Abstract
To date little is known about catabolic NO-dependent signaling systems in human skeletal muscle during early stages of gravitational unloading. The goal of the study was to analyze signaling pathways that determine the initial development of proteolytic events in human soleus muscle during short-term gravitational unloading (simulated microgravity). Gravitational unloading was simulated by 3-day head-out dry immersion. Before and after the immersion the samples of soleus muscle were taken under local anesthesia, using biopsy technique. The content of desmin, IRS-1, phospho-AMPK, total and phospho-nNOS in soleus of 6 healthy men was determined using Western-blotting before and after the dry-immersion. Three days of the dry immersion resulted in a significant decrease in desmin, phospho-nNOS and phospho-AMPK as compared to the pre-immersion values. The results of the study suggest that proteolytic processes in human soleus at the early stage of gravitational unloading are associated with inactivation of nNOS. Reduction in AMPK phosphorylation could serve as a trigger event for the development of primary atrophic changes in skeletal muscle.
Keywords: Dry Immersion, Soleus Muscle, Desmin, nNOS, AMPK, IRS-1
Introduction
Exposure to spaceflight or simulated microgravity profoundly compromises the structure and function of a mammalian skeletal muscle, especially affecting muscular strength[1-2], motor tasks as well as performance control[1]. Skeletal muscle changes are readily observed in simulated microgravity models such as dry immersion[1-3]. Both gravitational forces and amount of cutaneous mechanosensitive pressure gradients of the floating body (head-out) in dry immersion (via interaction of the floating trunk and limbs covered by a thin waterproof fabric with water) are much smaller than, for example, in bed rest models (via interaction of head-down tilt body at supine position with mattress)[4]. The rate at which muscle strength and transverse stiffness alter, and the pattern of recruiting of motor units in voluntary movements are also clearly different between these two body deconditioning models[2]. Dry immersion therefore appears to be a model of choice for studying skeletal muscle responses on ground under conditions of whole body unloading with little mechanical support to the body surface which is very similar to real spaceflight.
Earlier, we did not observe any significant signs of atrophy (reduction in cross-sectional area of muscle fibers) in human soleus following 3 days of dry immersion (unpublished data). However, it has been previously shown that a 7-day exposure to dry immersion may lead to a series of changes in human postural muscle at the cellular level, specifically, 7-day immersion induced a decrease in diameter and maximum force of isometric tension in permeabilized soleus muscle fibers (MF), reduced calcium sensitivity and transverse stiffness of MF, decreased cross-sectional area of, predominantly, type I MF, as well as reduced relative content of titin, nebulin, desmin and alpha-actinin in soleus muscle[5-7]. Thus, we can see that by the 7th day under conditions of support elimination, basic structural and a number of functional parameters in human soleus muscle are already changed. These changes developed against the background of the profound decline in the electrical activity of postural muscles[8-9]. In earlier studies, it was shown that by the 3rd day of dry immersion slow type motor units had reduced activity or were “switched off”[10]. In a rat experiment a decreased EMG activity of m. soleus was observed already during the first hours of support elimination[11]. It is most likely that during the first hours/days under eliminated support slow motor units disabling would lead to the activation of the catabolic pathways, activating proteolytic processes and causing the destruction of cytoskeletal and contractile proteins. It also has been shown that during the first days of hindlimb suspension calcium ions concentration in myoplasm in rat soleus is increased[12-14], and activation of calpains is observed leading to degradation of a number of cytoskeletal proteins[15-16]. The degradation of desmin, a typical substrate of μ-calpains, is observed in rat soleus after 3 days of hindlimb suspension[17-18]. At the same time, one of the most powerful endogenous inhibitors of μ-calpains is nitric oxide (NO)[19]. NO is produced in MF by neuronal NO-synthase (nNOS) as well as by endothelial NO-synthase (eNOS). Interestingly, the activity of S-nitrosylated μ-calpain molecules is significantly lower than the activity of calpain molecules, which were not subjected to the action of NO[20]. At the same time, in experiments with hindlimb-suspended rats and under space flight conditions as well as during head-down tilt bedrest in humans there was a decrease in nNOS content, and nNOS was translocated from sarcolemma to cytosol[6,21-24]. In addition, in the experiment with tail-suspended rats a general decrease in NO content in m. soleus was reported[25]. A general reduction of NO-synthase under conditions of gravitational unloading (and NOS translocation to the cytoplasm, for example, due to disruption of chemical bonds with syntrophins) could be associated with the action of μ-calpains[26-27]. However, it is still unclear why unloading-induced proteolytic effect of calpains on NO-synthase is more powerful than inhibitory effect of NO on the activity of calpains. It is not excluded, that a decreased nNOS activity is followed by calpain-dependent breakdown of nNOS. It is obvious, that reduction of nNOS activity (as well as eNOS) is possible not only due to breakdown of this enzyme, but also due to its dephosphorylation[28]. It is known that nNOS phosphorylation can occur through insulin/IGF-I-dependent protein kinases[29] and via AMP-activated protein kinase (AMPK)[28]. It has been shown that inactivation of insulin/IGF-I-dependent pathway under unloading is, primarily, caused by the degradation of the insulin receptor substrate - 1 (IRS-1)[30], so, the study of IRS-1 content as well as the level of AMPK phosphorylation in human soleus at the early stage of unloading may be of great scientific interest.
