Abstract
Purpose of Review
Autoimmune-mediated destruction of insulin-producing β-cells within the pancreas results in type 1 diabetes (T1D), which is not yet preventable or curable. Previously, our understanding of the β-cell specific T cell repertoire was based on studies of autoreactive T cell responses in the peripheral blood of patients at risk for, or with, T1D; more recently, investigations have included immunohistochemical analysis of some T cell specificities in the pancreas from organ donors with T1D. Now, we are able to examine live, islet-infiltrating T cells from donors with T1D.
Recent Findings
Analysis of the T cell repertoire isolated directly from the pancreatic islets of donors with T1D revealed pro-inflammatory T cells with targets of known autoantigens, including proinsulin and glutamic acid decarboxylase, as well as modified autoantigens.
Summary
We have assayed the islet-infiltrating T cell repertoire for autoreactivity and function directly from the inflamed islets of T1D organ donors. Design of durable treatments for prevention of or therapy for T1D requires understanding this repertoire.
Keywords: Human, T cell, Autoreactivity, Islets of Langerhans
Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by the activation of lymphocytes that infiltrate and destroy insulin-producing beta (β)-cells within the pancreatic islets [1]. Loss of β cell number and function results in insulin deficiency, and requires life-long insulin administration. Because people with T1D require frequent blood glucose monitoring along with intensive insulin therapy and the disease incidence is dramatically increasing, especially in young children [2], there is an urgent need for effective therapies. With this goal in mind, many well-designed clinical trials have been completed using immune therapies to prevent or stop β-cell destruction, before or soon after disease onset [3]. To date, no therapies have proven to be clinically beneficial. The need exists to understand the immune cells infiltrating islets of human patients with T1D to define specific targets for therapies and biomarkers of disease activity.
The human pancreas, and the islets which are naturally embedded throughout the organ, needs to be accessed to effectively study and characterize islet-infiltrating lymphocytes and fully understand the mechanisms of insulitis/β-cell death. However, the pancreas is a retroperitoneal organ, predominantly involved in the exocrine production of enzymes for the digestion of proteins, carbohydrates, and fat, making it extremely difficult to access or biopsy. A recent trial involving pancreatic biopsies of patients with new-onset T1D was halted because of surgical complications [4]. Attempts to visualize or image islets using non-invasive methods are being developed, but have not fully resolved nor defined insulitis, which is the lymphocytic infiltration of the islets in T1D [5–7]. By necessity, the study of autoreactive T cells in human T1D has come from the analysis of autoantigen-specific T cells from peripheral blood [8–16]. Almost all of the studies evaluating cellular infiltrates in the islets are gleaned from histology sections from cadavers.
Compared to humans, animal models of autoimmune diabetes provide straightforward access to the target organs. The non-obese diabetic (NOD) mouse model of spontaneous autoimmune diabetes shares many similarities with humans including MHC genes conferring disease risk, the development of insulin autoantibodies and insulitis [17]. Experiments evaluating NOD mouse islet-infiltrating T cells indicate the vast majority are specific for β-cell antigens and transfer diabetes to immune deficient (lacking T cells and B cells) NOD mice [18, 19]. By mutating or knocking out β-cell antigens, such as insulin or chromogranin A, CD4+ T cell responses to these proteins have been shown to be necessary for the development of autoimmune diabetes [20, 21]. Additionally, islet-derived CD8+ T cells have been shown to be a major driver of β-cell destruction by directly targeting and killing the β-cells [22, 23]. Furthermore, only β-cell antigen-specific CD8+ T cells infiltrate NOD mouse islets. In another animal model of T1D, the biobreeding (BB) rat, a variety of lymphoid cells infiltrate the islets following a viral infection or innate immune system activation [24].
Studying immune cells from the inflamed islets in animal models has led to many therapies capable of preventing and even reversing diabetes. Importantly, using antigens and peptides that stimulate islet-infiltrating T cells, known as antigen-specific immunotherapy, can induce long-lasting diabetes remission. Unfortunately, the therapeutic results in animal models have not translated to humans [25]. Studying the functional biology of human islet-infiltrating T cells “at the scene of the crime” will provide powerful new insights into the autoimmune basis of human disease with the potential to improve prevention efforts.
Pancreatic Histology and Autoreactive T cells in T1D
The unusual infiltration of cells in the islets of a young child who died from ketoacidosis was noted over a hundred years ago (the history of insulitis is comprehensively reviewed in [26]). In this review, In’t Veld makes the salient point that as of 2011, our knowledge of the histology and composition of insulitis comes from approximately only 150 donors, and that insulitic lesions are rare and heterogeneous, even in recent onset donors. Here, we will highlight the donor tissue collections that have aided in implicating islet-infiltrating immune cells, including CD4+ and CD8+ T cells, as the mediators of pathogenesis in T1D.
The Willy Gepts collection consists of 22 pancreas tissue samples from new or recent onset donors with T1D in Brussels, Belgium in the 1960s (11 with insulitis) [27]. This collection remains an important resource for studies into the disease processes leading to T1D [27–30]. Stained pancreatic sections from this collection are available in a digitized format as part of the Diabetes BioBank Brussels (http://www.diabetesbiobank.org/).
The Alan Foulis collection is a collection of autopsy pancreas samples recovered from nearly 200 individuals with new or recent onset T1D, from across the UK, who died shortly after receiving a diagnosis of T1D. This collection is now housed at the University of Exeter Medical School. Examples of infiltrated islets from cases can be seen here (http://foulis.vub.ac.be/index.php). From this collection, immune dysregulation was seen within the islets from new-onset donors with T1D by noting the upregulation of human leukocyte antigen (HLA) class II, and especially HLA class I, within inflamed islets and the pro-inflammatory phenotype of immune cells infiltrating the islets [31–36]. This collection is still in use today.
The Network of Pancreatic Organ Donors with Diabetes (nPOD) (http://www.jdrfnpod.org/) was established by the Juvenile Diabetes Research Foundation (JDRF) in 2007 in order to collect tissue from donors with T1D and to distribute these tissue to investigators while fostering collaboration and interaction to understand the etiology of human T1D. To date, there are over 160 cases of donors with T1D across the disease spectrum: islet autoantibody positive (30 donors) in the absence of diabetes, recent onset and established disease (130 donors. For a complete list of donors see, http://www.jdrfnpod.org/for-investigators/donor-groups/. In histopathological analyses of the pancreata from donors with T1D, detection of insulitis was rare (in 3–18% of islets) [37, 38]. Insulitis can be found in both islets containing insulin and islets without insulin and restricted to a single pancreatic lobe or located in several lobes of different areas of the pancreas [39, 40]. The current histopathological definition of insulitis is “the presence of ≥ 15 CD45+ leukocytes/islet (alternatively ≥ 6 CD3+ lymphocytes) in three islets with the presence of pseudoatrophic (insulin-negative) islets” [38], though continuing discussions and efforts examine this issue [41•].
Pancreas Tissue From Living Patients
Two studies (the Osaka Study and the DiViD Study) have sought to examine pancreas tissue from living donors; the most recent of these was terminated due to adverse events suffered by the donors [4]. While these studies were not without controversy [42], they generated important information on the nature of the pancreas, islets, and infiltrates in patients with recent onset T1D, which is quite rare.
