Abstract
Physical activity has been shown to positively influence a number of parameters in chronic schizophrenia, including cognition, social well being, and quality of life. Here, we present a systematic review of randomized controlled trials reporting on reduction of positive and negative symptoms using PANSS grading after the implementation of a physical activity protocol. Review of 64 articles yielded 6 relevant to our discussion. We found that physical activity significantly improved aggregate total PANSS score as well as positive symptoms PANSS score. While negative PANSS score showe a trend toward improvement, this was nonsignificant. Overall, we find the various forms of physical activity discussed within to be an appropriate adjunct to standard pharmacotherapy for the reduction of symptoms in chronic schizophrenia.
Keywords: schizophrenia, physical activity, PANSS
Introduction
Schizophrenia is a severe, chronic, and progressive disorder affecting nearly 1% of individuals in the United States.1 While its etiology remains largely unknown, it is characterized by a predictable natural progression. Positive symptoms, such as hallucinations, delusions, and paranoia are readily treated with antipsychotic pharmacotherapy – with acute rates of resolution approaching 70%.2 Despite this, resolution of positive symptoms alone does not appear to substantially improve function. Rather, degree of impairment in memory, executive function, attention, and concentration is a function of negative symptom control.2–4 These symptoms include withdrawal, decreased communication, and social isolation – they thus represent the most debilitating aspects of schizophrenia. Indeed, the negative symptoms of schizophrenia are largely responsible for its substantial economic and social burden.5 The utility of physical activity in treatment of other neuropsychiatric disorders, including depression, Alzheimer’s disease, and Parkinson’s disease, has previously been established.6–9 Additionally, the role of physical activity in schizophrenia treatment has been explored in a number of studies with varying conclusions. To date, the effect of physical activity specifically on the neurocognitive symptoms of chronic schizophrenia remains in question. The purpose of this systematic review is to determine the degree to which physical activity, when compared to standard treatment methods, improves neurocognitive symptoms in chronic schizophrenia. Our review contains only data from randomized controlled trials that report symptom outcomes using the standardized Positive and Negative Syndrome Scale (PANSS).10
Methods
We present a systematic review compliant with the guidelines outlined in the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA).11 Eligible studies for this review included only randomized controlled trials, in which patients diagnosed with chronic schizophrenia underwent treatment with standard pharmacotherapy, a predetermined exercise regimen, or a combination of the two. Eligible studies were full-text, published in the English language, in peer-reviewed journals, between January 2007 and January 2017. Individuals within included articles must have been diagnosed with schizophrenia according to the definitions outlined within the Diagnostic and Statistical Manual (DSM).12 Studies involving individuals with other mental disorders, including depression, bipolar disorder, and schizoaffective disorder were strictly excluded. Additionally, any study that did not report Total PANSS score pre and post intervention was excluded. Finally, all patient populations with comorbid medical or psychiatric conditions were excluded.
