Abstract
Early mortality (EM; death ≤ 6 months from diagnosis) has been reported in several newly diagnosed multiple myeloma (NDMM) trials. Before the era of novel agents, the incidence was 10%‐14%. Causes of death included infections/pneumonia, renal failure, refractory disease, and cardiac events. Staging systems, such as the revised International Staging System (r‐ISS), and prognostic factors including cytogenetics, lactate dehydrogenase levels, and myeloma‐specific factors, are useful to assess overall prognosis; however, they cannot predict EM. We evaluated patients treated with novel agents in the Connect MM® Registry and identified risk factors of the EM cohort. Eligible patients were enrolled in the registry within 60 days of diagnosis. Univariate and multivariate analyses were conducted to evaluate associations between baseline characteristics and EM. Prediction matrices for EM were constructed from a logistic model. Between September 2009 and December 2011, 1493 patients were enrolled in the registry and had adequate follow‐up. Of these patients, 102 (6.8%) had EM and 1391 (93.2%) survived for > 180 days. Baseline factors significantly associated with increased EM risk included age > 75 years, higher Eastern Cooperative Oncology Group performance status, lower EQ‐5D mobility score, higher ISS stage, lower platelet count, and prior hypertension. Renal insufficiency trended toward increased EM risk. These risk factors were incorporated into a prediction matrix for EM. The EM prediction matrix uses differential weighting of risk factors to calculate EM risk in patients with NDMM. Identifying patients at risk for EM may provide new opportunities to implement patient‐specific treatment strategies to improve outcomes.
1. INTRODUCTION
Even as the new era of multiple myeloma (MM) treatment has gone beyond novel agents to include aggressive incorporation of stem cell transplant and monoclonal antibodies, early mortality (EM) remains a major clinical issue that physicians need to be able to recognize the risk for and to prevent among their patients with newly diagnosed MM (NDMM). EM in NDMM is often described within the literature as death that occurs 2 to 12 months after diagnosis.1, 2 Among patients with NDMM, EM can be influenced by patient age, comorbidities, PS, therapy, and disease biology.1, 2, 3, 4, 5, 6
Risk factors of EM in patients with MM have been investigated in several studies, including a study that used data from the IFM 2005–01 phase 3 trial to develop a prognostic index of EM.7 That study constructed a prognostic index of early MM progression‐related death within 2 years of treatment initiation based on 3 independent prognostic variables: lactate dehydrogenase levels higher than normal, International Staging System (ISS) stage III, and adverse cytogenetics (t[4;14] and/or del[17p]).7 However, that index did not allow for differential weighting of each of the 3 prognostic variables, and its applicability is limited to transplant‐eligible patients (who comprise approximately 40% of patients with NDMM) who would meet the strict eligibility criteria of clinical trials in MM. By contrast, prediction matrix models similar to those created for cardiovascular disease and rheumatoid arthritis8, 9 can be used to calculate the risk of specific outcomes, such as mortality, and allow for differential weighting for the various model‐specific risk factors. Identifying patients with an increased risk of EM is critical for physicians to consider implementing different therapeutic approaches. Prediction tools that analyze both biological and clinical features have also been successfully used in several hematologic malignancies to predict EM. In acute myeloid leukemia, age, performance status (PS), and intensity of treatment have motivated the development of novel treatment paradigms.10 In diffuse large B‐cell lymphoma, molecular classification and positron emission tomography scans have introduced earlier decisions in the clinical treatment course to improve outcomes.11, 12
Much of what is currently known about risk factors for EM in patients with NDMM is based on data from clinical trial populations, which often differ from unselected patient populations due to exclusion of patients who are older, have comorbidities, are in poorer health, or are often ineligible for stem cell transplant. An observational registry offers the unique opportunity to report a broad set of baseline characteristics and treatment outcomes while assessing associations between NDMM biology, patient characteristics, comorbidities, and treatment selection with outcomes and overall survival. Thus, the Connect MM Registry investigators sought to review the registry patient population to get a comprehensive look at the incidence and factors associated with EM. The registry included patients independent of selection bias, age, therapy, PS, and comorbidities, which allowed for a cross‐sectional evaluation of patients with NDMM. Using data from a largely unselected patient population reflective of the heterogeneous patients seen in routine clinical practice, the registry investigators were able to develop a prognostic tool to assess the risk of EM that allows for differential weighting of risk factors. Here, we describe the construction of an early mortality prediction matrix (EMPM) in a population that includes both elderly and non–transplant‐eligible patients.
2. METHODS
The Connect MM REGISTRY is a US‐based, multicenter, prospective, observational, cohort registry study designed to describe patterns of care in NDMM in clinical practice and to analyze patient outcomes in correlation with baseline characteristics and treatments. Patients were enrolled into the registry consecutively per protocol to minimize selection bias. The study design and patient population have been previously described.13 Briefly, patients were eligible for enrollment within 2 months of a symptomatic MM diagnosis if aged ≥ 18 years, able to provide signed informed consent, and able to complete patient assessment questionnaires with no or minimal assistance from caregivers. Each participant enrolled in the Connect MM Registry signed an informed consent form. Study sites obtained central or local institutional review board approval. No exclusion criteria were applied. Treatment was at the discretion of each physician, and patient data were collected at baseline and quarterly thereafter using electronic case report forms. Collected data were reviewed and queries issued for consistency. Patients were censored at the discontinuation date and the data cutoff date. The Connect MM Registry is registered with ClinicalTrials.gov (NCT01081028).
