Abstract
Objectives
To determine the relationship between mismatch repair (MMR) classification and clinicopathologic features including tumor volume, and explore outcomes by MMR class in a contemporary cohort.
Methods
Single institution cohort evaluating MMR classification for endometrial cancers (EC). MMR immunohistochemistry (IHC) +/− microsatellite instability (MSI) testing and reflex MLH1 methylation testing was performed. Tumors with MMR abnormalities by IHC or MSI and MLH1 methylation were classified as epigenetic MMR deficiency while those without MLH1 methylation were classified as probable MMR mutations. Clinicopathologic characteristics were analyzed.
Results
466 endometrial cancers were classified; 75% as MMR proficient, 20% epigenetic MMR defects, and 5% as probable MMR mutations. Epigenetic MMR defects were associated with advanced stage, higher grade, presence of lymphovascular space invasion, and older age. MMR class was significantly associated with tumor volume, an association not previously reported. The epigenetic MMR defect tumors median volume was 10,220mm3 compared to 3,321 mm3 and 2,846mm3, for MMR proficient and probable MMR mutations respectively (p<.0001). Higher tumor volume was associated with lymph node involvement. Endometrioid EC cases with epigenetic MMR defects had significantly reduced recurrence-free survival (RFS). Among advanced stage (III/IV) endometrioid EC the epigenetic MMR defect group was more likely to recur compared to the MMR proficient group (47.7% vs 3.4%) despite receiving similar adjuvant therapy. In contrast, there was no difference in the number of early stage recurrences for the different MMR classes.
Conclusions
MMR testing that includes MLH1 methylation analysis defines a subset of tumors that have worse prognostic features and reduced RFS.
Keywords: endometrial cancer, DNA mismatch repair, lymph node excision, Lynch syndrome, epigenomics
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in the United States with an estimated 61,380 new cases in 2017 [1]. EC usually presents at an early stage and as such patients with EC tend to have good outcomes with a 5-year relative survival over 80% [2]. Approximately 3–5% of ECs are attributable to Lynch syndrome (LS), a hereditary cancer predisposition that is caused by mutations in the mismatch repair (MMR) genes. Women with LS have up to a 60% life-time risk for developing EC as well as significant risks for colorectal, ovarian, stomach and other cancers [3,4,5]. Given the clinical benefit of identifying families with LS and intensified cancer surveillance for mutation carriers, universal tumor screening of EC has been recommended [6,7].
MMR deficiency is common in EC, occurring in 20–40% of cases [8,9,10,11,12,13]. The most common cause of MMR deficiency is epigenetic silencing of MLH1, associated with MLH1 promoter methylation [8,9,11,12]. Since MMR deficiency (evidenced by microsatellite instability (MSI) or immunohistochemistry (IHC)) plays an important role in colorectal cancer management there has been substantial interest in determining what role MMR defects play in EC [14]. Our group recently reported on the clinicopathologic significance of MMR classes in a large cohort of endometrioid endometrial cancer (EEC) cases from the Gynecologic Oncology Group 210 (GOG210) study. EEC cases in that study were classified as MMR normal (proficient), epigenetic MMR defect (MMR defect associated with MLH1 methylation), and probable MMR mutation (MMR defect not attributable to MLH1 methylation). The epigenetic MMR defect group was associated with several poor prognostic indicators that are routinely used to make decisions for adjuvant therapy use in EEC, the most common histologic subtype [15,16]. Despite these poor prognostic features associated with epigenetic MMR defects, outcomes were similar between MMR classes in the GOG210 study [11].
In light of the fact that MMR testing is used by many institutions, we set out to evaluate the role of MMR classification beyond LS screening. We sought to determine the relationship between MMR classification and clinicopathologic features including tumor volume, which has not been previously reported. We hypothesized that patients in the epigenetic MMR defect group would have larger tumors, as an additional poor prognostic feature and that these larger tumors would have a corresponding higher rate of lymph node involvement. We also set out to explore patient outcomes by MMR class in a large single institution cohort with uniform and contemporary treatment of EC.
Methods
Patient Cohort and Classification of MMR status
In 2013, The Ohio State University Comprehensive Cancer Center initiated the Ohio Colorectal Cancer Prevention Initiative (OCCPI). This statewide initiative was created to reduce colorectal cancer incidence in Ohio by identifying newly diagnosed colorectal cancer patients with hereditary predisposition and also included EC patients in the Division of Gynecologic Oncology at Ohio State University. Subjects had universal tumor screening including MMR IHC, MSI testing and MLH1 methylation testing, as previously described [17,18]. MLH1 methylation testing was reserved for those with absent MLH1/PMS2 on IHC and/or MSI-high tumors with normal IHC findings. Patients with MMR deficiency not attributable to MLH1 methylation were tested for germline mutations in 24 cancer susceptibility genes.
