prolonged antigen presentation [28]. In these studies, antigen persistence required CD11c+ LN-resident DCs and macrophages and was critical for the optimal expansion of protective CD8+ T cell responses directed against the CS protein [28]. Given the importance of the DLNs in the generation and maintenance of protective immunity against malaria sporozoites, our laboratory and others have worked to characterize host-parasite interactions in this organ.
Sporozoites that gain access to the DLN are first found in the subcapsular sinus [5,10,17], a region of the LN populated by CD169+ macrophages specialized in trapping pathogens and initiating adaptive immune responses (reviewed in [29]). Additionally, sporozoites and parasite-derived antigens were found in association with CD11c+ DCs 5 to 8 hours after sporozoite migration to the DLN [5,17]. However, the contribution of these interactions to CD8+ T cell priming was unknown until recently. Using genetically manipulated malaria parasites, as well as antibody-mediated immobilization of sporozoites, we determined that sporozoite access to the DLNs is required for robust CD8+ T cell responses [17] (Fig. 1). In further support of a limited role for migratory skin-DCs, static and dynamic in vivo imaging revealed the formation of CD8+ T cell-DC clusters (a surrogate for antigen presentation) in the draining LN paracortex as early as 8 hours after sporozoite immunization. These early interactions between LN-resident DCs and CD8+ T cells correlated with T cell activation because we observed the up-regulation of CD69, an early activation marker, and the production of IFN-γ by CS-specific CD8+ T cells at 8 and 16 hours post-inoculation, respectively. In addition, we provided direct evidence of antigen presentation by LN-resident CD8α+ DCs to CD8+ T cells using histo-cytometry, an analytical microscopy method that allows for the quantification of cellular interactions in situ [30,31]. Moreover, malaria-specific CD8+ T cell responses were significantly reduced in Batf3−/− mice that possess substantial defects in their numbers of CD8α+ DCs [32,33]. It is worth noting that other studies have demonstrated a requirement for CD8α+ DCs in the presentation of Plasmodium blood and liver stage antigens to CD8+ T cells [34–37]. In summary, the innate and adaptive immune responses elicited by malaria sporozoites that migrate to the DLNs are essential for the induction of CD8+ T cell-mediated immunity.
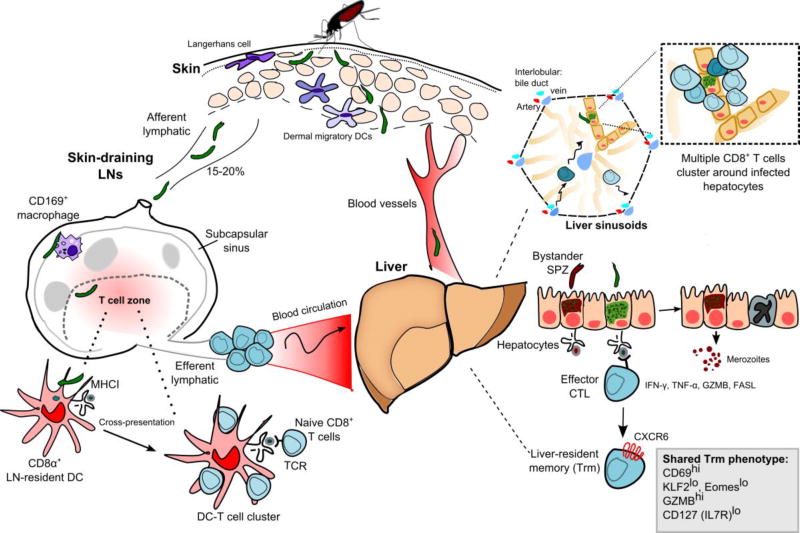
Fig. 1.
Anopheline mosquitoes inject Plasmodium sporozoites (SPZ) into the skin of their mammalian host, a tissue populated by epidermal Langerhans cells and dermal DCs. Sporozoites must enter the bloodstream to continue their development in the liver; however, a proportion of inoculated sporozoites (~15–20%) reach the DLN. Sporozoites that migrate to the DLN are first found in the subcapsular sinus in association with CD169+ macrophages. Within the T-cell zone, CD8α+ LN-resident DCs uptake and cross-present SPZ-derived antigens to naive CD8+ T cells. Following their activation in the DLN, malaria-specific CD8+ T cells enter the blood circulation and reach peripheral tissues, including the liver. Antigen-specific CD8+ T cells patrol along the liver sinusoids before forming large clusters of multiple effector T cells around an infected hepatocyte. The elimination of parasite-infected hepatocytes by CD8+ T cells is a cell-contact dependent process that requires the recognition of cognate parasite-derived peptides presented by MHC I molecules on infected hepatocytes. Liver-resident memory CD8+ T cells exhibit a tissue-resident memory T cell phenotype (Trm) and depend on the chemokine receptor CXCR6 for their long-term maintenance in this organ.