Osaka University Collection
While acknowledging that difference in HLA genotypes, age, or other pathogenic factors may play a role in the etiology of T1D, these studies from Japan are included here as these collections are rare. In addition to observation of rare insulitis and frequent lymphocytic infiltrate in the exocrine tissue in the pancreas from donors with T1D [43, 44], researchers from Osaka University Medical School recovered by pancreas tissue by biopsy from patients with newly diagnosed T1D and found detectable insulitis in approximately 50% of donors [45, 46]. The insulitis was composed of CD4+ and CD8+ T cells, B cells, and macrophages with noted HLA class I molecule hyperexpression in islets, intercellular adhesion molecule-1 (ICAM-1) expression on endothelial cells [47], and markers of an activated and pro-inflammatory environment were detected on infiltrating immune cells. Specifically, the costimulatory molecules CD80 and CD86 were expressed on CD3+ islet-infiltrating T cells [45] and Fas ligand was expressed by islet and endothelial cells within the islets [48]. Specific T cell receptor (TCR) clonotypes were over-represented in infiltrated islets with interferon-γ (IFN-γ) mRNA present [49]. CXC chemokine ligand 10 (CXCL10) was expressed on insulin+ cells within islets and CXCR3 on infiltrating CD3+ T cells [50]. In addition, tumor necrosis factor alpha (TNF-α) expressing, islet-infiltrating macrophages, and dendritic cells were seen [51].
In the DiViD study [4, 52•], insulitis was detected in all six recent onset donors with 5–58%of the insulin-expressing islets having insulitis of ≥ 15 T cells/islet [53•] with different patterns of B cell composition of the insulitis [54•]. The CD8+ T cells were tissue resident memory T cells (TRM) (CD8+CD69+CD103+), but without expression of mRNA species associated with acute cytotoxicity or inflammation [55•]. In contrast, interferon-stimulated genes and CXCL10 were shown to be upregulated in the islet core as compared to peri-islet tissue [56•].
HLA Associations in T1D
Genetic association studies have revealed that the HLA class II region has the strongest impact on risk of T1D [57•]. HLA-DQ2 (DQA*05:01, DQB*02:01) and DQ8 (DQ*A03:01, DQB*03:02) confer the greatest risk of developing T1D of any HLA alleles [58]. Individuals who are heterozygous for HLA-DQ2 and HLA-DQ8 are at greater risk of developing T1D than those with either HLA-DQ2 or HLA-DQ8 alone [59]. Antigen-presenting cells from HLA-DQ2, DQ8 heterozygous individuals express an HLA-DQ8 transdimer composed of the DQ2α chain paired with the HLA-DQ8β chain (DQA*05:01; DQB*03:02) and a DQ2 transdimer where the DQ8β pairs with DQ2α (DQA1*03:01; DQB1*02:01) [60]. These transdimers may promote β-cell autoimmunity by presenting unique diabetogenic epitopes, or the high density of T1D-promoting HLA molecules (DQ2, DQ8, DQ2trans and DQ8trans) may promote autoimmune CD4+ T cell responses against β-cell antigens [60, 61].
It is now clear that T cell responses to (pro)insulin are essential for the development of T1D in the NOD mouse [21, 62, 63]. Evidence continues to accumulate to support the role of (pro)insulin as an autoantigen in human T1D [64]. Genetic association studies have implicated proinsulin because a T1D susceptibility locus maps to a polymorphism of variable number of tandem repeats upstream of the insulin gene [65, 66]. This polymorphism is believed to modulate proinsulin expression in the thymus affecting central tolerance to this molecule [65–67]. Many studies have attempted to detect proinsulin specific CD4+ T cell responses in the peripheral blood mononuclear cells (PBMC) of patients with T1D and healthy control subjects [16]. Using sensitive methods capable of detecting very rare T cells, some investigators could detect weak responses to proinsulin peptides [68]. However, these CD4+ T cells could not be analyzed in detail. Furthermore, T cells isolated from the pancreatic lymph nodes (PLN) of deceased tissue donors with T1D were reported to be insulin specific and HLA-DR4 restricted [69].
Autoreactive CD8+ T Cells Detected In Situ in Islets
The antigen specificity of human islet-infiltrating T cells was first addressed by Coppetiers et al. [39] by using HLA-A2 tetramers loaded with known β-cell epitopes to stain pancreas sections from organ donors with T1D. This seminal work showed for the first time that CD8+ T cells infiltrated human islets in T1D and that these T cells were specific for epitopes of human glutamic acid decarboxylase 65 (GAD65), islet antigen 2 (IA-2, previously known as ICA-512), insulin and islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP). However, this study did not address the specificity of all islet-infiltrating CD8+ T cells or the T cell receptor (TCR) genes used by these cells.
Cloning Islet-Infiltrating T Cells
Identification of clinically relevant T cells and their antigens/epitopes has progressed slowly because β-cell antigen specific T cells are present at low frequencies in peripheral blood, pushing even the most sensitive assays to their limits [13, 16]. Moreover, it requires knowledge of the antigen or access to quantities of human islets. Evidence for a T1D-associated response in PBMC were invariably weak [14, 70, 71]. Isolating CD4+ T cell clones, based on their responses to β-cell antigens [11], allowed for some epitopes to be defined in detail [8, 12, 15]. It has become clear that a detailed understanding of the T cell responses against β-cells within the pancreatic islets would be essential to gain insights into the immunopathogenesis of human T1D. In doing so, one must consider the sample purity and T cell source when interpreting data derived from such studies since islets from control individuals [72•] and acinar tissue [73••] in pancreata can contain immune cells.
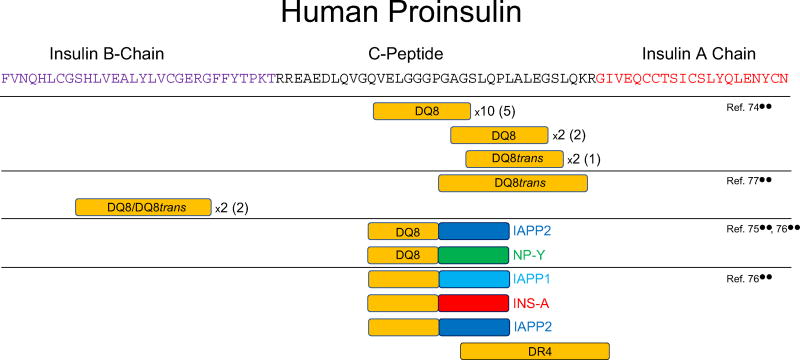
The first report describing the isolation and analysis of viable human islet-infiltrating CD4+ T cells was published in 2015 [74••]. Islets isolated from a 19-year-old tissue donor who had T1D for 3 years were cultured with IL-2 and IL-15 for 10 days. Under these conditions, T cells emerged from some of the islets. These T cells were cloned by fluorescent-activated cell sorting (FACS) sorting. Cloned cells were screened against a panel of overlapping peptides that mimicked the entire sequence of proinsulin. In addition, 26 peptides from GAD65, IA-2, IGRP, zinc transporter 8 (ZnT8), and heat-shock protein 6 (HSP-6) that were previously reported to be CD4+ T cell epitopes in earlier studies were tested, but none of them stimulated any of the T cell clones. Remarkably, all of the CD4+ T cell clones for which an epitope could be identified were restricted by HLA-DQ8, or HLA-DQ8 transdimers—HLA molecules strongly implicated in the pathogenesis of human T1D (Table 1, Fig. 1 and [74••]).′
Table 1.
