Summary
Objectives
To put data from our recent systematic review of phase 3 studies of anti‐proprotein convertase subtilisin/kexin type 9 (PCSK9) antibodies into the context of clinical practice.
Methods
Data from studies previously identified by a systematic review of phase 3 studies of alirocumab and evolocumab and additional references from non‐systematic literature searches were used. We evaluated the hypothetical cardiovascular (CV) benefit in cases of typical patients in whom anti‐PCSK9 antibodies may be recommended, using preliminary major CV event (CVE) rates from long‐term clinical trials of anti‐PCSK9 antibodies and from extrapolations derived from correlation between low‐density lipoprotein cholesterol (LDL‐C) reduction and CV benefit with other lipid‐lowering therapies (LLTs).
Results
Rapid (within 1‐2 weeks) and persistent (8‐74 weeks) reductions in LDL‐C levels were achieved with anti‐PCSK9 antibodies. When combined with statins (± ezetimibe), high rates of LDL‐C goal achievement were observed (41%‐87% with alirocumab and 63%‐100% with evolocumab). In long‐term alirocumab and evolocumab studies, reductions in major CVEs of 48% and 53%, respectively, were observed. For every 38.7 mg/dL (1 mmol/L) reduction in LDL‐C, a 22% reduction in relative CVE risk is predicted. Applying these assumptions to typical patients who have high–very high risk (15%‐60% absolute 10‐year CVE risk) and elevated LDL‐C despite maximally tolerated statins, the 10‐year number needed to treat with an anti‐PCSK9 antibody to prevent one additional CVE varies from 4 to 26, depending on baseline LDL‐C levels and residual absolute CVE risk.
Conclusions
Anti‐PCSK9 antibodies effectively lower LDL‐C levels in a broad patient population. While awaiting comprehensive data from CV outcome trials, these agents should be considered in very high risk patients, such as those in secondary prevention and those with familial hypercholesterolaemia who are already receiving maximally tolerated LLTs, have not achieved their LDL‐C goal and require substantial reductions in LDL‐C.
Review criteria
Most of the studies included in this review were identified by a systematic literature review of phase three clinical trials of anti‐proprotein convertase subtilisin/kexin type 9 (PCSK9) antibodies. Additional references were included from non‐systematic literature searches. Hypothetical case studies were used to put the low‐density lipoprotein cholesterol (LDL‐C) reductions achieved in clinical trials into the context of clinical practice.
Message for the clinic
Anti‐PCSK9 antibodies effectively reduce LDL‐C levels and are well tolerated in a broad range of patient populations, either as a monotherapy or in combination with other lipid‐lowering therapies. Strong, preliminary evidence suggests that anti‐PCSK9 antibodies reduce cardiovascular (CV) morbidity by approximately 50%. While awaiting comprehensive data from CV outcome trials, these agents may be suitable for patients in secondary prevention with very high CV risk and high LDL‐C.
1. INTRODUCTION
Cardiovascular (CV) disease (CVD) is a major cause of death worldwide; in 2012, 17.5 million people died as a result of CVD.1, 2 While increasing age, male gender and unfavourable genes are non‐modifiable risk factors for cardiovascular events (CVEs),3 hypercholesterolaemia is a major modifiable risk factor4 that can be corrected by reducing low‐density lipoprotein cholesterol (LDL‐C) levels. It has been shown that decreasing LDL‐C levels is an effective way to lower CV risk.5, 6
The Sixth Joint Task Force of the European Society of Cardiology proposes LDL‐C goals that are dependent on overall CVE risk; for patients with very high, high and moderate CVE risk, LDL‐C goals of <70 mg/dL (<1.8 mmol/L), <100 mg/dL (<2.6 mmol/L) and <115 mg/dL (<3.0 mmol/L), respectively, are recommended.3 Reductions in LDL‐C of at least 50% should be achieved in patients at very high and high CV risk when baseline LDL‐C values in drug‐naïve patients are between 70 and 135 mg/dL (1.8‐3.5 mmol/L) and 100 and 200 mg/dL (2.6‐5.1 mmol/L), respectively.3 In order to achieve these goals, European guidelines for the management of hypercholesterolaemia recommend lifestyle changes such as dietary modifications and weight loss as initial measures.3, 7 Even after dietary adjustments, however, LDL‐C goals are often not attained.8, 9 For patients who have continually elevated LDL‐C levels, statins are recommended as a first‐line therapeutic intervention, and as a second line, the cholesterol absorption inhibitor ezetimibe and bile sequestrants can be added to statins.3, 7 However, these therapies may not be sufficient in all patients. In a study in 2012, almost half of patients with hypercholesterolaemia in Europe and Canada did not achieve their LDL‐C goals, despite receiving statins.10 This may be due to the fact that some patients are statin‐intolerant and stop taking their medication. Additionally, some patients may have very high baseline LDL‐C concentrations and the maximum tolerated statin dose is insufficient to reduce LDL‐C to goal levels; therefore, alternative lipid‐lowering therapies (LLTs) may be needed in certain individuals. Long‐term persistence with statins is important for efficacy11; however, 50% or more of patients discontinue statin treatment within 1 year of initiation.12
A new target for LDL‐C lowering is the enzyme proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 binds to the receptor for LDL (unbound or bound with LDL‐C) in the liver, and this complex is endocytosed and targeted to the lysosome, rather than recycled back to the cell surface. This reduces the number of available LDL receptors and increases circulating LDL‐C levels13 (Figure 1). Monoclonal antibodies to PCSK9 can inhibit this interaction by binding to circulating PCSK9, meaning that LDL receptors are not degraded and are therefore available to bind LDL particles and promote their removal from the circulation.13 In 2015, the first anti‐PCSK9 antibodies, alirocumab and evolocumab, were approved for use in Europe and the USA.14, 15, 16, 17 This review discusses the implications of using these new agents in clinical practice by using data on their efficacy and safety as reported in studies previously identified by our systematic review of phase 3 studies of anti‐PCSK9 antibodies in patients with hypercholesterolaemia.18 To put the efficacy data from phase 3 clinical studies into the context of clinical practice, hypothetical case studies are discussed, together with safety data from LLTs.
Figure 1.
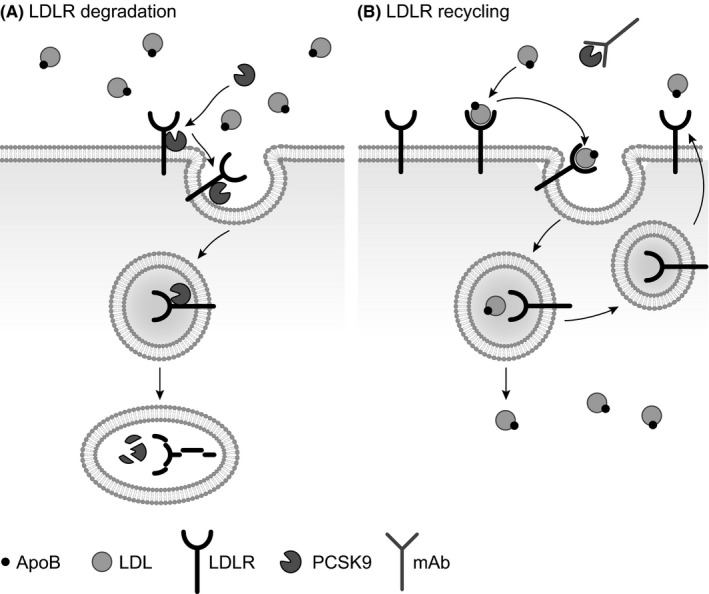
A, The role of PCSK9 in LDL receptor degradation and B, the mechanism of action of an anti‐PCSK9 antibody. PCSK9 binds to the receptor for LDL, and this complex is endocytosed and degraded intracellularly, thus reducing the number of available LDL receptors. Monoclonal antibodies targeted at PCSK9 can inhibit this interaction, by binding to circulating PCSK9 and thus facilitating the recycling of LDL receptors back to the cell surface. Therefore, the LDL‐receptors are made available to recognise and bind ApoB, a protein embedded in the phospholipid bilayer of LDL,13 the ApoB–LDL–LDL receptor complex is internalised and targeted for intracellular degradation, and thus LDL is removed from the circulation. ApoB, apoprotein B100; LDL, low‐density lipoprotein; LDLR, low‐density lipoprotein receptor; mAb, monoclonal antibody; PCSK9, proprotein convertase subtilisin/kexin type 9
2. METHODS
The previous systematic review identified 12 phase 3 studies of alirocumab and 9 phase 3 studies of evolocumab, together including more than 10 000 patients with elevated cholesterol levels (and hence, increased CV risk).18 Additional references were identified from non‐systematic literature searches. No head‐to‐head trials have compared the efficacy and safety of alirocumab and evolocumab. Therefore, the current review used data from the identified studies to analyse reductions in LDL‐C, rates of LDL‐C goal achievement and rates of adverse events (AEs). As a measure of the efficacy of these agents in clinical practice, we calculated the number needed to treat (NNT), using the following formula19:
where n=LDL‐C reduction in mmol/L and X represents the hazard ratio of CVE risk for each 1 mmol/L reduction in LDL‐C.5
Number needed to treat values were rounded up to the nearest whole figure to avoid overestimating effectiveness.20 In a large meta‐analysis of data from clinical trials of statins (with a follow‐up of 5 years), the Cholesterol Treatment Trialists’ (CTT) collaboration estimated that X, the hazard ratio of CVE risk for each 1 mmol/L reduction in LDL‐C, to be 0.78.5 Data from the IMPROVE‐IT trial21 also showed that the LDL‐C reduction associated with the addition of ezetimibe to statins followed the same correlation. In both treated and untreated non‐elderly individuals, the incidences of CVEs and CVE‐related mortality are linear over time, as demonstrated in the 20‐year follow‐up of the West of Scotland Coronary Prevention Study (WOSCOP)22 and in the Scandinavian simvastatin survival study (4S; up to 8 years follow‐up).23 It is therefore currently accepted to use the same reduction in relative risk to calculate NNTs over different time periods19, 24, 25; a follow‐up period of 10 years was used in the our paper. When considering the NNT estimates in our paper, it is important to note that the populations included in the CTT analysis differ slightly from those enrolled in the anti‐PCSK9 trials. Moreover, the recently published FOURIER study provides evidence for a delay in the onset of risk reduction with evolocumab.26 Hence, the NNT estimations presented here may be overestimated.