Univariate and multivariate regression modeling was used to evaluate association of baseline characteristics with survival. Variables were not preselected and represented an exhaustive set of baseline characteristics. A series of univariate logistic regression models were used to identify variables significantly associated with mortality ≤ 180 days after enrollment. This cutoff to define EM was selected by the Connect MM Steering Committee based on survival patterns seen in previous noninterventional studies of MM and allowed for assessment of clinical care and treatment. 5, 6, 14, 15, 16
Missing data are inherent to registries due to their noninterventional nature. The Connect MM Registry allowed for diagnostic data to be collected at the discretion of the physician, which resulted in missing data. Patient referrals from one center to another may have also resulted in missing data. Variables with < 60% missing data were included. Characteristics with P < .15 in univariate analysis were entered in order of decreasing significance into a series of multivariate models. The best multivariate model was selected by considering whether the added variable was significant at the 0.1 level using the Wald χ2 test with 1 degree of freedom. Multiple imputations, based on the Markov chain Monte Carlo method under the multivariate normal model,17 were generated for significant variables with 70% nonmissing data. The final model parameters were estimated for each imputed data set, and the results, across the imputed data sets, were combined using the Rubin method.
Construction of prediction matrices was based on a logistic model for mortality ≤ 180 days after enrollment. Internal cross‐validation of the model was performed with bootstrap resampling (100 samples) from the empirical data, using the Harrell C‐index18 to determine the concordance probability. For the logistic model, the concordance probability is identical to the area under the receiver operating characteristic curve for the model. A prediction matrix is a summary of an estimated logistic regression that illustrates the predicted probability of the modeled outcome at constituent levels of each of the predictors. In this way, the prediction matrix offers insight into the relative importance of each covariate and can be used to highlight the differential impact of predictors at different constituent levels.
External validation of the logistic model was also performed using data from the phase 3 MM‐015 trial, which investigated the safety and efficacy of lenalidomide in combination with melphalan, and prednisone in patients aged ≥ 65 years with NDMM.19 The EQ‐5D mobility score, which was not collected in the MM‐015 study, was estimated using Karnofsky PS (Karnofsky PS scores of ≤ 50 were mapped to the “confined to bed” mobility item, scores between 60 and 80 were mapped to “some problem walking about,” and scores of ≥ 90 were mapped to “no problem in walking about”). The resulting classification of patients from MM‐015 (0%, 55.9%, and 44.1%, respectively) was similar to that of patients with nonmissing data in the Connect MM Registry (1.0%, 56.4%, and 42.6%, respectively). Similarly, Eastern Cooperative Oncology Group (ECOG) PS was estimated using conversion criteria for Karnofsky PS scores recommended by the European Society for Medical Oncology.20, 21 A second external validation was conducted using data from the phase 3 FIRST (Frontline Investigation of Lenalidomide + Dexamethasone Versus Standard Thalidomide) trial (MM‐020), which investigated the safety and efficacy of lenalidomide plus low‐dose dexamethasone given until disease progression in transplant‐ineligible patients with NDMM.22 Finally, the model was validated utilizing data from a second cohort of patients with ≥ 10 months of follow‐up (n = 1492) enrolled in the Connect MM REGISTRY from December 2012 to April 2016. All 7 variables identified as predictors of EM in the logistic model developed from the Connect MM Registry data were collected in the FIRST study. Both external validations used the same 7 variables identified from the Connect MM data set and were conducted using the rms package of the R statistical programming language using the methods of Harrell.18
3. RESULTS
3.1. Patients
A total of 1493 protocol‐eligible patients were consecutively enrolled in the registry between September 2009 and December 2011 (Cohort 1) and had adequate baseline and postbaseline data. At the data cutoff date of November 30, 2014, the median time of follow‐up in the Connect MM Registry was 33.8 months (range, 0–59.8 months). Of the 1493 patients analyzed, 102 (6.8%) had EM (survival of ≤ 180 days) and 1391 (93.2%) survived for > 180 days or were censored. The median time from diagnosis to enrollment was 25 days. Patient characteristics between those who enrolled early (< 25 days from diagnosis) vs those who enrolled later (≥ 25 from diagnosis) were similar. A logistic regression analysis indicated that there was no bias in the probability of EM due to the lag time between diagnosis and enrollment (early vs late enrollers; odds ratio 1.19; 95% CI: 0.8–1.79; P = 0.390). Furthermore, 14 patients were hospitalized when they signed or immediately after they signed the informed consent document, indicating that very sick patients enrolled into the registry.
3.2. Baseline characteristics
At baseline, patients with EM were older than patients who survived for > 180 days (median age, 71.5 vs 66.0 years, respectively) and had a history of more comorbidities, including diabetes (31.0% vs 18.1%) and hypertension (75.8% vs 56.9%) (Table 1). A greater proportion of patients with EM had renal insufficiency (33.3% vs 17.1%), hypercalcemia (14.7% vs 6.8%), and low platelet counts (37.0% vs 21.0%) as well as poorer PS (ECOG PS ≥ 2 in 45.1% vs 14.7%). High‐risk disease features were more common in patients with EM. These features included extramedullary disease (62.5% vs 35.1%), low serum albumin levels (65.2% vs 51.5%), and elevated β2‐microglobulin concentrations (≥ 5.5 mg/L; 66.7% vs 34.6%). A greater proportion of patients with EM had ISS stage III disease (68.4% vs 35.2%). Similarly, del(17p) by cytogenetic analysis (25.0% vs 14.0%) and del(17p) by fluorescence in situ hybridization (FISH) analysis (34.5% vs 25.6%) were more common in patients with EM than patients who survived for > 180 days.
