Abstract
Chimeric antigen receptor (CAR) T cell therapy has shown promising efficacy against hematologic malignancies. Antitumor activity of CAR T cells, however, needs to be improved to increase therapeutic efficacy in both hematologic and solid cancers. Limitations to overcome are ‘on-target, off-tumor’ toxicity, antigen escape, short CAR T cell persistence, little expansion, trafficking to the tumor and inhibition of T cell activity by an inhibitory tumor microenvironment. Here we will discuss how optimizing the design of CAR T cells through genetic engineering addresses these limitations and improves the antitumor efficacy of CAR T cell therapy in pre-clinical models.
Keywords: Chimeric antigen receptor, Armored CAR T cells, Genetic engineering, Cytokines, Costimulation, Tumor microenvironment
1. Introduction
Chimeric antigen receptor (CAR) T cell therapies show great promise for haematologic malignancies, but less so for solid cancers. CARs consist of an extracellular antigen recognition domain, often the single-chain Fragment variant (scFv) derived from an antibody, a transmembrane domain and the intracellular T cell activation domain of CD3ζ. Second generation CARs add to this a co-stimulatory domain, commonly CD28 or 4-1BB. The CARs are designed to target a tumor specific protein and engineered into autologous T cells, which are subsequently given back to the patient. The most well-studied and successful CARs thus far are targeted against CD19, which is present on normal B cells and B-cell malignancies. Second generation CD19-targeting CARs with either a CD28 (CD28/CD3ζ, or 28z) or 4-1BB (4-1BB/CD3ζ, or BBz) costimulatory domain have shown antitumor activity in several clinical trials, most profoundly against B-cell acute lymphoblastic leukemia (B-ALL) (Brentjens et al., 2011, 2013; Kalos et al., 2011; Kochenderfer et al., 2012).
However, in spite of this success in B-ALL patients, a subset of patients that achieve complete remission relapses with CD19-negative leukemia (Grupp et al., 2013; Lee et al., 2015; Maude et al., 2014). Investigation of underlying causes for CD19 loss showed the presence of a CD19 isoform that lacked exon 2 (Δex2), caused by either genetic alterations or low expression of splicing factor SRSF3 (Sotillo et al., 2015). This CD19-Δex2 was more stable than full-length CD19 and retains protein function, but cannot be detected by their flow cytometry antibody and fails to trigger a CD19-targeted CAR T cell response. Recently, two B-ALL patients with rearrangements in the mixed lineage leukemia (MLL) gene have been reported of which the relapsed tumor after CD19 CAR T cell therapy demonstrated lineage switching to a CD19-negative myeloid phenotype (Gardner et al., 2016). Jacoby et al. (2016) showed in syngeneic murine pre-B-ALL that CD19 CAR T cell therapy could drive lineage switching and as a result loss of CD19 expression.
In addition to antigen loss in relapsed tumors post CAR T cell therapy, it is expected that tumors are heterogeneous for expression of the targeted antigen. To achieve complete tumor eradication, epitope spreading (Beatty et al., 2014) and a more broad immune response need to be triggered by the CAR T cells, which would result in elimination of antigen-negative tumor cells as well. Heterogeneity might especially play a role in solid cancers. Several targets have been identified for CAR T cell therapy in a variety of solid malignancies (Jackson, Rafiq, & Brentjens, 2016). Thus far, however, clinical trials with CAR T cells in solid cancers have reported only limited antitumor efficacy. Adoptive cell therapy against solid cancers have, in addition to target antigen selection, T cell proliferation and persistence, also specific hurdles to overcome, such as trafficking of the T cells into the tumor and the ability to overcome an immune suppressive microenvironment (Beatty & O’Hara, 2016).
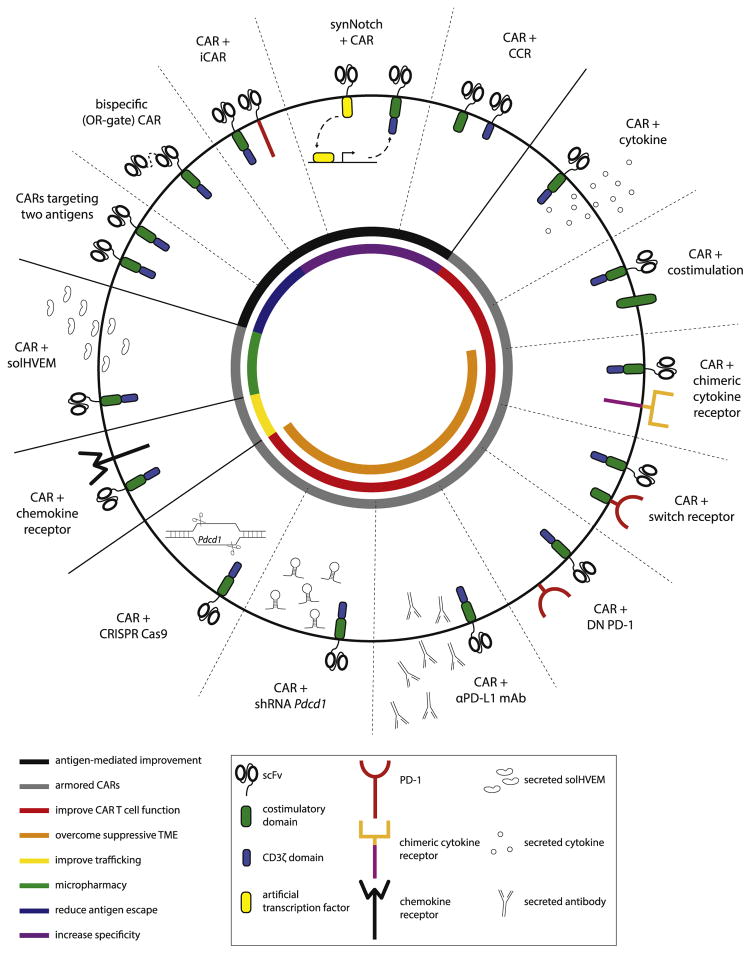
In this review we discuss how further genetic engineering of the CAR T cells can be used to improve their functionality, safety and antitumor efficacy, in both hematologic and solid malignancies (Fig. 1).
Fig. 1.
Summary of genetic strategies to improve CAR T cell function and antitumor activity. CAR: chimeric antigen receptor, iCAR: inhibitory CAR, CCR: chimeric costimulatory receptor, DN: dominant negative, PD-1: programmed cell death protein 1, αPD-L1 mAb: anti-PD-1 ligand 1 monoclonal antibody, shRNA: small hairpin RNA, solHVEM: soluble HVEM ectodomain protein, TME: tumor microenvironment.