3. RESULTS
3.1. LDL‐C reductions with alirocumab and evolocumab in different patient populations
Among patients with hypercholesterolaemia or mixed dyslipidaemia, rapid (within 1‐2 weeks) and persistent (20‐74 weeks with alirocumab27, 28, 29, 30, 31, 32, 33, 34, 35 and 8‐38 weeks with evolocumab36, 37, 38, 39, 40, 41, 42) reductions in LDL‐C and total cholesterol, compared with the control arms of clinical trials, were achieved with alirocumab and evolocumab.18 The LDL‐C reductions in patients with hypercholesterolaemia or mixed dyslipidaemia with alirocumab or evolocumab monotherapy were 45.0%‐47.2% and 56.1%‐57.0%, respectively.18 In most studies, anti‐PCSK9 antibodies were used in combination with other LLTs (statins and/or ezetimibe). LDL‐C reductions of 45.7%‐57.9% were observed with alirocumab, and 59.2%‐76.3% with evolocumab in patients with hypercholesterolaemia.18 For patients with heterozygous familial hypercholesterolaemia (HeFH), LDL‐C reductions ranged from 45.7% to 57.9% with alirocumab, and from 55.7% to 61.3% with evolocumab.18 Evolocumab has been investigated in patients with homozygous familial hypercholesterolaemia (HoFH), but alirocumab has not. HoFH is a rare but serious genetic disorder, and in these patients baseline LDL‐C levels can be very high (greater than 500 mg/dL [12 mmol/L]); therefore, management of LDL‐C is extremely challenging. Mean reductions in LDL‐C with evolocumab in patients with HoFH ranged from 18.6% to 23.4%, which is less than in HeFH.39, 43 Anti‐PCSK9 antibodies are also effective in LDL‐C lowering in patients with type 2 diabetes mellitus: pooled analyses of clinical trials of alirocumab or evolocumab in these patients revealed mean LDL‐C reductions of 58.3% and 60% compared with placebo, respectively.44, 45
For patients with high and very high CV risk (excluding those with HoFH), alirocumab and evolocumab treatment resulted in mean LDL‐C reductions of 44.1%‐61.0% and 55.7%‐75.9%, respectively.18 In patients with moderate to very high CV risk receiving alirocumab, LDL‐C reductions of 47.2%‐58.7% were observed.18 For those with low to high CV risk, evolocumab treatment reduced mean LDL‐C levels by 53.3%‐64.0%.18 Anti‐PCSK9 antibodies can also induce substantial reductions in LDL‐C in patients who are statin‐intolerant: mean reductions of 45.0% and 55.3%‐56.1% were observed in studies of alirocumab and evolocumab, respectively.32, 42
These data illustrate consistently large reductions in LDL‐C levels with anti‐PCSK9 antibodies across patient groups, regardless of baseline LDL‐C levels and background LLT. In addition, a meta‐analysis of 25 randomised controlled trials of anti‐PCKS9 antibodies reported slightly better efficacy in terms of LDL‐C lowering with evolocumab than with alirocumab.46 While the favourable effects of evolocumab and alirocumab were observed largely in patients receiving background statin treatment, such effects were also seen in patients not receiving additional LLTs.46 Therefore, background statin use appears to contribute to LDL‐C lowering to a minor degree only, with consistent LDL‐C reductions observed across groups, regardless of background LLT.18
Substantial, but transient, LDL‐C reductions can be achieved using lipoprotein apheresis in patients with HoFH,47 and there are data from patients with HeFH that show that switching from apheresis to evolocumab is effective in maintaining the LDL‐C‐lowering effect of apheresis.48 A small population of LLT‐intolerant patients with elevated LDL‐C levels may be eligible for apheresis. This procedure is widely used in Germany, but less so in other European countries and the USA.49 Anti‐PCSK9 antibodies could replace apheresis in some patients: 48‐week treatment with evolocumab (420 mg every 2 weeks) in 34 patients with HoFH resulted in 21% reduction in mean LDL‐C and 15% of patients stopping or reducing the frequency of apheresis.50 A phase 3 randomised study in 62 patients with HeFH undergoing regular apheresis therapy found that alirocumab (150 mg every 2 weeks) reduced the need for apheresis by 75% from week 7 to 18; apheresis was stopped in 63.4% of patients.51 At week 18, mean LDL‐C reductions from baseline were 42.5% in the alirocumab group vs 1.6% in the placebo group.51 How these data will translate into clinical practice is under debate; less than half of patients had not exhausted all statin options before enrolling in the study and apheresis was stopped once patients’ LDL‐C levels were at least 30% lower than at pre‐apheresis baseline, which may not be sufficient to achieve LDL‐C goal among patients very high LDL‐C levels at baseline.52 Notwithstanding, German reimbursement rules currently in place require initiation of anti‐PCSK9 antibodies before approval of apheresis treatment. This is legitimate since PCSK9 inhibition may result in improved quality of life at significantly lower costs compared with apheresis.48 A phase 3 randomised study assessing evolocumab compared with apheresis in patients with hypercholesterolaemia who were already receiving LDL‐C apheresis is ongoing (NCT02585895).
3.2. LDL‐C goal achievement with alirocumab and evolocumab in different patient populations
In the included studies, goal achievement was measured according to the 2011 European Atherosclerosis Society/European Society of Cardiology guidelines (<70 mg/dL [1.8 mmol/L] for patients at very high CV risk or <100 mg/dL [2.6 mmol/L] for patients at high CV risk).18, 53 High rates of LDL‐C goal achievement were observed when an anti‐PCSK9 antibody was combined with a statin: 41.0%‐87.2% with alirocumab plus statin and 63.0%‐100% with evolocumab plus statin.18 Goal achievement with monotherapy was also high: 42.0%‐65.0% with alirocumab and 69.0%‐84.0% with evolocumab.18 Overall, there was an apparent inverse relationship between baseline LDL‐C level and the proportion of patients achieving their LDL‐C goals.18 In most studies, LDL‐C goals were achieved by a large proportion of patients, regardless of the presence of comorbidities, type 2 diabetes mellitus, HeFH and CVD; and irrespective of baseline characteristics such as age, gender and use (and dose) of statin or other LLT.18
Table 1 provides an overview of the proportion of patients achieving LDL‐C goals according to baseline characteristics. For patients with HeFH, 72.2%‐81.4% of patients achieved their goals with alirocumab, and 63.0%‐68.0% with evolocumab.18 In another study of patients with HeFH and high CV risk, 41.0% of patients achieved their LDL‐C goal.54 In studies of patients with HoFH the proportions of patients achieving their LDL‐C goals were not reported. A meta‐analysis of patient data from individuals with type 2 diabetes mellitus receiving evolocumab found that goal achievement was 87.0%‐88.0%.45 Goal achievement was not reported for similar alirocumab studies. The high proportion of patients with type 2 diabetes mellitus achieving their LDL‐C goals may relate to the fact that baseline LDL‐C levels in these patients is slightly lower than in those without type 2 diabetes mellitus.
Table 1.
