Abstract
Genomic carrier screening can identify more disease-associated variants than existing carrier screening methodologies, but its utility from patients’ perspective is not yet established. A randomized controlled trial for preconception genomic carrier screening provided an opportunity to understand patients’ decisions about whether to accept or decline testing. We administered a survey to potential genomic carrier screening recipients who declined participation (N = 240) to evaluate their reasons for doing so. Two thirds of women declined participation. We identified major themes describing reasons these individuals declined to participate; the most common were time limitation, lack of interest, not wanting to know the information, and potential cause of worry or anxiety. Most women eligible for genomic carrier screening indicated that their reasons for opting out were due to logistical issues rather than opposing the rationale for testing. As expanded carrier screening and genomic sequencing become a more routine part of clinical care, it is anticipated there will be variable uptake from individuals for this testing. Thus, the advancement of clinical carrier screening from single genes, to expanded screening panels, to an exome- or genome-wide platform, will require approaches that respect individual choice to receive genetic testing for reproductive risk assessment.
Keywords: Genome sequencing, Genomic medicine, Preconception expanded carrier screening, Patient testing decisions, Declining genetic testing, Reproductive decisions, Qualitative research
Introduction
Traditional carrier screening assesses reproductive risk by evaluating a small number of highly penetrant genes associated with significant childhood disorders, including life-shortening conditions and those associated with serious health implications, all with relatively well-defined phenotypes. Professional practice guidelines in the United States provide guidance about offering traditional carrier screening for certain conditions based on ethnic background and family history (ACOG Committee on Genetics 2005; 2007; 2009a; b; 2010; American College of Medical Genetics and American College of Obstetricians and Gynecologists 2001; American College of Obstetricians and Gynecologists Committee on Genetics 2011). The recent development of commercial expanded carrier screening panels increases the number of conditions available for screening into the dozens and hundreds (Lazarin et al. 2013). While there are currently no professional guidelines recommending the addition of specific conditions for carrier screening among all couples planning a pregnancy, educational resources for clinicians and laboratories have been developed. These resources include information to consider regarding informed consent, given the increased availability of expanded carrier screening in the clinical setting (Edwards et al. 2015; Grody et al. 2013). It has been recommended that expanded carrier screening processes continue to uphold patient choice (Henneman et al. 2016).
Carrier screening using whole-genome or whole-exome sequencing (which we refer to as “genomic carrier screening”) can identify more disease-associated variants than existing carrier screening methodologies (traditional and expanded panels), increasing the likelihood of identifying carriers of genetic conditions as well as couples at risk of having a child with a genetic condition (Bell et al. 2011). The perceived advantages and disadvantages of genomic carrier screening compared to traditional carrier screening have been explored with individuals, highlighting the importance of individual choice when deciding whether or not to pursue testing (Schneider et al. 2016). Evaluating the use of clinical genome sequencing technology for carrier status provides an opportunity to study the influence of this technology with regard to value-based reproductive planning decisions for individuals and couples (Wilfond and Goddard 2015).
Understanding why individuals decline genome- or exome-wide testing platforms in different patient populations can shed light on the utility of this sequencing technology. One study assessed participation rates for diagnostic whole exome sequencing in a phenotypically affected pediatric cancer population, with 83% of eligible families choosing to participate (Scollon et al. 2014). Another study determined decliner rates for whole genome sequencing in two adult cohorts, primary care and cardiology, and found that about half declined to participate (Robinson et al. 2016). No studies have explored specifically why individuals decline genome sequencing related to reproductive planning.
Purpose of the Study
The ability to perform genomic carrier screening provides both a responsibility and an opportunity to evaluate individuals’ decision-making within the context of their own values – including the option to decline testing. In the context of a randomized controlled trial for preconception genomic carrier screening, we administered a survey to potential genomic carrier screening recipients who declined participation to evaluate their reasons for doing so. We defined the characteristics of both participants and decliners within this population to better understand participation biases in an effort to contribute to the overall assessment of patient preferences with regard to genome sequencing.
Methods
Study Population
As part of the National Human Genome Research Institute (NHGRI) Clinical Sequencing Exploratory Research (CSER) consortium, we conducted a randomized controlled trial to investigate the clinical implementation of preconception genomic carrier screening for over 750 autosomal recessive, X-linked, and mitochondrial conditions, and about 100 medically actionable incidental findings (Leo et al. 2016). The study population was drawn from members of Kaiser Permanente Northwest (KPNW), an integrated healthcare delivery system that serves approximately 540,000 health plan members throughout the Portland, Oregon metropolitan area and more rural locations in both Washington and Oregon. The study procedures were reviewed and approved by the Kaiser Permanente Northwest Institutional Review Board. This research received a waiver of the requirement to obtain signed consent. Verbal consent was obtained from all participants.