2. Specificity and safety of CAR T cell therapy
2.1. Targeting two tumor-associated antigens to reduce antigen escape
Expression of multiple tumor-specific targets reduces the chance of antigen escape by mutating or reducing expression of the target antigen, as both targets would have to be lost simultaneously. Two combinations have shown to prevent CD19-negative tumor escape: CD19/ C123 and CD19/CD20-dual targeting CAR T cells. CD123 is present on B-ALL blasts and CD19-negative relapsed tumors (Ruella et al., 2016). Simultaneous expression of CD123 and CD19-targeting CARs on T cells was more potent than treatment with pooled CAR T cells expressing either CAR. Zah, Lin, Silva-Benedict, Jensen, and Chen (2016) generated a bispecific CAR that gets activated upon binding of either CD19 or CD20 (so-called OR-gate CAR). This CAR showed enhanced antitumor efficacy in a pre-clinical B-cell lymphoma model, as the outgrowth of CD19-negative tumor cells was abrogated. Co-expressing CD19 CARs with other CARs targeting B-cell malignancies, such as against CD22 (Haso et al., 2013), ROR1 (Hudecek et al., 2010) and immunoglobulin kappa chain (Igκ) (Vera et al., 2006), might have the potential to prevent CD19 antigen escape as well. CAR recognition of two TAA’s potentially increases the risk of ‘on-target, off-tumor’ side effects. This extra toxicity will be minimal when both targets are restricted to the same cell type, such as CD19 and CD20. In addition, a lower affinity of the CAR to each target might lead to a strong CAR T cell activation when both targets are present and only weak against single-target expressing non-tumor cells. Duong, Westwood, Berry, Darcy, and Kershaw (2011) showed in in vitro models that T cells expressing two different CARs targeting folate-binding protein and HER2, were more responsive against tumor cells expressing both targets than against normal cells expressing only either one of the targets. This phenomenon was also demonstrated in glioblastoma xenograft tumors with HER2 and IL13Rα2-targeting CARs (Hegde et al., 2013). T cells expressing both CARs improved the antitumor response compared to pooled T cells expressing HER2 CARs or IL13Rα2 CARs. The same group generated a tandem CAR recognizing HER2 and IL13Rα2 simultaneously by inducing heterodimerization of the two targets (Hegde et al., 2016). This tandem CAR (tanCAR) further reduced antigen escape and had increased antitumor efficacy in pre-clinical models.
2.2. Targeting two tumor-associated antigens to increase specificity and safety
Targeting a specific combination of two antigens can also be exploited to increase specificity and reduce on-target, off-tumor side effects, even though the requirement of two antigens for CAR T cell activation increases the chance of antigen escape. CD19 CAR T cells target normal B-cells, leading to B-cell aplasia (Brentjens et al., 2011, 2013; Kalos et al., 2011; Porter, Levine, Kalos, Bagg, & June, 2011), which can be treated with monthly infusions of immunoglobulin. But a case report about a patient treated with HER2-targeting CAR T cells who experienced severe toxicity and died because of low levels of HER2 on lung epithelium (Morgan et al., 2010), demonstrates the need for careful design of CAR constructs, as targets that are completely tumor-specific are scarce.
Increased tumor specificity is achieved by separating the T cell activation signals over two antigen recognition molecules (Kloss, Condomines, Cartellieri, Bachmann, & Sadelain, 2013; Lanitis et al., 2013; Wilkie et al., 2012). The antigens do not need to be truly tumor specific, as long as the combination of the two garners tumor specificity. Kloss et al. (2013) describes CAR-mediated recognition of prostate stem cell antigen (PSCA) with an intracellular CD3ζ domain. Costimulation is provided by prostate specific membrane antigen (PSMA)-specific scFv coupled to CD28 and 4-1BB costimulatory domains (a chimeric costimulatory receptor). Lanitis et al. (2013) showed that transactivated CAR T cells (anti-mesothelin-CD3ζ plus anti-folate receptor-CD28) have similar antitumor efficacy against tumors expressing both antigens compared to cis-activated CAR T cells (anti-mesothelin-CD28-CD3ζ) and show less activity against single-positive tumors. This approach is expected to reach highest specificity when the CAR-mediated antigen recognition is relatively inefficient and T cells are only fully activated in presence of the antigen targeted by the chimeric costimulatory receptor.
Recently the lab of Wendell Lim has developed a different system in which two antigens are similarly needed for full CAR T cell activation: an AND-gate CAR, termed synNotch (Morsut et al., 2016; Roybal, Rupp, et al., 2016; Roybal, Williams, et al., 2016). This synthetic molecule consists of an engineered antigen-recognition domain, a Notch core and an artificial transcription factor, which gets cleaved off and activated upon antigen stimulation. This transcription factor specifically induces expression of the CAR, so the CAR and therefore the T cells only get activated when both antigens are present. This system works orthogonally and does not require an intermediate signaling molecule, creating a flexible tool to regulate specific signal-response cascades in a variety of applications. It remains to be investigated, however, whether the non-human transcription factors are immunogenic.
Another strategy to decrease on-target, off-tumor reactivity of CAR T cells is to co-express an inhibitory CAR (iCAR) that recognizes an antigen expressed on non-tumor tissues. The iCAR consists of an antigen-recognition domain coupled to the intracellular domain of T-cell checkpoint proteins programmed cell death protein 1 (PD-1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). Cells that express the iCAR target, even in the presence of the activating CAR antigen, do not activate T cells, thereby reducing damage to normal tissue (Fedorov, Themeli, & Sadelain, 2013). In this way, off-tumor toxicities are prevented rather than treated while the CAR T cells retain their antitumor activity. This is in contrast to the activation of suicide genes when toxicity is apparent (discussed below).
2.3. Suicide genes to control CAR T cell presence
CAR T cell therapy frequently results in severe side effects, such as the above-discussed on-target, off-tumor toxicity, neurotoxicity and cytokine release syndrome (CRS) with fevers and high levels of serum cytokines (Davila & Sadelain, 2016). CRS toxicity was found to correlate with tumor burden (Davila et al., 2014) and requires treatment with steroids and/or the anti-IL6 receptor antibody tocilizumab. CAR T cells may be selectively ablated by engineered expression of suicide genes, which can be activated in the case of acute toxicity or to reverse B-cell aplasia due to long-term CAR T cell survival as seen in the setting of CD19-targeted CAR T cells.
Transduction of allogeneic lymphocytes with herpes simplex virus thymidine kinase (HSV-tk) enables elimination of the lymphocytes by administration of ganciclovir when graft-versus-host disease (GVHD) developed in lymphoma patients (Bonini et al., 1997) and can be used for positron emission tomography (PET) imaging of the infused cells with a 18F-radiolabeled ganciclovir analog (Keu et al., 2017). This virus-specific gene, however, might be immunogenic. Indeed, HSV-tk-specific CD8+ and CD4+ T cell responses developed in patients after hematopoietic cell transfer (Berger, Flowers, Warren, & Riddell, 2006). A similar immunogenic response can be anticipated against the new doxycycline-inducible Tet-On CAR expression systems (Sakemura et al., 2016). Another well-studied suicide gene for adoptive cell transfer is inducible caspase 9 (iCasp9), consisting of the intracellular domain of the pro-apoptotic human caspase 9 protein fused to a human FK506 binding protein (FKBP), allowing for dimerization induced by small molecules such as AP1903 (Straathof et al., 2005). Activation of iCasp9 in patients who developed GVHD after genetically engineered T cell transplantation rapidly induced T cell apoptosis and reversed the GVHD (Di Stasi et al., 2011). Pre-clinical studies have observed efficient T cell elimination in the context of iCasp9-engineered CAR T cells (Budde et al., 2013; Hoyos et al., 2010; Minagawa et al., 2016) and the first clinical trials with iCasp9 in combination with anti-GD2 CAR T cell therapy are currently open to accrual (NCT02992210, NCT02107963).
