Abstract
Moderate hyperhomocysteinemia‐induced low folate status is an independent risk factor for cardiovascular disease, dementia, and depression. Folate is an essential cofactor in the one‐carbon metabolism pathway and is necessary in amino acid metabolism, purine and thymidylate synthesis, and DNA methylation. In the folate cycle and homocysteine metabolism, folate, vitamin B12, vitamin B6, and vitamin B2 are important cofactors. Many enzymes are involved in folate transport and uptake, the folate pathway, and homocysteine (Hcy) metabolism, and various polymorphisms have been documented in these enzymes. Serum folate and total Hcy (tHcy) levels are influenced by folate intake and genetic polymorphisms in 5,10‐methylenetertahydrofolate reductase (MTHFR) such as C677T. The prevalence of the MTHFR 677TT genotype varies across ethnic groups and regions, with a frequency of approximately 15% in Japanese populations. Individuals with the TT genotype have significantly higher tHcy levels and lower folate levels in serum than those with the CT and TT genotypes. However, administration of folic acid has been shown to eliminate these differences. Moreover, data have suggested that interventions based on genotype may be effective for motivating individuals to change their lifestyle and improve their nutrition status. Accordingly, in this review, we discuss the effects of MTHFR C677T polymorphisms on serum tHcy and folate levels with folic acid intervention and evaluate approaches for overcoming folic acid deficiency and related symptoms.
Keywords: folate, methylenetetrahydrofolate reductase, personalized nutrition, polymorphism
Introduction
The classical symptom of folate deficiency in humans is megaloblastic anemia. Inadequate folate intake constitutes a leading cause of folate deficiency, which involves decreased serum or plasma folate concentrations followed by increased serum or plasma total homocysteine (tHcy) concentrations and reduced red blood cell (RBC) folate levels. Elevated tHcy represents a major risk factor of cardiovascular and cerebrovascular diseases (Homocysteine Studies Collaboration 2002). Moreover, lower serum folate and higher plasma tHcy may also be causes of neural tube defects (NTDs) (Smithells et al. 1976; Daly et al. 1995), cognitive dysfunction (Seshadri et al. 2002), and depression (Bottiglieri 2005).
Folate is an essential cofactor in the folate‐mediated one‐carbon metabolism pathway and is essential in many biochemical processes, such as amino acid metabolism, purine and thymidylate synthesis, and DNA methylation (Brody and Shane 2001). The folate pathway is also closely associated with Hcy metabolism. Hcy itself is located at a branch‐point of metabolic pathways: remethylation and the trans‐sulfuration pathway, in which B‐vitamins, i.e. folate, vitamin B12, vitamin B6, and vitamin B2, are required as cofactors. Therefore, inadequate levels of these vitamins can raise plasma tHcy levels.
Several studies have identified associations between genetic polymorphisms related to the folate pathway and Hcy metabolism. The common C677T variant in the gene encoding the folate‐metabolizing enzyme methylenetetrahydrofolate reductase (MTHFR) is the most well‐known genetic factor influencing folate status. MTHFR catalyzes the conversion of 5,10‐methylenetetrahydorfolate to 5‐methyltetrahydrofolate in an irreversible reaction. This enzyme is critical for the regulation of available folate in the remethylation of Hcy. Carriers of the T allele have lower enzyme activity (Frosst et al. 1995), leading to elevated Hcy concentrations (Tsang et al. 2015). The frequency of this polymorphism is known to vary among different ethnic groups and geographical regions (Binia et al. 2014); for example, the allele frequency in the Japanese population is 0.391 (Iida et al. 2009) and the frequency of the homozygous mutant TT genotype is approximately 15% in Japanese individuals (Sadewa et al. 2002; Hiraoka 2004), but about 10% in individuals worldwide (Wilcken et al. 2003). Thus, for prevention of various diseases associated with elevated tHcy, it is important to consider nutrient‐gene interactions.
In this review, we will explore the evidence linking polymorphism‐related folate‐Hcy metabolism with folate status and describe examples of health promotion programs with personalized nutritional intervention based on MTHFR C677T polymorphisms.
Folate and Hcy Metabolism
Dietary folates predominantly exist as polyglutamates, which have to be deconjugated to monoglutamates prior to absorption in order to be transported. The enzyme responsible for this deconjugation in the gut is folyl poly‐γ‐glutamate carboxypeptidase (FGCP), which is anchored to the intestinal apical brush border and is encoded by the glutamate carboxypeptidade II (GCP1) gene, also known as folate hydrolase 1 (FOLH1) (Chandler et al. 1991). In comparison, synthetic folic acid (pteroylmonoglutamic acid [PteGlu]) has a fully oxidized pteridine ring conjugated to a single glutamate residue.
Folate monoglutamates including PteGlu are absorbed in the duodenum and upper part of the jejunum by the high‐affinity proton‐coupled folate transporter PCFT1 (SLC46A1) (Qiu et al. 2006). However, PteGlu requires an additional step in order to enter folate metabolism, as it must first be reduced to dihydrofolate (DHF) and then to the active form, 5‐methyltetrahydrofolate (5‐methyl‐THF). Once folate has entered the blood stream, 5‐methyl‐THF constitutes the main form as it can enter the cell through folate receptor (FR)‐α. FR‐α is a glycosylphosphatidylinositol‐linked glycoprotein with a high affinity for the monoglutamate 5‐methyl‐THF (Wang et al. 1992) and is expressed in a limited number of epithelial cells, predominantly in the renal proximal tubules, choroid plexus, uterus, and placenta (Kamen & Smith 2004). FR‐β and FR‐γ have a much lower affinity for 5‐methyl‐THF than FR‐α. 5‐Methyl‐THF can also enter the cell by carrier‐mediated transport via the ubiquitously expressed reduced folate carrier (RFC), although this has a lower affinity for 5‐methyl‐THF than FR‐α.
