Abstract
Objective:
To determine the feasibility and acceptability of using peak power and force, measured by jumping mechanography (JM), to detect early age-related features of sarcopenia in older women.
Methods:
Community-dwelling women aged 71-87 years were recruited into this cross-sectional study. Physical function tests comprised the short physical performance battery (SPPB), grip strength and, if SPPB score≥6, JM. JM measured peak weight-adjusted power and force from two-footed jumps and one-legged hops respectively. Questionnaires assessed acceptability.
Results:
463 women were recruited; 37(8%) with SPPB<6 were ineligible for JM. Of 426 remaining, 359(84%) were able to perform ≥1 valid two-footed jump, 300(70%) completed ≥1 valid one-legged hop. No adverse events occurred. Only 14% reported discomfort. Discomfort related to JM performance, with inverse associations with both power and force (p<0.01). Peak power and force respectively explained 8% and 10% of variance in SPPB score (13% combined); only peak power explained additional variance in grip strength (17%).
Conclusions:
Peak power and force explained a significant, but limited, proportion of variance in SPPB and grip strength. JM represents a safe and acceptable clinical tool for evaluating lower-limb muscle power and force in older women, detecting distinct components of muscle function, and possibly sarcopenia, compared to those evaluated by more established measures.
Keywords: Grip Strength, Short Physical Performance Battery, Gait Speed, Chair Rise, COSHIBA
Introduction
Age-related declines in muscle strength characterise sarcopenia which is thought to explain many facets of morbidity in older people including increased falls and fracture risk[1]. Due to difficulties in objectively measuring muscle strength outside a laboratory setting, clinical definitions of sarcopenia are often based upon the functional consequences of impaired muscle strength on physical performance, with differing definitions proposed[1,2]. For example, gait speed, chair-rise time and balance all predict falls[3,4] and, combined into the validated and well established composite short physical performance battery (SPPB)[5], can identify individuals with sarcopenia[6].
Upper-limb grip strength is widely used in clinical studies of older people as a measure of muscle strength (defined as the exertion of force to overcome resistance), and relates not only to functional measures like the SPPB[7], but to a range of clinical sequelae including falls and fractures[8-10]. Lower-limb muscle strength, which potentially has a more direct relationship to falls and fractures, is generally only measured as part of research studies. Jumping mechanography (JM), which unlike dynamometers for testing isometric muscle strength, can be used outside of a laboratory setting, and has been successfully used in child and adolescent research[11,12]; however, relatively few studies have examined this method in older adults[13-15]. In one recent US study, substantial age-related changes were observed in lower-limb muscle power, assessed by JM, which occurred earlier than deficits in grip strength or functional measures, suggesting lower-limb power may represent an important research, and possibly clinical, tool for detecting early deficits in muscle function[16]. This study detected weak positive relationships between lower-limb muscle power and grip strength (r=0.34) and SPPB (r=0.27), consistent with the suggestion that JM can detect early changes of sarcopenia.
JM calculates peak power as the product of force and velocity[15,17], typically measured during standing two-legged countermovement jumps. JM also measures peak force directly through the force platform in Newtons, ideally during multiple one-legged hopping because peak forces are typically greater during one-legged serial hopping than either two-legged serial hopping or countermovement or squat jumps[18]. Whilst peak power is thought to reflect muscle activity at the hip, knee and calf, peak force reflects additional joint bio-mechanics and tensile tendon properties (principally the Achilles). Hence, peak muscle power is strongly determined by muscle conditioning, whereas additional factors, such as tendon elasticity which may remain more constant over the life-course, influence muscle force. This may explain why peak lower-limb muscle power declines with age to a greater extent than muscle force[19]. Consequently, the more demanding one-legged serial hopping component of JM, required for assessing peak force, may provide minimal additional information regarding detection of early age-related sarcopenia. Muscle weakness, principally assessed as strength, is a well-established risk factor for incident and recurrent falls[20]; lower limb muscle power assessed by JM has been associated with self-reported falls in one cross-sectional study of older women[21]. Data linking measured muscle power and force to fractures however, are limited. Isometric lower limb muscle strength predicted incident fractures in one small Finnish cohort[22], and lower limb muscle power (measured using the Nottingham power rig) predicted incident hip fractures in a large population of older men, but not after adjustment for bone density[23].
We aimed to examine the feasibility and acceptability of evaluating components of sarcopenia based upon direct measurement of lower-limb muscle function by JM, in a community-based population of older women. In addition, to determine whether JM detects distinct aspects of muscle function compared to more established measures, we aimed to confirm that directly measured lower-limb muscle power and force only show a limited relationship with grip strength and SPPB. Finally, we aimed to extend previous observations suggesting peak lower-limb muscle power measured by JM can detect early sarcopenia, by examining the contribution made by peak muscle force to traditional measures of muscle function, namely grip strength and SPPB.
Materials and methods
Study participants
The VIBE (Vertical Impacts in Bone in the Elderly) study is a UK-based research program investigating the role of physical activity on musculoskeletal health in older adults with ranging functional capabilities[24]. This cross-sectional study, performed in 2015, re-recruited participants from an earlier population-based cohort study (Cohort for Skeletal Health in Bristol and Avon; COSHIBA) (Figure 1). The original COSHIBA participants (n=3200) were all female, born 1 January 1927-31 December 1942 and recruited through Bristol and Avon general practitioner registries during 2007-2009, with no exclusion criteria[25,26]. Of 1286 who had previously consented to contact regarding future studies, 1064 (83%) were still alive and resident within Bristol/Avon in 2015 and were invited to participate in the VIBE study, of whom 463 (43.5%) attended for assessment (invited alphabetically until appointments full).
Figure 1.