Thus, our study was aimed at the analysis of signaling processes that determine the initial development of proteolytic events in human soleus muscle during short-term dry-immersion. The results of the present study revealed that by the 3-rd day of dry immersion there are signs of calpain-dependent proteolysis due to reduced total and phosphorylated nNOS content. We have also observed a significant decrease in AMPK phosphorylation.
Materials and methods
Dry immersion model, subjects, ethics
In dry immersion, the subject is immersed in a thermostat-controlled water bath while being protected from water with a thin waterproof fabric much larger than the area of the bath container. Thus, the subject´s body (with head-out) almost freely floats in warm water without getting a wet skin. Hydrostatic pressure is exerted equally on all body surfaces, nearly balancing the gravity force and thereby creating almost non-reaction-force conditions (Figure 1). The study group consisted of 6 healthy voluntary males with mean age of 22 (±2.23) and mean weight of 67.7 (±6.12) kg. Using microbiopsy technique[31] samples of soleus muscle were taken before (pre-immersion) and after 3 days of dry immersion (post-immersion). The muscle samples were quickly blotted with gauze to remove superficial blood, frozen in liquid N2 for 20s, and then stored at -80°C until analysis. After the protocol of the experiment had been explained to volunteers, all the participants provided signed informed consent. The study protocol and consent form were approved by Biomedicine Ethics Committee of the Institute of Biomedical Problems, Russian Academy of Sciences (protocol no. 302, 25.07.2012) and all procedures conformed to the Declaration of Helsinki, International and Russian Law.
Figure 1.
The scheme of dry immersion.
Immunoblot analysis
Skeletal muscle tissue (30 mg) was homogenized in ice-cold RIPA lysis buffer: 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.004% sodium azide, and 5 mM EDTA, supplemented with 1 mM DTT, 1 mM PMSF, and 5 μl/ml pepstatin (Sigma-Aldrich, St. Louis, MO, USA), mammalian protease inhibitor cocktail (Amresco, Solon, OH, USA), and phosphatase inhibitor cocktail B (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The total protein concentration of the lysates was determined by incubation for 20 min at 4°C and centrifugation for 10 min at 12,000 g. Protein content of supernatants was quantified using an assay based on a modification of the Lowry protocol (RC DC Protein Assay; Bio-Rad Laboratories, Hercules, CA, USA). Bovine serum albumin was used as a standard. The samples were diluted in Laemmli buffer. Total protein (20-50 μg) was subjected to SDS-PAGE, and the proteins were then transferred to nitrocellulose membrane (Bio-Rad Laboratories, CA, USA). Membranes were blocked for 1 h at room temperature with blocking buffer (4% nonfat milk powder; TBS, pH 7.4; and 0.1% Tween 20) and incubated overnight at 4°C with primary monoclonal antibodies against IRS-1 (diluted 1:900; sc-559, Santa Cruz Biotechnology, CA, USA), desmin (diluted 1:1000, Santa Cruz Biotechnology, CA, USA), anti-phospho-AMPKα1/2 (Thr172) (diluted 1:10000; Santa Cruz Biotechnology, CA, USA), anti-phospho-nNOS (Ser1417) (diluted 1:100000; Millipore Chemicals, MA, USA), nNOS (diluted 1:10000; BD Transduction Laboratories, NJ, USA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; diluted 1:4000; Abcam, MO, USA). Antibodies were diluted in the blocking buffer. Three 10-min washes with TBS-Tween (TBS and 0.1% Tween 20) were then performed, after which membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies to rabbit or mouse immunoglobulins (diluted 1:200,000; Bio-Rad Laboratories, CA, USA). The membranes were washed again in TBS-Tween 3 times for 10 min, incubated in Immun-Star HRP Chemiluminescent system (Bio-Rad Laboratories, CA, USA), and exposed to X-ray film (Eastman Kodak, Rochester, NY, USA); the images were scanned then. The protein bands were quantified using densitometry scanning (GS-800; Quantity-One software, Bio-Rad Laboratories, CA, USA). Protein density was normalized to GAPDH. Western blots were performed at least 3 times.
Statistical analysis
The Wilcoxon signed rank test was used to compare proteins content before and after dry-immersion. The level of significance was set at p<0.05. The general content of the studied proteins was expressed as the median and interquartile range (25-75 percentiles).
Results
Three-day dry immersion resulted in 10% significant decrease (p<0.05) in cytoskeletal protein desmin general content in soleus muscle as compared to baseline values (Figure 2A). And in four of the six subjects decreased desmin concentrations were ranged from 12 to 24% (Figure 2B-C).
Figure 2.
