Abstract
Background
Despite significant advances in type 1 diabetes (T1D) management, achieving targeted glycemic control in pediatric patients remains a struggle. Continuous glucose monitoring (CGM) with remote access holds the promise to address this challenge by allowing caregivers to monitor glucose, even when the child is not directly under their supervision.
Objective
To explore real-time and remote CGM practices in homes and schools, including caregiver expectations regarding this technology.
Subjects
Parents and daytime caregivers.
Methods
Respondents answered an anonymous survey assessing characteristics of CGM use. Cross-sectional data were collected and analyzed using quantitative and qualitative methods.
Results
Thirty-three parents and 17 daytime caregivers responded. Threshold alerts (alerts when patients reached certain pre-set high or low limits) were used most frequently, followed by rate of change alerts. Most parents and daytime caregivers responded to low and high threshold CGM alerts by confirming with a glucose meter prior to treatment; while about one-third endorsed treating lows without a confirmatory test. Most parents expected their child’s daytime caregiver to respond to CGM alerts and daytime caregivers felt the parent’s expectations of them were reasonable. All parents and most caregivers reported decreased overall worry/stress. Parents felt positive about CGM use and daytime caregivers felt comfortable with CGM.
Conclusion
The positive and collaborative management reported by parents and daytime caregivers sets the stage for CGM to play an important role in the management of children with T1D both in the home and school settings.
Keywords: Type 1 diabetes, school, continuous glucose monitor
Introduction
Despite significant advances in management of type 1 diabetes (T1D), most youth with the condition fail to meet prescribed glycemic targets (1, 2). While the ultimate goal of achieving target hemoglobin A1c (HbA1c) levels through intensive treatment is to minimize the risk of micro and macrovascular complications, many patients and families struggle to implement the prescribed, rigorous treatment regimen into their daily routine (3). Mothers of children with T1D believe the practice of “constant vigilance” is required for diabetes management in order to identify behaviors in their children indicative of hypoglycemia or hyperglycemia (4). However, the psychological distress experienced by parents of children with T1D who are striving to achieve optimal control of their child’s diabetes often negatively affects the family’s quality of life (3), leaving caregivers and medical providers searching for ways to improve management.
Real-time continuous glucose monitoring (CGM) is the newest in an arsenal of tools aimed at improving the care of individuals living with T1D. Use of CGM has been shown to lower HbA1c levels in youth with T1D (5) if the devices are used on a nearly daily basis (6), but few families with children with T1D were able to maintain such a high frequency of use in early clinical trials of this technology (7, 8). Moreover, initial enrollment data in the T1D Exchange Clinic Registry indicated limited use of CGM in clinical practice, especially in children (9). However, more recent studies have indicated that advances in CGM technology over the past few years, including longer wear time, improved accuracy, and remote monitoring capabilities, have resulted in a 2–3 fold increase in CGM use in pre-school, school-aged, and adolescent patients (2, 10).
While the increased pediatric use and remote monitoring capabilities of current CGM systems has led to incorporation of these devices into the classroom environment, current practices involving CGM use in schools in the US, particularly CGM with remote monitoring capabilities, have not been described. Hence, this investigation sought to examine the real-time and remote CGM practices at home and in schools, attitudes regarding its use, and the expectations of parents and caregivers in using this diabetes management tool. This information is critical as diabetes technology will continue to evolve in the coming years and use of these devices will undoubtedly increase.
METHODS
Participants
Parents of pediatric patients with T1D who attend the Yale Children’s T1D Clinic were invited to participate in the study, if their child was currently using or had previously worn a CGM. The only exclusion criterion was inability to read or write English. Patient age, current HbA1c level and frequency of CGM use did not affect participation in the study. The protocol was approved by the institutional review board at Yale University.
Procedures
Participants were asked to complete a survey assessing the use of CGM devices and remote monitoring, as well as their reasons, goals, attitudes and expectations of CGM use. Participants were also asked to distribute a separate survey to their child’s daytime caregiver (i.e. school nurse, daycare teacher, nanny). The surveys collected limited demographic data to provide anonymity to encourage open and truthful responses. Daytime caregivers anonymously returned the surveys directly to the research team using pre-paid envelopes. Parents returned surveys using the above method or anonymously via a collection box if completed in person.