After entering the cell, 5‐methyl‐THF functions as a methyl donor for Hcy remethylation (Fig. 1). THF can directly be converted into 5,10‐methylene‐THF by the vitamin B6‐dependent enzyme serine hydroxymethyltransferase (SHMT). Conversion of THF into 5,10‐methylene‐THF via 10‐formyl‐THF and 5,10‐methenyl‐THF is catalyzed by the trifunctional enzyme methylene‐THF dehydrogenase (MTHFD), which exhibits formyl‐THF synthase, methenyl‐THF cyclohydrolase, and methenyl‐THF dehydrogenase activities (Hum et al. 1988). 10‐Formyl‐THF can act as a one‐carbon donor for the synthesis of purines. 5,10‐Methylene‐THF can donate a methylene group for the conversion of dUMP into dTMP. This reaction is catalyzed by thymidylate synthase (TYMS) and produces dihydrofolate (DHF), which is reduced back to THF by the action of DHF reductase (DHFR). 5,10‐Methylene‐THF can be further reduced to 5‐methyl‐THF by the riboflavin (vitamin B2)‐dependent enzyme methylene‐THF reductase (MTHFR). This enzyme is critical for the regulation of available 5‐methyl‐THF for Hcy remethylation.
Figure 1.
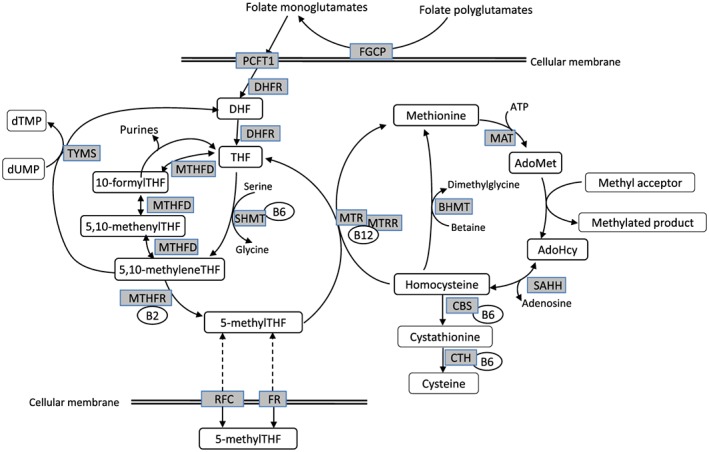
Simplified overview of the folate pathway and homocysteine metabolism. AdoHcy, S‐adenosylhomocysteine; AdoMet, S‐adenosylmethionine; BHMT, betaine‐homocysteine methyltransferase; CBS, cystathionine β‐synthase; CTH, γ‐cystathionase; DHF, dihydrofolate; FGCP, folyl poly‐γ‐glutamate carboxypeptidase; FR, folate receptor; MAT, methionine adenosyltransferase; MTHFD, methylenetetrahydrofolate dehydrogenase; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; MTRR, methionine synthase reductase; PCFT1, proton‐coupled folate transporter; RFC, reduced folate carrier; SAHH, S‐adenosylhomocysteine hydrolase; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate; B2, vitamin B2; B6, vitamin B6; B12, vitamin B12.
Remethylation of Hcy by methionine synthase (MTR) involves the donation of a methyl group from 5‐methyl‐THF to Hcy, leading to the formation of methionine and THF. MTR requires vitamin B12 (cobalamin) as a cofactor, and the resulting complex, Cbl(I)MTR, binds the methyl group of 5‐methyl‐THF to form methylcbl(III)MTR. The transfer of a methyl group to Hcy results in formation of the Cbl(I)MTR complex. The Cbl(I)MTR complex is also sensitive to oxidation into the active Cbl(II)MTR complex, which can be reactivated to the functional methylcbl(III)MTR by methionine synthase reductase (MTRR) using S‐adenosylmethionine (AdoMet) as a methyl donor. Whereas the MTR enzyme is ubiquitously expressed, another Hcy remethylation system, betaine‐Hcy methyltransferase (BHMT), is mainly expressed in the liver and kidneys.
Methionine adenosyltransferase (MAT) catalyzes the biosynthesis of AdoMet from methionine and ATP. AdoMet is the ultimate donor of methyl groups for methylation reactions; for example, those in DNA, RNA, proteins, and neurotransmitters. Each of these reactions produces S‐adenosylhomocysteine (AdoHcy), an allosteric inhibitor of methylation. AdoHcy is hydrolyzed to adenosine and Hcy by the enzyme AdoHcy hydrolase (SAHH). Because the equilibrium of this reversible reaction favors AdoHcy formation, Hcy and adenosine need to be metabolized to maintain low AdoHcy levels.
In the trans‐sulfuration pathway, Hcy is irreversibly metabolized to cysteine via reactions catalyzed by cystathionine β‐synthase (CBS) and γ‐cystathionase, both of which require vitamin B6 (pyridoxal phosphate) as a cofactor.
Genetic Polymorphisms Related to Folate and Thcy Metabolism and their Effects on Folate Status
In the folate and Hcy metabolic pathways (Fig. 1), MTHFR acts as a key enzyme, as are MTR and CBS, which require B vitamins as cofactors. The genes that encode the proteins/enzymes related to folate uptake and metabolism include many polymorphisms, among which MTHFR C677T, CBS 844ins68, GCPII H475Y, MTR A2756G, and MTRR A66G are known to affect folate and vitamin B12 intake (Uauy et al. 2014). Genetic polymorphisms related to the folate pathway have been shown to be associated with functional implications and specific conditions including NTDs (van der Linden et al. 2006), cardiovascular disease (Klerk et al. 2002), and some types of cancers, such as colorectal, breast, and lung cancers (Lee 2009). To date, most studies have shown that the MTHFR C677T genotype is related to biomarkers, such as serum folate, tHcy concentration, and folate intake. Other polymorphisms in the folate pathway have been found to be associated with a variety of complex traits and disorders including MTHFR A1298C, MTRR A66G, MTR A2756G, MTHFD1 G1958A, FOLH1 T484C, SLC19A1 A80G, and transcobalamin II (TCN2) C776G, the transport protein of vitamin B12 (Weisberg et al. 1998; Gaughan et al. 2001; Laverdiere et al. 2002; Kluijtmans et al. 2003; Dervieux et al. 2004; Martinelli et al. 2006; Moskau et al. 2007; Silva et al. 2011; Pangilinan et al. 2012; Xie et al. 2012). Table 1 summarizes the distribution of serum tHcy, folate, vitamin B6, and vitamin B12 according to polymorphisms related to folate and tHcy metabolism (Hiraoka et al. 2004; Hiraoka et al. 2009).
