Abstract
Objective
To investigate the efficacy and safety of lesinurad in combination with febuxostat in a 12‐month phase III trial in patients with tophaceous gout.
Methods
Patients with serum urate (UA) ≥8.0 mg/dl (≥6.0 mg/dl with urate‐lowering therapy) and ≥1 measurable target tophus were given febuxostat 80 mg/day for 3 weeks before randomization to receive lesinurad (200 or 400 mg daily) or placebo in addition to the febuxostat. The primary end point was the proportion of patients achieving a serum UA level of <5.0 mg/dl (month 6). The key secondary end point was the proportion of patients with complete resolution of ≥1 target tophus (month 12). Other end points included the percentage change in total target tophi area. Safety assessments included adverse events and laboratory data.
Results
Patients (n = 324) were predominantly male, with a mean age of 54.1 years. Significantly more patients achieved the serum UA target by month 6 with the addition of lesinurad 400 mg (76.1%; P < 0.0001), but not 200 mg (56.6%; P = 0.13), to the febuxostat therapy as compared with febuxostat alone (46.8%). At all other time points, significantly more patients in the lesinurad 200 mg group achieved the serum UA target. The number of patients with complete tophus resolution was not different between groups. Treatment with lesinurad (200 mg and 400 mg) plus febuxostat reduced the total target tophi area as compared with febuxostat alone (50.1% and 52.9% versus 28.3%, respectively; P < 0.05). Safety was generally comparable with that of febuxostat alone, except for higher rates of predominantly reversible elevations in the serum creatinine level, particularly with lesinurad 400 mg.
Conclusion
Treatment with lesinurad in combination with febuxostat demonstrated superior lowering of serum UA levels as compared with febuxostat alone, with clinically relevant added effects on tophi and an acceptable safety profile with lesinurad 200 mg in patients with tophaceous gout warranting additional therapy.
Current rheumatology guidelines for long‐term treatment of gout recommend maintenance of serum urate (UA) levels of <6.0 mg/dl or <5.0 mg/dl in patients with greater disease severity through a combination of lifestyle management and pharmacotherapy 1, 2, 3. The recommended first‐line urate‐lowering therapy is a xanthine oxidase inhibitor, either allopurinol or febuxostat 1, 2, to inhibit urate production 4. However, in clinical trials, only ∼40% of patients treated with allopurinol 300 mg/day achieved serum UA levels that were <6.0 mg/dl 5, 6, 7, 8. With febuxostat 80 mg/day, 67–75% of patients achieved a serum UA level of <6.0 mg/dl 5, 7, 8, 9, but only 48% were able to sustain it for 3 consecutive months 8. If target serum UA levels cannot be achieved with an appropriate dose of xanthine oxidase inhibitor, treatment guidelines recommend adding a uricosuric agent to the xanthine oxidase inhibitor 1, 2.
Lesinurad is a novel selective urate anion reabsorption inhibitor approved in the US and Europe for the treatment of gout in combination with a xanthine oxidase inhibitor for patients in whom target levels of serum UA are not achieved with a xanthine oxidase inhibitor 10. Lesinurad inhibits the uric acid transporter URAT1, which is responsible for most reabsorption of urate anion from the renal tubule 11. By inhibiting URAT1, lesinurad increases the excretion of uric acid and lowers the serum UA level 12. Therefore, lesinurad in combination with a xanthine oxidase inhibitor provides a dual mechanism of action for lowering serum UA levels by increasing renal excretion of uric acid and reducing urate production 13, 14.
A phase Ib clinical study of lesinurad plus febuxostat demonstrated greater reduction in serum UA levels than febuxostat alone 12. The aims of the current phase III study were to examine the benefits and risks of lesinurad (200 mg or 400 mg oral, once daily) in combination with febuxostat 80 mg in patients with tophaceous gout.
PATIENTS AND METHODS
Patients
Men or women (ages 18–85 years; body mass index <45 kg/m2) with a diagnosis of gout according to the criteria of the American College of Rheumatology 15 were eligible for the study. Eligible patients included those receiving urate‐lowering therapy currently or in the past as well as those who had never taken a urate‐lowering drug. Serum UA levels were required to be ≥8.0 mg/dl in patients not taking urate‐lowering therapy and ≥6.0 mg/dl in those taking urate‐lowering therapy. The presence of ≥1 measurable tophus on the hands/wrists and/or feet/ankles that was ≥5 mm and ≤20 mm in the longest diameter (length), as measured using Vernier calipers 16, was required for study entry.
Exclusion criteria included an estimated creatinine clearance of <30 ml/minute, as calculated via the Cockcroft‐Gault formula using ideal body weight. A history of kidney stones was not an exclusion criterion. Complete inclusion/exclusion criteria are provided in Supplementary Table 1 (available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40159/abstract) and were similar to those used in recent trials of hyperuricemia and gout treatments.
Trial design
Treatment procedures
The Combination Treatment Study in Subjects with Subcutaneous Tophaceous Gout with Lesinurad and Febuxostat (CRYSTAL) was a phase III multicenter, multinational, randomized, double‐blind, placebo‐controlled combination study evaluating the efficacy and safety of lesinurad 200 mg or 400 mg orally in combination with febuxostat 80 mg orally compared with placebo in combination with febuxostat 80 mg (ClinicalTrials.gov identifier NCT01510769). The study, which was conducted in North America, Europe, Australia, and New Zealand, included an ∼35‐day screening period (including a run‐in period of ∼21 days), a 12‐month double‐blind treatment period, and a follow‐up period of ≤3.5 months (Supplementary Figure 1, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40159/abstract). Regardless of urate‐lowering therapy type at screening, all patients began febuxostat 80 mg once daily as the sole urate‐lowering therapy along with gout flare prophylaxis on day −21. Gout flare prophylaxis consisted of colchicine (0.5 or 0.6 mg once daily, per protocol, and as locally available) or a nonsteroidal antiinflammatory drug (NSAID) if patients had an intolerance/contraindication to colchicine. Gout flare prophylaxis was continued through month 5 unless patients became intolerant or developed toxicity to prophylaxis.
After 3 weeks of febuxostat 80 mg treatment, patients were randomized 1:1:1 to receive placebo plus febuxostat 80 mg, lesinurad 200 mg plus febuxostat 80 mg, or lesinurad 400 mg plus febuxostat 80 mg. Randomization at all study sites used a centralized Interactive Voice Response System/Interactive Web Response System.