An “intervention” was defined to be any predetermined mild-moderate physical activity regimen occurring for a minimum of 150 minutes per week as defined by the United States Department of Health and Human Services Physical Activity Guidelines for Americans.13 These activities includes yoga, martial arts, organized team sports (basketball, soccer, etc), gymnastics, jogging, swimming, biking, and resistance training, among others. Interventions were compared to the control – the current gold standard pharmacotherapy regimen for schizophrenia, typical and atypical antipsychotics. The primary outcome measure was post intervention improvement in Total PANSS. The PANSS is a rigorously validated, standardized, and uniform medical scale that establishes presence and severity of both positive and negative symptoms in schizophrenia. Because, assessment is conducted by a trained interviewer within a set time period, results are reliable and reproducible.10
A literature search of Web of Science, Medline/Pubmed, Embase, and PsychINFO was conducted by an experienced librarian. A representative search strategy for Pubmed is shown below:
“(“Schizophrenia” [Mesh] OR (schizophren* [Title/Abstract] OR schizophren* [Other Term] OR
Dementia Praecox [Title/Abstract] OR Dementia Praecox [Other Term])) AND ((“Exercise” [Mesh] OR “Exercise Therapy” [Mesh] OR “Exercise Movement Techniques” [Mesh] OR “Sports” [Mesh]) OR (exercis* [Title/Abstract] OR exercis* [Other Term] OR pilates [Title/Abstract] OR pilates [Other Term] OR Qigong [Title/Abstract] OR
danc* [Title/Abstract] OR qigong [Other Term] OR danc* [Title/Abstract] OR danc* [Other Term] OR Tai chi [Title/Abstract] OR Tai chi [Other Term] OR Tai ji [Title/Abstract] OR Tai ji [Other Term] OR T’ai chi [Title/Abstract] OR T’ai chi [Other Term] OR Yoga [Title/Abstract] OR Yoga [Other Term] OR Martial art* [Title/Abstract] OR Martial art* [Other Term] OR Judo [Title/Abstract] OR Judo [Other Term] OR Karate [Title/Abstract] OR Karate [Other Term] OR Sport* [Title/Abstract] OR Sport* [Other Term] OR Athlet* [Title/Abstract] OR Athlet* [Other Term] OR Gym* [Title/Abstract] OR Gym* [Other Term] OR Running [Title/Abstract] OR Running [Other Term] OR Jogging [Title/Abstract] OR Jogging [Other Term] OR Swim* [Title/Abstract] OR Swim* [Other Term] OR Walk* [Title/Abstract] OR Walk* [Other Term] OR Weight lifting [Title/Abstract] OR Weight lifting [Other Term] OR lift* AND weights [Title/Abstract] OR lift* AND weights [Other Term] OR Resistance training [Title/Abstract] OR Resistance training [Other Term] OR Motion therapy [Title/Abstract] OR Motion therapy [Other Term] OR Plyometrics [Title/Abstract] OR Plyometrics [Other Term] OR Stretching [Title/Abstract] OR Stretching [Other Term] OR Gymnastic* [Title/Abstract] OR Gymnastic* [Other Term] OR Interval training [Title/Abstract] OR Interval training [Other Term] OR bicycling [Other Term] OR bicycling [Title/Abstract] OR
bicycle* [Other Term] OR bicycle* [Title/Abstract] OR Stair climbing [Title/Abstract] OR Stair climbing [Other Term] OR climb* AND stair* [Title/Abstract] OR Circuit training [Title/Abstract] OR climb* AND stair* [Other Term] OR Circuit training [Other Term] OR Calisthenic* [Title/Abstract] OR Calisthenic* [Other Term] OR biking [Title/Abstract] OR biking [Other Term] OR bike* [Title/Abstract] OR bike* [Other Term] OR Weight bearing strengthening [Title/Abstract] OR Weight bearing strengthening [Other Term] OR Strength training [Title/Abstract] OR Strength training [Other Term] OR Tae Kwon Do [Title/Abstract] OR Tae Kwon Do [Other Term] OR Kung fu [Title/Abstract] OR Kung fu [Other Term] OR Gong fu [Title/Abstract] OR Aikido [Title/Abstract] OR Aikido [Other Term] OR Wushu [Title/Abstract] OR Jujitsu [Title/Abstract])) AND (“Randomized Controlled Trial” [Publication Type] OR “Randomized Controlled Trials as Topic” [Mesh]) AND (positive and negative syndrome scale [Title/Abstract] OR positive and negative syndrome scale [Other Term] OR positive and negative symptom scale [Title/Abstract] OR positive and negative symptom scale [Other Term] OR PANSS [Title/Abstract] OR PANSS [Other Term]) NOT (systematic review [Title/Abstract] OR meta-analysis [Publication Type])”
The above search strategy was altered according to each specific database. Title and abstract review were conducted by 2 independent reviewers to determine eligible articles. These then underwent full text review to determine whether the above defined inclusion criteria were met. These same reviewers independently extracted data in a similar fashion. Extracted data included patient demographic information, physical activity intervention, standard control methods, and outcome measures. Additionally, pre and post intervention Total PANSS scores were extracted. Data were consolidated using fixed effects model statistics.