LDL‐C goal achievement (according to the 2011 EAS/ESC guidelines53) with alirocumab and evolocumab, by patient population
| Study | Proportion of patients achieving LDL‐C goal at primary end‐point according to the EAS/ESC guidelines (<70 mg/dL/<1.8 mmol/L [patients at very high CV risk] or <100 mg/dL/<2.6 mmol/L [patients at high CV risk])18, 53 | Study duration (weeks) | Concomitant therapy (all treatment arms) | |
|---|---|---|---|---|
| Anti‐PCSK9 antibody group | Comparator group | |||
| Heterozygous FH | ||||
| ODYSSEY FH I30 | Alirocumab 75 mg Q2W: 72.2% | Placebo: 2.4% | 78 |
82.7%‐85.3% received high‐intensity statin therapy (atorvastatin 40‐80 mg QD, rosuvastatin 20‐40 mg QD or simvastatin 80 mg QD) 56.0%‐59.5% received ezetimibe |
| ODYSSEY FH II 30 | Alirocumab 75 mg Q2W: 81.4% | Placebo: 11.3% | 78 |
86.8%‐91.5% received high‐intensity statin therapy (atorvastatin 40‐80 mg QD, rosuvastatin 20‐40 mg QD or simvastatin 80 mg QD) 64.6%‐67.1% received ezetimibe |
| ODYSSEY HIGH FH54 , a | Alirocumab 150 mg Q2W: 41.0% | Placebo: 5.7% | 24 | 100% received maximally tolerated statin ± other LLT |
| RUTHERFORD‐240 |
Evolocumab 140 mg Q2W: 68.0% Evolocumab 420 mg QM: 63.0% |
Placebo: 2% | 12 |
100% received statins: 87% received high‐intensity statin (simvastatin 80 mg QD, atorvastatin ≥40 mg QD, rosuvastatin ≥20 mg QD or any dose of statin together with ezetimibe) 62% received ezetimibe |
| Type 2 diabetes mellitus | ||||
| Pooled meta‐analysis of four studies45, 79 |
Evolocumab 140 mg Q2W: 88.0% Evolocumab 420 mg Q4W: 87.0% |
Ezetimibe 10 mg + placebo Q2W: 36.0% Ezetimibe 10 mg + placebo QM: 29.0% Placebo Q2W: 23.0% Placebo QM: 16.0% |
12 |
MENDEL‐238: no background therapy LAPLACE‐280: 29% received high‐intensity statin therapy (atorvastatin >40 mg QD or rosuvastatin >20 mg QD, simvastatin 80 mg or any statin plus ezetimibe), 41% received non‐intensive statin therapy, 30% received no statin therapy RUTHERFORD‐240: 87% received high‐intensity statin therapy (simvastatin 80 mg QD, atorvastatin ≥40 mg QD, rosuvastatin ≥20 mg QD or any dose of statin together with ezetimibe 10 mg QD), 62% received ezetimibe 10 mg QD GAUSS‐242: 33% received LLT, 18% received low‐dose statin therapy |
| Low‐to‐high CV risk | ||||
| DESCARTES36 , b |
Evolocumab 420 mg Q4W + diet + background LLT: 83.6% Evolocumab 420 mg Q4W + diet + atorvastatin 10 mg QD: 90.1% Evolocumab 420 mg Q4W + diet + atorvastatin 80 mg QD: 80.8% Evolocumab 420 mg Q4W + diet + atorvastatin 80 mg QD + ezetimibe 10 mg QD: 67.0% Overall: 82.3% Goal defined as LDL‐C <70 mg/dL (<1.8 mmol/L) for all patients |
Placebo Q4W+ diet + background LLT: 3.2% Placebo Q4W + diet + atorvastatin 10 mg QD: 5.3% Placebo Q4W + diet + atorvastatin 80 mg QD: 6.1% Placebo Q4W + diet + atorvastatin 80 mg QD + ezetimibe 10 mg: 11.1% Overall: 6.4% |
52 | None |
| Moderate‐to‐very high CV risk | ||||
| ODYSSEY MONO34 , c | Alirocumab 75 mg Q2W (up titrated to 150 mg Q2W): 65.0% | Ezetimibe: 10 mg QD: 2%‐3% | 24 | None |
| ODYSSEY CHOICE I35 , d |
Alirocumab 300 mg Q4W: 78.9% Alirocumab 300 mg Q4W + statin: 85.2% Alirocumab 75 mg Q2W: 84.9% Alirocumab 75 mg Q2W + statin: 82.5% |
Placebo: 9.4% Placebo + statin: 22.2% |
24 |
No statin group: 0–1.4% received atorvastatin, rosuvastatin or simvastatin; 32.4%‐45.2% received other LLT Statin group: 99.4%‐100% received atorvastatin, rosuvastatin or simvastatin 32.4%‐45.2% received other LLT |
| ODYSSEY CHOICE II81 , d |
Alirocumab 150 mg Q4W: 63.9% Alirocumab 75 mg Q2W: 70.3% |
Placebo Q2W: 1.8% | 24 |
30.2%‐34.5% received diet alone 59.3%‐60.3% received ezetimibe 5.2%‐10.3% received fenofibrate |
| High CV risk | ||||
| ODYSSEY COMBO I31 , e | Alirocumab 75 mg Q2W: 75.0% | Placebo: 9% | 24 |
99.5–100% received statins (high dose: 61.7%‐64.5%; atorvastatin 40‐80 mg QD or rosuvastatin 20‐40 mg QD or simvastatin 80 mg QD) 7.2%‐10.3% received ezetimibe 38.3%‐49.5% received other LLT |
| ODYSSEY COMBO II 28 , f | Alirocumab 75 mg Q2W: 77.0% | Ezetimibe 10 mg QD: 45.6% | 24 |
99.8%‐100% received statins 66.4%‐66.8% received high‐intensity statin therapy (atorvastatin 40‐80 mg QD or rosuvastatin 20‐40 mg QD) |
| ODYSSEY LONG TERM 33 , f , g |
Alirocumab 150 mg Q2W (high or very high risk): 80.7% Regardless of risk: 79.3% |
Placebo: (high or very high risk):8.5% Regardless of risk: 8.0% |
24 (secondary end‐point) |
100% received maximum tolerated daily statin therapy ≥99.9% received statins 46.7%‐46.8% received high‐intensity therapy (atorvastatin 40‐80 mg QD, rosuvastatin 20‐40 mg QD or simvastatin 80 mg QD) 13.9%‐15.0% received ezetimibe 27.9%‐28.1% received other LLT |
| YUKAWA‐237 , h |
Evolocumab 140 mg Q2W + atorvastatin 5 mg QD: 98.0%‐100% Evolocumab 420 QM + atorvastatin 5 mg QD: 96.0%‐100% Evolocumab 140 Q2W + atorvastatin 20 mg QD: 96.0%‐100% Evolocumab 420 mg QM + atorvastatin 20 mg QD: 98.0%‐100% Goal defined as LDL‐C <70 mg/dL (<1.8 mmol/L) for all patients |
Placebo Q2W + atorvastatin 5 mg QD: 0.0%‐29.0% Placebo QM + atorvastatin 5 mg QD: 4.0%‐42.0% Placebo Q2W + atorvastatin 20 mg QD: 20.0%‐59.0% Placebo QM + atorvastatin 20 mg QD: 20.0%‐88.0% |
12 | 100% received statins (atorvastatin 5 mg or 20 mg QD) |
| High‐to‐very high CV risk | ||||
| ODYSSEY OPTIONS I 27 , i | Alirocumab 75 mg Q2W + atorvastatin 20/40 mg QD: 84.6%‐87.2% |
Ezetimibe 10 mg QD + atorvastatin 20/40 mg QD: 65.1%‐68.4% Rosuvastatin 40 mg QD: 62.2% Atorvastatin 40/80 mg QD: 18.5%‐34.5% |
24 | 100% received statins (atorvastatin 20 mg or 40 mg QD) |
| ODYSSEY OPTIONS II29 , i | Alirocumab 75 mg Q2W + rosuvastatin 10/20 mg QD: 66.7%‐84.9% |
Ezetimibe 10 mg QD + rosuvastatin 10/20 mg QD: 43.1%‐57.2% Rosuvastatin 20/40 mg QD: 29.9%‐45.0% |
24 | 100% received statins (rosuvastatin 10 mg or 20 mg QD) |
| Statin intolerant | ||||
| ODYSSEY ALTERNATIVE 32 | Alirocumab 75 mg Q2W (up titrated to 150 mg Q2W): 41.9%‐51.2% |
Ezetimibe 10 mg QD: 4.4%‐5.6% Atorvastatin 20 mg QD: not reported |
24 |
37.3%‐54.0% received LLT other than statins 32.5%‐49.2% received LLT other than nutraceuticals 5.6%‐13.6% received nutraceuticals |
| GAUSS‐242 |
Evolocumab 140 mg Q2W + placebo QD (week 12): 50.5% Evolocumab 420 mg QM+ placebo QD (week 12): 37.5% Goal defined as LDL‐C <70 mg/dL (<1.8 mmol/L) for all patients |
Ezetimibe 10 mg QD + placebo Q2W: 2.0% Ezetimibe 10 mg QD + placebo QM: 0.0% |
12 |
33% received any LLT (18% low dose statin) 4%‐12% received rosuvastatin 0%‐6% received simvastatin 1%‐4% received atorvastatin 3–7% received other statins 18% received a low‐dose statin |
Only studies that reported LDL‐C goal achievement are included. aPatients had baseline LDL‐C levels of ≥160 mg/dL (4.1 mmol/L) despite maximally tolerated statin ± other LLT. bCV risk was determined according to NCEP ATP III guidelines. High risk: 26.0% and 26.6% of patients receiving evolocumab and placebo, respectively. Moderately high risk: 9.3% and 9.6% of patients receiving evolocumab and placebo, respectively. Moderate risk: 33.9% and 32.1% of patients receiving evolocumab and placebo, respectively. Low risk: 30.7% and 32.1% of patients receiving evolocumab and placebo, respectively. cModerate CV risk: 10‐year fatal CVD risk SCORE of ≥1% and <5%. High CV risk: presence of at least one of the following—10‐year fatal CVD risk SCORE ≥5%; moderate chronic kidney disease; type 1 or type 2 diabetes mellitus without target organ damage; or FH. Very high CV risk: presence of one or more of the following—history of CHD, ischaemic stroke, peripheral artery disease, transient ischaemic attack, abdominal aortic aneurysm or carotid artery occlusion >50% without symptoms; carotid endarterectomy or carotid artery stent procedure; renal artery stenosis or renal artery stent procedure; or type 1 or type 2 diabetes mellitus with target organ damage. dCV risk definition not reported. eHigh CV risk: LDL‐C ≥70 mg/dL (≥1.8 mmol/L) and established CVD or LDL‐C ≥100 mg/dL (≥2.6 mmol/L) with CHD risk equivalents (eg, diabetes mellitus with other risk factors or chronic kidney disease) and LDL‐C ≥70 mg/dL (≥1.8 mmol/L). fHigh CV risk: heterozygous familial hypercholesterolaemia or established CHD or a CHD risk equivalent (ischaemic stroke, peripheral artery disease, moderate chronic kidney disease, or diabetes mellitus plus ≥2 additional risk factors). g17.7% of patients had heterozygous HF. hHigh CV was determined according to Japanese Atherosclerosis Society (JAS) classification criteria. Goal defined as LDL‐C <100 mg/dL (<2.6 mmol/L); a more stringent goal of LDL‐C <70 mg/dL (<1.8 mmol/L) was also assessed. iVery high CV risk: a history of CVD, including CHD, or type 2 diabetes mellitus with target organ damage and LDL‐C ≥70 mg/dL (≥1.8 mmol/L). High CV risk: no history of CVD or CHD but with other risk factors as follows—10‐year fatal CVD risk SCORE ≥5%; moderate chronic kidney disease; type 2 diabetes mellitus with no target organ damage; and LDL‐C ≥100 mg/dL (≥2.6 mmol/L). CHD, coronary heart disease; CV, cardiovascular; CVD, cardiovascular disease; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; FH, familial hypercholesterolaemia; LDL‐C, low‐density lipoprotein cholesterol; LLT, lipid‐lowering therapy; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; PCSK9, proprotein convertase subtilisin/kexin type 9; QD, every day; Q2W, every 2 weeks; Q4W, every 4 weeks; QM, every month; SCORE, Systemic Coronary Risk Evaluation.