Table 1.
Distribution of serum tHcy, folate, vitamin B6, and vitamin B12 concentrations in healthy young Japanese women according to polymorphisms of folate metabolisms
| Polymorphism | % |
tHcy (μmol/L) |
Folate (nmol/L) |
Vitamin B6 (μmol/L) |
Vitamin B12 (pmol/L) |
|
|---|---|---|---|---|---|---|
| (Mean ± SD) | ||||||
| All subjects (n = 250) | 100.0 | 9.1 ± 2.7 | 18.1 ± 7.5 | 74.5 ± 65.8 | 450 ± 154 | |
|
MTHFR
C677T rs1801133 |
CC | 32.8 | 8.8 ± 2.0 | 20.3 ± 9.3 * | 66.1 ± 47.5 | 450 ± 138 |
| CT | 51.6 | 8.9 ± 2.0 | 17.3 ± 6.3 | 69.4 ± 46.7 | 448 ± 162 | |
| TT | 15.6 | 10.9 ± 4.7 * | 16.1 ± 5.7 | 107.3 ± 119.8 | 474 ± 156 | |
|
MTHFR
A1298C rs1801131 |
AA | 68.8 | 9.4 ± 3.0 | 17.6 ± 6.1 | 78.0 ± 70.5 | 452 ± 157 |
| AC | 29.6 | 8.6 ± 1.8 | 19.4 ± 10.1 | 68.0 ± 55.8 | 444 ± 151 | |
| CC | 1.6 | 8.2 ± 1.4 | 17.3 ± 3.8 | 49.6 ± 11.9 | 442 ± 24 | |
|
MTR
A2756G rs1805087 |
AA | 67.2 | 9.2 ± 2.2 | 17.9 ± 7.6 | 75.4 ± 66.5 | 459 ± 154 |
| AG | 29.2 | 9.3 ± 3.6 | 18.4 ± 6.9 | 69.0 ± 60.8 | 421 ± 139 | |
| GG | 3.6 | 7.8 ± 2.1 | 19.4 ± 11.1 | 113.5 ± 100.2 | 513 ± 220 | |
|
MTRR
A66G rs1801394 |
AA | 55.6 | 9.2 ± 3.1 | 18.2 ± 6.5 | 72.3 ± 51.1 | 454 ± 163 |
| AG | 35.9 | 9.2 ± 2.1 | 17.9 ± 6.6 | 84.6 ± 93.0 | 440 ± 142 | |
| GG | 3.6 | 8.8 ± 2.1 | 19.2 ± 14.9 | 52.2 ± 17.3 | 455 ± 147 | |
|
SLC19A1
A80G rs1051266 |
AA | 33.6 | 9.1 ± 2.6 | 17.4 ± 5.9 | 81.5 ± 71.5 | 438 ± 137 |
| GA | 46.0 | 9.2 ± 2.9 | 18.9 ± 8.9 | 67.0 ± 52.2 | 473 ± 164 | |
| GG | 20.4 | 9.1 ± 2.2 | 17.4 ± 6.1 | 78.0 ± 80.0 | 415 ± 148 | |
|
CBS
844ins68 |
DD | 99.6 | 9.2 ± 2.7 | 18.1 ± 7.5 | 74.5 ± 66.0 | 450 ± 154 |
| ID | 0.4 | 7.7 | 15.1 | 69.2 | 351 | |
|
GCPII H475Y rs202676 |
CC | 100.0 | ||||
The common C677T variant in the gene that encodes MTHFR is a C to T transition at position 677, which causes the substitution of alanine with valine. This substitution results in a mildly dysfunctional thermolabile MTHFR enzyme and leads to a 30% decrease in enzyme activity in heterozygotes and a 60% decrease in homozygotes (Frosst et al. 1995). The C677T variant exhibits enhanced loss of the FAD cofactor, creating a thermolabile protein (Yamada et al. 2001) and resulting in decreased 5‐methyl‐THF concentrations and increased tHcy concentrations.
In a study of Japanese individuals (ages 20–73 years, n = 170, TT: 11.8%) with high dietary folate intake (> 255 μg/day) by Nishio et al. (2008), age‐, sex‐, and energy‐adjusted regression analyses revealed that serum folate levels were significantly lower in individuals with the TT genotype than in those with the CC genotype (P = 0.01). They suggested that individuals with the TT genotype may need to consume more folate (approximately 1.4 times more) to maintain serum folate levels similar to those found in individuals with the 677CC/CT genotypes. Similar results were obtained in Japanese women (ages 20–22 years, n = 252, TT: 15.5%) by Hiraoka (2004). In individuals with folate intake above 200 μg/day, those with the TT genotype exhibited lower serum folate concentrations (P < 0.001) and higher serum tHcy concentrations (P < 0.001) than those with the CC genotype (Fig. 2). These data suggested that the recommended dietary allowance (RDA) of 240 μg/day for Japanese individuals may not be sufficient for individuals with the TT genotype to maintain serum folate levels and serum tHcy levels similar to those with the 677CC/CT genotype. Moreover, the folate requirement to keep the serum folate levels above 4.4 ng/mL (10 nM), the cutoff point for the appearance of functional pathologies associated with increases tHcy (Selhub et al. 2008), was evaluated based on the equation of plotted folate intake versus serum folate: Y (folate intake, μg/day) = 211.314X (logarithmic transformed serum folate, n) + 62.334. The obtained value was 330 μg, after multiplying by 1.2 as the safety margin; this was nearly equal to the mean folate intake. Another study (Taguchi et al. 2012) in young Japanese women (ages 15.4 ± 0.1 years, n = 192, TT: 16.1%) also showed that the MTHFR C677T polymorphism influenced folate and tHcy status, similar to that in adults, even if the mean concentration of serum folate (4.8 ng/mL, 10.8 nM) in individuals with the TT genotype was lower than that of the overall Japanese population.