Antigen presentation requirements of sporozoite-specific CD8+ T cells
While it is clear that DCs are critical for the induction of CD8+ T cells, it is well appreciated that DC maturation is needed for the optimal priming of these cells [38]. DC maturation, their migration to secondary lymphoid organs, and cross-presentation of exogenous antigens is greatly enhanced following their recognition of microbial molecular patterns by pattern recognition receptors (PRRs) such as toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). TLRs recognize a diverse range of microbe-derived lipids, carbohydrates, peptides, and nucleic acid structures and elicit different signaling cascades depending on the engagement of the following intracellular adaptor proteins: myeloid differentiation factor 88 (MyD88), MyD88-adaptor-like (MAL), Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFN-β (TRIF), and TRIF-related adaptor molecule (TRAM) [39]. Like TLRs, NLRs respond to pathogen-associated molecular patterns (PAMPs), but are additionally capable of responding to endogenous molecules released during stress or infection such as ATP, reactive oxygen species, and potassium efflux [40]. The oligomerization of certain NLRs results in the assembly of the inflammasome, a large macromolecular complex that is critical for the maturation of the proinflammatory cytokines interleukin-1 beta (IL-1β) and IL-18 [40]. Although TLR and inflammasome agonists from P. falciparum and P. berghei blood stage parasites have been characterized [41,42], a sporozoite-derived PAMP, nor its contribution to protective CD8+ T cell responses, has not been described. To address this question, we used transgenic P. berghei parasites expressing SIINFEKL, the H-2Kb-restricted epitope of ovalbumin (P. berghei CS5M) described previously [43], and a panel of mice deficient in components of PRR signaling. Myd88−/− Trif−/− and Ipaf−/− Asc−/− mice were both protected against live sporozoite challenge after immunization by irradiated P. berghei CS5M-infected mosquito bites. In addition, there was no difference in the magnitude or function of endogenous sporozoite-specific CD8+ T cells primed in the absence of TLR or inflammasome (NLRP1, NLRP3, and IPAF)-dependent signaling [18]. Therefore, it is possible that protective CD8+ T cell responses against malaria parasites require PRR-mediated signaling through compensatory and redundant mechanisms or alternative PRRs.
An additional consideration is how DCs acquire, process, and present malaria antigens to naïve CD8+ T cells. DCs are especially adept at processing exogenous antigens derived from apoptotic, necrotic, or pathogen-infected cells in a major histocompatibility complex class (MHC) I-restricted manner via cross-presentation [44]. In contrast to T. gondii and L. major, Plasmodium sporozoites do not productively invade DCs, and instead, cross-presentation is required for the induction of CD8+ T cell responses directed against molecular determinants in the CS protein [25,45]. In subsequent studies, Cockburn et al. demonstrated that DCs cross-present malaria antigens to CD8+ T cells through an endosome-to-cytosol pathway [43]; however, the mechanism by which sporozoite antigens are deposited in the cytosol of DCs is largely unknown. Furthermore, the TAP-dependent pathway was also shown to be critical for the presentation of liver stage antigens by infected hepatocytes [43]. Therefore, both the induction and effector phases of anti-malaria CD8+ T cells require TAP-mediated delivery of CS epitopes from the cytosol to the ER in DCs and hepatocytes, respectively.
Trafficking and survival of tissue-specific CD8+ T cells
In order to continue their life cycle in the mammalian host, sporozoites must invade and replicate within hepatocytes. Importantly, infected hepatocytes present parasite-derived antigens to CD8+ T cells and are susceptible to CD8+ T cell-mediated killing [1,25,46]. Because parasites remain in the liver for only a short time, intrahepatic CD8+ T cells are probably key factors in protection against malaria. Therefore, a better understanding of the mechanisms regulating CD8+ T cells trafficking and survival in the liver is critical for malaria vaccine design. During activation, effector T cells can be “selectively imprinted” to express certain homing receptors that regulate their migration to peripheral organs [47]. While this process has been shown to be important for the preferential homing of effector CD8+ T cells to barrier tissues such as the intestine and skin (reviewed in [48]), it is unclear whether parasite-specific T cells are instructed for ‘liver-homing’ shortly after their priming in lymphoid organs. After sporozoite immunization, CS-specific effector CD8+ T cells were found in various peripheral organs, such as the liver, lungs and kidneys [49], suggesting that the initial homing pattern of CD8+ T cells is not a stringent process. The promiscuous trafficking of activated CS-specific CD8+ T cells is likely due to their simultaneous expression of several inflammatory chemokine receptors [50–52].