Measures
Parent surveys collected information regarding CGM use, alert settings and responses, frequency of real-time and retrospective sensor glucose review, and expectations of daytime caregivers. Open response answers assessed parents’ reasons for and goals of CGM use, and feelings regarding both CGM use and remote monitoring.
Daytime caregiver surveys collected data on alert responses, frequency of visualizing CGM trends, and use of remote monitoring. Comfort using CGM technology was assessed using a 10-point Likert Scale (1 = not at all comfortable, 10 = extremely comfortable). Open response questions asked daytime caregivers to describe their responsibilities regarding CGM alerts, tracings, and T1D management decisions, as well as their overall feelings about CGM and remote monitoring.
Parent surveys included 15 multiple choice and 9 open response questions. Daytime caregiver surveys included 10 multiple choice and 8 open response questions. The full surveys can be found in the supplementary materials.
Data Analyses
A mixed methods analysis was used to examine the data obtained. Characteristics of the cohort and CGM use were described using frequency, median, and mean. Spearman correlation was used to assess whether there was a relationship between the child’s age or number of students in a school and the comfort level with CGM expressed by the daytime caregiver. Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA).
For the qualitative analysis, code structure was accomplished using the Grounded Theory Approach (11). Briefly, a team of three researchers assigned themes to open response survey answers which reflected common concepts that emerged. Another researcher from our study group reviewed the theme categorizations assigned to each open response question for consistency.
RESULTS
Participants/Patients
Fifty-seven survey pairs were distributed, and 33 parent surveys and 17 daytime caregiver surveys were returned to the research team. The mean age of the children cared for by the respondents was 9.1 ± 4.0 years (range 2–17); 21 parents cared for elementary school age children (≤10 years) and 11 cared for middle or high school age (11–17 years) youth. Thirty-two patients wore Dexcom® G4 or G5 sensors (Dexcom, Inc. San Diego, CA) and one patient wore a Medtronic Enlite® Sensor (Medtronic, Inc. Northridge, CA). Frequency of sensor use was very high with 94% of respondents stating their child used the sensor 7 days a week. The duration of CGM use ranged from 2 weeks (0.5 months) to 7 years (84 months); the mean duration of use was 1.78 years (21 ±20.7 months). Twenty-three (68%) of the parents reported using remote monitoring. Parental use of remote monitory did not significantly correlate with the age of the child. However, use of remote monitoring was more common in elementary school children (age ≤10 years) (81%) as compared to those in middle school and beyond (50%).
CGM and Remote Monitor Settings, Use, and Responses
As demonstrated in Figure 1, the use of both high and low threshold alerts as well as rate of change alerts was consistent between the CGM device and remote monitor. The use of threshold alerts was nearly two times more common than rate of change alerts, a trend that remained consistent with remote monitoring.
Figure 1.
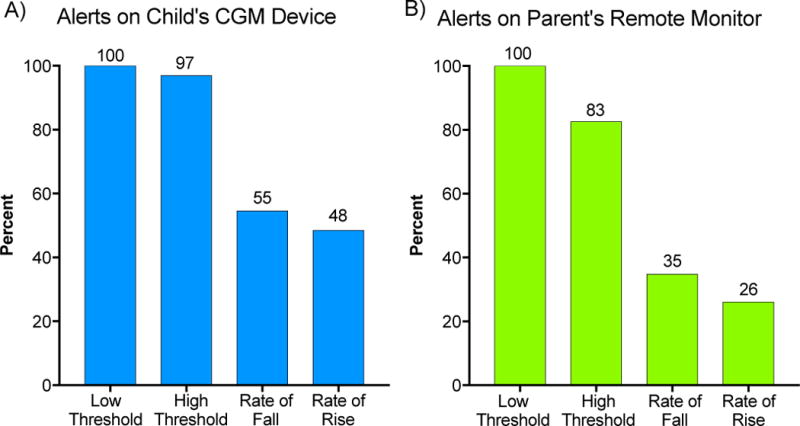
Alert settings on child’s CGM (A) and parent’s remote monitor (B) based on parental survey responses.
Both parents and daytime caregivers typically responded to high and low glucose alerts, by first checking the SMBG before treating (Figure 2). However, 39% of parents and 35% of caregivers indicated that they also treated lows without checking blood glucose levels, depending on the clinical situation. Additionally, more than one third of daytime caregivers reported contacting the child’s parent if a low or high glucose alert occurred.