In August 2014, The Ohio State University Comprehensive Cancer Center implemented reflex MLH1 methylation testing for all EC tumors with loss of expression of MLH1/PMS2 on IHC. Clinical records were reviewed for all cases of primary EC in the calendar year of 2015 (first full year with complete MMR data). The 2015 clinical series was combined with the OCCPI cohort (January, 2013–June, 2016). The studies were performed after approval by an institutional review board (IRB) (OSU IRB-approved protocols (OSU2012C0123 and OSU2016C0096)). For patients enrolled in OCCPI informed consent was obtained.
The MMR status of tumors from the OCCPI was classified as previously reported [11]. In brief, tumors with intact expression of MMR proteins were considered MMR proficient (normal). Patients’ tumors with loss of MLH1/PMS2 on IHC and/or MSI-high that were found to have methylation of the MLH1 promoter region were classified as having an epigenetic MMR defect, whereas tumors with abnormal IHC and/or MSI-high without MLH1 methylation were classified as MMR deficient due to a probable MMR mutation. Given the high concordance between MSI and IHC findings [19,20], we classified the 2015 clinical cohort based on IHC and MLH1 methylation only (clinical MSI testing not performed).
Women were excluded if their MMR IHC or methylation studies were not performed/failed or if they had received neo-adjuvant chemotherapy (NACT) which may impact intrauterine tumor volume. Demographic, clinical, follow-up and pathologic data were abstracted from clinical charts. For outcomes analysis those patients who had persistent disease (after primary surgery) were excluded since time to recurrence could not be calculated (n=3). Patients with synchronous malignancy were excluded from treatment and outcomes analysis if they received therapy based on the synchronous malignancy and not their EC (n=9). Intrauterine tumor volume was based on hysterectomy gross tumor specimen measurements recorded by the evaluating pathologist. The maximum tumor measurements for 3 lengths, length A (height), B (width) and C (length), were then used to calculate tumor volume with the equation (A × B × C / 2). This calculation assumed that most tumors have an ellipsoid configuration. Microscopic tumors (those too small for gross measurement), were assigned a tumor volume of 0.1mm3.
Analysis of Tumors/Samples
For those subjects enrolled in the OCCPI study, MMR IHC for MSH2, MSH6, MLH1 and PMS2 was performed as part of their routine clinical testing. Additionally, the OCCPI subjects’ tumors underwent MSI analysis and reflex MLH1 methylation performed, as previously described [17]. Patients in the 2015 cohort (those women who were not part of the OCCPI study) had tumor MMR IHC as part of their routine pathologic evaluation as well as reflex MLH1 methylation analysis performed.
Statistical Analysis
The relationship between MMR status and clinicopathologic data was assessed using X2 and Fisher’s exact test. Tumor volume measurements were compared using ANOVA (Kruskal Wallis) test and Wilcoxon rank sum test with continuity correction and were two-sided. Recurrence-free survival (RFS) was defined as time from surgery to recurrence; patients who did not recur were censored at their last encounter with a physician. EC and overall survival was defined as time from surgery until death from EC or other causes respectively. The Kaplan-Meier product limit was used to estimate survival. The log-rank test was used to test for differences in survival by MMR class. Significance was set at a P value of .05.
Results
A total of 493 EC cases were initially evaluated; 295 from the OCCPI study and an additional 198 clinical cases from the 2015 calendar year. Twenty-seven (5.5%) cases were excluded due to either incomplete MMR testing (n=15) or NACT (n=12). Median follow-up time was 18 months. Among the 466 eligible subjects, 350 (75.1%) had tumors classified MMR proficient, 94 (20.2%) epigenetic MMR defect and 22 (4.7%) as probable MMR mutation (Fig. 1).
Figure 1.
