Abstract
Objective:
To investigate the relationship between frailty and sarcopenia, by evaluating the prevalence of sarcopenia among frail, pre-frail and robust elderly nursing home residents in Belgium.
Methods:
This is an analysis of baseline data collected from the SENIOR (Sample of Elderly Nursing home Individuals: an Observational Research) cohort. All subjects received a sarcopenia evaluation, based on the definition proposed by the European Working Group on Sarcopenia in Older People (EWGSOP). The frailty evaluation was primarily based on FRIED’s definition but also on 9 other operational definitions.
Results:
A total of 662 subjects (73.1% of women) were included in this analysis (mean age: 83.2±8.99 years). The prevalence of sarcopenia was 38.1% whereas the prevalence of frail and pre-frail persons was respectively 24.7% and 61.4%. Among frail, pre-frail and robust subjects, respectively 47%, 38.9% and 16.3% were diagnosed sarcopenic. The prevalence of sarcopenia according to ten different operational definitions of frailty ranged between 32.8 % (i.e. Frail scale Status and Frailty Index) and 47% (i.e. Fried definition).
Conclusion:
This research highlights that over a third of nursing home residents are sarcopenic and the percentage is almost 50% among frail subjects; those latter constitute about 1 in 4 of the population of nursing home residents studied here.
Keywords: Sarcopenia, Frailty, Nursing Home, Prevalence
Introduction
Over the past ten years, frailty and sarcopenia have been recognized as major public health issues, because they are both prevalent in the elderly and associated with negative health-related events, the latter being both easily detectable clinically and relatively simple to prevent or treat[1,2]. The European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) set up a working group on sarcopenia and frailty because they saw a strong link between these conditions and the skeletal diseases that are that society’s focus[3]. Therefore, special attention has been given to frailty[4] and sarcopenia[5]. For some time now, these geriatric syndromes have been studied in parallel and have overlapping phenotypes, but the causal relationship existing between the two is still controversial[6]. Determining whether frailty is due to sarcopenia, or that sarcopenia is a clinical manifestation of frailty, is a current focus of research. Sarcopenia was initially described by Rosenberg as the universal loss of muscle mass and strength inherent in the ageing process[7]. It is now recognized as an independent condition by an International Classification of Disease (ICD-10-CM)[8]. Frailty is multi-system impairment associated with increased vulnerability to stressors and describes individuals who are at increased risk of adverse health outcomes[9]. Sarcopenia is considered an important factor in the frailty cycle because all factors that trigger its development are related to the musculoskeletal system[10]. Thus, there is a deeper connection between these geriatric syndromes since sarcopenia is involved, as a main factor, in the development of the frailty syndrome. Frisoli et al. showed that sarcopenia was individually associated with frailty, though not statistically significant [odds ratio (OR): 3.1; 95% confidence interval (CI): 0.88-11.1][11]. Beaudart et al. (2015) have also shown that there were significantly more frail subjects among sarcopenic subjects (34.2%) than among non-sarcopenic ones (12.6%) (p-value=0.03)[12]. These studies were performed in community-dwelling elderly people but, as yet, the association between frailty and sarcopenia in a nursing home setting has never been studied. Thus, the aim of this study was to investigate the relationship between frailty and sarcopenia, by evaluating the prevalence of sarcopenia among frail, pre-frail and robust elderly nursing home residents in Belgium.
Methods
Study design
This study was conducted using baseline data collected among the SENIOR (Sample of Elderly Nursing home Individuals: an Observational Research) cohort[13], a prospective longitudinal study of Belgian nursing home residents. The study was approved by the Ethics Committee of the University Teaching Hospital of Liège under number 2013/178 and was registered on clinicaltrials.gov.
Population
Residents included in the SENIOR cohort and from 28 different nursing homes in Belgium were included in this analysis if they met the selection criteria: (1) to be oriented, to provide informed consent (Mini-Mental State Examination (MMSE) score above 18), (2) to be able to stand and walk (walking aids allowed), (3) to be a volunteer. Some tests or devices also involve specific exclusion criteria. For example, those for Bioelectrical Impedance Analysis (BIA) devices are: presence of an electronic implant (heart pacemaker, brain stimulator), body mass index over 50 kg/m2 and limb amputation.
Data collected
Diagnosis of sarcopenia
The definition of sarcopenia proposed by the EWGSOP was applied for this research[14]. According to these experts, sarcopenia diagnosis is based on the evidence of low muscle mass plus either low muscle strength or low physical performance:
- Muscle mass was assessed using Bioelectrical Impedance Analysis (BIA) using an InBody S10, Biospace device (Biospace Co., Ltd, Korea/Model JMW140) which allows estimation of the appendicular lean mass divided by the square of body height. BIA is based on the notion that tissues rich in water and electrolytes (i.e. skeletal muscles) are less resistant to the passage of an electrical current than lipid-rich adipose tissues (i.e. bones)[15,16]. All BIA systems exploit these tissue-specific conductivity differences to quantify body-compartments. In bioimpedance measurements, the human body is divided into five inhomogeneous segments, two for the upper limbs, two for the lower limbs and one for the trunk[17]. This non-invasive and easy to use method estimates the volume of fat and lean body mass based on the relationship between the volume of a conductor and its electrical resistance. Volunteers were seated on a chair and tactile electrodes were placed at 8 points on the body to achieve a multi-segmental frequency analysis. Gelled tissues were used to obtain optimal electrical conductance (i.e. inBody Tissue, Biospace CO.n Ltd: NaCl0.9%, isothiazlin 15 ppm, DDAC 150ppm). Threshold criteria for sarcopenia, when using bio-electrical impedance analysis, were 8.87 kg/m2 for men and 6.42 kg/m2 for women[18] as recommended by the EWGSOP.