Islet donor characteristics and specific autoreactivity of islet-derived T cells
| Organ donor ID |
Age (years) |
Sex | T1D duration (years) |
Donor HLA | T cell | Autoantigen | HLA restriction | Reference |
|---|---|---|---|---|---|---|---|---|
| Donor A | 19 | M | 3 | A1, A2 | 8 CD4+ T cell clones | Proinsulin42–50 | DQ8 | [74••] |
| B8, B51 | 1 CD4+ T cell clone | Proinsulin41–51 | DQ8 | [74••] | ||||
| DR3, DR4 | 1 CD4+ T cell clone | Proinsulin41–49 | DQ8 | [74••] | ||||
| DQ2, DQ8 | 1 CD4+ T cell clone | Proinsulin50–59 | DQ8 | [74••] | ||||
| 1 CD4+ T cell clone | Proinsulin50–58 | DQ8 | [74••] | |||||
| 2 CD4+ T cell clones | Proinsulin52–62 | DQ8transa | [74••] | |||||
| 1 CD4+ T cell clone | HIP C-peptide: IAPP2b | DQ8 | [75••] | |||||
| 1 CD4+ T cell clones | HIP C-peptide: IAPP2b | DQ8 | [75••] | |||||
| nPOD6342 | 14 | F | 2 | A2, A68 | 1 CD4+ T-cell clone | GAD274–286 | DR4c | [76••] |
| DR1, DR4 DQ5, DQ8 | 1 CD4+ Transductant (GSE.20D11)d | Insulin B9–23 | DQ8 | [77••] | ||||
| nPOD6367 | 24 | M | 2 | A2, A29 DR4, DR7 DQ2, DQ8 | 1 CD4+ T cell line | HIP C-peptide:A-chainb | ND | [76••] |
| nPOD6268 | 12 | F | 3 | A2, A68 DR17, DR13 DQ2, DQ6 | 3 CD8+ T cell lines | Pooled insulin B10–18, IA-2797–805, IGRP265–273 tetramers | A*02:01 | [76••] |
| nPOD69 | 6 | F | 3 | A2, A26 | 1 CD4+ T cell line | Proinsulin76–90e | DR4c | [76••] |
| DR4, DR7 | 1 CD4+ T cell line | Chromogranin Af | ND | [76••] | ||||
| DQ2, DQ8 | 2 CD4+ T cell lines | Proinsulinf | ND | [76••] | ||||
| nPOD6323 | 22 | F | 6 | A1, A25 | 1 CD4+ T cell line | Chromogranin Af | ND | [76••] |
| DR4, DR17 | 1 CD4+ T cell line | HIP C-peptide:IAPP1b | ND | [76••] | ||||
| DQ2, DQ8 | 1 CD4+ T cell line | HIP C-peptide: IAPP2b | ND | [76••] | ||||
| 1 CD4+ T cell line | GRP78292–305(Arg-Cit 297) | ND | [76••] | |||||
| 1 CD4+ Transductant (GSE.8E3)d | Proinsulin49–65 | DQ8transa | [77••] | |||||
| 1 CD4+ Transductant (GSE.6H9)d | Insulin B9–23 | DQ8 | [77••] | |||||
| T1D.6 | 20 | M | 7 | A2, − | 1 CD4+ T cell line | GAD555–567 | DR4c | [76••] |
| DR17, DR4 Q2, DQ8 | 1 CD4+ T cell line | HIP C-peptide:NP-Yb | DQ8 | [75••, 76••] | ||||
| T1D.7 | 27 | M | 17 | A1, A3 | 1 CD4+ T cell clone | IAPP65–84(Arg-Cit 73, 81) | ND | [76••] |
| DR17, DR4 | 1 CD4+ T cell clone | GAD115–127 | ND | [76••] | ||||
| DQ2, DQ8 | 1 CD4+ T cell clone | IA-2545–562(Gln-Glu 548, 551, 556) | ND | [76••] |
The age, gender, HLA, and duration of T1D of the islet donors are shown along with the original citations. In this on-going project, these are the autoreactivities of islet-infiltrating T cell lines or clones identified, to date, and the donors from which the islet-infiltrating T cells were derived. (Adapted from: Babon JA, et al. Nat Med. 2016;22:1482–1487) [76••]
ND not determined
HLA-DQ8trans: DQA1*05:01/DQB1*03:02
HIP hybrid insulin peptide: fusion of a human insulin C-peptide fragment (N-terminus ELGGG) with a fragment of another peptide (A-chain insulin A-chain fragment, IAPP1 and 2 two islet amyloid polypeptide fragments, NP-Y neuropeptide Y fragment)
HLA-DR4 were all HLA-DRB1*04:01
Clonal CD4+ T-cell receptor transductant
Proinsulin76–90 (SLQPLALEGSLQKRG) is designated Proinsulin52–66 by numbering starting with the B chain
Epitopes not identified
Fig. 1.
Proinsulin-derived epitopes recognized by human islet-infiltrating CD4+ T cells. The boxes indicate regions of human proinsulin for which CD4+ T cell epitopes have been mapped examining human islet-infiltrating T-cells from multiple donors with T1D. Two-colored boxes indicate hybrid insulin peptides (HIPs) and are placed to align with the proinsulin part of the epitope, with the other half of the HIP is as labeled: islet amyloid polyprotein (IAPP), neuropeptide Y (NP-Y), insulin A-chain (INS-A). Horizontal lines indicate the epitopes described in each study (references shown on the right). For epitopes that an HLA restriction have been determined, the restricting HLA allele is shown within the box. In some cases, several clones have been isolated that recognize the same, or very similar epitopes indicated by the numbers (i.e., ×2). The number of unique TCRαβ sequences expressed by these clones is shown in parenthesis
Using a similar strategy [76••], the isolated islets from nine donors with T1D (2–20 years duration of T1D, received 2–5 days following brain death) were handpicked for increased purity and divided into two aliquots that were treated in two parallel methods. The first aliquot of 100 isolated handpicked islets were dispersed with enzyme, stained for viability and immune cell surface markers, and then immediately detected and sorted by FACS. By doing so, an “ex vivo” or “ex islet” profile of islet-infiltrating T cells could be seen along with single T cell sorting for expansion. From these donors, there were 202 ± 404 CD4+ T cells and 119 ± 189 CD8+ T cells (per 100 islets) for a CD4+:CD8+ ratio of 1.7:1. From the isolated, handpicked islets of seven control donors and from two donors with type 2 diabetes, a few CD8+ T cells were seen from only one of the control donors. The second aliquot of 100 handpicked islets was plated on a gel matrix with T cell receptor stimulation and cytokines for growth. After 10 days in culture, cellular outgrowths were seen only in the islets from donors with T1D, with an average of 26% of the plated islets. These outgrowths were collected, characterized for CD4+ and CD8+ T cells, and expanded.
The autoreactivity from 50 lines (grown from individual islets from donors) or from sorted clones from donor islets was tested with panels of known islet-protein associated peptide targets and to modified peptides using either HLA-matched Epstein Barr virus (EBV)-transformed B cells or autologous splenic EBV-transformed B cells. To date, we have identified the reactivity of 18 of the T cell lines or clones (Table 1, Fig. 1 and [76••]).
Ex vivo Sequencing of TCR From Islet - Infiltrating T Cells
An alternate, but complementary approach to study islet-infiltrating T cells was carried out by single cell sorting islet-infiltrating CD4+ and CD8+ T cells after short-term culture, followed by TCR sequencing of individual cells [77••]. Subsequently, the TCR α/β chains were transduced in a TCR null cell line, termed TCR transductants, and tested for antigen specificity to overlapping preproinsulin peptides and other well-characterized islet antigens. Isolated islets from three recent onset T1D organ donors were studied in this manner, all of which were also evaluated by Babon and colleagues by functional T cell analysis (Table 1). It was possible to isolate hundreds to thousands of T cells from 500 islet equivalents. Analysis of α/β TCR sequences revealed diversity within CD4+ T cells with about 15–20% of sequences detected more than two times from two separate donors [77••]. CD8+ TCR sequences revealed more clonality with 1/3 to 1/2 of all sequences in the same donor repeated > 2 times [77••]. Interestingly, the majority of repeatedly detected TCR sequences were found from separate islet preps in the same donor, indicating that clonally expanded T cells have the ability to migrate to different islets in the pancreas. None of the TCR sequences, CD4+ or CD8+, were shared between patients. This could be due to the fact that only three patients with slightly different HLA genes were studied and larger numbers may reveal more clustering of TCR usage.