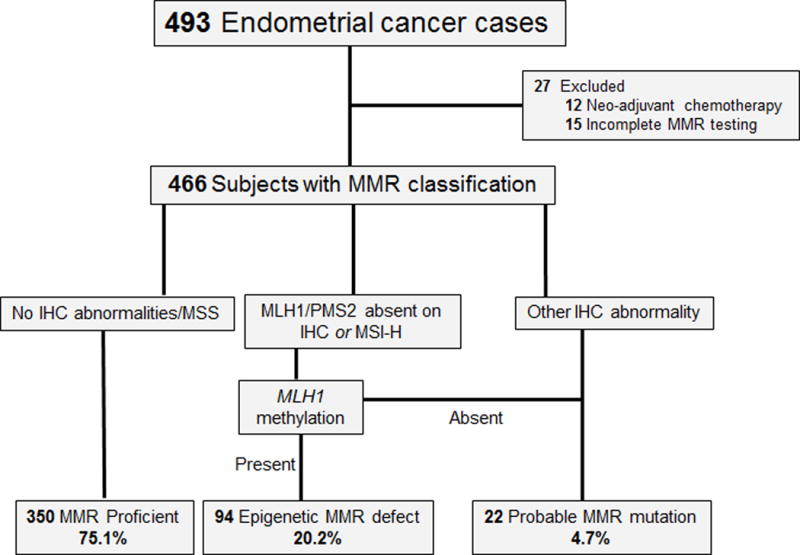
MMR testing algorithm and class distribution.
MMR status was significantly associated with age, with those women whose tumors were classified as having epigenetic MMR defects being older than MMR proficient or probable MMR mutation group, this association has been previously reported (Table 1) [8,11]. Body mass index (BMI) was not significantly associated with MMR class in our cohort. The frequency of synchronous malignancies (~4%) was similar for all the MMR classes, with ovarian or fallopian tube cancer being the most common (data not shown). Most tumors were endometrioid histology (83.7%). All uterine papillary serous histology tumors had intact MMR.
Table 1.
Demographic and clinicopathologic characteristics by MMR class
| MMR Proficient (n=350) | Epigenetic MMR Defect (n=94) | Probable MMR Mutation (n=22) | P valuea | ||
|---|---|---|---|---|---|
| Age at surgery, mean (SD) | 59.51 (10.92) | 64.14 (9.52) | 56.23 (9.00) | ||
| N (%) | N (%) | N (%) | |||
| <60 | 172 (49.1) | 28 (29.8) | 15 (68.2) | <.001 | |
| ≥60 | 178 (50.9) | 66 (70.2) | 7 (31.8) | ||
| BMI | .373 | ||||
| <25 | 36 (10.3) | 7 (7.4) | 4 (18.2) | ||
| ≥25–30 | 53 (15.1) | 16 (17.0) | 6 (27.3) | ||
| ≥30–35 | 78 (22.3) | 20 (21.3) | 5 (22.7) | ||
| ≥35 | 183 (52.3) | 51 (54.3) | 7 (31.8) | ||
| Race | 1.000 | ||||
| White | 331 (94.6) | 89 (94.7) | 22 (100.0) | ||
| Black | 11 (3.1) | 3 (3.2) | 0 (0.0) | ||
| Other | 8 (2.3) | 2 (2.1) | 0 (0.0) | ||
| Histology | .814 | ||||
| Endometrioid | 295 (84.3) | 77 (81.9) | 18 (81.8) | ||
| Non-Endometrioidb | 55 (15.7) | 17 (18.1) | 4 (18.2) | ||
| FIGO Grade | .002 | ||||
| 1 | 258 (73.7) | 52 (55.3) | 16 (72.7) | ||
| 2 | 32 (9.1) | 22 (23.4) | 1 (4.5) | ||
| 3/Highc | 60 (17.1) | 20 (21.3) | 5 (22.7) | ||
| Myometrial Invasion | .024 | ||||
| None | 93 (26.6) | 12 (12.8) | 6 (27.3) | ||
| Inner half | 163 (46.6) | 46 (48.9) | 12 (54.5) | ||
| Outer half | 94 (26.9) | 36 (38.3) | 4 (18.2) | ||
| LVSI | <.001 | ||||
| Absent | 284 (81.1) | 52 (55.3) | 17 (77.3) | ||
| Present | 66 (18.9) | 42 (44.7) | 5 (22.7) | ||
| Stage | .015 | ||||
| I/II | 293 (83.7) | 66 (70.2) | 17 (77.3) | ||
| III/IV | 57 (16.3) | 28 (29.8) | 5 (22.7) | ||
| Lymph Node Statusd | .005 | ||||
| Negative | 193 (55.5) | 55 (58.5) | 13 (59.1) | ||
| Positive | 35 (10.1) | 21 (22.3) | 3 (13.6) | ||
| Not performed | 120 (34.4) | 18 (19.2) | 6 (27.3) | ||
| Adjuvant Therapye | .045 | ||||
| Yes | 101 (29.0) | 39 (42.4) | 8 (36.4) | ||
| None | 247 (71.0) | 53 (57.6) | 14 (63.6) | ||
Abbreviations: MMR, mismatch repair; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LVSI, lymphovascular space invasion.
