Abstract
Objectives:
The aim was to determine whether daily muscle electrical stimulation (ES) and streptomycin treatment would have positive or negative effects on trabecular bone mass in disuse rats.
Methods:
Seven-week-old male F344 rats were randomly divided into five groups of eight animals each: an age-matched control group (CON); a sciatic denervation group (DN); a DN + direct electrical stimulation group (DN+ES); a DN + streptomycin treatment group (DN+SM); and a DN+ES+SM group. The tibialis anterior (TA) muscles in all ES groups were stimulated with 16mA at 10Hz for 30 min/day, six days/week, for one week. Bone volume and structure were evaluated using micro-CT, and histological examinations of the tibiae were performed.
Results:
Direct ES significantly reduced the disuse-induced trabecular bone loss. Osteoid thickness were also significantly greater in the ES groups than in the DN group. Micro CT and histomorphological parameters were significantly lower in the DN+ES+SM group than in the DN+ES group, while there were no significant differences between the DN and DN+SM groups.
Conclusions:
These results suggest that ES-induced muscle force reduced trabecular bone loss, and streptomycin treatment did not induce bone loss, but attenuated the effects of ES-induced muscle force on reducing the loss of disused bone.
Keywords: Electrical Stimulation, Muscle Contraction, Denervation, Bone Loss, Streptomycin, Osteoid
Introduction
Limb disuse due to denervation causes musculoskeletal atrophy accompanied by a large reduction in bone mass and changes in trabecular architecture. Findings from animal studies suggest that electric muscle stimulation (ES)-induced muscle force with an appropriate regimen reduces muscle deterioration caused by disuse and bone loss caused by denervation or suspension[1,2]. Muscle contraction affects various factors, such as mechanical, circulatory, and humoral factors, in muscles and bones. ES causes a muscle contraction force that results in mechanical loads to bone through the tendon-bone interface. In addition, muscle contraction induced by ES and/or exercise facilitates cytokine (myokine) production[3] and increases in bone blood flow and capillary vascularity[4,5]. Therefore, one or more of these factors may explain the effects of ES on the reduction of bone loss due to disuse.
Our previous study demonstrated that relatively small muscle contractions induced by ES could delay trabecular bone and muscle loss during the early stage of musculoskeletal atrophy due to disuse[6]. In addition, even low-magnitude mechanical stimuli increased bone and muscle mass in animal and human disuse studies[7-9]. Therefore, ES-induced muscle contraction force would have beneficial effects to reduce disuse-induced osteopenia, because mechanical loading is one of the major factors affecting bone remodeling[10,11].
In general, a higher intensity of ES could induce higher muscle force and then possibly produce adequate mechanical stimuli to bone, while higher muscle contraction and exercise that involves eccentric or lengthening contractions results in muscle damage. It has been reported that eccentric contractions cause damage to skeletal muscles in in vivo and ex vivo studies, characterized by increased intracellular calcium concentration and membrane permeability, decreased maximal muscle force, Z line disruption, and abnormal t-tube arrangement[12-16]. Muscle damage may lead to a decrease in the mechanical stress through the muscle damage-related force deficit. On the other hand, these events related to damage following ECC are reportedly reduced by blockers of stretch-activated channels (SACs), such as streptomycin, gadolinium (Gd3+), and GsMTx4[12,14-17]. SAC blockers may have therapeutic potential by reducing contraction-induced muscle damage[18]. Streptomycin, which was the first aminoglycoside antibiotic, has been used clinically to treat tuberculosis. In an in vivo study, streptomycin, which is one of the potent blockers of SACs, was used to reduce exercise-induced muscle injury and damage-related force deficit[12,14,16]. Combined treatment involving direct ES and streptomycin might prevent muscle injury and damage-related force deficit.
In bone tissue, osteoblasts and osteocytes also express SACs[19], and they act as mechanosensors during mechanical signal transmission. SAC blockade inhibits the increase in [Ca2+]i and the secretion of growth factors relating to bone formation induced by mechanical stress[16,17,19,20]. Therefore, it was hypothesized that SAC blockade has a potentially negative effect on bone formation by partially inhibiting the SACs of osteoblasts and osteocytes, whereas it may have a positive effect on reducing muscle damage-induced muscle force deficit. However, it remains unclear whether streptomycin treatment has positive or negative effects on bone and muscle mass with daily ES-induced muscle contraction force in disuse osteopenia. The aim of this study was to determine whether daily muscle ES and streptomycin treatment would have positive or negative effects on trabecular bone mass in disuse rats.