Although the initial trafficking of effector CD8+ T cell appears to be an indiscriminate process, the local persistence of memory CD8+ T cells is clearly dependent on specific environmental cues, e.g. chemokines and cytokines. For example, the transmembrane chemokine CXCL16 is abundantly expressed in the liver sinusoids and was shown to be crucial for the maintenance of hepatic NKT cells during steady-state [53]. Importantly, CXCR6, the chemokine receptor which recognizes CXCL16, was found to be significantly up-regulated by liver-resident CD8+ T cells several weeks after sporozoite immunization [51,52]. These findings hinted at a potential role for CXCR6 in the retention of these cells. Indeed, adoptive transfer studies using Cxcr6−/− CD8+ T cells demonstrated that CXCR6 was dispensable for the early recruitment, but important for the long-term survival, of memory CD8+ T cells in the livers of sporozoite-immunized mice [52]. A similar phenotype was recently observed in an experimental model of Listeria monocytogenes [54]. Though it is unclear how CXCR6 expression regulates the survival of CD8+ T cells in the liver, one possible mechanism is through the up-regulation of anti-apoptotic molecules such as BCL2. In support of this notion, Cxcr6−/− effector cells exhibited lower expression of BCL2 after malaria liver stage infection [52]. It is worth noting that studies in other models have shown that anti-apoptotic genes are highly expressed by resident CD8+ T cells in the skin and the brain [55,56]. Another nonexclusive possibility is that homeostatic cytokines, such as IL-7 and IL-15, mediate the lifespan of tissue-resident CD8+ T cells. Recently, the survival of liver-resident CD8+ T cells was shown to require IL-15 after immunization with P. berghei sporozoites [57].
Parasite-specific CD8+ T cells: A unique liver-resident memory population
While it is clear that environmental cues regulate the survival of parasite-specific memory CD8+ T cells in the liver, it was previously unknown whether this population was maintained through local proliferation or through the migration of memory cells from the periphery [58,59]. In the latter scenario, the CD8+ T cells recovered from the liver would exhibit an overlapping phenotype and molecular signature with those recovered from the lymphoid organs. To explore these possibilities, we used TCR transgenic mice specific for the H-2Kd-restricted P. yoelii epitope SYVSAEQI [60] and compared the gene transcription profiles of the clonal CD8+ T cell population from different tissues [51]. Memory CD8+ T cells recovered from the spleen were mostly CD62LHi and CCR7Hi, a phenotype of the central memory population (Tcm). In contrast, memory CD8+ T cells residing in the liver were CD62LLo and CCR7Lo, suggesting that these T cells are predominantly effector cells (Tem) that have entered non-lymphoid tissues from the circulation. A comparative transcriptome analysis of resident memory CD8+ T cells in the spleen versus the liver revealed striking differences between these T cell populations [51]. A large number of genes involved in transcription, cell cycle, trafficking, and signaling were differentially regulated in the liver-resident memory CD8+ T cell population and these cells underwent more vigorous homeostatic proliferation than their counterparts in the spleen. Importantly, liver-resident memory CD8+ T cells were homogenously CD69Hi and expressed lower levels of the transcription factors Eomes and KLF2, consistent with the tissue-resident (Trm) phenotype recently reported [61]. KLF2 is known to regulate the expression of S1PR1 and CD62L, two crucial molecules involved in lymphocyte recirculation. Subsequently, the loss of KLF2 was found to prevent the recirculation of CD8+ T cells and promote the establishment of a tissue-resident memory population [62]. Hence, the KLF2Lo memory population observed in the livers of sporozoite-immunized mice is likely distinct from the circulating Tem population, suggesting that this population resides in the liver and is not frequently renewed by lymphoid migrants (Fig. 1).
Protection mediated by local T cells: Direct or indirect killing?
Tissue-resident memory T cells (Trm) are critical for host resistance [63,64] and have been shown to provide better protection than other memory populations against vaccinia and influenza viruses [63,65]. Although virus-specific Trm can recruit circulating memory T cells [66], recruited cells likely contribute a minor role to protection against malaria parasites because of the brief period in which CD8+ T cells can limit their development in the liver (2 days in mice). For this reason, host resistance against malaria liver stages is probably mediated by tissue-resident CD8+ T cells. Indeed, we observed that mice with reduced numbers of liver-resident memory CD8+ T cells were less protected against live sporozoite challenge, despite having normal numbers of memory CD8+ T cells in the spleen [52].