- A hydraulic dynamometer, Saehan Corporation (MSD Europe Bvba, Belgium), was used to assess grip strength. It was calibrated for 10, 40 and 90 kg by the firm at the beginning of the recruitment period. Subjects were asked to grip the dynamometer as hard as they could three times with each hand. The maximum of the six measurements was recorded as the result, as recently recommended by Roberts[19]. The threshold points for the diagnosis of sarcopenia, defined by the EWGSOP group[14] - 30 kg for men and 20 kg for women - were used[20].
- To assess physical performance, the Short Physical Performance Battery test (SPPB) was used. It is a composite of three separate tests: balance, 4-metre gait speed and chair stand tests. Each test is weighted equally with a score between 0 and 4 points. Low physical performance threshold in the context of sarcopenia, scored on 12 points, is below or equal to 8 points[21].
Diagnosis of frailty
Frailty, as first measured by the Fried definition[10], is a function of deficits in some five domains. Thus, the phenotype of frailty was identified by the presence of three or more of the following components: weight loss (self-reported unintentional weight loss of more than 4.5 kg in the past year) or loss of appetite, weakness (dynamometer-measured grip strength below the established threshold based on sex and BMI), poor endurance and energy (self-reported exhaustion measured by two items from the Centre for Epidemiological Studies’ depression scale), slowness (walking speed over a distance of 4.5 m below the established threshold based on sex and height) and low physical activity level (self-reported time spent in physical activity in the past 7 days based on the Minnesota scale below the established threshold based on sex). The presence of one or two deficits indicates a pre-frail condition, and a total of three or more deficits indicate frailty, while the absence of deficits indicates a robust state.
Then, frailty was assessed using 9 other operational definitions: Clinical Frailty Scale (CFS)[22], Edmonton Frail Scale (EFS)[23], Frail Scale Status[24], Frailty index[25,26], Groningen Frailty Indicator (GFI)[27], Sega grid[28], Share Frailty Instrument (Share-FI)[29], Strawbridge questionnaire[30], Tilburg Frailty Indicator (TFI)[31].
Socio-demographic and clinical data
Other variables were also collected. The socio-demographic and clinical data include age, sex and Body Mass Index (BMI). The use of walking aids was recorded. The level of physical activity was evaluated by means of the short version of the Minnesota Leisure Time Physical Activity Questionnaire. This questionnaire asks participants about the types, frequency and duration of their leisure time activity (average hours/day engaged in the following four categories: walking, doing aerobics or workouts, sports and household activities). The number of calories burned per day was calculated using the activity metabolic index, which enables the calories burned to be measured using the metabolic equivalent of tasks[32]. Cognitive status was assessed with the Mini-Mental State Examination (MMSE), which consists of a 30-item questionnaire. A maximum score of 30 is attainable for a person without any neuropsychological impairment. Any score greater than or equal to 25 points indicates normal cognition. Below this threshold, scores can indicate severe (≤9 points), moderate (10-18 points) or mild (19-24 points) cognitive impairment[33]. Nutritional status was assessed using the Mini Nutritional Assessment (MNA). This test comprises two parts: a screening part followed by an assessment part. If the score obtained for the screening section was 12 or more points out of the 14 total possible points, the subject was classified as well-nourished and did not need to complete the assessment part. If the subject presented a screening score of 11 points or less, the assessment part had to be completed. The full evaluation is scored on 30 points. A score of 24 points or more indicates that the subject is well-nourished, a score between 17 and 23.5 points indicates a risk of malnutrition, and a score lower than 17 points indicates malnutrition[34]. Quality of life was assessed using the Short-Form 36 (SF-36) questionnaire. This is a 36-item questionnaire that measures quality of life (QoL) across eight domains that are both physically and emotionally based. The eight domains that the SF-36 measures are as follows: physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain and general health. In summary, an aggregate percentage score is produced for each of the eight domains that the SF-36 measures. The percentage scores range from 0% (lowest or worst possible level of functioning) to 100 % (highest or best possible level of functioning)[35]. Gait and body balance was assessed using 4 tests: (1) the Tinetti test[36] which consists of 16 items: 9 for body balance and 7 for gait. The maximum score is 16 for body balance, 12 for gait and thus 28 for the global score (balance + gait). A score below 19 indicates a high risk for falls, a score between 19 and 24 indicates a moderate risk for falls, and 28 points indicates no risk of falls. (2) The “Timed Up and Go” test[37] which measures basic mobility and capabilities of dynamic equilibrium in a complex task in the elderly. From a sitting position, the subject has to stand up, walk 3 m, turn over, walk back and sit down again. The time needed to complete the task is recorded and used for analyses[38]. A time of more than 30 seconds indicates a high level of dependence. A time of between 20 and 30 seconds indicates uncertain mobility and risk of falling. A time of less than 20 seconds indicates independence of the subject. (3) The “Short Physical Performance Battery” test[14] which is composed of three separate tests: balance, 4-metre gait speed and chair stand test. A score between 0 and 4 is assigned for each test, which are weighted equally. Therefore, the maximum score is 12 points. The cut-off value used to assess a poor physical performance is ≤8 points, according to the EWGSOP. (4) the gait speed test[14], is also proposed by the EWGSOP. A score <0.8 m/s for walking speed is considered as poor physical performance[14]. Patients were instructed to walk as fast as they could safely, without running from one cone to the other, placed at 4 m.