For patients with high LDL‐C at baseline (≥3.5 mmol/L [≥135 mg/dL]; excluding those with FH), goal achievement was reported in 64.0%‐87.2% of patients receiving alirocumab.18 In the YUKAWA‐1 study, 96%‐100% of patients with high LDL‐C at baseline achieved their LDL‐C goals with evolocumab.18, 37 Almost half (42.0%‐45.5%) of statin‐intolerant patients achieved their LDL‐C goals with alirocumab or evolocumab.18
3.3. Safety data for anti‐PCSK9 antibodies and other LLTs
Musculoskeletal events feature strongly in the adverse event (AE) profiles of LLTs. Rhabdomyolysis and myopathy have emerged, through decades of clinical experience, as the main AEs associated with statin treatment. These AEs are rare at the approved dose range, but increasing doses, interactions with other medications and genetic predisposition can increase the risk of a patient experiencing these events.55 Although the incidence of rhabdomyolysis in patients taking statin monotherapy is low (approximately 34 hospitalised cases per million person‐years), it is potentially fatal.55 Myalgias have been reported in up to 10% of patients receiving statins in clinical practice55, 56 and higher rates up to 18% were seen with high‐intensity statins.56 In terms of musculoskeletal events, as with the safety profile of statins in general, the risk of AEs is impacted minimally by the addition of ezetimibe.57
Musculoskeletal events such as back pain or arthralgia are also among the most common AEs reported across clinical studies of anti‐PCSK9 antibodies. Data from two large pooled analyses, one of phase 3 alirocumab trials and another of phase 2 and 3 open‐label extension studies of evolocumab, showed that the incidence of back pain was 5.3% and 4.2%, respectively (placebo or standard of care: 6.0% and 3.7%, respectively) and that arthralgia occurred in 5.1% and 4.8% of patients, respectively (placebo or standard of care: 6.5% and 3.2%, respectively).58, 59 Although there is some variation in the incidences of muscle‐related AEs reported in trials of anti‐PCSK9 antibodies, in general these are balanced between study arms. In the pooled analysis of open‐label extension studies of evolocumab, the incidence of muscle‐related AEs was 6.5% with evolocumab and 6.1% with standard of care.59 With alirocumab, the incidence was slightly higher because muscle‐related events were grouped with connective tissue disorders in the long‐term follow‐up trial ODYSSEY LONG TERM, but remained balanced with the placebo arm (alirocumab: 30.1%; placebo: 30.7%).33 Myalgia occurred in 3.0% of patients receiving evolocumab and 2.9% of those receiving the standard of care59 and in the long‐term follow‐up trial of alirocumab (ODYSSEY LONG TERM), the incidence of myalgia was 5.4% with alirocumab compared with 2.9% with placebo.33 In the open‐label extension studies of evolocumab, elevated creatinine kinase levels (>5×upper limit of the normal [ULN] range at baseline), a sign of muscle inflammation, were reported in 0.6% of patients receiving evolocumab (standard of care, 1.2%).59 In ODYSSEY LONG TERM, 3.7% of individuals receiving alirocumab and 4.9% of those receiving placebo had elevated levels of creatinine kinase (>3×ULN).33 For patients who were statin‐intolerant, elevated creatine kinase levels (>3×ULN) were observed in 2.4% of patients receiving alirocumab and in 1.6% and 4.8% of patients receiving ezetimibe and atorvastatin, respectively.32 In GAUSS‐2, 2.0% of statin‐intolerant patients receiving evolocumab 420 mg every month had elevated creatinine kinase levels (>5×ULN) compared with 0% in the ezetimibe group. Elevated levels of creatinine kinase were not observed in the evolocumab 140 mg every 2 weeks group, but were seen in the corresponding ezetimibe group (6%).42
There are case reports documenting mild and reversible impaired cognitive function in patients receiving statins.60 The results of several large meta‐analyses, however, seem to suggest no increase in risk, and a causal link with statin treatment has not been established.61 In 2014, the US Food and Drug Administration (FDA) requested that the feasibility of including analysis of neurocognitive AEs in late‐stage clinical trials of anti‐PCSK9 antibodies be assessed.62 In the randomised double‐blinded ODYSSEY LONG TERM trial of alirocumab in combination with statins, the rate of neurocognitive AEs at week 78 was not significantly different between the alirocumab and placebo arms (1.2% vs 0.5%, respectively; P=.17).33 In a pooled analysis of two open‐label randomised trials (OSLER‐1 and OSLER‐2), the incidences of neurocognitive events in patients treated with evolocumab plus standard therapy versus standard therapy alone were 0.9% and 0.3%, respectively, at week 48.41 No association has been found between the rate of these AEs and LDL‐C reduction in this or other studies.33, 41, 59 However, a recent meta‐analysis of 13 083 patients in phase 2 and 3 trials reported that anti‐PCSK9 antibodies were associated with an increased incidence of neurocognitive AEs compared with placebo (odds ratio [OR] 2.34; P=.02).63 This meta‐analysis was based on 55 neurocognitive events and resulted in a number needed to harm of 269 associated with a broad confidence interval (CI) ranging from 93 to 3257.64 The methodology of this meta‐analysis was criticised as some patients were counted more than once, owing to their participation in both the OSLER studies and the parent studies.65 The effect of anti‐PCSK9 antibodies on neurocognitive events or cognitive function remains unclear owing to the fact that the blood‐brain barrier cannot be crossed by monoclonal antibodies and that the brain produces its cholesterol by de novo synthesis.65 A 4‐year neurocognitive substudy of the FOURIER trial (EBBINGHAUS) is ongoing to prospectively assess the effects of evolocumab on specific cognitive aspects using a standardised, sensitive and validated test instrument.
Other potential AEs with statins include elevated liver enzymes, which may occur more frequently in patients receiving ezetimibe‐statin combination therapy than with statin monotherapy.57 Differences in levels of liver enzymes between anti‐PCSK9 antibody groups and control groups have not yet been observed in clinical studies. With alirocumab, elevated levels (>3×ULN) of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were observed in 1.8% and 1.4% of patients, respectively in the ODYSSEY LONG TERM study (placebo 2.1% and 2.3%, respectively).33 In the combined analysis of open‐label extension studies of evolocumab, elevated levels of ALT/AST were found in 1.0% of patients receiving evolocumab and in 1.2% of those receiving the standard of care.41, 59
Limited data are available on the use of anti‐PCSK9 antibodies in patients with hepatic or renal impairment. However, anti‐PCSK9 antibodies are proteins and are therefore not expected to be metabolised by the liver or kidneys; hence, no effects on the liver or kidneys are expected. For evolocumab, a small pharmacokinetics study reported no differences in efficacy or safety between healthy volunteers and in patients with mild or moderate hepatic impairment.66 Another evolocumab study reported no differences in LDL‐C lowering or safety among patients with normal renal function and those with severe renal impairment or end‐stage renal disease.67 The recently published FOURIER study included patients with renal impairment (glomerular filtration rate as low as 20 mL/min)26 and subgroup analyses examining the safety and efficacy of evolocumab in this population are expected. For alirocumab, a pooled analysis of data from patients with type 2 diabetes mellitus and chronic kidney disease enrolled in phase 3 trials showed no difference in LDL‐C lowering or safety signals between patients with mild or moderate kidney disease. Furthermore, no effect on chronic kidney disease progression was observed.68 Although larger studies in patients with hepatic or renal impairment are warranted, current evidence supports the use of evolocumab without dose adjustments in these patients.15 Similarly, with alirocumab, dose adjustments are not required for patients with renal or hepatic impairment, although no data are currently available for patients with hepatic impairment.17
The systematic review of phase 3 data for alirocumab and evolocumab reported that, aside from musculoskeletal events, the most common AEs were nasopharyngitis and upper respiratory tract infections.18 Injection site reactions were reported at similar frequencies in evolocumab and control treatment arms, whilst studies of alirocumab varied in whether injection site reactions occurred more often with the anti‐PCSK9 agent or comparators.18 Treatment‐emergent anti‐drug antibodies were rare, but were reported in five alirocumab studies (highest incidence 12%), and in one patient in a single evolocumab study.18 Treatment‐emergent neutralising antibodies were reported in a small number of patients (<1%) in four of the alirocumab studies that were included in the anti‐PCSK9 systematic review, but this did not impact on efficacy or safety.18 Of the evolocumab studies that assessed the formation of neutralising antibodies, such antibodies were not detected in any patient receiving evolocumab.18 Furthermore, no neutralising antibodies were detected in the pooled analysis of over 6000 patients who had participated in evolocumab clinical trials.59 Serious AEs, treatment‐related discontinuations and discontinuations owing to AEs were rare with anti‐PCSK9 antibody therapy.18
Importantly, no significant differences in the incidence AEs, both overall and within each category of AE were observed in patients achieving very low levels of LDL‐C and those with higher LDL‐C levels with evolocumab69 or with alirocumab.70
3.4. What benefits can be expected when using anti‐PCSK9 antibodies?
There is considerable evidence for a causal relationship between LDL‐C reduction and a decrease in the number of CVEs.71 The reduction in CV risk may, however, be specific to the mechanism of action of a drug: agents that increase the number of LDL receptors, such as statins, anti‐PCSK9 antibodies and ezetimibe, are likely to reduce CV risk.72 Drugs with a different mechanism of action, such as inhibitors of the cholesteryl ester transfer protein (CETP), decrease LDL‐C without any proven evidence of CVE risk reduction.73 The ongoing REVEAL trial is investigating the effect of anacetrapib, a CETP inhibitor, on CV risk.74
In 2005, the CTT's collaboration published a meta‐analysis in which data from over 90 000 patients were analysed to assess the effect of statin therapy on CV risk reduction.75 An update to this meta‐analysis assessing the impact of more or less intensive statin therapy was published in 2010.5 Both studies found that a 40 mg/dL (1 mmol/L) reduction in LDL‐C reduced the annual rate of major CVEs by approximately a fifth (21% in the 2005 study and 22% in the later study).5, 75 Crucially, the 2010 meta‐analysis demonstrated that, within the cholesterol range studied, the size of the proportional reduction in major CVEs was directly proportional to the absolute LDL reduction achieved. It was suggested that reducing LDL‐C by 80‐120 mg/dL (2‐3 mmol/L; as seen with anti‐PCSK9 antibodies) would reduce risk by 40%‐50%.5 More recently, the double‐blind, randomised IMPROVE‐IT trial compared statin plus ezetemibe with statin monotherapy in 18 144 patients who had been hospitalised for acute coronary syndrome.21 The primary end‐point of this study was a composite of CV death, major coronary event and non‐fatal stroke. Adding ezetimibe to statin therapy in patients with mean LDL‐C levels within guideline recommendations (<70 mg/dL [<1.8 mmol/L]) resulted in a further 12.8 mg/dL (0.33 mmol/L) reduction in LDL‐C compared with statin alone (based on imputation for missing data) after a 7 year follow‐up. This reduction in risk is consistent with that expected from the CTT meta‐analysis: a 38.7 mg/dL (1 mmol/L) LDL‐C reduction leads to a 22% reduction in CVEs; therefore a 12.8 mg/dL (0.33 mmol/L) LDL‐C reduction is expected to reduce CVEs by 7.9%.5, 75 This current study has demonstrated that adding LLTs to statins can reduce the risk of CVEs, consistent with the conclusion of the CTT meta‐analysis.5, 75 The nature of the relationship between intensive LDL‐C lowering and CVE rates warrants further investigation.
