Abstract
Treatment with high-dose melphalan chemotherapy supported by hematopoietic rescue with autologous stem cells produces high rates of hematologic responses and improvement in survival and organ function for patients with AL amyloidosis. Ongoing clinical trials explore pre-transplant induction regimens, post-transplant consolidation or maintenance approaches, and compare transplant to non-transplant regimens. To put these studies into context, we reviewed our recent experience with transplant for AL amyloidosis in the Amyloid Treatment and Research Program at Boston Medical Center and Boston University School of Medicine. Over the past 10 years, there was a steady reduction in rates of treatment-related mortality and improvement in 1-year survival, now approximately 5% and 90%, respectively, based upon an intention-to-treat analysis. Median overall survival of patients treated with this approach at our center exceeds 7.5 years.
Introduction
Treatment of AL amyloidosis patients with high-dose intravenous melphalan chemotherapy and autologous hematopoietic stem cell support (HDM/SCT) produces a high rate of hematologic complete responses and improvement in organ function. Nephrotic syndrome can resolve [1], myocardial wall thickness can decline (Meier-Ewert et al., this volume), and quality of life improves [2]. Good outcomes are dependent upon the safe application of the therapy. Our first large patient series, published in 2004, reported a 100 day treatment-related mortality (TRM) of 14% [3]. Since then, we have refined patient selection and transplantation techniques and seen a concomitant decline in the rate of serious complications.
Methods
Data on patients evaluated and treated in the Amyloid Treatment and Research Program at Boston University School of Medicine and Boston Medical Center were collected under protocols approved by the Boston University Medical Campus IRB after informed consent was signed. Data on patients enrolled into protocols using HDM/SCT from January 2000–December 2009 were reviewed retrospectively. Survival of all patients was determined through the end of December 2010, providing at least 1 year of follow-up on 100% of patients. Kaplan-Meier survival and confidence intervals for median overall and event-free survival were determined.
Results and discussion
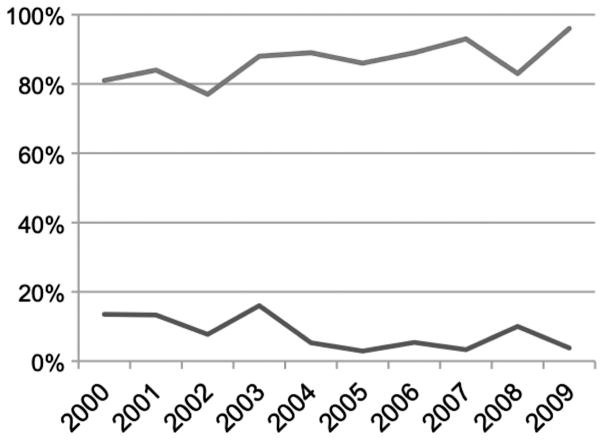
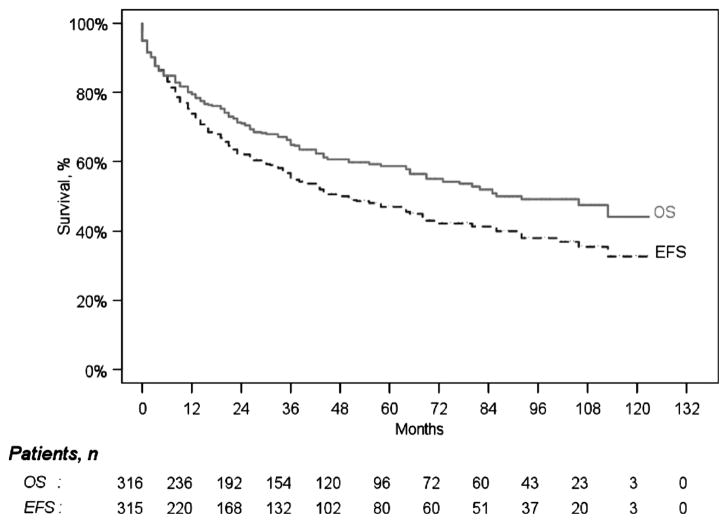
In the 10-year-period from January 2000 through December 2009, 323 patients began treatment on institutional or cooperative group protocols using HDM/SCT. The age range of enrolled patients was 28–80 years (median 57). The overall TRM was 8.4%, and the 1-year survival (1 YS) was 86.1% by intention-to-treat analysis. In the more recent 5-year-period from January 2005 through December 2009, 158 patients were treated; the age range was 28–77 years (median 57), the TRM was 5.1%, and the 1YS was 89.2%. Annual trends are plotted in Figure 1. The overall survival and progression free survival of all 323 patients are plotted in Figure 2.