Eligibility Criteria
Women eligible for inclusion were current members of the KPNW healthcare delivery system, were not pregnant, and stated they were planning future pregnancies. Women were excluded from participation if they were pregnant at the time of recruitment or consent visit, did not have access to email, had a known cognitive impairment, did not speak English, or were not between the ages of 21 and 50. Women were only eligible if they had previously completed preconception carrier screening through their clinical care at KPNW (98% of tests performed were for cystic fibrosis (CF) carrier status). Within KPNW, CF carrier screening is offered to all pregnant women who have not had this testing performed previously; this testing is usually offered through their OB-GYN healthcare provider during an early prenatal visit. There is variability in the offering of CF carrier screening in the preconception setting at KPNW; however, it is offered as part of an infertility evaluation to women.
Recruitment Process
Study staff reviewed the medical record of each potential participant to determine eligibility. Staff then contacted potential participants by phone to describe the study and to determine interest in participating. Women who were interested in participating after the initial informational phone call received a consent form by mail; recruitment staff then telephoned to schedule a consent visit with a genetic counselor.
Decliner Timing
Eligible women who declined to participate at any point were invited to complete a survey over the phone, which took less than five minutes to administer. If the initial phone contact resulted in a refusal from the potential participant and they completed the survey, they were considered early decliners. Any woman who was mailed the consent form but did not go on to consent for any reason was considered a late decliner. Women who attended a consent visit with a genetic counselor and decided not to consent were also considered late decliners.
Data Collection
The survey given to women declining participation was administered over the phone and directly entered into an online database by recruitment staff. Survey respondents were asked about reasons for declining participation via the open-ended question “Please tell me your reason or reasons for not choosing to participate in this study,” and all reasons given were recorded. We asked respondents if they already had a child, if they had a genetic condition in their family, if they had knowledge of families with a child with a genetic condition, and if so, the perceived effect of that condition on those families. These same questions were also asked of women who enrolled in the study through a participant survey completed at their consent visit. Demographic information including race/ethnicity, education level, current employment status, marital status, and annual income was requested from both participants and decliners. Data on age, insurance type (Medicaid or not) and clinical CF screening results (positive or negative) were collected from the electronic medical record. For all KPNW women pregnant during the study time period, we collected data on race/ethnicity, age, and insurance type (Medicaid or not) from the electronic medical record; educational level and annual income were inferred from geocoded census tract data.
Data Analysis
Quantitative Analysis
We reviewed descriptive statistics for both time points of decliners (early, late) and the enrolled population based on responses collected on the surveys completed by these women. We compared differences between decliners and enrolled participants on demographic characteristics (race/ethnicity, education, employment, age, marital status, income, and Medicaid status) with Fisher’s exact test. We used Fisher’s exact test to compare early decliners, late decliners, and enrolled participants on their responses to already having children, having a genetic condition in the family, knowledge of a family with a child with a genetic condition, and the perceived impact of having a child with a genetic condition. Due to a small amount of missing data from survey respondents not answering all questions (maximum 8% missing per question), we used pairwise deletion to handle missing data. Among those who completed the decliner survey, we examined the association between socioeconomic status (SES) and “declining because of privacy or discrimination concerns” using multivariable logistic regression because SES was defined as comprising three variables: education, income, and employment. We tested the association of other demographic characteristics with reasons for declining with Fisher’s exact test. We conducted all tests at a two-tailed alpha level of .05.
Qualitative Analysis
To examine the responses of decliners to the open-ended question “Please tell me your reason or reasons for not choosing to participate in this study,” we used qualitative content analysis to identify major reasons for declining (Bernard and Ryan 2010; Denzin and Lincoln 2011; Silverman 2009). In an effort to keep the open-ended comments grounded in the experiences of respondents, an investigator (JLS) with expertise in qualitative analyses who did not administer the survey led the analysis. To develop a coding dictionary, an initial reading of the responses was conducted to provide a general overview and understanding of the content, which was followed by a second reading to establish a draft list of codes (e.g., descriptive phrases that summarized the content). The draft list of codes was discussed with the project team until consensus was met on codes and their related definitions. Authors MJG and JLS individually re-read and applied the codes to the responses and reconciled discrepancies. The responses of decliners could reflect a single or multiple codes. The codes were summarized by JLS into themes representing reasons for declining, which were then tabulated by frequency. The themes were shared with the project team for comment and consensus.