Given the short window in which liver-stage parasites can be eliminated, T cells must be poised for an immediate antimicrobial response. It is well established that CD8+ T cells recognize pathogen-derived peptides presented by MHC I molecules on infected cells. Therefore, the elimination of target cells is generally considered to be a cell-contact dependent process. In fact, mouse studies using bone marrow chimeras revealed a requirement for direct peptide recognition between effector CD8+ T cells and hepatocytes as CD8+ T cells were unable to eliminate cells bearing a non-cognate MHC class I molecule [25]. Recently, the elimination of parasitized hepatocytes by effector CD8+ T cells was visualized using intravital imaging [46]. Strikingly, large clusters of CD8+ T cells formed around infected hepatocytes (Fig. 1). The initiation of these T cell clusters was strictly dependent on cognate interactions as CD8+ T cells specific for an irrelevant antigen did not initiate clustering around hepatocytes. More importantly, T cell clustering required G-protein coupled signaling and appeared to be crucial for the effective elimination of parasitized hepatocytes because the protective capacity of effector cells was greatly reduced following inhibition of T cell clustering with pertussis toxin [46]. Together, our in vivo imaging studies demonstrated that the killing of target cells is a prolonged process that requires the recruitment of multiple T cells to the infected hepatocyte. In addition to direct recognition of infected hepatocytes, malaria-specific CD8+ T cells mediate protection through the production of several effector cytokines, including IFN-γ and TNFα, and to some extent perforin/granzyme B [67,68]. Liver-resident memory CD8+ T cells were found to express higher levels of granzyme B and IFN-γ transcripts than their counterparts in other organs [51]. However, It is likely that these pathways work redundantly because CD8+ T cells lacking IFN-γ could still limit parasite growth [69] and mice lacking perforin and FasL mounted a protective response against Plasmodium parasites (reviewed in [70,71]).
Within the last few years, studies have highlighted the role of CD8+ T cells not only as efficient cytolytic T cells, but as ‘early sensors’ of foreign pathogens in lymphocytic choriomeningitis virus, herpes simplex virus, and vaccinia virus infection models. In these studies, IFN-γ produced by tissue-resident T cells triggered local anti-viral responses and facilitated protection against irrelevant viral antigens [72,73]. In light of these findings, we tested whether sporozoite-specific CD8+ T cells could eliminate parasitized hepatocytes through bystander killing independent of, or in addition to, the direct killing of target cells. To address these questions, mice were co-infected with two different P. berghei parasites in equal ratios, with only one of the strains carrying a CD8+ T cell epitope recognized by TCR transgenic cells that were adoptively transferred to these mice. In contrast to what was observed with viral pathogens, malaria-specific CD8+ T cells failed to eliminate the irrelevant ‘bystander’ parasites [74] (Fig. 1). These results suggest that sterile immunity likely requires the location and killing of every infected cell in a cell-contact dependent manner. Moreover, they further support the notion that CD8+ T cells recognize parasite-derived peptides directly on the surface of hepatocytes.
Because parasite-infected cells are eliminated in a cell-contact dependent manner involving multiple T cells, it is not surprising that the number of memory CD8+ T cells needed for protective immunity against malaria parasites far exceeds what is required for bacterial and viral infections (~100–1000 fold) [75]. A sufficiently high number of anti-parasite CD8+ T cells, particularly in the liver, is critical for early pathogen control since one schizont that escapes immunosurveillance can give rise to thousands of merozoites. Thus, early pathogen control in the liver is key to host resistance. These stringent requirements, in part, explain the challenges to achieving sterile protection against malaria parasites.
CD8+ T cell responses to blood-stage malaria
After an obligatory developmental stage within hepatocytes, Plasmodium parasites develop into merozoites and infect erythrocytes. Since erythrocytes lack the expression and processing machinery of MHC I molecules, CD8+ T cells are not generally thought to play a major role in controlling malaria blood stages. Nevertheless, studies have shown that CD8+ T cell depletion results in the delayed clearance of P. chabaudi blood stages in mice [76] and blood-stage specific CD8+ T cell responses have been described in rodents [77] and in P. falciparum-infected humans [78]. These studies suggest that CD8+ T cells, to some extent, contribute to protective immunity against blood-stage infection; however, CD8+ T cells have also been implicated in severe host pathology in experimental models. For a more detailed review of this topic, we direct the reader to the article written by Renia and colleagues in this special issue.