Fisher’s exact tests
MMR proficient: 24 serous, 15 mixed, 10 carcinosarcoma, 3 clear cell, 2 mucinous, 1 undifferentiated/dedifferentiated
Probable MMR mutation: 2 mixed, 1 clear cell, 1 undifferentiated/dedifferentiated
Epigenetic MMR defect: 5 undifferentiated/dedifferentiated, 5 mixed, 4 mucinous, 3 carcinosarcoma
Includes serous, clear cell, carcinosarcoma, undifferentiated/dedifferentiated and mixed histologies with a high grade component
Two patients in the MMR proficient group with lymph node involvement by synchronous malignancy were excluded
Two patients from MMR proficient and 2 patients from epigenetic MMR defect class had adjuvant therapy recommended but were lost to follow up
Epigenetic MMR deficient tumors are associated with poor prognostic features including larger tumor volume
The epigenetic MMR defect group was significantly associated with several poor prognostic indicators including higher grade, myometrial invasion, advanced stage (III/IV), and the presence of lymphovascular space invasion (LVSI) (Table 1), these associations have been previously reported [11,21,22,23,24]. The epigenetic MMR defect group had the highest rate of adjuvant therapy (42.4% of subjects receiving some form of adjuvant treatment).
MMR class was significantly associated with tumor volume, an association not previously reported. Tumors with epigenetic MMR defects were larger than the MMR proficient and probable MMR mutation tumors. The median volume [interquartile range] for the epigenetic MMR defect group was 10,220mm3 [3473.75, 20719.50], compared to 3,321mm3 [344.25, 13595.75] for the MMR proficient group and 2,846mm3 [754.88, 7606.50] for the probable MMR mutation group (P<.001, Fig. 2A). The MMR proficient tumors were not only smaller overall but were also more likely to be microscopic, 14.6%, compared to only 4.3% of the epigenetic MMR defects (P=.02, Fisher’s exact test).
Figure 2.
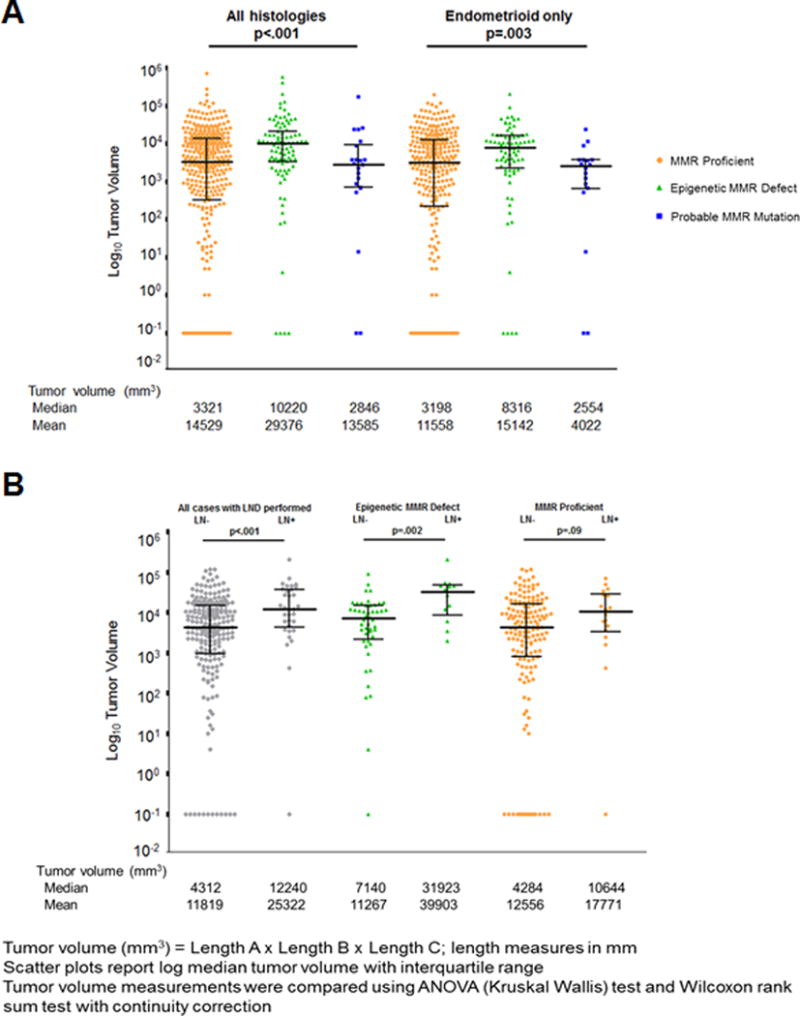
A) Tumor volume by MMR class for all histologies and for endometrioid histology only. B) Tumor volume and lymph node status for all endometrioid cases with lymphadenectomy performed and by epigenetic MMR defective and MMR proficient tumors.
