Abstract
Objectives:
Spaceflight back pain and intervertebral disc (IVD) herniations cause problems in astronauts. Purpose of this study was to assess changes in T2-relaxation-time through MRI measurements before and after head-down tilt bed-rest, a spaceflight analog.
Methods:
8 men participated in the bed-rest study. Subjects remained in 6° head down tilt bed-rest in two campaigns of 21 days, and received a nutritional intervention (potassium bicarbonate 90 mmol/d) in a cross-over design. MRI measurements were performed 2 days before bed-rest, as well as one and five days after getting up. Image segmentation and data analysis were conducted for the IVDs Th12/L1 to L5/S1.
Results:
7 subjects, average age of 27.6 (SD 3.3) years, completed the study. Results showed a significant increase in T2-time in all IVDs (p < 0.001), more pronounced in the nucleus pulposus than in the annulus fibrosus (p<0.001). Oral potassium bicarbonate did not show an effect (p=0.443). Pfirrmann-grade correlated with the T2-time (p<0.001).
Conclusions:
6° head-down tilt bed-rest leads to a T2-time increase in lumbar IVDs. Oral potassium bicarbonate supplementation does not have an effect on IVD T2-time.
Keywords: MRI, Intervertebral Disc, Bed-rest, Back Pain, Space Flight
Introduction
Chronic lower back pain is a widespread disease in the modern population and a main operational concern in space flight participants[1,2]. During space flight, weightlessness leads to an increase in height of intervertebral discs (IVDs), resulting in an elongation of the spine up to several centimeters[2,3]. Astronauts frequently report moderate to severe lower back pain that seems to be of discogenic origin and probably results from IVD expansion[1,3]. This back pain is typically located in the lumbar spine and its intensity usually decreases in the fetal tuck position (bending the knees to the chest)[1]. It is currently unknown whether the increase in disc volume is solely caused by water influx or if the amount of other tissue components, such as the protein content, changes in microgravity as well[4,5]. Atrophy of the paraspinal muscles has been observed in space flight participants and discussed to contribute to IVD pathologies[6].
In addition to in-flight back pain, astronauts have an increased risk of nucleus pulposus herniation, particularly in the immediate post-flight period[7]. In the literature, possible changes in IVD morphology such as tissue degeneration are discussed as causative factors. However, the underlying structural or pathophysiological processes within the IVD are currently not entirely understood[1,8].
The same type of back pain as experienced by astronauts during spaceflight has been reported in bed-rest studies with 6° head down tilt[9]. Bed-rest studies have proven to be a good analog to spaceflight in IVD research[4,10]. The T2 relaxation time has been shown to correlate with the amount of water, the glycosaminoglycan and collagen content, as well as the stage of degeneration of IVD tissue[11-15]. LeBlanc et al.[16] measured T2 relaxation times in the “hydrated portion of disc” in a five-week bed-rest study, and found a decrease in T2-time in L1/2 to L5/S1 throughout horizontal bed-rest. LeBlanc et al. later published data from spaceflight and bed rest that showed an increase in T2 time[17]. We recently investigated lumbar IVDs in bed-rest with the dGEMRIC-method[18]. Findings seem to indicate an increase in glycosaminoglycans (GAGs) throughout bed-rest, but some results were hard to interpret[18]. Diurnal variation in the hydration of IVDs has been known for a long time, and some studies have indicated a “redistribution” of fluid shifting between the annulus fibrosus and the nucleus pulposus throughout the loading/unloading-cycle[19,20]. When the opportunity arose to measure T2 relaxation times in a bed-rest-study conducted by the European Space Agency, and organized as a cross-over trial with two campaigns to test the effects of oral potassium bicarbonate on subjects undergoing bed-rest, we decided to perform measurements of T2 relaxation time to see if we can confirm LeBlanc et al.’s results. In addition we were interested in the effect of the oral potassium bicarbonate supplementation on IVD composition. To our knowledge it is currently unknown whether oral potassium bicarbonate supplementation has an effect on IVD physiology. In theory, the alkaline characteristics of the supplement might affect the pH environment and possibly lead to an outward water shift from the IVD tissue. We therefore hypothesized a decrease in T2-time to occur with the supplement[21], and an increase in T2-time through bed-rest.