Results
Study recruitment staff successfully reached by telephone 816 women eligible for participation (Fig. 1). A total of 540 (66%) of these women declined to participate in genomic carrier screening; of these women, 240 (44%) completed the survey. Women who completed this survey were categorized either as early decliners (who declined prior to receiving the consent form; 69%) or late decliners (who declined after receiving the consent form; 31%). Twenty-eight percent of late decliners had scheduled a consent visit and then either cancelled the visit or did not attend their consent appointment. Five late decliners (7%) attended the consent visit and then declined participation.
Fig. 1.
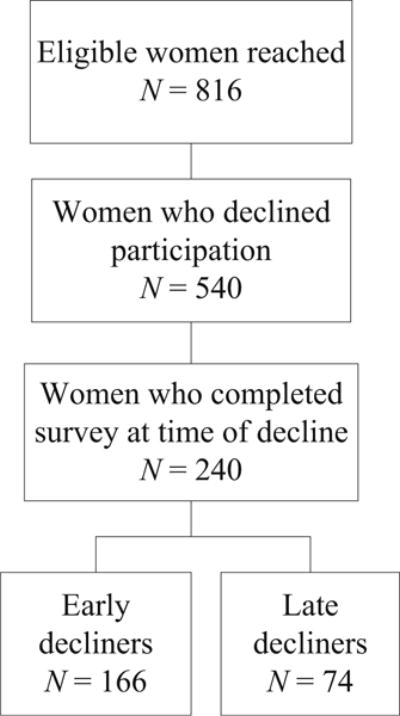
Flow chart for preconception genomic carrier screening recruitment
Women who declined to participate were significantly less educated, were younger, and had a lower income (Table 1) than those who enrolled. Women who declined were also significantly more likely to already have children and less likely to have a genetic condition in their family or to know someone with a child with a genetic condition. There were no statistically significant differences between the women who declined and those who enrolled by race/ethnicity, employment status, marital status, having a positive CF clinical carrier test result, or Medicaid enrollment status. Compared with all women who became pregnant at KPNW during the enrollment time frame, women who were eligible for the study (both those who enrolled and those who declined) and planning a pregnancy were more educated and older (Table 1). Additionally, women eligible for the study were less commonly enrolled in Medicaid compared with all women who became pregnant at KPNW.
Table 1.
Characteristics of participants and decliners of preconception genomic carrier screening
| Characteristic | KPNW pregnant women (N = 16,746) | Percentage of decliners
|
Percentage enrolled (N = 238) | P- valuea,b | ||
|---|---|---|---|---|---|---|
| Early (N = 166) | Late (N = 74) | Total (N = 240) | ||||
| Non-Hispanic white | 70% | 76% | 74% | 75% | 76% | 1.000 |
| Bachelor’s degree or higher | 30%d | 59% | 58% | 59% | 77% | <.001 |
| Employed for wages | NA | 85% | 75% | 82% | 86% | 0.378 |
| Age | <.001 | |||||
| <30 years | 53% | 34% | 46% | 38% | 18% | |
| ≥ 30 years | 47% | 66% | 54% | 63% | 82% | |
| Married | NA | 83% | 90% | 86% | 80% | 0.082 |
| Income | 0.001 | |||||
| < $40,000 | 28%d | 22% | 15% | 20% | 9% | |
| $40,000 to $79,999 | 30%d | 36% | 26% | 33% | 29% | |
| ≥ $80,000 | 42%d | 42% | 59% | 48% | 62% | |
| Enrolled in medicaid | 11% | 4% | 4% | 4% | 4% | 1.000 |
| Received positive clinical CF test results | NA | 1% | 1% | NA | 5% | 0.062 |
| Currently has children | NA | 45% | 55% | NA | 38% | 0.029 |
| Genetic condition in the family | NA | 15% | 21% | NA | 46%c | <.001 |
| Know of a family with a child with a genetic condition | NA | 21% | 24% | NA | 50% | <.001 |
| If know of a family with a child with a genetic condition, overall impact on the family | NA | NA | 0.103 | |||
| Strongly or slightly negative | 54% | 58% | 74% | |||
| None | 21% | 17% | 8% | |||
| Slightly or strongly positive | 25% | 26% | 19% | |||
Missing data (maximum 8%) excluded from analysis. All percentages are percent of non-missing responses. P Values in bold indicate statistically significant differences.