Approaches for antibody-mediated T cell ablation, through antibody-mediated cellular cytotoxicity (ADCC) and complement-mediated toxicity, include T cell transduction of CD20, targeted by rituximab (Griffioen et al., 2009; Vogler et al., 2010) and a truncated form of the epithelial growth factor receptor (EGFR), targeted by cetuximab (Koneru, O’Cearbhaill, Pendharkar, Spriggs, & Brentjens, 2015; Koneru, Purdon, Spriggs, Koneru, & Brentjens, 2015; Paszkiewicz et al., 2016; Wang et al., 2011). EGFRt-mediated CAR T cell elimination is incorporated in several CAR T cell clinical trials targeting CD171 (NCT02311621), CD19 (NCT02028455, NCT01865617, NCT02146924, NCT02051257), CD123 (NCT02159495) and MUC16ecto (NCT02498912).
3. Armored CARs to improve T cell function
The antitumor activity of CAR T cell therapy is not only determined by antigen specificity; also persistence and expansion of the CAR T cells and an immune-suppressive microenvironment play a role. Persistence of second generation CAR T cells is dependent on many factors, including the costimulatory domain(s) (Zhao et al., 2015), tumor burden (Brentjens et al., 2011), lymphodepletion prior to CAR T cell therapy (Turtle et al., 2016) and stimulation of activation-induced T-cell inhibitory proteins, such as PD-1 (Cherkassky et al., 2016). Interestingly, ibrutinib, a Bruton tyrosine kinase (BTK) inhibitor used for treatment of CLL, was shown to reduce PD-1 expression on CAR T cells and to increase persistence and antitumor activity (Fraietta et al., 2016). The importance of CAR T cell persistence is demonstrated by its correlation with survival in both hematologic (Porter et al., 2015) and solid cancers (Louis et al., 2011).
To further improve their function, CAR T cells can be engineered to co-express other molecules, such as cytokines and costimulatory molecules. This not only stimulates these ‘armored’ CAR T cells in an autocrine manner, but may also have a pro-inflammatory effect on other immune cells within the tumor microenvironment, such as tumor-associated macrophages, dendritic cells (DCs), natural killer (NK) cells and tumor-infiltrating lymphocytes (TILs). Below we discuss various approaches that have been studied in the pre-clinical setting.
3.1. Armored CAR T cells secreting cytokines
Several cytokines have been investigated to enhance adoptive transfer efficacy, both for TILs and CAR T cells, mainly to increase the ex vivo expansion of the infusion product. Systemic administration of recombinant cytokine(s) to stimulate T cell activity after adoptive cell transfer is often associated with severe toxicities. To avoid systemic side effects, we and others have investigated expression of cytokines by the T cells themselves to locally stimulate persistence and expansion in an autocrine manner. Here we will discuss expression of interleukin 12 (IL-12), IL-2 and IL-15.
IL-12 is most studied for its potent T cell activating effect. IL-12 is a pro-inflammatory cytokine that is produced by DCs, macrophages and neutrophils. It enhances cytotoxic activity of CD8+ T cells and NK cells and stimulates a Th1 helper T cell response. Because of its potent effect, cancer patients have been treated with systemic recombinant IL-12 (rIL-12). This led, however, to severe toxicity (Lacy et al., 2009) and treatment-related mortality (Leonard et al., 1997). To avoid these systemic side effects, several studies have engineered tumor-specific T cells to express IL-12, resulting in tumor-localized IL-12 secretion. In a B16 murine melanoma model, IL-12-secreting tumor-reactive pmel-1 CD8+ T cells still caused weight loss and decreased survival when more than 5 × 105 cells were administered (Kerkar et al., 2010). Lower cell numbers without side effects showed increased accumulation and antitumor activity in B16 melanomas. The pmel-1-reactive T cells, however, did not proliferate and, in fact, constitutive expression of IL-12 induced apoptosis. To circumvent this, the investigators generated inducible IL-12 under the promoter of nuclear factor of activated T cells (NFAT) (Zhang et al., 2011). These NFAT-IL-12 pmel-1 T cells retained antitumor activity without inducing toxicity. In the same pmel-1 model, local secretion of IL-12 was shown to increase cross-presentation of natural tumor antigens and reprogram myeloid-derived cells, including dendritic cells, macrophages and myeloid-derived suppressor cells (MDSCs), stimulating the CD8+ T cells (Kerkar et al., 2011). Fas expression on the tumor-resident myeloid-derived cells was shown to mediate the enhanced antitumor activity of IL-12-pmel-1 T cells (Kerkar et al., 2013). Patients with metastatic melanoma who received TILs transduced with NFAT-IL-12, however, experienced grade 3 and 4 toxicities (Zhang et al., 2015), similar to what has been observed after intratumor injection of rIL-12 (van Herpen et al., 2004). In the case of CAR T cells, inducible NFAT-IL-12 production also demonstrated improved antitumor activity in mice, at least in part mediated by macrophages, without apparent toxicities (Chinnasamy et al., 2012; Chmielewski, Kopecky, Hombach, & Abken, 2011).
We generated constitutive IL-12 production by engineering a bicistronic vector encoding for a CD19-targeting CAR and a murine IL-12 α and β subunit fusion. In an immune-competent EL4 thymoma mouse model expressing human CD19, IL-12 production by hCD19-targeting CAR T cells increased the cytotoxicity and cytokine production of the CAR T cells and led to tumor eradication in vivo (Pegram et al., 2012). This required autocrine IL-12 signaling and subsequent IFNγ production. Importantly, hCD19/IL-12 CAR T cells were able to overcome inhibition of CAR T cell activity by regulatory T cells (Tregs), eliminating the need for prior conditioning chemotherapy to remove endogenous T and B cells (Pegram et al., 2012). Also a CAR targeting the MUC16ecto domain coupled to IRES-IL-12 expression demonstrated improved cytokine secretion, expansion and in vivo antitumor efficacy (Koneru, Purdon, et al., 2015). In both models no toxicities were observed. The MUC16ecto CAR cells secreting IL-12 are currently being tested in a phase I clinical trial (NCT02498912). To increase safety a low starting dose, 3 × 105 CAR T cells/kg, is used for dose escalation. In addition, expression of the suicide gene EGFRt has been included to alleviate potential on-target, off-tumor or other toxicity (Koneru, O’Cearbhaill, et al., 2015).
Genetic engineered secretion of IL-2 has been studied in the context of TIL therapy. T cells normally produce IL-2 upon activation and IL-2 is required for a good T cell response. IL-2 has been well studied to enhance the efficacy of adoptive transfer. Systemic high-dose rIL-2 treatment of cancer patients, however, demonstrated a wide range of toxicities and treatment-related deaths (Rosenberg et al., 1989). To achieve local IL-2 production by adoptively transferred T cells for autocrine IL-2 signaling, melanoma-derived TILs were transduced to constitutively express IL-2. This resulted in prolonged T cell proliferation after antigen removal by tumor cell death and in absence of exogenous IL-2, while retaining tumor specificity (Liu & Rosenberg, 2003). In metastatic melanoma patients, however, IL-2 producing TILs caused toxicity and an increased clinical response was not observed compared to untransduced TILs (Heemskerk et al., 2008).