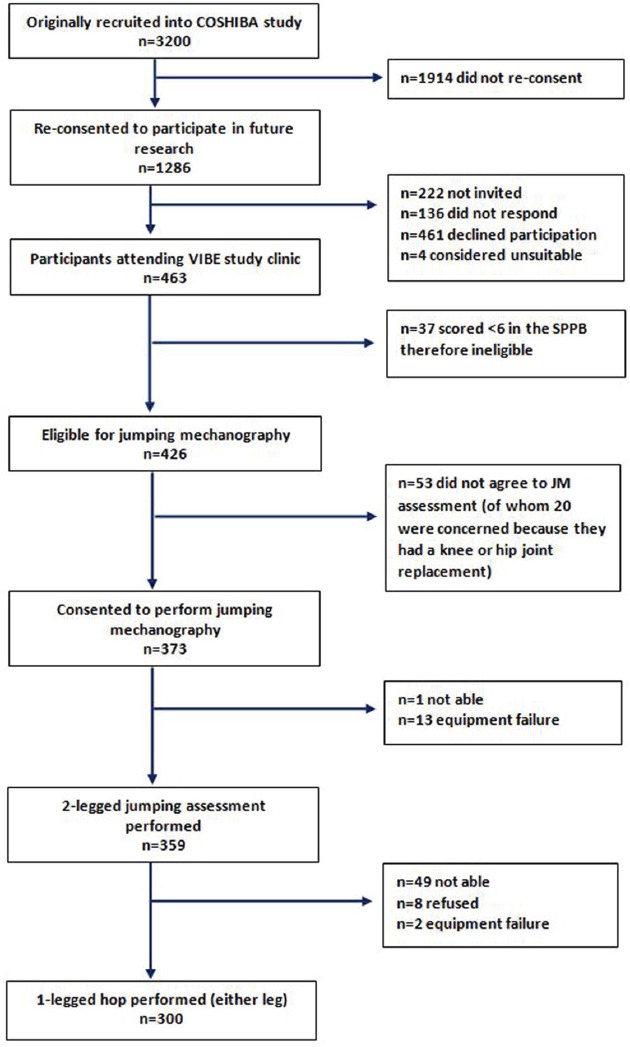
Flow chart explaining origin of study population and of jumping mechanography data collection.
Consenting participants attended our research clinic throughout 2015; physical function and musculoskeletal assessments were performed by fully trained staff working to standard operating procedures. All participants were eligible for physical function testing (i.e. SPPB and grip strength detailed below). After functional assessments, paper-based questionnaires provided demographic, socio-demographic, self-rated health and lifestyle data, and medical history (determining co-morbidities). Written informed consent was provided in line with the Declaration of Helsinki[27]. The study was approved by the Southwest Frenchay Research Ethics Committee (REC:14/SW/0138).
The short performance physical battery (SPPB)
The SPPB, used for pre-screening prior to JM (see below), was scored using standard methodology[28]; 3 components (i) gait speed: best of two 4-metre timed walks at normal pace, (ii) chair-rise time: 5 timed chair-rises performed as fast as possible (N.B. without use of upper limbs to rise from the chair), and (iii) tandem balance: stands held for maximum of 10 seconds with feet side-by-side, semi-tandem and then tandem. The time to perform each component was categorised, allocated a score; these 3 scores were summed to generate an overall SPPB score (minimum=zero, maximum=12)[29].
Jumping mechanography (JM)
We used a 1 m2 Leonardo Mechanograph Ground Reaction Force platform (weight 60 kg, area 1 m2 and height 7 cm) which consists of two plates with eight sensors underneath each corner which detect a voltage proportional to applied force (Figure S1). Sensor recordings are used to derive test-specific performance calculations using Leonardo software (version 4.2, Novotec Medical, Pforxheim, Germany). All staff completed standard training from Novotec Medical[13]. All participants scoring SPPB≥6 were eligible (i.e. physically capable and safe to jump) and, if consenting, proceeded with jumping assessments. Vertebral osteoporosis was not considered a contraindication to JM. If participants expressed anxiety about their balance during the assessment, additional safety measures were considered, namely a) having an extra staff member present, and b) the staff member positioning themselves in front with arms out-stretched and forefingers pointing toward the participant to give reassurance (without adding any upward propulsion).
Supplementary Figure S1.

Leonardo Mechanograph Ground Reaction Force platform used for jumping and hopping assessments in the VIBE Study. Here two-legged countermovement jump (2LJ) is demonstrated.
Participant footwear was checked for suitability (flat soft soles; no heels) and removed if unsuitable. Prior to each assessment, the platform was calibrated (zero adjustment) and basic data input, e.g. age, gender, height (measured by calibrated Harpenden stadiometer). Body mass index (BMI) was calculated as weight (kilograms)/height (square metres). Clinic workers demonstrated and gave verbal instructions on how to perform a 2-legged countermovement jump (2LJ) and one-legged hops (1LH). If necessary they stood beside the participant to assist with balance on landing. Participants could use their arms in any way that was comfortable and standard instructions were provided: “I would like you to stand as still as you can please. When I instruct you to do so, I would like you to jump once as high as possible using both legs. Land on your forefoot and then stand as still as possible on both feet”. For the single-legged hopping test (1LH), participants were encouraged to start with their most comfortable or dominant leg and attempt 6-8 hops with the standard instructions: “When I instruct you to begin, I would like you to hop on your forefoot, without landing on your heels using a stiff knee and follow the instructions as I provide them. Stand still to start on both legs”. 2LJ and 1LH were each attempted three times, unless there was an error, in which case a maximum of four attempts was allowed. 1LH was only attempted if 2LJ could be performed. Participants remained standing between jumps/hops and recommenced when they were recovered and ready. Reasons were recorded if jumps/hops were not performed (unable/equipment failure (n=15)/other) (Figure 1). Clinic workers recorded adverse events. Peak power (kW) from the maximal valid 2LJ and the maximal peak force (kN) from all 1LH were extracted. Isotonic peak power and force relative to body weight were derived using body mass measured using calibrated Tanita weighing scales (Tanita UK Ltd.), prior to JM assessment. Peak weight-adjusted maximum power (W/kg) and force (N/kg) were used in analyses.
Acceptability of jumping mechanography
To assess acceptability of JM in older people, participants were asked two questions upon completion of their jump assessment; 1) “how frequently have you performed these types of movements (jumping and hopping) in the past 12 months?” (options: never/rarely/every once in a while/often/very often), and 2) “how comfortable did you feel carrying out the movements” (very uncomfortable/uncomfortable/unsure/comfortable/very comfortable).