Implications for malaria vaccine effort
It is well established that live, but not dead, malaria sporozoites can induce protective CD8+ T cell responses after ID and intravenous (IV) routes of immunization [25,79]. Our recent studies demonstrating a requirement for direct parasite access to the DLNs for CD8+ T cell priming [17] provide an explanation for why ID injection of dead sporozoites, which do not reach the DLNs [5], elicit poor CD8+ T cell responses [25]. However, the requirement for live sporozoites following an IV route of immunization is less obvious. To elaborate, sporozoites injected IV have direct access to the spleen and we have previously shown that CD8+ T cells are first primed here after IV immunization [60]. Thus, it is possible that motile sporozoites deliver antigens that are efficiently presented by DCs in the lymphoid organs, e.g. shed or particulate antigens. Another possibility is that sporozoite traversal through host cells deposits antigens in the cytosol while triggering a cytokine microenvironment that favors CD8+ T cell priming. As with dead sporozoites, antibody-treated sporozoites injected ID do not reach the DLNs and fail to prime robust CS-specific CD8+ T cell responses [17,43]. These findings may have direct implications for vaccine efforts as they indicate that it might be difficult to generate CD8+ T cell responses in individuals with high-titer anti-CS antibodies.
The superior immunogenicity of live versus dead parasites is not unique to malaria, but was also observed in vaccination models of L. infantum chagasi and S. mansoni [80,81]. Furthermore, an experimental model of T. gondii infection recently revealed that antigens derived from direct infection, and not through phagocytosis of heat-killed or invasion-blocked parasites, were critical for optimal CD4+ and CD8+ T cell responses [82]. In addition to providing a diverse array of antigens, live attenuated parasites more efficiently access tissue and cellular compartments required for the induction of long-lasting immunity. Therefore, it appears that the strategies important for parasite infection—cell traversal and cell invasion—may also be critical for immune activation, and should be incorporated into whole parasite vaccine approaches.
Although live, radiation-attenuated parasites represent the ‘gold standard’ for malaria vaccination and form the rationale for a whole parasite vaccine [83,84], there are several technical limitations to this approach (reviewed in [85]). The recent success of the IV administered live, attenuated P. falciparum sporozoite vaccine is encouraging [83]; however, it remains to be seen whether large-scale IV vaccination is feasible and if this vaccine can provide long-term protection in humans. In addition to whole parasite vaccine approaches, vaccines designed to induce CD8+ T cell-mediated immunity through the use of attenuated viral vectors or synthetic constructs have yielded encouraging results in mouse studies but have yet to demonstrate significant efficacy in human trials. Finally, the observation that sterile protection against Plasmodium parasites requires a large population of intrahepatic memory CD8+ T cells raises several questions as to whether these numbers can be maintained in vaccinated humans. The demonstration of prolonged antigen presentation, and the subsequent activation of naive recent thymic emigrants [28], is promising because it suggests a potential mechanism by which the memory CD8+ T cell population may be renewed after vaccination with non-replicating organisms. Because it is clear that CD8+ T cells exert their protective function in the liver, further research is required to understand how to recruit and maintain these cells in this organ.
Conclusions
CD8+ T cells are critical for protective immunity against several medically important pathogens, especially the malaria parasite Plasmodium spp. Through the use of experimental models of malaria infection, transgenic animals, and high-resolution imaging we have gained valuable insight into the factors regulating the activation, survival, and effector functions of malaria-specific CD8+ T cells. Despite these advances, it is clear that protective immunity against malaria parasites is more stringent than for other pathogens, requiring the location and elimination of every infected cell in a cell-contact dependent manner. Therefore, an important future direction will be to translate the information obtained from experimental models to the development of effective vaccines in humans.
Acknowledgments
This work was supported in part by the Intramural Research Program, NIAID, NIH; AJR was supported by the Intramural Research Program of NIAID, NIH. FZ is supported by NIH grant AI44375.
References
We apologize that we are unable to include all relevant references due to space constraints.