Figure 2.
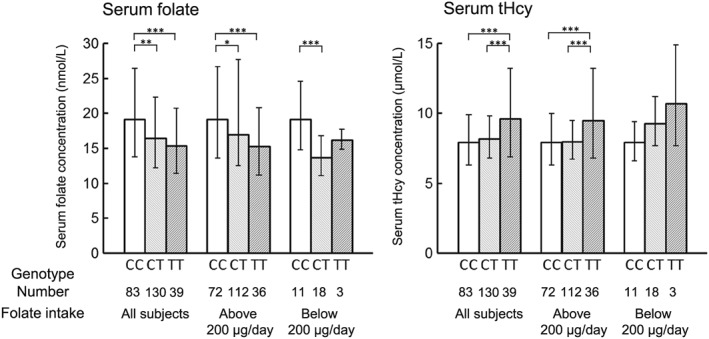
Distribution of serum folate and tHcy concentrations by folate intake according to MTHFR C677T genotype in young Japanese women (n = 252). The value of 200 μg/day represents the RDA of folate established according to the 6th edition of the RDA for Japanese to be used from April 2000 to March 2005. Values are the geometric mean and bars transformed from the logarithmic‐transformed values of the mean ± SD. Based on data from Hiraoka (2004). Significant difference between genotypes at P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***) for Bonferroni post hoc test of analysis of variance.
Tsang et al. (2015) performed a meta‐analysis of the association between the MTHFR C677T polymorphism and blood folate concentrations among women of reproductive age (12–49 years); the percent differences in blood folate concentrations measured by microbiological assays (MAs) between genotypes showed a clear pattern of CC > CT > TT (Table 2). The percent differences in plasma folate were greatest for CC > TT (13%), followed by CC > CT (7%) and CT > TT (6%), and those in RBC folate were in the order of CC > TT (16%), CC > CT (8%), and CT > TT (9%). Plasma folate concentrations measured with protein‐binding assays (PBAs) also showed this pattern, albeit to a greater extent (e.g., CC > TT: 20%). In contrast, RBC folate concentrations measured with PBAs exhibited a clear reverse pattern of CC < CT < TT, as shown by the negative estimated percent differences for genotype comparisons of CC versus TT and CT and CT versus TT. These data supported previous studies, which also suggested that PBAs may not reflect true whole blood folate concentrations without adjustments for differential folate recovery and MTHFR C677T genotype (Fazili et al. 2008). Differential recoveries across assay methods and differential distributions across MTHFR C677T genotypes for individual folate species could result in substantial variations in total blood concentrations. MTHFR C677T polymorphisms have been shown to alter the mix of circulating folate species in RBCs. Median 5‐methyl‐THF concentrations in individuals with the CC genotype have been reported as 80–100% for RBC folate; in contrast, those in individuals with the TT genotype are only 58–70%. Formyl‐THF species have been observed at up to 59% of the total in those with the TT genotype (Bagley and Selhub 1998). However, no differences in folate species by genotype were found for serum folate (Fazili et al. 2008).
Table 2.
Blood folate concentrations in women aged 12–49y by MTHFR C677T genotypes and assay method from results of the meta‐analysis
| Genotype | Serum/Plasma folate, nmol/L | RBC folate, nmol/L | ||
|---|---|---|---|---|
|
MA (n = 1590) |
PBA (n = 10 283) |
MA (n = 1590) |
PBA (n = 1146) |
|
| CC | 15 (10, 25) | 15 (14, 17) | 694 (546, 939) | 468 (304, 687) |
| CT | 14 (10, 23) | 14 (12, 15) | 639 (504, 863) | 484 (315, 708) |
| TT | 13 (9, 22) | 12 (11, 13) | 579 (455, 783) | 531 (345, 783) |
|
Concentration pattern |
CC > CT > TT | CC > CT > TT | CC > CT > TT | CC < CT < TT |
Values are the estimated median (95% CrI). 95% Equal tailed credible intervals (CrI) defined by the 2.5th and 97.5th percentiles of the posterior distributions for the estimated values. MA, microbiological assay; PBA, protein‐binding assay;
Adapted from Tsang et al. (2015).
Protein‐binding assays rely on folate binding protein (FBP) for detection of the various folate species. Depending on their source, FBPs may have different binding affinities for different folate species. MAs completely recovered folates added to whole blood hemolysates, except for THF, which was recovered at 46.4%. However, PBAs showed poor recovery of 5‐methyl‐THF (51%) and 5‐formyl‐THF (18%) and excellent recovery of THF (152%) (Fazili et al. 2008). Tsang et al. (2015) suggested that researchers should use caution when interpreting RBC folate concentrations assessed by PBAs. Standardizing blood folate assay methods is necessarily in order to avoid compromising interpretation.
The prevalence of the MTHFR 677TT genotype varies across ethnic groups and regions. In Europe, Asia, Central America, and South America, the prevalence of the MTHFR 677TT genotype ranges from 10% to 32%, whereas different African populations have a prevalence of only 0–3% (Wilcken et al. 2003). Binia et al. (2014) reported that the prevalence rates of the MTHFR 677TT genotype were 25% and 57% in Mexican Mestizo and American‐Indian populations, respectively. Yang et al. (2013) showed regional differences in the frequencies of this genotype among Chinese Han populations; the frequencies of the 677TT genotype were significantly higher among northern populations and ranged from 6.4% in Hainan (southern) to 40.8% in Shandong (northern). In Japanese populations, the frequency of the 677TT genotype was found to be approximately 15% (Sadewa et al. 2002; Hiraoka 2004). Thus, these data suggested that the estimated average requirement for folate and the necessity of folic acid and vitamin B supplementation may be increased in some regions around the world.