The lowering of LDL‐C achieved with anti‐PCSK9 antibody therapy may also reduce CV risk. Over periods of a year or more, alirocumab or evolocumab have been reported to signficantly reduce the rate of CVEs when added to standard therapy (Table 2).33, 41 Although these studies were not designed or powered to examine efficacy in terms of CV benefit, it is interesting to observe that the reductions in incidence of CVEs with anti‐PCSK9 antibody therapy fit well with theoretical reductions predicted on the basis of data from the CTT meta‐analysis.5, 75 Based on a predicted reduction in CVE rate of 22% for every 38.7 mg/dL (1 mmol/L) reduction in LDL‐C in statin and ezetimibe trials, and the LDL‐C reductions reported (at 24 and 12 weeks in two anti‐PCSK9 antibody trials, ODYSSEY LONG TERM and OSLER‐1 and ‐2, respectively33, 41), a reduction of around 40% in major CVEs would be expected. Notably, the observed reductions in major CVEs in clinical trials of 48% and 53% with alirocumab33 and evolocumab,41 respectively (Table 2), even slightly surmounted the expected decreases. The FOURIER trial, in which patients with atherosclerotic cardiovascular disease and elevated LDL‐C levels were treated with either placebo or evolocumab (on a background of maximally tolerated statins), is the first to report on the impact of anti‐PCSK9 therapies on the incidence of CVEs.26 After a median follow‐up of 2.2 years, compared with placebo evolocumab reduced LDL‐C levels by 59% and reduced the relative risk of the composite CVE end‐point (CV death, myocardial infarction, stroke, hospitalisation for unstable angina and coronary revascularisation) by 15%.26 The magnitude of risk reduction increased over time from 12% in the first 12 months to 19% beyond the first year.26 This reduction is largely consistent with the benefit observed with statins, based on the per‐micromole‐per‐litre reductions in LDL‐C levels observed in the CTT study.26
Table 2.
Observed and predicted reductions in cardiovascular events in ODYSSEY LONG TERM and OSLER‐1 and ‐2
| Alirocumab | Evolocumab | |
|---|---|---|
| Study | ODYSSEY LONG TERM33 | OSLER‐1 and ‐241 |
| Number of patients | 2341 (1553 alirocumab/788 placebo) | 4465 (2976 evolocumab + standard therapy; 1489 standard therapy alone) |
| Follow‐up duration | 78 weeks | 48–56 weeks |
| Percentage reduction in LDL‐C (placebo‐corrected) | 62% (−70.6 mg/dL [−1.8 mmol/L] at week 24); P<.001 | 61% (−73.4 mg/dL [−1.9 mmol/L] at week 12); P<.001 |
| Predicted annual hazard ratio in CVEs based on CTT regression analysis | 0.64 (−36%) | 0.63 (−37%) |
| Observed major CVEs | 1.7% (alirocumab) vs 3.3% (placebo)a | 0.95% (evolocumab) vs 2.11% (standard therapy)b |
| Observed hazard ratio in major CVEs | 0.52 (95% CI 0.31‐0.90); P=.002) (−48%) | 0.47 (95% CI 0.28‐0.78); P=.003) (−53%) |
CI, confidence interval; CTT, Cholesterol Treatment Trialists; CVE, cardiovascular event. aIncludes death from coronary heart disease, non‐fatal myocardial infarction, fatal or non‐fatal ischaemic stroke, and unstable angina requiring hospitalisation. bIncludes death, major coronary events and major cerebrovascular events.
3.5. Hypothetical patient cases
We have used the hypothetical principles of anti‐PCSK9 antibody‐mediated LDL‐C lowering (assuming that the same benefit is observed as with statin and ezetimibe) to predict 10‐year NNTs with anti‐PCSK9 antibodies to prevent one CVE for patients with varying baseline LDL‐C levels and absolute 10‐year CVE risks (Figure 2). As the 10‐year risk of a CVE increased, the NNT to prevent one CVE decreased. For example, for patients with high baseline LDL‐C (160 mg/dL [4.1 mmol/L]), an anti‐PCSK9 antibody is expected to decrease LDL‐C level by 96 mg/dL (2.5 mmol/L; ie, 60% LDL reduction). If the patient has a 10‐year absolute risk of a CVE of 15%, the predicted 10‐year NNT to prevent one CVE was 15. At the same baseline LDL‐C level, the 10‐year NNT to prevent one CVE was less than 8 if the 10‐year risk of a CVE was higher than 30%. For patients with very high 10‐year CVE risk (above 30%) and LDL‐C levels of 160 mg/dL (4.1 mmol/L) or more, the predicted 10‐year NNT (ranging from 3 to 8) might be considered as very efficient. Predicted 10‐year NNTs to prevent one CVE with anti‐PCSK9 antibodies for patients with various baseline LDL‐C levels and absolute 10‐year risks of CVEs are shown in Table 3.
Figure 2.
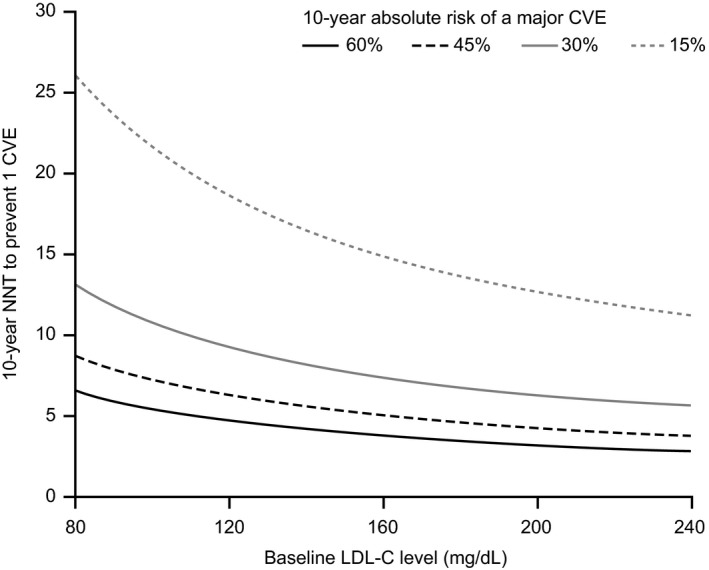
Relationship between 10‐year NNT to prevent one CVE and LDL‐C levels in hypothetical patients with various levels of residual CVE risk and who are receiving an anti‐PCSK9 antibody as add‐on therapy to statin and ezetimibe. CVE, cardiovascular event; LDL‐C, low‐density lipoprotein cholesterol; NNT, number needed to treat; PCSK9, proprotein convertase subtilisin/kexin type 9. An estimated absolute reduction in LDL‐C levels from baseline of 60% was considered to predict the 10‐year NNT
Table 3.
Predicted 10‐year NNT with an anti‐PCSK9 antibody (with a predicted 60% LDL‐C reduction from baseline) to prevent 1 CVE for various absolute 10‐year risk of major CVEs and various baseline LDL‐C levels
| Absolute 10‐year risk of a major CVEa | Baseline LDL‐C levels, mg/dL (mmol/L) | Predicted LDL‐C reductions achieved with anti‐PCSK9 antibody treatment, mg/dL (mmol/L) | Predicted LDL‐C levels following anti‐PCSK9 antibody treatment, mg/dL (mmol/L) | Predicted 10‐year absolute risk of a major CVE with anti‐PCSK9 antibody treatmentb | 10‐year NNT to prevent 1 CVEc |
|---|---|---|---|---|---|
| 60% | 200 (5.2) | 120 (3.1) | 80 (2.1) | 28% | 4 |
| 160 (4.1) | 96 (2.5) | 64 (1.7) | 32% | 4 | |
| 120 (3.1) | 72 (1.7) | 48 (1.2) | 38% | 5 | |
| 80 (2.1) | 48 (1.2) | 32 (0.83) | 44% | 6 | |
| 30% | 200 (5.2) | 120 (3.1) | 80 (2.1) | 14% | 6 |
| 160 (4.1) | 96 (2.5) | 64 (1.7) | 16% | 7 | |
| 120 (3.1) | 72 (1.7) | 48 (1.2) | 19% | 9 | |
| 80 (2.1) | 48 (1.2) | 32 (0.83) | 22% | 13 | |
| 15% | 200 (5.2) | 120 (3.1) | 80 (2.1) | 7% | 13 |
| 160 (4.1) | 96 (2.5) | 64 (1.7) | 8% | 15 | |
| 120 (3.1) | 72 (1.7) | 48 (1.2) | 9% | 18 | |
| 80 (2.1) | 48 (1.2) | 32 (0.83) | 11% | 26 |
CTT, Cholesterol Treatment Trialists; CVE, cardiovascular event; LDL‐C, low‐density lipoprotein‐cholesterol; NNT, number needed to treat; PCSK9, proprotein convertase subtilisin/kexin type 9. aPatients may or may not be receiving LLT. bUsing data from the CTT meta‐analysis.5 cNNT for 10 years to prevent one CVE=100/([1−0.78n]×10‐year CVE risk in %), where n=LDL‐C reduction in mmol/L and 0.78 represents the decrease in CVD risk for each 1 mmol/L reduction in LDL‐C.5, 19
High 10‐year absolute CVE risk (>30%) might be expected in some patients, such as: those in secondary prevention; those aged over 30 years with FH and other risk factors; those aged over 40 years and with type 2 diabetes mellitus and other risk factors or microalbuminuria; patients aged over 60 years without type 2 diabetes mellitus, but with multiple other risk factors and a SCORE (ie, 10‐year risk of fatal CVD) of 10% or higher. Additional risk factors, such as smoking, high blood pressure, chronic kidney disease, family history of CVD, high levels of lipoprotein(a) or triglycerides may also contribute to increased CVE risk.3 These high‐risk patient groups, together with those who are intolerant to statins, could benefit from anti‐PCSK9 antibody treatment. However, with limited CV outcomes data, considering the costs associated with treatment, and in line with recent German recommendations, anti‐PCSK9 antibody therapy should currently only be considered for secondary prevention in patients who have high LDL‐C levels despite receiving maximally tolerated LLT and at least two of the following additional conditions: FH; previous myocardial infarction, progressive coronary heart disease or atherosclerosis; type 2 diabetes mellitus; moderate‐to‐severe chronic kidney disease (glomerular filtration rate <60 mL/min/1.73 m2); or heart failure (New York Heart Association classification III–IV) (Table 4).76
Table 4.