Doses of febuxostat, lesinurad, or placebo were taken once daily in the morning with food and 1 cup of water. Patients were encouraged to drink 2 liters of fluid/day and to remain well hydrated, consistent with the American College of Rheumatology guidelines for the management of gout 1. Compliance with study medication was assessed using the medication dispensing records, with verification of the returned medication packaging and any remaining medication during each study visit. Concomitant medication use was recorded at each study visit.
The study was conducted in accordance with Independent Ethics Committee E6, Good Clinical Practice, the Declaration of Helsinki (October 2008), and all applicable local regulatory requirements. Patients were permitted to withdraw from treatment or from the study at any time. The study was conducted between February 2012 and April 2014.
Evaluations
The primary efficacy end point was the proportion of patients in each treatment group with a serum UA level of <5.0 mg/dl by month 6. Secondary serum UA efficacy end points included mean serum UA levels recorded at each visit. Prespecified sensitivity and supportive analyses included the proportion of patients with the following 3 conditions: 1) serum UA level of <5.0 mg/dl at the 4‐, 5‐, and 6‐month assessments; 2) serum UA levels of <5.0, <4.0, and <3.0 mg/dl at each monthly visit; and 3) a median serum UA level of <5.0 mg/dl, as well as the proportion of patients with a serum UA level >5.0 mg/dl at baseline and <5.0 mg/dl by month 6.
Key secondary end points were the proportion of patients with complete resolution (100% decrease in the area of a tophus) of ≥1 target tophus by month 12 and the proportion of patients with a best tophus response for ≥1 target tophus of complete or partial (≥50% decrease in area) resolution by month 12. An additional tophus end point was the mean percentage change from baseline in the sum of the areas of all target tophi at each visit. Tophi were measured using digital calipers to capture both the longest diameter (length) and longest perpendicular measurement.
Other secondary end points included the proportion of patients with gout flares requiring treatment at each month and the mean rate of gout flares from the end of month 6 to the end of month 12. Gout flares were reported using a daily electronic patient diary (e‐diary) that elicited the duration and extent of pain, the presence of warmth, swelling, and tenderness, and any change in medication used to treat the flare.
Serum UA levels were determined at baseline (day 1), week 2, and months 1–6, 8, 10, and 12. Tophi were measured every 3 months.
Safety assessments included treatment‐emergent adverse events (TEAEs; coded according to the Medical Dictionary for Regulatory Activities version 14.0), clinical laboratory data, physical examination findings, electrocardiogram results, and vital signs. Adverse events (AEs) of special interest included renal and cardiovascular (CV) safety assessments. Renal safety assessments were included because renal impairment is a common comorbid condition in patients with gout 17. Renal safety is of special interest because of the increased uric acid excretion caused by lesinurad. Increases in urinary uric acid excretion have the potential to induce microcrystallization of uric acid in renal tubules and/or the urinary system 18, which could manifest clinically as kidney stones and/or changes in kidney function. Assessments of renal safety included renal‐related and kidney stone TEAEs (Supplementary Table 2, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40159/abstract) and clinical laboratory findings including the serum creatinine level, the urinary protein‐to‐creatinine ratio, and the estimated creatinine clearance.
Review of CV safety was conducted by an independent Cardiovascular Events Adjudication Committee. AEs were routinely assessed for a potential CV relationship, with categorization into major adverse CV event (MACE) and non‐MACE end points (Supplementary Table 3, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40159/abstract) 19.
Patients who completed the double‐blind treatment were eligible to enroll in an extension study of lesinurad plus febuxostat. Patients who did not enter the extension study completed a follow‐up visit within 14 days of completing the double‐blind treatment.
Statistical analysis
The study consisted of a 12‐month treatment period, with the primary end point evaluated at month 6 and key secondary end points evaluated at month 12. The primary end point was the proportion of patients with serum UA levels of <5.0 mg/dl by month 6. All randomized patients who received ≥1 dose of randomized study medication were included in the intent‐to‐treat (ITT) population, the primary population for efficacy and safety assessments. Comparisons of response rates based on serum UA levels between each lesinurad group and the febuxostat plus placebo group were performed using the Cochran‐Mantel‐Haenszel test statistic, stratified according to renal function on day −7 (estimated creatinine clearance ≥60 ml/minute versus <60 ml/minute) and serum UA level on day −7 (≥6.0 versus <6.0 mg/dl). A Bonferroni correction was used for the primary end point for each of the 2 treatment comparisons with placebo at an alpha level of 0.025. Results for serum UA response were expressed as proportions, corresponding adjusted 95% confidence intervals (95% CIs) of the difference between response rates, and P values. Nonresponder imputation analysis was the primary analysis method, in which patients who were missing their month 6 serum UA result were considered nonresponders. If the null hypothesis for the primary end point for 1 dose was rejected at the 0.025 level, hierarchical testing of the key secondary end points for the surviving dose was performed at an alpha level of 0.025.
All other efficacy end points were evaluated at the level of α = 0.05 (nominal P value), 2‐sided without adjustment for multiple comparisons. For the primary analyses of response rates at each level, nonresponder imputation was used for all visits. Secondary end points were analyzed by negative binomial regression (gout flares) or Cochran‐Mantel‐Haenszel test (tophus response). Mean rates of gout flares were adjusted for day −7 renal function and serum UA level and duration of exposure to randomized study medication.
Safety data were listed by treatment group and were not subjected to statistical hypothesis testing. TEAEs were coded by system organ class and preferred term and were listed according to the incidence, severity, relation to study medication, and relation to discontinuation. Baseline serum creatinine was defined as the highest value within 14 days prior to the first dose of study medication. The relative increase in serum creatinine levels (i.e., ≥1.5 times and ≥2.0 times the baseline level at any time) was selected as the most clinically relevant assessment 20, 21. Resolution of serum creatinine elevation was defined as a serum creatinine value that returned to ≤1.2 times baseline.
Approximately 315 patients were planned to be recruited, for an allocation of ∼105 to each treatment group. This sample size was calculated to provide >90% power to detect a difference in response rates between treatment groups if the placebo group had a 40% response rate and the lesinurad groups had response rates as low as 65% using Fisher's exact test and adjusting for multiplicity with an alpha level of 0.025, 2‐sided, for each test.