Response of Folate Status to Folate Intake and Supplementation
Folate status can be assessed using either serum/plasma folate or RBC folate concentrations, as short‐term and long‐term indicators, respectively. Clinical folate deficiency (associated with megaloblastic anemia) is generally defined as RBC folate concentrations of less than 135 ng/mL (305 nM) and serum concentrations of less than 3.1 ng/mL (7.0 nM) (Green 2008). The cutoff points for the appearance of functional pathologies associated with increased tHcy are greater than 4.4 ng/mL (10 nM) folate in serum and greater than 150 ng/mL (340 nM) folate in RBCs (Selhub et al. 2008). However, RBC folate concentrations should be above 400 ng/mL (906 nM) in women of reproductive age, in order to achieve the greatest reduction of NTDs (Daly et al. 1995; WHO 2015).
Marchetta et al. (2015) determined the associations between folate intake from natural food alone and blood folate concentrations using a meta‐analysis of studies identified through a systematic literature review (Fig. 3). A 10% increase in natural food folate intake was associated with a 6–7% increase in both serum and RBC folate concentrations. Using modeled results, they estimated that a folate intake of 450 μg dietary folate equivalents (DFE)/day or higher from natural food could achieve the lowest bound of RBC folate concentrations (~1050 nM) associated with a low risk of NTDs.
Figure 3.
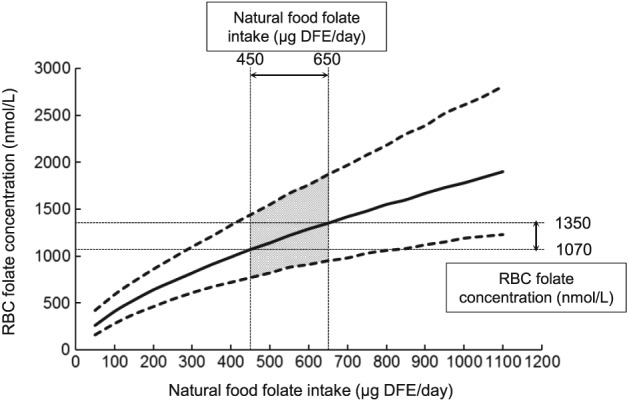
Natural food folate intake (μg/day of DFE) and associated RBC folate concentrations (nmol/L) based on Bayesian modeling of the association between natural food folate intake and RBC folate concentration as analyzed by microbiological assay. Solid line represents the median value under the assumed model. Dotted lines represent the 95% credible interval. The shaded area (natural food intakes between 450 μg DFE/day and 650 g DFE/day) refer to the range of intakes and RBC folate concentrations associated with the lowest population risk for a NTD according to Crider et al. (2014). Based on data from Marchetta et al. (2015). DFE, dietary folate equivalent.
Both environmental and genetic factors contribute to the development of NTDs. Significantly lower levels of folate are observed in pregnant mothers whose fetuses exhibit NTDs (Kirke et al. 1993) and preconception folic acid supplementation has led to a significant reduction in the risk of NTDs by up to 75% (MRC Vitamin Study Research Group 1991; Czeizel and Dudás 1992; Berry et al. 1999). Genetic factors also contribute to the occurrence of NTDs. The most widely studied genetic risk factor is MTHFR C677T polymorphisms. Indeed, a significantly higher frequency of the MTHFR 677TT genotype has been observed in cases of NTDs in many populations (Botto and Yang 2000).
The first government‐mandated folic acid fortification program was implemented in the United States of America (USA) beginning in 1998; 79 other countries have also implemented similar programs as of November 2016 (Food Fortification Initiative. 2017). The level of fortification differs among countries; however, in all cases, these programs are designed to reduce the prevalence of pregnancies with NTDs. In the USA and Canada, these folic supplementation programs were thought to be successful because the population folate status was improved (Ray et al. 2002) and reduced rates of NTDs were noted (Honein et al. 2001; Williams et al. 2002), although the results varied depending on ethnicity (Williams et al. 2005). Data from the USA reported from the NHANES trial (Pfeiffer et al. 2012) showed that the mean concentration of serum and RBC folate increased dramatically from the prefortification period (1988–1994) to the postfortification period (1990–2010), from 16.7 ± 0.5 to 41.0 ± 0.3 nM and from 747 ± 10 to 1120 ± 7 nM, respectively, resulting in a 31% reduction in the occurrence of NTDs (Williams et al. 2002).