Typical characteristics of patients in secondary prevention with high LDL‐C levels despite receiving maximally tolerated LLT who may be considered for treatment with anti‐PCSK9 antibodies.76 At least two of the following factors should be present
| Familial hypercholesterolaemia |
| Previous myocardial infarction, progressive CVD or atherosclerosis |
| Type 2 diabetes mellitus |
| Moderate‐to‐severe chronic kidney disease (GFR<60 mL/min/1.73 m2) |
| Heart failure (New York Heart Association classification III‐IV) |
CVD, cardiovascular disease; GFR, glomerular filtration rate; LDL‐C, low‐density lipoprotein cholesterol; LLT, lipid‐lowering therapy; PCSK9, proprotein convertase subtilisin/kexin type 9.
Two examples of individuals who might visit their primary care physician and have a very high CVE risk and high levels of LDL‐C are presented in Table 5 (see also Figure 3). These patients could be considered good candidates for anti‐PCSK9 antibody therapy, either as monotherapy (in the case of statin intolerance) or in combination with other LLTs.
Table 5.
Predicted outcomes in a hypothetical case of a 54‐year old male patient who had previously experienced a myocardial infarction and has very high baseline LDL‐C levels (scenario A) despite receiving maximally tolerated statin plus ezetimibe and (scenario B) under ezetimibe treatment in the scenario of statin intolerance. On the basis of the overall results from ODYSSEY COMBO II,28 an absolute reduction in LDL‐C levels of at least 100 mg/dL (2.6 mmol/L) would be anticipated in scenario A following the addition of an anti‐PCSK9 antibody to a statin plus ezetimibe. Using estimates from the CTT trial, this would translate into a 47% reduction in relative risk of major CVEs over 10 years.5 Assuming a predicted 10‐year absolute risk of CV death of 20% for this patient (Figure 3), the use of ezetimibe plus maximally tolerated statin would half this risk (9%). Anti‐PCSK9 antibody as an add‐on therapy would further reduce this risk to 5%. In terms of absolute 10‐year risk of a major CVE (fatal and non‐fatal), an anti‐PCSK9 antibody with ezetimibe and maximally tolerated statin would reduce the 10‐year absolute CVE risk from 60% to 15%. Addition of an anti‐PCSK9 antibody to statin and ezetimibe gives a NNT of 8. We have applied the same principles to another scenario (scenario B). This patient is similar to that described above, but has lower baseline LDL‐C (191 mg/dL [4.9 mmol/L]) and is statin intolerant. In this case, and using data from the ODYSSEY ALTERNATIVE trial32 and the CTT meta‐analysis,5 ezetimibe slightly reduced the 10‐year absolute risk of CV death from 20% to 17%; treatment with anti‐PCSK9 antibodies was predicted to drastically reduce this risk to 8% (a reduction in predicted absolute 10‐year risk of a CVE from 60% to 24%). The NNT for the addition of an anti‐PCSK9 antibody to ezetemibe therapy was 4, compared with 10 with ezetimibe alone. In both scenarios, it was assumed that treatments were well tolerated and that patients were adherent to treatment
| Scenario A | |||
|---|---|---|---|
| Parameter | Baseline | Ezetimibe + maximally tolerated statin | Anti‐PCSK9 antibody + ezetimibe + maximally tolerated statin |
| LDL‐C, mg/dL (mmol/L) | 261 (6.7) | 141 (3.6) | 41 (1.1) |
| Predicted reduction in LDL‐C (% change vs previous therapy)a | – | −46% | −71% |
| Predicted reduction in LDL‐C, mg/dL (mmol/L)a | – | 120 (3.1) | 100 (2.6) |
| Predicted annual risk reduction in major CVEs (%)b | – | −54% vs baseline |
−47% vs ezetemibe + statins 76% vs baseline |
| Predicted absolute 10‐year risk of a major CVE (%)c | 60% | 28% | 15% |
| Predicted 10‐year risk of CV death (%)c | 20% | 9% | 5% |
| NNT for 10 years to prevent one CVEd | – | 4 vs baseline |
3 vs baselinee
8 vs previous treatmentf |
| Scenario B: Statin intolerance | |||
| Parameter | Baseline without statins | Ezetimibe alone | Anti‐PCSK9 antibody + ezetimibe |
| LDL‐C, mg/dL (mmol/L) | 191 (4.9) | 163 (4.2) | 51 (1.3) |
| Predicted reduction in LDL‐C (% change vs previous therapy)a | – | 15% | 69% |
| Predicted reduction in LDL‐C, mg/dL (mmol/L) vs previous therapya | – | 28 (0.72) | 112 (2.9) |
| Predicted annual risk reduction in major CVEs (%) vs previous therapyb | – | 16% vs baseline |
51% vs ezetemibe 59% vs baseline |
| Predicted absolute 10‐year risk of a major CVE (%)c | 60% | 50% | 24% |
| Predicted 10‐year risk of CV death (%)c | 20% | 17% | 8% |
| NNT for 10 years to prevent one CVEd | – | 11 |
3 vs baselinee
4 vs ezetemibef |
CTT, Cholesterol Treatment Trialists; CV, cardiovascular; CVE, cardiovascular event; LDL‐C, low‐density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9. aPredicted reductions in LDL‐C are based on data from the ODYSSEY COMBO II trial.28 bPredicted risk reduction in CVEs calculated based on data from the CTT meta‐analysis.5 cAbsolute 10‐year risk of a major CVE=baseline risk×(1−risk reduction), as calculated on Figure 2. dNNT for 10 years to prevent one CVE=100/([1−0.78n]×10‐year CVE risk in %), where n=LDL‐C reduction in mmol/L and 0.78 represents the decrease in CVD risk for each 1 mmol/L reduction in LDL‐C.5, 19 eThe 10‐year NNT was calculated using the reduction in LDL‐C from baseline. fThe 10‐year NNT was calculated when adding an anti‐PCSK9 antibody to previous therapy
Figure 3.

Predicted LDL‐C reductions and corresponding CV risk that may be expected when using a statin and then an anti‐PCSK9 antibody as an add‐on therapy in a patient with a 10‐year absolute risk of a major CVE of 60%: a hypothetical case example of a male patient aged 54 years who has experienced a previous myocardial infarction. CV, cardiovascular; CVE, cardiovascular event; LDL‐C, low‐density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9
3.6. Approved indications for alirocumab and evolocumab
Alirocumab and evolocumab have both been approved in Europe for use as an adjunct to diet in adults with primary hypercholesterolaemia (HeFH or non‐familial hypercholesterolaemia) or mixed dyslipidaemia who do not achieve their LDL‐C goal with other LLTs, or in those who are statin‐intolerant.15, 17 Evolocumab is also approved for use in combination with other LLTs in patients aged 12 years and older who have HoFH.15 Alirocumab is administered subcutaneously at a recommended starting dose of 75 mg once every 2 weeks or 300 mg once every 4 weeks, and it may be adjusted to 150 mg every 2 weeks.17 The dose of alirocumab can be tailored to the individual and may be up‐ or down‐titrated as needed.17 Evolocumab is administered subcutaneously at a recommended dose of 140 mg every 2 weeks or 420 mg every month: both doses are considered clinically equivalent.15 For patients with HoFH, the dose of 420 mg every month may be up‐titrated to 420 mg every 2 weeks if a clinically meaningful response is not achieved after 12 weeks of treatment.15 Owing to the high cost of these therapies, their use is expected to be limited to patients considered to be at very high risk, in whom LDL‐C goals are not achieved with alternative maximally tolerated LLT.