RESULTS
Patient disposition
Of 1,045 patients screened, 330 were randomized at 102 study sites (Figure 1). The remaining 715 patients were withdrawn prior to randomization, including 667 screening failures and 48 who withdrew consent. Of the reasons for the screening failures, 443 were related to the inclusion criteria, 168 to the exclusion criteria, 48 to both the inclusion/exclusion criteria, and 8 to other. A total of 324 patients received ≥1 dose of randomized study medication. A total of 74 patients (22.8%) withdrew from the study prior to completion: 20.2% of all patients in the febuxostat group, 25.5% in the lesinurad 200 mg plus febuxostat group, and 22.9% in the lesinurad 400 mg plus febuxostat group. The most common reasons were TEAEs (10.5%) and noncompliance/protocol violation (9.0%). Study medication was completed by 86.2%, 82.1%, and 80.7% of patients in the respective groups at 6 months and by 76.1%, 71.7%, and 69.7%, respectively, at 12 months.
Figure 1.
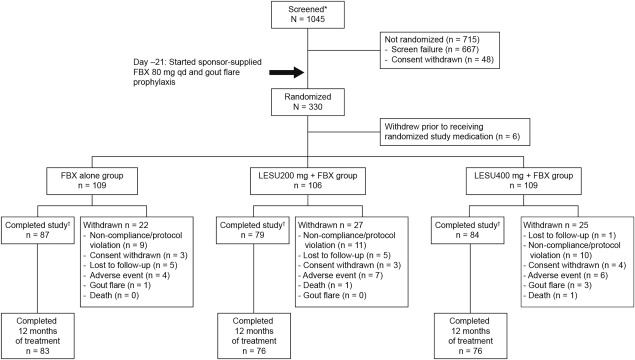
Flow chart showing the distribution of study patients from screening through study completion. * Signed an informed consent form. † Completed the study, with or without completing the randomized study medication. FBX = febuxostat; qd = once daily; LESU = lesinurad.
Baseline demographics and clinical history
Demographic and baseline disease characteristics were similar between treatment groups (Table 1). Patients were predominantly male (95.4%) and white (79.9%), with a mean ± SD age of 54.1 ± 11.0 years, and a mean ± SD time since gout diagnosis of 14.7 ± 10.9 years. Mean ± SD serum UA levels were 8.7 ± 1.6 mg/dl at screening and 5.3 ± 1.6 mg/dl at randomization (baseline), after 3 weeks of treatment with febuxostat 80 mg. At baseline, the mean ± SD number of tophi was 1.8 ± 1.2, and the mean ± SD area of target tophi was 293.6 ± 234.6 mm2. Patients reported the occurrence of a mean ± SD of 6.7 ± 8.2 gout flares in the 12 months prior to study entry.
Table 1.
Demographic and baseline clinical characteristics of the study patients, intent‐to‐treat populationa
| Placebo plus febuxostat (n = 109) | Lesinurad 200 mg plus febuxostat (n = 106) | Lesinurad 400 mg plus febuxostat (n = 109) | Total (n = 324) | |
|---|---|---|---|---|
| Age, mean ± SD years | 54.6 ± 10.9 | 54.2 ± 11.0 | 53.3 ± 11.2 | 54.1 ± 11.0 |
| Male, no. (%) | 107 (98.2) | 100 (94.3) | 102 (93.6) | 309 (95.4) |
| Race, no. (%) | ||||
| Asian | 6 (5.5) | 8 (7.5) | 6 (5.5) | 20 (6.2) |
| Black/African American | 8 (7.3) | 14 (13.2) | 13 (11.9) | 35 (10.8) |
| White | 94 (86.2) | 80 (75.5) | 85 (78.0) | 259 (79.9) |
| Other | 1 (0.9) | 4 (3.8) | 5 (4.6) | 10 (3.3) |
| Ethnicity, no. (%) | ||||
| Hispanic/Latino | 9 (8.3) | 7 (6.6) | 5 (4.6) | 21 (6.5) |
| Not Hispanic/Latino | 100 (91.7) | 99 (93.4) | 104 (95.4) | 303 (93.5) |
| Body weight, mean ± SD kg | 99.4 ± 21.0 | 110.3 ± 19.5 | 98.8 ± 21.4 | 99.5 ± 20.6 |
| Body mass index, mean ± SD kg/m2 | 32.0 ± 5.6 | 32.4 ± 5.6 | 31.6 ± 5.7 | 32.0 ± 5.6 |
| Duration since gout diagnosis, mean ± SD years | 15.2 ± 10.9 | 15.8 ± 11.0 | 13.2 ± 10.6 | 14.7 ± 10.9 |
| No. of target tophi at baseline, mean ± SD | 1.9 ± 1.3 | 1.8 ± 1.3 | 1.8 ± 1.2 | 1.8 ± 1.2 |
| Total area of target tophi at baseline, mean ± SD mm2 | 291.1 ± 246.4 | 310.1 ± 227.9 | 280.3 ± 230.3 | 293.6 ± 234.6 |
| No. of gout flares in previous 12 months, mean ± SD | 6.1 ± 5.1 | 6.9 ± 11.2 | 7.0 ± 7.4 | 6.7 ± 8.2 |
| Gout flare prophylaxis at baseline, no. (%) | ||||
| Colchicine | 87 (79.8) | 95 (89.6) | 94 (86.2) | 276 (85.2) |
| NSAIDs | 22 (20.2) | 9 (8.5) | 15 (13.8) | 46 (14.2) |
| Renal function (estimated CrCl) at baseline, no. (%) | ||||
| ≥90 ml/minute | 31 (28.4) | 37 (34.9) | 42 (38.5) | 110 (34.0) |
| 60 to <90 ml/minute | 53 (48.6) | 41 (38.7) | 45 (41.3) | 139 (42.9) |
| <60 ml/minute | 25 (22.9) | 28 (26.4) | 22 (20.2) | 75 (23.1) |
| Thiazide/thiazide‐like diuretic at baseline, no. (%) | 11 (10.1) | 15 (14.2) | 18 (16.5) | 44 (13.6) |
| Serum UA, mean ± SD mg/dl | ||||
| At screening | 8.8 ± 1.5 | 8.7 ± 1.6 | 8.6 ± 1.8 | 8.7 ± 1.6 |
| At baseline | 5.2 ± 1.5 | 5.4 ± 1.7 | 5.3 ± 1.6 | 5.3 ± 1.6 |
| Any CV comorbidity or CV disease history (combined), no. (%)b | 80 (73.4) | 81 (76.4) | 79 (72.5) | 240 (74.1) |
| Hypertension | 65 (59.6) | 70 (66.0) | 62 (56.9) | 197 (60.8) |
| Hyperlipidemia | 46 (42.2) | 42 (39.6) | 50 (45.9) | 138 (42.6) |
| Diabetes mellitus | 17 (15.6) | 21 (19.8) | 14 (12.8) | 52 (16.0) |
| Myocardial infarction | 7 (6.4) | 5 (4.7) | 7 (6.4) | 19 (5.9) |
| Kidney stones | 16 (14.7) | 15 (14.2) | 11 (10.1) | 42 (13.0) |
NSAIDs = nonsteroidal antiinflammatory drugs; CrCl = creatinine clearance; UA = uric acid; CV = cardiovascular.