Table 1.
Baseline characteristics and treatment
|
Mortality within 180 days (n = 102) |
Mortality > 180 days or censored (n = 1391) |
|
|---|---|---|
| Patient specific | ||
| Median age, years (range) | 71.5 (38–91) | 66.0 (24–94) |
| Male, n (%) | 53 (52.0) | 801 (57.6) |
| Race, n/N (%)a | ||
| White | 84/102 (82.4) | 1137/1390 (81.8) |
| Black | 11/102 (10.8) | 186/1390 (13.4) |
| Other | 7/102 (6.9) | 67/1390 (4.8) |
| Median body mass index, kg/m2 (range) | 27.4 (14.7–52.6) | 27.8 (13.5–58.7) |
| ECOG performance status ≥ 2, n/N (%)a | 32/71 (45.1) | 144/982 (14.7) |
| History of diabetes, n/N (%)a | 31/100 (31.0) | 247/1368 (18.1) |
| History of hypertension requiring treatment, n/N (%)a | 75/99 (75.8) | 771/1354 (56.9) |
| History of VTE, n/N (%)a | 6/88 (6.8) | 59/1273 (4.6) |
| Disease specific | ||
| del(17p) from cytogenetics, n/N (%)a | 4/16 (25.0) | 16/114 (14.0) |
| del(17p) from FISH, n/N (%)a | 10/29 (34.5) | 91/356 (25.6) |
| t(4;14) from FISH, n/N (%)a | 3/24 (12.5) | 53/297 (17.8) |
| History of MGUS, n/N (%)a | 10/92 (10.9) | 151/1308 (11.5) |
| History of smoldering myeloma, n/N (%)a | 5/92 (5.4) | 81/1312 (6.2) |
| Hyperdiploid, n/N (%)a | 7/13 (53.8) | 81/127 (63.8) |
| Lactic acid dehydrogenase > 300 g/dL, n/N (%)a | 9/42 (21.4) | 103/609 (16.9) |
| Extramedullary plasmacytoma, n/N (%)a | 10/16 (62.5) | 59/168 (35.1) |
| Immunoglobulin G ≥ 5 g/dL, n/N (%)a | 13/80 (16.3) | 196/1116 (17.6) |
| Albumin ≤ 3.5 g/dL, n/N (%)a | 60/92 (65.2) | 667/1294 (51.5) |
| ISS stage III (calculated), n/N (%)a, b | 52/76 (68.4) | 373/1061 (35.2) |
| Myeloma bone involvement, n/N (%)a | 73/101 (72.3) | 1070/1389 (77.0) |
| Hypercalcemia (serum calcium ≥ 11.5 mg/dL), n/N (%)a | 15/102 (14.7) | 94/1382 (6.8) |
| Renal insufficiency (serum creatinine > 2 mg/dL), n/N (%)a | 34/102 (33.3) | 237/1384 (17.1) |
| Anemia (hemoglobin < 10 g/dL or > 2 below LLN), n (%) | 51 (50.0) | 619 (44.5) |
| Platelet count, n/N (%)a | ||
| ≤ 150 × 109/L | 37/100 (37.0) | 291/1388 (21.0) |
| > 150 × 109/L | 63/100 (63.0) | 1097/1388 (79.0) |
| IMWG risk category, n/N (%)a | ||
| High risk | 19/60 (31.7) | 234/875 (26.7) |
| Standard risk | 39/60 (65.0) | 553/875 (63.2) |
| Low risk | 2/60 (3.3) | 88/875 (10.1) |
| β2‐microglobulin (≥ 5.5 mg/L), n/N (%)a | 52/78 (66.7) | 373/1077 (34.6) |
| HRQOL from EQ‐5D, n/N (%)a | ||
| Self‐care from EQ‐5D (unable to wash or dress) | 10/98 (10.2) | 21/1381 (1.5) |
| Mobility from EQ‐5D (confined to bed) | 5/99 (5.1) | 10/1384 (0.7) |
| Treatment | ||
| Setting, n (%) | ||
| Community | 82 (80.4) | 1129 (81.2) |
| Academic | 18 (17.6) | 245 (17.6) |
| Government | 2 (2.0) | 17 (1.2) |
| Timing, n (%) | ||
| ≤ 60 days | 93 (91.2) | 1330 (95.6) |
| > 60 days | 0 (0.0) | 27 (1.9) |
| Treatment not started | 9 (8.8) | 34 (2.4) |
| Novel therapy | ||
| 1, n/N (%)a | 72/87 (82.7) | 890/1268 (70.2) |
| ≥ 2, n/N (%)a | 15/87 (17.2) | 378/1268 (29.8) |
| Combination therapy | ||
| Doublet therapy (use of 2 therapies), n/N (%)a | 52/93 (56.0) | 606/1357 (44.6) |
| Triplet therapy (use of 3 therapies), n/N (%)a | 28/93 (30.0) | 607/1357 (44.7) |
| Unknown, n (%) | 9 (8.8) | 34 (2.4) |
| Radiation therapy for myeloma, n/N (%)a | 24/98 (24.5) | 211/1377 (15.3) |
HRQOL indicates health‐related quality of life; LLN, lower limit of normal; MGUS, monoclonal gammopathy of undetermined significance; and VTE, venous thromboembolism.