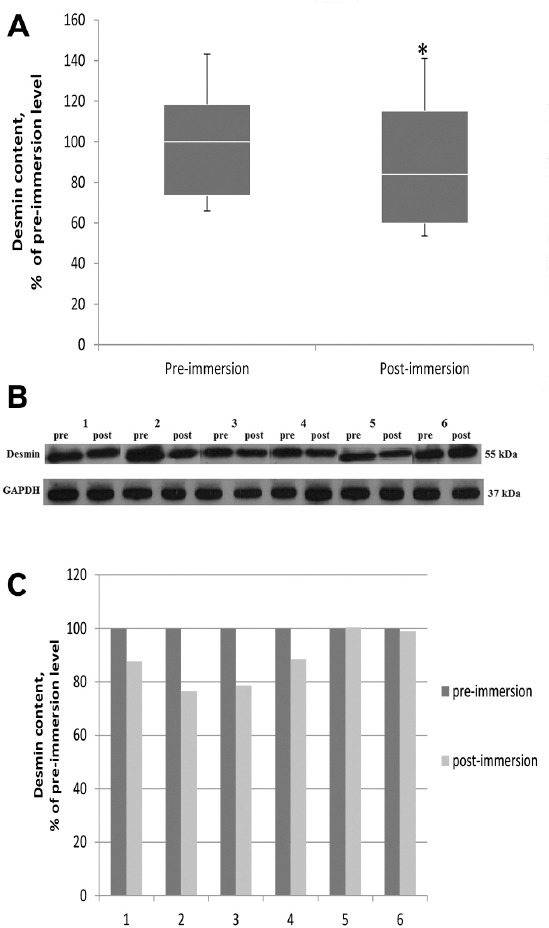
Effect of 3 day dry immersion on desmin content in human soleus muscle. A. Quantification of general desmin content in soleus muscle before and after 3 day dry immersion (n=6), normalized to GAPDH. Box plots show 25-75 percentiles and median values and the whiskers represent the minimum and the maximum, * - difference from the pre-immersion level (p<0.05). B. Representative immunoblots of desmin and GAPDH in soleus muscle of each individual before (pre) and after (post) 3 day dry immersion. C. Quantification of desmin content of each individual before (pre) and after (post) 3 day dry immersion, normalized to GAPDH.
We did not find any significant changes in total nNOS content in soleus muscle after 3-day immersion (Figure 3A-C). However there was a 43% significant decrease (p<0.05) in the content of phosphorylated nNOS (Figure 4A-C).
Figure 3.
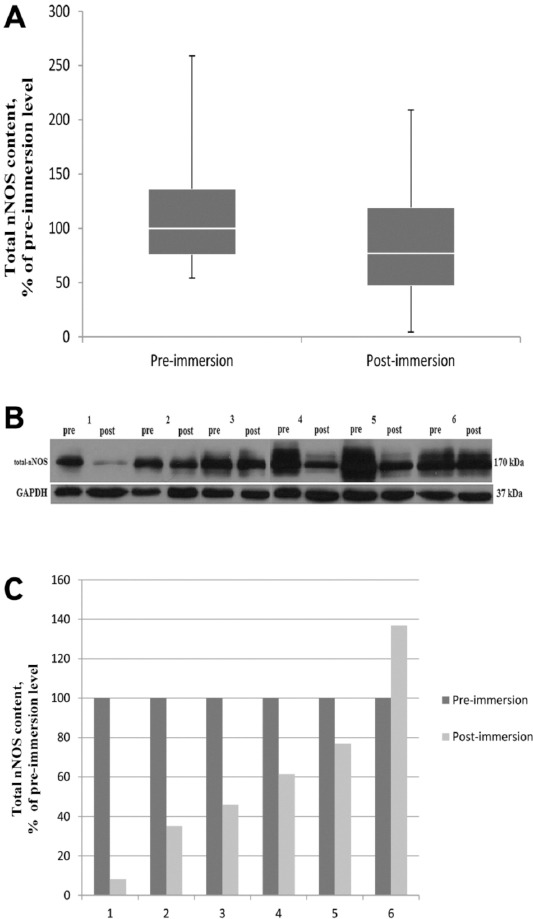
Effect of 3 day dry immersion on total-NOS content in human soleus muscle. A. General total-NOS content in soleus muscle before and after 3 day dry immersion (n=6), normalized to GAPDH. Box plots show 25-75 percentiles and median values and the whiskers represent the minimum and the maximum. B. Representative immunoblots of total NOS and GAPDH content in soleus muscle of each individual (n=6) before and after 3 day dry immersion. C. Quantification of total-NOS content of each individual before (pre) and after (post) 3 day dry immersion, normalized to GAPDH.
Figure 4.