Includes hypertension, hyperlipidemia (hypercholesterolemia, hypertriglyceridemia), diabetes mellitus, kidney stones, myocardial infarction, angina pectoris, heart failure, peripheral vascular disease, stroke, and transient ischemic attack.
Compliance with study medications
The overall proportion of patients demonstrating ≥80% compliance with the study medications was 99.1%, 97.2%, and 92.7% in the febuxostat, lesinurad 200 mg plus febuxostat, and lesinurad 400 mg plus febuxostat groups, respectively.
Efficacy assessments
Primary end point of serum UA response and secondary serum UA end points
The proportion of patients who achieved a serum UA level of <5.0 mg/dl by month 6 was 46.8% in the febuxostat group, 56.6% in the lesinurad 200 mg plus febuxostat group, and 76.1% in the lesinurad 400 mg plus febuxostat group (Figure 2). Significantly more patients treated with lesinurad 400 mg plus febuxostat achieved the primary end point compared with febuxostat alone (P < 0.0001), while the difference was not significant for the lesinurad 200 mg plus febuxostat group (P = 0.13).
Figure 2.
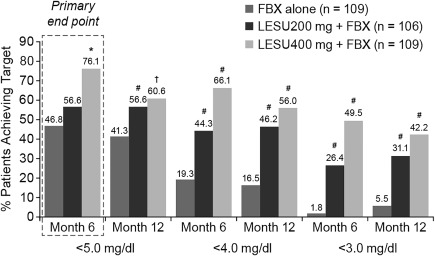
Proportion of patients achieving serum uric acid (UA) targets of <5.0 mg/dl, <4.0 mg/dl, and <3.0 mg/dl at month 6 and month 12 (intent‐to‐treat population). The primary end point was the proportion of patients achieving a serum UA level of <5.0 mg/dl at month 6, with nonresponder imputation. ∗ = P < 0.0001; # = P < 0.0001 versus febuxostat (FBX) alone, adjusted for multiple comparisons; † = P < 0.01 versus febuxostat alone, without adjustment for multiple comparisons. LESU = lesinurad.
The mean serum UA levels by visit are shown in Figure 3. Although the difference in serum UA levels was not statistically significantly different for the lesinurad 200 mg plus febuxostat group by month 6, superior treatment effects were observed for this group at all other time points assessed (months 1, 2, 3, 4, 5, 8, 10, and 12; P ≤ 0.0281). In addition, prespecified sensitivity and supporting analyses showed differences favoring lesinurad 200 mg plus febuxostat versus febuxostat alone (Supplementary Figure 2, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40159/abstract). These included achieving serum UA levels that were <5.0 mg/dl for each of 3 consecutive months (months 4, 5, and 6), a median serum UA level of <5.0 mg/dl, and a serum UA level of <4.0 and <3.0 mg/dl (Figure 2 shows months 6 and 12).
Figure 3.
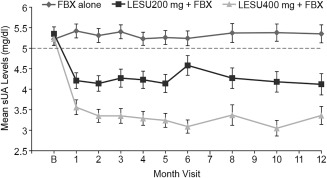
Mean serum urate (sUA) levels at each study visit in the observed cases (intent‐to‐treat population). Values are the mean ± SEM. For the lesinurad (LESU) plus febuxostat (FBX) groups, differences at all time points are P < 0.0001 versus baseline (B), except for month 6 for the lesinurad 200 mg plus febuxostat group, which is P = 0.0002.
Included in the prespecified analyses was the subgroup of patients with serum UA levels ≥5.0 mg/dl after 3 weeks of febuxostat treatment (n = 161 [49.7%]). In this subgroup, 23.5% of patients were at goal by month 6 with febuxostat treatment alone, 44.1% with lesinurad 200 mg plus febuxostat, and 70.6% with lesinurad 400 mg plus febuxostat (P = 0.024 and P < 0.0001 versus febuxostat alone). The proportion of patients who achieved a serum UA level of <5.0 mg/dl was greater in both lesinurad plus febuxostat groups than in the febuxostat alone group at all time points assessed (Supplementary Figure 3, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40159/abstract). Also, more patients achieved a serum UA level of <4.0 mg/dl by month 6 with lesinurad 200 mg plus febuxostat (28.8%, P = 0.005) and lesinurad 400 mg plus febuxostat (56.9%, P < 0.0001) than with febuxostat (7.8%).
Key secondary and secondary end points
Tophus resolution
The proportion of subjects with complete resolution of ≥1 target tophus was numerically greater in both the lesinurad 200 mg plus febuxostat (25.5%) and lesinurad 400 mg plus febuxostat (30.3%) groups compared with the febuxostat group (21.1%), although the differences were not statistically significant (P = 0.45 and P = 0.11, respectively). The proportion of patients with complete or partial resolution of ≥1 target tophus also was numerically greater in the lesinurad 200 mg plus febuxostat (49.1%) and lesinurad 400 mg plus febuxostat (51.4%) groups compared with the febuxostat group (45.9%) at month 12, but the differences were not significant.
At months 3, 6, 9, and 12 when tophi were measured, each of the lesinurad plus febuxostat treatment groups had a higher mean percentage reduction from baseline in the sum of the areas of all target tophi as compared with febuxostat alone (Figure 4). At month 12, a 50.1% and 52.9% reduction in target tophi area was observed with the lesinurad 200 mg plus febuxostat and lesinurad 400 mg plus febuxostat groups, respectively, compared with febuxostat alone (28.3%) (P < 0.05 and P < 0.01, respectively).
Figure 4.