N = patients with data available.
Calculated using albumin and β2‐microglobulin levels from diagnosis or if not present from enrollment.
3.3. Treatment
The majority of patients in both groups (approximately 81%) were enrolled within community practices, and approximately 18% were enrolled in academic centers (Table 1). The timing of initiating treatment was similar for both patient groups. A smaller proportion of patients with EM vs longer‐surviving patients received ≥ 2 novel therapies (17.2% vs 29.8%) and triplet therapy (30.0% vs 44.7%). Radiation therapy for myeloma was administered to a higher proportion of patients with EM (24.5% vs 15.3%).
3.4. Causes of death
For the 102 patients with EM, 39.2% (2.7% of the total enrolled patients) of deaths were related to MM progression and 32.4% were related to nonmyeloma causes. Common causes of death in patients with EM included heart failure, pneumonia, other infection, and renal failure, and 28.4% were listed as other or unknown causes (Table 2). For the 1391 patients surviving for > 180 days, 31.5% died within the time frame of this analysis. The majority of these deaths (58.2%) were directly attributed to myeloma progression and 5% were due to nonmyeloma causes. Common causes of death included renal failure, heart failure, pneumonia, and other infections; in 25.8% of patients, the cause of death was listed as other or unknown.
Table 2.
Primary cause of death
|
Mortality within 180 days (n = 102) |
Mortality > 180 days or censored (n = 1391) |
|
|---|---|---|
| Total deaths, n | 102 | 438 |
| Directly attributed to myeloma progression, n (%) | 40 (39.2) | 255 (58.2) |
| Not directly attributed to myeloma progression, n (%) | 33 (32.4) | 70 (5.0) |
| Heart failure, n (%)a | 11 (33.3) | 17 (24.3) |
| Other infection, n (%)a | 7 (21.2) | 12 (17.1) |
| Pneumonia, n (%)a | 6 (18.2) | 18 (25.7) |
| Renal failure, n (%)a | 4 (12.1) | 19 (27.1) |
| Sudden death, n (%)a | 2 (6.1) | 0 (0.0) |
| Pulmonary embolus, n (%)a | 2 (6.1) | 1 (1.4) |
| Vascular event, n (%)a | 1 (3.0) | 2 (2.8) |
| Bleeding, n (%)a | 0 (0.0) | 1 (1.4) |
| Other, n (%) | 20 (19.6)b | 65 (14.8) |
| Unknown, n (%) | 9 (8.8) | 48 (11.0) |
Proportion due to deaths not directly attributed to myeloma progression.
Other deaths included sepsis (n = 7), cardiac related (n = 3), lung and brain cancer (n = 2), and single occurrences of death due to aspiration pneumonitis, complications from anasarca, emphysema, gastrointestinal hemorrhage, liver failure, multiorgan system failure, perforated diverticulum, and respiratory arrest.
3.5. Logistic regression analysis
In the univariate analysis, 15 baseline factors were statistically associated with EM (Table 3). The 15 factors were included in the multivariate logistic regression analysis and following an iterative variable selection process, 6 variables were identified as significantly associated with a higher likelihood of EM: age (> 75 years), prior hypertension, higher ECOG PS, higher ISS stage, lower platelet count, and lower EQ‐5D mobility score (Table 3). Additionally, a trend toward increased likelihood of EM was associated with renal insufficiency (serum creatinine > 2 mg/dL), making renal insufficiency a seventh factor. The 7 factors identified in the multivariate regression analysis were used to construct a prediction matrix that estimated the probability of EM (Figure 1A).
Table 3.