4. CONCLUSIONS AND CLINICAL IMPLICATIONS
Anti‐PCSK9 antibodies are safe, well tolerated and efficacious in lowering LDL‐C in a broad range of patient populations, and are suitable as an add‐on therapy to a statin or ezetimibe, or as a monotherapy for patients who are intolerant to statins. Current data suggest that they not only lower LDL‐C, but also reduce the risk of CVEs. Studies that look at CV outcomes as a primary end‐point are ongoing, and long‐term data from outcome studies are awaited. These anti‐PCSK9 antibodies are currently costly as they are intended for only a narrow population; however, once CV outcomes data are available the indication may expand and costs may therefore decrease. In Germany, the annual cost for treating one patient per year with an anti‐PCSK9 antibody is approximately €8500.76 Therefore, the use of anti‐PCSK9 antibodies may be limited by payers to patients with an extremely high CV risk and high LDL‐C levels after exhausting all other LLTs at maximally tolerated doses (Table 4).76 According to this reasoning, approximately 60 000 patients would be eligible for anti‐PCSK9 antibody treatment in Germany.76
Safety data from phase 3 clinical trials of anti‐PCSK9 antibodies have shown that they are associated with few serious AEs or treatment discontinuations and have a safety profile very comparable to placebo. Formal interaction testing is not required as monoclonal antibodies are not expected to have drug–drug interactions.77 Dose adjustments are not required for patients with mild to moderate renal or hepatic impairment. Additionally, dose adjustments are not needed for body weight making it simple for physicians to administer the correct dose. Following discontinuation of evolocumab, LDL‐C levels gradually return to baseline over a period of up to 12 weeks (less rapidly than with statins), with no evidence of a rebound effect.78
Estimates of 10‐year NNT to prevent one CVE are well‐supported by theoretical rationale and preliminary observations from clinical studies. However, we are aware that there may be limitations in using the hazard ratio observed with other LLTs and extrapolating these estimates to predict treatment effects of anti‐PCSK9 antibodies. One of the limitations of calculating NNTs is the assumption that the treatment effect for statins compared with no statins will be the same as for anti‐PCSK9 antibody plus statins compared with statin alone, which remains to be confirmed. Secondly, a 38.7 mg/dL (1 mmol/L) reduction in the context of the high baseline LDL‐C levels seen in the CTT meta‐analysis trials (143 mg/dL, 3.7 mmol/L) could have accounted for an overestimation of the NNT as compared with the effect of starting at a lower baseline of 88 mg/dL (2.3 mmol/L). The fact that anti‐PCSK9 antibodies are add‐on therapies to statins may also account for limitations in using the same NNT calculation for anti‐PCSK9 antibodies and for statins alone. Because the LDL‐C reductions in the IMPROVE‐IT trial were small compared with reductions associated with anti‐PCSK9 antibodies, caution is needed when extrapolating the effects observed in this trial to anti‐PCSK9 antibodies. However, there is currently a strong level of evidence that the relationship between LDL‐C level and CVE risk is similar between drugs that specifically target the LDL receptor pathway (eg, ezetimibe and various statins). This contrasts with other drugs that modulate LDL‐C levels by other pathways (eg, fibrates and CETP inhibitors) with which no such relationship has been observed. Anti‐PCSK9 antibodies primarily act on the LDL receptor pathways, therefore it would be reasonable to expect that the LDL‐C reductions induced by these agents would have the same proportional effect on CVE risk as statins and ezetimibe. Furthermore, preliminary data demonstrating a CVE risk benefit in anti‐PCSK9 antibody trials, albeit limited study durations33, 41 supports the predictions of CVE risk benefits made in this review.
While we await further data from anti‐PCSK9 antibody trials that evaluate CV outcomes as primary end‐points (rather than surrogate markers such as LDL‐C levels), we propose that an anti‐PCSK9 antibody should be considered for use in secondary prevention patients with high LDL‐C levels despite receiving maximally tolerated LLT and at least two additional CVE risk factors.
AUTHOR CONTRIBUTIONS
All authors contributed to the concept and design of the manuscript, drafting and critical revision of the article.
DISCLOSURES
U. Fraass is an employee of Amgen and owns stocks. R. Dent was an employee of Amgen at time of this work and owns stocks in Amgen and Esperion Therapeutics. I. Gouni‐Berthold has received personal fees from Sanofi/Genzyme, Amgen, AstraZeneca, Bristol‐Myers Squibb and Eli Lilly. O.S. Descamps has received grants, personal fees and non‐financial support from Merck Sharp & Dohme, Amgen and Sanofi; grants and personal fees from AstraZeneca; grants from Pfizer; and personal fees from Abbott. W. März has received grants and personal fees from Aegerion Pharmaceuticals, Amgen, AstraZeneca, BASF, Danone Research, Numares AG, Pfizer, Sanofi/Genzyme and Siemens Diagnostics; grants from Abbott Diagnostics; personal fees from Alexion, Hoffmann‐La Roche, Merck Sharp & Dohme, Sanofi; and has been employed by Synlab Holding Deutschland GmbH.
ACKNOWLEDGEMENTS
Medical writing support was provided by Liz Hartfield PhD of Oxford PharmaGenesis, Oxford, UK, which was funded by Amgen (Europe) GmbH. Editorial support was provided by Carine Thual of Amgen (Europe) GmbH. Mary Elliott, biostatistician at Amgen Ltd, completed a quality check of the number‐needed‐to‐treat calculations and methodology used in this manuscript. Alexander Dressel PhD, DACH Society for the Prevention of Cardiovascular Disease, Hamburg, provided statistical advice.
Descamps OS, Fraass U, Dent R, März W, Gouni‐Berthold I. Anti‐PCSK9 antibodies for hypercholesterolaemia: Overview of clinical data and implications for primary care. Int J Clin Pract. 2017; 71:e12979 https://doi.org/10.1111/ijcp.12979
REFERENCES
- 1. Townsend N, Nichols M, Scarborough P, Rayner M. Cardiovascular disease in Europe – epidemiological update 2015. Eur Heart J. 2015;36:2696‐2705. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Cardiovascular diseases (CVDs). Fact sheet No. 317, 2015. http://www.who.int/mediacentre/factsheets/fs317/en/. Accessed December 11, 2015.
- 3. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Donnell CJ, Elosua R. Cardiovascular risk factors. Insights from Framingham Heart Study. Rev Esp Cardiol. 2008;61:299‐310. [PubMed] [Google Scholar]
- 5. Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ford ES. Trends in the risk for coronary heart disease among adults with diagnosed diabetes in the U.S.: findings from the National Health and Nutrition Examination Survey, 1999–2008. Diabetes Care. 2011;34:1337‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635‐1701. [DOI] [PubMed] [Google Scholar]
- 8. Aquilani R, Tramarin R, Pedretti RF, et al. Despite good compliance, very low fat diet alone does not achieve recommended cholesterol goals in outpatients with coronary heart disease. Eur Heart J. 1999;20:1020‐1029. [DOI] [PubMed] [Google Scholar]
- 9. Hunninghake DB, Stein EA, Dujovne CA, et al. The efficacy of intensive dietary therapy alone or combined with lovastatin in outpatients with hypercholesterolemia. N Engl J Med. 1993;328:1213‐1219. [DOI] [PubMed] [Google Scholar]
- 10. Gitt AK, Drexel H, Feely J, et al. Persistent lipid abnormalities in statin‐treated patients and predictors of LDL‐cholesterol goal achievement in clinical practice in Europe and Canada. Eur J Prev Cardiol. 2012;19:221‐230. [DOI] [PubMed] [Google Scholar]
- 11. Shalev V, Chodick G, Silber H, et al. Continuation of statin treatment and all‐cause mortality: a population‐based cohort study. Arch Intern Med. 2009;169:260‐268. [DOI] [PubMed] [Google Scholar]
- 12. Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long‐term statin therapy? Curr Atheroscler Rep. 2013;15:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seidah NG. Proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors in the treatment of hypercholesterolemia and other pathologies. Curr Pharm Des. 2013;19:3161‐3172. [DOI] [PubMed] [Google Scholar]
- 14. Amgen Inc . REPATHA® (evolocumab) highlights of prescribing information. http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/repatha/repatha_pi_hcp_english.ashx. Accessed January 10, 2017.
- 15. Amgen . Repatha® (evolocumab) summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003766/WC500191398.pdf. Accessed May 19, 2016.
- 16. Regeneron Pharmaceuticals Inc. and Sanofi‐Aventis US . PRALUENT® (alirocumab) highlights of prescribing information. http://products.sanofi.us/praluent/praluent.pdf. Accessed January 10, 2017.
- 17. Sanofi‐Regeneron . Praluent® (alirocumab) summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003882/WC500194521.pdf. Accessed May 19, 2016.
- 18. Gouni‐Berthold I, Descamps OS, Fraass U, et al. Systematic review of published phase 3 data on anti‐PCSK9 monoclonal antibodies in patients with hypercholesterolaemia. Br J Clin Pharmacol. 2016;82:1412‐1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soran H, Schofield JD, Durrington PN. Cholesterol, not just cardiovascular risk, is important in deciding who should receive statin treatment. Eur Heart J. 2015;36:2975‐2983. [DOI] [PubMed] [Google Scholar]
- 20. McQueen D. Numbers‐needed‐to‐treat analysis. Adv Psychiatr Treat. 2011;17:158. [Google Scholar]
- 21. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387‐2397. [DOI] [PubMed] [Google Scholar]
- 22. Ford I, Murray H, McCowan C, Packard CJ. Long‐term safety and efficacy of lowering low‐density lipoprotein cholesterol with statin therapy: 20‐year follow‐up of West of Scotland Coronary Prevention Study. Circulation. 2016;133:1073‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pedersen TR, Wilhelmsen L, Faergeman O, et al. Follow‐up study of patients randomized in the Scandinavian simvastatin survival study (4S) of cholesterol lowering. Am J Cardiol. 2000;86:257‐262. [DOI] [PubMed] [Google Scholar]
- 24. Egan BM, Li J, White K, et al. ACC/AHA cholesterol guideline and implications for healthy people 2020 cardiovascular disease prevention goals. J Am Heart Assoc. 2013;2016:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robinson JG, Ray K. Counterpoint: low‐density lipoprotein cholesterol targets are not needed in lipid treatment guidelines. Arterioscler Thromb Vasc Biol. 2016;36:586‐590. [DOI] [PubMed] [Google Scholar]
- 26. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713‐1722. https://doi.org/10.1056/nejmoa1615664. [DOI] [PubMed] [Google Scholar]
- 27. Bays H, Gaudet D, Weiss R, et al. Alirocumab as add‐on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015;100:3140‐3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cannon CP, Cariou B, Blom D, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farnier M, Jones P, Severance R, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular‐risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138‐146. [DOI] [PubMed] [Google Scholar]
- 30. Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996‐3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169:906‐915,e13. [DOI] [PubMed] [Google Scholar]
- 32. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin‐intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9:758‐769. [DOI] [PubMed] [Google Scholar]
- 33. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489‐1499. [DOI] [PubMed] [Google Scholar]
- 34. Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double‐blind, randomized Phase 3 trial. Int J Cardiol. 2014;176:55‐61. [DOI] [PubMed] [Google Scholar]
- 35. Roth EM, Moriarty PM, Bergeron J, et al. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add‐on to statin: ODYSSEY CHOICE I. Atherosclerosis. 2016;254:254‐262. [DOI] [PubMed] [Google Scholar]
- 36. Blom DJ, Hala T, Bolognese M, et al. A 52‐week placebo‐controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809‐1819. [DOI] [PubMed] [Google Scholar]
- 37. Kiyosue A, Honarpour N, Kurtz C, et al. A phase 3 study of evolocumab (AMG 145) in statin‐treated Japanese patients at high cardiovascular risk. Am J Cardiol. 2016;117:40‐47. [DOI] [PubMed] [Google Scholar]
- 38. Koren MJ, Lundqvist P, Bolognese M, et al. Anti‐PCSK9 monotherapy for hypercholesterolemia: the MENDEL‐2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531‐2540. [DOI] [PubMed] [Google Scholar]
- 39. Raal FJ, Hovingh GK, Blom D, et al. Long‐term treatment with evolocumab in patients with homozygous familial hypercholesterolaemia (HoFH): interim results from the Trial Assessing long‐term Use of PCSK9 Inhibition in Subjects with Genetic LDL disorders (TAUSSIG) study. Presented at 17th International Symposium on Atherosclerosis, Amsterdam, Netherlands, 23–25 May, 2015.