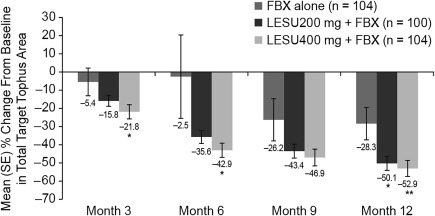
Percentage change in the sum of the areas of all target tophi versus baseline (mm2) at each study visit in the last observation carried forward imputation (intent‐to‐treat population). Values are the mean ± SEM. ∗ = P < 0.05; ∗∗ = P < 0.01 versus febuxostat (FBX) alone. LESU = lesinurad.
Gout flares requiring treatment
The mean ± SD rates of gout flares requiring treatment over the 6‐month period from the end of month 6 to the end of month 12 were 1.2 ± 2.7, 1.4 ± 2.5, and 0.7 ± 1.2 per patient per 6 months in the febuxostat, lesinurad 200 mg plus febuxostat, and lesinurad 400 mg plus febuxostat groups, respectively (P = 0.55 and P = 0.04 versus febuxostat alone). The proportion of patients with gout flares requiring treatment at the end of month 1 was higher in both lesinurad plus febuxostat groups (25.5% in those taking 200 mg and 35.8% in those taking 400 mg) than the febuxostat group (17.4%). The proportion of patients with gout flares requiring treatment generally declined throughout the study, with the lowest proportions at the end of month 11 to the end of month 12 (9.2% in those taking febuxostat, 10.1% in those taking lesinurad 200 mg plus febuxostat, and 6.0% in those taking lesinurad 400 mg plus febuxostat).
Safety assessments
Adverse events
The proportion of patients with TEAEs throughout the study was 72.5% in the febuxostat group, 82.1% in the lesinurad 200 mg plus febuxostat group, and 82.6% in the lesinurad 400 mg plus febuxostat group (Table 2). The majority of patients in each group had TEAEs with maximum severity of grade 1 or 2, based on the Rheumatology Common Toxicity Criteria 22. TEAEs led to discontinuation of study medication in 8.3%, 8.5%, and 13.8% of patients in the febuxostat, lesinurad 200 mg plus febuxostat, and lesinurad 400 mg plus febuxostat groups, respectively. The most common individual TEAEs in the febuxostat, lesinurad 200 mg plus febuxostat, and lesinurad 400 mg plus febuxostat groups, respectively, were nasopharyngitis (8.3%, 9.4%, and 13.8%), hypertension (7.3%, 5.7%, and 11.0%), headache (7.3%, 9.4%, and 5.5%), extremity pain (3.7%, 5.7%, and 8.3%), and back pain (4.6%, 7.5%, and 5.5%).
Table 2.
Overall summary of treatment‐emergent adverse events and renal‐related adverse events (safety population)*
| Adverse event category | Placebo plus febuxostat (n = 109) | Lesinurad 200 mg plus febuxostat (n = 106) | Lesinurad 400 mg plus febuxostat (n = 109) |
|---|---|---|---|
| Any TEAE | 79 (72.5) | 87 (82.1) | 90 (82.6) |
| Any TEAE with RCTC toxicity of grade 3 or 4 | 13 (11.9) | 11 (10.4) | 11 (10.1) |
| Any TEAE possibly related to randomized study medication | 22 (20.2) | 25 (23.6) | 28 (25.7) |
| Any serious TEAE | 10 (9.2) | 6 (5.7) | 9 (8.3) |
| Any fatal TEAE | 0 | 1 (0.9) | 1 (0.9) |
| Any TEAE leading to discontinuation of randomized study medication | 9 (8.3) | 9 (8.5) | 15 (13.8) |
| Any TEAE leading to study withdrawal | 4 (3.7) | 7 (6.6) | 7 (6.4) |
| Renal‐related AEs | |||
| Any renal‐related AEs | 6 (5.5) | 9 (8.5) | 11 (10.1) |
| Serious renal‐related AEs | 1 (0.9) | 0 (0) | 2 (1.8) |
| Acute renal failure | 1 (0.9) | 0 | 1 (0.9) |
| Chronic renal failure | 0 | 0 | 1 (0.9) |
| Kidney stones | 4 (3.7) | 1 (0.9) | 2 (1.8) |
| Serum creatinine elevation | |||
| ≥1.5 times baselinea | 3 (2.8) | 5 (4.7) | 11 (10.1) |
| Cases unresolved at last study visit†‡ | 0 | 1 | 1 |
| ≥2.0 times baseline | 0 (0) | 3 (2.8) | 6 (5.5) |
| Cases unresolved at last study visitb | 0 | 1 | 1 |
Values are the number (%). TEAE = treatment‐emergent adverse event; RCTC = Rheumatology Common Toxicity Criteria.
All ≥2.0 times baseline elevations were captured in the ≥1.5 times baseline elevations group.
Serum creatinine resolution was defined as return of an elevated serum creatinine level to ≤1.2 times baseline.
Serious TEAEs were reported in 9.2% of patients in the febuxostat group, 5.7% in the lesinurad 200 mg plus febuxostat group, and 8.3% in the lesinurad 400 mg plus febuxostat group (Table 2). No single serious TEAE occurred in >1 patient. One death was reported in the lesinurad 200 mg plus febuxostat group (due to cardiac arrest) and 1 in the lesinurad 400 mg plus febuxostat group (due to congestive heart failure).
Renal safety analyses
Renal‐related AEs occurred in 5.5% of patients in the febuxostat group, 8.5% in the lesinurad 200 mg plus febuxostat group, and 10.1% in the lesinurad 400 mg plus febuxostat group (Table 2). No patients in the lesinurad 200 mg plus febuxostat group had a renal‐related serious AE, while 2 patients in the lesinurad 400 mg plus febuxostat group (1.8%; renal failure acute; renal failure chronic) and 1 patient in the febuxostat group (0.9%; acute renal failure) had renal‐related serious AEs.† All were considered by the investigator to be either unlikely to be related or unrelated to the study medication. Kidney stone TEAEs were reported in 3.7%, 0.9%, and 1.8% of the patients in the febuxostat, lesinurad 200 mg plus febuxostat, and lesinurad 400 mg plus febuxostat groups, respectively.