Others have studied TCRβ chain usage among islet-infiltrating T cells from histologic sections [78] and isolated islets [79] or from the PLN [69] from donors with T1D, finding some skewing of certain TCRVβ families with higher frequencies than in the spleen or peripheral blood. Some skewing of TCRVα chains has been seen [49]. The largest effort to profile TCR sequences from donor tissues from individuals with T1D comes from Brusko and colleagues within the nPOD consortium [80••]. Tissue donors with T1D (n = 18) and non-diabetic controls (n = 9) had PLN, nonpancreatic lymph nodes, spleen, and peripheral blood FACS sorted into T cell subsets and TCRVβ chains sequenced. Within a single individual, there was evidence of TCR clonal expansion that could be traced from PLN to spleen to peripheral blood, especially within CD8+ T cells; however, there was limited TCR clonal sharing across T1D donors. However, the TCRVβ CDR3 region of a known GAD-restricted CD4+ T cell clone [81] was identified within the PLN of seven donors. From these studies, it appears that larger numbers of HLA-matched patients need to be studied with a focus on targeted searched for antigen-specific T cells.
Autoantigen Specificity of Islet-Infiltrating T Cells
Proinsulin Epitopes
Proinsulin, the precursor of insulin, has been a strong candidate antigen in the pathogenesis of human T1D for many years [64, 82, 83]. Several lines of evidence suggest that proinsulin is recognized by the adaptive immune response that drives β-cell destruction. For example, autoantibodies to (pro)insulin precede the onset of T1D [84] and genetic polymorphisms in the insulin promoter modulate risk of T1D [65]. Now that several human islet-infiltrating CD4+ T cell clones specific for proinsulin epitopes have been described, the evidence against proinsulin is very strong. All but one epitope recognized by human islet-infiltrating CD4+ T cells derived from the C-peptide region of proinsulin, which is not present in administered insulin (Table 1 and Fig. 1). Two CD4+ TCR transductants responding to insulin B chain amino acids 9–23 (B:9–23) presented by either DQ8 or DQ8trans were identified from two separate tissue donors with T1D [77••]. This epitope is known to play a critical role in NOD mouse diabetes development [21] and has been well-characterized from the peripheral blood of patients with T1D [85–87]. Another identical antigen-specific CD4+ T cell response has been reported to amino acids 19–35 within C-peptide presented by DQ8trans from two separate patients identified in different laboratories [74••, 77••] (Table 1 and Fig. 1). This indicates the distinct possibility of common epitopes stimulating islet-derived CD4+ T cells, even after the clinical onset of T1D.
Additional Known Islet Epitopes
A large bank of T cells directly sorted from or directly grown from individual islets of nine donors with T1D includes a total of 236 lines or clones: 111 CD4+ T cell lines or clones, 23 CD8+ T cell lines or clones, and 102 lines grown from individual islets that were mixtures of both CD4+ T cells and CD8+ T cells. Initial analysis found a broad repertoire of T cell autoreactivity to a number of known target epitopes and to a number of modified epitopes [76••]. To date, we have identified the reactivity of 15 CD4+ T cell lines or clones and three CD8+ T cells lines (Table 1). Proinsulin was the target of four of the islet-infiltrating lines: a CD4+ T cell line reactive with an HLA DRB1*04:01 restricted proinsulin76–90 epitope and two CD4+ T cell lines reactive with as-yet-unidentified proinsulin epitope(s). Other known CD4+ targets included three epitopes ofGAD65 and a CD4+ T cell line reactive with an unidentified epitope of chromogranin A. Three CD8+ T cell lines reacted with pools of HLA-A2 multimers loaded with previously identified [88] peptides from insulin, IA-2 and IGRP. It should be noted that all donors (Table 1) were recovered after diagnosis of T1D and have been on an insulin regimen since diagnosis. In addition, we must consider epitope spreading as a mechanism of multiple targets of autoimmunity after diagnosis [89]. The remaining islet-infiltrating CD4+ T cell and CD8+ T cell lines and clones from this bank are under current investigation.
Post-Translationally Modified Epitopes
Epitopes generated by post-translational modification have been implicated in the pathogenesis of many autoimmune diseases [90], including T1D [91, 92]. Epitopes formed by post-translational disulfide bond rearrangement in insulin [12], glutamine deamidation of several islet-associated proteins [93•], and the conversion of arginine to citrulline have all be reported for GAD65 [91]. From the large bank of islet-infiltrating T cells [76••], islet-derived CD4+ T cell lines and clones were reactive to an epitope of glucose-regulated protein 78 (GRP78) with an arginine to citrulline modification (GRP78292–305(Arg-Cit 297)), an epitope of islet amyloid polypeptide (IAPP) with two arginine to citrulline modifications (IAPP65–84 (Arg-Cit 73,81)), and an epitope of IA-2 with three glutamine to glutamic acid deamidations (IA-2545–562(Gln-Glu 548, 551, 556)).
Hybrid Insulin Peptide (HIP) Epitopes
A new type of post-translation modification, the formation of hybrid peptides by transpeptidation, was recently reported to generate neo-epitopes recognized by NOD mouse and human CD4+ T cells [75••]. Human islet-infiltrating CD4+ T cells, isolated fromthe residual pancreatic islets of deceased organ donors who suffered from T1D were found to recognize hybrid insulin peptides (HIPs). Two HIPs were shown to be the targets of a human islet-derived CD4+ T cell clone and a human islet-derived CD4+ T cell line: a C-peptide: IAPP2 and a C-peptide:neuropeptide-Y HIP, respectively [75••]. Synthetic peptides of these sequences were very also potent stimulators of these T cells, with responses being detected at low nanomolar concentrations. Interestingly, some of these clones are restricted by HLA-DQ8, which is strongly associated with risk of T1D in humans [57•, 58].
The presence of human HIP specific CD4+ T cells in the pancreatic islets of organ donors who suffered from T1D was confirmed recently. Babon et al. [76••] reported that T cell responses to HIPs formed by the fusion of C-peptide and peptides from IAPP1, IAPP2, or insulin A-chain could all be detected in CD4+ T cell lines derived the islets of organ donors who suffered from T1D.
Function of Islet-Infiltrating T Cells
In addition to the autoantigenic reactivity of islet-infiltrating T-cells, identification of their effector functions is critical for understanding and intervening with their function in potential therapies. In examining the autoreactive CD4+ T cells from the large bank of islet-infiltrating T cells from nine donors with T1D, we found that, upon stimulation with specific peptide-pulsed HLA-matched or autologous EBV-transformed B cells, all autoreactive CD4+ T cell lines or clones secreted interleukin (IL)-2, IFN-γ and/or TNF-α and none of lines or clones secreted any detectable IL-4, IL-5, IL-10, or IL-17a [76••]; this was done with low passage number lines and clones. This will be an important line of investigation to continue with the inclusion of a variety of methods to fully understand the function of the islet-infiltrating T cells.
Pathogenicity
There is a strong “circumstantial” case to be made that human islet-infiltrating T cells cause T1D. Recurrence of autoimmunity has been seen following islet transplantation [94–96], indicating that autoimmunity must be controlled in those with long-term T1D for whom islet regeneration or replacement may be a therapeutic option. The best possible evidence linking human T cells to the development of T1D is to analyze them directly from infiltrated islets. This has the advantage that no bias, due to selection based on antigen specificity, is introduced: T cells are selected solely by their location within the affected tissue of individuals with the disease.