Baseline characteristics associated with mortality within 180 days by univariate and multivariate logistic regression analyses
| Univariate Analysis | |||
|---|---|---|---|
| Estimate | 95% CI | P a | |
| Patient specific | |||
| Age (≤ 75 vs > 75 years) | 2.09 | 1.37–3.19 | .001 |
| Body mass index, kg/m2 | 1.06 | 0.84–1.34 | .606 |
| ECOG performance status | 7.02 | 3.30–14.9 | < .001 |
| History of diabetes | 2.04 | 1.31–3.18 | .002 |
| History of hypertension | 2.36 | 1.47–3.79 | < .001 |
| History of VTE | 1.51 | 0.63–3.59 | .356 |
| Disease specific | |||
| del(17p) from FISH and cytogenetic forms | 1.80 | 0.90–3.57 | .095 |
| t(4;14) from FISH | 0.87 | 0.26–2.93 | .822 |
| History of MGUS | 0.93 | 0.47–1.84 | .845 |
| History of smoldering myeloma | 0.87 | 0.34–2.21 | .775 |
| Hyperdiploid | 1.53 | 0.66–3.57 | .321 |
| Lactic acid dehydrogenase (≤ 300 vs > 300 g/dL) | 1.34 | 0.62–2.89 | .454 |
| Extramedullary plasmacytoma (yes/no) | 1.42 | 0.81–2.49 | .227 |
| Immunoglobulin G class (< 5 g/dL vs ≥ 5 g/dL) | 0.91 | 0.49–1.68 | .765 |
| Albumin (≤ 3.5 g/dL vs > 3.5 g/dL)b | 1.62 | 1.06–2.49 | .027 |
| ISS disease stage (calculated) | 4.00 | 2.42–6.59 | < .001 |
| Myeloma bone involvement | 0.78 | 0.49–1.22 | .276 |
| Hypercalcemia (serum calcium ≥ 11.5 mg/dL) | 2.41 | 1.34–4.34 | .003 |
| Renal insufficiency (serum creatinine > 2 mg/dL) | 2.45 | 1.59–3.79 | < .001 |
| Anemia (hemoglobin < 10 g/dL or > 2 below LLN) | 1.25 | 0.84–1.88 | .269 |
| Platelet count (≤ 150 × 109/L vs > 150 × 109/L) | 2.66 | 1.77–4.02 | < .001 |
| IMWG risk | 1.42 | 0.90–2.23 | .133 |
| β2–microglobulin (≥ 5.5 mg/L)b | 2.86 | 1.91–4.30 | < .001 |
| HRQOL from EQ‐5D | |||
| Self‐care from EQ‐5D | 2.18 | 1.53–3.09 | < .001 |
| Mobility from EQ‐5D | 3.22 | 2.04–5.10 | < .001 |
| Novel therapy | |||
| Novel therapy use (0, 1) vs ≥ 2 | 0.46 | 0.26–0.81 | .007 |
| Multivariate Analysis c | |||
| Estimate | 95% CI | P | |
| Patient specific | |||
| Age (≤ 75 vs > 75 years) | 1.70 | 1.09–2.67 | .020 |
| ECOG performance status | 3.89 | 1.67–9.05 | .002 |
| History of hypertension | 1.96 | 1.19–3.22 | .008 |
| Disease specific | |||
| ISS disease stage (calculated) | 1.85 | 1.18–2.90 | .007 |
| Renal insufficiency (serum creatinine > 2 mg/dL) | 1.59 | 0.98–2.60 | .062 |
| Platelet count (≤ 150 × 109/L vs > 150 × 109/L) | 2.29 | 1.49–3.53 | < .001 |
| HRQOL from EQ‐5D | |||
| Mobility from EQ‐5D | 2.42 | 1.48–3.94 | < .001 |
HRQOL indicates health‐related quality of life; LLN, lower limit of normal; MGUS, monoclonal gammopathy of undetermined significance; and VTE, venous thromboembolism.
Baseline characteristics with P values in bold were considered significant at P < .15 in the univariate analysis.
Albumin and β2‐microglobulin levels were analyzed as independent variables separate from ISS stage to understand their individual impact in the final model.
Baseline characteristics for which multivariate data are not presented are those that were screened out because either they had univariate P > .15 or they were not significant in the variable selection step.
Figure 1.
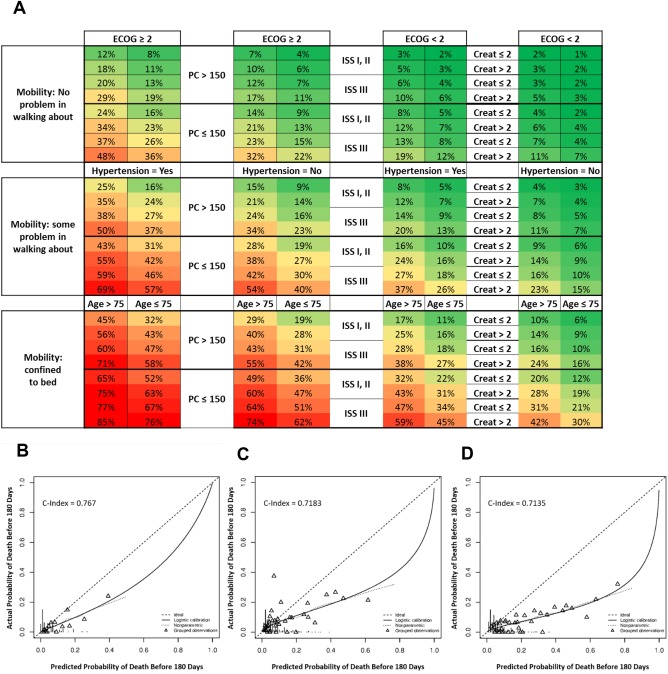
EMPM with estimated probability of mortality within 180 days (A) and external validation model using data from previous trials (B, C, and D). (A) Green, yellow, and red shading represent lower, intermediate, and higher probabilities of EM, respectively. As an example in the practical use of the EMPM, consider a patient with NDMM enrolled in the Connect MM Registry. This patient answered “I have some problems walking about” to the EQ‐5D mobility question, and his platelet count was 150 × 109/L. This patient had an ECOG PS of 2, had a history of hypertension, and was 56 years old. He had ISS stage III disease and a serum creatinine level of 3.19 mg/dL. Entering these baseline factors into the EMPM showed that this patient had a 57% chance of mortality within the first 180 days on study and, in actuality, the patient died at 26 days. Creat indicates serum creatinine and PC, platelet count. (B) Data from MM‐015, (C) data from the FIRST trial, and (D) data from the second cohort of the Connect MM registry. Triangles represent observations in groups of 30 (groups ordered from most probable to least probable) for whom the actual probabilities (from the MM‐015, FIRST trial, or Cohort 2 data) are plotted against the predicted probabilities (based on the Connect MM logistic model). The solid line and the curved dotted line (nonparametric curve) are the fitted curves for the plot of actual and predicted probabilities. The C‐index or concordance probability is the probability that a randomly selected pair of patients in the independent external data set, one with a poorer survival outcome than the other, will be correctly differentially identified based on inputting the 2 patients’ baseline prognostic characteristics in the fitted model obtained from the Connect MM Registry.