Results
Our search yielded a total of 64 initial results. Upon removal of duplicates, as well as title, abstract, and full text review, a total of 6 studies met full inclusion criteria.14-19 Details of study selection can be found in Figure 1.
Figure 1.
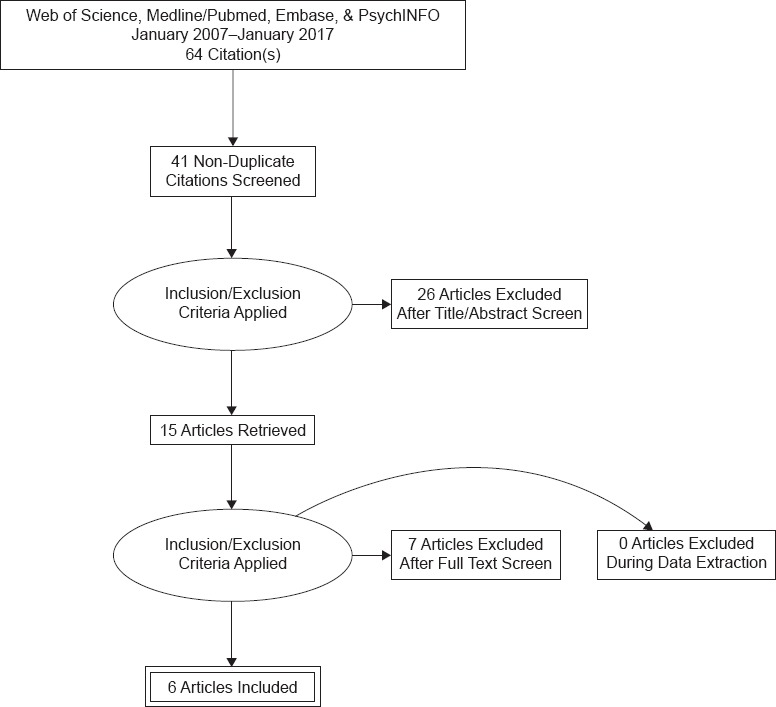
Summary of Search Results
Of the 6 included randomized controlled trials, 2 compared the effects of a previously outlined yoga intervention to standard pharmacotherapy, 1 study compared a regimen of traditional greek dance to standard pharmacotherapy, 1 compared cardiovascular exercise to occupational therapy, and 2 studies compared both yoga and aerobic exercise to standard treatments. Together, these comprised 8 total interventions, 7 of which were directly compared to the current gold standard treatment. Mean follow-up ranged from 2 to 6 months, with 66.7% of studies reporting outcomes after 8 weeks of experimental intervention. Patient were relatively young, with a mean age of participating subjects was 35.1 +/− 8.19 years. Of note, 4 of the 6 studies had patients with a mean age between 29 and 32 years, with the other two study populations were aged above 40 years. A total of 301 patients represented populations from the following countries: India, Greece, Japan, The Netherlands, and The United States.
Reviewed interventions in this study included various types of yoga, dance, aerobic and resistance training. Yoga included pranayama (breathing exercises), gentle movements of major muscle groups and joint rotations, asana posturing (back bends, twists, inversions, standing, and balancing postures), and nidra (deep relaxation). The dancing intervention consisted of Greek dance taught by a professional instructor to achieve 60–70% maximal heart rate calculated individually for each patient. Finally, aerobic and resistance training for major muscle groups followed the recommendations of the American College of Sports Medicine.