Similar to IL-2, IL-15 stimulates activity and expansion of CD8+ cytotoxic T cells and NK cells and is produced by monocytes, macrophages and DCs (Waldmann, Dubois, & Tagaya, 2001). Autocrine IL-15 signaling largely increased the proliferative capacity of IL-15-transduced lymphocytes after external cytokine withdrawal, while retaining antigen recognition (Hsu et al., 2005). Ex vivo IL-15 stimulation of CAR T cells also increased proliferation and effector function and systemic treatment with rIL-15 after CAR T cell treatment in the SKOV3 ovarian cancer model slightly decreased tumor growth (Xu et al., 2016), potentially via reducing CD8+ T cell senescence (Weng et al., 2016). Further studies by Hsu et al. (2007), however, showed that IL-15-transduced T cells from one donor (out of 23) proliferated exponentially ex vivo for more than a year, indicating that expression of an IL-15 transgene could be leukemogenic. ShRNA-mediated knockdown of the IL-15 receptor showed a decrease in proliferation, demonstrating that the effect was indeed mediated by IL-15 signaling. To avoid the leukemogenic effect of IL-15 transduction, systemic treatment could be considered for this cytokine. Recombinant IL-15 could be safely administered to metastatic cancer patients (Conlon et al., 2015), although it is not clear whether intratumor IL-15 concentrations will be high enough to stimulate adoptively transferred TILs or CAR T cells. Co-expression of a suicide gene makes it possible to retain the local T cell-specific stimulation by IL-15 transduction, while preventing uncontrolled T cell proliferation (Hoyos et al., 2010; Quintarelli et al., 2007). Hoyos et al. (2010) generated a tricistronic vector expressing a CD19-targeting CAR construct combined with the IL-15 transgene and iCasp9 suicide gene. 19CAR/IL-15/ iCasp9 T cells produced IL-15 in response to antigen stimulation and demonstrated increased persistence, expansion and antitumor activity in the Raji (Burkit lymphoma) and Daudi (B cell lymphoma) xenograft models. Treatment with AP20187, the substrate for iCasp9, eliminated the CAR T cells in mice, indicating a safe approach to abolish uncontrolled T cell proliferation or other adverse effects.
3.2. Armored CAR T cells expressing (chimeric) cytokine receptors
Cytokines are powerful mediators to drive a pro or anti-inflammatory immune response. Responsiveness of CAR T cells to cytokines can be influenced by engineered expression of cytokine receptors. Taking this a step further, one can transform binding of an anti-inflammatory Th2 cytokine into a pro-inflammatory Th1 signal by fusing the ectodomain and endodomain of two different cytokine receptors. Combining the extracellular part of the Th2 cytokine Il-4 receptor α (IL-4Rα) with the intracellular β-subunit of the IL-2 and IL-15 receptors, creating the chimeric cytokine receptor 4αβ, has shown to largely increase T cell expansion in vitro in the presence of IL-4, more than with standard IL-2 treatment (Wilkie et al., 2010). 4αβ-T1E28z CAR T cells (called T4 immunotherapy), targeting ErbB dimers, also demonstrated in vivo antitumor activity (van der Stegen et al., 2013), although it is unclear how this compares to T1E28z CAR T cells. Interestingly, the toxicity profile of T4 immunotherapy depends on the route of CAR T cell administration; intraperitoneal administration induces CRS, whereas intratumoral and intravenous administration does not (van der Stegen et al., 2013). Intratumoral administration of T4 immunotherapy is currently being investigated in head and neck cancer patients (NCT01818323) (van Schalkwyk et al., 2013).
Leen et al. (2014) generated another chimeric cytokine receptor with IL-4, using the intracellular signaling domain of the IL-7 receptor. IL-7 is important for T cell homeostasis and expansion, but IL-7R levels are low on stimulated cytotoxic T cells (Vera et al., 2009). Expression of this IL-4/7 receptor on Epstein-Barr Virus (EBV)-specific T cells promotes a Th1 response instead of an IL-4-induced Th2 response and increases antitumor activity in an IL-4 producing xenograft tumor model. Conversely, genetically engineered expression of the IL-7 receptor α (IL-7Rα) itself in T cells restores their responsiveness to IL-7 (Vera et al., 2009). Perna et al. (2014) generated a bicistronic vector encoding a GD2-targeting CAR and IL-7Rα. Expression of both molecules on EBV-specific T cells stimulates in vitro proliferation and in vivo antitumor activity in the presence of IL-7 and overcomes inhibition by Tregs, which do not respond to IL-7. Importantly, treatment with recombinant IL-7 is well tolerated in patients (Rosenberg et al., 2006; Sportès et al., 2008), in contrast to IL-12 and IL-2, making this a feasible strategy for the clinic.
3.3. CAR T cells armored with additional costimulation
T cell receptor (TCR)-mediated T cell activation is amplified via co-stimulatory signaling. This reduces T cell anergy (unresponsiveness) and exhaustion. Initially, CAR constructs contained an intracellular CD3ζ chain for T cell activation (first generation). Second generation CARs contain an additional costimulatory domain (van der Stegen, Hamieh, & Sadelain, 2015), mostly CD28 or 4-1BB, which results in CAR T cell expansion and cytokine secretion upon target antigen exposure (Imai et al., 2004; Maher, Brentjens, Gunset, Rivière, & Sadelain, 2002). Many costimulatory and coinhibitory molecules have been identified to date, which are tightly regulated in space and time to achieve different functional outcomes (Chen & Flies, 2013). In an immune suppressive microenvironment, however, T cells may not be exposed to sufficient costimulation. Tumor cells often do not express costimulatory ligands CD80 and CD86 and expression of costimulatory receptors on DCs is inhibited (Zou, 2005). Here we discuss two costimulatory molecules, CD40L and 4-1BBL, genetically engineered to be constitutively expressed on armored CAR T cells to provide additional costimulation independent of antigen-presenting cells (APCs).
CD40 ligand (CD40L, CD154) is a type II transmembrane protein and member of the tumor necrosis factor (TNF) receptor superfamily. CD40L was initially found to be expressed on activated T and B cells and later also identified on a variety of immune and non-immune cell types (Armitage et al., 1992; Schönbeck & Libby, 2001). CD40L binds to its receptor CD40, which is also expressed on multiple cell types, including DCs, macrophages and lymphocytes (Schönbeck & Libby, 2001). CD40 activation on DCs by binding to CD40L upregulates the costimulatory molecules CD80 and CD86 and is essential for production of IL-12, which in turn stimulates a Th1 T cell response (Cella et al., 1996). CD40/CD40L interaction also stimulates the generation of CD8+ T cell memory (Bourgeois, Rocha, & Tanchot, 2002) and reverses CD8+ T cell exhaustion (Bhadra, Gigley, & Khan, 2011). Interestingly, CD40 is expressed on several hematological and solid tumors. CD40 agonists have a direct antitumor effect and activate APCs to generate an antitumor specific cytotoxic T cell response (Khong, Nelson, Nowak, Lake, & Robinson, 2012). CLL patients that were treated with CLL cells transduced with murine CD40L adenovirus demonstrated increased plasma levels of IL-12 and IFNγ, tumor-specific T cells and a reduced tumor burden (Wierda et al., 2000). Together, this indicates that the CD40/CD40L axis can be used to elicit a direct as well as an APC-mediated antitumor response. When T cells are transduced with CD40L and co-cultured with either CD40+ tumor cells or DCs, this increases the tumor immunogenicity, DC maturation and IL-12 secretion (Curran et al., 2015). Furthermore, a bicistronic vector was generated to constitutively express CD40L on CD19-targeting CAR T cells. CD40L expression increased the CAR T cell cytotoxicity in vitro and prolonged survival of DOHH2 lymphoma-bearing mice. CAR T cells armored with CD40L expression is a promising approach to increase the antitumor efficacy of CAR T cell therapy.