- 1.Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341(6240):323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 2.Weiss WR, Berzofsky JA, Houghten RA, Sedegah M, Hollindale M, Hoffman SL. A T cell clone directed at the circumsporozoite protein which protects mice against both Plasmodium yoelii and Plasmodium berghei. J Immunol. 1992;149(6):2103–2109. [PubMed] [Google Scholar]
- 3.Wang XN, McGovern N, Gunawan M, Richardson C, Windebank M, Siah TW, Lim HY, Fink K, Li JL, Ng LG, Ginhoux F, Angeli V, Collin M, Haniffa M. A three-dimensional atlas of human dermal leukocytes, lymphatics, and blood vessels. J Invest Dermatol. 2014;134(4):965–974. doi: 10.1038/jid.2013.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol. 2013;14(10):978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 5.Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Menard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12(2):220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- 6.Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, Prevost MC, Ishino T, Yuda M, Menard R. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe. 2008;3(2):88–96. doi: 10.1016/j.chom.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9(5):1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gueirard P, Tavares J, Thiberge S, Bernex F, Ishino T, Milon G, Franke-Fayard B, Janse CJ, Menard R, Amino R. Development of the malaria parasite in the skin of the mammalian host. P Natl Acad Sci USA. 2010;107(43):18640–18645. doi: 10.1073/pnas.1009346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voza T, Miller JL, Kappe SH, Sinnis P. Extrahepatic exoerythrocytic forms of rodent malaria parasites at the site of inoculation: clearance after immunization, susceptibility to primaquine, and contribution to blood-stage infection. Infect immun. 2012;80(6):2158–2164. doi: 10.1128/iai.00246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mac-Daniel L, Buckwalter MR, Berthet M, Virk Y, Yui K, Albert ML, Gueirard P, Menard R. Local immune response to injection of Plasmodium sporozoites into the skin. J Immunol. 2014;193(3):1246–1257. doi: 10.4049/jimmunol.1302669. [DOI] [PubMed] [Google Scholar]
- 11.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321(5891):970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498(7454):371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tvinnereim AR, Hamilton SE, Harty JT. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. J Immunol. 2004;173(3):1994–2002. doi: 10.4049/jimmunol.173.3.1994. [DOI] [PubMed] [Google Scholar]
- 14.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143(3):416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10(12):1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 16.Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35(2):260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radtke AJ, Kastenmuller W, Espinosa DA, Gerner MY, Tse SW, Sinnis P, Germain RN, Zavala FP, Cockburn IA. Lymph-node resident CD8alpha+ dendritic cells capture antigens from migratory malaria sporozoites and induce CD8+ T cell responses. PLoS pathogens. 2015;11(2):e1004637. doi: 10.1371/journal.ppat.1004637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radtke AJ. The antigen presentation requirements of CD8+ T cell-mediated immunity against malaria liver stages. Dissertation. Johns Hopkins University; 2013. [Google Scholar]
- 19.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194(9):1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe. 2011;9(6):451–462. doi: 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy SC, Kas A, Stone BC, Bevan MJ. A T-cell response to a liver-stage Plasmodium antigen is not boosted by repeated sporozoite immunizations. P Natl Acad Sci USA. 2013;110(15):6055–6060. doi: 10.1073/pnas.1303834110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumkate S, Jenkins GR, Paveley RA, Hogg KG, Mountford AP. CD207+ Langerhans cells constitute a minor population of skin-derived antigen-presenting cells in the draining lymph node following exposure to Schistosoma mansoni. Int J Parasitol. 2007;37(2):209–220. doi: 10.1016/j.ijpara.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritter U, Meissner A, Scheidig C, Korner H. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol. 2004;34(6):1542–1550. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- 24.Chtanova T, Han SJ, Schaeffer M, van Dooren GG, Herzmark P, Striepen B, Robey EA. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. 2009;31(2):342–355. doi: 10.1016/j.immuni.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13(9):1035–1041. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 26.Boyd MF, Kitchen SF. The demonstration of sporozoites in human tissues. Am J Trop Med Hyg. 1939;s1–19(1):27–31. [Google Scholar]