Analysis of the overall effect of all physical activity on post-intervention symptoms revealed mean reduction in Total PANSS score of −8.61 +/− 7.41 points with physical activity versus −1.93 +/− 2.62 points for the control (P = 0.0221). All but one study with yoga-only interventions reported a minimum reduction in Total PANSS of greater than 10%. The exercise-only arms of studies comparing both yoga and exercise unanimously report no reduction of either positive or negative symptoms (P < 0.05). The single study examining twice weekly cardiovascular exercise training reports a reduction in Total PANSS of over 20%, compared to a 3.3% reduction in the control (P < 0.001). Physical activity additionally reduced the aggregate positive symptom PANSS score when compared to the aggregate control (−2.33 +/− 1.93 vs −0.68 +/− 1.18; P = 0.0302). With regards to negative symptom PANSS, a non-significant trend favored physical activity (reduction of −1.91 +/− 2.07) to the control (reduction 0.40 +/− 1.24) (P = 0.059). Table 1 contains a summary of included studies.
TABLE 1. SUMMARY OF INCLUDED STUDIES.
| TITLE | AUTHOR | PATIENTS (N, MEAN AGE +/− STANDARD DEVIATION OR AGE RANGE) | INTERVENTION | CONTROL | RESULTS | LIMITATIONS |
|---|---|---|---|---|---|---|
| Yoga therapy as an adjunctive treatment for schizophrenia: A randomized, controlled pilot study14 | Viscegila et al. 2011 | 18, 42.0 +/− 13.5 | 8 week Yoga therapy program | Standard pharmacotherapy | Reduction in all PANSS measures except thought disturbance (P = 0.34), including positive symptoms (P = 0.02), Negative symptoms (P = 0.01), general psychopathy (P = 0.00), anergia (P = 0.03), activation (P = 0.04), paranoia/belligerence (P = 0.01), depression (0.02), and total PANSS score (P = 0.00) | Small sample size, No active control group |
| Effect of yoga therapy on facial emotion recognition deficits, symptoms and functioning in patients with schizophrenia15 | Behere et al. 2011 | 27, 31.3 +/− 9.3 (Yoga arm); 17, 30.2 +/− 8.0 (Exercise arm) | Antipsychotic medication + Home yoga module following professional instruction session; Antipsychotic medication + home exercise module | Standard pharmacotherapy | In the yoga group, positive and negative symptoms were significantly reduced at 2 and 4 month follow up (P = 0.008 and P = 0.002 respectively). Exercise did not reduce PANSS score in the same time frame. | Limited supervision of interventions. Yoga and exercise were unscheduled; highly motivated individuals, or those with reliable caregivers, may independently be more likely to follow through with the intervention. |
| Effects of exercise training with traditional dancing on functional capacity and quality of life in patients with schizophrenia: a randomized controlled study16 | Kaltsatou et al. 2015 | 31.59 ± 14.1 | 8-month Greek traditional dancing program | Standard pharmacotherapy | At 8 month f/u intervention group PANSS total score was 77.0 +/− 23.1 vs. 82.0 +/− 24.4, for the control (P < 0.05) | Volunteer population, no long term follow-up, small sample size |
| Effects of weekly one-hour hatha yoga therapy on resilience and stress levels in patients with schizophrenia-spectrum disorders: An eight-week randomized controlled trial17 | Ikai et al. 2014 | 50, 50.9 ± 11.3 | Weekly 1-hour Hatha yoga sessions + antipsychotic medication for 8 weeks | Standard pharmacotherapy | No significant difference in PANSS from baseline to week 8 were found between the two groups (changes in the yoga group versus the control group) | Yoga sequence varied among patients; not all participants completed intervention as older patients were physically limited |
| Exercise therapy improves mental and physical health in schizophrenia: a randomized controlled trial18 | Scheewe et al. 2013 | 63, 29.2 ± 7.2 | 1 hour cardiovascular exercise twice weekly | 1 hour occupational therapy sessions twice weekly | Total PANSS for the intervention decreased by 20.7% over 6 months, whereas for the control group PANSS increased by 3.3% (P < 0.001) | Significant dropout and noncompliance rate; only 39 patients followed given protocol |
