Abstract
Emerging evidence suggests that the airway microbiota plays an important role in viral bronchiolitis pathobiology. However, little is known about the combined role of airway microbiota and CCL5 in infants with bronchiolitis. In this multicenter prospective cohort study of 1,005 infants (age <1 year) hospitalized for bronchiolitis during 2011–2014, we observed statistically-significant interactions between nasopharyngeal airway CCL5 levels and microbiota profiles with regard to the risk of both intensive care use (Pinteraction=0.02) and hospital length-of-stay ≥3 days (Pinteraction=0.03). Among infants with lower CCL5 levels, the Haemophilus-dominant microbiota profile was associated with a higher risk of intensive care use (OR, 3.20; 95%CI, 1.18–8.68; P=0.02) and hospital length-of-stay ≥3 days (OR, 4.14; 95%CI, 2.08–8.24; P<0.001) compared to the Moraxella-dominant profile. Conversely, among those with higher CCL5 levels, there were no significant associations between the microbiota profiles and these severity outcomes (all P≥0.10).
Keywords: airway, bronchiolitis, CCL5/RANTES, microbiota, severity
Bronchiolitis is the most common lower respiratory infection in young children, accounting for 18% of infant hospitalizations in the US [1]. While bronchiolitis is traditionally viewed as viral-induced inflammation of bronchioles, recent studies suggest the presence of a complex interplay among the virus pathogens, airway microbiome, and host immune response systems [2–5]. Of the various components of the host immune systems, CCL5 is a β-chemokine chemoattractant that, via virus- (e.g., respiratory syncytial virus [RSV])[6] and bacteria-induced activation of Toll-like receptors in airway epithelia, recruits a variety of innate and adaptive immune cells [7]. Studies have also shown that specific gut microbiota induce CCL5-driven inflammation in animal models [8, 9]. Additionally, we recently identified, among infants hospitalized for bronchiolitis (“severe bronchiolitis”), that those with specific nasopharyngeal airway microbiota (Haemophilus-dominant profile) had the highest disease severity [4], and that this association was observed only in those with low circulating cathelicidin levels [5]. However, interrelations between the microbiota and host immune response (e.g., CCL5) within the airway niche were not investigated. In the current analysis, we studied the same cohort of infants with severe bronchiolitis to determine the interaction between the nasopharyngeal microbiota and CCL5 with regard to the disease severity.
METHODS
We analyzed data from a multicenter prospective cohort study of infants with severe bronchiolitis. Details of the study design, setting, population, data collection and testing, and analysis may be found in the Online Supplement (Supplemental Methods). Briefly, this cohort study, the 35th Multicenter Airway Research Collaboration (MARC-35)[4], enrolled 1,016 infants (aged <12 months) hospitalized for bronchiolitis at 17 sites across 14 US states (Table E1) during the 2011–2014 winter seasons. Bronchiolitis was defined by the American Academy of Pediatrics guidelines [10]. The institutional review board of every participating hospital approved the study. Written informed consent was obtained from the infant’s parent or guardian.
In addition to the collection of phenotypic data through structured interview and medical record review, investigators also collected nasopharyngeal aspirates within 24 hours of hospitalization using a standardized protocol. Nasopharyngeal CCL5 concentration was quantified by using an enzyme-linked immunosorbent assay. Composition of nasopharyngeal microbiota was characterized by sequencing the bacterial 16S rRNA gene V4 region on the Illumina MiSeq platform. The details of these testing may be found in Supplemental Methods. As previously described [4], by using a unbiased clustering approach (partitioning around medoids method), we derived four distinct nasopharyngeal microbiota profiles: Haemophilus-dominant, Moraxella-dominant, Streptococcus-dominant, and mixed profiles (Figure E1).
For the present analysis, we used the median level to divide the nasopharyngeal CCL5 levels into two groups: low CCL5 (≤42.0 pg/ml) and high CCL5 (>42.0 pg/ml). The primary outcome was intensive care use (defined as admission to intensive care unit and/or use of mechanical ventilation); the secondary outcome was hospital length-of-stay of ≥3 days [4]. To examine the difference in microbiota-outcome associations by CCL5 status, we fit a generalized linear mixed model adjusting for 14 potential confounders (age, sex, race/ethnicity, history of premature birth and breathing problems, attendance of daycare, cohabiting siblings, breastfeeding status, history of antibiotic and corticosteroid use in lifetime, antibiotic use at pre-hospitalization visit, detected respiratory viruses, and serum LL-37 and 25-hydroxyvitamin D levels [4, 5]) within each CCL5 stratum (low and high) with the Moraxella-dominant microbiota profile as the reference profile.