Materials and methods
Study setting
The “NUC”-study was funded by the European Space Agency and took place in the Institute of Aerospace Medicine of the German Aerospace Center (DLR) in Cologne, Germany. The study was registered with the Clinical Trials Registry http://www.clinicaltrials.gov (Number: NCT01509456) and approved by the ethics committee of North Rhine Medical Association (Ärztekammer Nordrhein, no. 2007405). It was designed and performed in compliance with the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Study design
Eight healthy, non-smoking, male human subjects were immobilized in 6° head-down-tilt bed-rest for 21 days (HDT-1 to HDT-21). The entire bed-rest-study included two campaigns for each subject, with a wash-out period of 154 days in between. The aim was to investigate a nutritional countermeasure for bone loss (oral application of potassium bicarbonate 30mmol/tablet three times a day) in a cross-over design[9,22,23]. The measurements presented here were conducted in both campaigns (schedule of the measurement times in each campaign: Figure 1). For organisational reasons, the measurements pre-bed-rest (BDC-2) and 5 days after bed-rest (R+5) were taken in the afternoon while the measurement one day after bed-rest (R+1) was performed in the morning that follows the day subjects got up again.
Figure 1.
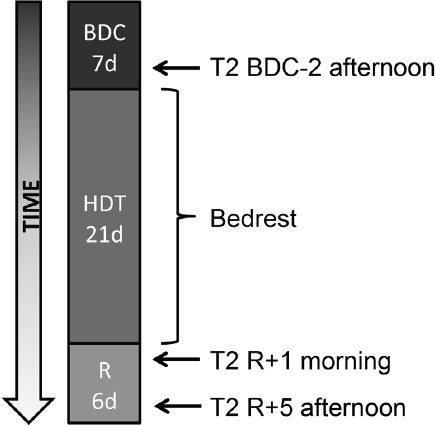
Study schedule and measurements. Two campaigns were performed with the same design. The nutritional intervention was given to half of the subjects in each campaign (cross-over design).
During baseline data collection and recovery, subjects could move free inside the lab (baseline: 7 days, BDC-7 to BDC-1; recovery: 6 days, R+0 to R+5). Reambulation from bed-rest took place in the morning of R+0. Throughout bed-rest, participants remained in bed 24h/day with at least one shoulder on the mattress at any time. All hygienic procedures, food intake and experiments took place in this position without exception. Compliance with the protocol was ensured by video surveillance and by staff. Subjects did not undergo exercise or training. Psychologists gave psychological support and medical doctors looked after the subjects in daily ward rounds. Weighing all ingredients, food items and beverages for each test subject to prevent changes in bodyweight strictly controlled the dietary intake.
Subject selection
Only male subjects were recruited for this study because the European Space Agency expected hormonal variations in female subjects to mimic effects in some of the experiments. Subject recruitment was announced on the institution’s website as well as by flyers and posters in several universities and research institutes. Subject selection included a variety of clinical and laboratory tests, as well as a psychological examination. Inclusion criteria were: male gender, age 20-45 years, body mass index 20-26 kg/m2, body height 158-190 cm (62-75 inches), body weight 65-85 kg and willingness to participate in the entire study. Exclusion criteria included abuse of drugs, medicine, nicotine or alcohol, regular medication, vegetarians and vegans, history of mental illness, rheumatic diseases, chronic hypertension, diabetes, obesity, arthritis, hyperlipidaemia, renal dysfunction, thyroid dysfunction, hepatic disease, disorders of calcium and bone metabolism, exercising more than four times a week, chronic back pain, a history of intervertebral disc prolapse, muscle and joint disease, family history of thrombosis and blood clotting disorders (Tests performed included AT III, Lupus-PTT, ferritin, Factors II, IV and V Leiden).