Upper-limb grip strength
Isometric grip strength was measured by JAMAR electronic handgrip dynamometery[30], whilst standing with one arm fully extended beside the body, with a stiff wrist and a 2 cm gap between arm and body. Standardised participant instructions based upon the Southampton approach ensured comparability in grip strength assessments[31]; maximal grip strength was taken from six attempts on alternating sides with the instruction “I want you to squeeze as hard as you can for as long as you can until I say stop. Squeeze, squeeze, squeeze, stop”. Grip strength was recorded to the nearest 0.1 kg; if not performed, reasons were recorded.
Statistical analyses
Descriptive statistics were compared between participants able to perform 2LJ and 1LH and the remaining clinic attendees. Data distributions were transformed where necessary. Continuous variables were summarised as mean (standard deviation:SD) and categorical variables as counts (percentages). Co-morbidities, identified by questionnaire, were deemed a priori to have a potential impact on musculoskeletal health.
In analysing the relationship between age, muscle power, force and grip strength, analyses were restricted to participants with at least one valid jump and hop measurement. Multivariable linear regression models examined associations between weight-adjusted peak power and force with grip strength, overall SPPB score, gait speed and chair-rise time. Multivariable logistic regression models examined associations between power/force and (binary) tandem balance (87.7% could balance for the maximum 10secs). Adjusted analyses included the a priori confounders age, height and co-morbidities (co-morbidities vs.≥1 co-morbidity).
To assess the relative contributions of power and force in predicting grip strength and SPPB components, r2 values (logistic regression pseudo r2) and a fit statistic (AIC=Akaike information criterion[32]) were calculated from adjusted regression models, with each exposure assessed independently and in combination. The AIC statistic is used to compare the ‘goodness of fit’ between similar models and penalizes for additional variables, thus identifying the model with ‘best fit’, with a minimum number of parameters. Lower AIC values indicate better ‘goodness of fit’.
All continuous exposure and outcome variables were standardized for regression analyses. Standardised beta coefficients, with 95% confidence intervals (CI), are presented representing a SD change in outcome per SD change in exposure. Data were collected using a secure online database (REDcap) with inbuilt data checks; analyses performed using Stata 13.1.
Results
Participant characteristics
Of 1064 women invited, 463(44%) attended the VIBE study clinic; they were mean 77 years old with BMI 26.5 kg/m2 (Figure 1). From inception of the cohort in 2007-2009, those subsequently attending the 2015 VIBE clinic were younger, less likely to smoke, but more likely to consume alcohol, with higher qualification attainment and lower BMI, than those who did not attend (Table S1).
Supplementary Table S1.
Characteristics of women recruited into the original COSHIBA study (2007-09) who did and did not participate in the subsequent 2015 VIBE study.
| Historic COSHIBA population who did not participate in the VIBE study | All VIBE study participants | ||||
|---|---|---|---|---|---|
| n | mean (SD) | n | mean (SD) | p value | |
| Age | 2737 | 73.2 (4.3) | 463 | 69.7 (2.9) | <0.001 |
| Height (cm) | 2433 | 160.0 (6.5) | 422 | 161.0 (6.4) | 0.004 |
| Weight (kg) | 2589 | 69.6 (13.4) | 452 | 68.6 (12.0) | 0.167 |
| n | % | n | % | p value | |
| Smoker | 0.015 | ||||
| Current | 219 | 8.1 | 23 | 5.0 | |
| Past | 1058 | 39.2 | 165 | 36.2 | |
| Never | 1421 | 52.7 | 268 | 58.8 | |
| Weekly alcohol consumption | <0.001 | ||||
| None | 1219 | 45.2 | 155 | 33.7 | |
| Few glasses per week | 1153 | 42.7 | 235 | 51.1 | |
| 1 drink daily | 236 | 8.7 | 48 | 10.4 | |
| >1 drink daily | 82 | 3.0 | 20 | 4.4 | |
| Don’t know/can’t remember | 10 | 0.4 | 2 | 0.4 | |
| Educational level | <0.001 | ||||
| School education until 16 years old | 1485 | 56.3 | 214 | 47.3 | |
| School education until 18 years old | 247 | 9.4 | 77 | 17.0 | |
| Apprenticeship | 613 | 23.3 | 98 | 21.7 | |
| University degree | 116 | 4.4 | 46 | 10.2 | |
| Other | 50 | 1.9 | 6 | 1.3 | |
| Don’t know/can’t remember | 126 | 4.8 | 11 | 2.4 | |
P; p value for difference. SD; Standard Deviation, COSHIBA; Cohort for Skeletal Health in Bristol and Avon, VIBE; Vertical Impacts in Bone in the Elderly. Data recorded at COSHIBA baseline.
Feasibility of jumping mechanography
Of 463 women attending the VIBE clinic, 37(8%) with SPPB<6 were ineligible to perform JM and 53(11%) declined, often due to fear of jumping on a hip/knee joint replacement; of those eligible to perform JM, 61(74%) with a joint replacement consented vs. 311(91%) without (p<0.01). Of the remaining 373 individuals, 359(96%) were able to perform at least one valid 2LJ (Figure S1), and 300(80%) also completed at least one valid 1LH. 14 participants required assistance with balance on landing from the clinic worker stood beside the jump platform. No adverse events occurred during JM for any participant. The 300 who successfully completed both JM measurements were all community-dwelling women (97% white British) with mean age 76 years (range: 71-87) and BMI 26.2 kg/m2 (range: 16.5-41.0) (Table 1). Self-rated health was deemed good or very good by 90%; 68% self-reported ≥1 co-morbidity, the most prevalent being arthritis (37%) and cancer (21%). The 163 who attended the VIBE clinics but were ineligible, unwilling or unable to generate JM data were generally older (mean age 78), had higher mean BMI (29.3 kg/m2), lower self-rated health (61% good or very good) and scored lower on physical function tests.
Table 1.