| Therapeutic efficacy of add-on yogasana intervention in stabilized outpatient schizophrenia: Randomized controlled comparison with exercise and waitlist19 | Varambally et al. 2012 | 95, 30.6 ± 7.3 | 25 sessions per month of 45 minute session of either yogasana or exercise | Standard pharmacotherapy | Total PANSS improved significantly for yoga group (P < 0.001), but not for exercise group (P = 0.10) when compared to control | Few patients completed logs according to original protocol; difficult to confirm regularity of either yogasana or exercise sessions; patients received subsidization for travel in interventional groups |
Discussion
Here, we report that physical activity, specifically yoga and aerobic activity, appears to contribute to modest reductions in the positive and negative symptoms of chronic schizophrenia in young and otherwise healthy individuals. Specifically, using the Positive and Negative Syndrome Scale, we find that individualized physical activity over the course of 2–4 months yields a significant decrease in Total PANSS and Positive PANSS scores, and reveals a trend towards decreased negative PANSS as well. In conjunction with appropriate pharmacotherapy, physical activity appears to complement today’s well-established pharmacological management of chronic schizophrenia.
The PANSS was first developed by SR Kay in 1987 for the purpose of characterizing symptomatology during clinical trials for antipsychotic pharmaceuticals. The PANSS assessment is part of a 40–60 minute interview process conducted by a trained interviewer. It consists of 3 main arms, which form the basis for symptom characterization – the Positive, Negative, and General Psychopathy scales. The Positive and Negative scales each consist of 7 symptoms, graded on a severity of 1–7, and the General psychopathy scale consists of 16 similarly graded symptoms. The total minimum and maximum for a PANSS assessment are 30 and 210 points respectively. Intraobserver reliability is reported to be excellent, and a number of recent studies have continued to confirm the validity of PANSS.10
A number studies have reported on the effect of physical activity on cognition, quality of life, global functioning, development of other psychiatric disorders, and symptom reduction in schizophrenia.20–23 A 2016 systematic review of 26 articles reported that among individuals with a history of schizophrenia, schizophreniform, schizoaffective, delusional, or psychotic disorder not otherwise specified (NOS), exercise was successful at improving quality of life, depressive symptoms, and global functioning. Additionally, they report a reduction in total symptom severity as well as positive and negative symptoms, as measured by PANSS or the brief psychiatric rating scale (BPRS). While their total sample size was large at over 1,000 patients, included studies reporting symptomatic changes involved a total of about 60% of that number. Processing speed, long term and working memory, and executive function were not affected by exercise intervention, with the exception of those studies reporting on yoga therapy specifically. Yoga was found to enhance cognitive long term memory. Similar to physical activity interventions discussed within our review, Dauwan et al. report on studies that primarily use the standard of aerobic exercise totaling 90–120 minutes per week, and yoga techniques involving deep breathing, posturing, meditation, and relaxation.20
A previous 2013 systematic review of yoga interventions on schizophrenia also reported on quality of life, cognitive and social function, hospitalization rate, and symptoms. Among their reported 6 randomized controlled trials (totaling 337 patients), Cramer et al. found no evidence for short or long-term improvement in positive or negative symptoms. While moderate evidence was found for improvement on short term quality of life, these effects were in isolated, high-bias studies and thusly disputed by the authors of the review. Additionally, no significant difference was determined to be present between yoga and exercise therapies with regards to social function, quality of life, or adverse events.21 While these findings differed substantially from our own, they highlight the a key feature of the current literature on yoga-based intervention for schizophrenia. Namely, the total pool of eligible RCTs incorporated in their analysis was small, and thus the derived effect size estimates were highly unstable. Still, such a review provides valuable insight into the role of non-traditional physical activity-based interventions in treating schizophrenia.