Materials and Methods
Animals and treatment
Forty male Fischer 344 rats (CLEA, Tokyo, Japan) were maintained under constant temperature (23±2°C) and humidity (55±5%) and 12-h:12-h light-dark cycles. The rats were housed individually in standard cages and provided with a rodent chow (CE-2, CLEA) ad libitum. At 7 weeks of age (body weight, 135±16 g), the animals were randomly divided into the following five groups of eight animals each: an age-matched control group (CON); sciatic denervation group (DN); a DN + direct electrical stimulation group (DN+ES); a DN + streptomycin treatment group (DN+SM); and a DN+ES+SM group. The rats in the DN groups were anesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg body weight). The skin covering the buttock was cut on the left side, and the sciatic nerve was exposed and carefully separated from the surrounding tissue. The sciatic nerve was frozen by contact with a stainless steel rod (5-mm-diameter) that had been cooled in liquid nitrogen[2,6,21,22]. This freezing procedure uniformly damages nerve fibers, although they are more likely to become re-innervated using this procedure compared to others, such as nerve crushing, cutting, or transection with a suture[21-23]. The incision was then closed with sutures, and each animal was kept in a standard cage. The rats in the ES groups were administered direct muscle ES from the day after DN surgery for 1 week. The rats in the DN+SM and DN+ES+SM groups were treated with streptomycin starting 6 days before DN surgery and continuing to the end of the experimental period[12]. All procedures were performed in accordance with the guidelines presented in the Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences, published by the Physiological Society of Japan. This study was approved by the Animal Study Committee of the National Institute of Fitness and Sports and Niigata University of Health and Welfare.
Streptomycin treatment
The rats in the DN+SM and DN+ES+SM groups were treated with streptomycin in the drinking water (4 g/L)12, while the others were given normal drinking water using a feeding bottle with a ball point tube. The daily amount of drinking was measured using a precision balance. The mean streptomycin intakes in the DN+SM and DN+ES+SM groups were 686±112 and 723±107 mg/kg/day, respectively (no significant difference).
Direct ES procedures and evoked muscle contraction force measurement
The stimulation protocol was delivered as described previously[2]. The tibialis anterior (TA) muscles in all ES groups were stimulated electrically under isoflurane inhalation (1.5-2.5%) anesthesia. Bipolar silver surface electrodes were attached on the shaved anterior surface of the left leg of the rats to stimulate the left TA muscle. Direct muscle stimulation was applied using an electrostimulator and isolator (SEM-4201, SS-201, Nihon Kohden, Tokyo, Japan) with current intensity of 16 mA at a frequency of 10 Hz, pulse width of 250 μs, for 30 min per day, 6 days per week, for 1 week. The ES regimen was carried out with 2-sec stimulation followed by 6-sec rest. Although it did not cause a maximal contraction (~26% maximal contraction force)2 in denervated TA muscle, it evoked visible toe flexion. Age-matched control, DN, and DN+SM rats were also anesthetized with isoflurane inhalation (1.5-2.5%) for the same time period as the ES rats.
TA muscle tension was measured at 8 weeks of age in the DN+ES and DN+ES+SM groups under the same stimulus conditions with the daily ES regimen to determine mechanical factors evoked by direct ES as previously described (n=8/group)[2, 22]. Briefly, the lower limbs of rats anesthetized by continuous isoflurane inhalation were secured and stabilized on the working platform with restraining bars and pins at the knee and ankle joints. The distal tendon of the TA was oriented along the natural pull of the muscle and attached to an isometric transducer (TB-654T, Nihon Kohden) that was secured with a 4-0 silk suture on a three-dimensional drive precision stage[24, 25]. Isometric contraction force and force-time integrals were measured under the same stimulation conditions with the daily direct ES regimen. The muscle tension signal was sampled at 2 kHz through a PowerLab 8SP A/D converter (ADInstruments, Nagoya, Japan).
Functional evaluation of motor denervation
TA muscle denervation was confirmed by electromyography (EMG) testing of nerve-to-muscle transmission at 8 weeks of age in the denervated rat groups (n=4-8/group). Under anesthesia, each of the left and right sciatic nerves was stimulated with bipolar hook electrodes connected to the stimulator and isolator (SEM-4201, SS-102J, Nihon Kohden) using supramaximal (~10 V) square wave pulses, 0.1 msec in duration[24]. On the denervated hindlimb, the stimulation points lay proximal and distal to the lesion site, while on the contralateral hindlimb, they were at mid-thigh level. Surface EMG electrodes (3-mm-diameter) were attached to the shaved anterior surface on the TA muscle and used to check whether muscle action potentials were induced by nerve stimulation (Figure 1D).
Figure 1.
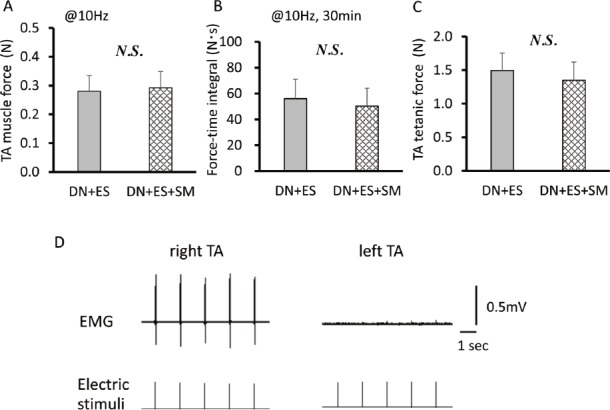
TA muscle force (A) and force-time integrals (B) induced by direct muscle electrical stimulation (ES) at an intensity of 16 mA at 10 Hz, and TA tetanic force (C) in ES intervention rats. Representative EMG recordings from the right contralateral and left denervated TA muscles during sciatic nerve stimulation after 1 week of denervation (D). Note that no significant differences are observed in muscle force output induced by the same condition with the daily ES regimen between the DN+ES and DN+ES+SM groups. No EMG recordings are observed in the denervated TA. Values are means ± SD. DN, denervation; SM, streptomycin treatment.