Isometric strength of 8 different muscle groups
The relative isometric strength (i.e. strength per kg) of 8 different muscle groups (i.e. knee flexors and extensors, ankle flexors and extensors, hip abductors and extensors and elbow flexors and extensors) was assessed using a hand-held dynamometer according to the protocol defined by Buckinx[39]. The protocol consisted of three consecutive maximal contractions for each muscle group, preceded by 3 warm-up trials. The three measurements were performed with 30 seconds intervals between contractions and the highest performance was considered for analysis. Subjects were asked to gradually increase their muscle force to a maximum effort which had to be sustained for six seconds. Measurements were performed on the dominant side (writing hand and kicking leg). The testing positions have been described elsewhere[39].
Statistical analysis
Quantitative variables that were normally distributed were expressed as a mean ± standard deviation (SD), and quantitative variables that were not normally distributed were reported as the median and percentiles (P25-P75). Shapiro-Wilk’s test verified the normal distribution of all parameters. Qualitative variables were reported as absolute and relative frequencies (%). The prevalence of frail and sarcopenic subjects was calculated by means of the descriptive statistics. Logistic regressions analysis, adjusted on confounding variables (age, sex and number of comorbidities) was used to test the association between the presence of sarcopenia and frailty. Then a comparison between characteristics of sarcopenic and non-sarcopenic subjects was performed using the Student t test (or non-parametric Mann-Whitney test) for continuous variables and by means of the Chi squared test for categorical variables. The data analyses were performed using Statistica 12 software. Bonferonni corrections were used for the pairwise comparison. The results were considered statistically significant when the 2-tailed p values were less than 0.05.
Results
Population
A total of 662 subjects from the SENIOR cohort and living in 28 nursing homes in the area of Liège, Belgium, were included in this analysis. The mean age of the population was 83.2 ± 8.99 years and 73.1% of the residents were women. The mean BMI was 25.9 ± 5.52 kg/m2. In this population, the main components of sarcopenia were evaluated and the mean SPPB score was 5.56 ± 3.23 points, the mean grip strength was 18.6 ± 10.9, the mean appendicular lean mass divided by the square of body height was 8.46 ± 6.47 kg/m2. The totality of the baseline characteristics of the subjects are presented elsewhere[13].
Prevalence of frailty and sarcopenia
In the study population, 24.7% of the subjects were frail (i.e. 27.3% of women and 19.2% of men) and 61.4% were pre-frail (i.e. 59.6% of women and 62.4% of men), according to Fried’s definition. The prevalence of sarcopenia was 38.1% in this population (i.e. 36.2% of women and 43.1 % of men).
The prevalence of sarcopenia according to the frailty status (i.e. Fried definition) showed that47% of frail subjects were sarcopenic. Among pre-frail subjects, 38.9% were sarcopenic and the proportion of sarcopenia among robust subjects was 16.3%.
After adjustment on age, sex and number of co-morbidities, the probability of being sarcopenic when the patient is frail is increased by 2.36 (OR= 2.36, 95% CI=1.31-4.13; p=0.004). Conversely, the probability of being frail when the patient is sarcopenic is increased by 2.33 (OR=2.33, 95%CI=1.31-4.14; p=0.003).
Table 1 shows the number of subjects below the cut-off defined by the EWGSOP for each component of sarcopenia (i.e. muscle mass, muscle strength and physical performances), among the total population, frail and pre-frail subjects. Low muscle mass is observed among 26.7% of the men and among 76% of the women. Dynapenia or low muscle strength is observed among 87.7% of the women and among 20.5% of the men. A low physical performance is observed among 66.2% of the population.
Table 1.
Prevalence of each component of sarcopenia in the total population, among frail and among pre-frail subjects.
| Total population n (%) | Prevalence of Frailty n (%) | Prevalence of pre-frailty n (%) | |
|---|---|---|---|
| Muscle mass: | |||
| Men: ALM/ht2<8.87 kg/m2 | 43 (26.7) | 10 (23.2) | 24 (55.8) |
| Women: ALM/ht2<6.42 kg/m2 | 19 (76) | 5 (56.3) | 11 (57.9) |
| Muscle Strength: | |||
| Men: <30 kg | 118 (20.5) | 32 (27.1) | 80 (67.8) |
| Women: <20 kg | 398 (87.7) | 122 (30.7) | 253 (63.6) |
| Physical performances: | |||
| SPPB<8 points | 438 (66.2) | 144 (33.8) | 264 (60.3) |
Figure 1 shows the prevalence of each component of frailty (i.e. Fried definition) among frail, pre-frail and sarcopenic subjects. Regarding Fried’s five component, the majority of sarcopenic subjects have weakness and almost 50% of them have a low level of physical activity. These are also the components most found among frail subjects.
Figure 1.
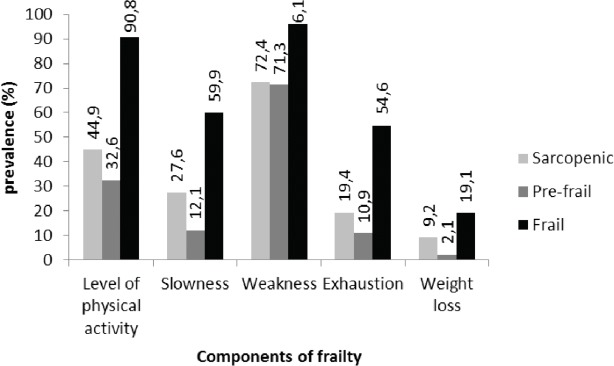
Prevalence of each component of frailty among frail, pre-frail and sarcopenic subjects.