Elevations in the serum creatinine level ≥1.5 times the baseline values occurred in 2.8% (n = 3), 4.7% (n = 5), and 10.1% (n = 11) of patients in the febuxostat, lesinurad 200 mg plus febuxostat, and lesinurad 400 mg plus febuxostat groups, respectively. A total of 100%, 60%, and 85.7% of cases resolved without interruption in study medication. Elevations in the serum creatinine level ≥2.0 times baseline occurred in 0.0%, 2.8% (n = 3), and 5.5% (n = 6) of the respective groups. There was only 1 unresolved elevation in both the lesinurad 200 mg plus febuxostat and lesinurad 400 mg plus febuxostat groups at the last study assessment.
Mean ± SD changes in the serum creatinine levels between baseline and the last assessment were 0.00 ± 0.19, 0.03 ± 0.18, and −0.09 ± 0.21 mg/dl in the febuxostat, lesinurad 200 mg plus febuxostat, and lesinurad 400 mg plus febuxostat groups, respectively. There were no clinically meaningful changes in the mean estimated creatinine clearance or protein‐to‐creatinine ratio (Supplementary Table 4, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40159/abstract).
Cardiovascular safety analyses
TEAEs classified as CV events were reported in 1.8%, 5.7%, and 3.7% of patients in the febuxostat, lesinurad 200 mg plus febuxostat, and lesinurad 400 mg plus febuxostat groups, respectively, with serious CV events in 0.9%, 2.8%, and 3.7%, respectively. All patients reporting CV events had ≥1 baseline CV comorbidity and/or CV disease history. The Cardiovascular Events Adjudication Committee determined that criteria for MACE were met by 1 patient (1 event) in the febuxostat group, 2 patients (2 events) in the lesinurad 200 mg plus febuxostat, and 2 patients (4 events) in the lesinurad 400 mg plus febuxostat. Non‐MACE CV end points were reported in 0 patients, 2 patients (2 events), and 2 patients (3 events), respectively. There were no notable changes from baseline in electrocardiography parameters in any group.
Findings of other clinical laboratory tests and vital signs
Clinical laboratory test results, including hematology, serum chemistry (excluding renal laboratory results reported above), and urinalysis, assessed over time demonstrated no notable differences between treatment groups. There were no notable changes in vital signs, including blood pressure, from baseline during the study in any group.
DISCUSSION
The CRYSTAL trial investigated the efficacy and safety of lesinurad in combination with febuxostat in patients with tophaceous gout. Lesinurad, 200 mg or 400 mg, in combination with febuxostat 80 mg increased the proportions of patients achieving a serum UA level of <5.0 mg/dl by month 6 (the primary end point) compared with febuxostat alone. The difference in proportions was significant only for the lesinurad 400 mg group. However, additional prespecified analyses showed that lesinurad 200 mg plus febuxostat was effective in more patients reaching the target serum UA level.
More importantly, in the prespecified analysis of the subset of patients with a baseline serum UA level of ≥5 mg/dl (i.e., those not at target after 3 weeks of febuxostat alone), the addition of lesinurad 200 mg enabled more patients to achieve serum UA levels of <5 mg/dl at all time points through month 12, including month 6. This is a clinically meaningful result because this is the group of patients with the greatest need for additional treatment options, as they failed to respond to febuxostat 80 mg, the highest dose of febuxostat approved in the US.
Some patients in the study achieved serum UA levels of <3 mg/dl. The clinical benefits and risks of these very low serum urate levels are currently uncertain. Although both clinical trial data and observational studies have shown benefit in flare reduction and tophus regression with very low serum urate concentrations 16, 23, the European League Against Rheumatism has recommended against lowering UA to these levels for more than several years 2.
Lesinurad 200 and 400 mg in combination with febuxostat resulted in increases in the proportions of patients with complete resolution of ≥1 target tophus by month 12 compared with febuxostat alone, but the differences were not statistically significant. A similar positive, but not statistically significant, trend was noted for the proportion of patients with complete or partial resolution of ≥1 target tophus by month 12. However, there was an almost 50% greater reduction in target tophi area with lesinurad 200 mg plus febuxostat and lesinurad 400 mg plus febuxostat treatments compared with febuxostat alone. This is the first study of an oral agent to show benefits in tophus regression by month 12 of therapy.
The mean rate of gout flares requiring treatment during the 6‐month period from the end of month 6 through month 12 was reduced by nearly 50% in the lesinurad 400 mg plus febuxostat group compared with febuxostat alone, whereas the rate with lesinurad 200 mg plus febuxostat was similar to that of febuxostat alone. The proportion of patients with gout flares requiring treatment declined over 52 weeks, similar to that observed in other studies with febuxostat or allopurinol 5, 6, 8. Longer‐term treatment may be needed to further reduce gout flares, as well as to dissolve baseline tophi, particularly to demonstrate treatment effect differences 24, 25.
Lesinurad was generally well tolerated, particularly at the 200 mg dose. Although the incidence of overall TEAEs was higher with lesinurad 200 mg and 400 mg in combination with febuxostat as compared with febuxostat alone, the majority of events were grade 1 or 2, and the incidences of serious AEs and TEAEs that led to study withdrawal were comparable across the 3 treatment groups. Patients treated with lesinurad 400 mg in combination with febuxostat had a higher incidence of TEAEs that led to discontinuation of the randomized study medication as compared with lesinurad 200 mg in combination with febuxostat or febuxostat alone.
In renal safety analyses, patients treated with lesinurad 200 mg or 400 mg in combination with febuxostat had a higher incidence of renal‐related TEAEs compared with those treated with febuxostat alone. Elevations of the serum creatinine levels occurred at higher rates in the lesinurad groups as compared with febuxostat alone, particularly with lesinurad 400 mg, a dosage which has not been approved for treatment. The majority of serum creatinine elevations resolved by the time of the next assessment; most resolved without interruption of randomized study medication and without adverse effects on renal function during the study. The mechanism underlying elevated serum creatinine levels has not been completely elucidated but may be due to increased excretion and microcrystallization of urinary UA in renal tubules. NSAIDs were allowed as prophylaxis in patients who were not able to tolerate colchicine, and NSAID use was low (∼17% of patients). Evaluation of CV and renal safety in this subgroup did not demonstrate notable treatment differences. The safety profile of lesinurad 200 mg daily in combination with a xanthine oxidase inhibitor is being further characterized in a randomized controlled clinical trial with a planned duration of 2 years.
Other therapies that inhibit URAT1 have been associated with the development of kidney stones 18, 26. Few kidney stone TEAEs were reported during the study. This may be explained by the fact that febuxostat, through decreasing urate production, reduces the amount of uric acid excreted by the kidneys, as previously reported for xanthine oxidase inhibitor therapies 27, 28.