Tissue preparations
The rats were anesthetized with sodium pentobarbitone (50 mg/kg body weight) at the conclusion of the experiment (at 8 weeks of age). The TA, extensor digitorum (EDL), and soleus (Sol) muscles in each rat were harvested and weighed, and their weights were normalized by body weight (BW). Then, a mixed fixative of 2% paraformaldehyde and 2% glutaraldehyde in 0.01 M sodium cacodylate buffer (pH 7.35) was injected via the abdominal aorta, and perfusion fixation was allowed to occur at room temperature for 30 minutes. The tibiae were removed and preserved in 70% ethanol at 4°C.
Micro-computed tomography (μCT)
Trabecular bone volume and skeletal microarchitecture of the tibiae were measured by a high-resolution micro CT scanner using specific software (SkyScan 1076, SkyScan, Kontich, Belgium)[2]. Briefly, each scan was performed with a source voltage of 70 kV, current of 141 μA, rotation step of 0.6°, and full rotation of over 180°, with a 1-mm aluminum filter for beam hardening reduction. The pixel size was 17.67 μm, and exposure time was 0.54 seconds. Three-dimensional (3D) microstructural image data were reconstructed using NRecon software (SkyScan). Morphometric parameters were calculated using the SkyScan CT Analyzer (CTAn) software for trabecular bone in the tibiae. Semiautomated contouring was used to select the region of interest (ROI) in the trabecular bone within the proximal tibiae. The volume of interest (VOI) started at a distance 1 mm from the lower end of the growth plate and extended distally for 114 cross sections (2 mm in height) comprising trabecular bone and the marrow cavity. An upper threshold of 255 and a lower threshold of 50 were used to delineate each pixel as “bone” or “non-bone”. Bone volume fraction (BV/TV, %), trabecular number (Tb. N), trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp), and connectivity density (Conn.D) were measured according to the guidelines for assessment of bone microstructure in rodents using micro CT[26].
Bone histological staining
After micro CT scanning, the tibia was cut at the proximal and distal ends and divided into three parts, i.e., proximal, middle, and distal. For paraffin-embedded block preparation, the other side of the proximal tibia was decalcified in 0.1 mol/L ethylenediaminetetraacetic acid (pH 7.4) at 4°C for 2-3 weeks. The samples were dehydrated through a graded series of ethanol before being embedded in paraffin. Longitudinal sections (6 μm) per block were created from the paraffin-embedded blocks using a microtome. Hematoxylin and eosin (H-E) staining according to the Goland-Yoshiki method[27] was used to stain osteoid in the decalcified specimens, and osteoid thickness (O. Th), osteoid area (O. Ar), and osteoid number in the osteoid area were determined using a light/fluorescence microscope (BX60; Olympus, Tokyo, Japan) and image analysis software (Image-Pro Premier 9.0; Media Cybernetics, Rockville, MD, USA), as reported previously[2,6]. Bone histomorphometric analysis was performed at a minimum of eight optic fields with 400-fold magnification within the area of interest (ROI) that started at a distance of 1 mm from the lower end of the growth plate and extended distally 2 mm. The ROI comprised only trabecular bone and the marrow cavity in the metaphysis of the proximal tibiae. More than eight separate regions per ROI for calculating mean O. Th, O. Ar, and osteoid number were measured.
Statistical analysis
All data are expressed as means ± standard deviation. One-way analysis of variance followed by the post hoc Bonferroni test was applied to assess significant differences among the groups. Muscle force data in the ES groups were analyzed by unpaired t-tests. TA and Sol muscle weights (MWs) were normalized by body weight (BW) and are expressed as a ratio of MW/BW. The values of micro CT parameters in the left (denervation side: DN) tibiae were normalized by the corresponding values of the right (contralateral: CL) tibiae and are expressed as DN/CL ratios (Figures 2 and 3). The mean values of micro CT parameters and osteoid thickness in the DN, DN+ES, and DN+ES+SM groups were used to calculate the degree that streptomycin treatment abolished the effect of ES on the reduction of bone loss due to denervation (Figures 2 and 3), as follows:
Figure 2.