NA not applicable
P Values in italics are for the combined early and late decliners vs. enrolled
From Fisher’s exact tests
Response of Missing/Don’t Know for 123 (52%) of participants enrolled
Estimated from geocoded census data based on home address. Income categories listed are from survey data. For census data, top group is >$75,000
Of the 240 women who provided responses about their reasons for not participating, 112 (47%) gave one reason, 91 (38%) gave two reasons, and 37 (15%) gave three or more reasons. We identified 12 themes to describe reasons the decliners refused to participate. The most common themes were time limitation (48%), lack of interest (27%), not wanting to know the information (22%), anxiety or worry (17%), and travel limitations (15%) (Table 2). Additional reasons for declining included privacy or discrimination concerns, research study barriers, and other health issues. Less frequently, participants cited their partner’s resistance, already having prior genetic screening experience, sufficient healthcare, and future pregnancy planning status (undecided about plans for having children) as reasons for declining participation. We evaluated the additional reasons for declining given by women who cited the rather general but frequently cited reasons of time limitations or lack of interest. We found that the pattern of their additional reasons mirrored those in Table 2.
Table 2.
Reasons given for declining participation in preconception genomic carrier screening
| Theme | Code definition | Decliners
|
Quotes | ||
|---|---|---|---|---|---|
| Early N = 166 (%) | Late N = 74 (%) | Total N = 240 (%) | |||
| Time limitations | Lack of time due to work, young children, school, or generally busy | 85 (51%) | 30 (41%) | 115 (48%) | “I really want to participate, but with a baby, going to school, and trying to find work, just too busy!” |
| Lack of interest | Not relevant/no concerns about family history/won’t change choices/general lack of interest | 54 (33%) | 10 (14%) | 64 (27%) | “I’m not interested in genetic testing—we would still have the baby no matter what.” |
| Don’t want to know | Uncertain information would be of help or of value/ethically don”t want to know | 32 (19%) | 21 (28%) | 53 (22%) | “We don’t want to know—Feel it’s up to God to determine, and we’ll take whatever hand is dealt.” |
| Anxiety or worry | Too much information/potentially creates too much stress and worry | 24 (14%) | 16 (22%) | 40 (17%) | “If I was found to be a carrier I would obsess over it and worry too much.” |
| Travel limitations | Inconvenient location/unable to travel easily/barriers to travel | 23 (14%) | 14 (19%) | 37 (15%) | “Too far from work, it would take too much time to get there [study location].” |
| Privacy or discrimination concerns | Concerns for life or health insurance discrimination, privacy in health record | 8 (5%) | 19 (26%) | 27 (11%) | “I don’t want to be denied coverage because of what’s in my medical record.” |
| Research study barriers | Dislike blood draws/not enough compensation for commitment/dislike research in general | 13 (8%) | 10 (14%) | 23 (10%) | “[I] don’t like blood tests, and don’t think the compensation is worth it to cover the time to drive and gas.” |
| Health issues | Other health issues or concerns for self or others take precedence at moment | 15 (9%) | 6 (8%) | 21 (9%) | “[I] have lots of appointments for fibroids in uterus and trying to fix that [first] so can get pregnant.” |
| Partner resistance | Partner not interested/joint decision with partner to decline | 3 (2%) | 11 (15%) | 14 (6%) | “My husband didn’t want to participate – [I] was on the fence too so that helped me to decide not to.” |
| Prior genetic screening experience | Completed or declined prior genetic testing such as CF | 9 (5%) | 2 (3%) | 11 (5%) | “[My] CF test was negative so I don’t want to have any more tests.” |
| Sufficient healthcare | Satisfied with current care/does not desire more “testing” | 7 (4%) | 3 (4%) | 10 (4%) | “[I] feel the information [I] get from the doctor is fine.” |
| Conception status | Undecided if having children/more children | 4 (2%) | 3 (4%) | 7 (3%) | “We are deciding not to have more children for the time being.” |
Our qualitative analysis revealed some differences in reasons between the two groups of decliners (early, late). Early decliners more commonly described lack of interest than did late decliners (early, 33%; late, 14%). Late decliners more commonly expressed uncertainty about wanting to know the information (early, 19%; late, 28%), emotional reasons of anxiety or worry (early, 14%; late, 22%), concerns about privacy or discrimination (early, 5%; late, 26%), and partner resistance (early, 2%; late, 15%) (Table 2).
To assess whether previous knowledge of or experience with a genetic condition could influence rates of participation, we asked if there were genetic conditions in their family. When survey respondents reported that there were, we also asked them what condition or conditions were in their family. Most of the examples provided were actually for multifactorial conditions, rather than monogenic or chromosomal conditions (such as CF or Down syndrome). Regardless of the condition described, women who reported a “genetic condition” in the family were more likely to participate.