The costimulatory receptor 4-1BB is also a type II transmembrane protein and TNF receptor superfamily member (Schwarz, Tuckwell, & Lotz, 1993). It is expressed on activated T cells and binding of 4-1BB ligand (4-1BBL) increases T cell proliferation, cytokine secretion and cytolytic capacity (Shuford et al., 1997; Takahashi, Mittler, & Vella, 1999). 4-1BB plays a role in maintenance of CD8+ T cell memory (Sabbagh, Snell, & Watts, 2007). In addition to T cells, 4-1BB has been found on other lymphoid cells, monocytes, DCs and other non-hematological cells, but not on cancer cells (Drenkard et al., 2007; Melero, Johnston, Shufford, Mittler, & Chen, 1998; Schwarz, Valbracht, Tuckwell, von Kempis, & Lotz, 1995; von Kempis, Schwarz, & Lotz, 1997; Wilcox et al., 2002; Zhang et al., 2010). DCs are activated by 4-1BBL binding to 4-1BB and secrete pro-inflammatory cytokines, such as IL-12 and IL-6 (Futagawa et al., 2002). To provide 4-1BB costimulation to CAR T cells and the tumor microenvironment, two pre-clinical studies have investigated the effects of armored CAR T cells with constitutive 4-1BBL expression on CAR T cell activity and antitumor efficacy.
Stephan et al. (2007) transduced PSMA-targeting first generation CAR T cells (PSMA/CD3ζ, Pz1) with CD80 and/or 4-1BBL. Constitutive coexpression of CD80 and 4-1BBL on Pz1 CAR T cells showed increased proliferation upon stimulation with PSMA+CD80−CD86−4-1BBL−LNCaP prostate cancer cells. This increase was more modest when CD80 and 4-1BBL were expressed on the cancer cells instead of on the CAR T cells. CAR T cells armored with both CD80 and 4-1BBL were able to eradicate PSMA+ tumors in vivo, whereas CAR T cells expressing either CD80 or 4-1BBL showed no benefit compared to Pz1 CAR T cells alone. Furthermore, the investigators were able to show that coexpression of CD80 and 4-1BBL provides costimulation to the CAR T cell itself and, importantly, transcostimulation to other tumor-reactive T cells, not expressing CD80 or 4-1BBL. This transcostimulation may enhance the antitumor response of TILs that are reactive to other TAAs, thereby broadening the antitumor immune response and reducing the chance of tumor antigen escape.
In order to elucidate optimal costimulatory signaling in CD19-targeting CAR T cells, Zhao et al. (2015) investigated various combinations of CD28 and 4-1BB receptors and their respective ligands CD80 and 4-1BBL. The initial comparison between second generation CAR T cells with either CD28 (1928z) or 4-1BB (19BBz) costimulatory domains showed different kinetics for CAR T cell proliferation and antitumor efficacy. Four different possibilities to combine CD28 and 4-1BB signaling in the CAR T cells were investigated, where the receptors are incorporated in the CAR molecule and the ligands coexpressed: 1928BBz, 1928z/4-1BBL, 19BBz/CD80 and 19z1/CD80/4-1BBL. Of these, 4-1BBL-armored 1928z CAR T cells showed the highest CD8/CD4 ratio and the best in vivo antitumor activity, which was in part mediated by IRF7, a transcription factor for type I interferons. The resulting increased IFNβ production could potentially increase antigen cross-presentation on intratumoral DCs (Yang et al., 2014), disrupt the tumor vasculature (Spaapen et al., 2014) and inhibit activation and proliferation of Tregs (Srivastava, Koch, Pepper, & Campbell, 2014). The authors hypothesize that together with transcostimulation in the tumor microenvironment (discussed above (Stephan et al., 2007)) this may be responsible for the increased antitumor activity of 1928z/4-1BBL armored CAR T cells. Together, these studies demonstrate how smart design of engineered costimulation has a big impact on behavior and antitumor efficacy of CAR T cells.
3.4. Armored CAR T cells to overcome an inhibitory tumor microenvironment
CAR T cell antitumor activity may be limited by an immune suppressive TME; not only through inhibitory cell types, such as MDSCs and Tregs (which may be targeted by IL-12, see 2.1), but also the expression and stimulation of inhibitory receptors. Normally, activated T cells express these checkpoint proteins, such as PD-1, CTLA-4, T-cell membrane protein 3 (TIM-3) and lymphocyte activating gene 3 (LAG-3), to control T cell activation and prevent autoimmunity, and are therefore often used as markers of T cell exhaustion. Cancer cells frequently express the PD-1 ligands PD-L1 and PD-L2, thereby preventing an effective anti-tumor immune response. PD-L1 can also bind to CD80, and thus prevent it from binding to CD28, adding another layer to T cell inhibition. Antibodies that block CTLA-4 (ipilimumab) or PD-1 (nivolumab and pembrolizumab) have been successful in patients with metastatic melanoma (Larkin et al., 2015; Wolchok et al., 2013) and non-small cell lung cancer (Borghaei et al., 2015; Brahmer et al., 2015; Garon et al., 2015) and are being tested in many other cancer types.
In syngeneic pre-clinical models, adoptively transferred T cells show upregulation of PD-1, TIM-3 and LAG-3 and combining cell therapy with PD-1 blockade increases antitumor efficacy of CAR T cells (John et al., 2013) and TILs (Moon et al., 2016). Important results are expected from the first clinical trial combining CAR T cells with CTLA-4 blockade (NCT00586391). A recent case study reported a patient with PD-L1+ diffuse large B cell lymphoma who progressed after CAR T cell therapy and received pembrolizumab 26 days after CAR T cell infusion (Chong et al., 2017). PD-1 blockade led to an increase in circulating CAR T cells and reduced size of tumor lesions. Although it is unclear whether this was mediated by CAR T cells or endogenous TILs or both, it is a promising response and a phase I/II clinical trial has been initiated for pembrolizumab treatment in CD19 CAR T-cell refractory or relapsing patients (NCT02650999).
Treatment with checkpoint inhibitors can cause severe immune-related adverse events, which can be treated with steroids or anti-TNFα antibodies (Michot et al., 2016). Even though treatment of the adverse events does not affect treatment efficacy or overall survival (Horvat et al., 2015), local checkpoint blockade might be able to prevent systemic side effects and further improve CAR T cell efficacy. Several approaches have been developed to prevent inhibitory signaling via the PD-1 receptor on CAR T cells. Secretion of anti-PD-L1 antibodies by armored CAR T cells against carbonic anhydrase IX (CAIX) increases the antitumor CAR T cell efficacy in an orthotopic xenograft model (Suarez et al., 2016). Cherkassky et al. (2016) used an anti-mesothelin CAR in an orthotopic pleural mesothelioma model and tested CAR T cells with either CD28 (M28z) or 4-1BB (MBBz) costimulation, of which the latter performed better than M28z at lower doses. M28z CAR T cells showed stronger PD-1 upregulation in vivo and they used three different approaches to disrupt PD-1-mediated CAR T cell inhibition. Both systemic administration of an αPD-1 antibody and especially coexpression of a dominant negative PD-1 receptor, which binds to PD-L1 but does not signal, improved tumor control by CAR T cells. Thirdly, coexpression of a PD-1-targeting short hairpin RNA improved CAR T cell function in vitro, but not in vivo, possibly due to incomplete knockdown of PD-1. In addition, disruption of PD-1 with the CRISPR/Cas9 system enhanced antitumor activity of PSCA CAR T cells and also in CD19 CAR T cells with additional disruption of TCR and beta-2-microglobulin (required for HLA class I expression) to allow for allogeneic use of the CAR T cells (Ren et al., 2016).