The prevalence of sarcopenia among frail subjects, diagnosed according to ten different operational definitions of frailty, ranged between 32.8 % (i.e. Frail Scale status and Frailty Index) and 47% (i.e. Fried definition). The prevalence of sarcopenia among frail subjects, stratified by gender and age, according to the median age of the population (i.e. 85 years), is presented in Table 2.
Table 2.
prevalence of sarcopenia, stratified by age and gender, among frail subjects, diagnosed using 10 different operational definition.
| Operational definition of frailty | General population (n=662) | Men | Women | ||||
|---|---|---|---|---|---|---|---|
| <85 years (n=122) | ≥ 85 years (n=60) | Total (n=182) | <85 years (n=136) | ≥ 85 years (n=282) | Total (n=480) | ||
| Clinical Frailty Scale | 37.3 | 25 | 62.5 | 40 | 34.4 | 41.5 | 39.9 |
| Edmonton Frail Scale | 38.3 | 28.8 | 66.7 | 41.2 | 30.5 | 38.1 | 35.5 |
| Frail Scale Status | 32.8 | 25 | 60 | 33.3 | 43.5 | 47.3 | 46.2 |
| Frailty Index | 32.8 | 100 | 0 | 11 | 50 | 28.6 | 33.3 |
| Fried definition | 47 | 39.1 | 50 | 42.9 | 51.3 | 41.5 | 44.3 |
| Groningen Frailty Indicator | 39.9 | 29.3 | 67.5 | 41.2 | 30.8 | 41.3 | 37.6 |
| Sega grid | 40.6 | 23.3 | 77.8 | 35.9 | 24.6 | 42.9 | 37.9 |
| Share Frailty Instrument | 39.7 | 31.3 | 58.3 | 42.9 | 28.7 | 38.3 | 35.6 |
| Strawbridge questionnaire | 40.6 | 32.8 | 56.3 | 40.6 | 33.3 | 39.5 | 37.8 |
| Tilburg Frailty Indicator | 41.9 | 36.9 | 68.4 | 46.2 | 29.4 | 43.6 | 38.8 |
Comparison between sarcopenic and non-sarcopenic subjects among frail, pre-frail and robust subjects
As expected, certain clinical and demographic characteristics differ between subjects with sarcopenia and those without sarcopenia (Table 3). Indeed, sarcopenic subjects have a lower BMI than non-sarcopenic ones and this is true among frail (25.1±4.89 vs. 27.5±5.17 kg/m2; p=.02), pre-frail (23.3±5.33 vs. 27.3±5.02 k/m2; p<.0001) and robust subjects (22.6±5.53 vs. 26.4±4.48 k/m2; p=.004). Among pre-frail and robust subjects, sarcopenic are older than non-sarcopenic (85.9±8.14 vs. 82.1±9.12; p<0.0001 and 82.6±5.63 vs.76.7±8.8, p=.02; respectively). Robust subjects with sarcopenia have globally lower physical and muscular performances than subjects without sarcopenia. The first group have a lower Tinetti score (6.79±2.49 vs. 9.03±1.94 points; p<.0001), a higher time to perform the Timed Up and go test (17.3±7.59 vs 16.6±4.36 seconds; p=.01). Their isometric strength of the ankle extensors is also lower compared to non-sarcopenic subjects, among frail subjects (p=0.02).
Table 3.
Differences regarding characteristics of sarcopenic subjects and non-sarcopenic ones among frail and non-frail subjects (n=662).
| Characteristics | Frail (n=163) | Pre-frail (n=406) | Robust (n=93) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sarcopenic (n=77) | Non-sarcopenic (n=86) | p-value | Sarcopenic (n=158) | Non-sarcopenic (n=248) | p-value | Sarcopenic (n=15) | Non-sarcopenic (n=78) | p-value | |
| Age (years) | 85.8±6.46 | 85.9±9.23 | .92 | 85.9±8.14 | 82.1±9.12 | <.0001 | 82.6±5.63 | 76.7±8.80 | .02 |
| Nursing home and care (yes) | 23 (31.5) | 20 (25) | .75 | 25 (16.9) | 28 (11.9) | .32 | 4 (26.7) | 1 (1.37) | .16 |
| BMI (kg/m2) | 25.1±4.89 | 27.5±5.17 | .02 | 23.3±5.33 | 27.3±5.02 | <.0001 | 22.6±5.53 | 26.4±4.48 | .004 |
| Walking support (no) | 14 (19.2) | 15 (18.8) | .97 | 66 (44.6) | 114 (48.5) | .29 | 9 (60) | 57 (78.1) | .39 |
| Comorbidities (number) | 6.61±4.21 | 5.91±3.12 | .33 | 5.71±3.25 | 4.76±3.48 | .03 | 7.25±3.95 | 4.44±3.85 | .01 |