3.6. Validation
Internal cross‐validation of this model found a 2.2% reduction in the concordance probability in the test bootstrap resampling estimate compared with the training bootstrap estimate. The training optimism‐adjusted concordance probability of the fitted logistic model was estimated as 74.3% (95% CI: 68.7%‐80.0%); a concordance probability significantly greater than 50% is indicative of a good predictive model. Consistent with these results, external validation using data from patients with NDMM enrolled in the MM‐015 phase 3 trial resulted in a concordance probability of 76.7% for the logistic model (Figure 1B). A second external validation was conducted using data from patients with NDMM enrolled in the phase 3 FIRST trial, which demonstrated a concordance probability of 71.8% for the logistic model (Figure 1C). Validation using data from the 1492 Cohort 2 patients who had ≥ 10 months of follow‐up resulted in a concordance probability of 71.4% for the logistic model (Figure 1D). Patient characteristics were similar between Cohort 1 and Cohort 2: median age was 67 years for both cohorts; 57% and 58% were male, respectively; 28% and 27% had ISS stage III disease, respectively. A full report on the second cohort of patients (n = 1518) enrolled from December 2012 to April 2016 into the Connect MM REGISTRY will be provided at a later date.
4. DISCUSSION
Before the era of novel agents, the incidence of EM was 10% to 14% in patients with NDMM.1, 5, 15, 23 Advances in myeloma treatment (i.e., therapeutic approaches and supportive care) have improved long‐term outcomes and survival. However, despite increasing awareness of its phenomenon, EM remains an area of unmet medical need and not all patients benefit from the long‐term impact of novel agents. EM was further brought to light by the impassioned management issues with newly diagnosed patients by Gonsalves et al.24 The authors defined the incidence of EM from phase 3 trials and outlined key management strategies for patients with NDMM to help mitigate EM, while also touching on the patients most at risk for EM. The EMPM described here offers clinicians the parameters to identify and treat patients with NDMM at risk for EM and the opportunity to address EM in clinical trials.
The prognosis of NDMM depends on staging, patient features, disease biology, and treatment outcomes.3 Current tools designed for risk stratification use the revised ISS (r‐ISS) for MM from the International Myeloma Working Group (IMWG); the r‐ISS is important for long‐term prognosis but has not been used to identify patients at risk for EM.25 Because the r‐ISS is a point‐based system, it is less possible to assess the relative contribution of each element. In addition, the r‐ISS is predominantly based on disease‐specific risk factors and does not account for patient‐specific risk factors. Similar to the r‐ISS, the frailty score is a point‐based system that combines age, functional status, and comorbidities to predict long‐term survival and feasibility of a treatment regimen in elderly patients with NDMM.26 Combining the frailty score with the r‐ISS stage improves the prognostic value of each score individually to predict long‐term survival, but neither score combined or alone has been used to identify patients at the highest risk for EM.
Prognostic studies have provided physicians with a better understanding of the relationship between aggressiveness of disease and survival in patients with NDMM. However, significant gaps remain in our understanding of the best methods of stratifying risk in these patients, incorporating patient‐ and disease‐specific risk factors and the relative contributions of individual risk factors. The existing point‐based systems make it difficult to accurately predict outcomes in patients who have a mixture of high‐ and standard‐risk characteristics.14, 25, 26, 27 Furthermore, the point‐based models are primarily based on data from interventional clinical trials and may not represent the general MM patient population. Therefore, using data from a patient registry, we set out to create an EMPM model that allows differential weighting of the impact of individual patient‐ and disease‐specific risk factors.
Patient comorbidities have been associated with higher mortality in various clinical trials of patients with MM.4, 16, 28, 29, 30, 31, 32, 33, 34 For some patients with MM, comorbidities are a direct cause of death, but they also put patients at risk for early disease‐related mortality by compromising the ability to effectively give treatment.4, 28, 30, 31 Although the 6.8% incidence of EM observed in this study suggests the benefit of novel agents and improved supportive care, there are several issues to be noted. Patients in our study with EM were older and in generally poorer health, with higher rates of comorbidities (including diabetes and hypertension), greater burden of disease, and high‐risk features such as ISS stage III and the presence of del(17p) cytogenetics. Consistent with these observations, the median time from diagnosis to enrollment was 25 days, indicating that this study did not likely select for a healthy population of patients with MM. In our prediction matrix, lower mobility score, age > 75 years, history of hypertension, lower platelet count, higher ECOG PS, high ISS disease stage, and renal insufficiency were associated with a higher likelihood of EM. Although identified as significant in the univariate analysis, albumin level, β2‐microglobulin level, degree of anemia, history of diabetes, hypercalcemia, IMWG risk, del(17p) mutation, and self‐care score from EQ‐5D were not found to be independently associated with EM in the multivariate analysis. In addition, patients with EM required more radiation, which likely limited their ability to receive more aggressive therapy (≥ 2 novel therapies or triplet therapy) or potentially led to a delay in initiating systemic therapy. It is important to note that although a lower proportion of patients with EM were treated with ≥ 2 novel therapies, this was not a significant factor for EM in the multivariate analysis. Lastly, the current analysis of patients with high‐risk disease was limited by too few patients with conventional cytogenetic and FISH data.13 However, as the data mature, a future analysis can be conducted to characterize patients with high‐risk disease who did not die within 180 days compared with patients with non–high‐risk disease who had a longer survival time.