We also evaluated the potential associations between reasons for declining and demographic characteristics. We explored whether racial/ethnic minority status was associated with having concerns about privacy or discrimination. We also explored whether already having children was associated with interest in genomic carrier screening and whether marital status was associated with partner resistance as a reason for declining participation. Finally, we questioned whether reported anxiety about genetic screening or health issues was associated with age. We did not find an association between concerns with privacy/discrimination and racial/ethnic status. In addition, lack of interest in genomic carrier screening was similar for people with or without children. Women who were married expressed similar concerns about partner resistance to join the study compared to women who were not married. Finally, age was not significantly associated with anxiety about receiving genomic carrier screening results as a reason for declining participation. In the multivariable logistic regression, SES was associated with privacy/discrimination concerns (p < .001). After controlling for education and employment status, those with higher income were more likely to endorse privacy/discrimination concerns as a reason, OR = 1.44, 95% CI [1.03, 2.01].
Discussion
We evaluated women’s uptake of preconception genomic carrier screening to assess their reasons for deciding to decline research preconception genomic carrier screening. Two thirds of women (66%) successfully contacted by recruitment staff declined to participate. The percentage of women who declined genome sequencing in this carrier screening setting is higher than in a previously described study of phenotypically affected pediatric cancer patients (children and their parents – trios) offered diagnostic exome sequencing; in that setting, only 17% of eligible families declined participation (Scollon et al. 2014). The decliner rate identified in a genome sequencing study with two adult cohorts, primary care (ostensibly healthy adults ages 40–65) and cardiology (adults at any age with a personal diagnosis of hypertrophic or dilated cardiomyopathy), was also lower than in our described population (about 50% overall); however, it is important to note that in their healthy cohort, an unaffected population more similar to our population, there was a higher decliner rate than in their cardiology cohort (Robinson et al. 2016). These results suggest that healthy individuals may perceive the information gained from genome- or exome-wide testing platforms to be less valuable, or they are less willing to overcome barriers to participation than those within a phenotypically affected population.
The most common reasons for women declining to participate were a lack of time or a lack of interest. Early decliners were more likely to mention a lack of interest as a reason for not participating in the study; this suggests that sometimes potential participants assessed the value of testing offered through our study relatively quickly. This reveals a distinction many women may have made between clinical care they previously accepted (completing clinical carrier screening was an eligibility criterion) and additional testing beyond what was offered by their healthcare provider. However, these stated reasons, lack of time and lack of interest, are quite general, and could mask an implicit reason not mentioned; the ability to determine more specific meaning from these broad responses is limited. Less frequently cited reasons for declining participation included travel limitations and research study barriers. A previous genome sequencing study indicated the most frequently cited reasons for study decline (59%) in adults were time constraints and study logistics (Robinson et al. 2016). Similarly, a systematic review of factors affecting decisions related to CF carrier screening noted perceived barriers, such as lack of time, as the factor most frequently associated with decisions to decline carrier screening (Chen and Goodson 2007). This suggests that barriers related to logistical challenges such as lack of time may apply to both the single gene carrier screening and the broader genomic carrier screening.
We identified other reasons for declining genomic carrier screening including not wanting to know the information and anxiety or worry. It is interesting to note that more women in the late decliner group indicated they did not want to know the information that could be gained through testing or that it caused them anxiety or worry to consider proceeding with potentially receiving the testing. Women declining to participate after receiving the consent form were considered late decliners. While we cannot know for certain if a given female decliner actually read the consent form in detail, it could be that the information in the consent form guided their reasons for declining participation. These results indicate that some women are choosing to forgo genomic carrier screening due to possible perceived implications of the testing being offered. These women previously accepted clinical CF carrier screening; perhaps the extensive amount of information provided prior to genomic carrier screening compared to clinical CF carrier screening influenced their willingness to utilize the genomic carrier screening, as well as their reason(s) for opting out.
A small portion of individuals chose to not participate due to health issues. These ongoing health issues varied, but upon review of the coded responses, many included issues around infertility evaluations or treatments. Given that these women had clinical carrier testing performed prior to study recruitment efforts, sometimes during an initial infertility evaluation, this tendency to forgo testing due to ongoing other health issues may be biased by our sample population. The most commonly cited reason for declining exome sequencing in a phenotypically affected pediatric cancer population (specifically trios) included the family being overwhelmed by the recent diagnosis of cancer (Scollon et al. 2014). This suggests that the level of interest and ability to cope with the complexity of potential exome- or genome-wide platform testing may vary depending on whether there are ongoing health issues and when the given health issue was first brought to light for the individual or family.