While the exact mechanism by which physical activity may influence the positive and negative symptoms of chronic schizophrenia remains unknown, a number are proposed mechanisms currently exist within the literature. Among these, the most frequently cited is the concept of enhanced synaptic efficacy and plasticity via Brain-Derived Neurotrophic Factor (BDNF). BDNF is a molecule belonging to the neurotrophin family of growth factors. Its action on both central and peripheral neurons is to enhance neuron survival, synaptic growth, and differentiation. In mammalian models, BDNF has been shown to increase neurogenesis, and to play a vital role in the formation of long term and working memory through its action on glutaminergic and GABAergic signaling. Loss of vital neuron survival signal appears to be at least partially implicated in the neuro-atrophy observed in schizophrenia.22 A number of studies, including a 2011 meta-analysis, report that compared to healthy control subjects, patients with psychotic-spectrum disorders have reduced serum and plasma BDNF levels. Furthermore, reductions were present in both medicated, and drug-naive individuals, suggesting that pharmacological control does not necessarily improve peripheral BDNF levels.23 While it is well established that physical activity increased baseline and peak BDNF levels in healthy populations, a growing body of evidence supports the idea that this increase in nearly twice as large among psychiatric populations.24 Current research is centered on establishing exercise regimens for people with chronic schizophrenia that specifically target peripheral BDNF levels.
This study has a number of limitations that may hinder drawing concrete conclusions on the current role of physical activity in the treatment of the symptoms of chronic schizophrenia. The scope of this review is admittedly narrow, which is reflected in the obtained search results. It was the hope of the authors that by utilizing only one form of outcome measure, and by providing a narrow sampling of a specific subset of the literature, that the aggregated data obtained would be homogeneous and thus more conclusive. The total sample size in this study is small, at just over 300 individuals. Additionally, lack of unification in physical activity protocol may further add distance between the data and definitive recommendations. Despite these limitations, physical activity as an adjunctive therapy appears to positively impact the symptom profile of chronic schizophrenia. Further research should be geared towards unifying physical activity protocols for this population, developing protocols that target BDNF enhancement, and producing more long term outcomes data.
Acknowledgments
The authors would like to extend their sincere gratitude to librarian Karen Sorensen; without her help this review would not have been possible.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflicts of Interest
On behalf of both authors, the corresponding author states that there are no conflicts of interest or sources of funding to declare.
References
- 1.Abi-Dargham A. Schizophrenia: overview and dopamine dysfunction. J Clin Psychiatry. 2014;75:e31. doi: 10.4088/JCP.13078tx2c. [DOI] [PubMed] [Google Scholar]
- 2.Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: A meta-analysis. Schizophrenia Research. 2009;113(2-3):189–199. doi: 10.1016/j.schres.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strassnig MT, Raykov T, O’Gorman C et al. Determinants of different aspects of everyday outcome in schizophrenia: the roles of negative symptoms, cognition, and functional capacity. Schizophr Res. 2015;165:76–82. doi: 10.1016/j.schres.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stubbs B, Chen L-J, Chung M-S, Ku P-W. Physical activity ameliorates the association between sedentary behavior and cardiometabolic risk among inpatients with schizophrenia: A comparison versus controls using accelerometry. Comprehensive Psychiatry. 2017;74:144–150. doi: 10.1016/j.comppsych.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Zeidler J, Slawik L, Fleischmann J, Greiner W et al. The costs of schizophrenia and predictors of hospitalisation from the statutory health insurance perspective. Health Econ Rev. 2012;2:9. doi: 10.1186/2191-1991-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramer H, Anheyer D, Lauche R, Dobos G. A systematic review of yoga for major depressive disorder. Journal of Affective Disorders. 