MRI measurements
After obtaining routine localizer images, we prescribed a midline sagittal image by placing a cursor on the coronal image and angling it if necessary. Sagittal T2 weighted multi echo images of the spine were acquired using a 1.5 T MRI scanner (Sonata, Siemens Medical Systems, Erlangen) with TR: 2500 ms, 15 x TE: 10,3 ms - 164,8 ms, 256 × 256 pixels in FOV: 330 mm X 330 mm, 3 mm slice thickness, 6 mm interslice gap with a dedicated spine coil.
For each IVD, a stage from I to V was assigned by an experienced radiologist on the basis of Pfirrmann et al.[24] to quantify the amount of IVD degeneration.
Segmentation
Automated segmentation of the lumbar IVDs in the MRI data was performed in three steps (Figure 2). The first was the detection of the spine. For this purpose a horizontal symmetry transformation, based on the paper of Reisfeld et al.[24], was carried out with subsequent tracking of higher symmetry values along the y-axis. For smoothing a Bézier curve was adapted to the detected points of the centerline of the spine.
Figure 2.
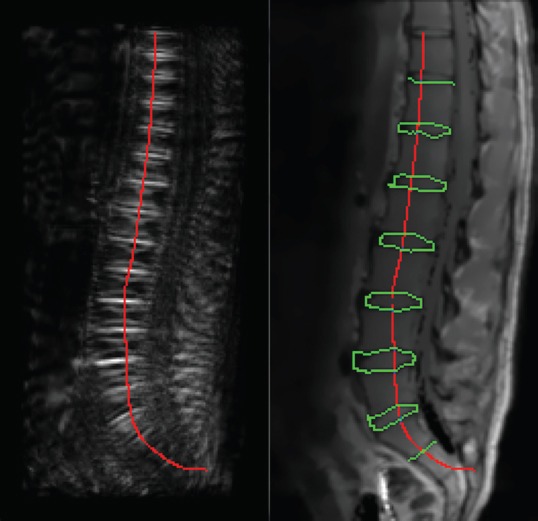
Image processing and segmentation. Left: Horizontal symmetry transformation and localization of the spine. Right: IVDs detection with contour tracing combined with graph search for final completion of segmentation.
The second step was the localization of the contour points of the IVDs in the gray value profile along the detected centerline with a threshold-algorithm. Based on the detected starting points of the IVDs in the third step contour tracing combined with graph search was realized for final segmentation. Similar to Stelzenender et al.[19], we subdivided the IVDs into five segments for a more detailed analysis of the annulus fibrosus and nucleus pulposus tissue (Figure 3). Further details on the segmentation procedure were published by Pohle-Fröhlich et al.[26].
Figure 3.
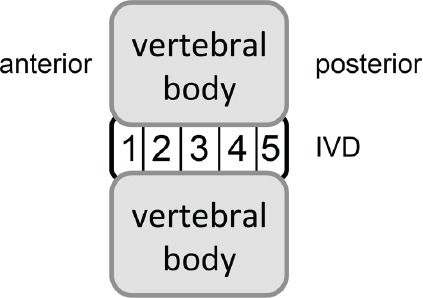
Segmentation of IVDs in five segments from anterior to posterior for analysis.
Statistical analysis
“SPSS” (IBM SPSS Statistics release 20.0.0, NY, USA) was used to calculate Linear Mixed Effect (LME) models with segment, measurement, campaign and Pfirrmann-grade as well as their interaction as fixed effects and subjects as a random effect (variance components). Significance was assumed at p<0.05. Data are presented as counts and as means and their SD. “SigmaPlot” (version 12.0) was used for plotting of data. T2-time was the primary outcome measure of this study. Effects of the nutritional countermeasure on the IVD were analysed.