Figure 2.
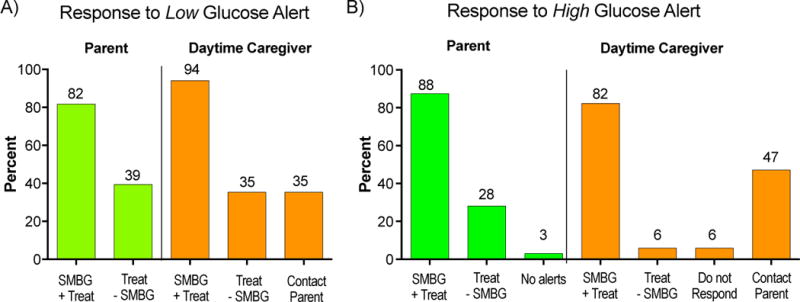
Parental and Daytime Caregiver responses to low (A) and high (B) glucose alerts. Self-monitored blood glucose (SMBG) + Treat= confirm blood glucose by a (SMBG) test before treatment with glucose or insulin. Treat - SMBG= Treatment with glucose or insulin without confirmatory SMBG.
CGM data were primarily used in real-time and not for retrospective review (Figure 3), with 56% of parents reporting checking the remote monitor either constantly or hourly. In contrast, only 38% reported retrospective review of uploaded data more than quarterly.
Figure 3.
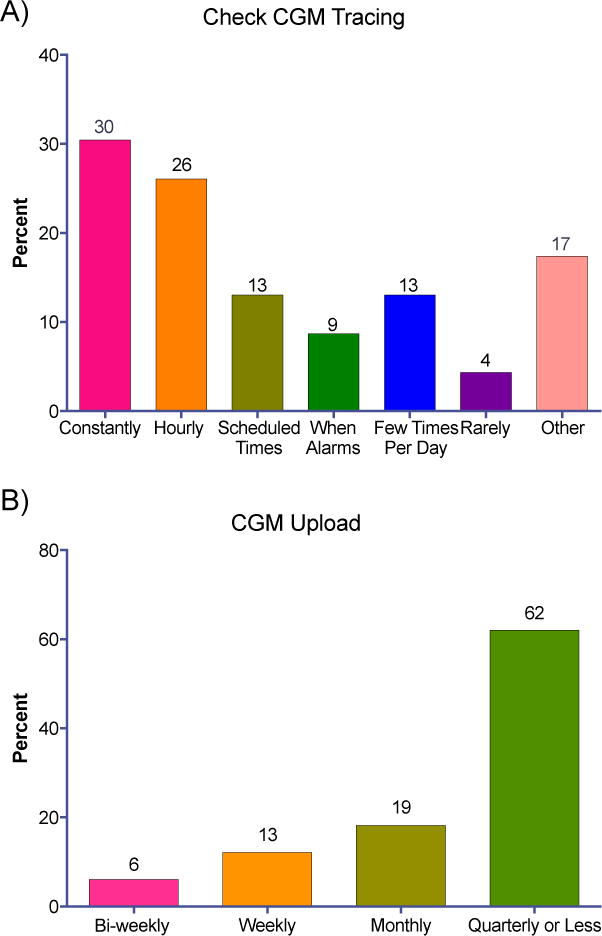
Frequency with which parents check remote monitor CGM tracing daily (A) and upload CGM for dose adjustment (B).
Eighty-five percent of parents expected their child’s daytime caregiver to respond to CGM alerts, and 61 % felt the caregiver should use the CGM data to make decisions. The majority (65%) of parents indicated that they wanted their child’s caregiver to be in contact with them in responding to CGM alerts. Eighty-nine percent of daytime caregivers felt the parents’ expectations on how they should use CGM data were reasonable. All parents surveyed and 78% of caregivers reported that use of the system decreased their worry or stress. When asked about their comfort level with using CGM technology, daytime caregivers reported an average comfort level of 7.8 (range 5–10) on a 10 point Likert scale. The median number of students to daytime caregivers was reported to be 455 (Interquartile Range: 301, 611). There was no significant relationship between CGM comfort and number of children for whom the daytime caregiver was responsible (r=0.07, p=0.80) or the age of the child (r=-0.15, p=0.59).
Qualitative Findings
Review of open-ended responses led to the emergence of 9 themes that fell into two categories: 1) Overall Feelings of CGM and 2) Reasons for and Goals of CGM (Table 1).