Concerns about privacy or discrimination were more common reasons given by late decliners explaining their rationale for declining participation. Five respondents ultimately did not consent to be in the study after attending their consent appointment with a genetic counselor; four of them were specifically concerned about possible discrimination related to incidental findings, health insurance, or life insurance and long-term care. For these four women, concerns of discrimination were not alleviated after receiving additional information about the study via the consent form or a consent visit with a study genetic counselor. It may be that the low number of women attending a consent visit and ultimately declining suggests our recruitment process was truly facilitating informed consent. We employed a recruitment process with multiple opportunities for potential participants to learn information about the study and ask questions; a similar multistep recruitment approach was implemented in another study for genome sequencing, where they also had a very low decliner rate at or after the consent visit (Robinson et al. 2016).
Partner resistance was another theme identified in the women declining genomic carrier screening. This reason was more common among late decliners, possibly because they were initially interested when recruitment staff first approached them, and then with time, perhaps after reviewing the consent form and discussing it with their partner, they became less interested and specifically described their partner as influencing their decision. This highlights the potential dynamic within a couple around carrier screening decisions and that our strategy of recruitment in the study (men were only recruited to join the study if their female partners were found to be carriers of autosomal recessive conditions) could be influencing participation rates.
Our decliner population had already received carrier testing in the context of their clinical care; it was an eligibility criterion for recruiting to the study. Only 4% of decliners expressed that the reason they were not interested in the study was that they were already receiving sufficient clinical care. It is not clear whether they were not interested in receiving expanded carrier screening because it was beyond currently offered usual clinical care or because it was provided outside the context of their usual healthcare. A very small portion of decliners chose not to participate because they were unlikely to have future children. This was an eligibility criterion, suggesting that some potential participants changed their minds or more explicitly expressed their perspective on future reproductive planning later in the recruitment process after the determination of eligibility was made by recruitment staff. This observed refinement of a small number of women’s perspectives reinforces the value of our study design, which involved mailing study information (the consent form) and allowing potential participants to think about their future reproductive plans in greater depth before agreeing to additional carrier screening.
Additionally, we defined the characteristics of both participants and decliners within our study population to better understand participation biases with regard to genome sequencing. Characteristics of women in our study who declined preconception genomic carrier screening were similar to previously reported characteristics of women who declined CF carrier screening; they tended to be less educated, have lower incomes, and be more likely to already have children (Ioannou et al. 2014). It is well established that CF carrier screening uptake in the preconception period remains low (Chen and Goodson 2007); our findings suggest that this trend of lower uptake for traditional preconception carrier screening may translate to expanded preconception carrier screening. Potential participants with a higher income were more likely to endorse privacy or discrimination concerns as a reason for not participating in the preconception genomic carrier screening study. Further research addressing this potential correlation may be warranted.
When asking survey respondents (both study participants and decliners) if they had a genetic condition in their family, we observed that the responses often detailed multifactorial conditions such as diabetes, heart disease, and autism. Respondent perceptions of these being “genetic conditions” highlights broad inclusivity, using “genetic conditions that run in their family” to mean any condition that occurs in their family, regardless of whether it was a single-gene disorder with a potential disease-associated variant identifiable by genomic carrier screening. Decliners were less likely than participants to have a genetic condition in the family or to know of a family with a child with a genetic condition. Perhaps there is an assumption that receiving genomic carrier screening could identify the condition in the family, increasing the perceived value of genomic carrier screening. It is also possible that women without a family history of a genetic condition are overall less concerned about risks for a future pregnancy or child; they may not think the information that could be gained by genomic carrier screening is relevant to them.
Study Limitations
The survey for decliners was administered only to eligible women whom our recruitment staff could reach; study refusers who did not complete the decliner survey, especially those who received the consent form, may have had other reasons for not participating that are not reflected in our results. There are several aspects of this study that limit the generalizability of our findings. First, the diversity of our study population reflects the Portland area’s demographics. Although a majority of KPNW patients speak English, we did exclude women from participation if they did not speak English. Also, we assessed reasons women declined to participate in a research study offering genomic carrier screening rather than the testing itself; the reasons provided are confounded with investing in the research. We were able to assess only the reasons for declining participation in a study offering genomic carrier screening and the characteristics of that population in individuals who had already received clinical CF carrier screening. We were not able to capture or evaluate reasons for declining genomic carrier screening among KPNW members who had already declined or had not been offered clinical preconception carrier screening. As our data illustrate, there are differences in the demographic characteristics of women who were eligible to join the study because they had preconception carrier screening compared with all women who became pregnant during the recruitment time frame. In addition, we anticipate the level of interest and reasons for declining will differ among women who declined clinical preconception carrier screening and thus, were not eligible to be enrolled in the study. There are additional factors that may be barriers in a clinic environment that are not present in the research environment, and thus, were not explored here. For instance, in clinical care, health insurance coverage and the costs related to genomic carrier screening would likely be a barrier for many patients as well as a financial burden to the healthcare system. Another barrier in the clinical setting might be the availability or capacity of genetic counseling services. In addition to the carrier screening, we offered medically actionable incidental findings as an optional part of testing in this study. While we are not able to confirm whether this influenced the participation rate in our study, no women mentioned this as a reason for declining participation. Finally, we did not describe reasons for declining genomic carrier screening for potential male participants in the study population, who may have different reasons for declining to participate.