When prescribing lesinurad, physicians should take into consideration that patients with gout typically present with comorbidities, may also be taking other renal‐acting medications, and may be poorly hydrated. The prescribing information 10 recommends that patients take lesinurad with food and water and maintain appropriate hydration via daily water intake. It also states that physicians should monitor patients' renal function before they start and while they are taking lesinurad, particularly in patients with an estimated creatinine clearance of <60 ml/minute or with serum creatinine elevations 1.5–2 times the starting value, and evaluate for signs and symptoms of acute uric acid nephropathy. Lesinurad should not be started in patients with an estimated creatinine clearance of <45 ml/minute.
In CV safety analyses, CV comorbidities and risk factors were present in 74% of the patients at baseline, reflecting the high rates of CV disease in gout patients. Nonserious CV TEAEs were observed at low frequencies in this population at high risk of a CV event, with small increases in rates with the lesinurad treatment groups. All patients had ≥1 baseline CV comorbid condition or CV disease history. Independently adjudicated MACE occurred in 1 patient in the febuxostat group and 2 patients in each lesinurad plus febuxostat group. Results of database analyses have indicated no change in CV risk upon initiation of xanthine oxidase inhibitor therapy 29 and similar risks of CV AEs for allopurinol and febuxostat versus placebo 30, 31.
Limitations of CRYSTAL include the small number of women in the study and the high percentage of patients who had already achieved serum UA levels of <5.0 mg/dl at randomization after 3 weeks of febuxostat treatment. However, analysis of patients whose serum UA level was not at goal at the time of randomization showed that lesinurad (200 or 400 mg) plus febuxostat increased the percentage of patients at goal compared with febuxostat alone. The relatively short length of the study limited the potential amount of resolution of tophi and decline in gout flares. Previous studies with febuxostat or allopurinol showed that much longer treatment, longer than 12 months, was needed to show a treatment difference 24, 25. An extension of CRYSTAL has recently been completed that included an additional 1 year follow‐up for assessment of resolution of tophi and decline in gout flares.
For patients whose disease is not controlled with an appropriate dose of a xanthine oxidase inhibitor for whom a uricosuric is recommended, there is a need for additional treatment options. Lesinurad 200 mg is a novel selective uric acid reabsorption inhibitor approved for treatment of gout in combination with a xanthine oxidase inhibitor for those not at target serum UA levels with a xanthine oxidase inhibitor alone. Combination therapy with lesinurad and febuxostat provides a dual mechanism, addressing both uric acid excretion and urate production, and may represent a treatment option for patients with tophaceous gout who are taking febuxostat and warrant additional therapy.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Dalbeth had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Dalbeth, Storgard, Baumgartner, Perez‐Ruiz.
Acquisition of data
Dalbeth, Jones, Terkeltaub, Khanna, Kopicko, Bhakta, Adler, Fung, Storgard, Baumgartner, Perez‐Ruiz.
Analysis and interpretation of data
Dalbeth, Jones, Terkeltaub, Khanna, Kopicko, Bhakta, Adler, Fung, Storgard, Baumgartner, Perez‐Ruiz.
ROLE OF THE STUDY SPONSOR
Ardea Biosciences, a subsidiary of AstraZeneca, had a role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, the review and approval of the manuscript, and the decision to submit the manuscript for publication. Editorial support was provided by Tom Claus, PhD (PAREXEL, a clinical research organization), and was funded by AstraZeneca. Publication of this article was contingent upon approval by Ardea Biosciences.
Supporting information
Supplemental Table 1. Complete inclusion/exclusion criteria for CRYSTAL
Supplemental Table 2. Renal‐related and kidney stone TEAEs
Supplemental Table 3. Major and non‐major adverse cardiovascular events
Supplemental Table 4. Change from baseline to month 12 (or to last value) in eCrCl and change from baseline to month 12 in the urinary protein/creatinine ratio.
Supplemental Figure 1. Study Design
Supplemental Figure 2. Summary of analyses showing a response that favors lesinurad 200 mg + febuxostat over placebo + febuxostat. NRI, nonresponder imputation; LOCF, last observation carried forward; sUA, serum uric acid; LESU, lesinurad; PBO, placebo; FBX, febuxostat; CI, confidence interval.
Supplemental Figure 3. Proportion of patients with a sUA <5.0 mg/dl by visit – Nonresponder imputation (ITT population subgroup with baseline sUA ≥5.0 mg/dl). *P ≤ 0.025 for difference in proportions versus PBO + FBX. sUA = serum uric acid; PBO = placebo; FBX = febuxostat; LESU = lesinurad.
ClinicalTrials.gov identifier: NCT01510769.
Supported by Ardea Biosciences (a member of the AstraZeneca group).
Dr. Dalbeth has received consulting fees, speaking fees, and/or honoraria from Menarini, AstraZeneca, Takeda, Fonterra, Pfizer, Cymabay, and Crealta (less than $10,000 each) and research grants from AstraZeneca. Dr. Jones has received consulting fees, speaking fees, and/or honoraria from UCB, Roche, Janssen, AbbVie, Novartis, Mundipharma, Amgen, Bristol‐Myers Squibb, Pfizer, and Hospira (less than $10,000 each) and research grants from AbbVie, Ardea, Novartis, and Auxilium. Dr. Terkeltaub has received consulting fees from AstraZeneca, Takeda, Revive, Relburn, and UCB (less than $10,000 each). Dr. Khanna has received consulting fees from AstraZeneca and Takeda (less than $10,000 each) and a research grant from AstraZeneca. Dr. Perez‐Ruiz has received consulting fees, speaking fees, and/or honoraria from AstraZeneca, Menarini, Metabolex, Novartis, Pfizer, and Sobi (less than $10,000 each).
Footnotes
Correction added on August 21, 2017, after online publication: 21.8% was changed to 1.8% in the preceding sentence.