2017;213:70–77. doi: 10.1016/j.jad.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Müller J, Chan K, Myers JN. Association between exercise capacity and late onset of dementia, Alzheimer disease, and cognitive impairment. Mayo Clinic Proceedings. 2017;92(2):211–217. doi: 10.1016/j.mayocp.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Farran CJ, Etkin CD. Effect of moderate to vigorous physical activity intervention on improving dementia family caregiver physical function: A Randomized controlled trial. Journal of Alzheimer’s Disease & Parkinsonism. 2016;6(4) doi: 10.4172/2161-0460.1000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaseda Y, Ikeda J, Sugihara K, Yamawaki T, Kohriyama T, Matsumoto M. Therapeutic effects of intensive inpatient rehabilitation in advanced Parkinson’s disease. Neurology and Clinical Neuroscience. 2016;5(1):18–21. doi: 10.1111/ncn3.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A. The PRISMA statement for reporting systematic reviews and Meta-Analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine. 2009;151(4):W. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 12.Washington, DC: American Psychiatric Association; 2013. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders – DSM-IV-TR. 5. [Google Scholar]
- 13.9th United States: Wolters Kluwer Health; Aug 1, 2013. Wilkins LW &. Acsm’s guidelines for exercise testing and prescription. + essential clinical. [Google Scholar]
- 14.Visceglia E, Lewis S. Yoga therapy as an Adjunctive treatment for schizophrenia: A Randomized, controlled pilot study. The Journal of Alternative and Complementary Medicine. 2011;17(7):601–607. doi: 10.1089/acm.2010.0075. [DOI] [PubMed] [Google Scholar]
- 15.Behere RV, Arasappa R, Jagannathan A et al. Effect of yoga therapy on facial emotion recognition deficits, symptoms and functioning in patients with schizophrenia. Acta Psychiatrica Scandinavica. 2010;123(2):147–153. doi: 10.1111/j.1600-0447.2010.01605.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaltsatou A, Kouidi E, Fountoulakis K et al. Effects of exercise training with traditional dancing on functional capacity and quality of life in patients with schizophrenia: A randomized controlled study. Clinical Rehabilitation. 2014;29(9):882–891. doi: 10.1177/0269215514564085. [DOI] [PubMed] [Google Scholar]
- 17.Ikai S, Suzuki T, Uchida H et al. Effects of weekly One-Hour Hatha yoga therapy on resilience and stress levels in patients with schizophrenia-spectrum disorders: An Eight-Week Randomized controlled trial. The Journal of Alternative and Complementary Medicine. 2014;20(11):823–830. doi: 10.1089/acm.2014.0205. [DOI] [PubMed] [Google Scholar]
- 18.Scheewe TW, Backx FJG, Takken T et al. Exercise therapy improves mental and physical health in schizophrenia: A randomised controlled trial. Acta Psychiatrica Scandinavica. 2012;127(6):464–473. doi: 10.1111/acps.12029. [DOI] [PubMed] [Google Scholar]
- 19.Varambally S, Thirthalli J, Venkatasubramanian G et al. Therapeutic efficacy of add-on yogasana intervention in stabilized outpatient schizophrenia: Randomized controlled comparison with exercise and waitlist. Indian Journal of Psychiatry. 2012;54(3):227. doi: 10.4103/0019-5545.102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dauwan M, Begemann MJH, Heringa SM, Sommer IE. Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: A systematic review and Meta-analysis. Schizophrenia Bulletin. 2015;42(3):588–599. doi: 10.1093/schbul/sbv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramer H, Lauche R, Klose P, Langhorst J, Dobos G. Yoga for schizophrenia: A systematic review and meta-analysis. BMC Psychiatry. 2013;13(1) doi: 10.1186/1471-244x-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. British Journal of Pharmacology. 2009;153(S1):S310–S324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: A systematic review with meta-analysis. Molecular Psychiatry. 2010;16(9):960–972. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt RH, Nickerson JM, Boatright JH. Exercise as Gene Therapy: BDNF and DNA Damage Repair. Asia-Pacific Journal of Ophthalmology. 2016;5(4):309–311. doi: 10.1097/apo.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]