Characteristics of 463 women who attended the VIBE study clinics who were and were not eligible/able to produce valid jumping and/or hopping measurements by jumping mechanography.
| Participants attending VIBE study clinic eligible and able to produce valid jump/hop data | Participants attending VIBE study clinic either ineligible or unable to produce valid jump/hop data4 | ||||
|---|---|---|---|---|---|
| N | % or mean ±SD | N | % or mean ±SD | P value | |
| Total | 300 | 65 | 163 | 35 | |
| Age | 300 | 6.4±2.6 | 163 | 7.7±3.6 | <0.01 |
| Height (cms) | 300 | 159.4±6.0 | 163 | 157.4±6.1 | <0.01 |
| Weight (kgs) | 300 | 66.6±10.5 | 163 | 2.7±14.6 | <0.01 |
| BMI (kg/m2) | 300 | 26.2±.83 | 163 | 29.3±5.70 | <0.01 |
| Self-rated health | <0.01 | ||||
| Very good | 92 | 31.1% | 20 | 12.4% | |
| Good | 174 | 58.8% | 78 | 48.5% | |
| Fair | 28 | 9.5% | 56 | 34.8% | |
| Poor | 2 | 0.7% | 7 | 4.4% | |
| Very poor | 0 | 0% | 0 | 0% | |
| Co-morbidities | <0.01 | ||||
| No co-morbidities | 97 | 32.3% | 19 | 11.7% | |
| At least one co-morbidity1 | 203 | 67.7% | 144 | 88.3% | |
| Current smoker | 12 | 4.0% | 8 | 4.9% | 0.66 |
| Consumed alcohol in the past year | 257 | 86.5% | 109 | 66.9% | <0.01 |
| At least one fall in the past year | 77 | 26.3% | 76 | 47.8% | <0.01 |
| Taking vitamin D and/or calcium supplements | 90 | 30.6% | 82 | 51.9% | <0.01 |
| Lower-limb JM Maximum Power (W/kg)2 | 300 | 20.5±5.1 | |||
| Lower-limb JM Maximum Force (N/kg)3 | 300 | 20.2±4.7 | |||
| Upper limb grip strength (kg) | 300 | 21.6±4.9 | 158 | 19.3±5.6 | <0.01 |
| SPPB score (max of 12 points) | 300 | 10.6±1.4 | 155 | 8.3±2.8 | <0.01 |
| Gait speed time (secs) | 300 | 4.01±0.9 | 146 | 5.3±2.2 | <0.01 |
| Chair-rise time (secs) | 299 | 12.9±4.2 | 127 | 15.5±6.2 | <0.01 |
| Tandem balance held for the maximum of 10 seconds | 263 | 87.7% | 88 | 54.0% | <0.01 |
P=p value for difference. BMI=Body Mass Index. SD=Standard Deviation. SPPB=Short Physical Performance Battery, JM=Jumping Mechanography.
At least one of the following: Chronic non-specific lung disease(13%), cardiovascular disease (10%), peripheral arterial disease (1%), Diabetes mellitus (5%), Stroke (3%), Cancer (21%), Osteoporosis (11%), Arthritis (37%), Chronic liver/kidney disease (2%), Thyroid condition (10%), Coeliac disease(1%).
Maximum power generated from two legged jump, relative to weight.
Maximum force generated from one footed hop, relative to weight.
Full details shown in Figure 1.
Acceptability of jumping mechanography
Information about the comfort of performing JM was available in 279(93%) participants. The majority (86%) did not find the assessment uncomfortable; 64% even reporting JM to be comfortable or very comfortable (Table 2). The assessment was similarly acceptable for women with self-reported co-morbidities; JM was comfortable or very comfortable in 62% with ≥1 co-morbidity, 60% with arthritis and 57% with lung disease. Discomfort was not associated with self-reported hip and/or knee replacement. Of the 300 women completing both JM components, most (83%) reported they had not often performed jumping and hopping movements over the previous twelve months.
Table 2.
Comfort performing JM and frequency over the past 12 months of jumping/hopping activity amongst study participants according to co-morbidities.
| All women | Women with Arthritis | Women with Lung disease | Women with ≥1 comorbidity | |
|---|---|---|---|---|
| N=279 | n (%) | n (%) | n (%) | n (%) |
| Comfort performing jumps/hops | ||||
| Very uncomfortable/uncomfortable | 38 (13.6) | 12 (11.3) | 7 (20.0) | 26 (13.5) |
| Not sure | 64 (22.9) | 30 (28.3) | 8 (22.9) | 48 (25.0) |
| Comfortable | 155 (55.6) | 56 (52.8) | 18 (51.4) | 106 (55.2) |
| Very comfortable | 22 (7.9) | 8 (7.6) | 2 (5.7) | 12 (6.3) |
| Frequency of jump/hop activity in past 12 months | ||||
| Never | 92 (33.0) | 36 (34.0) | 13 (37.1) | 70 (36.5) |
| Rarely | 76 (27.2) | 31 (29.3) | 8 (22.9) | 59 (30.7) |
| Every once in a while | 63 (22.6) | 21 (19.8) | 11 (31.4) | 36 (18.8) |
| Often | 37 (13.3) | 14 (13.2) | 2 (5.7) | 21 (10.9) |
| Very often | 11 (3.9) | 4 (3.8) | 1 (2.9) | 6 (3.1) |
JM: Jumping mechanography.
Reported comfort while performing JM was related to JM performance, with positive associations observed with both power and force (test for trend p<0.01) (Table 3). A similar relationship was also observed with reported frequency of jumping and hopping over the previous year.
Table 3.