REFERENCES
- 1. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda‐Sanabria J, et al. 2016 updated EULAR evidence‐based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42. [DOI] [PubMed] [Google Scholar]
- 3. Kiltz U, Smolen J, Bardin T, Cohen SA, Dalbeth N, Doherty M, et al. Treat‐to‐target (T2T) recommendations for gout. Ann Rheum Dis 2016;76:632–8. [DOI] [PubMed] [Google Scholar]
- 4. Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 2006;58:87–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450–61. [DOI] [PubMed] [Google Scholar]
- 6. Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, et al. The urate‐lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010;12:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becker MA, Fitz‐Patrick D, Choi HK, Dalbeth N, Storgard C, Cravets M, et al. An open‐label, 6‐month study of allopurinol safety in gout: the LASSO study. Semin Arthritis Rheum 2015;45:174–83. [DOI] [PubMed] [Google Scholar]
- 8. Schumacher HR Jr, Becker MA, Wortmann RL, MacDonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28‐week, phase III, randomized, double‐blind, parallel‐group trial. Arthritis Rheum 2008;59:1540–8. [DOI] [PubMed] [Google Scholar]
- 9. Edwards NL. Treatment‐failure gout: a moving target. Arthritis Rheum 2008;58:2587–90. [DOI] [PubMed] [Google Scholar]
- 10.Zurampic (lesinurad) prescribing information. Wilmington (DE): AstraZeneca; 2015.
- 11. Yeh L, Shen Z, Kerr B. RDEA594: a potent URAT1 inhibitor without affecting other important renal transporters, OAT1 and OAT3 [abstract]. Ann Rheum Dis 2009;68:320. [Google Scholar]
- 12. Fleischmann R, Kerr B, Yeh LT, Suster M, Shen Z, Polvent E, et al. Pharmacodynamic, pharmacokinetic and tolerability evaluation of concomitant administration of lesinurad and febuxostat in gout patients with hyperuricaemia. Rheumatology (Oxford) 2014;53:2167–74. [DOI] [PubMed] [Google Scholar]
- 13. Iwanaga T, Kobayashi D, Hirayama M, Maeda T, Tamai I. Involvement of uric acid transporter in increased renal clearance of the xanthine oxidase inhibitor oxypurinol induced by a uricosuric agent, benzbromarone. Drug Metab Dispos 2005;33:1791–5. [DOI] [PubMed] [Google Scholar]
- 14. Stocker SL, Graham GG, McLachlan AJ, Williams KM, Day RO. Pharmacokinetic and pharmacodynamic interaction between allopurinol and probenecid in patients with gout. J Rheumatol 2011;38:904–10. [DOI] [PubMed] [Google Scholar]
- 15. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977;20:895–900. [DOI] [PubMed] [Google Scholar]
- 16. Perez‐Ruiz F, Calabozo M, Pijoan JI, Herrero‐Beites AM, Ruibal A. Effect of urate‐lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Rheum 2002;47:356–60. [DOI] [PubMed] [Google Scholar]
- 17. Richette P, Perez‐Ruiz F, Doherty M, Jansen TL, Nuki G, Pascual E, et al. Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol 2014;10:654–61. [DOI] [PubMed] [Google Scholar]
- 18. Perez‐Ruiz F, Hernandez‐Baldizon S, Herrero‐Beites AM, Gonzalez‐Gay MA. Risk factors associated with renal lithiasis during uricosuric treatment of hyperuricemia in patients with gout. Arthritis Care Res (Hoboken) 2010;62:1299–305. [DOI] [PubMed] [Google Scholar]
- 19. White WB, Faich G, Borer JS, Makuch RW. Cardiovascular thrombotic events in arthritis trials of the cyclooxygenase‐2 inhibitor celecoxib. Am J Cardiol 2003;92:411–8. [DOI] [PubMed] [Google Scholar]
- 20. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woodworth T, Furst DE, Alten R, Bingham C, Yocum D, Sloan V, et al. Standardizing assessment and reporting of adverse effects in rheumatology clinical trials II: the Rheumatology Common Toxicity Criteria v.2.0. J Rheumatol 2007;34:1401–14. [PubMed] [Google Scholar]
- 23. Sundy JS, Baraf HS, Yood RA, Edwards NL, Gutierrez‐Urena SR, Treadwell EL, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 2011;306:711–20. [DOI] [PubMed] [Google Scholar]
- 24. Becker MA, Schumacher HR, MacDonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol 2009;36:1273–82. [DOI] [PubMed] [Google Scholar]
- 25. Schumacher HR Jr, Becker MA, Lloyd E, MacDonald PA, Lademacher C. Febuxostat in the treatment of gout: 5‐yr findings of the FOCUS efficacy and safety study. Rheumatology (Oxford) 2009;48:188–94. [DOI] [PubMed] [Google Scholar]
- 26. Bach MH, Simkin PA. Uricosuric drugs: the once and future therapy for hyperuricemia? Curr Opin Rheumatol 2014;26:169–75. [DOI] [PubMed] [Google Scholar]
- 27. Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 1986;315:1386–9. [DOI] [PubMed] [Google Scholar]
- 28. Goldfarb DS, MacDonald PA, Gunawardhana L, Chefo S, McLean L. Randomized controlled trial of febuxostat versus allopurinol or placebo in individuals with higher urinary uric acid excretion and calcium stones. Clin J Am Soc Nephrol 2013;8:1960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim SC, Schneeweiss S, Choudhry N, Liu J, Glynn RJ, Solomon DH. Effects of xanthine oxidase inhibitors on cardiovascular disease in patients with gout: a cohort study. Am J Med 2015;128:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh JA. Advances in gout: some answers, more questions. Arthritis Res Ther 2010;12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tayar JH, Lopez‐Olivo MA, Suarez‐Almazor ME. Febuxostat for treating chronic gout. Cochrane Database Syst Rev 2012;11:CD008653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Complete inclusion/exclusion criteria for CRYSTAL
Supplemental Table 2. Renal‐related and kidney stone TEAEs
Supplemental Table 3. Major and non‐major adverse cardiovascular events
Supplemental Table 4. Change from baseline to month 12 (or to last value) in eCrCl and change from baseline to month 12 in the urinary protein/creatinine ratio.
Supplemental Figure 1. Study Design
Supplemental Figure 2. Summary of analyses showing a response that favors lesinurad 200 mg + febuxostat over placebo + febuxostat. NRI, nonresponder imputation; LOCF, last observation carried forward; sUA, serum uric acid; LESU, lesinurad; PBO, placebo; FBX, febuxostat; CI, confidence interval.
Supplemental Figure 3. Proportion of patients with a sUA <5.0 mg/dl by visit – Nonresponder imputation (ITT population subgroup with baseline sUA ≥5.0 mg/dl). *P ≤ 0.025 for difference in proportions versus PBO + FBX. sUA = serum uric acid; PBO = placebo; FBX = febuxostat; LESU = lesinurad.