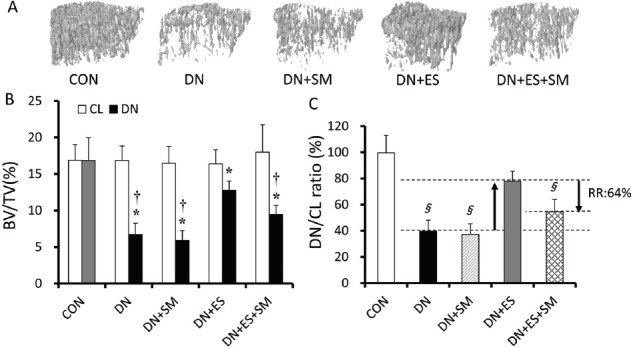
Representative 3D micro CT images of trabecular bone at the analysis site of the tibiae (A), bone volume fraction (BV/TV) (B), and relative BV/TV (denervated side, DN / contralateral side, CL) (C). Values are means ± SD. *P<0.05, vs CL, †P<0.05, vs CON, §P<0.05, vs DN+ES. CON, age-matched control; DN, denervation; ES, electrical stimulation; SM, streptomycin treatment; RR, reduction ratio.
Figure 3.
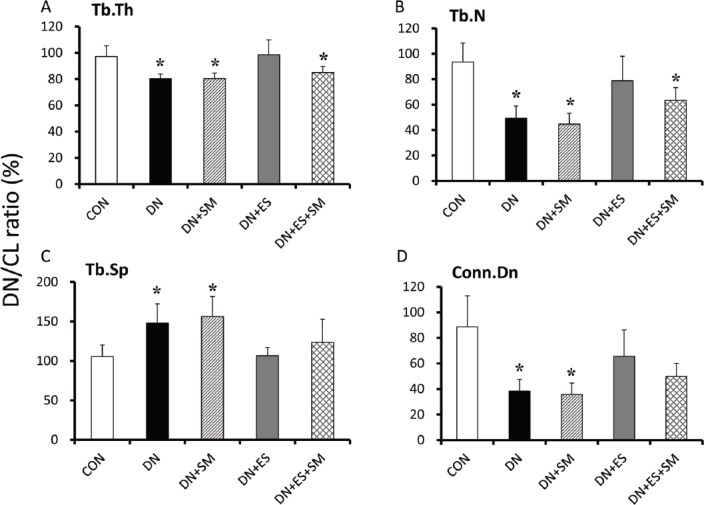
Streptomycin abolishes the majority of the beneficial effects of ES on reducing bone loss and impairment of trabecular microarchitecture. Relative (DN/CL ratio) values of trabecular thickness (Tb.Th) (A), trabecular number (Tb.N) (B), trabecular separation (Tb.Sp) (C), and connectivity density (Conn.D) (D). *P<0.05, vs DN+ES. Values are means ± SD. RR, reduction ratio.
Reduction ratio (RR: %) = | (DN+ES) – (DN+ES+SM) | / | (DN+ES) – (DN) | *100
Values of P<0.05 were considered significant for all analyses.
Results
Body weight
The BWs of the rats in the five groups before the experiment were not significantly different. The denervation, electrical stimulation, and streptomycin-treated groups had 1.3-8.6% lower BWs than the age-matched control group at 1 week after denervation, but no significant difference was observed among the groups (Table 1).
Table 1.
Body weight (BW) and muscle weights of the TA, EDL, and Sol muscles.
| CON | DN | DN+SM | DN+ES | DN+ES+SM | |
|---|---|---|---|---|---|
| Body weight (g) | 166 ± 17 | 153 ± 21 | 165 ± 6 | 152 ± 23 | 155 ± 16 |
| Muscle weight (mg/BW*100) | |||||
| TA | 1.92 ± 0.17 | 1.31 ± 0.07* | 1.28 ± 0.10* | 1.47 ± 0.14*† | 1.36 ± 0.09* |
| EDL | 0.48 ± 0.08 | 0.39 ± 0.02* | 0.39 ± 0.02* | 0.38 ± 0.05* | 0.38 ± 0.02* |
| Sol | 0.39 ± 0.04 | 0.25 ± 0.04* | 0.24 ± 0.03* | 0.27 ± 0.02* | 0.25 ± 0.03* |
P<0.05, vs CON,
P<0.05, vs DN. Values are means ± SD.
Muscle weight and ES-evoked muscle contraction force
At 1 week after denervation, EMGs could be recorded in the contralateral TA muscle but not in the denervated TA muscle during nerve stimulation, even when supramaximal stimulation intensities of over 10 V were applied to the sciatic nerves (Figure 1D). TA, EDL, and Sol MWs relative to BW (MW/BW) decreased significantly (P<0.05) following denervation compared to the CON group and contralateral side muscles (Table 1).
However, relative TA MW was significantly (P<0.05) higher in the ES groups than in the DN group. The streptomycin-treated groups (DN+SM and DN+ES+SM) did not have different TA MWs compared to the DN and DN+ES groups, respectively. Relative Sol MWs were not different among the denervated rat groups. In the ES groups, the tetanic force of TA was lower by 9.7% in the DN+ES+SM group than in the DN+ES group; however, there were no significant differences in the peak force and the force-time integrals evoked by the same conditions of the daily ES regimen (i.e., at 10 Hz, 16 mA, for 30 min) between the DN+ES and DN+ES+SM groups (Figure 1A-C).