| Level of physical activity (kcal) | 340.5±250.2 | 546.1±171.4 | .02 | 857.3±647.7 | 938.4±898.9 | .34 | 1355.5±1080.1 | 1364.5±982 | .97 |
| MMSE (/30) | 22.8±5.22 | 23.9±4.45 | .14 | 24±4.05 | 24.5±5.45 | .36 | 23.6±5.87 | 25.9±2.97 | .04 |
| Normal nutritional status | 31 (42.5) | 47 (48.7) | .17 | 58 (39.2) | 39 (16.6) | <.0001 | 4 (26.7) | 58 (79.5) | .003 |
| Risk of malnutrition | 31 (42.5) | 29 (36.3) | .32 | 54 (36.6) | 37 (15.7) | <.0001 | 4 (26.7) | 10 (13.7) | .31 |
| Malnutrition | 5 (6.85) | 3 (3.75) | .70 | 4 (2.7) | 2 (0.85) | .75 | 0 | 0 | 1 |
| QoL: EQ-5D (%) | 42.9±26.4 | 44.9±25.7 | .63 | 59.1±22.3 | 58.7±21.2 | .84 | 69.1±0.19 | 0.67±0.19 | .80 |
| QoL : SF-36 physical function(%) | 27.4±23 | 24.7±21.9 | .46 | 65.7±22.7 | 49.2±26.9 | .38 | 74±22.6 | 65.7±20.3 | .22 |
| QoL : SF-36 social function(%) | 80.1±26.7 | 78.1±25.5 | .63 | 89.3±18.9 | 92.7±16.4 | .07 | 94.2±14.2 | 87.5±20.4 | .14 |
| QoL : SF-36 physical role functioning (%) | 71.6±44.6 | 64.2±45.7 | .31 | 92±23.9 | 89.4±27.9 | .33 | 92.7±25 | 92.3±27.7 | .96 |
| SF-36 : emotional role functioning (%) | 86.2±34.5 | 80.6±37.8 | .33 | 95.8±12.8 | 95.5±18.9 | .84 | 74.4±38. | 897.7±14.1 | <.0001 |
| SF-36 : mental health (%) | 56.2±22.6 | 53.8±19.3 | .49 | 64.1±20.6 | 63.4±21.7 | .91 | 69.5±19.8 | 63.1±23.8 | .30 |
| SF-36 : vitality (%) | 39.6±22.2 | 39.6±18 | .99 | 49.1±19.3 | 51.1±29.1 | .55 | 55.3±17.3 | 57.3±22.1 | .72 |
| SF-36 : pain (%) | 61.6±32.2 | 59.5±31.1 | .71 | 72.8±28.3 | 74.1±28.3 | .68 | 72.6±32.1 | 76.8±37.2 | .72 |
| SF-36 : general health (%) | 57.7±19.9 | 57.3±20.2 | .90 | 66.1±17.7 | 65.1±19.1 | .99 | 73.1±17.5 | 66.5±13.8 | .21 |
| SF-36 : change health | 37.5±23.1 | 39.9±23.2 | .52 | 48.5±22.2 | 44.4±22.1 | .08 | 60±56.9 | 51.9±27.9 | .62 |
| Katz (points) | 12.9±3.44 | 14.2±4.88 | .08 | 10.6±2.64 | 11.3±3.07 | .03 | 10.1±2.04 | 10.4±4.03 | .83 |
| Tinetti (/28) | 17.1±6.86 | 17.4±6.67 | .74 | 23.4±4.88 | 23.6±5.22 | .84 | 25.9±3.11 | 26.9±3.34 | .15 |
| SPPB (/12) | 2.89±2.12 | 2.83±2.21 | .88 | 5.79±2.81 | 6.07±3.03 | .37 | 6.79±2.49 | 9.03±1.94 | <.0001 |
| TUG (sec) | 35.5±24.3 | 35.9±20.6 | .93 | 24.7±15.7 | 23.9±19.2 | .68 | 17.3±7.59 | 16.6±4.36 | .01 |
| Gait speed (m/sec) | 0.43±0.18 | 0.49±0.19 | .46 | 0.73±0.32 | 0.76±0.34 | .29 | 0.91±0.29 | 1.07±0.32 | .07 |
| Grip strength (kg) | 14±6.47 | 13.8±6.47 | .84 | 17.3±9.22 | 18.7±9.29 | .14 | 22.9±13.7 | 31.3±16.5 | .06 |
| Peak flow (ml/min) | 114.8±59.9 | 123.1±68.5 | .43 | 138.6±80.4 | 147.7±99.8 | .36 | 165.4±82.2 | 190.7±82.3 | .32 |
| IS : knee extensors (N/kg) | 1.27±0.62 | 1.31±0.66 | .75 | 1.60±1.04 | 1.72±0.63 | .28 | 2.03±0.59 | 2.36±0.88 | .24 |
| IS : knee flexors (N/kg) | 1.05±0.44 | 1.16±0.49 | .23 | 1.34±0.49 | 1.42±0.47 | .22 | 1.51±0.59 | 1.61±0.43 | .68 |
| IS : ankle flexors (N/kg) | 0.99±0.47 | 1.18±2.017 | .52 | 1.20±0.47 | 1.16± 0.51 | .50 | 1.42±0.39 | 1.57±0.92 | .69 |
| IS : ankle extensors (N/kg) | 1.06±0.41 | 1.29±0.55 | .02 | 1.4±0.82 | 1.45±0.62 | .59 | 1.77±0.66 | 1.75±0.63 | .94 |
| IS : hip extensors (N/kg) | 0.93±0.48 | 1.04±0.46 | .27 | 1.26±0.62 | 1.38±0.65 | .13 | 1.07±0.67 | 1.77±0.72 | .83 |
| IS : hip abductors (N/kg) | 0.88±0.38 | 1.07±0.53 | .08 | 1.18±0.52 | 1.28±0.46 | .14 | 1.58±0.57 | 1.67±0.47 | .73 |
| IS : elbow flexors (N/kg) | 1.05±0.41 | 1.18±0.5 | .15 | 1.41±0.49 | 1.44±0.52 | .63 | 1.69±0.54 | 1.73±0.53 | .86 |
| IS : elbow extensors (N/kg) | 0.78±0.30 | 0.89±0.36 | .07 | 1.02±0.36 | 0.99±0.36 | .50 | 1.07±0.39 | 1.22±0.34 | .32 |
| ALM/height2 | 7.13±1.01 | 9.61±5.97 | .007 | 7.08±1.12 | 9.11±3.11 | <.0001 | 7.65±1.02 | 8.56±1.49 | .04 |
IS: Isometric strength. QoL: Quality of life.