Another approach to not only abolish PD-1-mediated inhibition, but even turn inhibitory PD-1 signaling into a pro-inflammatory signal, Liu et al. (2016) coexpressed a “switch receptor” with the PD-1 extracellular domain and CD28 intracellular domain (Prosser, Brown, Shami, Forman, & Jensen, 2012) in CAR T cells. In two different solid tumor models PD-1/CD28-armored CAR T cells with 4-1BB costimulation showed increased antitumor activity and less exhaustion, compared to mice that received CAR T cells plus the PD-1 inhibitor pembrolizumab, or armored CAR T cells with a truncated non-functional PD-1.
Together, these studies demonstrate an increased CAR T cell antitumor activity when the PD-1/PD-L1 inhibitory axis is blocked or converted into a pro-inflammatory T cell signal. Most approaches, except the secretion of an anti-PD-L1 antibody (Suarez et al., 2016), do not affect inhibitory checkpoint signaling on endogenous TILs. Secreting a checkpoint inhibitor in the local tumor microenvironment might trigger a broader antitumor T cell response and increase efficacy of the CAR T cell therapy. Thus far, targeting other checkpoint proteins, such as CTLA-4, LAG-3 or TIM-3, have not been studied in the context of CAR T cell therapy. Dual blockade of PD-1 and a second checkpoint protein, however, might further optimize therapy as upregulation of TIM-3 has been demonstrated on PD-1-blocked T cells in murine and human lung tumors that acquired resistance to anti-PD-1 therapy (Koyama et al., 2016).
4. Other applications of armored CAR T cells
4.1. Armored CAR T cells to improve trafficking to tumor
In order to reach their full potential of killing tumor cells, CAR T cells have to migrate to and infiltrate into the tumor tissue. Especially for solid tumors, CAR T cell infiltration can present a major obstacle, as endogenous T cells are often excluded from the tumor microenvironment as well (Joyce & Fearon, 2015). Local delivery of CAR T cell can greatly enhance their therapeutic efficacy (Adusumilli et al., 2014). This is, however, not feasible for all tumor sites and metastatic lesions. To enhance CAR T cell migration to the tumor several studies have expressed receptors of tumor-secreted chemokines on CAR T cells to enhance migration to the tumor and efficacy of CAR T cell therapy.
Di Stasi et al. (2009) showed that Reed-Stemberg cells of Hodgkin lymphoma produce CCL17 and CCL22. These bind to CCR4, which is expressed by Tregs and Th2 cells, but not by activated cytotoxic T cells. Co-expression of CCR4 on CD30-targeting CAR T cells improved CAR T cell migration to the tumor site and antitumor efficacy. A similar increase in CAR T cell infiltration and antitumor activity has been demonstrated for CCR2b expression in anti-GD2 (Craddock et al., 2010) and anti-mesothelin (Moon et al., 2011) CAR T cells and for CXCR2 expression in pmel-1 T cells (Peng et al., 2010). Collectively, these pre-clinical findings indicate that CAR T cell trafficking to the tumor can be enhanced through engineered expression of tumor-associated chemokine receptors.
4.2. CAR T cells as micropharmacies
As discussed above, CAR T cells can be engineered to express anything to stimulate an antitumor T cell function or manipulate the tumor microenvironment. In addition, the tumor-specific nature of CAR T cell therapy can be used to deliver molecules that target the tumor cells directly. Boice et al. (2016) found that in follicular lymphomas (FL) the interaction between the tumor suppressor HVEM and BTLA is frequently lost due to loss of one of the two molecules, leading to B cell proliferation, accompanied by an increase in BTLA-expressing follicular helper T cells. Treatment with soluble HVEM ectodomain protein (solHVEM) in HVEM-deficient FL activated the inhibitory BTLA receptor and reduced proliferation of lymphoma cells in vitro. Intratumoral injections of solHVEM in subcutaneous lymphomas inhibited tumor growth in vivo, but less so with 10-fold more systemic solHVEM administration. To address this limitation, the investigators generated CD19 CAR T cells that secrete solHVEM and showed reduced tumor growth compared to non-solHVEM-producing CAR T cells. This indicates that CAR T cells can be used for local delivery of tumor-targeting factors to increase the antitumor efficacy of both CAR T cell therapy and targeted cancer therapy.
5. Concluding remarks
Clinical studies have demonstrated great potential of CAR T cell therapy, especially in B-ALL. Further improving the antitumor activity of CAR T cells, however, will be important to be successful in other hematologic and solid malignancies. The generation of armored CAR T cells is a powerful strategy to achieve local expression and secretion of a variety of molecules to stimulate CAR T cell function, manipulate tumor-infiltrating immune cells and target the tumor cells directly. Local production of cytokines and checkpoint inhibitors is expected to reduce side effects that are observed with systemic administration. Synthetic molecules such as the CAR itself, chimeric costimulatory receptors and synNotch increase tumor specificity and reduce antigen escape. Furthermore, genetic engineering of chimeric receptors allows for converting inhibitory signals into a stimulating response and vice versa, as discussed for the iCAR, switch receptor and chimeric cytokine receptors, thereby increasing tumor specificity, safety and reducing immune suppressive signaling. Pre-clinical studies in syngeneic tumor models will help to elucidate which of the discussed strategies will be optimal, which likely differs per target antigen and tumor (sub)type. Ultimately, clinical trials with armored CAR T cells will provide crucial information to make CAR T cell therapy more safe and effective in a broad range of malignancies.
Abbreviations
- 28z
CD28 + CD3ζ
- 4-1BBL
4-1BB ligand
- B-ALL
B cell acute lymphatic leukemia
- BBz
4-1BB + CD3ζ
- CAR
chimeric antigen receptor
- CD40L
CD40 ligand
- CRS
cytokine release syndrome
- CTLA-4
cytotoxic T-lymphocyte associated protein 4
- DCs
dendritic cells
- GVHD
graft-versus-host disease
- iCAR
inhibitory chimeric antigen receptor
- iCasp9
inducible caspase 9
- IL
interleukin
- NFAT
nuclear factor of activated T cells
- NK cells
natural killer cells
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death 1 ligand 1
- PSCA
prostate stem cell antigen
- PSMA
prostate specific membrane antigen
- TAA
tumor-associated antigen
- TILs
tumor-infiltrating lymphocytes
- Tregs
regulatory T cells
Footnotes
Conflict of interest statement
R.J.B. is a co-founder, stockholder and consultant for Juno Therapeutics Inc. The authors declare no other conflicts of interest.