Table 1.
Themes that emerged from open-ended responses from parents and daytime caregivers.
| Overall Feeling of CGM and Remote Monitoring | Reasons for and Goals of CGM and Remote Monitoring |
|---|---|
| Overall Positive Feelings and Satisfaction with CGM | Effective Diabetes Management/Data |
| Life Changing | Access to Real-time Monitoring |
| Peace of Mind and Sense of Security | Ability to See Blood Glucose Trends |
| Increase in Child’s Independence | Address Concerns About Overnight High and Low Blood Glucose |
| Address Concerns About School/Out of Parent Care |
Overall Feelings of CGM
A recurrent opinion expressed by parents was that CGM with remote monitoring brought them peace of mind and a sense of security. The response from one parent indicated that this was true for both she and her daughter: “Not only has it given me more peace of mind…it has given her peace of mind to know that someone else is watching and worrying about her blood sugar so she doesn’t have to. She can be a carefree kid again (kind of).” Even when parents noted the technical difficulties in using CGM, they indicated that the benefits of current devices outweighed the hassles of using them: “Although the CGM poses some problems - twice I’ve had to send [it] back … and sometimes the CGM reading is pretty off from the actual BG. However, the CGM gives me a sense of peace and empowerment. I feel I can better care for my daughter.” Additionally, many described use of the CGM and remote monitor as life changing, “It is amazing how much it has changed our lives. Our child has so much more freedom now!”
Parental responses also highlighted how use of these systems allowed their children to participate in more activities and to be more independent: “CGM is a powerful tool in managing my child’s diabetes…CGM allows her to have more independence with her peers. Safety for time away is imperative in her lifelong care. Love it!”
Parents also commented on their role in remote monitoring alongside a secondary caregiver, stating “I think its helpful for babysitters and home caregivers. At school, I leave it to the school nurse because she takes excellent care of my child and we touch base daily.” Another parent noted “I like the layer of awareness it gives to others. If his alarm for a low goes off on my phone while he is at school, I can see if he is being addressed.”
Reasons for and Goals of Remote Monitoring
In the 23 parents who used CGM on a nearly daily basis, the integral role that CGM data could play in achieving targeted glycemic control was commonly endorsed: “It (CGM and remote monitoring) is essential to diabetes care and should be provided to all patients (with T1D).” A daytime caregiver stated: “I feel quite comfortable doing daily tasks along with the CGM. It gives me a good idea of where the student is and what I need to do next.”
Use of real time monitoring at school was a common reason parents initiated this technology. They noted that CGM with remote monitoring would benefit “us and school nurses” and helped to address “concern[s] about school and nights.” The importance of real-time alerts was also key among respondents: “[I use CGM] to alert me to my child’s highs and lows, and to try to stabilize her BG before she gets too high or too low.” Daytime caregivers also found that the detection of blood glucose trends was helpful: “[CGM] helps me to plan our day and keep an eye on what we can do next.” Another daytime caregiver remarked, “I actually seem to rely on the CGM. I feel it is a better way to manage the blood glucose.” Parents also found that the use of CGM and remote monitoring allowed them to address concerns with overnight highs/low and concerns when the child was outside of their care.
Discussion
The overarching outcome of this study of CGM and remote monitoring in children and adolescents with T1D who wear CGM regularly, was congruency of care and positive attitudes towards this technology, which was expressed by both parents and daytime caregivers. Indeed, despite the rigors of implementing management with new technology, both parents and caregivers were satisfied with the use of CGM in and out of the home and felt that these systems helped to decrease stress and worry. Importantly, many of the daytime caregivers felt that use of these systems allowed them to work in a collaborative manner with parents to provide intensive diabetes management.
Both parents and daytime caregivers had similar strategies for managing CGM alerts, with the vast majority indicating that they confirmed sensor alerts by performing SMBG prior to treatment, reflecting that standard education provided prior to FDA approval of non-adjunctive CGM use (12) was followed in the outpatient home and school settings by those studied. On the other hand, more than one-third of parents and daytime caregivers also indicated that they treated a hypoglycemic alert without a confirmatory blood glucose measurement. It should be noted that all but one of the children in the study were using Dexcom sensors and that the FDA recently approved use of Dexcom G5 sensors to guide diabetes treatment decisions without confirmatory blood glucose measurements (12). This recommendation was based in great measure by the improved accuracy and precision of the current Dexcom sensor compared to earlier CGM devices. We expect that this action is likely to increase adoption of CGM in pediatric and adult patients with T1D, as well as improve the cost-effectiveness of using these devices (13).