Future Directions
This is an on-going analysis of large banks of islet-infiltrating T cells from a number of donors across three laboratories that will include analyses such as epitope discovery, functional analyses, and transcriptome analyses for both CD4+ and CD8+ islet-derived T cell clones, lines, and transductants. These analyses will most likely expand to other laboratories as additional techniques and expertise is required to obtain a comprehensive analysis of the islet-infiltrating T cell repertoire. For example, these studies can be paired with in situ staining of pancreata from the same donors for global phenotype and specific autoreactivity with HLA multimers of islet-infiltrating T cells and transcriptome analyses of islets recovered by laser microcapture.
We anticipate the recovery of more donors with T1D. In order to begin to define common antigens that may be targeted early in the disease process, through the efforts of nPOD, the recovery of donors with circulating T1D-associated autoantibody, but without a diagnosis of T1D will be pursued. Nonetheless, the isolation of islet-infiltrating T cells from these samples may be challenging [97–100].
A major goal of any immunotherapy is to monitor patients’ responses to that therapy, which can only be done by sampling peripheral blood. However, to perform the correct comparison of the T cell repertoire infiltrating a donor’s islets to the repertoire found in that donor’s peripheral blood is a challenge. For these tissue donors, peripheral blood is either unavailable or in quantities insufficient for current analyses. To overcome this, we must first understand the islet-infiltrating T cell repertoire and then examine the peripheral blood of HLA-matched individuals at risk for T1D and at different stages of T1D. Ultimately, we will apply this knowledge to develop biomarkers of disease activity and improve antigen-specific therapy.
Conclusions
Through the collaborative efforts of many individuals, consortia, institutions, and families of donors, we are now able, for the first time, to directly assay the repertoire and function of islet-infiltrating immune cells. Here, across three laboratories, we have isolated both CD4+ and CD8+ T cells directly from the islets of donors with T1D and have seen remarkable similarity in CD4+ autoreactivity to known islet-associated proteins (peptides from proinsulin, GAD65, and, chromogranin A), with post-translationally modified peptides, with argininecitrulline modifications or deamidations, peptides from islet-associated proteins (GRP78, IAPP, IA-2), or to a number of hybrid insulin peptides. Both CD4+ and CD8+ T cell clonality has been observed, but with noted diversity of the TCR from islet-infiltrating T cells. To date, the islet-infiltrating CD4+ T cells have exhibited a pro-inflammatory phenotype. This is an active, on-going investigation that will yield critical information on the repertoire and function of islet-infiltrating T cells and inform the design of therapies for T1D.
Acknowledgments
This research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/for-partners/npod-partners/. We thank the families of the donors. We acknowledge the support of the Tom Mandel Islet Transplantation Program and the Australian Islet Transplantation Consortium. We thank Dr. Alvin C. Powers (Vanderbilt University) and Drs. Mark A. Atkinson and Clayton Mathews (University of Florida, Gainesville). We thank Dr. M. Nakayama (Barbara Davis Center for Childhood Diabetes, University of Colorado), Professor Tom Kay, and Associate Professor Helen Thomas (St. Vincent’s Institute of Medical Research, Melbourne, Australia).
The following funding sources supported this research: the Helmsley Charitable Trust 2015PG-T1D057 (SCK), National Institutes of Health/National Institute of Allergy and Infectious Diseases AI126189 (SCK), and the Human Islet Research Network (HIRN) Opportunity Pool Fund National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases U01 DK104162 (SCK), R01 DK108868 (AWM), DP3 DK110845 (AWM), and UC4 DK104223 (AWM), The Australian National Health and Medical Research Council (NHMRC) GNT1123586 (SIM), Diabetes Australia Research Program Y17M1-MANS (SIM) JDRF, America Diabetes Association, 1-15-ACE-14 (SIM) and JDRF 5-CDA-2014-210-A-N (SIM), and Juvenile Diabetes Research Foundation Postdoctoral Fellowship 3-PDF-201703740A-N (JABB).
Footnotes
Conflicts of Interest Sally C. Kent, Jenny Aurielle B. Babon, and Stuart I. Mannering declare that they have no conflict of interest.
Aaron W. Michels reports conflicts outside the submitted work, including a patent Compounds That Modulate Autoimmunity and Methods of Using the Same licensed to ImmunoMolecular Therapeutics, a patent Methods of Preventing and Treating Autoimmunity licensed to ImmunoMolecular Therapeutics, and a patent Insulin Mimotopes and Methods of Using the Same pending. And he is the scientific founder and owns shares in ImmunoMolecular Therapeutics, LLC.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of each institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376:1419–29. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons KM, Gottlieb PA, Michels AW. Immune intervention and preservation of pancreatic beta cell function in type 1 diabetes. Curr Diab Rep. 2016;16:97. doi: 10.1007/s11892-016-0793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krogvold L, Edwin B, Buanes T, Ludvigsson J, Korsgren O, Hyoty H, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia. 2014;57:841–3. doi: 10.1007/s00125-013-3155-y. [DOI] [PubMed] [Google Scholar]
- 5.Christoffersson G, von Herrath MG. A deeper look into type 1 diabetes—imaging immune responses during onset of disease. Front Immunol. 2016;7:313. doi: 10.3389/fimmu.2016.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Signore A, Capriotti G, Chianelli M, Bonanno E, Galli F, Catalano C, et al. Detection of insulitis by pancreatic scintigraphy with 99mTc-labeled IL-2 and MRI in patients with LADA (Action LADA 10) Diabetes Care. 2015;38:652–8. doi: 10.2337/dc14-0580. [DOI] [PubMed] [Google Scholar]
- 7.Gaglia JL, Harisinghani M, Aganj I, Wojtkiewicz GR, Hedgire S, Benoist C, et al. Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc Natl Acad Sci U S A. 2015;112:2139–44. doi: 10.1073/pnas.1424993112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007;148:1–16. doi: 10.1111/j.1365-2249.2006.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallone R, Kochik SA, Laughlin EM, Gersuk VH, Reijonen H, Kwok WW, et al. Differential recognition and activation thresholds in human autoreactive GAD-specific T-cells. Diabetes. 2004;53:971–7. doi: 10.2337/diabetes.53.4.971. [DOI] [PubMed] [Google Scholar]
- 10.Mallone R, Martinuzzi E, Blancou P, Novelli G, Afonso G, Dolz M, et al. CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56:613–21. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- 11.Mannering SI, Dromey JA, Morris JS, Thearle DJ, Jensen KP, Harrison LC. An efficient method for cloning human autoantigen-specific T cells. J Immunol Methods. 2005;298:83–92. doi: 10.1016/j.jim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Mannering SI, Harrison LC, Williamson NA, Morris JS, Thearle DJ, Jensen KP, et al. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. J Exp Med. 2005;202:1191–7. doi: 10.1084/jem.20051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannering SI, Morris JS, Jensen KP, Purcell AW, Honeyman MC, van Endert PM, et al. A sensitive method for detecting proliferation of rare autoantigen-specific human T cells. J Immunol Methods. 2003;283:173–83. doi: 10.1016/j.jim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Mannering SI, Morris JS, Stone NL, Jensen KP, van Endert PM, Harrison LC. CD4+ T cell proliferation in response to GAD and proinsulin in healthy, pre-diabetic, and diabetic donors. Ann N Y Acad Sci. 2004;1037:16–21. doi: 10.1196/annals.1337.003. [DOI] [PubMed] [Google Scholar]