References
- Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Science Translational Medicine. 2014;6(261):261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunology Research. 2014;2(2):112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, O’Hara M. Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps. Pharmacology & Therapeutics. 2016;166:30–39. doi: 10.1016/j.pharmthera.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Flowers ME, Warren EH, Riddell SR. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107(6):2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra R, Gigley JP, Khan IA. Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. Journal of Immunology. 2011;187(9):4421–4425. doi: 10.4049/jimmunol.1102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice M, Salloum D, Mourcin F, Sanghvi V, Amin R, Oricchio E, et al. Loss of the HVEM tumor suppressor in lymphoma and restoration by modified CAR-T cells. Cell. 2016;167(2):405–418. e13. doi: 10.1016/j.cell.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276(5319):1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. The New England Journal of Medicine. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297(5589):2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. The New England Journal of Medicine. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science Translational Medicine. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde LE, Berger C, Lin Y, Wang J, Lin X, Frayo SE, et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PloS One. 2013;8(12):e82742. doi: 10.1371/journal.pone.0082742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of inter-leukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. The Journal of Experimental Medicine. 1996;184(2):747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nature Reviews Immunology. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. The Journal of Clinical Investigation. 2016;126(8):3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clinical Cancer Research. 2012;18(6):1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Research. 2011;71(17):5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine B, et al. PD-1 blockade modulates chimeric antigen receptor (CAR) modified T cells and induces tumor regression: Refueling the CAR. Blood. 2017;129(8):1039–1041. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. Journal of Clinical Oncology. 2015;33(1):74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. Journal of Immunotherapy. 2010;33(8):780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran KJ, Seinstra BA, Nikhamin Y, Yeh R, Usachenko Y, van Leeuwen DG, et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Molecular Therapy. 2015;23(4):769–778. doi: 10.1038/mt.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science Translational Medicine. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila ML, Sadelain M. Biology and clinical application of CAR T cells for B cell malignancies. International Journal of Hematology. 2016;104(1):6–17. doi: 10.1007/s12185-016-2039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113(25):6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi A, Tey S-K, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. The New England Journal of Medicine. 2011;365(18):1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenkard D, Becke FM, Langstein J, Spruss T, Kunz-Schughart LA, Tan TE, et al. CD137 is expressed on blood vessel walls at sites of inflammation and enhances monocyte migratory activity. FASEB Journal. 2007;21(2):456–463. doi: 10.1096/fj.05-4739com. [DOI] [PubMed] [Google Scholar]
- Duong CP, Westwood JA, Berry LJ, Darcy PK, Kershaw MH. Enhancing the specificity of T-cell cultures for adoptive immunotherapy of cancer. Immunotherapy. 2011;3(1):33–48. doi: 10.2217/imt.10.81. [DOI] [PubMed] [Google Scholar]
- Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Science Translational Medicine. 2013;5(215):215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117–1127. doi: 10.1182/blood-2015-11-679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. International Immunology. 2002;14(3):275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- Gardner R, Wu D, Cherian S, Fang M, Hanafi L-A, Finney O, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged BALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406–2410. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England Journal of Medicine. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- Griffioen M, van Egmond EHM, Kester MGD, Willemze R, Falkenburg JHF, Heemskerk MHM. Retroviral transfer of human CD20 as a suicide gene for adoptive T-cell therapy. Haematologica. 2009;94(9):1316–1320. doi: 10.3324/haematol.2008.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England Journal of Medicine. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lym-phoblastic leukemia. Blood. 2013;121(7):1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk B, Liu K, Dudley ME, Johnson LA, Kaiser A, Downey S, et al. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Human Gene Therapy. 2008;19(5):496–510. doi: 10.1089/hum.2007.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde M, Corder A, Chow KKH, Mukherjee M, Ashoori A, Kew Y, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Molecular Therapy. 2013;21(11):2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. The Journal of Clinical Investigation. 2016;126(8):3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat TZ, Adel NG, Dang T-O, Momtaz P, Postow MA, Callahan MK, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with Ipilimumab at Memorial Sloan Kettering cancer center. Journal of Clinical Oncology. 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C, Hughes MS, Zheng Z, Bray RB, Rosenberg SA, Morgan RA. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cy-tokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. Journal of Immunology. 2005;175(11):7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C, Jones SA, Cohen CJ, Zheng Z, Kerstann K, Zhou J, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007;109(12):5168–5177. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudecek M, Schmitt TM, Baskar S, Lupo-Stanghellini MT, Nishida T, Yamamoto TN, et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116(22):4532–4541. doi: 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai C, Mihara K, Andreansky M, Nicholson IC, Pui C-H, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nature Reviews Clinical Oncology. 2016;13(6):370–383. doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby E, Nguyen SM, Fountaine TJ, Welp K, Gryder B, Qin H, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nature Communications. 2016;7:12320. doi: 10.1038/ncomms12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LB, Devaud C, Duong CPM, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clinical Cancer Research. 2013;19(20):5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science Translational Medicine. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. The Journal of Clinical Investigation. 2011;121(12):4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkar SP, Leonardi AJ, van Panhuys N, Zhang L, Yu Z, Crompton JG, et al. Collapse of the tumor stroma is triggered by IL-12 induction of Fas. Molecular Therapy. 2013;21(7):1369–1377. doi: 10.1038/mt.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Research. 2010;70(17):6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keu KV, Witney TH, Yaghoubi S, Rosenberg J, Kurien A, Magnusson R, et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Science Translational Medicine. 2017;9(373) doi: 10.1126/scitranslmed.aag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong A, Nelson DJ, Nowak AK, Lake RA, Robinson BWS. The use of agonistic anti-CD40 therapy in treatments for cancer. International Reviews of Immunology. 2012;31(4):246–266. doi: 10.3109/08830185.2012.698338. [DOI] [PubMed] [Google Scholar]
- Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nature Biotechnology. 2013;31(1):71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. Journal of Translational Medicine. 2015;13:102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4(3):e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with up-regulation of alternative immune checkpoints. Nature Communications. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy MQ, Jacobus S, Blood EA, Kay NE, Rajkumar SV, Greipp PR. Phase II study of interleukin-12 for treatment of plateau phase multiple myeloma (E1A96): A trial of the eastern cooperative oncology group. Leukemia Research. 2009;33(11):1485–1489. doi: 10.1016/j.leukres.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, et al. Chimeric antigen receptor T cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunology Research. 2013;1(1):43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. The New England Journal of Medicine. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Sukumaran S, Watanabe N, Mohammed S, Keirnan J, Yanagisawa R, et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Molecular Therapy. 2014;22(6):1211–1220. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90(7):2541–2548. [PubMed] [Google Scholar]