- 27.Obeid M, Franetich JF, Lorthiois A, Gego A, Grüner AC, Tefit M, Boucheix C, Snounou G, Mazier D. Skin- draining lymph node priming is sufficient to induce sterile immunity against pre- erythrocytic malaria. EMBO Mol Med. 2013;5:250–263. doi: 10.1002/emmm.201201677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cockburn IA, Chen YC, Overstreet MG, Lees JR, van Rooijen N, Farber DL, Zavala F. Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathogens. 2010;6(5):e1000877. doi: 10.1371/journal.ppat.1000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray EE, Cyster JG. Lymph node macrophages. Innate Immun. 2012;4(5–6):424–436. doi: 10.1159/000337007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;37(2):364–376. doi: 10.1016/j.immuni.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerner MY, Torabi-Parizi P, Germain RN. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 2015;42(1):172–185. doi: 10.1016/j.immuni.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207(4):823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jobe O, Donofrio G, Sun G, Liepinsh D, Schwenk R, Krzych U. Immunization with radiation-attenuated Plasmodium berghei sporozoites induces liver cCD8alpha+DC that activate CD8+ T cells against liver-stage malaria. PloS One. 2009;4(4):e5075. doi: 10.1371/journal.pone.0005075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundie RJ, de Koning-Ward TF, Davey GM, Nie CQ, Hansen DS, Lau LS, Mintern JD, Belz GT, Schofield L, Carbone FR, Villadangos JA, Crabb BS, Heath WR. Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8α+ dendritic cells. P Natl Acad Sci USA. 2008;105(38):14509–14514. doi: 10.1073/pnas.0806727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piva L, Tetlak P, Claser C, Karjalainen K, Renia L, Ruedl C. Cutting edge: Clec9A+ dendritic cells mediate the development of experimental cerebral malaria. J Immunol. 2012;189(3):1128–1132. doi: 10.4049/jimmunol.1201171. [DOI] [PubMed] [Google Scholar]
- 37.Lau LS, Fernandez-Ruiz D, Mollard V, Sturm A, Neller MA, Cozijnsen A, Gregory JL, Davey GM, Jones CM, Lin Y-H, Haque A, Engwerda CR, Nie CQ, Hansen DS, Murphy KM, Papenfuss AT, Miles JJ, Burrows SR, de Koning-Ward T, McFadden GI, Carbone FR, Crabb BS, Heath WR. CD8+ T cells from a novel T cell receptor transgenic mouse induce liver-stage immunity that can be boosted by blood-stage infection in rodent malaria. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 41.Ropert C, Franklin BS, Gazzinelli RT. Role of TLRs/MyD88 in host resistance and pathogenesis during protozoan infection: lessons from malaria. Semin Immunopathol. 2008;30(1):41–51. doi: 10.1007/s00281-007-0103-2. [DOI] [PubMed] [Google Scholar]
- 42.Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS pathogens. 2009;5(8):e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cockburn IA, Tse SW, Radtke AJ, Srinivasan P, Chen YC, Sinnis P, Zavala F. Dendritic cells and hepatocytes use distinct pathways to process protective antigen from Plasmodium in vivo. PLoS Pathogens. 2011;7(3):e1001318. doi: 10.1371/journal.ppat.1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 45.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cockburn IA, Amino R, Kelemen RK, Kuo SC, Tse S-W, Radtke A, Mac-Daniel L, Ganusov VV, Zavala F, Menard R. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. P Natl Acad Sci U S A. 2013;110(22):9090–9095. doi: 10.1073/pnas.1303858110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400(6746):776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 48.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9(3):153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 49.Morrot A, Hafalla JCR, Cockburn IA, Carvalho LH, Zavala F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J Exp Med. 2005;202(4):551–560. doi: 10.1084/jem.20042463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tse S-W. Differentiation and migration of CD8 T cells against liver-stage Plasmodium. Dissertation. Johns Hopkins University; 2012. [Google Scholar]
- 51.Tse S-W, Cockburn IA, Zhang H, Scott AL, Zavala F. Unique transcriptional profile of liver-resident memory CD8+ T cells induced by immunization with malaria sporozoites. Genes Immun. 2013;14(5):302–309. doi: 10.1038/gene.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tse S-W, Radtke AJ, Espinosa DA, Cockburn IA, Zavala F. The Chemokine receptor CXCR6 is required for the maintenance of liver memory CD8+ T cells specific for infectious pathogens. J Infect Dis. 2014;210(9):1508–1516. doi: 10.1093/infdis/jiu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3(4) doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heesch K, Raczkowski F, Schumacher V, Hünemörder S, Panzer U, Mittrücker H-W. The function of the chemokine receptor CXCR6 in the T cell response of mice against Listeria monocytogenes. PLoS ONE. 2014;9(5) doi: 10.1371/journal.pone.0097701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14(12):1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 56.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of tesidence. P Natl Acad ScI USA. 2010;107(42):17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarling S, Berenzon D, Dalai S, Liepinsh D, Steers N, Krzych U. The survival of memory CD8 T Cells that is mediated by IL-15 correlates with sustained protection against malaria. J Immunol. 2013;190(10):5128–5141. doi: 10.4049/jimmunol.1203396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obhrai JS, Oberbarnscheidt MH, Hand TW, Diggs L, Chalasani G, Lakkis FG. Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs. J Immunol. 2006;176(7):4051–4058. doi: 10.4049/jimmunol.176.7.4051. [DOI] [PubMed] [Google Scholar]