- 40. Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD‐2): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:331‐340. [DOI] [PubMed] [Google Scholar]
- 41. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500‐1509. [DOI] [PubMed] [Google Scholar]
- 42. Stroes E, Colquhoun D, Sullivan D, et al. Anti‐PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS‐2 randomized, placebo‐controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541‐2548. [DOI] [PubMed] [Google Scholar]
- 43. Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:341‐350. [DOI] [PubMed] [Google Scholar]
- 44. Ginsberg HN, Farnier M, Robinson JG, et al. Efficacy and safety of alirocumab: pooled analyses of 1048 individuals with diabetes mellitus from five placebo‐controlled phase 3 studies of at least 52 weeks duration. Circulation. 2015;132:Abstr 17070. [Google Scholar]
- 45. Sattar N, Preiss D, Robinson JG, et al. Lipid‐lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta‐analysis of individual patient data. Lancet Diabetes Endocrinol. 2016;4:403‐410. [DOI] [PubMed] [Google Scholar]
- 46. Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti‐PCSK9 antibodies: a meta‐analysis of 25 randomized, controlled trials. BMC Med. 2015;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146‐2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lappegård K, Enebakk T, Thunhaug H, Hovland A. Transition from LDL apheresis to evolocumab in heterozygous FH is equally effective in lowering LDL, without lowering HDL cholesterol. Atherosclerosis. 2016;251:119‐123. [DOI] [PubMed] [Google Scholar]
- 49. Wang A, Richhariya A, Gandra SR, et al. Systematic review of low‐density lipoprotein cholesterol apheresis for the treatment of familial hypercholesterolemia. J Am Heart Assoc. 2016;5:e003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stein E, Sampietro T, Santos R, et al. Long‐term treatment with evolocumab homozygous familial hypercholesterolemia patients: results from the trial assessing long‐term use of PCSK9 inhibition in subjects with genetic LDL disorders (TAUSSIG). Presented at the 84th European Atherosclerosis Society Congress, Innsbruck, Austria May 29–June 1 2016.
- 51. Moriarty PM, Parhofer KG, Babirak SP, et al. Alirocumab in patients with heterozygous familial hypercholesterolaemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur Heart J 2016;37:3588‐3595. https://doi.org/10.1093/eurheartj/ehw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bruckert E. Effect of alirocumab on the frequency of lipoprotein apheresis: a randomised phase III trial. Discussant review presented at the European Society of Cardiology Congress, Rome, Italy 27–31 August 2016.
- 53. Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769‐1818. [DOI] [PubMed] [Google Scholar]
- 54. Ginsberg HN, Rader DJ, Raal FJ, et al. ODYSSEY HIGH FH: efficacy and safety of alirocumab in patients with severe heterozygous familial hypercholesterolemia. Circulation. 2014;130. [Google Scholar]
- 55. Hu M, Cheung BM, Tomlinson B. Safety of statins: an update. Ther Adv Drug Saf. 2012;3:133‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high‐dosage statin therapy in hyperlipidemic patients – the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403‐414. [DOI] [PubMed] [Google Scholar]
- 57. Luo L, Yuan X, Huang W, et al. Safety of coadministration of ezetimibe and statins in patients with hypercholesterolaemia: a meta‐analysis. Intern Med J. 2015;45:546‐557. [DOI] [PubMed] [Google Scholar]
- 58. Farnier M, Gaudet D, Valcheva V, et al. Efficacy of alirocumab in high cardiovascular risk populations with or without heterozygous familial hypercholesterolemia: pooled analysis of eight ODYSSEY phase 3 clinical program trials. Int J Cardiol. 2016;223:750‐757. [DOI] [PubMed] [Google Scholar]
- 59. Toth PP, Descamps O, Genest J, et al. A pooled safety analysis of over 6000 patients from double‐blind and open‐label extension studies with evolocumab. J Am Coll Cardiol. 2016;67:1865. [DOI] [PubMed] [Google Scholar]
- 60. US Food and Drug Administration . FDA expands advice on statin risk, 2014. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm293330.htm. Accessed December 14, 2016.
- 61. Gauthier JM, Massicotte A. Statins and their effect on cognition: let's clear up the confusion. Can Pharm J (Ott). 2015;148:150‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Swiger KJ, Martin SS. PCSK9 inhibitors and neurocognitive adverse events: exploring the FDA directive and a proposal for N‐of‐1 trials. Drug Saf. 2015;38:519‐526. [DOI] [PubMed] [Google Scholar]
- 63. Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin‐kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta‐analysis. Eur Heart J. 2016;37:536‐545. [DOI] [PubMed] [Google Scholar]
- 64. Santos RD. Review: PCSK9 inhibitors reduce mortality but increase neurocognitive events in hypercholesterolemia. Ann Intern Med 2016;164:Jc31. [DOI] [PubMed] [Google Scholar]
- 65. Kolodziejczak M, Navarese EP. Role of PCSK9 antibodies in cardiovascular disease: critical considerations of mortality and neurocognitive findings from the current literature. Atherosclerosis. 2016;247:189‐192. [DOI] [PubMed] [Google Scholar]
- 66. Gibbs JP, Slatter JG, Egbuna O, et al. Evaluation of evolocumab (AMG 145), a fully human anti‐PCSK9 IgG2 monoclonal antibody, in subjects with hepatic impairment. J Clin Pharmacol. 2017;57:513‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee E, Gibbs J, Wasserman SM, et al. Pharmacokinetics and pharmacodynamics of evolocumab in patients with renal impairment. Eur Heart J. 2016;37(Abstract Supplement):343.26553538 [Google Scholar]
- 68. Dwyer JP, Colhoun HM, Tinahones FJ, et al. Abstract 16850: Effect of alirocumab in patients with diabetic dyslipidemia and chronic kidney disease. Circulation. 2016;134:A16850. [Google Scholar]
- 69. Koren MJ, Blom D, Giugliano RP, et al. Safety and tolerability of very low LDL‐C levels in patients treated with 52 weeks of evolocumab (AMG 145). Circulation. 2014;130:A16865. [Google Scholar]
- 70. Robinson JG, Rosenson RS, Farnier M, et al. Safety of very low low‐density lipoprotein cholesterol levels with alirocumab: pooled data from randomized trials. J Am Coll Cardiol. 2017;69:471‐482. [DOI] [PubMed] [Google Scholar]
- 71. Jarcho JA, Keaney JF Jr. Proof that lower is better – LDL cholesterol and IMPROVE‐IT. N Engl J Med. 2015;372:2448‐2450. [DOI] [PubMed] [Google Scholar]
- 72. Grundy SM. Dyslipidaemia in 2015: advances in treatment of dyslipidaemia. Nat Rev Cardiol. 2016;13:74‐75. [DOI] [PubMed] [Google Scholar]
- 73. Charlton‐Menys V, Durrington PN. Human cholesterol metabolism and therapeutic molecules. Exp Physiol. 2008;93:27‐42. [DOI] [PubMed] [Google Scholar]
- 74. ClinicalTrials.gov . REVEAL: Randomized EValuation of the Effects of Anacetrapib Through Lipid‐modification (REVEAL). https://clinicaltrials.gov/ct2/show/NCT01252953. Accessed May 20, 2016.
- 75. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267‐1278. [DOI] [PubMed] [Google Scholar]
- 76. März W, Scharnagl H, Gouni‐Berthold I, et al. LDL‐cholesterol: standards of treatment 2016 ‐ a German perspective. Am J Cardiovasc Drugs. 2016;16:323‐336. [DOI] [PubMed] [Google Scholar]
- 77. Seitz K, Zhou H. Pharmacokinetic drug‐drug interaction potentials for therapeutic monoclonal antibodies: reality check. J Clin Pharmacol. 2007;47:1104‐1118. [DOI] [PubMed] [Google Scholar]
- 78. Koren MJ, Giugliano RP, Raal FJ, et al. Efficacy and safety of longer‐term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52‐week results from the Open‐Label Study of Long‐Term Evaluation Against LDL‐C (OSLER) randomized trial. Circulation. 2014;129:234‐243. [DOI] [PubMed] [Google Scholar]
- 79. Sattar NA, Djedjos CS, Robinson JG, et al. Efficacy and safety of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes. Diabetes. 2015;64:A68. [DOI] [PubMed] [Google Scholar]
- 80. Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate‐ or high‐intensity statin therapy on LDL‐C lowering in patients with hypercholesterolemia: the LAPLACE‐2 randomized clinical trial. JAMA. 2014;311:1870‐1882. [DOI] [PubMed] [Google Scholar]
- 81. Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5:e003421. [DOI] [PMC free article] [PubMed] [Google Scholar]