- Liu K, Rosenberg SA. Interleukin-2-independent proliferation of human melanoma-reactive T lymphocytes transduced with an exogenous IL-2 gene is stimulation dependent. Journal of Immunotherapy. 2003;26(3):190–201. doi: 10.1097/00002371-200305000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Research. 2016;76(6):1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nature Biotechnology. 2002;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England Journal of Medicine. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cellular Immunology. 1998;190(2):167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. European Journal of Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Minagawa K, Jamil MO, Al-Obaidi M, Pereboeva L, Salzman D, Erba HP, et al. In vitro pre-clinical validation of suicide Gene modified anti-CD33 redirected chimeric antigen receptor T-cells for acute myeloid leukemia. PloS One. 2016;11(12):e0166891. doi: 10.1371/journal.pone.0166891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EK, Carpenito C, Sun J, Wang L-CS, Kapoor V, Predina J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clinical Cancer Research. 2011;17(14):4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EK, Ranganathan R, Eruslanov E, Kim S, Newick K, O’Brien S, et al. Blockade of programmed death 1 augments the ability of human T cells engineered to target NY-ESO-1 to control tumor growth after adoptive transfer. Clinical Cancer Research. 2016;22(2):436–447. doi: 10.1158/1078-0432.CCR-15-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, et al. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell. 2016;164(4):780–791. doi: 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkiewicz PJ, Fräßle SP, Srivastava S, Sommermeyer D, Hudecek M, Drexler I, et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. The Journal of Clinical Investigation. 2016;126(11):4262–4272. doi: 10.1172/JCI84813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Ye Y, Rabinovich BA, Liu C, Lou Y, Zhang M, et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clinical Cancer Research. 2010;16(22):5458–5468. doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna SK, Pagliara D, Mahendravada A, Liu H, Brenner MK, Savoldo B, et al. Interleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T-cell inhibition. Clinical Cancer Research. 2014;20(1):131–139. doi: 10.1158/1078-0432.CCR-13-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Hwang W-T, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Science Translational Medicine. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England Journal of Medicine. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser ME, Brown CE, Shami AF, Forman SJ, Jensen MC. Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T cells modified to express a PD1: CD28 chimeric receptor. Molecular Immunology. 2012;51(3–4):263–272. doi: 10.1016/j.molimm.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GMP, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110(8):2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clinical Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Annals of Surgery. 1989;210(4) doi: 10.1097/00000658-198910000-00008. 474-484-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Sportès C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. Journal of Immunotherapy. 2006;29(3):313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164(4):770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, et al. Engineering T cells with customized therapeutic response programs using synthetic Notch receptors. Cell. 2016;167(2):419–432. e16. doi: 10.1016/j.cell.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. The Journal of Clinical Investigation. 2016;126(10):3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh L, Snell LM, Watts TH. TNF family ligands define niches for T cell memory. Trends in Immunology. 2007;28(8):333–339. doi: 10.1016/j.it.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Sakemura R, Terakura S, Watanabe K, Julamanee J, Takagi E, Miyao K, et al. A Tet-on inducible system for controlling CD19-chimeric antigen receptor expression upon drug administration. Cancer Immunology Research. 2016;4(8):658–668. doi: 10.1158/2326-6066.CIR-16-0043. [DOI] [PubMed] [Google Scholar]
- Schönbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cellular and Molecular Life Sciences. 2001;58(1):4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz H, Tuckwell J, Lotz M. A receptor induced by lymphocyte activation (ILA): A new member of the human nerve-growth-factor/tumor-necrosis-factor receptor family. Gene. 1993;134(2):295–298. doi: 10.1016/0378-1119(93)90110-o. [DOI] [PubMed] [Google Scholar]
- Schwarz H, Valbracht J, Tuckwell J, von Kempis J, Lotz M. ILA, the human 4-1BB homologue, is inducible in lymphoid and other cell lineages. Blood. 1995;85(4):1043–1052. [PubMed] [Google Scholar]
- Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. The Journal of Experimental Medicine. 1997;186(1):47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discovery. 2015;5(12):1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaapen RM, Leung MYK, Fuertes MB, Kline JP, Zhang L, Zheng Y, et al. Therapeutic activity of high-dose intratumoral IFN-β requires direct effect on the tumor vasculature. Journal of Immunology. 2014;193(8):4254–4260. doi: 10.4049/jimmunol.1401109. [DOI] [PubMed] [Google Scholar]
- Sportès C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. The Journal of Experimental Medicine. 2008;205(7):1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Koch MA, Pepper M, Campbell DJ. Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. The Journal of Experimental Medicine. 2014;211(5):961–974. doi: 10.1084/jem.20131556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nature Medicine. 2007;13(12):1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105(11):4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez ER, Chang DK, Sun J, Sui J, Freeman GJ, Signoretti S, et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7(23):34341–34355. doi: 10.18632/oncotarget.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. Journal of Immunology. 1999;162(9):5037–5040. [PubMed] [Google Scholar]
- Turtle CJ, Hanafi L-A, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Science Translational Medicine. 2016;8(355):355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stegen SJC, Davies DM, Wilkie S, Foster J, Sosabowski JK, Burnet J, et al. Preclinical in vivo modeling of cytokine release syndrome induced by ErbB-retargeted human T cells: Identifying a window of therapeutic opportunity? Journal of Immunology. 2013;191(9):4589–4598. doi: 10.4049/jimmunol.1301523. [DOI] [PubMed] [Google Scholar]
- van der Stegen SJC, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nature Reviews Drug Discovery. 2015;14(7):499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herpen CM, Looman M, Zonneveld M, Scharenborg N, de Wilde PC, van de Locht L, et al. Intratumoral administration of recombinant human interleu-kin 12 in head and neck squamous cell carcinoma patients elicits a T-helper 1 profile in the locoregional lymph nodes. Clinical Cancer Research. 2004;10(8):2626–2635. doi: 10.1158/1078-0432.ccr-03-0304. [DOI] [PubMed] [Google Scholar]
- van Schalkwyk MCI, Papa SE, Jeannon J-P, Guerrero Urbano T, Spicer JF, Maher J. Design of a phase I clinical trial to evaluate intratumoral delivery of ErbB-targeted chimeric antigen receptor T-cells in locally advanced or recurrent head and neck cancer. Human Gene Therapy Clinical Development. 2013;24(3):134–142. doi: 10.1089/humc.2013.144. [DOI] [PubMed] [Google Scholar]
- Vera JF, Hoyos V, Savoldo B, Quintarelli C, Giordano Attianese GMP, Leen AM, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Molecular Therapy. 2009;17(5):880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108(12):3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler I, Newrzela S, Hartmann S, Schneider N, von Laer D, Koehl U, et al. An improved bicistronic CD20/tCD34 vector for efficient purification and in vivo depletion of gene-modified T cells for adoptive immunotherapy. Molecular Therapy. 2010;18(7):1330–1338. doi: 10.1038/mt.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kempis J, Schwarz H, Lotz M. Differentiation-dependent and stimulus-specific expression of ILA, the human 4-1BB-homologue, in cells of mesenchymal origin. Osteoarthritis and Cartilage. 1997;5(6):394–406. doi: 10.1016/s1063-4584(97)80044-1. [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: Implications for immunotherapy. Immunity. 2001;14(2):105–110. [PubMed] [Google Scholar]
- Wang X, Chang W-C, Wong CW, Colcher D, Sherman M, Ostberg JR, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118(5):1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Moriarty KE, Baio FE, Chu F, Kim S-D, He J, et al. IL-15 enhances the antitumor effect of human antigen-specific CD8+ T cells by cellular senescence delay. OncoImmunology. 2016;5(12):e1237327. doi: 10.1080/2162402X.2016.1237327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood. 2000;96(9):2917–2924. [PubMed] [Google Scholar]
- Wilcox RA, Chapoval AI, Gorski KS, Otsuji M, Shin T, Flies DB, et al. Cutting edge: Expression of functional CD137 receptor by dendritic cells. Journal of Immunology. 2002;168(9):4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- Wilkie S, Burbridge SE, Chiapero-Stanke L, Pereira ACP, Cleary S, van der Stegen SJC, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. The Journal of Biological Chemistry. 2010;285(33):25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie S, van Schalkwyk MCI, Hobbs S, Davies DM, van der Stegen SJC, Pereira ACP, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. Journal of Clinical Immunology. 2012;32(5):1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England Journal of Medicine. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-J, Song D-G, Poussin M, Ye Q, Sharma P, Rodríguez-García A, et al. Multiparameter comparative analysis reveals differential impacts of various cytokines on CART cell phenotype and function ex vivo and in vivo. Oncotarget. 2016;7(50):82354–82368. doi: 10.18632/oncotarget.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, et al. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell. 2014;25(1):37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zah E, Lin M-Y, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunology Research. 2016;4(6):498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Molecular Therapy. 2011;19(4):751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Morgan RA, Beane JD, Zheng Z, Dudley ME, Kassim SH, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clinical Cancer Research. 2015;21(10):2278–2288. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Voskens CJ, Sallin M, Maniar A, Montes CL, Zhang Y, et al. CD137 promotes proliferation and survival of human B cells. Journal of Immunology. 2010;184(2):787–795. doi: 10.4049/jimmunol.0901619. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28(4):415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Reviews Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]