- 59.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrançois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20(5):551–562. doi: 10.1016/S1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 60.Sano G, Hafalla JC, Morrot A, Abe R, Lafaille JJ, Zavala F. Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages. J Exp Med. 2001;194(2):173–180. doi: 10.1084/jem.194.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skon CN, Lee J-Y, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14(12):1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483(7388):227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 65.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol. 2013;14(5):509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 68.Nussler A, Pied S, Goma J, Rénia L, Miltgen F, Grau GE, Mazier D. TNF inhibits malaria hepatic stages in vitro via synthesis of IL-6. Int Immunol. 1991;3(4):317–321. doi: 10.1093/intimm/3.4.317. [DOI] [PubMed] [Google Scholar]
- 69.Chakravarty S, Baldeviano GC, Overstreet MG, Zavala F. Effector CD8+ T lymphocytes against liver stages of Plasmodium yoelii do not require gamma interferon for antiparasite activity. Infect Immun. 2008;76(8):3628–3631. doi: 10.1128/iai.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tse SW, Radtke AJ, Zavala F. Induction and maintenance of protective CD8+ T cells against malaria liver stages: implications for vaccine development. Mem I Oswaldo Cruz. 2011;(106 Suppl 1):172–178. doi: 10.1590/s0074-02762011000900022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morrot A, Zavala F. Effector and memory CD8+ T cells as seen in immunity to malaria. Immunol Rev. 2004;201(1):291–303. doi: 10.1111/j.0105-2896.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 72.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song J-Y, Jacobs H, Haanen JB, Schumacher TN. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346(6205):101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 74.Cockburn IA, Tse S-W, Zavala F. CD8+ T cells eliminate liver-stage Plasmodium berghei parasites without detectable bystander effect. Infect Immun. 2014;82(4):1460–1464. doi: 10.1128/IAI.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, Gilbert SC, Hill AV, Bartholomay LC, Harty JT. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. P Natl Acad Sci USA. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Podoba JE, Stevenson MM. CD4+ and CD8+ T lymphocytes both contribute to acquired immunity to blood-stage Plasmodium chabaudi AS. Infect Immun. 1991;59(1):51–58. doi: 10.1128/iai.59.1.51-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lau LS, Ruiz DF, Davey GM, Koning-Ward TFd, Papenfuss AT, Carbone FR, Brooks AG, Crabb BS, Heath WR. Blood-stage Plasmodium berghei infection generates a potent, specific CD8+ T-cell response despite residence largely in cells lacking MHC I processing machinery. J Infect Dis. 2011;204(12):1989–1996. doi: 10.1093/infdis/jir656. [DOI] [PubMed] [Google Scholar]
- 78.Woodberry T, Pinzon-Charry A, Piera KA, Panpisutchai Y, Engwerda CR, Doolan DL, Salwati E, Kenangalem E, Tjitra E, Price RN, Good MF, Anstey NM. Human T cell recognition of the blood stage antigen Plasmodium hypoxanthine guanine xanthine phosphoribosyl transferase (HGXPRT) in acute malaria. Malaria J. 2009;8:122. doi: 10.1186/1475-2875-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hafalla JC, Rai U, Morrot A, Bernal-Rubio D, Zavala F, Rodriguez A. Priming of CD8+ T cell responses following immunization with heat-killed Plasmodium sporozoites. Eur J Immunol. 2006;36(5):1179–1186. doi: 10.1002/eji.200535712. [DOI] [PubMed] [Google Scholar]
- 80.Bruhn KW, Birnbaum R, Haskell J, Vanchinathan V, Greger S, Narayan R, Chang PL, Tran TA, Hickerson SM, Beverley SM, Wilson ME, Craft N. Killed but metabolically active Leishmania infantum as a novel whole-cell vaccine for visceral leishmaniasis. Clin Vaccine Immunol: CVI. 2012;19(4):490–498. doi: 10.1128/cvi.05660-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hewitson JP, Hamblin PA, Mountford AP. Immunity induced by the radiation-attenuated schistosome vaccine. Par immunol. 2005;27(7–8):271–280. doi: 10.1111/j.1365-3024.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- 82.Dupont CD, Christian DA, Selleck EM, Pepper M, Leney-Greene M, Harms Pritchard G, Koshy AA, Wagage S, Reuter MA, Sibley LD, Betts MR, Hunter CA. Parasite fate and involvement of infected cells in the induction of CD4+ and CD8+ T cell responses to Toxoplasma gondii. PLoS pathogens. 2014;10(4):e1004047. doi: 10.1371/journal.ppat.1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 84.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science. 2011;334(6055):475–480. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 85.Butler NS, Vaughan AM, Harty JT, Kappe SH. Whole parasite vaccination approaches for prevention of malaria infection. Trends in Immunol. 2012;33(5):247–254. doi: 10.1016/j.it.2012.02.001. [DOI] [PubMed] [Google Scholar]