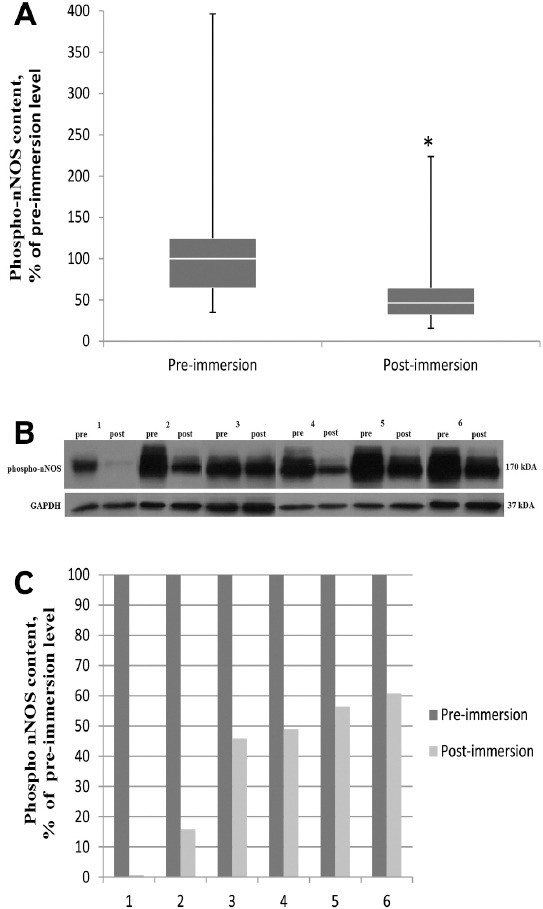
Effect of 3 day dry immersion on phospho-NOS content in human soleus muscle. A. General phospho-NOS (Ser1417) content in soleus muscle before and after 3 day dry immersion (n=6), normalized to GAPDH. Box plots show 25-75 percentiles and median values and the whiskers represent the minimum and the maximum, * - difference from the pre-immersion level (p<0.05). B. Representative immunoblots of phospho-NOS(Ser1417) and GAPDH content in soleus muscle of each individual (n=6) before and after 3 day dry immersion. C. Quantification of phospho-NOS (Ser1417) content of each individual before (pre) and after (post) 3 day dry immersion, normalized to GAPDH.
Since a decrease in the content of phosphorylated nNOS can be caused by reduced protein-kinase activity of IGF-I/IRS-1/Akt pathway, an important factor in this case is IRS-I content. In general, the content of IRS-1 in soleus muscle after 3-day dry immersion did not differ from the pre-immersion level, except for two subjects, which revealed an increase in the content of this protein (Figure 5A-C).
Figure 5.
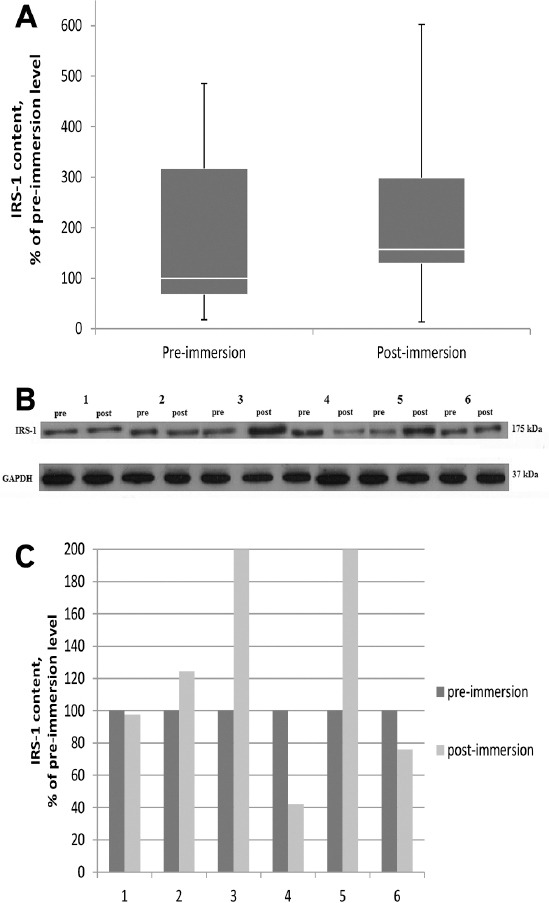
Effect of 3 day dry immersion on IRS-1 content in human soleus muscle. A. General IRS-1content in soleus muscle before and after 3 day dry immersion (n=6), normalized to GAPDH. Box plotsshow 25-75 percentiles and median values and the whiskers represent the minimum and the maximum. B. Representative immunoblots of IRS1 and GAPDH content in soleus muscle of each individual (n=6)before and after 3 day dry immersion. C. Quantification of IRS1 content of each individual before (pre) and after (post) 3 day dry immersion, normalized to GAPDH.
Another factor contributing to the reduction of nNOS phosphorylation could be the level of AMPK phosphorylation. Three days of dry immersion resulted in significantly reduced (by 36%) content of phosphorylated AMPK (Figure 6A) as compared to pre-immersion values (p<0.05). In five of the six subjects decreased phospho-AMPK content was in the range from 26 to 84%, and one subject revealed an increased level of this protein (Figure 6B-C).