Parents and daytime caregivers reported a high frequency of use of “High” and “Low’ threshold alerts. Use of these alerts to alter insulin delivery has the potential to mitigate glycemic variation and improve HbA1c, an important issue in school age children, who have more glucose variability than adults (14). On the other hand, both groups reported a much lower frequency of using rate of change alerts, which may be related to attempts to prevent alert fatigue or to limit the number of alert disturbances both in the classroom and overnight. Alternatively, parents and daytime caregivers may not have felt comfortable using these data to adjust insulin doses.
Even though disturbances due to CGM alerts at school might be a concern of school personnel, a study investigating teachers’ opinions of CGM use in the classroom found teachers felt CGM use in schools was not disruptive, but rather useful (15). The sentiments expressed by daytime caregivers in our study echo this finding. Parents and youth who are unsure about using CGM in the classroom may find relief in knowing teachers, school nurses, and daytime caregivers feel comfortable using CGM. Previous literature investigating T1D management in young children suggests each child should establish a diabetes health care plan for the school nurse or daytime caregiver to follow (16).
Responses from daytime caregivers illustrate devotion to ensuring safe and effective diabetes management during the school day, and demonstrate the collaborative management that can be achieved in youngsters while using CGM during the day. Furthermore, CGM can be used in large as well as small schools, with the median number of pupils under a daytime caregivers’ supervision being over 400 in this study. While the investigators anticipated that use of CGM might be perceived as burdensome to daytime caregivers, we were pleased that this concern was not supported by our findings. Instead, daytime caregivers’ comments highlighted that CGM use was beneficial and far exceeded the technical difficulties that may be encountered. As adoption of CGM into the treatment plan for youth living with T1D continues to increase, specific instructions in standardized school orders may need to be developed and implemented.
One clear area for improvement identified in the present study was the limited use of retrospective data review to make insulin dose adjustments. While most parents reported checking the remote monitor frequently in real time; fewer than 40% of parents reported uploading their child’s CGM more than quarterly. It is possible these quarterly uploads may represent those conducted during scheduled office visits, since our group sees patients in follow up at least every 3 months. Thus, it appears families would benefit from encouragement regarding the benefits of retrospective data review and guidance on how to interpret reports and adjust insulin doses.
The results of this study were limited by its relatively small sample size and response rate of 58% amongst parents and one-third of daytime caregivers, thus, it’s possible the study could be biased towards positive responses if those who were satisfied with CGM were more likely to return the surveys. Importantly, our respondents were extremely adherent to sensor technology, with nearly continuous sensor wear reported; thus interpretation must be used when extrapolating these results to those who utilize CGM less frequently. Our small sample size may have limited our ability to detect a relationship between remote CGM use and age of the child. To protect the anonymity of our study respondents and encourage uninhibited responses, demographic and other information by which participants could be identified, including HbA1c, were not collected. Therefore, although participants extolled the benefits of CGM, correlation with degree of glycemic control was not possible, nor could we confirm that the self-reported CGM use was accurate. However, the cross sectional data collected provides a basis on which to develop further hypotheses and interventions to improve glycemic control in children with T1D.
Previous studies have indicated that the benefits of CGM are directly correlated with the frequency of daily CGM use (16–18). In the JDRF CGM trial, durable improvements in metabolic control were only observed in the 21% of children and adolescents who used CGM on a nearly daily basis throughout the 12 months of the study (8). In contrast, in this study, nearly all respondents indicated that CGM was being used 7-days a week, with a mean duration of use of CGM of 21 months. Given the high frequency of use reported in this study, the sample may not represent the experiences of youth who use CGM fewer days per week. Though patient acceptance and usefulness of CGM may be a barrier to effective use (17), the improvements that have been made in the accuracy of CGM systems, ease of use of these devices, and ability to monitor data remotely, have contributed to the greater long-term commitment to this technology that families of children with T1D are beginning to exhibit.