Several studies have reported the effects of the MTHFR C677T genotype on the response to folic acid supplementation. A large, population‐based double‐blind folic intervention trial was conducted in northern China by Crider et al. (2011). They examined the response of plasma and RBC folate concentrations and plasma tHcy concentrations to consumption of 100, 400, or 4000 μg folic acid/day or to a single dose of 4000 μg folic acid/week during 3‐ and 6‐month periods after discontinuation of supplementation and assessed responses according to the MTHFR C677T genotype. Northern Chinese women (n = 932) of childbearing age were enrolled. Plasma and RBC folate and tHcy concentrations were shown to be associated with the MTHFR C677T genotype throughout the supplementation trial, regardless of folic acid dose. Within each folic acid dose, the trend of plasma folate concentrations was CC > CT > TT, and that of RBC folate concentrations was CC > TT. Anderson et al. (2013) reported a randomized, double‐blind, controlled, crossover study of different doses of folic acid supplementation and a 30‐week washout period. Volunteers (n = 142; ages 18–69 years) were randomized to receive two of three doses (0, 200, or 400 μg/day) of folic acid for a 12‐week period. Serum folate concentrations were responsive to modest increases in folic acid intake, and increases in RBC folate concentrations with 400 μg folic acid supplementation occurred within each MTHFR C677T genotype, with individuals with the 677TT genotype having larger responses than those with the CC or CT genotype. Our group (Hiraoka et al. 2004) reported the effects of controlled folate intake among Japanese women (n = 100) with various combinations of the four single nucleotide polymorphisms (SNPs) associated with folate metabolism; i.e., MTHFR C677T, MTHFR A1298C, MTR A2756G, and SLC19A1 A80G. Although there were significant differences in serum folate and tHcy concentrations in individuals with the MTHFR C677T genotype at baseline, supplementation with 400 μg/day folic acid for 4 weeks eliminated these differences in individuals with the MTHRF C677T genotype (Fig. 4). The effects of the three other SNPs were not as apparent. In a randomized, double‐blind, placebo‐controlled study of healthy male Japanese workers, Miyaki et al. (2005) showed that folic acid supplementation significantly lowered tHcy concentrations for all MTHFR C677T genotypes, and the degree to which the tHcy concentration was lowered from baseline was almost the same at 1 and 3 months after supplementation with 1 mg folic acid/day. The effect size of tHcy reduction in the TT genotype was estimated to be 2.4‐fold compared with that in the CC genotype. The inconsistent response of serum and RBC folate or tHcy could be explained by the observation that the baseline folate status appeared to influence changes in these markers (Farrell et al. 2013). These metabolic data regarding folate in individuals with SNPs should be useful for the development of personalized nutrition programs to prevent cardiovascular diseases and dementia.
Figure 4.
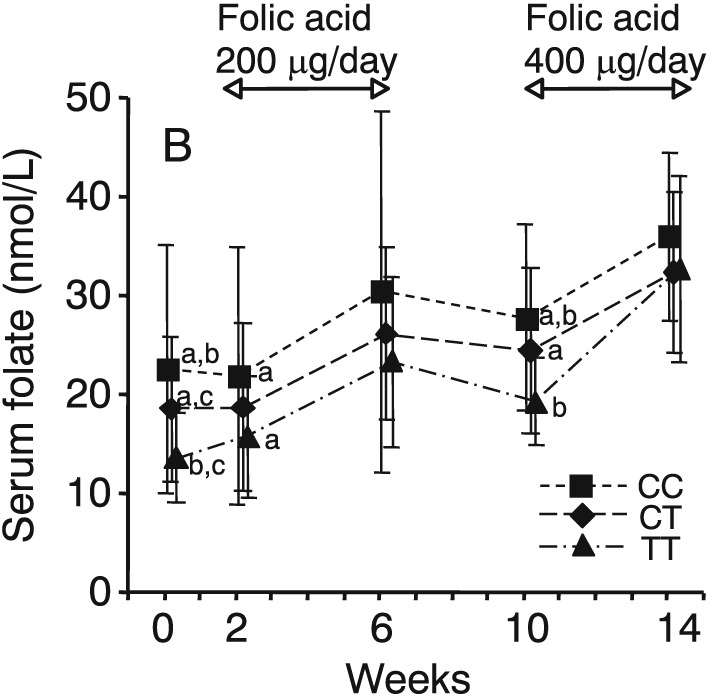
Serum folate concentrations in healthy young Japanese women with differing MTHFR C677T genotypes (CC, n = 36; CT, n = 47; TT, n = 17) at baseline and before and after supplementation with 200 and 400 μg/day folic acid. a, b, c MTHFR genotypes with the same superscripts differ significantly; P < 0.05. Reproduced from Hiraoka et al. (2004) with permission.
Personalized Nutritional Intervention in Folate Status for Individuals with the MTHFR C677T Genotype
As described above, folic acid supplementation (400 μg/day) can help overcome the negative health effects of MTHRF C677T polymorphisms. Our group implemented the health promotion program designated the “Sakado Folate Project”, which aimed to achieve personalized nutritional intervention based on the MTHFR C677T genotype for the prevention of various diseases including cardiovascular diseases and dementia (Kagawa et al. 2017). The project was implemented by a local government in Sakado city and Kagawa Nutrition University, Japan from 2006 to 2015. Participants (n = 836, including 179 men and 657 women, age: 62 ± 10 years) were notified of their MTHFR C677T genotype and given nutritional guidance. Significant increases in serum folate concentrations and significant decreases in serum tHcy concentrations were observed at 4 months and 1 year after the onset of this intervention. In individuals with the TT genotype, these changes were particularly obvious and intake of green leafy vegetables, which are rich in folate, was increased in individuals with the TT genotype. An increase in the consumption of folic acid‐fortified foods was also observed. These results suggested that intervention of folate status by notification of genotype was effective in motivating individuals to change their lifestyle and improve nutrition status. Similar results were reported in a randomized control trial demonstrating that genotype‐based personalized dietary advice was understood better and was more likely to be followed than general dietary advice (Nielsen and El‐Sohemy 2012).
Conclusion
MTHFR C677T polymorphisms are major factors influencing folate status. Individuals with the TT genotype have lower serum folate concentrations and higher serum tHcy concentrations than those with the CC genotype; hence, folate intake of 400 μg/day above the Japanese current RDA (240 μg/day) is recommended to maintain serum folate and Hcy levels similar to those found in individuals with the 677CC/CT genotypes. Intervention of folate status based on personalized nutrition by notifying individuals of their genotype could be effective in motivating individuals to change their lifestyle and improve their nutrition status, particularly in individuals with the TT genotype. Folic acid‐fortified foods may allow individuals to more easily consume adequate folate to prevent diseases.
Disclosure
None.
Acknowledgments
This work was supported by JSPS KAKENHI (grant number: JP16K00865).
Hiraoka, M. , and Kagawa, Y. (2017) Genetic polymorphisms and folate status. Congenital Anomalies, 57: 142–149. doi: 10.1111/cga.12232.