Bone microarchitecture analysis
Significant loss in trabecular bone and porosity of architecture in the tibiae were evident in the DN rats (Figure 2). The denervation procedure significantly (P<0.05) reduced the relative (DN/CL ratio) BV/TV, Tb.Th, Tb.N, and Conn.D, and increased trabecular separation (Tb. Sp) in the DN group compared with the CON group (Figures 2C and 3A-D). Relative BV/TV, Tb.Th, and Tb.N, as well as Conn.D, were higher (P<0.05) in DN+ES than in DN rats. However, these parameters were significantly (P<0.05) lower in the DN+ES+SM group than in the DN+ES group. No significant differences were observed between the DN and DN+SM groups in all parameters. The reduction ratios were 64%, 69%, 58%, 42%, and 57% for BV/TV, Tb.Th, Tb.N, Tb.Sp, and Conn.D, respectively (Figures 2C and 3A-D).
Histology
Osteoid thickness (O. Th), osteoid area (O. Ar), and number of osteoid-osteocytes in the osteoid area (Ot. N) were also significantly (P<0.05) lower in the DN groups as compared to CON, except for the DN+ES group (Figure 4A-C). Compared to the DN group, the DN+ES group had significantly (P<0.05) greater O.Th, O.Ar, and Ot. N, but the DN+SM and DN+ES+SM groups did not. O.Th, O.Ar, and Ot. N were significantly (P<0.05) lower in the DN+ES+SM group than in the DN+ES group, and the reduction ratios were 75%, 93%, and 71%, respectively.
Figure 4.
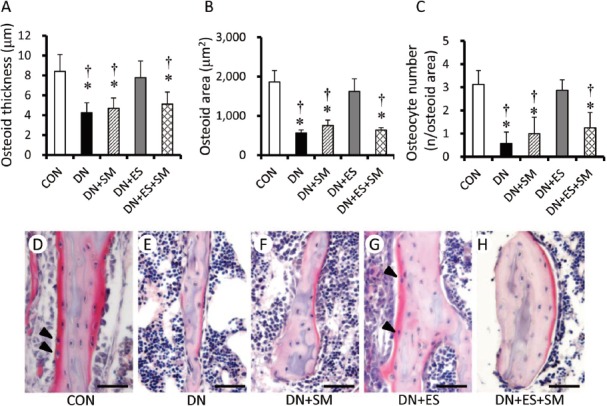
Histomorphometric analyses for mean osteoid thickness (A), osteoid area (B), and osteocyte numbers embedded in the osteoid area (C), and a representative image of osteoid (D-H) following DN of 1 week. Micrographs show distinct eosinophilia of the osteoid matrix at trabecular bone stained with H-E and osteocytes embedded in osteoid (arrowhead in D and G). Bar = 50 μm *P< 0.05, vs CON, †P<0.05, vs ES. Values are means ± SD.
Discussion
The main findings of the present study were: (1) direct electrical stimulation (ES) reduced disuse muscle and bone loss at the early stage of disuse atrophy; and (2) streptomycin treatment did not induce bone loss, but it attenuated the effects of ES-induced muscle force on reducing the loss of disused bone.
We have previously reported that direct ES to denervated muscles could lessen the denervation-induced osteopenia in the early stage of disuse atrophy[2], but the mechanism remains unknown. The effects of ES on the reduction of bone loss due to disuse would be explained by the induction of mechanical and humoral factors by muscle contraction and ES. The adaptive response of bone to mechanical loading is essentially linear between the peak dynamic load and the changes that occur in trabecular bone after denervation[1]1, and increased bone formation was evident even with low-magnitude mechanical stimulation[9,28]. Direct ES treatment with 16 mA at 10 Hz for 30 min/day for one week reportedly reduced the decrease in BV/TV, Tb.Th, Tb.N, Conn.D, and osteoid thickness and the increase in Tb.Sp in disuse rats with muscle and bone atrophy[2]. In the present micro CT and histomorphological data, daily ES intervention under the same conditions as in the above-mentioned report reduced the disuse-induced decrease in BV/TV, Tb.Th, Tb.N, Conn.D, and osteoid formation, and the disuse-induced increase in Tb.Sp in the early stage of disuse musculoskeletal atrophy. The positive effects of ES-induced muscle contraction on the trabecular bone mass and structure in disuse rats were characterized by reduction of the disuse-induced decrease in Tb.Th, Tb.N, Conn.D, and osteoid formation and the disuse-induced increase in Tb.Sp. In addition, micro CT and histomorphological parameters were significantly lower in the DN+ES+SM group than in the DN+ES group, while there were no significant differences between the DN and DN+SM groups. This suggests that streptomycin treatment itself does not induce bone loss, but attenuates the ES effect of reduction of disuse bone loss.
Although the magnitude of tibial bone strain induced by electrical muscle stimulation was not measured, it should be noted that bone strain is the direct modulator of mechano-adaptive osteogenesis. Sugiyama et al. reported that peak strain at the tibial mid-shaft could be determined during walking (~300 με), jumping (~600 με), and applying dynamic load (0-14 N) with strain gauges attached to the bone surface in vivo [11]. In the tibia of a neurectomized limb, application of a peak dynamic load of 2 N resulted in a similar level of peak strain during walking. The minimum effective strain (MES) value to maintain current trabecular bone volume (BV/TV) has been reported to be around 1000 με (6.6 N)11 in mouse and rat tibiae[29]. In the present study, BV/TV of denervated side tibia was still about 20% lower than that of the contralateral side in the DN+ES group. Therefore, we speculated that the tibia bone strain induced by daily intervention with ES-induced muscle contraction would be lower by 1000 με in the present study.