Conclusion, Research Recommendations, and Practice Implications
Most women declined genomic carrier screening due to logistical issues rather than opposition to the rationale for testing. Logistical reasons for declining screening could also reflect a trade-off between effort required to participate and a low perceived potential value of the genomic carrier screening being offered. In clinical care, carrier testing usually occurs as part of a clinical visit at a location most convenient for the patient. While the research study was limited to one location and required a visit outside of the context of usual medical care, logistical reasons for declining testing in the clinical arena could provide evidence for a low perceived value in receiving genomic carrier screening. Thus, in some cases, the work to minimize logistical barriers by genetic counselors may be counterproductive to what the patient prefers.
It will be important to expand upon our findings and determine if trends observed with regard to receptiveness of genomic carrier screening and reasons for declining are similar in a broader and more diverse population (socioeconomic, racial/ethnic, education level) than we were able to access. Additionally, determining interest in genomic carrier screening when offered to women who have not already completed clinical carrier screening would minimize the potential bias inherent in our study design. While we did not recruit women who declined clinical CF testing and acknowledge this bias, we hypothesize that these women are less likely to accept broad scale carrier screening if it were offered. Expanding the population to prenatal patients, where most clinical carrier testing is performed, could be valuable as it would allow us to more fully assess the clinical utility of genomic carrier screening in the reproductive setting. Our results suggest that a multistep recruitment and consent process should be explored further to understand if and how it effectively facilitates informed consent for genome sequencing with various populations.
As expanded carrier screening and genomic sequencing become more integrated into clinical care, we will likely continue to observe variable uptake from individuals. Patient perceptions of the value of genomic carrier screening have been evaluated showing a range of perspectives, with some individuals indicating they desire all possible information, while others express caution regarding its value (Schneider et al. 2016). The progression of clinical carrier screening from single genes, to expanded gene panels to, ultimately, an exome-or genome-wide platform, will necessitate that we continue to respect individual choice to receive expanded carrier screening, and reduce logistical barriers to enable individuals to obtain the information if they desire it.
Acknowledgments
This work was supported by a grant from the National Human Genome Research Institute (UM1HG007292; co-PIs: Wilfond, Goddard) with additional support from the Coordinating Center (U01HG007307; PI: Jarvik) as part of the Clinical Sequencing Exploratory Research (CSER) consortium. The authors thank the other members of the study team for their many useful insights and discussion about the study.
Footnotes
Conflict of Interest Marian J. Gilmore, Jennifer Schneider, James V. Davis, Tia L. Kauffman, Michael C. Leo, Kellene Bergen, Jacob A. Reiss, Patricia Himes, Elissa Morris, Carol Young, Carmit McMullen, Benjamin S. Wilfond, and Katrina A.B. Goddard declare that they have no conflict of interest.
Human Studies and Informed Consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Animal Studies No animal studies were carried out by the authors for this article.