Figure 6.
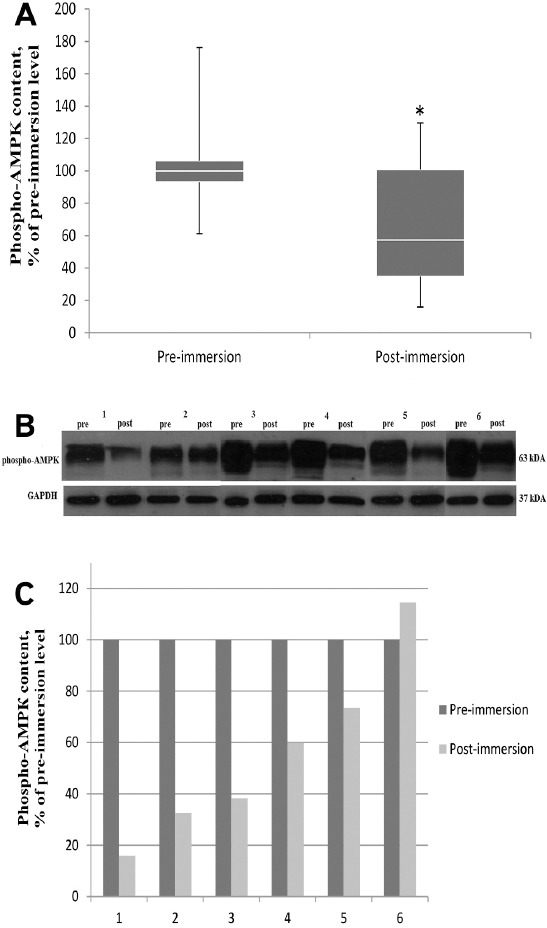
Effect of 3 day dry immersion on phospho-AMPKcontent in human soleus muscle. A. General phospho-AMPK (Thr 172) content in soleus muscle before and after 3 day dry immersion (n=6), normalized to GAPDH. Box plots show 25-75 percentiles and median values and the whiskers represent the minimum and the maximum, * - difference from the pre-immersion level (p<0.05). B. Representative immunoblots of phospho- AMPK(Thr 172) and GAPDH content in soleus muscle of each individual (n=6) before and after 3 day dry immersion. C. Quantification of phospho- AMPK(Thr 172) content of each individual before (pre) and after (post) 3 day dry immersion, normalized to GAPDH.
Discussion
Exposure of humans or animals to short-term simulated microgravity is usually accompanied by enhanced proteolytic activity of calpains that can be indirectly assessed in vivo by determination of content of some calpains substrates, cytoskeletal proteins, such as desmin[16]. Previous studies in our laboratory have shown that 7-day dry immersion leads to a decrease in desmin content in human soleus by about 30%[7]. In the present study after 3 days of dry immersion there was a little, but significant decrease in desmin content (by 10%). So, it can be suggested that degradation of cytoskeletal proteins, which are μ-calpains substrates, takes place already at the early phase of gravitational unloading. We also observed a reduced nNOS phosphorylation during early period of unloading in human soleus muscle. Since NO is an inhibitor of calpain activities, the reduced nNOS phosphorylation may lead to possible activation of calpain-dependent processes in muscle cell. The decrease in the content of NO-synthase under unloading conditions was previously observed in leg muscles of both humans and laboratory animals[22-24]. However, these observations were carried out during the longer periods of exposure to unloading conditions. So, we were the first who found a decrease in nNOS phosphorylation in soleus muscle during short-term exposure of human to dry immersion.
We know that NO is one of the effective μ-calpains inhibitors[19,32]. However NO-synthase at the initial stage of the unloading seems to be unable to prevent an enhanced calpains activity. Obviously, at this period the activity of NOS is reduced due to changes in its phosphorylation which was shown in the previous reports[15,33]. Indeed, in the present study the content of the phosphorylated nNOS in soleus muscle is significantly reduced (by more than 40%). It is known that a similar effect (a decrease in NOS phosphorylation level and NO production) can be induced by inactivation of AMPK[28], protein kinase D[34] as well as by inactivation of IGF-I /insulin/Akt/mTOR signaling pathway[29]. In the present study we did not observe any significant changes in IRS-I content in soleus of the volunteers after 3 days of dry immersion. In the study of Nakao et al.[30] it was previously found that 5 and 10 days of gravitational unloading (rat hindlimb suspension) resulted in IRS-I breakdown due to activity of ubiquitin-ligase Cbl-b. Reduced IRS-I content in rat soleus was also observed after 14 days of hindlimb suspension[35]. However, to date, any studies concerning the content of IGF-I/mTOR cascade key elements in a human postural muscle during early stages of the gravitational unloading have not been carried out yet. The fact that we did not observe any changes in IRS-1 content after 3 days of gravitational unloading in humans means not only that the trigger mechanism of E3 ubiquitin-ligases up-regulation (which may be associated with IRS-I breakdown) is not typical for the early stages of unloading, but it also may indirectly show that the decrease in nNOS phosphorylation level is likely not caused by the changes in the activity of the canonical cascade kinases.