The EMPM presented here was developed based on a test cohort of the first approximately 1500 patients enrolled in the Connect MM Registry and has been validated by bootstrap resampling for internal cross‐validation. Additionally, a high degree of concordance was observed when we applied 2 external validations of the model using data from patients enrolled in the phase 3 MM‐015 trial and the phase 3 FIRST trial, despite more restrictive sets of inclusion/exclusion criteria in those studies. Although external validation was one of the strengths of this study, a potential limitation was the use of well‐controlled phase 3 randomized trials and not a similar, general‐patient population. Therefore, we also applied the model to a second cohort of patients who enrolled in the Connect MM Registry between December 2012 and April 2016. Despite the difference in enrollment periods between the first cohort (2009–2011) and the second cohort, which enrolled during the era of novel agents and a rapidly changing treatment landscape in multiple myeloma, there was a high degree of concordance between predicted and actual probabilities of EM. Future analyses could also include additional data to distinguish between comorbidities related to frailty vs advanced disease, which could help inform management decisions. Thus, with further validation and analyses, the myeloma EMPM has the potential to be a clinically useful tool not only for physicians to identify patients with NDMM who are at risk for EM but also for analyses of specific patient populations, selection of therapy, identification of new targets for treatment, and standardized comparisons between trials.
There are several inherent limitations to using data from patient registries. Because data collection is more passive than collection of interventional trial data, there is an increased likelihood of missing data.35 If a patient is new to a clinic, complete medical and clinical history may not be available. Furthermore, data is as reported by the treating physician and it is often difficult to interpret missing data.36 To reduce data missingness and inaccuracies, we conducted comprehensive data review, including issuing queries to sites and conducting limited site monitoring. Registry patients are also treated in community practices where delivery and choice of care is at physician discretion. Finally, although generalizability of registry data to a disease population as a whole is considered a strength, it is dependent on the source population from which the registry enrolls patients.37
In conclusion, high‐quality systematic research to identify patients at risk for EM have been limited. The EMPM is the first weight‐based model that accounts for both patient‐ and disease‐specific risk factors. Importantly, our study highlighted common causes of EM, including cardiac and infectious complications. This model can facilitate early recognition of patients with high‐risk disease and help physicians introduce personalized treatment, such as incorporation of early consultation with a cardiologist, avoidance of nephrotoxic agents, careful monitoring of steroid dosing in patients with diabetes, prompt and early initiation of doublet or triplet therapy with limited radiation fields, and the use of prophylactic antibiotics. This model also supports additional studies in these subsets of patients to evaluate potential strategies to reduce the risk of EM. Other strengths of the EMPM are ease of use and the ability to rapidly implement into routine clinical practice and consider risk‐adapted approaches to disease management.
ACKNOWLEDGMENTS
The authors acknowledge financial support for this study from Celgene Corporation. The authors thank Yasir Nagarwala, MD, and Jayesh Mehta, MD, for their contributions to this manuscript. The authors received editorial assistance from MediTech Media, Ltd (Nicola Hanson, PhD, and Peter J. Simon, PhD), sponsored by Celgene Corporation.
Terebelo H, Srinivasan S, Narang M, et al. Recognition of early mortality in multiple myeloma by a prediction matrix. Am J Hematol. 2017;92:915–923. https://doi.org/10.1002/ajh.24796
REFERENCES
- 1. Murakami H, Hayashi K, Hatsumi N, et al. Risk factors for early death in patients undergoing treatment for multiple myeloma. Ann Hematol. 2001;80:452–455. [DOI] [PubMed] [Google Scholar]
- 2. Kastritis E, Terpos E, Roussou M, et al. Very early death (< 2 months) in myeloma is associated with advanced age, poor performance status and reduced use of novel agents, while early death within 12 months is associated with high risk features of both the disease and the patient. Blood. 2013;122:[abstract 3195]. [Google Scholar]
- 3. Biran N, Jagannath S, Chari A. Risk stratification in multiple myeloma, part 1: characterization of high‐risk disease. Clin Adv Hematol Oncol. 2013;11:489–503. [PubMed] [Google Scholar]
- 4. Larocca A, Bringhen S, Petrucci M, et al. Early mortality in elderly newly diagnosed multiple myeloma patients treate with novel agents: a pooled analysis of two large randomized pahse III trials. Haematologica. 2015;100:[abstract P270]. 25261096 [Google Scholar]
- 5. Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002–Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219–9226. [DOI] [PubMed] [Google Scholar]
- 6. Rana V, Srivastava G, Hayman SR, et al. Factors predicting early mortality in patients with newly diagnosed multiple myeloma. Blood. 2011;118:[abstract 3981]. [Google Scholar]