Discussion
This study made it possible to assess the relationship between frailty and sarcopenia in a population of nursing home residents, by evaluating the prevalence of sarcopenia among frail, pre-frail and robust elderly nursing home residents in Belgium.
First, this study highlights that, in the population studied, the prevalence of sarcopenia is 38.1% whereas the prevalence of frailty and pre-frailty is respectively 24.7% and 61.4%. Another Belgian study, the Belfrail study, has shown a prevalence of sarcopenia of 12.5% among subjects aged 80 years and over[40]. In the SarcoPhAge study, a Belgian cohort of volunteer subjects aged 65 years or more, the prevalence of sarcopenia was very close to that obtained in Belfrail (i.e. 13.7%). Prevalence of sarcopenia in the present study, the SENIOR cohort, is about 3 times higher, which can be explained by the particular population that was studied. Indeed, both Belfrail and SarcoPhAge are focused on community-dwelling elderly people whereas SENIOR is focused on nursing home residents. It is admitted that the prevalence of sarcopenia increases in a nursing home setting[41]. There is a close association of the degree of sarcopenia with dependence among residents[42]. The pathophysiology of sarcopenia in this population is strongly influenced by comorbidity and often there is a significant overlap with the cachexia syndrome, which could explain such a high prevalence[14]. Landi et al (2012) found a prevalence of sarcopenia among nursing home residents comparable with the one in our study (i.e. 32.8%)[43]. In a Spanish multi-centre study, sarcopenia was demonstrated in 37% of subjects (15% men, 46% women), which is also quite close to our prevalence[44]. In addition, an Australian study performed in 11 nursing homes identified 40.2% of sarcopenic subjects[45]. Among a Turkish cohort, the prevalence of sarcopenia (i.e. 29%) is also comparable to that observed in the SENIOR cohort[46]. When regarding the prevalence of frailty, it varies from 4.0% to 59.1% in community-dwelling elderly adults[47]. According to Fried, the mean prevalence of frailty among subjects aged 80 years or more is 16.3%[10]. In the SENIOR cohort, we observed a slightly higher prevalence (i.e. 24.7%) but this is in agreement with the scientific literature which reports a higher prevalence among nursing home residents than in community-dwelling people[1]. A Polish cohort reports a prevalence of frailty of 34.9% in nursing homes assessed with the Clinical Frail Scale which is around 3% lower than that found in the present study with the same definition[48]. A Canadian cohort shows a prevalence of 48% in the same environment[49]. The comparison between studies is difficult because the lack of consensus and the variation in the operational definition used to diagnose frailty.
Thus, to the best of our knowledge, this study is the first to evaluate the prevalence of sarcopenia according to the frailty status in a population of nursing home residents. The results highlight that among frail, pre-frail and robust subjects, respectively 47%, 38.9% and 16.3% were diagnosed sarcopenic. This suggests that frail subjects are more at risk of being sarcopenic and it confirms the assumption that sarcopenia is a major component in the development of frailty[50]. Note that the definition used to asses frailty (i.e. Fried’s definition) has not been validated in a population of nursing home residents. To the best of our knowledge, all existing tools to assess frailty have not been tested in this specific population. Because Fried’s definition is the most widespread in the literature, we chose to use it. Moreover, this study has shown that sarcopenic subjects have around a twofold increased probability of frailty compared to non-sarcopenic subjects. This corroborate those of a recent study which demonstrated that pre-frail elderly individuals were significantly more likely to have sarcopenia than non-frail elderly individuals [odds ratio (OR): 2.77, 95% confidence interval (CI): 1.05-9.26][51]. The main component of physical frailty found in sarcopenic subjects from the SENIOR cohort is weakness. This seems obvious since the 2 geriatric syndromes included this measure in their definition. Weakness is the most common first manifestation of the frailty syndrome[52], which explains its high frequency. Indeed, it affects respectively 96.1% and 72.4% of frail and sarcopenic subjects in this research. Given that weakness is the cardinal feature of the frailty syndrome, sarcopenia is likely a key pathophysiologic contributor to frailty[53]. Investigators in Europe and Asia consider sarcopenia research a potentially useful initial step towards interventional studies of the frailty syndrome[54,55].