Results
One subject dropped out of the study due to personal reasons during the bed-rest-phase of the second campaign. While the R+1 and R+5 measurements of this subject are missing, the acquired data was used for the present analysis. All measurements of the other seven subjects with an average age of 27.6 (SD 3.3) years and an average body mass of 78.6 (SD 6.4) kg were collected and analyzed successfully. There were no adverse events in connection with the MRI measurements.
The main finding was a significant increase in T2-time within the IVDs Th12/L1 to L5/S1 throughout 21 d of 6°HDT bed rest (p<0.001). T2-time (averaged over all IVDs, Figure 4a) increased significantly between BDC-2 and R+1 (p<0.001). Between R+1 and R+5, T2-time in turn decreased significantly (p<0.001), but still remained above baseline levels even at R+5 (p=0.001). Similar changes in T2-time were observed in all investigated IVDs (Th12/L1 to L5/S1). Overall, T2-time was longer in the more cranial IVDs, shortening towards the more caudal discs (Figure 4b). Within the IVDs (segmentation according to figure 3), T2-time was longest in segment 3 that consists of nucleus pulposus tissue, shorter in segments 2 and 4 that contain both partially nucleus pulposus and anulus fibrosus, and shortest in segments 1 and 5 representing anulus fibrosus tissue (Figure 4c). Differences between segments were significant (p<0.001). The bed-rest-effect on T2-time was more pronounced in the nucleus pulposus than in the anulus fibrosus (p<0.001). The average Pfirrmann-grade was 2.0 (SD 0.95). Figure 4d shows the Pfirrmann-grade plotted against T2-time. Intervertebral discs with a Pfirrmann-grade >2 were excluded from the analysis because there was only one case each. T2-time significantly differed between Pfirrmann-grades (p<0.001). No pattern, function or linearity, however, could be found that shows a connection of the Pfirrmann-grade to the extent of the bed-rest-effect.
Figure 4.
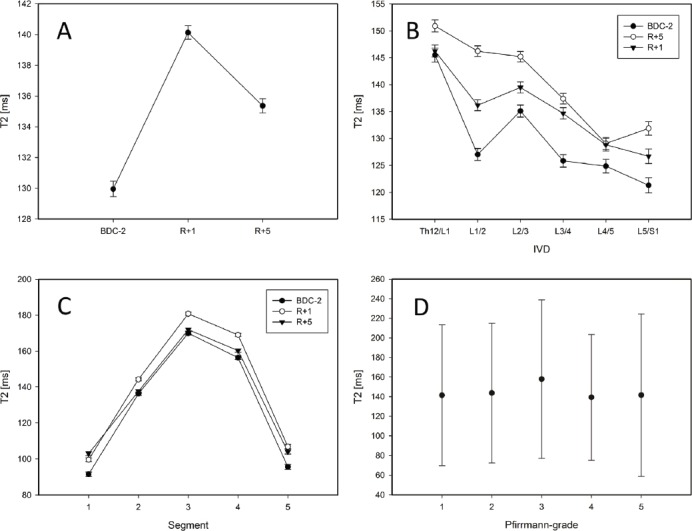
Results. A: Differences between all measurements were significant (p<0.001). B: Average values separated by IVDs and measurements. C: Segments separated by measurements. D: Differences in T2-values between Pfirrmann-grades are significant (p<0.001).
There was no carry-over effect between campaigns (no statistical difference, p=0.416). Furthermore, there was no intervention effect. The intervention with oral potassium bicarbonate given throughout bed-rest did not lead to a significant difference in T2-values at R+1 (p=0.443). T2-values were significantly higher prior to the intervention in the intervention group compared to the group without intervention (BDC-2, p<0.001). T2-time was significantly shorter on R+5 (p=0.008) in the intervention group compared to the group without intervention.