Figure 1.
Trends in TRM (lower line) and 1YS (upper line) each year in the 10-year-period from 2000 through the end of 2009, for a total of 323 patients.
Figure 2.
Overall and event-free survival for 323 patients based upon intention-to-treat. The median overall survival was 7.7 years (95% CI: 69–120 months) and the event-free survival was 4.2 years (95% CI: 36–69 months).
We attribute these improvements to two factors: firstly, rigorous selection of appropriate patients; and secondly, experienced multidisciplinary management during the peri-transplant period.
Appropriate patient selection requires a comprehensive multidisciplinary clinical evaluation. The key evaluation components are summarized below. Chronological age is not a critical factor [4]. Patients who are potential candidates for HDM/SCT are assessed by sub-specialists highly familiar with the clinical manifestations of amyloidosis and with the complications of HDM/SCT.
Cardiopulmonary function
Many studies have demonstrated that outcomes for patients with AL amyloidosis are driven by cardiac disease, defined by clinical parameters, echocardiographic parameters, and biomarkers for cardiac function. We use all of these to judge cardiac risk, including careful review of symptoms, physical examination for signs of heart failure, electrocardiography and arrhythmia screening, assessment of ejection fraction by echocardiography/and or cardiac magnetic resonance imaging, walking oximetry, pulmonary function testing, and cardiopulmonary exercise testing. The cardiologist must integrate these data to assess risk for HDM/SCT. Patients with cardiac dysfunction at high risk of early mortality may need to be evaluated for combined orthotopic heart transplantation and HDM/SCT if other organ dysfunction does not preclude this possibility (Chung et al., submitted) [5]. During the 10-year-period under consideration, the proportion of patients with cardiac involvement was consistently 45–50%.
Renal function
Renal dysfunction in amyloidosis can present in two ways, either as proteinuria, and/or azotemia. In our experience, renal failure itself does not preclude safe treatment with HDM/SCT [5]. However, nephrotic syndrome can predispose to hypotension, anasarca, and to the development of pleural and pericardial effusions that increase risk of complications during HDM/SCT. Diuretics are the mainstay of management for symptoms related to nephrotic syndrome and amyloid-induced congestive heart failure, but not effective in all patients.
Gastrointestinal function and nutrition
These factors have been difficult to quantify, but are clearly important. Patients with amyloid deposition in the GI tract with ulceration or active bleeding are at high risk of catastrophic bleeding during treatment-related pancytopenia. Patients with malabsorption, GI dysmotility, and poor nutrition tend to have poor performance status and difficulty recovering from infectious and other complications of HDM/SCT, and also should be excluded from this treatment.
Neurologic function
Significant peripheral neuropathy affects performance status and raises treatment risk. More importantly, autonomic dysfunction leading to hypotension can predispose to morbidity and mortality during stem cell mobilization and collection as well as after chemotherapy.
Psychosocial factors
A commitment to self-care, and conscientious caregiver support, are important contributors to successful treatment. Patients and caregivers must be committed to following instructions for medications, nutrition, exercise, and monitoring for signs of infection or other complications, particularly in an outpatient transplant program. Adherence to the plan of care can be achieved with detailed instructions and treatment calendars for patients and caregivers, and with support of social workers and psychiatrists through a very stressful period.
Peri-transplant management
The transplant team must assess the patient and respond to any changes in clinical condition throughout the peri-transplant period. For patients with other blood diseases undergoing stem cell transplantation, monitoring focuses upon infection or bleeding complications necessitating appropriate antibiotic and blood product support. For patients with organ dysfunction due to AL amyloidosis, additional monitoring is required to detect and respond appropriately to changes in cardiac, renal, GI, or neurologic function during transplant. Careful attention to fluid balance to maintain a constant weight and avoidance of drugs known to exacerbate cardiac and renal dysfunction in AL amyloidosis are some of the critical issues. During stem cell mobilization and collection, diuretic requirements often increase. During the administration of high-dose melphalan, oral cryotherapy with ice reduces blood flow to the oral mucosa and minimizes mucositis. Post-transplant, insensible losses increase dramatically, and diuretics must be reduced or eliminated (and intravenous fluids administered) to maintain blood pressure and prevent dehydration. Patients should be assessed daily throughout the transplant period by specialized amyloid transplant nurses and doctors, and by amyloid sub-specialists as needed. That being said, we believe that performing transplants on an outpatient basis minimizes exposure to resistant hospital organisms and also allows patients to adhere to medication and activity protocols that can be at odds with the protocols on inpatient units. Recently, benefits of outpatient transplantation have been demonstrated for other diseases [6].