At the same time, in our study we observed a significant decrease in the content of phosphorylated AMPK (Thr 172) in human soleus muscle. Earlier, an altered level of AMPK phosphorylation on this site in rat soleus was registered in the two studies with two-week hindlimb-suspension[35-36]. In a report by Han et al.[35] a decreased AMPK phosphorylation was shown but Hilder and co-workers[36], on the contrary, observed an increased phosphorylation level of AMPK. In any case, we did not find any reports about changes of AMPK phosphorylation in animals or humans during short-term unloading. It is known that the level of AMPK phosphorylation is regulated by both systemic factors (e.g., interleukin-637) and intracellular mechanisms, first of all, the ratio between phosphorylated and non-phosphorylated high energy nucleotides (ATP/ADP/AMP)[38]. Wakatsuki et al. (1994)39 showed that 10-day hindlimb suspension brought about the slight but significant increase in the basal CrP levels in rat soleus muscle. And this increase (although very small) was found in spite of the fact that the longer periods of the unloading were usually accompanied by the slowly increasing EMG activity (i.e. contractile activity)[40]. So, one could expect the accumulation of the high-energy phosphates at the early stages of unloading, when no EMG activities were recorded. This accumulation might induce the reduction in the AMPK phosphorylation level, which was found in the present study. The AMPK phosphorylation is known to be a main up-stream mechanism of the PGC1α expression[41], therefore, the reduced level of phospho-AMPK during unloading might be followed by the reduced PGC1α expression which was recently found by Cannavino et al. (2015)[42]. The lowered level of AMPK phosphorylation may also lead to a decrease in nNOS (as well as eNOS) phosphorylation level and reduced NO production[28].
Thus, short-term (3 days) exposure of human to dry immersion (gravitational unloading) resulted in the activation of proteolytic processes, accompanied by substantial inactivation of nNOS, one of the most important components of the proteolytic negative control mechanisms. Three-day dry-immersion also revealed a reduction in AMPK phosphorylation, which could serve as a trigger event for the development of primary atrophic changes in skeletal muscle.
Acknowledgements
The authors express their gratitude to the scientific staff headed by the corresponding member of the RAS I. B. Kozlovskaya for preparation and carrying out dry immersion experiment and Dr. Ilya Rukavishnikov for the excellent performing the microbiopsy procedure.
The work was supported by RFBR grant 13-04-00888.
Footnotes
Edited by: J. Rittweger
References
- 1.Kozlovskaya I, Dmitrieva L, Grigorieva L, Kreydich Y. Gurfinkel VS, Ioffe MYe, Massion J, editors. Stance and Motion. Gravitational mechanisms in the motor system. Studies in real and simulated weightlessness. 1988;1088:37–48. [Google Scholar]
- 2.Kozlovskaya IB, Grigorieva LS, Gevlich GI. Comparative analysis of the weightlessness and its simulations effects on the force-velocity properties and tone of human skeletal muscles. Kosm Biol Aviakosm Med. 1984;18:22–26. [PubMed] [Google Scholar]
- 3.Shenkman BS, Nemirovskaya TL, Cheglova IA, Belozerova IN, Kozlovskaya IB. Morphological characteristics of human m. vastus lateralis in supportlessness. Dokl Ross Akad Nauk. 1999;364:563–565. [PubMed] [Google Scholar]
- 4.Navasiolava NM, Custaud MA, Tomilovskaya ES, Larina IM, Mano T, et al. Long-term dry immersion:review and prospects. Eur J Appl Physiol. 2011;111:1235–60. doi: 10.1007/s00421-010-1750-x. [DOI] [PubMed] [Google Scholar]
- 5.Litvinova KS, Vikhlyantsev IM, Kozlovskaya IB, Podlubnaya ZA, Shenkman BS. Effects of artificial support stimulation on fiber and molecular characteristics of soleus muscle in men exposed to 7-day dry immersion. J Gravit Physiol. 2004;11:131–2. [PubMed] [Google Scholar]
- 6.Moukhina M, Shenkman BS, Blottner D, Nemirovskaya T, Lemesheva Y, et al. Effects of support stimulation on human soleus fiber characteristics during exposure to «dry»immersion. J Gravit Physiol. 2004;11:137–138. [PubMed] [Google Scholar]
- 7.Ogneva IV, Ponomareva EV, Kartashkina NL, Altaeva EG, Fokina NM, et al. Decrease of contractile properties and transversal stiffness of single fibers in human soleus after 7-day “dry” immersion. Acta Astronaut. 2011;68:1478–1485. [Google Scholar]