Discussion
The aim of the study was to assess possible changes in T2-time within the lumbar IVDs before and after 21 days of 6° head down tilt bed-rest. Furthermore, the effect of an intervention with oral potassium bicarbonate on T2-time in the lumbar IVDs was approached in a cross-over design.
Results of the present study showed a significant increase in T2-time after bed-rest in Th12/L1 to L5/S1, and a partial decrease after four days reambulation, but no effect of oral potassium bicarbonate supplementation on the T2-time measured in IVD tissue. The hypothesis that T2-time increases in lumbar IVDs throughout bed rest could not be rejected. The second hypothesis that T2-time decreases through oral supplementation of potassium bicarbonate was rejected.
The strengths of the study were its well-controlled test environment in the bed-rest facility, including standardized nutrition and enforcement of strict bed-rest, a very high standard in subject selection including medical and psychological parameters, and good quality of the acquired data. A weakness was the low number of subjects due to the complex nature of bed-rest studies.
T2- relaxation time has been shown to correlate with the amount of water and the glycosaminoglycan content of IVD tissue[11-13]. Detiger et al. demonstrated a linear decrease in T2-time with increasing IVD degeneration[11]. Marinelli et al.[12] found that T2 relaxation times correlate strongly with the IVD water content and weakly with proteoglycan content. In addition, T2 relaxation times in lumbar IVDs correlate with the stage of degeneration and with patient age[13-15].
The increase in T2-time throughout bed-rest may be interpreted as an increase in the amount of free water within the IVDs. This finding is in accordance with other studies where an increase in lumbar IVD height was found[3,4,10], including LeBlanc et al.[16] We could not confirm the findings of LeBlanc et al. from 1988[16] who measured a decrease in T2-time and interpreted it as a loss of water of IVD tissue, but our results are in line with later findings of LeBlanc et al.[17].
The intervention with oral potassium bicarbonate did not show an effect on IVD T2-time. Buehlmeier et al.[21] showed that oral potassium bicarbonate can influence bone and protein physiology in terms of a reduction of bone loss and protein catabolism during bed-rest.
The present results showed significant differences in T2-times between different Pfirrmann-grades. A correlation that can be expressed as a function, however, could not be found. We can therefore not confirm the results of Detiger et al.[10] who found a linear decrease in T2 relaxation time with increasing Pfirrmann grade. The reason is probably the low number of participants in our study.
Summarizing the study results, an increase in T2-relaxation time was found after bedrest. This increase may be interpreted as an increase in free water and/or glycosaminoglycans/collagen within the IVDs tissue. In case an increase in glycosaminoglycans throughout bed-rest can be confirmed with other methods, controlled bed-rest schemes (not necessarily en-bloc, but possibly for a few hours per day) might be considered a treatment option in IVD pathologies, under consideration of comorbidities such as coagulopathy and cardiovascular or pulmonary disease. Future research should not only consider further methods, but also focus on the thoracic and cervical spine.
Acknowledgements
We would like to express our gratitude towards the subjects for their willingness to participate in the NUC-study. We also acknowledge the support of Dr. Oliver Angerer of ESA, as well as Alexandra Noppe, Dr. Joachim Latsch and Dr. Francisca May (all DLR) and the study management team of DLR.
Footnotes
The authors have no conflict of interest.