These results and comparable results from other centers leads to a set of provisional conclusions [1]. Patients should be carefully selected for treatment with HDM/SCT through a multi-disciplinary evaluation process. Treatment decisions should be made on a highly personalized basis, within the context of available clinical trials whenever possible [2]. Centers providing HDM/SCT, and other treatments, to patients with AL amyloidosis should have the volume and experience to provide expert multidisciplinary care and to follow carefully developed standard operating procedures [3]. Incorporation of HDM/SCT into a treatment algorithm, and comparison of HDM/SCT to other therapies, is scientifically and ethically justified when morbidity and mortality is minimized using these or other strategies.
Acknowledgments
We acknowledge and honor the participation of our patients in research and clinical trials. The Amyloid Treatment and Research Program patient database has been maintained recently by Kip Bodi and Anna Badiee, with support of NIH grant P01 HL68705. Patient evaluations and insurance approval for HDM/SCT are coordinated by Janis Johnson, Natasha Yancey, and Carol Antonelli. In addition to the authors, clinical care is provided by highly skilled chemotherapy and pheresis nurses and staff and physicians of Boston Medical Center.
References
- 1.Dember LM, Sanchorawala V, Seldin DC, Wright DG, Lavalley M, Berk JL, Falk RH, Skinner M. Effect of dose-intensive intravenous melphalan and autologous blood stem-cell transplantation on al amyloidosis-associated renal disease. Ann Intern Med. 2001;134:746–753. doi: 10.7326/0003-4819-134-9_part_1-200105010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Seldin DC, Anderson JJ, Sanchorawala V, Malek K, Wright DG, Quillen K, Finn KT, Berk JL, Dember LM, Falk RH, et al. Improvement in quality of life of patients with AL amyloidosis treated with high-dose melphalan and autologous stem cell transplantation. Blood. 2004;104:1888–1893. doi: 10.1182/blood-2004-01-0089. [DOI] [PubMed] [Google Scholar]
- 3.Skinner M, Sanchorawala V, Seldin DC, Dember LM, Falk RH, Berk JL, Anderson JJ, O’Hara C, Finn KT, Libbey CA, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85–93. doi: 10.7326/0003-4819-140-2-200401200-00008. [DOI] [PubMed] [Google Scholar]
- 4.Seldin DC, Anderson JJ, Skinner M, Malek K, Wright DG, Quillen K, Finn K, Oran B, Sanchorawala V. Successful treatment of AL amyloidosis with high-dose melphalan and autologous stem cell transplantation in patients over age 65. Blood. 2006;108:3945–3947. doi: 10.1182/blood-2006-06-029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dey BR, Chung SS, Spitzer TR, Zheng H, Macqillivray TE, Seldin DC, McAfee S, Ballen K, Attar E, Wang T, et al. Cardiac transplantation followed by dose-intensive melphalam and autologous stem-cell transplantation for light-chain amyloidosis and heart failure. Transplantation. 2010;90:905–911. doi: 10.1097/TP.0b013e3181f10edb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casserly LF, Fadia A, Sanchorawala V, Seldin DC, Wright DG, Skinner M, Dember LM. High-dose intravenous melphalan with autologous stem cell transplantation in AL amyloidosis-associated end-stage renal disease. Kidney Int. 2003;63:1051–1057. doi: 10.1046/j.1523-1755.2003.00813.x. [DOI] [PubMed] [Google Scholar]
- 7.McDiarmid S, Hutton B, Atkins H, Bence-Bruckler I, Bredeson C, Sabri E, Huebsch L. Performing allogeneic and autologous hematopoietic SCT in the outpatient setting: effects on infectious complications and early transplant outcomes. Bone Marrow Transplant. 2010;45:1220–1226. doi: 10.1038/bmt.2009.330. [DOI] [PubMed] [Google Scholar]