- 15.Mannering SI, Pang SH, Williamson NA, Naselli G, Reynolds EC, O'Brien-Simpson NM, et al. The A-chain of insulin is a hotspot for CD4+ T cell epitopes in human type 1 diabetes. Clin Exp Immunol. 2009;156:226–31. doi: 10.1111/j.1365-2249.2009.03907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannering SI, Wong FS, Durinovic-Bello I, Brooks-Worrell B, Tree TI, Cilio CM, et al. Current approaches to measuring human islet-antigen specific T cell function in type 1 diabetes. Clin Exp Immunol. 2010;162:197–209. doi: 10.1111/j.1365-2249.2010.04237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaparro RJ, Di Lorenzo TP. An update on the use of NOD mice to study autoimmune (type 1) diabetes. Expert Rev Clin Immunol. 2010;6:939–55. doi: 10.1586/eci.10.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lennon GP, Bettini M, Burton AR, Vincent E, Arnold PY, Santamaria P, et al. T cell islet accumulation in type 1 diabetes is a tightly regulated, cell-autonomous event. Immunity. 2009;31:643–53. doi: 10.1016/j.immuni.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Tsai S, Shameli A, Yamanouchi J, Alkemade G, Santamaria P. In situ recognition of autoantigen as an essential gatekeeper in autoimmune CD8+ T cell inflammation. Proc Natl Acad Sci U S A. 2010;107:9317–22. doi: 10.1073/pnas.0913835107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker RL, Bradley B, Wiles TA, Lindsay RS, Barbour G, Delong T, et al. Cutting edge: nonobese diabetic mice deficient in chromogranin A are protected from autoimmune diabetes. J Immunol. 2016;196:39–43. doi: 10.4049/jimmunol.1501190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–3. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata M, Santamaria P, Kawamura T, Utsugi T, Yoon JW. Evidence for the role of CD8+ cytotoxic T cells in the destruction of pancreatic beta-cells in nonobese diabetic mice. J Immunol. 1994;152:2042–50. [PubMed] [Google Scholar]
- 23.Utsugi T, Yoon JW, Park BJ, Imamura M, Averill N, Kawazu S, et al. Major histocompatibility complex class I-restricted infiltration and destruction of pancreatic islets by NOD mouse-derived beta-cell cytotoxic CD8+ T-cell clones in vivo. Diabetes. 1996;45:1121–31. doi: 10.2337/diab.45.8.1121. [DOI] [PubMed] [Google Scholar]
- 24.Bortell R, Yang C. The BB rat as a model of human type 1 diabetes. Methods Mol Biol. 2012;933:31–44. doi: 10.1007/978-1-62703-068-7_3. [DOI] [PubMed] [Google Scholar]
- 25.Reed JC, Herold KC. Thinking bedside at the bench: the NOD mouse model of T1DM. Nat Rev Endocrinol. 2015;11:308–14. doi: 10.1038/nrendo.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.In't Veld P. Insulitis in human type 1 diabetes: the quest for an elusive lesion. Islets. 2011;3:131–8. doi: 10.4161/isl.3.4.15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–33. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 28.Gepts W, In't Veld PA. Islet morphologic changes. Diabetes Metab Rev. 1987;3:859–72. doi: 10.1002/dmr.5610030403. [DOI] [PubMed] [Google Scholar]
- 29.Gepts W, Lecompte PM. The pancreatic islets in diabetes. Am J Med. 1981;70:105–15. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- 30.LeCompte PM, Legg MA. Insulitis (lymphocytic infiltration of pancreatic islets) in late-onset diabetes. Diabetes. 1972;21:762–9. doi: 10.2337/diab.21.6.762. [DOI] [PubMed] [Google Scholar]
- 31.Foulis AK, Farquharson MA. Aberrant expression of HLA-DR antigens by insulin-containing beta-cells in recent-onset type I diabetes mellitus. Diabetes. 1986;35:1215–24. doi: 10.2337/diab.35.11.1215. [DOI] [PubMed] [Google Scholar]
- 32.Foulis AK, Farquharson MA, Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987;30:333–43. doi: 10.1007/BF00299027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foulis AK, Farquharson MA, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet. 1987;2:1423–7. doi: 10.1016/s0140-6736(87)91128-7. [DOI] [PubMed] [Google Scholar]
- 34.Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia. 1986;29:267–74. doi: 10.1007/BF00452061. [DOI] [PubMed] [Google Scholar]
- 35.Foulis AK, McGill M, Farquharson MA. Insulitis in type 1 (insulin-dependent) diabetes mellitus in man—macrophages, lymphocytes, and interferon-gamma containing cells. J Pathol. 1991;165:97–103. doi: 10.1002/path.1711650203. [DOI] [PubMed] [Google Scholar]
- 36.Foulis AK, Stewart JA. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia. 1984;26:456–61. doi: 10.1007/BF00262221. [DOI] [PubMed] [Google Scholar]
- 37.Campbell-Thompson M. Organ donor specimens: what can they tell us about type 1 diabetes? Pediatr Diabetes. 2015;16:320–30. doi: 10.1111/pedi.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell-Thompson ML, Atkinson MA, Butler AE, Chapman NM, Frisk G, Gianani R, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia. 2013;56:2541–3. doi: 10.1007/s00125-013-3043-5. [DOI] [PubMed] [Google Scholar]
- 39.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gianani R, Campbell-Thompson M, Sarkar SA, Wasserfall C, Pugliese A, Solis JM, et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia. 2010;53:690–8. doi: 10.1007/s00125-009-1642-y. [DOI] [PubMed] [Google Scholar]
- 41•.Campbell-Thompson ML, Atkinson MA, Butler AE, Giepmans BN, von Herrath MG, Hyoty H, et al. Re-addressing the 2013 consensus guidelines for the diagnosis of insulitis in human type 1 diabetes: is change necessary? Diabetologia. 2017;60:753–5. doi: 10.1007/s00125-016-4195-x. This report is a continuing discussion of the definition of human insulitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atkinson MA. Pancreatic biopsies in type 1 diabetes: revisiting the myth of Pandora’s box. Diabetologia. 2014;57:656–9. doi: 10.1007/s00125-013-3159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakanishi K, Kobayashi T, Miyashita H, Okubo M, Sugimoto T, Murase T, et al. Relationships among residual beta cells, exocrine pancreas, and islet cell antibodies in insulin-dependent diabetes mellitus. Metabolism. 1993;42:196–203. doi: 10.1016/0026-0495(93)90035-m. [DOI] [PubMed] [Google Scholar]
- 44.Waguri M, Hanafusa T, Itoh N, Miyagawa J, Imagawa A, Kuwajima M, et al. Histopathologic study of the pancreas shows a characteristic lymphocytic infiltration in Japanese patients with IDDM. Endocr J. 1997;44:23–33. doi: 10.1507/endocrj.44.23. [DOI] [PubMed] [Google Scholar]
- 45.Imagawa A, Hanafusa T, Itoh N, Miyagawa J, Nakajima H, Namba M, et al. Islet-infiltrating T lymphocytes in insulin-dependent diabetic patients express CD80 (B7-1) and CD86 (B7-2) J Autoimmun. 1996;9:391–6. doi: 10.1006/jaut.1996.0053. [DOI] [PubMed] [Google Scholar]
- 46.Imagawa A, Hanafusa T, Tamura S, Moriwaki M, Itoh N, Yamamoto K, et al. Pancreatic biopsy as a procedure for detecting in situ autoimmune phenomena in type 1 diabetes: close correlation between serological markers and histological evidence of cellular autoimmunity. Diabetes. 2001;50:1269–73. doi: 10.2337/diabetes.50.6.1269. [DOI] [PubMed] [Google Scholar]