Associations between comfort performing JM and past 12-month frequency of jumping/hopping with peak power and force.
| N=279 | N (%) | Power1 | Force1 | ||||
|---|---|---|---|---|---|---|---|
| β | 95% CI | P value | β | 95% CI | P value | ||
| Comfort of jump/hop movements | |||||||
| Very uncomfortable/uncomfortable (ref) | 38 (13.6) | - | - | <0.01 | - | - | <0.01 |
| Not sure | 64 (22.9) | -0.04 | -0.21, 0.12 | -0.16 | -0.49, 0.18 | ||
| Comfortable | 155 (55.6) | 0.07 | -0.08, 0.21 | 0.07 | -0.23, 0.37 | ||
| Very comfortable | 22 (7.9) | 0.36 | 0.15, 0.58 | 0.21 | -0.23, 0.65 | ||
| Frequency of jump/hop movements | |||||||
| Never (ref) | 92 (33.0) | - | - | <0.01 | - | - | <0.01 |
| Rarely | 76 (27.2) | 0.11 | -0.02, 0.23 | 0.08 | -0.18, 0.33 | ||
| Every once in a while | 63 (22.6) | 0.23 | 0.09, 0.36 | 0.22 | -0.05, 0.49 | ||
| Very often/often | 48 (17.2) | 0.15 | 0.01, 0.30 | 0.37 | 0.07, 0.66 | ||
P; p value for trend. CI; Confidence Interval. JM: Jumping mechanography. SD: Standard Deviation.
Force and power relative to weight. Standardized beta coefficients represent SD difference in outcome for each category of exposure compared with the reference category (ref).
Power and force vs. age
Whilst power was lower with age (unadjusted standardized β [95% CI] -0.19 [-0.25, -0.12], p<0.01), force was not (0.07 [-0.31, 0.45], p=0.71). Grip strength was inversely associated with age (-0.15 [-0.26, -0.03], p=0.01) (Figure 2).
Figure 2.
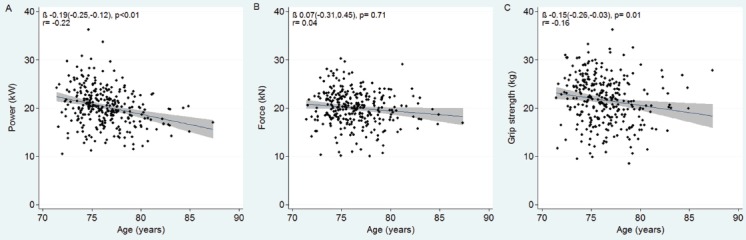
Scatter plots with linear regression lines and 95% confidence intervals, and correlation coefficients shown to illustrate the relationships between muscle power (A), force (B), and grip strength (C) and age within our study population.
Power and force vs. SPPB and grip strength
Power and force were both positively associated with SPPB, with the stronger association seen for power (unadjusted standardized β 0.41 [0.10, 0.72], p<0.01) (Table S2). Power was also the stronger predictor of faster gait speed (β -0.47 [-0.22, -0.72], p<0.01), quicker chair-rise time (β -0.46 [-0.18, -0.74], p<0.01) and increased odds of holding the tandem balance for 10 seconds (OR 12.4 [3.43, 45.1], p<0.01). Whilst power showed a moderate association with grip strength (β 0.24 [0.10, 0.38], p<0.01), force did not (Figure S2). After adjustment for age, height and co-morbidities, results were largely unchanged. After accounting for age, height and co-morbidities, power and force accounted for similar proportions of variance in SPPB (r2=0.08, 0.10 respectively), and combined explained 13% (Table 4). Power demonstrated the higher r2 and lowest AIC for gait speed (r2=0.12) and tandem balance models (pseudo r2=0.09), whilst force provided the best ‘goodness of fit’ for chair-rise time (r2=0.09). Power accounted for 17% of variance in grip strength, which was unchanged by the addition of force.
Supplementary Table S2.
Associations between jumping mechanography peak power and force with grip strength, the SPPB and its three components1.
| Grip Strength | SPPB score | Gait speed | Chair-rise time | Tandem balance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposures | Adjustment | β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | OR | 95% CI | P |
| Power3 | Unadjusted | 0.24 | 0.10, 0.38 | <0.01 | 0.41 | 0.10, 0.72 | 0.01 | -0.47 | -0.72, -0.22 | <0.01 | -0.46 | -0.74, -0.18 | <0.01 | 12.44 | 3.43, 45.1 | <0.01 |
| Adjusted 2 | 0.21 | 0.06, 0.35 | <0.01 | 0.39 | 0.08, 0.70 | 0.01 | -0.44 | -0.70, -0.18 | <0.01 | -0.42 | -0.70, -0.13 | <0.01 | 15.16 | 3.99, 57.6 | <0.01 | |
| Force3 | Unadjusted | 0.01 | -0.05, 0.07 | 0.68 | 0.22 | 0.11, 0.34 | <0.01 | -0.13 | -0.23, -0.03 | 0.01 | -0.24 | -0.38, -0.11 | <0.01 | 1.79 | 1.08, 2.96 | 0.02 |
| Adjusted 2 | 0.04 | -0.01, 0.09 | 0.14 | 0.22 | 0.12, 0.32 | <0.01 | -0.13 | -0.21, -0.05 | <0.01 | -0.23 | -0.34, -0.11 | <0.01 | 1.87 | 1.12, 3.13 | 0.02 | |
P; p value. CI; Confidence Interval. OR; Odds Ratio. SD: Standard Deviation.
n=300 for analyses with SPPB score, gait speed & tandem balance. n=299 for chair-rise time.
All continuous exposure and outcome variables standardized. Standardized beta coefficients represent SD change in outcome per SD change in exposure.
Adjusted for age, height and comorbidities.
power and force relative to weight.
Supplementary Figure S2.
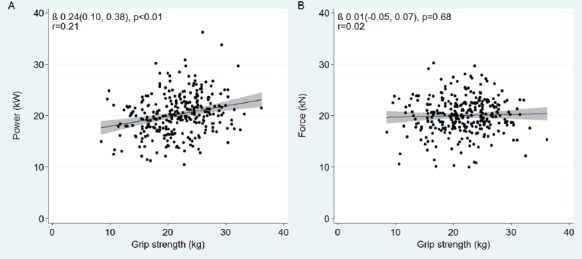
Scatter plots with linear regression lines with 95% confidence intervals, and correlation coefficients shown to illustrate the relationships between muscle power (A) and force (B), with grip strength.
Table 4.