Edited by: F. Rauch
References
- 1.Kerstman EL, Scheuring RA, Barnes MG, DeKorse TB, Saile LG. Space Adaptation Back Pain: A retrospective Study. Aviat Space Environ Med. 2012;83:2–7. doi: 10.3357/asem.2876.2012. [DOI] [PubMed] [Google Scholar]
- 2.Wing PC, Tsang IK, Susak L, Gagnon F, Gagnon R, Potts JE. Back pain and spinal changes in microgravity. Orthop Clin North Am. 1991;22(2):255–62. [PubMed] [Google Scholar]
- 3.Sayson JV, Hargens AR. Pathophysiology of Low Back Pain during Exposure to Microgravity. Aviat Space Environ Med. 2008;79:365–73. doi: 10.3357/asem.1994.2008. [DOI] [PubMed] [Google Scholar]
- 4.Belavý DL, Bansmann PM, Böhme G, Frings-Meuthen P, Heer M, Rittweger J, et al. Changes in intervertebral disc morphology persist 5 mo after 21-day bed rest. J Appl Physiol. 2011;111:1304–1314. doi: 10.1152/japplphysiol.00695.2011. [DOI] [PubMed] [Google Scholar]
- 5.Paajanen H, Lehto I, Alanen A, Erkintalo M, Komu M. Diurnal Fluid Changes of Lumbar Discs Measured Indirectly by Magnetic Resonance Imaging. J Orthop Res. 1994;12(4):509–14. doi: 10.1002/jor.1100120407. [DOI] [PubMed] [Google Scholar]
- 6.Chang DG, Healey RM, Snyder AJ, Sayson JV, Macias BR, Coughlin DG, Bailey JF, Parazynski SE, Lotz JC, Hargens AR. Lumbar Spine Paraspinal Muscle and Intervertebral Disc Height Changes in Astronauts after Long-duration Spaceflight on the International Space Station. Spine (Phila Pa 1976) 2016;41(24):1917–1924. doi: 10.1097/BRS.0000000000001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston SL, Campbell MR, Scheuring R, Feiveson AH. Risk of Herniated Nucleus Pulposus Among U.S. Astronauts. Aviat Space Environ Med. 2010;81:566–74. doi: 10.3357/asem.2427.2010. [DOI] [PubMed] [Google Scholar]
- 8.Belavy DL, Adams M, Brisby H, Cagnie B, Danneels L, Fairbank J, Hargens AR, Judex S, Scheuring RA, Sovelius R, Urban J, van Dieën JH, Wilke HJ. Disc herniations in astronauts: What causes them, and what does it tell us about herniation on earth? Eur Spine J. 2016;25(1):144–54. doi: 10.1007/s00586-015-3917-y. [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson KJ, Watenpaugh DE, Murthy G, Convertino VA, Hargens AR. Back pain during 6 degrees head-down tilt approximates that during actual microgravity. Aviat Space Environ Med. 1995;66(3):256–9. [PubMed] [Google Scholar]
- 10.Belavý DL, Armbrecht G, Felsenberg D. Incomplete recovery of lumbar intervertebral discs 2 years after 60-day bed rest. Spine. 2012;37(14):1245–51. doi: 10.1097/BRS.0b013e3182354d84. [DOI] [PubMed] [Google Scholar]
- 11.Detiger SE, Holewijn RM, Hoogendoorn RJ, van Royen BJ, Helder MN, Berger FH, Kuijer JP, Smit TH. MRI T2*mapping correlates with biochemistry and histology in intervertebral disc degeneration in a large animal model. Eur Spine J. 2015;24(9):1935–43. doi: 10.1007/s00586-014-3498-1. [DOI] [PubMed] [Google Scholar]
- 12.Marinelli NL, Haughton VM, Munoz A, Anderson PA. T2 relaxation times of intervertebral disc tissue correlated with water content and proteoglycan content. Spine. 2009;34:520–524. doi: 10.1097/BRS.0b013e318195dd44. [DOI] [PubMed] [Google Scholar]
- 13.Marinelli NL, Haughton VM, Anderson PA. T2 relaxation times correlated with stage of lumbar intervertebral disk degeneration and patient age. AJNR Am J Neuroradiol. 2010;31(7):1278–82. doi: 10.3174/ajnr.A2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoppe S, Quirbach S, Mamisch TC, Krause FG, Werlen S, Benneker LM. Axial T2 mapping in intervertebral discs: a new technique for assessment of intervertebral disc degeneration. Eur Radiol. 2012;22(9):2013–9. doi: 10.1007/s00330-012-2448-8. [DOI] [PubMed] [Google Scholar]