- 47.Itoh N, Hanafusa T, Miyazaki A, Miyagawa J, Yamagata K, Yamamoto K, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest. 1993;92:2313–22. doi: 10.1172/JCI116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moriwaki M, Itoh N, Miyagawa J, Yamamoto K, Imagawa A, Yamagata K, et al. Fas and Fas ligand expression in inflamed islets in pancreas sections of patients with recent-onset type I diabetes mellitus. Diabetologia. 1999;42:1332–40. doi: 10.1007/s001250051446. [DOI] [PubMed] [Google Scholar]
- 49.Yamagata K, Nakajima H, Tomita K, Itoh N, Miyagawa J, Hamaguchi T, et al. Dominant TCR alpha-chain clonotypes and interferon-gamma are expressed in the pancreas of patients with recent-onset insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1996;34:37–46. doi: 10.1016/s0168-8227(96)01328-9. [DOI] [PubMed] [Google Scholar]
- 50.Uno S, Imagawa A, Saisho K, Okita K, Iwahashi H, Hanafusa T, et al. Expression of chemokines, CXC chemokine ligand 10 (CXCL10) and CXCR3 in the inflamed islets of patients with recent-onset autoimmune type 1 diabetes. Endocr J. 2010;57:991–6. doi: 10.1507/endocrj.k10e-076. [DOI] [PubMed] [Google Scholar]
- 51.Uno S, Imagawa A, Okita K, Sayama K, Moriwaki M, Iwahashi H, et al. Macrophages and dendritic cells infiltrating islets with or without beta cells produce tumour necrosis factor-alpha in patients with recent-onset type 1 diabetes. Diabetologia. 2007;50:596–601. doi: 10.1007/s00125-006-0569-9. [DOI] [PubMed] [Google Scholar]
- 52•.Skog O, Korsgren S, Wiberg A, Danielsson A, Edwin B, Buanes T, et al. Expression of human leukocyte antigen class I in endocrine and exocrine pancreatic tissue at onset of type 1 diabetes. Am J Pathol. 2015;185:129–38. doi: 10.1016/j.ajpath.2014.09.004. The detection of HLA Class I hyperexpression from pancreatic biopsy samples from recent-onset T1D patients confirms old and new reports of immune-related dysregulation in the pancreas of individuals with T1D. [DOI] [PubMed] [Google Scholar]
- 53•.Krogvold L, Wiberg A, Edwin B, Buanes T, Jahnsen FL, Hanssen KF, et al. Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia. 2016;59:492–501. doi: 10.1007/s00125-015-3820-4. This report shows detection of insulitis pancreatic biopsy samples from recent-onset T1D patients. [DOI] [PubMed] [Google Scholar]
- 54•.Leete P, Willcox A, Krogvold L, Dahl-Jorgensen K, Foulis AK, Richardson SJ, et al. Differential insulitic profiles determine the extent of beta-cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65:1362–9. doi: 10.2337/db15-1615. This report shows two different immunohistopathological patterns can be seen in the pancreata of individuals with T1D based on their age at diagnosis. [DOI] [PubMed] [Google Scholar]
- 55•.Kuric E, Seiron P, Krogvold L, Edwin B, Buanes T, Hanssen KF, et al. Demonstration of tissue resident memory CD8 T cells in insulitic lesions in adult patients with recent-onset type 1 diabetes. Am J Pathol. 2017;187:581–8. doi: 10.1016/j.ajpath.2016.11.002. From laser microcapture studies and gene expression anaylses from pancreatic biopsy donors, this report showed CD8+ Tcells derived from islets to express tissue resident markers with a lack of transcription of cytotoxic or acute inflammatory molecules. [DOI] [PubMed] [Google Scholar]
- 56•.Lundberg M, Krogvold L, Kuric E, Dahl-Jorgensen K, Skog O. Expression of interferon-stimulated genes in insulitic pancreatic islets of patients recently diagnosed with type 1 diabetes. Diabetes. 2016;65:3104–10. doi: 10.2337/db16-0616. From laser microcapture studies and gene expression anaylses from pancreatic biopsy donors, this report showed an over-expression of IFN-stimulated genes in islets with insulitis as compared to islets from control donors. [DOI] [PubMed] [Google Scholar]
- 57•.Noble JA. Immunogenetics of type 1 diabetes: a comprehensive review. J Autoimmun. 2015;64:101–12. doi: 10.1016/j.jaut.2015.07.014. An essential review of T1D immunogenetics. [DOI] [PubMed] [Google Scholar]
- 58.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 59.Sheehy MJ, Scharf SJ, Rowe JR, Neme de Gimenez MH, Meske LM, Erlich HA, et al. A diabetes-susceptible HLA haplotype is best defined by a combination of HLA-DR and -DQ alleles. J Clin Invest. 1989;83:830–5. doi: 10.1172/JCI113965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nepom BS, Schwarz D, Palmer JP, Nepom GT. Transcomplementation of HLA genes in IDDM. HLA-DQ alpha-and beta-chains produce hybrid molecules in DR3/4 heterozygotes. Diabetes. 1987;36:114–7. doi: 10.2337/diab.36.1.114. [DOI] [PubMed] [Google Scholar]
- 61.van Lummel M, van Veelen PA, Zaldumbide A, de Ru A, Janssen GM, Moustakas AK, et al. Type 1 diabetes-associated HLA-DQ8 transdimer accommodates a unique peptide repertoire. J Biol Chem. 2012;287:9514–24. doi: 10.1074/jbc.M111.313940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest. 2006;116:3258–65. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krishnamurthy B, Mariana L, Gellert SA, Colman PG, Harrison LC, Lew AM, et al. Autoimmunity to both proinsulin and IGRP is required for diabetes in nonobese diabetic 8.3 TCR transgenic mice. J Immunol. 2008;180:4458–64. doi: 10.4049/jimmunol.180.7.4458. [DOI] [PubMed] [Google Scholar]
- 64.Mannering SI, Pathiraja V, Kay TW. The case for an autoimmune aetiology of type 1 diabetes. Clin Exp Immunol. 2016;183:8–15. doi: 10.1111/cei.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bennett ST, Lucassen AM, Gough SC, Powell EE, Undlien DE, Pritchard LE, et al. Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nat Genet. 1995;9:284–92. doi: 10.1038/ng0395-284. [DOI] [PubMed] [Google Scholar]
- 66.Pugliese A, Zeller M, Fernandez A, Jr, Zalcberg LJ, Bartlett RJ, Ricordi C, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–7. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 67.Durinovic-Bello I, Wu RP, Gersuk VH, Sanda S, Shilling HG, Nepom GT. Insulin gene VNTR genotype associates with frequency and phenotype of the autoimmune response to proinsulin. Genes Immun. 2010;11:188–93. doi: 10.1038/gene.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113:451–63. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kent SC, Chen Y, Bregoli L, Clemmings SM, Kenyon NS, Ricordi C, et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224–8. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- 70.Ott PA, Herzog BA, Quast S, Hofstetter HH, Boehm BO, Tary-Lehmann M, et al. Islet-cell antigen-reactive T cells show different expansion rates and Th1/Th2 differentiation in type 1 diabetic patients and healthy controls. Clin Immunol. 2005;115:102–14. doi: 10.1016/j.clim.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Roep BO. T-cell responses to autoantigens in IDDM. The search for the holy grail. Diabetes. 1996;45:1147–56. doi: 10.2337/diab.45.9.1147. [DOI] [PubMed] [Google Scholar]
- 72•.Radenkovic M, Uvebrant K, Skog O, Sarmiento L, Avartsson J, Storm P, et al. Characterization of resident lymphocytes in human pancreatic islets. Clin Exp Immunol. 2017;187:418–27. doi: 10.1111/cei.12892. Both effector and central memory CD4+ and CD8+ (tissue resident marker+) are in the islets of donors without T1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73••.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63:3880–90. doi: 10.2337/db14-0549. Immune cell infiltrate is readily seen in the acinar tissue of pancreata of donors with T1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Pathiraja V, Kuehlich JP, Campbell PD, Krishnamurthy B, Loudovaris T, Coates PT, et al. Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes. 2015;64:172–82. doi: 10.2337/db14-0858. The first report of isolation and analysis of live, islet-derived T cells from a donor with T1D. The islet-derived CD4+ T cell response target epitopes of proinsulin and were restricted by high-risk HLA alleles. [DOI] [PubMed] [Google Scholar]