Finally, differences between sarcopenic subjects and non-sarcopenic ones have been highlighted in the present study. This confirms, in a particular population of nursing home residents, findings from previous work in other populations. The same differences between sarcopenic and healthy subjects were observed in the SarcoPhAge study[12]. We found that BMI was lower in sarcopenic subjects than in non-sarcopenic subjects, which was to be expected in so far as sarcopenic subjects presented a lower amount of muscle mass. In this study, among robust subjects, the quality of life related to emotional role functioning of subjects affected by sarcopenia had deteriorated. By now, few studies have reported data concerning quality of life for sarcopenic subjects. This is probably due to the lack of specific tools to measure the quality of life of sarcopenic subjects. Beaudart et al. 2015 have developed a questionnaire for this purpose which can be used in future studies[56]. As expected, our study indicates that sarcopenic subjects show lower physical and muscular performances. This is not surprising since these elements are part of the definition of sarcopenia that we used. It is also encouraging because if physical and muscular performances are strategically addressed, could reduce the burden of sarcopenia and frailty. This had been recently demonstrated among community-dwelling older people and could be tested among nursing home residents[57]. This study included an original measure of relative isometric muscle strength and highlighted that relative muscle strength of the ankle extensors, among frail subjects, was significantly different between sarcopenic and non-sarcopenic subjects. Among the 8 muscle groups explored, this one seems the most discriminating for sarcopenia. Among pre-frail and robust subgroups, sarcopenic subjects have also a higher number of comorbidities than non-sarcopenic ones. This is probably due to the overlapping between cachexia and sarcopenia observed among frail subjects. Indeed, the cause behind the loss of muscle mass (whether cachexia or sarcopenia) may, however, be indistinguishable in clinical practice[58]. It is admitted that sarcopenia is often related to multiple pathologies and comorbidities which can also compromise the measurement of its prevalence[2].
This study has a number of strengths. Not only was it conducted on a large sample of nursing home residents, but it also took into account a considerable number of socio-demographic and clinical variables. However, the study is unfortunately limited in its external validity because of the non-representativeness of the sample. The sample is composed of volunteer subjects, able to walk and not disoriented. Then, the lack of medical data of the population should be taken into account to obtain more precise data on prevalence of frailty and sarcopenia. Then, a single definition of sarcopenia was used. However, there are many such definitions in the literature and the choice of the definition undoubtedly influences the measure of prevalence. Finally, BIA is sensitive to water and food intake prior the measurement. This has not been standardized, which may have influenced muscle mass measurements. Nevertheless, this bias is limited in nursing homes setting because residents receive similar meals at identical time. Note that no currently technique serves all the requirements for the measurement of muscle mass. Each has limitations and in particular, there is dearth of information on accuracy. Major disadvantages of CT scan, MRI or DXA are limited acces to the radiological department which operate it, considerably higher cost than BIA and radiation exposure.
In practice, more attention should be given to frail subjects for the prevention of sarcopenia because there is a link between these two diseases. It would be important to facilitating the translation of the two conditions in the clinical arena. Freeing the concepts of sarcopenia and frailty from what can be perceived as only indirectly related to the target organ (i.e., skeletal muscle) may indeed represent a possible solution for combining them into a unique, objective, standardized, and clinically relevant definition. This is a way to explore in future research.
In conclusion, this research highlights that over a third of nursing home residents are sarcopenic and the percentage is almost 50% among frail subjects who constituted about 1 in 4 of the population of nursing home residents studied here. Thus, sarcopenia seems to be associated with many socio-demographic and clinical components making this geriatric syndrome a potential public health issue.
Acknowledgements
The authors would like to thank all the participants who participated in this study. We also thank directory and healthcare staff from the nursing homes for their collaboration in the study.
Footnotes
Funding statement
Fanny Buckinx is supported by a Fellowship from the FNRS (Fonds National de la Recherche Scientifique de Belgique — FRSFNRS. www.frs-fnrs.be).
The authors have no conflict of interest.
Edited by: A. Ireland
References
- 1.Buckinx F, et al. Burden of frailty in the elderly population: perspectives for a public health challenge. Arch Public Health. 2015;73(1):19. doi: 10.1186/s13690-015-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaudart C, et al. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014;72(1):45. doi: 10.1186/2049-3258-72-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper C, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012;23(7):1839–48. doi: 10.1007/s00198-012-1913-1. [DOI] [PubMed] [Google Scholar]
- 4.Janssen I, et al. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–5. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 5.Clegg A, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cederholm T. Overlaps between Frailty and Sarcopenia Definitions. Nestle Nutr Inst Workshop Ser. 2015;83:65–9. doi: 10.1159/000382063. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990s–991s. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 8.Cao L, Morley JE. Sarcopenia Is Recognized as an Independent Condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. J Am Med Dir Assoc. 2016;17(8):675–7. doi: 10.1016/j.jamda.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Abellan van Kan G, et al. Clinical trials on sarcopenia: methodological issues regarding phase 3 trials. Clin Geriatr Med. 2011;27(3):471–82. doi: 10.1016/j.cger.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried LP, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 11.Frisoli A, Jr, et al. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women’s Health and Aging Study (WHAS) II. Bone. 2011;48(4):952–7. doi: 10.1016/j.bone.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Beaudart C, et al. Quality of life and physical components linked to sarcopenia: The SarcoPhAge study. Exp Gerontol. 2015;69:103–110. doi: 10.1016/j.exger.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Buckinx F, et al. Relationship between frailty, physical performance and quality of life among nursing home residents: the SENIOR cohort. Aging Clin Exp Res. 2016 doi: 10.1007/s40520-016-0616-4. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymsfield SB, et al. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015;74(4):355–66. doi: 10.1017/S0029665115000129. [DOI] [PubMed] [Google Scholar]