References
- Anderson CA, Beresford SA, McLerran D et al. 2013. Response of serum and red blood cell folate concentrations to folic acid supplementation depends on methylenetetrahydrofolate reductase C677T genotype: results from a crossover trial. Mol Nutr Food Res 57:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley PJ, Selhub J. 1998. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci U S A 95:13217–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry RJ, Li Z, Erickson JD, Li S et al. 1999. Prevention of neural‐tube defects with folic acid in China. China‐U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med 341:1485–1490 Erratum in: N Engl J Med 1999;341:1864. [DOI] [PubMed] [Google Scholar]
- Binia A, Contreras AV, Canizales‐Quinteros S, Alonzo VA, Tejero ME, Silva‐Zolezzi I. 2014. Geographical and ethnic distribution of single nucleotide polymorphisms within genes of the folate/homocysteine pathway metabolism. Genes Nutr 9:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottiglieri T. 2005. Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry 29:1103–1112. [DOI] [PubMed] [Google Scholar]
- Botto LD, Yang Q. 2000. 5,10‐Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol 151:862–877. [DOI] [PubMed] [Google Scholar]
- Brody T, Shane B. 2001. Folic acid In: Rucker RB, Suttie JW, McCormic DB, Machin LJ, editors. Handbook of Vitamins. New York: Marcel Dekker, Inc. p 427–462. [Google Scholar]
- Chandler CJ, Harrison DA, Buffington CA, Santiago NA, Halsted CH. 1991. Functional specificity of jejunal brush‐border pteroylpolyglutamate hydrolase in pig. Am J Physiol 260:G865–G872. [DOI] [PubMed] [Google Scholar]
- Crider KS, Zhu JH, Hao L et al. 2011. MTHFR 677C‐>T genotype is associated with folate and homocysteine concentrations in a large, population‐based, double‐blind trial of folic acid supplementation. Am J Clin Nutr 93:1365–1372. [DOI] [PubMed] [Google Scholar]
- Crider KS, Devine O, Hao L et al. 2014. Population red cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ 349:g4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE, Dudás I. 1992. Prevention of the first occurrence of neural‐tube defects by periconceptional vitamin supplementation. N Engl J Med 327:1832–1835. [DOI] [PubMed] [Google Scholar]
- Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. 1995. Folate levels and neural tube defects. Implications for prevention JAMA 274:1698–1702. [DOI] [PubMed] [Google Scholar]
- Dervieux T, Kremer J, Lein DO et al. 2004. Contribution of common polymorphisms in reduced folate carrier and gamma‐glutamylhydrolase to methotrexate polyglutamate levels in patients with rheumatoid arthritis. Pharmacogenetics 14:733–739. [DOI] [PubMed] [Google Scholar]
- Farrell CJ, Kirsch SH, Herrmann M. 2013. Red cell or serum folate: what to do in clinical practice? Clin Chem Lab Med 51:555–569. [DOI] [PubMed] [Google Scholar]
- Fazili Z, Pfeiffer CM, Zhang M, Jain RB, Koontz D. 2008. Influence of 5,10‐methylenetetrahydrofolate reductase polymorphism on whole‐blood folate concentrations measured by LC‐MS/MS, microbiologic assay, and Bio‐Rad radioassay. Clin Chem 54:197–201. [DOI] [PubMed] [Google Scholar]
- Frosst P, Blom HJ, Milos R et al. 1995. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–113. [DOI] [PubMed] [Google Scholar]
- Gaughan DJ, Kluijtmans LA, Barbaux S et al. 2001. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis 157:451–456. [DOI] [PubMed] [Google Scholar]
- Green R. 2008. Indicators for assessing folate and vitamin B12 status and for monitoring the efficacy of intervention strategies. Food Nutr Bull 29:S52–S63 discussion S64‐66. [DOI] [PubMed] [Google Scholar]
- Hiraoka M. 2004. Folate intake, serum folate, serum total homocysteine levels and methylenetetrahydrofolate reductase C677T polymorphism in young Japanese women. J Nutr Sci Vitaminol 50:238–245. [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Kato K, Saito Y, Yasuda K, Kagawa Y. 2004. Gene‐nutrient and gene‐gene interactions of controlled folate intake by Japanese women. Biochem Biophys Res Commun 316:1210–1216. [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Kageyama M, Yurimoto M et al. 2009. Tailor‐made nutrition based on polymorphisms of folate metabolism: Sakado Folate Projects. Vitamins 83:26–274 (in Japanese.) [Google Scholar]
- Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. 2001. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 285:2981–2986 Erratum in: JAMA 2001;286:2236. [DOI] [PubMed] [Google Scholar]
- Hum DW, Bell AW, Rozen R, MacKenzie RE. 1988. Primary structure of a human trifunctional enzyme. Isolation of a cDNA encoding methylenetetrahydrofolate dehydrogenase‐methenyltetrahydrofolate cyclohydrolase‐formyltetrahydrofolate synthetase. J Biol Chem 263:15946–15950. [PubMed] [Google Scholar]
- Iida K, Tomita K, Okada R et al. 2009. Applicability of allele/genotype frequency from documented controls for case‐control studies on genotypes among Japanese: MTHFR C677T as an example. Asian Pac J Cancer Prev 10:231–236. [PubMed] [Google Scholar]
- Kagawa Y, Hiraoka M, Kageyama M et al. 2017. Medical cost savings in Sakado City and worldwide achieved by preventing disease by folic acid fortification. Congenit Anom [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. 1993. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med 86:703–708. [PubMed] [Google Scholar]
- Klerk M, Verhoef P, Clarke R et al., MTHFR Studies Collaboration Group . 2002. MTHFR 677C‐‐>T polymorphism and risk of coronary heart disease: a meta‐analysis. JAMA 288:2023–2031. [DOI] [PubMed] [Google Scholar]
- Kluijtmans LA, Young IS, Boreham CA et al. 2003. Genetic and nutritional factors contributing to hyperhomocysteinemia in young adults. Blood 101:2483–2488. [DOI] [PubMed] [Google Scholar]