- 75••.Delong T, Wiles TA, Baker RL, Bradley B, Barbour G, Reisdorph R, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351:711–4. doi: 10.1126/science.aad2791. This report was the first to describe and characterize T cell autoreactivity to a new class of neoautoantigens, hybrid isulin peptides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76••.Babon JA, DeNicola ME, Blodgett DM, Crevecoeur I, Buttrick TS, Maehr R, et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med. 2016;22:1482–7. doi: 10.1038/nm.4203. This report examined the largest bank to date of islet-derived T cells from 9 donors with T1D and saw islet-derived T cells targeting known antigens, modified antigens, and hybrid insulin peptides with a pro-inflammatory profile. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77••.Michels AW, Landry LG, McDaniel KA, Yu L, Campbell-Thompson M, Kwok WW, et al. Islet-derived CD4 T cells targeting proinsulin in human autoimmune diabetes. Diabetes. 2017;66:722–34. doi: 10.2337/db16-1025. This report was the first to generate TCR trandsuctants from single islet-infiltrating T cells from donors with T1D. They showed clonal expansion of both CD4+ and CD8+ islet-derived T cells and CD4+ autoreactivity targeting peptides of insulin and proinsulin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Somoza N, Vargas F, Roura-Mir C, Vives-Pi M, Fernandez-Figueras MT, Ariza A, et al. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V beta usage, and cytokine profile. J Immunol. 1994;153:1360–77. [PubMed] [Google Scholar]
- 79.Codina-Busqueta E, Scholz E, Munoz-Torres PM, Roura-Mir C, Costa M, Xufre C, et al. TCR bias of in vivo expanded T cells in pancreatic islets and spleen at the onset in human type 1 diabetes. J Immunol. 2011;186:3787–97. doi: 10.4049/jimmunol.1002423. [DOI] [PubMed] [Google Scholar]
- 80••.Seay HR, Yusko E, Rothweiler SJ, Zhang L, Posgai AL, Campbell-Thompson M, et al. Tissue distribution and clonal diversity of the T and B cell repertoire in type 1 diabetes. JCI Insight. 2016;1:e88242. doi: 10.1172/jci.insight.88242. This is the first report to examine the TCR (and B cell receptor) repertoire across several tissues, including islets, from a large bank of donors with T1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reijonen H, Mallone R, Heninger AK, Laughlin EM, Kochik SA, Falk B, et al. GAD65-specific CD4+ T-cells with high antigen avidity are prevalent in peripheral blood of patients with type 1 diabetes. Diabetes. 2004;53:1987–94. doi: 10.2337/diabetes.53.8.1987. [DOI] [PubMed] [Google Scholar]
- 82.Narendran P, Mannering SI, Harrison LC. Proinsulin-a pathogenic autoantigen in type 1 diabetes. Autoimmun Rev. 2003;2:204–10. doi: 10.1016/s1568-9972(03)00009-0. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol. 2008;20:111–8. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–9. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alleva DG, Crowe PD, Jin L, Kwok WW, Ling N, Gottschalk M, et al. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107:173–80. doi: 10.1172/JCI8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakayama M, McDaniel K, Fitzgerald-Miller L, Kiekhaefer C, Snell-Bergeon JK, Davidson HW, et al. Regulatory vs. inflammatory cytokine T-cell responses to mutated insulin peptides in healthy and type 1 diabetic subjects. Proc Natl Acad Sci U S A. 2015;112:4429–34. doi: 10.1073/pnas.1502967112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang J, Chow IT, Sosinowski T, Torres-Chinn N, Greenbaum CJ, James EA, et al. Autoreactive T cells specific for insulin B: 11–23 recognize a low-affinity peptide register in human subjects with autoimmune diabetes. Proc Natl Acad Sci U S A. 2014;111:14840–5. doi: 10.1073/pnas.1416864111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Velthuis JH, Unger WW, Abreu JR, Duinkerken G, Franken K, Peakman M, et al. Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers. Diabetes. 2010;59:1721–30. doi: 10.2337/db09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mallone R, van Endert P. T cells in the pathogenesis of type 1 diabetes. Curr Diab Rep. 2008;8:101–6. doi: 10.1007/s11892-008-0019-9. [DOI] [PubMed] [Google Scholar]
- 90.Zavala-Cerna MG, Martinez-Garcia EA, Torres-Bugarin O, Rubio-Jurado B, Riebeling C, Nava A. The clinical significance of posttranslational modification of autoantigens. Clin Rev Allergy Immunol. 2014;47:73–90. doi: 10.1007/s12016-014-8424-0. [DOI] [PubMed] [Google Scholar]
- 91.McGinty JW, Marre ML, Bajzik V, Piganelli JD, James EA. T cell epitopes and post-translationally modified epitopes in type 1 diabetes. Curr Diab Rep. 2015;15:90. doi: 10.1007/s11892-015-0657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roep BO, Kracht MJ, van Lummel M, Zaldumbide A. A roadmap of the generation of neoantigens as targets of the immune system in type 1 diabetes. Curr Opin Immunol. 2016;43:67–73. doi: 10.1016/j.coi.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 93•.van Lummel M, Duinkerken G, van Veelen PA, de Ru A, Cordfunke R, Zaldumbide A, et al. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes. 2014;63:237–47. doi: 10.2337/db12-1214. This report defines the naturally processed epitopes with modifications of islet autoantigens in T1D. [DOI] [PubMed] [Google Scholar]
- 94.Sibley RK, Sutherland DE, Goetz F, Michael AF. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Investig. 1985;53:132–44. [PubMed] [Google Scholar]
- 95.Vendrame F, Hopfner YY, Diamantopoulos S, Virdi SK, Allende G, Snowhite IV, et al. Risk factors for type 1 diabetes recurrence in immunosuppressed recipients of simultaneous pancreas-kidney transplants. Am J Transplant. 2016;16:235–45. doi: 10.1111/ajt.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59:947–57. doi: 10.2337/db09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gianani R, Putnam A, Still T, Yu L, Miao D, Gill RG, et al. Initial results of screening of nondiabetic organ donors for expression of islet autoantibodies. J Clin Endocrinol Metab. 2006;91:1855–61. doi: 10.1210/jc.2005-1171. [DOI] [PubMed] [Google Scholar]
- 98.In't Veld P, Lievens D, De Grijse J, Ling Z, Van der Auwera B, Pipeleers-Marichal M, et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56:2400–4. doi: 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- 99.Wagner R, McNally JM, Bonifacio E, Genovese S, Foulis A, McGill M, et al. Lack of immunohistological changes in the islets of nondiabetic, autoimmune, polyendocrine patients with beta-selective GAD-specific islet cell antibodies. Diabetes. 1994;43:851–6. doi: 10.2337/diab.43.7.851. [DOI] [PubMed] [Google Scholar]
- 100.Wiberg A, Granstam A, Ingvast S, Harkonen T, Knip M, Korsgren O, et al. Characterization of human organ donors testing positive for type 1 diabetes-associated autoantibodies. Clin Exp Immunol. 2015;182:278–88. doi: 10.1111/cei.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]