References
- ACOG Committee on Genetics. ACOG committee opinion Number. Vol. 318. Washington: ACOG; 2005. Oct, 2005. Screening for Tay-Sachs disease. [Google Scholar]
- ACOG Committee on Genetics. ACOG practice bulletin no. 78: hemoglobinopathies in pregnancy. Obstetrics and Gynecology. 2007;109(1):229–237. doi: 10.1097/00006250-200701000-00055. [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Genetics. ACOG committee opinion no. 432: spinal muscular atrophy. Obstetrics and Gynecology. 2009a;113(5):1194–1196. doi: 10.1097/AOG.0b013e3181a6d03a;00006250-200905000-00046. [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Genetics. ACOG Committee opinion no. 442: preconception and prenatal carrier screening for genetic diseases in individuals of eastern European Jewish descent. Obstetrics and Gynecology. 2009b;114(4):950–953. doi: 10.1097/AOG.0b013e3181bd12f4. [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Genetics. ACOG Committee opinion no. 469: carrier screening for fragile X syndrome. Obstetrics and Gynecology. 2010;116(4):1008–1010. doi: 10.1097/AOG.0b013e3181fae884. [DOI] [PubMed] [Google Scholar]
- American College of Medical Genetics and American College of Obstetricians and Gynecologists. Preconception and prenatal carrier screening for cystic fibrosis: clinical and laboratory guidelines. Washington, DC: ACOG; Bethesda: ACMG; 2001. [Google Scholar]
- American College of Obstetricians and Gynecologists Committee on Genetics. ACOG Committee opinion no. 486: update on carrier screening for cystic fibrosis. Obstetrics and Gynecology. 2011;117(4):1028–1031. doi: 10.1097/AOG.0b013e31821922c2. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Dinwiddie DL, Miller NA, Hateley SL, Ganusova EE, Mudge J, et al. Carrier testing for severe childhood recessive diseases by next-generation sequencing. Sci Transl Med. 2011;3(65):65ra64. doi: 10.1126/scitranslmed.3001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H, Ryan G. Analyzing qualitative data: systematic approaches. Los Angeles: Sage Publications; 2010. [Google Scholar]
- Chen LS, Goodson P. Factors affecting decisions to accept or decline cystic fibrosis carrier testing/screening: a theory-guided systematic review. Genetics in Medicine. 2007;9(7):442–450. doi: 10.1097/gim.0b013e3180986767. [DOI] [PubMed] [Google Scholar]
- Denzin N, Lincoln Y. The sage handbook of qualitative research. Thousand Oaks: Sage Publications; 2011. [Google Scholar]
- Edwards JG, Feldman G, Goldberg J, Gregg AR, Norton ME, Rose NC, et al. Expanded carrier screening in reproductive medicine-points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of genetic counselors, perinatal quality foundation, and Society for Maternal-Fetal Medicine. Obstetrics and Gynecology. 2015;125(3):653–662. doi: 10.1097/aog.0000000000000666. [DOI] [PubMed] [Google Scholar]
- Grody WW, Thompson BH, Gregg AR, Bean LH, Monaghan KG, Schneider A, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genetics in Medicine. 2013;15(6):482–483. doi: 10.1038/gim.2013.47. [DOI] [PubMed] [Google Scholar]
- Henneman L, Borry P, Chokoshvili D, Cornel MC, van El CG, Forzano F, et al. Responsible implementation of expanded carrier screening. European Journal of Human Genetics. 2016;24(6):e1–e12. doi: 10.1038/ejhg.2015.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou L, McClaren BJ, Massie J, Lewis S, Metcalfe SA, Forrest L, et al. Population-based carrier screening for cystic fibrosis: a systematic review of 23 years of research. Genetics in Medicine. 2014;16(3):207–216. doi: 10.1038/gim.2013.125. [DOI] [PubMed] [Google Scholar]
- Lazarin GA, Haque IS, Nazareth S, Iori K, Patterson AS, Jacobson JL, et al. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: results from an ethnically diverse clinical sample of 23, 453 individuals. Genetics in Medicine. 2013;15(3):178–186. doi: 10.1038/gim.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo MC, McMullen C, Wilfond BS, Lynch FL, Reiss JA, Gilmore MJ, et al. Patients’ ratings of genetic conditions validate a taxonomy to simplify decisions about preconception carrier screening via genome sequencing. American Journal of Medical Genetics Part A. 2016;170(3):574–582. doi: 10.1002/ajmg.a.37477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JO, Carroll TM, Feuerman LZ, Perry DL, Hoffman-Andrews L, Walsh RC, et al. Participants and study Decliners’ perspectives about the risks of participating in a clinical trial of whole genome sequencing. Journal of Empirical Research on Human Research Ethics. 2016;11(1):21–30. doi: 10.1177/1556264615624078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JL, Goddard KA, Davis J, Wilfond B, Kauffman TL, Reiss JA, et al. “is it worth knowing?” focus group Participants’ perceived utility of genomic preconception carrier screening. Journal of Genetic Counseling. 2016;25(1):135–145. doi: 10.1007/s10897-015-9851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollon S, Bergstrom K, Kerstein RA, Wang T, Hilsenbeck SG, Ramamurthy U, et al. Obtaining informed consent for clinical tumor and germline exome sequencing of newly diagnosed childhood cancer patients. Genome Medicine. 2014;6(9):69. doi: 10.1186/s13073-014-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. Doing qualitative research. Thousand Oaks: Sage Publications; 2009. [Google Scholar]
- Wilfond B, Goddard KAB. It’s complicated: criteria for policy decisions for the clinical integration of genome scale sequencing for reproductive decision-making. Molecular Genetics and Genomic Medicine. 2015;3(4):239–242. doi: 10.1002/mgg3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]