With the rapid advancements in CGM technology, its increased use among children with T1D, and commercialization of a closed loop insulin delivery system, the demand to implement CGM in school environments will continue to rise quickly. The positive experiences of daytime caregivers who have integrated CGM technology into routine management and the congruency of parental and daytime caregiver responses to CGM alerts demonstrates the commitment of both groups in striving for the best care possible for youth with T1D. These findings support the theory that school personnel will be also be receptive to future technologies in the care of youth with T1D and will be willing to integrate them into school management plans.
Supplementary Material
Acknowledgments
The authors thank the parents and daytime caregivers who participated in this study, the health care professionals and staff of the Yale Children’s Diabetes Program. This publication was possible through the support of grants from the NIDDK Medical Student Research Program in Diabetes (5 T32 DK 7058-41), National Institutes of Health (K12-DK-094714), the Juvenile Diabetes Research Foundation (5-ECR-2014-112-A-N), the Yale CTSA grant UL1TR000142 from the National Center for Advancing Translational Science (NCATS), NIH and through the Stephen I. Morse Fund.
References
- 1.ADA. Children and adolescents. Sec. 11 In Standards of Medical Care in Diabetes. Diabetes Care. 2016;39(Supplement 1):S86–S93. [Google Scholar]
- 2.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current State of Type 1 Diabetes Treatment in the U.S.: Updated Data From the T1D Exchange Clinic Registry. Diabetes Care. 2015;38(6):971–8. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 3.Whittemore R, Jaser S, Chao A, Jang M, Grey M. Psychological experience of parents of children with type 1 diabetes: a systematic mixed-studies review. Diabetes Educ. 2012;38(4):562–79. doi: 10.1177/0145721712445216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan-Bolyai S, Deatrick J, Gruppuso P, Tamborlane W, Grey M. Constant vigilance: mothers’ work parenting young children with type 1 diabetes. J Pediatr Nurs. 2003;18(1):21–9. doi: 10.1053/jpdn.2003.4. [DOI] [PubMed] [Google Scholar]
- 5.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G. Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32(8):1378–83. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G. Beck RW, Buckingham B, Miller K, Wolpert H, Xing D, et al. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32(11):1947–53. doi: 10.2337/dc09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–76. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 8.Chase HP, Beck RW, Xing D, Tamborlane WV, Coffey J, Fox LA, et al. Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther. 2010;12(7):507–15. doi: 10.1089/dia.2010.0021. [DOI] [PubMed] [Google Scholar]
- 9.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA, et al. The T1D Exchange clinic registry. The Journal of clinical endocrinology and metabolism. 2012;97(12):4383–9. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 10.Miller KM, Foster NC, DeSalvo D, DiMeglio LA, Laffel L, Tamborlane W, et al. Continuous Glucose Monitoring (CGM) Use in Type 1 Diabetes: An Update from the T1D Exchange Clinic Registry; Presented at the International Society for Pediatric and Adolescent Diabetes (ISPAD) Meeting; Valencia, Spain. 2016. [Google Scholar]
- 11.Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758–72. doi: 10.1111/j.1475-6773.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. Web site 2017 [FDA letter regarding Dexcom G5 Mobile Continuous Glucose Monitoring System request to expand the indications to include replacement of fingerstick blood glucose testing for diabetes treatment decisions] Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf12/P120005S041a.pdf.
- 13.Huang ES, O’Grady M, Basu A, Winn A, John P, Lee J, et al. The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2010;33(6):1269–74. doi: 10.2337/dc09-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forlenza GP, Pyle LL, Maahs DM, Dunn TC. Ambulatory glucose profile analysis of the juvenile diabetes research foundation continuous glucose monitoring dataset-Applications to the pediatric diabetes population. Pediatr Diabetes. 2016 doi: 10.1111/pedi.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benassi K, Drobny J, Aye T. Real-time continuous glucose monitoring systems in the classroom/school environment. Diabetes Technol Ther. 2013;15(5):409–12. doi: 10.1089/dia.2012.0314. [DOI] [PubMed] [Google Scholar]
- 16.Bratina N, Battelino T. Insulin pumps and continuous glucose monitoring (CGM) in preschool and school-age children: how schools can integrate technology. Pediatr Endocrinol Rev. 2010;7(Suppl 3):417–21. [PubMed] [Google Scholar]
- 17.Acerini C. The rise of technology in diabetes care. Not all that is new is necessarily better. Pediatr Diabetes. 2016;17(3):168–73. doi: 10.1111/pedi.12366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