Contributions of peak power and force to grip strength and each component of the SPPB.
| Outcome | Model1 | R2 | AIC |
|---|---|---|---|
| Grip strength (kg) | Base model | 0.134 | 349.61 |
| Base model +power | 0.165 | 340.87 | |
| Base model +force | 0.139 | 349.95 | |
| Base model +power +force | 0.166 | 342.58 | |
| SPPB score | Base model | 0.021 | 588.05 |
| Base model +power | 0.076 | 572.59 | |
| Base model +force | 0.100 | 564.67 | |
| Base model +power +force | 0.130 | 556.52 | |
| Gait speed (metres/second) | Base model | 0.030 | 515.45 |
| Base model +power | 0.118 | 488.77 | |
| Base model +force | 0.067 | 505.78 | |
| Base model +power +force | 0.134 | 485.40 | |
| Chair-rise time (seconds) | Base model | 0.032 | 723.58 |
| Base model +power | 0.071 | 713.19 | |
| Base model +force | 0.086 | 708.43 | |
| Base model +power +force | 0.108 | 703.15 | |
| Tandem balance2 | Base model | 0.008 | 230.31 |
| Base model +power | 0.089 | 214.12 | |
| Base model +force | 0.036 | 226.00 | |
| Base model +power +force | 0.093 | 215.34 |
Base model is adjusted for age, height and co-morbidities.
AIC=Akaike information criterion.
Force and power relative to weight.
Binary outcome, includes pseudo r2 results.
NB Power and force were weakly correlated r=0.24.
N=300 for all except chair-rise time (n=299).
Discussion
Feasibility and acceptability of JM
We aimed to assess the feasibility and acceptability of JM in community-dwelling older women. We used the SPPB to screen out individuals in whom the risk of falls and other adverse events was deemed excessive, which only excluded a small proportion from assessment (8%). This strategy was effective, since no participant undergoing JM experienced an adverse event. The safety of performing JM in older individuals, providing suitable precautions are in place, is supported by two smaller US studies: no injuries were reported in 60 adults, aged 55-75 years, where JM was performed provided a physician had given medical clearance[33], nor in a further 40 men and women aged >60 years[34].
Unlike previous studies assessing JM safety in older people, we did not exclude those with co-morbidities in order to maximize generalisability of our findings; in fact, two thirds had at least one co-morbidity. Ours is the first study to seek participant opinion regarding JM acceptability. Whilst most women reported infrequently performing jumping and hopping movements day-to-day, the majority felt comfortable with our JM assessment, with similar acceptability amongst those with co-morbidities, including arthritis and lung disease. That said, we observed a positive relationship between comfort levels while performing JM, and peak power and force achieved. Therefore, in those older individuals able to perform JM despite some discomfort, results may be artefactually reduced due to technical difficulties in test performance.
Peak muscle force, measured by one-footed hopping, is more physically demanding than two-legged jumping measuring peak muscle power, and is less commonly used; although 84% of those able to perform two-legged jumps were also able to perform one-footed hopping. One previous JM study used a 2-legged jump to calculate force in 30 women and men aged 80+[19], which may be a more feasible approach in older individuals with a tendency towards balance impairment. Furthermore, to what extent peak muscle force shows equivalent age-related declines to those previously reported for muscle power[16], and reflects early sarcopenic changes, is currently unclear. In addition, muscle force may be less helpful clinically as, despite the relatively narrow age range, peak power was inversely related to age whilst peak force was not.
Relationships between peak power, peak force and other measures of muscle function
The extent to which peak muscle power and force show differing relationships with functional tests may also provide a rationale for their added clinical utility. Peak power principally predicted grip strength; peak force had no additional explanatory value. However, peak muscle power and force together explained a greater proportion of variance in SPPB than either did alone, suggesting both measures reflect different components of muscle function in older women. Our finding that peak muscle power predicts SPPB is consistent with studies in older US[16] and Italian[35] populations; ours is the first UK study. However, we are not aware of previous studies assessing the differential contributions of lower-limb muscle power and force to SPPB.
Regarding individual SPPB components, we found peak power best explained gait speed and tandem balance, whilst peak force best explained chair-rise time. Power, a function of force and velocity, perhaps unsurprisingly best explained gait speed; however, this contrasts with only weak correlations between jumping power and gait speed (r=0.10) previously reported in older community-dwelling US adults[16]. We further identified peak power as the strongest predictor of tandem balance; power, thought to reflect lower-limb muscle activity, can de-condition with age. Power training improves balance in healthy older Australians[36]. We are not aware of previous reports linking peak muscle force to chair-rise time; however, the latter has been associated with the related measure of rate of force development[37,38].
Our observation that peak muscle power and force are related to widely used measures of muscle function, namely SPPB, including the individual components thereof, supports the clinical relevance of measures obtained from JM. Furthermore, these measures relate to other health outcomes; peak power was associated cross-sectionally with falls amongst women aged 60-85[21,39]. In men and women (mean age 56), peak power was positively related to hip bone density, whereas peak force was related to bone size[40]. JM has the advantage of precisely measuring specific elements of muscle function, whereas the SPPB is a composite measure of task-specific neuro-muscular function. This precision may be of clinical relevance, providing greater sensitivity with which to detect early changes associated with sarcopenia.
Peak lower-limb muscle power and force, together explaining only 13% of variance in SPPB, suggests that SPPB reflects components of physical function which are in part independent of lower-limb muscle power and force. This is consistent with previous observations that lower-limb muscle power and jumping height, measured by JM, show earlier age-related changes compared to SPPB[16]. Therefore, whilst JM lower-limb muscle function measures are related to clinical and functional outcomes such as SPPB, they may provide additional information regarding early sarcopenia. The same conclusion applies to grip strength, for which JM only explained 17% of variance. Whilst peak muscle power has been reported to show more rapid age-related changes compared to grip strength[16], in our study, age-related associations with these two parameters were similar.