MW, muscle force, and force-time integrals induced by the same ES conditions with daily ES intervention (10 Hz, 16 mA, 30 min) were not different between the DN+ES and DN+ES+SM groups. This suggests that streptomycin treatment would not inhibit the effects of ES-induced muscle force output under the ES conditions used in this study, and the resulting mechanical loads to the bone would not be different between DN+ES and DN+ES+SM. Although it was not possible to examine the effects of muscle-derived factors, such as myokines, induced by ES-induced muscle contraction on bone tissue, the positive effects of ES-induced muscle contraction on reducing disuse bone loss would be attenuated in the DN+ES+SM group if the ES effects were mainly elicited by a mechanical factor.
On the other hand, electrical muscle stimulation has a potential to elicit osteogenesis by direct electrical stimulation to bone. Reportedly, direct electrical current stimulation to bone caused osteogenesis in an in vivo study[30]. Based on the present muscle data, no ES effect of reducing muscle weight loss was observed in the EDL, which lies in a deeper layer than the TA. We speculated that the electrical stimulation with the intensity used in the present study could not effectively reach the deeper muscle, much less the tibial bone. Thus, mechanical stimulation by muscle contraction might be the more dominant factor in the results of the present study.
Generally, once osteoid is formed by osteoblasts, calcification is believed to progress rapidly, within one week or 10 days, with rapid disappearance of the osteoid[31]. Osteoblasts forming new bone matrix, in part, can become embedded in their own osteoid and differentiate into osteocytes[32]. Therefore, the observed osteoid and osteocyte numbers embedded in osteoid areas seem to reflect the osteoblastic activity of bone matrix formation mostly in the experimental period of 1 week in the present study. Streptomycin treatment resulted in about a 70-90% reduction in the effects of the bone anabolic response to ES-induced muscle contraction in the present study. Many studies have reported that streptomycin is potentially one of the SAC blockers[16,17], and SACs are included in osteoblasts and osteocytes[19,33,34]. It was speculated that the resulting osteoblastic osteogenesis maintained by ES-induced muscle contraction might be caused in part through activation of mechanosensors in bone tissue.
Although there was no direct evidence about the difference in the effect of streptomycin on SACs of cells in bone tissue between the streptomycin-treated groups, some studies in which oral streptomycin was used to inhibit SACs reported a 10% decline in tetanic muscle tension force, while there was no difference in muscle force induced by ES of 20 Hz (incomplete tetanus) in the streptomycin-treated animals[14]. The present muscle data also showed a similar trend of a 9.7% decline in tetanic tension in the DN+ES+SM group compared to the DN+ES group, but there was no difference in muscle force induced by ES of 10 Hz (incomplete tetanus) between the two groups. Additionally, the daily dose of streptomycin was checked, and there was no significant difference between the DN+SM and DN+ES+SM groups. In vivo studies reported the effects of oral treatment with streptomycin that reduced stretch-induced muscle damage[12,14,16,35]. The streptomycin treatment regimen of the present study was in accordance with their studies.
On the other hand, with respect to the possibility of a systemic effect of streptomycin on decreasing the bone volume, it would be unlikely, at least in denervated rats, because there were no significant differences in bone volume data between the DN and the DN+SM groups, as well as among contralateral sides in all groups. This also suggests that streptomycin treatment itself does not cause the bone loss, but attenuates the effect of reduction of disuse bone loss by ES-induced muscle contraction.
How streptomycin treatment affects osteoclast activity with bone loss in a denervated rat is unknown. Concerning the possibility that the reduction of ES effect in the DN+ES+SM group was caused by activation of osteoclasts rather than suppression of osteoblasts by streptomycin, it seems to be quite unlikely that streptomycin affected osteoclasts directly according to the results in the present study in which streptomycin treatment did not result in lower BV/TV and osteoid formation in DN+SM rats compared to DN rats. In addition, there was no significant difference in BV/TV on the contralateral side between streptomycin-treated and non-treated rats. As another possibility, it would be conceivable that streptomycin treatment affects osteoclast activity via osteocytes. The osteocyte is recognized as a major orchestrator of skeletal activity, capable of sensing and integrating mechanical and chemical signals from its environment to regulate both bone formation and resorption[36]. Osteocytes are also the source of molecules that regulate the generation and activity of osteoclasts, such as osteoprotegerin (OPG) and receptor activator of nuclear factor-kappaB ligand (RANKL)[37]. In fact, SACs are involved in osteocytes, which are inhibited by Gd3+ [19,38].