- 8.Mitarai G, Mano T, Mori S, and Jijiwa H. Electromyographic study on human standing posture in experimental hypograve state. Annu Rep Res Inst Environ Med Nagoya Univ. 1972;19:1–9. [PubMed] [Google Scholar]
- 9.Miller TF, Saenko IV, Popov DV, Vinogradova OL, Kozlovskaya IB. Effect of mechanical stimulation of the support zones of soles on the muscle stiffness in 7-day dry immersion. J Gravit Physiol. 2004;11:135–6. [PubMed] [Google Scholar]
- 10.Kirenskaya AV, Kozlovskaya IB, Sirota MG. Influence of the immersion hypokinesia on the characteristics of the rhythmic activity of soleus motor units. Fiziol Chel. 1986;12:617–632. [PubMed] [Google Scholar]
- 11.Kawano F, Ishihara A, Stevens JL, Wang XD, Ohshima S, et al. Tension- and afferent input-associated responses of neuromuscular system of rats to hindlimb unloading and/or tenotomy. Am J Physiol Regul Integr Comp Physiol. 2004;287:R76–86. doi: 10.1152/ajpregu.00694.2003. [DOI] [PubMed] [Google Scholar]
- 12.Ingalls CP, Warren GL, Armstrong RB. Intracellular Ca2+transients in mouse soleus muscle after hindlimb unloading and reloading. J Appl Physiol. 1999;87:386–390. doi: 10.1152/jappl.1999.87.1.386. [DOI] [PubMed] [Google Scholar]
- 13.Ingalls CP, Wenke JC, Armstrong RB. Time course changes in [Ca2+]i, force, and protein content in hindlimb-suspended mouse soleus muscles. Aviat Space Environ Med. 2001;72:471–476. [PubMed] [Google Scholar]
- 14.Shenkman BS, Nemirovskaya TL. Calcium-dependent signaling mechanisms and soleus fiber remodeling under gravitational unloading. J Muscle Res Cell Motil. 2008;29:221–230. doi: 10.1007/s10974-008-9164-7. [DOI] [PubMed] [Google Scholar]
- 15.Enns DL, Belcastro AN. Early activation and redistribution of calpain activity in skeletal muscle during hindlimb unweighting and reweighting. Can J Physiol Pharmacol. 2006;84:601–609. doi: 10.1139/y06-013. [DOI] [PubMed] [Google Scholar]
- 16.Enns DL, Raastad T, Ugelstad I, Belcastro AN. Calpain/calpastatin activities and substrate depletion patterns during hindlimb unweighting and reweighting in skeletal muscle. Eur J Appl Physiol. 2007;100:445–455. doi: 10.1007/s00421-007-0445-4. [DOI] [PubMed] [Google Scholar]
- 17.Ogneva IV. The transversal stiffness of fibers and the desmin content in the leg muscles of rats under gravitational unloading of various duration. J Appl Physiol. 2010;109:1702–1709. doi: 10.1152/japplphysiol.00793.2010. [DOI] [PubMed] [Google Scholar]
- 18.Mirzoev TM, Shenkman BS, Ushakov IB, Ogneva IV. Desmin and α-actinin-2 content in rat soleus muscle in the dynamics of gravitational unloading and subsequent reloading. Dokl Biochem Biophys. 2012;444:144–146. doi: 10.1134/S1607672912030052. [DOI] [PubMed] [Google Scholar]
- 19.Michetti M, Salamino F, Melloni E, Pontremoli S. Reversible inactivation of calpain isoforms by nitric oxide. Biochem Biophys Res Commun. 1995;207:1009–1014. doi: 10.1006/bbrc.1995.1285. [DOI] [PubMed] [Google Scholar]
- 20.Samengo G, Avik A, Fedor B, Whittaker D, Myung KH, et al. Age-related loss of nitric oxide synthase in skeletal muscle causes reductions in calpain S-nitrosylation that increase myofibril degradation and sarcopenia. Aging Cell. 2012;11:1036–45. doi: 10.1111/acel.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tidball JG, Lavergne E, Lau KS, Spencer MJ, Stull JT, et al. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am J Physiol. 1998;275:C260–266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]
- 22.SandonàD Desaphy JF, Camerino GM, Bianchini E, Ciciliot S, et al. Adaptation of mouse skeletal muscle to long-term microgravity in the MDS mission. PLoS One. 2012;7:e33232. doi: 10.1371/journal.pone.0033232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudnick J, Püttmann B, Tesch PA, Alkner B, Schoser BG, et al. Differential expression of nitric oxide synthases (NOS 1-3) in human skeletal muscle following exercise countermeasure during 12 weeks of bed rest. FASEB. 2004;18:1228–1230. doi: 10.1096/fj.03-0792fje. [DOI] [PubMed] [Google Scholar]
- 24.Sun LW, Blottner D, Luan HQ, Salanova M, Wang C, et al. Bone and muscle structure and quality preserved by active versus passive muscle exercise on a new stepper device in 21 days tail-suspended rats. J Musculoskelet Neuronal Interact. 2013;13:166–177. [PubMed] [Google Scholar]