- Laverdiere C, Chiasson S, Costea I, Moghrabi A, Krajinovic M. 2002. Polymorphism G80A in the reduced folate carrier gene and its relationship to methotrexate plasma levels and outcome of childhood acute lymphoblastic leukemia. Blood 100:3832–3834. [DOI] [PubMed] [Google Scholar]
- Lee SA. 2009. Gene‐diet interaction on cancer risk in epidemiological studies. J Prev Med Public Health 42:360–370. [DOI] [PubMed] [Google Scholar]
- Marchetta CM, Devine OJ, Crider KS et al. 2015. Assessing the association between natural food folate intake and blood folate concentrations: a systematic review and Bayesian meta‐analysis of trials and observational studies. Forum Nutr 7:2663–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli M, Scapoli L, Palmieri A et al. 2006. Study of four genes belonging to the folate pathway: transcobalamin 2 is involved in the onset of non‐syndromic cleft lip with or without cleft palate. Hum Mutat 27:294. [DOI] [PubMed] [Google Scholar]
- Moskau S, Farmand S, Semmler A et al. 2007. The methionine synthase polymorphism c.2756A > G (D919G) influences diastolic blood pressure. J Hum Hypertens 21:418–420. [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group . 1991. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group Lancet 338:131–137. [PubMed] [Google Scholar]
- Nielsen DE, El‐Sohemy A. 2012. A randomized trial of genetic information for personalized nutrition. Genes Nutr 7:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangilinan F, Molloy AM, Mills JL et al. 2012. Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects. BMC Med Genet 13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer CM, Hughes JP, Lacher DA et al. 2012. Estimation of trends in serum and RBC folate in the U.S. population from pre‐ to postfortification using assay‐adjusted data from the NHANES 1988‐2010. J Nutr 142:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Jansen M, Sakaris A et al. 2006. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127:917–928. [DOI] [PubMed] [Google Scholar]
- Ray JG, Vermeulen MJ, Boss SC, Cole DE. 2002. Increased red cell folate concentrations in women of reproductive age after Canadian folic acid food fortification. Epidemiology 13:238–240. [DOI] [PubMed] [Google Scholar]
- Sadewa AH, Sunarti SR, Hayashi C et al. 2002. The C677T mutation in the methylenetetrahydrofolate reductase gene among the Indonesian Javanese population. Kobe J Med Sci 48:137–144. [PubMed] [Google Scholar]
- Selhub J, Jacques PF, Dallal G, Choumenkovitch S, Rogers G. 2008. The use of blood concentrations of vitamins and their respective functional indicators to define folate and vitamin B12 status. Food Nutr Bull 29:S67–S73. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J et al. 2002. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med 346:476–483. [DOI] [PubMed] [Google Scholar]
- Silva LM, Silva JN, Galbiatti AL et al. 2011. Head and neck carcinogenesis: impact of MTHFD1 G1958A polymorphism. Rev Assoc Med Bras 57:194–199. [DOI] [PubMed] [Google Scholar]
- Smithells RW, Sheppard S, Schorah CJ. 1976. Vitamin deficiencies and neural tube defects. Arch Dis Child 51:944–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T, Mori H, Hamada A, Yamori Y, Mori M. 2012. Serum folate, total homocysteine levels and methylenetetrahydrofolate reductase 677C>T polymorphism in young healthy female Japanese. Asia Pac J Clin Nutr 21:291–295. [PubMed] [Google Scholar]
- The Homocysteine Studies Collaboration . 2002. Homocysteine and risk of ischemic heart disease and stroke: a meta‐analysis. JAMA 288:2015–2022. [DOI] [PubMed] [Google Scholar]
- Tsang BL, Devine OJ, Cordero AM et al. 2015. Assessing the association between the methylenetetrahydrofolatereductase (MTHFR) 677C>T polymorphism and blood folate concentrations: a systematic review and meta‐analysis of trials and observational studies. Am J Clin Nutr 101:1286–1294. [DOI] [PubMed] [Google Scholar]
- Uauy R, Hawkesworth S, Dangour AD. 2014. Food‐based dietary guidelines for healthier populations: international considerations In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Modern Nutrition in Health and Disease, 11th edn. Philadelphia, PA, USA: Lippincott Williams & Wilkins; p 1518–1532. [Google Scholar]
- van der Linden IJ, Afman LA, Heil SG, Blom HJ. 2006. Genetic variation in genes of folate metabolism and neural‐tube defect risk. Proc Nutr Soc 65:204–215. [DOI] [PubMed] [Google Scholar]
- Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. 1998. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 64:169–172. [DOI] [PubMed] [Google Scholar]
- Wilcken B, Bamforth F, Li Z et al. 2003. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. J Med Genet 40:619–625 Erratum in: J Med Genet 2004;41:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LJ, Mai CT, Edmonds LD. 2002. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology 66:33–39. [DOI] [PubMed] [Google Scholar]
- Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. 2005. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995‐2002. Pediatrics 116:580–586. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2015. Guideline: Optimal Serum and Red Blood Cell Folate Concentrations in Women of Reproductive Age for Prevention of Neural Tube Defects. Geneva: World Health Organization. [PubMed] [Google Scholar]
- Xie H, Guo J, Wang J et al. 2012. Glutamate carboxypeptidase II gene polymorphisms and neural tube defects in a high‐risk Chinese population. Metab Brain Dis 27:59–65. [DOI] [PubMed] [Google Scholar]
- Yamada K, Chen Z, Rozen R, Matthews RG. 2001. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Natl Acad Sci U S A 98:14853–14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Liu Y, Li Y et al. 2013. Geographical distribution of MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in China: findings from 15357 adults of Han nationality. PLoS One 8 e57917. [DOI] [PMC free article] [PubMed] [Google Scholar]