One limitation of this study is that it could not examine whether muscle-derived factors such as insulin-like growth factor (IGF) and fibroblast growth factor (FGF) significantly affect bone formation[39]. Our previous study, which used the same ES regimen as the present study, demonstrated the upregulation of IGF-1 mRNA expression in stimulated TA muscle[23]. However, contralateral tibial bone volumes in the ES groups were not significantly different from those in the CON and DN without ES groups in the present study. The present study provided no evidence that the treatment is likely to have broad systemic effects on bone tissue under the ES conditions used.
In conclusion, the present study demonstrated that ES-induced muscle force reduces trabecular bone loss in the early stage of disuse atrophy, and this effect was suppressed by streptomycin treatment. Streptomycin treatment did not alter the muscle force production induced by direct ES at 10 Hz, but it partially abolished the effects of ES-induced muscle force on reducing the loss of disused bone. Activation of mechanical factors might thus explain why ES-induced muscle contraction reduces bone loss in the denervated rat hindlimb. This experimental evidence suggests that ES-induced muscle contractions without streptomycin treatment can have more beneficial effects on bone health in the early stage of disuse bone loss.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (project nos. 25350829 and 25282163), and by a Grant-in-Aid for Developed Research (B) from the Niigata University of Health and Welfare. The authors are grateful to the Niigata Bone Science Institute for the technical support.
Footnotes
Edited by: S. Warden
References
- 1.Lam H, Qin YX. The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone. 2008;43:1093–100. doi: 10.1016/j.bone.2008.07.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamaki H, Tomori K, Yotani K, Ogita F, Sugawara K, Kirimto H, Onishi H, Yamamoto N, Kasuga N. Electrical stimulation of denervated rat skeletal muscle retards trabecular bone loss in early stages of disuse musculoskeletal atrophy. J Musculoskelet Neuronal Interact. 2014;14:220–8. [PubMed] [Google Scholar]
- 3.Scheler M, Irmler M, Lehr S, Hartwig S, Staiger H, Al-Hasani H, Beckers J, de Angelis MH, Haring HU, Weigert C. Cytokine response of primary human myotubes in an in vitro exercise model. Am J Physiol Cell Physiol. 2013;305:C877–86. doi: 10.1152/ajpcell.00043.2013. [DOI] [PubMed] [Google Scholar]
- 4.Stabley JN, Moningka NC, Behnke BJ, Delp MD. Exercise training augments regional bone and marrow blood flow during exercise. Med Sci Sports Exerc. 2014;46:2107–12. doi: 10.1249/MSS.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viboolvorakul S, Niimi H, Wongeak-in N, Eksakulkla S, Patumraj S. Increased capillary vascularity in the femur of aged rats by exercise training. Microvasc Res. 2009;78:459–63. doi: 10.1016/j.mvr.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Tamaki H, Yotani K, Ogita F, Takahashi H, Kirimto H, Onishi H, Yamamoto N. Changes over time in structural plasticity of trabecular bone in rat tibiae immobilized by reversible sciatic denervation. J Musculoskelet Neuronal Interact. 2013;13:251–8. [PubMed] [Google Scholar]
- 7.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–74. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 8.Hannan MT, Cheng DM, Green E, Swift C, Rubin CT, Kiel DP. Establishing the compliance in elderly women for use of a low level mechanical stress device in a clinical osteoporosis study. Osteoporos Int. 2004;15:918–26. doi: 10.1007/s00198-004-1637-y. [DOI] [PubMed] [Google Scholar]
- 9.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15:2225–9. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 10.Frost HM. Bone “mass” and the “mechanostat”:a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama T, Meakin LB, Browne WJ, Galea GL, Price JS, Lanyon LE. Bones’adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J Bone Miner Res. 2012;27:1784–93. doi: 10.1002/jbmr.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBride TA, Stockert BW, Gorin FA, Carlsen RC. Stretch-activated ion channels contribute to membrane depolarization after eccentric contractions. J Appl Physiol (1985) 2000;88:91–101. doi: 10.1152/jappl.2000.88.1.91. [DOI] [PubMed] [Google Scholar]
- 13.Takekura H, Fujinami N, Nishizawa T, Ogasawara H, Kasuga N. Eccentric exercise-induced morphological changes in the membrane systems involved in excitation-contraction coupling in rat skeletal muscle. J Physiol. 2001;533:571–83. doi: 10.1111/j.1469-7793.2001.0571a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willems ME, Stauber WT. Streptomycin and EDTA decrease the number of desmin-negative fibers following stretch injury. Muscle Nerve. 2005;32:310–5. doi: 10.1002/mus.20370. [DOI] [PubMed] [Google Scholar]