- 15.Welsch GH, Trattnig S, Paternostro-Sluga T, Bohndorf K, Goed S, Stelzeneder D, Mamisch TC. Parametric T2 and T2*mapping techniques to visualize intervertebral disc degeneration in patients with low back pain: initial results on the clinical use of 3.0 Tesla MRI. Skeletal Radiol. 2011;40(5):543–51. doi: 10.1007/s00256-010-1036-8. [DOI] [PubMed] [Google Scholar]
- 16.LeBlanc AD, Schonfeld E, Scheider VS, Evans HJ, Taber KH. The spine: changes in T2 relaxation times from disuse. Radiology. 1988;169(1):105–7. doi: 10.1148/radiology.169.1.3420243. [DOI] [PubMed] [Google Scholar]
- 17.LeBlanc AD, Evans HJ, Schneider VS, Wendt RE, 3rd, Hendrick TD. Changes in intervertebral disc cross-sectional area with bed rest and space flight. Spine (Phila Pa 1976) 1994;19(7):812–7. doi: 10.1097/00007632-199404000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Koy T, Zange J, Rittweger J, Pohle-Fröhlich R, Hackenbroch M, Eysel P, Ganse B. Assessment of Lumbar Intervertebral Disc Glucosaminoglycan Content by Gadolinium-Enhanced MRI before and after 21 days of Head-Down-Tilt Bedrest. PLoS One. 2014;9(11):e112104. doi: 10.1371/journal.pone.0112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludescher B, Effelsberg J, Martirosian P, Steidle G, Markert B, Claussen C, Schick F. T2- and diffusion-maps reveal diurnal changes of intervertebral disc composition: an in-vivo MRI study at 1.5 Tesla. J Magn Reson Imaging. 2008;28(1):252–7. doi: 10.1002/jmri.21390. [DOI] [PubMed] [Google Scholar]
- 20.Stelzeneder D, Kovács BK, Goed S, Welsch GH, Hirschfeld C, Paternostro-Sluga T, Friedrich KM, Mamisch TC, Trattnig S. Effect of short-term unloading on T2 relaxation time in the lumbar intervertebral disc - in vivo magnetic resonance imaging study at 3.0 tesla. Spine J. 2012;12(3):257–64. doi: 10.1016/j.spinee.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buehlmeier J, Frings-Meuthen P, Remer T, Maser-Gluth C, Stehle P, Biolo G, Heer M. Alkaline salts to counteract bone resorption and protein wasting induced by high salt intake: results of a randomized controlled trial. J Clin Endocrinol Metab. 2012;97(12):4789–97. doi: 10.1210/jc.2012-2857. [DOI] [PubMed] [Google Scholar]
- 22.Frings-Meuthen P, Boehme G, Liphardt AM, Baecker N, Heer M, Rittweger J. Sclerosin and DKK1 levels during 14 and 21 days of bed rest in healthy young men. J Musculoskelet Neuronal Interact. 2013;13(1):45–52. [PubMed] [Google Scholar]
- 23.Kelsen J, Bartels LE, Dige A, et al. 21 Days head-down bed rest induces weakening of cellmediated immunity - Some spaceflight findings confirmed in a ground-based analog. Cytokine. 2012;59:403–9. doi: 10.1016/j.cyto.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 24.Pfirrmann CWA, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic Resonance Classification of Lumbar Intervertebral Disc Degeneration. Spine (Phila Pa 1976) 2001;26(17):1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 25.Reisfeld D, Wolfson H, Yeshurun Y. Context-free attentional operators: The generalized symmetry transform. Int J Computer Vis. 1995;14(2):119–130. [Google Scholar]
- 26.Pohle-Fröhlich R, Brandt C, Koy T. Segmentierung der lumbalen Bandscheiben in MRT-Bilddaten. Informatik Aktuell. 2013:63–68. [Google Scholar]