RESULTS
Of 1,016 infants with bronchiolitis, 1,005 (98.9%) met the 16S rRNA gene sequencing quality control requirements for the analysis. Overall, the median age was 3.2 months (IQR, 1.6–5.9 months), 40.0% were female, and 53.6% were non-Hispanic black or Hispanic. The median CCL5 level in the nasopharyngeal samples was 42.0 pg/ml (IQR, 18.3–109.1 pg/ml). While most patient characteristics and clinical variables, including the severity outcomes, did not differ significantly by CCL5 status (Table E2), the infants with lower CCL5 levels were younger, more likely to have received antibiotics during their pre-hospitalization visit, and had lower RSV genomic loads (all P<0.05) compared to those with higher CCL5 levels. There were no significant between-group differences in the detected viruses, serum LL-37 levels, nasopharyngeal microbiota profiles (Table E2), or relative abundance of major bacteria genera (all P≥0.10; Table E3). Additionally, there was no significant correlation between the nasopharyngeal CCL5 and serum LL-37 levels (P=0.76; Figure E2).
By contrast, we found statistically significant interactions between nasopharyngeal CCL5 status and microbiota profiles with regard to the risk of both intensive care use (Pinteraction=0.02) and hospital length-of-stay ≥3 days (Pinteraction=0.03), indicating that the microbiota-severity associations differ by CCL5 status. For example, among infants with lower CCL5 levels, the multivariable-adjusted model demonstrated that the Haemophilus-dominant microbiota profile was associated with a higher risk of intensive care use (adjusted OR, 3.20; 95%CI, 1.18–8.68; P=0.02) compared to the Moraxella-dominant profile (Figure 1). Streptococcus-dominant and mixed profiles were associated with a non-significantly higher risk of intensive care use compared to the Moraxella-dominant profile. In contrast, among those with higher CCL5 levels, there were no significant associations between the microbiota profiles and risk of intensive care use (all P≥0.10).
Figure 1. Multivariable associations of nasopharyngeal microbiota profiles with risks of intensive care use and longer hospital length-of-stay, stratified by nasopharyngeal CCL5 level.
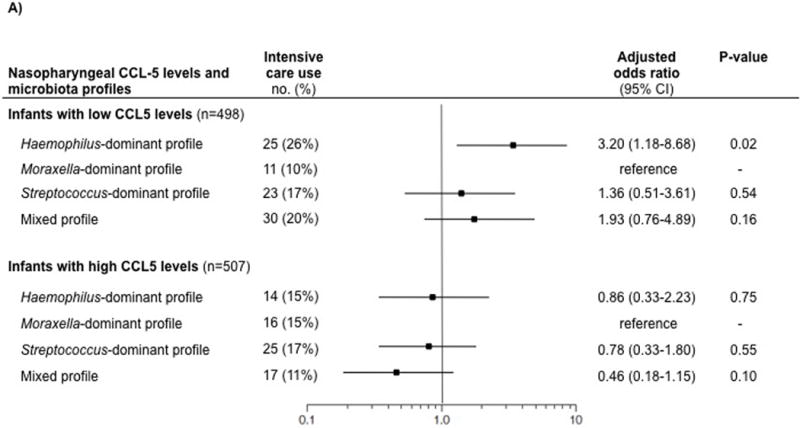
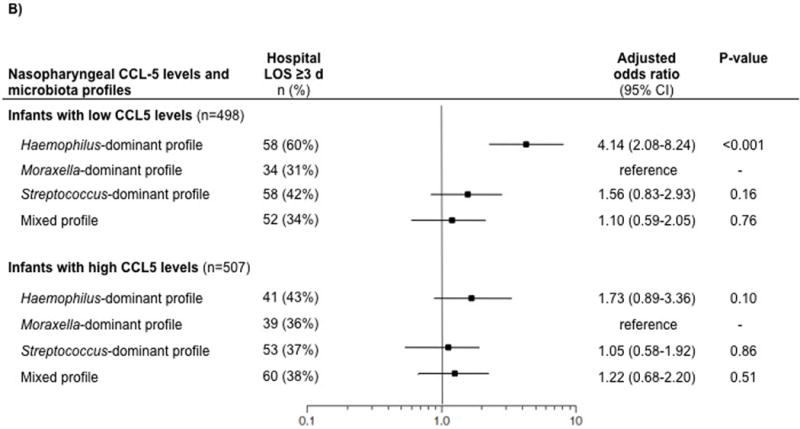
Generalized linear mixed model was constructed for each strata (infants with low nasopharyngeal CCL5 levels [≤42.0 pg/ml] and those with high CCL5 levels [>42.0 pg/ml)]), with the Moraxella-dominant microbiota profile as the reference profile. The models adjusted for age, sex, race/ethnicity, history of premature birth, history of breathing problems, attendance of daycare, cohabiting siblings at home, breastfeeding status, lifetime history of systemic antibiotic use, lifetime history of corticosteroid use, systemic antibiotic use at the pre-hospitalization visit, respiratory viruses detected by PCR, and serum LL-37 and 25-hydroxyvitamin D levels. The models also accounted for patient clustering within each hospital.