- 15.Yeung EW, Head SI, Allen DG. Gadolinium reduces short-term stretch-induced muscle damage in isolated mdx mouse muscle fibres. J Physiol. 2003;552:449–58. doi: 10.1113/jphysiol.2003.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–80. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belus A, White E. Streptomycin and intracellular calcium modulate the response of single guinea-pig ventricular myocytes to axial stretch. J Physiol. 2003;546:501–9. doi: 10.1113/jphysiol.2002.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung EW, Allen DG. Stretch-activated channels in stretch-induced muscle damage:role in muscular dystrophy. Clin Exp Pharmacol Physiol. 2004;31:551–6. doi: 10.1111/j.1440-1681.2004.04027.x. [DOI] [PubMed] [Google Scholar]
- 19.Miyauchi A, Notoya K, Mikuni-Takagaki Y, Takagi Y, Goto M, Miki Y, Takano-Yamamoto T, Jinnai K, Takahashi K, Kumegawa M, Chihara K, Fujita T. Parathyroid hormone-activated volume-sensitive calcium influx pathways in mechanically loaded osteocytes. J Biol Chem. 2000;275:3335–42. doi: 10.1074/jbc.275.5.3335. [DOI] [PubMed] [Google Scholar]
- 20.Sakai K, Mohtai M, Iwamoto Y. Fluid shear stress increases transforming growth factor beta 1 expression in human osteoblast-like cells:modulation by cation channel blockades. Calcif Tissue Int. 1998;63:515–20. doi: 10.1007/s002239900567. [DOI] [PubMed] [Google Scholar]
- 21.Sakakima H, Kawamata S, Kai S, Ozawa J, Matsuura N. Effects of short-term denervation and subsequent reinnervation on motor endplates and the soleus muscle in the rat. Arch Histol Cytol. 2000;63:495–506. doi: 10.1679/aohc.63.495. [DOI] [PubMed] [Google Scholar]
- 22.Takekura H, Tamaki H, Nishizawa T, Kasuga N. Plasticity of the transverse tubules following denervation and subsequent reinnervation in rat slow and fast muscle fibres. J Muscle Res Cell Motil. 2003;24:439–51. doi: 10.1023/a:1027356912404. [DOI] [PubMed] [Google Scholar]
- 23.Tomori K, Ohta Y, Nishizawa T, Tamaki H, Takekura H. Low-intensity electrical stimulation ameliorates disruption of transverse tubules and neuromuscular junctional architecture in denervated rat skeletal muscle fibers. J Muscle Res Cell Motil. 2010;31:195–205. doi: 10.1007/s10974-010-9223-8. [DOI] [PubMed] [Google Scholar]
- 24.Tamaki H, Murata F, Takekura H. Histomorphological evidence of muscle tissue damage and recording area using coiled and straight intramuscular wire electrodes. Eur J Appl Physiol. 2006;98:323–7. doi: 10.1007/s00421-006-0278-6. [DOI] [PubMed] [Google Scholar]
- 25.Tamaki H, Yotani K, Yuki A, Kirimoto H, Sugawara K, Onishi H. Magnetic field strength properties in bone marrow during pulsed electromagnetic stimulation. Journal of Biomedical Science and Engineering. 2010;3:1156–60. [Google Scholar]
- 26.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 27.Yoshiki S. A simple histological method for identification of osteoid matrix in decalcified bone. Stain Technol. 1973;48:233–8. doi: 10.3109/10520297309116630. [DOI] [PubMed] [Google Scholar]
- 28.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603–4. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 29.Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9:87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]
- 30.Yonemori K, Matsunaga S, Ishidou Y, Maeda S, Yoshida H. Early effects of electrical stimulation on osteogenesis. Bone. 1996;19:173–80. doi: 10.1016/8756-3282(96)00169-x. [DOI] [PubMed] [Google Scholar]
- 31.Demer LL. A skeleton in the atherosclerosis closet. Circulation. 1995;92:2029–32. doi: 10.1161/01.cir.92.8.2029. [DOI] [PubMed] [Google Scholar]
- 32.Dallas SL, Prideaux M, Bonewald LF. The osteocyte:an endocrine cell and more. Endocr Rev. 2013;34:658–90. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Liu X, Tong J, Sun L, Xu H, Shi L, Zhang J. Fluid shear stress induces calcium transients in osteoblasts through depolarization of osteoblastic membrane. J Biomech. 2014;47:3903–8. doi: 10.1016/j.jbiomech.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Zanello LP, Norman AW. Multiple molecular mechanisms of 1 alpha,25(OH)2-vitamin D3 rapid modulation of three ion channel activities in osteoblasts. Bone. 2003;33:71–9. doi: 10.1016/s8756-3282(03)00162-5. [DOI] [PubMed] [Google Scholar]
- 35.Allen DG, Whitehead NP, Yeung EW. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle:role of ionic changes. J Physiol. 2005;567:723–35. doi: 10.1113/jphysiol.2005.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes:master orchestrators of bone. Calcif Tissue Int. 2014;94:5–24. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94:25–34. doi: 10.1007/s00223-013-9774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyauchi A, Gotoh M, Kamioka H, Notoya K, Sekiya H, Takagi Y, Yoshimoto Y, Ishikawa H, Chihara K, Takano-Yamamoto T, Fujita T, Mikuni-Takagaki Y. AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. J Bone Miner Metab. 2006;24:498–504. doi: 10.1007/s00774-006-0716-x. [DOI] [PubMed] [Google Scholar]
- 39.Hamrick MW. The skeletal muscle secretome:an emerging player in muscle-bone crosstalk. Bonekey Rep. 2012;1:60. doi: 10.1038/bonekey.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]