- 25.Lomonosova YN, Kalamkarov GR, Bugrova AE, Shevchenko TF, Kartashkina NL, et al. Role of NO-synthase in regulation of protein metabolism of stretched rat m. soleus muscle during functional unloading. Biochemistry (Mosc) 2012;77:208–16. doi: 10.1134/S0006297912020137. [DOI] [PubMed] [Google Scholar]
- 26.Lainé R, de Montellano PR. Neuronal nitric oxide synthase isoforms alpha and mu are closely related calpain-sensitive proteins. Mol Pharmacol. 1998;54:305–12. doi: 10.1124/mol.54.2.305. [DOI] [PubMed] [Google Scholar]
- 27.Crosbie RH, Barresi R, Campbell KP. Loss of sarcolemma nNOS in sarcoglycan-deficient muscle. FASEB J. 2002;16:1786–91. doi: 10.1096/fj.02-0519com. [DOI] [PubMed] [Google Scholar]
- 28.Chen ZP, McConell GK, Belinda J, Snow RJ, Canny BJ, et al. AMPK signaling in contracting human skeletal muscle:acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202–1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- 29.Hinchee-Rodriguez K, Garg N, Venkatakrishnan P, Roman MG, Adamo ML, et al. Neuronal nitric oxide synthase is phosphorylated in response to insulin stimulation in skeletal muscle. Biochem Biophys Res Commun. 2013;435:501–5. doi: 10.1016/j.bbrc.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakao R, Hirasaka K, Goto J, Ishidoh K, Yamada C, et al. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol Cell Biol. 2009;29:4798–811. doi: 10.1128/MCB.01347-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayot M, Michaud A, Koechlin C, Caron MA, Leblanc P, et al. Skeletal muscle microbiopsy:a validation study of a minimally invasive technique. Eur Respir J. 2005;25:431–440. doi: 10.1183/09031936.05.00053404. [DOI] [PubMed] [Google Scholar]
- 32.Koh, Tidball Nitric oxide inhibits calpain-mediated proteolysis of talin in skeletal muscle cell. Am J Physiol Cell Physiol. 2000;279:806–812. doi: 10.1152/ajpcell.2000.279.3.C806. [DOI] [PubMed] [Google Scholar]
- 33.Ma XW, Li Q, Xu PT, Zhang L, Li H, Yu ZB. Tetanic contractions impair sarcomeric Z-disk of atrophic soleus muscle via calpain pathway. Mol Cell Biochem. 2011;354:171–180. doi: 10.1007/s11010-011-0816-3. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Ruiloba L, Aicart-Ramos C, García-Guerra L, Pose-Utrilla J, Rodríguez-Crespo I, et al. Protein Kinase D Interacts with Neuronal Nitric Oxide Synthase and Phosphorylates the Activatory Residue Serin e1412. PLoS One. 2014;9:e95191. doi: 10.1371/journal.pone.0095191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han B, Zhu MJ, Ma C, Du M. Rat hindlimb unloading down-regulates insulin like growth factor-1 signaling and AMP-activated protein kinase, and leads to severe atrophy of the soleus muscle. Appl Physiol Nutr Metab. 2007;32:1115–23. doi: 10.1139/H07-102. [DOI] [PubMed] [Google Scholar]
- 36.Hilder TL, Baer LA, Fuller PM, Fuller CA, Grindeland RE, et al. Insulin-independent pathways mediating glucose uptake in hindlimb-suspended skeletal muscle. J Appl Physiol. 2005;99:2181–8. doi: 10.1152/japplphysiol.00743.2005. [DOI] [PubMed] [Google Scholar]
- 37.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, et al. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes. 2006;55:S48–54. doi: 10.2337/db06-s007. [DOI] [PubMed] [Google Scholar]
- 38.Hardie DG, Ashford ML. AMPK:regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 2014;29:99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakatsuki T, Ohira Y, Yasui W, Nakamura K, Asakura T, Ohno H, Yamamoto M. Responses of contractile properties in rat soleus to high-energy phosphates and/or unloading. Jpn J Physiol. 1994;44:193–204. doi: 10.2170/jjphysiol.44.193. [DOI] [PubMed] [Google Scholar]
- 40.Alford EK, Roy RR, Hodgson JA, Edgerton VR. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hind limb suspension. Exp Neurol. 1987;96:635–49. doi: 10.1016/0014-4886(87)90225-1. [DOI] [PubMed] [Google Scholar]
- 41.Correia JC, Ferreira DM, Ruas JL. Intercellular:local and systemic actions of skeletal muscle PGC-1s. Trends Endocrinol Metab. 2015;26:305–314. doi: 10.1016/j.tem.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Cannavino J, Brocca L, Sandri M, Grassi B, Bottinelli R, Pellegrino MA. The role of alterations in mitochondrial dynamics and PGC-1αover-expression in fast muscle atrophy following hindlimb unloading. J Physiol. 2015;593(8):1981–95. doi: 10.1113/jphysiol.2014.286740. [DOI] [PMC free article] [PubMed] [Google Scholar]