A) Intensive care use (primary outcome)
B) Hospital length-of-stay of ≥3 days (secondary outcome)
Abbreviations: CI, confidence interval; LOS, length-of-stay
Likewise, among infants with lower CCL5 levels, the Haemophilus-dominant profile was associated with a higher risk of hospital length-of-stay ≥3 days (adjusted OR, 4.14; 95%CI, 2.08–8.24; P<0.001) compared to the Moraxella-dominant profile (Figure 1). By contrast, among those with higher CCL5 levels, there were no significant associations (all P≥0.10).
DISCUSSION
In this multicenter cohort study of 1,005 infants with severe bronchiolitis, we found a statistically-significant interaction between nasopharyngeal airway CCL5 and microbiota profiles with regard to the disease severity. Specifically, among infants who had low CCL5 levels, the Haemophilus-dominant microbiota profile was associated with a higher risk of intensive care use and longer hospital length-of-stay. These observed interactions were independent from viral pathogens and circulating cathelicidin levels. By contrast, in those with high CCL5 levels, there were no associations between the microbiota profiles and disease severity.
Emerging evidence suggests that a complex interplay among the respiratory viruses, airway microbiome, and host immune response influences the pathogenesis of respiratory infection in children. For example, we have previously identified integrated contributions of the nasopharyngeal microbiota and circulating (not airway) cathelicidin to the disease severity in infants with bronchiolitis [5]. Additionally, an analysis of the nasopharyngeal microbiota and whole blood transcriptome in a single-center study of 106 children (age <2 years) with RSV infection showed microbiota-specific systemic immune responses – e.g., children with RSV infection and Haemophilus-dominant microbiota had upregulation of the genes linked to Toll-like receptors and macrophage activation in the whole blood [2]. The present study corroborates these prior reports and extends them by demonstrating, for the first time, the interaction between the microbiota and host immune response (particularly CCL5) within the airway niche with regard to the respiratory infection severity.
The nature of the observed microbiota-CCL5-severity interrelation warrants elucidation. It is possible that a specific function (not structure) of the airway microbiota, through altering the expression of CCL5 locally, influences bronchiolitis severity. Indeed, Elinav et al., in mouse models, reported that an aberrant gut microbiota changes local epithelial induction of CCL5 transcription as a downstream mechanism, and leads to an exaggerated inflammatory response locally (i.e., exacerbation of colitis)[8]. Another mechanism is that airway CCL5, by recruiting a variety of immune cells, modifies the airway microbiota and jointly contributes to the bronchiolitis pathobiology (and host resilience). Furthermore, these mechanisms are not mutually exclusive. Despite this complexity, the identification of significant interactions between the microbiota and CCL5 within the airway of infants with bronchiolitis is an important finding. Our data should facilitate mechanistic investigations into the complex crosstalk among the respiratory viruses, microbiota, and host immune systems, and help to define their integrated role in bronchiolitis pathobiology.
This study has several potential limitations. First, our data are based on the nasopharyngeal microbiota and CCL5 level while bronchiolitis involves inflammation of the lower airways. Yet, lower airway sampling in infants is technically- and ethically-challenging. Furthermore, the literature has demonstrated that there are significant correlations between upper and lower airway inflammatory mediators, including CCL5 [11], and that upper airway microbiota is reliable representation of bronchoalveolar lavage microbiota in young children [12]. Second, the current analytic design precluded investigations into the relation between the airway microbiome succession and respiratory health in children (e.g., incident asthma). To address this question, the MARC-35 cohort is currently being followed up to 6 years of age, with airway sampling at multiple time-points. Third, despite a rigorous adjustment for confounding factors (e.g., age, prior use of systemic antibiotics), the observed associations might be confounded by unmeasured factors. Fourth, our study did not include the data of a “control” group (e.g., asymptomatic infants). Nevertheless, the study aim is not to evaluate the role of airway microbiota and CCL5 on the development of bronchiolitis but to investigate their integrated contributions to the disease severity among infants with bronchiolitis. Lastly, even with our racially/ethnically- and geographically-diverse US sample, the inferences might not be generalizable beyond infants who had severe bronchiolitis. Still, our data remain directly relevant for 130,000 hospitalized US children each year [1].