- 16.Buckinx F, et al. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: a cross-sectional study. BMC Musculoskelet Disord. 2015;16:60. doi: 10.1186/s12891-015-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil SF, Mohktar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors (Basel) 2014;14(6):10895–928. doi: 10.3390/s140610895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc. 2008;56(9):1710–5. doi: 10.1111/j.1532-5415.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 19.Roberts HC, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–9. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 20.Lauretani F, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95(5):1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Rolfson DB, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35(5):526–9. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–8. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Searle SD, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.K Rockwood AM. How might deficit accumulation give rise to frailty? J Frailty Aging. 2012;1(1):8–12. doi: 10.14283/jfa.2012.2. [DOI] [PubMed] [Google Scholar]
- 27.Baitar A, et al. Evaluation of the Groningen Frailty Indicator and the G8 questionnaire as screening tools for frailty in older patients with cancer. J Geriatr Oncol. 2013;4(1):32–8. doi: 10.1016/j.jgo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Schoevaerdts Didier BS, Malhomme Brigitte, Rezette Céline, Gillet Jean-Bernard, vanpee Dominique, Cornette Pascale. SWINE Christian, Identification précoce du profil gériatrique en salle d’urgences: présentation de la grille SEGA. La Revue de Gériatrie. 2004;29(3):169–178. [Google Scholar]
- 29.Romero-Ortuno R, et al. A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE) BMC Geriatr. 2010;10:57. doi: 10.1186/1471-2318-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strawbridge WJ, et al. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998;53(1):S9–16. doi: 10.1093/geronb/53b.1.s9. [DOI] [PubMed] [Google Scholar]
- 31.Gobbens RJ, et al. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc. 2010;11(5):344–55. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Taylor HL, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 33.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 34.Vellas B, et al. Overview of the MNA - Its history and challenges. J Nutr Health Aging. 2006;10(6):456–63. discussion 463-5. [PubMed] [Google Scholar]
- 35.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 36.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119–26. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 37.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 38.Bruyere O, et al. Controlled whole body vibration to decrease fall risk and improve health-related quality of life of nursing home residents. Arch Phys Med Rehabil. 2005;86(2):303–7. doi: 10.1016/j.apmr.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Buckinx F, et al. Reliability of muscle strength measures obtained with a hand-held dynamometer in an elderly population. Clinical Physiology and Functional Imaging. 2015 doi: 10.1111/cpf.12300. accepted for publication. [DOI] [PubMed] [Google Scholar]
- 40.Legrand D, et al. The relationship between grip strength and muscle mass (MM), inflammatory biomarkers and physical performance in community-dwelling very old persons. Archives of Gerontology & Geriatrics. 2013;57(3):345–51. doi: 10.1016/j.archger.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Landi F, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci. 2012;67(1):48–55. doi: 10.1093/gerona/glr035. [DOI] [PubMed] [Google Scholar]
- 42.Bauer JM, Kaiser MJ, Sieber CC. Sarcopenia in nursing home residents. J Am Med Dir Assoc. 2008;9(8):545–51. doi: 10.1016/j.jamda.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Landi F, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13(2):121–6. doi: 10.1016/j.jamda.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Salva A, et al. Prevalence of sarcopenia in Spanish nursing homes: Comparison of the results of the ELLI study with other populations. Rev Esp Geriatr Gerontol. 2016;51(5):260–4. doi: 10.1016/j.regg.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Senior HE, et al. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas. 2015;82(4):418–23. doi: 10.1016/j.maturitas.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Yalcin A, et al. Sarcopenia prevalence and factors associated with sarcopenia in older people living in a nursing home in Ankara Turkey. Geriatr Gerontol Int. 2016;16(8):903–10. doi: 10.1111/ggi.12570. [DOI] [PubMed] [Google Scholar]
- 47.Collard RM, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 48.Matusik P, et al. Severe frailty and cognitive impairment are related to higher mortality in 12-month follow-up of nursing home residents. Arch Gerontol Geriatr. 2012;55(1):22–4. doi: 10.1016/j.archger.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 49.Freiheit EA, et al. Operationalizing frailty among older residents of assisted living facilities. BMC Geriatr. 2011;11:23. doi: 10.1186/1471-2318-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolland Y, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12(7):433–50. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishiguchi S, et al. Differential association of frailty with cognitive decline and sarcopenia in community-dwelling older adults. J Am Med Dir Assoc. 2015;16(2):120–4. doi: 10.1016/j.jamda.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433–41. doi: 10.2147/CIA.S45300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rockwood K, Bergman H. Frailty: A Report from the 3(rd) Joint Workshop of IAGG/WHO/SFGG, Athens, January 2012. Can Geriatr J. 2012;15(2):31–6. doi: 10.5770/cgj.15.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu LK, et al. Age-related skeletal muscle mass loss and physical performance in Taiwan: implications to diagnostic strategy of sarcopenia in Asia. Geriatr Gerontol Int. 2013;13(4):964–71. doi: 10.1111/ggi.12040. [DOI] [PubMed] [Google Scholar]
- 56.Beaudart C, et al. Development of a self-administrated quality of life questionnaire for sarcopenia in elderly subjects: the SarQoL. Age Ageing. 2015;44(6):960–6. doi: 10.1093/ageing/afv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan DD, et al. Integrated care for geriatric frailty and sarcopenia: a randomized control trial. J Cachexia Sarcopenia Muscle. 2017;8(1):78–88. doi: 10.1002/jcsm.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rolland Y, et al. Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care. 2011;14(1):15–21. doi: 10.1097/MCO.0b013e328340c2c2. [DOI] [PubMed] [Google Scholar]