Limitations
Despite being the largest study of its kind to date, there are several limitations. Analysis of only women limits generalisability of findings. Our final study population represents a small proportion of the original historic population-based COSHIBA cohort and reduces generalisability of results; participants were younger and better educated than non-participants. Frailer, older women unable to consent due to cognitive impairment were excluded. Self-reported co-morbidities were subject to recall bias, hence we used non-specific terms such as ‘arthritis’; however, we expect any misclassification to be non-differential. Whilst our policy of using an SPPB<6 to screen out those at high falls risk was effective at avoiding adverse events, it does limit the utility of JM for all older people. However, as JM may be best placed to identify early sarcopenic changes in advance of other traditional tools, and as individuals with SPPB<6 are more likely to have established sarcopenia, this restriction in application in conjunction with the SPPB may be beneficial. Currently though, utility may be predominantly restricted by the cost of JM equipment (~15,000 euros).
Conclusions
Our results suggest that jumping mechanography is a feasible, safe, and acceptable method for evaluating lower-limb muscle function in the majority of community-dwelling older women (aged 71-87 years), using a pre-screening strategy of a SPPB≥6 threshold. In addition to measurement of peak power through two-legged jumps, peak force from one-legged hops is also feasible in the majority, and provides additional information regarding physical function, given its relationships with SPPB. Despite the strong associations of both peak muscle power and force with SPPB, these only explain a relatively small proportion of overall variance in SPPB, suggesting JM detects distinct components of physical function compared to those evaluated by more functional measures, possibly including those aspects affected in early sarcopenia. Further studies are justified to examine to what extent peak power and force, as measured by JM, have additional functional consequences not detected by conventional measures such as SPPB and grip strength, and/or provide useful prognostic information for selecting individuals for preventative measures such as exercise programmes.
Acknowledgements
Funding
This work was supported by the Medical Research Council, grant ref MR/K024973/1. CLG is funded as a Clinician Scientist Fellowship by Arthritis Research UK (grant number 20000). The COSHIBA study was funded via a Clinician Scientist Fellowship for EMC from Arthritis Research UK (grant number 17823). AH is funded by the Wellcome Trust (grant ref 203738/Z/16/Z).
Author contributions
Authors’ roles: Study Design KH, EM, JT Study Conduct KH, AH, JHT Data Collection KH, JT Data analysis KH, JT, CG, Data interpretation KH, AH, AS, JT, CG, Drafting manuscript KH, JT, CG Revising manuscript EM, AH, AS, JT, CG, Approving final version KH, EM, AH, AS, JT, CG. KH takes responsibility for the integrity of data analysis.
Sponsor’s role
The design, methods, subject recruitment, data collections, analysis and preparation of manuscript were all conducted independently of the sponsor.
We are extremely grateful to all our study participants, our clinic workers, laboratory technicians and research scientists. The research was supported by the NIHR Newcastle Biomedical Research Centre. To discuss access to the VIBE data, please contact Professor Jon Tobias: Academic Rheumatology, Musculoskeletal Research Unit, University of Bristol, Southmead Hospital, Bristol BS10 5NB, UK. Jon.Tobias@bristol.ac.uk.
Footnotes
The authors have no conflict of interest.
Edited by: S. Warden
References
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JrM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veronese N, Bolzetta F, Toffanello ED, et al. Association between Short Physical Performance Battery and falls in older people: the Progetto Veneto Anziani Study. Rejuvenation Res. 2014;17:276–284. doi: 10.1089/rej.2013.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward RE, Leveille SG, Beauchamp MK, et al. Functional Performance as a Predictor of Injurious Falls in Older Adults. J Am Geriatr Soc. 2015;63:315–320. doi: 10.1111/jgs.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 6.Beaudart C, Reginster JY, Slomian J, et al. Estimation of sarcopenia prevalence using various assessment tools. Experiment Gerontol. 2015;61:31–37. doi: 10.1016/j.exger.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Legrand D, Adriaensen W, Vaes B, Mathei C, Wallemacq P, Degryse J. The relationship between grip strength and muscle mass (MM), inflammatory biomarkers and physical performance in community-dwelling very old persons. Arch Gerontol Geriatr. 2013;57:345–351. doi: 10.1016/j.archger.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Karkkainen M, Rikkonen T, Kroger H, et al. Association between functional capacity tests and fractures: an eight-year prospective population-based cohort study. Osteoporos Int. 2008;19:1203–1210. doi: 10.1007/s00198-008-0561-y. [DOI] [PubMed] [Google Scholar]
- 9.Albrand G, Munoz F, Sornay-Rendu E, Duboeuf F, Delmas PD. Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the OFELY study. Bone. 2003;32:78–85. doi: 10.1016/s8756-3282(02)00919-5. [DOI] [PubMed] [Google Scholar]
- 10.Stalenhoef PA, Diederiks JP, Knottnerus JA, Kester AD, Crebolder HF. A risk model for the prediction of recurrent falls in community-dwelling elderly: a prospective cohort study. J Clin Epidemiol. 2002;55:1088–1094. doi: 10.1016/s0895-4356(02)00502-4. [DOI] [PubMed] [Google Scholar]
- 11.Ward KA, Das G, Roberts SA, et al. A Randomized, Controlled Trial of Vitamin D Supplementation upon Musculoskeletal Health in Postmenarchal Females. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2009-2725. [DOI] [PubMed] [Google Scholar]
- 12.Busche P, Rawer R, Rakhimi N, Lang I, Martin DD. Mechanography in childhood: references for force and power in counter movement jumps and chair rising tests. J Musculoskelet Neuronal Interact. 2013;13:213–226. [PubMed] [Google Scholar]