In summary, on the basis of the multicenter cohort of 1,005 infants hospitalized for bronchiolitis, we identified a statistically-significant interaction between the nasopharyngeal microbiota profiles and CCL5 status with regard to the risk of intensive care use and longer hospital length-of-stay. These findings indicate that the microbiota-severity associations differ by airway CCL5 levels. Although the clinical relevance of these findings is not yet determined, our data should underscore the importance of understanding the integrated contributions of respiratory viruses, airway microbiome, and immune systems to the pathobiology of bronchiolitis.
Supplementary Material
Acknowledgments
We thank the MARC-35 study hospitals and research personnel for their ongoing dedication to bronchiolitis and asthma research (Table E1 in the Online Supplement). We also thank Ashley F. Sullivan, MS, MPH and Janice A. Espinola, MPH (Massachusetts General Hospital, Boston, MA) as well as Alkis Togias, MD (National Institute of Allergy and Infectious Diseases) for their contributions to the study.
Financial Support: This work was supported by the grants UG3 OD-023253, U01 AI-087881, R01 AI-114552, R01 AI-108588, R01 AI-127507, and R21 HL-129909 from the National Institutes of Health (Bethesda, MD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: Dr. Mansbach has provided bronchiolitis-related consultation for Regeneron. Drs. Ajami and Petrosino own shares at Diversigen Inc., a microbiome research company. Dr. Piedra provided bronchiolitis-related consultation for Gilead, Novavax, and Regeneron. The other authors have no financial relationships relevant to this article to disclose.
Author Contributions: KH and CAC conceived the study. JMM, RJF, and SJT contributed to data collection. CAC obtained research funding and supervised the conduct of the study. AJA, JFP and PAP handled the samples and generated the virology, microbiota, and cytokine data. KH and NJA analyzed the data. KH drafted the manuscript, and all authors contributed substantially to its revision. KH takes responsibility for the paper as a whole.
References
- 1.Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA., Jr Trends in bronchiolitis hospitalizations in the United States, 2000–2009. Pediatrics. 2013;132(1):28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Steenhuijsen Piters WA, Heinonen S, Hasrat R, Bunsow E, Smith B, Suarez-Arrabal MC, Chaussabel D, Cohen DM, Sanders EA, Ramilo O, et al. Nasopharyngeal microbiota, host transcriptome and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansbach JM, Hasegawa K, Henke DM, Ajami NJ, Petrosino JF, Shaw CA, Piedra PA, Sullivan AF, Espindola PA, Camargo CA., Jr Respiratory syncytial virus and rhinovirus severe bronchiolitis are associated with distinct nasopharyngeal microbiota. J Allergy Clin Immunol. 2016;137(6):1909–1913. doi: 10.1016/j.jaci.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasegawa K, Mansbach JM, Ajami NJ, Espinola JA, Henke DM, Petrosino JF, Piedra PA, Shaw CA, Sullivan AF, Camargo CA., Jr Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalized for bronchiolitis. Eur Respir J. 2016;48:1329–1339. doi: 10.1183/13993003.00152-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa K, Mansbach JM, Ajami NJ, Petrosino JF, Freishtat RJ, Teach SJ, Piedra PA, Camargo CA., Jr Serum cathelicidin, nasopharyngeal microbiota, and disease severity in infants hospitalized with bronchiolitis. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.09.037. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacharier LB, Cohen R, Schweiger T, Yin-Declue H, Christie C, Zheng J, Schechtman KB, Strunk RC, Castro M. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130(1):91–100 e103. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berube J, Bourdon C, Yao Y, Rousseau S. Distinct intracellular signaling pathways control the synthesis of IL-8 and RANTES in TLR1/TLR2, TLR3 or NOD1 activated human airway epithelial cells. Cellular signalling. 2009;21(3):448–456. doi: 10.1016/j.cellsig.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B, Elinav E, Huber S, Strowig T, Hao L, Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC, et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci U S A. 2013;110(24):9862–9867. doi: 10.1073/pnas.1307575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, Johnson DW, Light MJ, Maraqa NF, Mendonca EA, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 11.McDougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ, Walsh GM. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008;39(5):560–568. doi: 10.1165/rcmb.2007-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsh RL, Kaestli M, Chang AB, Binks MJ, Pope CE, Hoffman LR, Smith-Vaughan HC. The microbiota in bronchoalveolar lavage from young children with chronic lung disease includes taxa present in both the oropharynx and nasopharynx. Microbiome. 2016;4(1):37. doi: 10.1186/s40168-016-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