- 7. Moreau P, Cavo M, Sonneveld P, et al. Combination of International Scoring System 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front‐line autologous stem‐cell transplantation at high risk of early MM progression‐related death. J Clin Oncol. 2014;32:2173–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 9. Vastesaeger N, Xu S, Aletaha D, et al. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rhematology. 2009;48:1114–1121. [DOI] [PubMed] [Google Scholar]
- 10. Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large‐B‐cell lymphoma. N Engl J Med. 2002;346:1937–1947. [DOI] [PubMed] [Google Scholar]
- 12. Lin C, Itti E, Haioun C, et al. Early 18F‐FDG PET for prediction of prognosis in patients with diffuse large B‐cell lymphoma: SUV‐based assessment versus visual analysis. J Nucl Med. 2007;48:1626–1632. [DOI] [PubMed] [Google Scholar]
- 13. Rifkin RM, Abonour R, Terebelo H, et al. Connect MM registry: the importance of establishing baseline disease characteristics. Clin Lymphoma Myeloma Leuk. 2015;15:368–376. [DOI] [PubMed] [Google Scholar]
- 14. Ozaki S, Harada T, Saitoh T, et al. Survival of multiple myeloma patients aged 65–70 years in the era of novel agents and autologous stem cell transplantation. A multicenter retrospective collaborative study of the Japanese Society of Myeloma and the European Myeloma Network. Acta Haematol. 2014;132:211–219. [DOI] [PubMed] [Google Scholar]
- 15. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Costa LJ, Gonsalves WI, Kumar SK. Early mortality in multiple myeloma. Leukemia. 2015;29:1616–1618. [DOI] [PubMed] [Google Scholar]
- 17. Bernaards CA, Belin TR, Schafer JL. Robustness of a multivariate normal approximation for imputation of incomplete binary data. Stat Med. 2007;26:1368–1382. [DOI] [PubMed] [Google Scholar]
- 18. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for some tradtional and novel measures. Epidemiology. 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759–1769. [DOI] [PubMed] [Google Scholar]
- 20. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 21. European Society for Medical Oncology (ESMO) . Performance scales: Karnofsky & ECOG scores. http://oncologypro.esmo.org/Guidelines-Practice/Practice-Tools/Performance-Scales. Accessed October 27, 2015.
- 22. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant‐ineligible patients with myeloma. N Engl J Med. 2014;371:906–917. [DOI] [PubMed] [Google Scholar]
- 23. Beksac M, Haznedar R, Firatli‐Tuglular T, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86:16–22. [DOI] [PubMed] [Google Scholar]
- 24. Gonsalves WI, Godby K, Kumar SK, Costa LJ. Limiting early mortality: do's and don'ts in the management of patients with newly diagnosed multiple myeloma. Am J Hematol. 2016;91:101–108. [DOI] [PubMed] [Google Scholar]
- 25. Palumbo A, Avet‐Loiseau H, Oliva S, et al. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma: an International Myeloma Working Group report. Blood. 2015;125:2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehta J, Cavo M, Singhal S. How I treat elderly patients with myeloma. Blood. 2010;116:2215–2223. [DOI] [PubMed] [Google Scholar]
- 28. Dimopoulos MA, Delimpasi S, Katodritou E, et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol. 2014;25:195–200. [DOI] [PubMed] [Google Scholar]
- 29. Zomas A, Terpos E, Kastritis E, et al. Hypercalcemia remains an adverse prognostic factor for newly diagnosed patients with symptomatic multiple myeloma in the era of novel anti‐myeloma therapies, independently of age, ISS stage and treatment type: an analysis of 2129 patients. Blood. 2014;124:[abstract 2113]. [Google Scholar]
- 30. Kumar S. Risk of early death in multiple myeloma. Clin Adv Hematol Oncol. 2012;10:172–174. [PubMed] [Google Scholar]
- 31. Holmstrom MO, Gimsing P, Abildgaard N, et al. Causes of early death in multiple myeloma patients who are ineligible for high‐dose therapy with hematopoietic stem cell support: a study based on the nationwide Danish Myeloma Database. Am J Hematol. 2015;90:E73–E74. [DOI] [PubMed] [Google Scholar]
- 32. Mey UJM, Leitner C, Driessen C, et al. Improved survival of older patients with multiple myeloma in the era of novel agents. Hematol Oncol. 2016;34:217–223. [DOI] [PubMed] [Google Scholar]
- 33. Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28:269–277. [DOI] [PubMed] [Google Scholar]
- 34. Mikhael JR, Dingli D, Roy V, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk‐Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013;88:360–376. [DOI] [PubMed] [Google Scholar]
- 35. Levine MN, Julian JA. Registries that show efficacy: good, but not good enough. J Clin Oncol. 2008;26:5316–5319. [DOI] [PubMed] [Google Scholar]
- 36. Gliklich R, Dreyer N, Leavy M and eds. Registries for Evaluating Patient Outcomes: A User's Guide. Third edition. Two volumes. (Prepared by the Outcome DEcIDE Center [Outcome Sciences, Inc., a Quintiles company] under Contract No. 290 2005 00351 TO7.) AHRQ Publication No. 13(14)‐EHC111. Rockville, MD: Agency for Healthcare Research and Quality. April 2014. http://www.effectivehealthcare.ahrq.gov/registries-guide-3.cfm.
- 37. Yoshida K, Radner H, Kavanaugh A, et al. Use of data from multiple registries in studying biologic discontinuation: challenges and opportunities. Clin Exp Rheumatol. 2013;31:S28–S32. [PMC free article] [PubMed] [Google Scholar]