- 13.Buehring B, Krueger D, Binkley N. Jumping mechanography: a potential tool for sarcopenia evaluation in older individuals. J Clin Densitom. 2010;13:283–291. doi: 10.1016/j.jocd.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Buehring B, Krueger D, Fidler E, Gangnon R, Heiderscheit B, Binkley N. Reproducibility of jumping mechanography and traditional measures of physical and muscle function in older adults. Osteoporos Int. 2015;26:819–825. doi: 10.1007/s00198-014-2983-z. [DOI] [PubMed] [Google Scholar]
- 15.Rittweger J, Schiessl H, Felsenberg D, Runge M. Reproducibility of the jumping mechanography as a test of mechanical power output in physically competent adult and elderly subjects. J Am Geriatr Soc. 2004;52:128–131. doi: 10.1111/j.1532-5415.2004.52022.x. [DOI] [PubMed] [Google Scholar]
- 16.Siglinsky E, Krueger D, Ward RE, et al. Effect of age and sex on jumping mechanography and other measures of muscle mass and function. J Musculoskelet Neuronal Interact. 2015;15:301–308. [PMC free article] [PubMed] [Google Scholar]
- 17.Ward KA, Das G, Berry JL, et al. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94:559–563. doi: 10.1210/jc.2008-1284. [DOI] [PubMed] [Google Scholar]
- 18.Anliker E, Rawer R, Boutellier U, Toigo M. Maximum ground reaction force in relation to tibial bone mass in children and adults. Med Sci Sports Exerc. 2011;43:2102–2109. doi: 10.1249/MSS.0b013e31821c4661. [DOI] [PubMed] [Google Scholar]
- 19.Dietzel R, Gast U, Heine T, Felsenberg D, Armbrecht G. Cross-sectional assessment of neuromuscular function using mechanography in women and men aged 20-85 years. J Musculoskelet Neuronal Interact. 2013;13:312–319. [PubMed] [Google Scholar]
- 20.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004;52:1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 21.Dietzel R, Felsenberg D, Armbrecht G. Mechanography performance tests and their association with sarcopenia, falls and impairment in the activities of daily living - a pilot cross-sectional study in 293 older adults. J Musculoskelet Neuronal Interact. 2015;15:249–256. [PMC free article] [PubMed] [Google Scholar]
- 22.Shigematsu R, Rantanen T, Saari P, et al. Motor speed and lower extremity strength as predictors of fall-related bone fractures in elderly individuals. Aging Clin Experiment Res. 2006;18:320–324. doi: 10.1007/BF03324666. [DOI] [PubMed] [Google Scholar]
- 23.Cawthon PM, Fullman RL, Marshall L, et al. Physical performance and risk of hip fractures in older men. J Bone Miner Res. 2008;23:1037–1044. doi: 10.1359/JBMR.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deere KC, Hannam K, Coulson J, et al. Quantifying Habitual Levels of Physical Activity According to Impact in Older People: Accelerometry Protocol for the VIBE Study. J Aging Phys Act. 2016;24:290–295. doi: 10.1123/japa.2015-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark EM, Gould V, Morrison L, Ades AE, Dieppe P, Tobias JH. Randomized controlled trial of a primary care-based screening program to identify older women with prevalent osteoporotic vertebral fractures: Cohort for Skeletal Health in Bristol and Avon (COSHIBA) J Bone Miner Res. 2012;27:664–671. doi: 10.1002/jbmr.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark EM, Gould VC, Morrison L, Masud T, Tobias J. Determinants of fracture risk in a UK-population-based cohort of older women: a cross-sectional analysis of the Cohort for Skeletal Health in Bristol and Avon (COSHIBA) Age Ageing. 2012;41:46–52. doi: 10.1093/ageing/afr132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.59th General Assembly Seoul. World Medical Assembly Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. Seoul, Korea. 2008 [Google Scholar]
- 28.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 29.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton GF, McDonald C, Chenier TC. Measurement of grip strength: validity and reliability of the sphygmomanometer and jamar grip dynamometer. J Ortho Sport Phys Therapy. 1992;16:215–219. doi: 10.2519/jospt.1992.16.5.215. [DOI] [PubMed] [Google Scholar]
- 31.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 32.Aho K, Derryberry D, Peterson T. Model selection for ecologists: the worldviews of AIC and BIC. Ecology. 2014;95:631–636. doi: 10.1890/13-1452.1. [DOI] [PubMed] [Google Scholar]
- 33.Singh H, Kim D, Kim E, et al. Jump test performance and sarcopenia status in men and women, 55 to 75 years of age. J Geriatric Phys Therapy. 2014;37:76–82. doi: 10.1519/JPT.0b013e3182a51b11. [DOI] [PubMed] [Google Scholar]
- 34.Buehring B, Krueger D, Binkley N. Jumping Mechanography: A Potential Tool for Sarcopenia Evaluation in Older Individuals. J Clin Densitom. 2010;13:283–291. doi: 10.1016/j.jocd.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the inCHIANTI study: Which influences mobility more? J Gerontol. 2003;58:728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 36.Orr R, de Vos NJ, Singh NA, Ross DA, Stavrinos TM, Fiatarone-Singh MA. Power training improves balance in healthy older adults. J Gerontol. 2006;61:78–85. doi: 10.1093/gerona/61.1.78. [DOI] [PubMed] [Google Scholar]
- 37.Regterschot GR, Folkersma M, Zhang W, Baldus H, Stevens M, Zijlstra W. Sensitivity of sensor-based sit-to-stand peak power to the effects of training leg strength, leg power and balance in older adults. Gait & posture. 2014;39:303–307. doi: 10.1016/j.gaitpost.2013.07.122. [DOI] [PubMed] [Google Scholar]
- 38.Hannam K, Deere K, Worrall S, Hartley A, Tobias JH. Characterization of Vertical Accelerations Experienced by Older People Attending an Aerobics Class Designed to Produce High Impacts. J Aging Phys Act. 2016;24:268–274. doi: 10.1123/japa.2016-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benichou O, Lord SR. Rationale for Strengthening Muscle to Prevent Falls and Fractures: A Review of the Evidence. Calcif Tissue Int. 2016;98:531–545. doi: 10.1007/s00223-016-0107-9. [DOI] [PubMed] [Google Scholar]
- 40.Hardcastle SA, Gregson CL, Rittweger J, Crabtree N, Ward K, Tobias JH. Jump power and force have distinct associations with cortical bone parameters: findings from a population enriched by individuals with high bone mass. J Clin Endocrinol and Metab. 2014;99:266–275. doi: 10.1210/jc.2013-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]