When the analyses were limited to EECs, all of the associations with demographic and clinicopathologic features seen for the entire cohort remained significant (Supplemental Table 1, Fig. 2A). Adjuvant therapy usage was significantly higher in EECs with epigenetic MMR defects, 33.3% compared to 19% in the MMR proficient group (P=.01, Fisher’s exact test). The rate of adjuvant therapy for non-EEC tumors was 82% (data not shown).
Lymph node positivity is associated with tumor volume and epigenetic MMR defects
Two hundred and fifty-five (65.4%) of EEC cases were informative for lymph node status (lymphadenectomy performed). Lymph node sampling differed among the three molecular classes, with 39% of cases with MMR proficient tumors not having lymph node dissections compared to only 19% of the epigenetic MMR defect group. Lymph node positive tumors had significantly higher median tumor volumes 12,240mm3 compared to 4,312mm3 for lymph node negative cases (P<.001, Fig. 2B). The significant difference in tumor volume and lymph node status was most pronounced for the epigenetic MMR defective group, 31,923 mm3 compared to 7,140 mm3 (P=.002). The tumor volumes for the MMR proficient group on the other hand were more similar for lymph node positive cases 10,644mm3 compared to 4,284mm3 in lymph node negative cases (P=.09).
Women with epigenetic MMR deficient EEC had more than double the rate of lymph node positivity than the MMR proficient group (22% versus 10%, Supplemental Table 1).
Differences in recurrence by MMR class
Recurrence-free survival (RFS) was significantly reduced for women in the epigenetic MMR defect group (Fig. 3A, P<.001). Even when the non-endometrioid histologies (which are at higher risk for recurrence compared with the EECs) were included in the outcome analysis, the epigenetic MMR defect group demonstrated a trend towards reduced RFS (P=.054, data not shown). Endometrial cancer specific and overall survival for women with endometrioid tumors and epigenetic MMR defects was reduced, however did not reach statistical significance (P=.076 and .066, respectively; data not shown).
Figure 3.
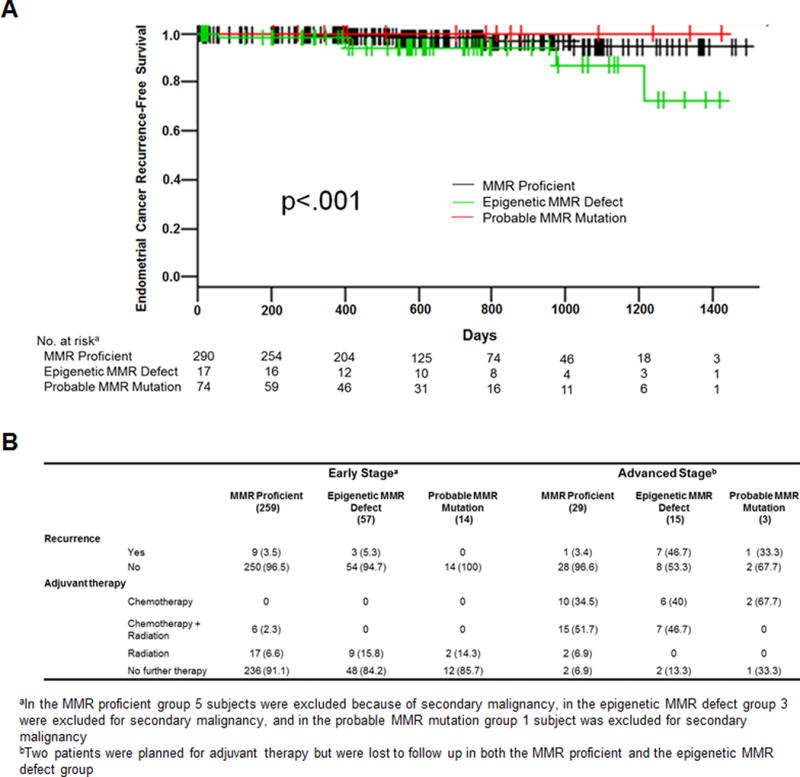
A) Recurrence-free survival by MMR class for subjects with endometrioid histology tumors. B) Recurrence and adjuvant therapy use by stage and MMR class.
The overall rate of disease recurrence in the cohort was 9%. For EECs, the rate of recurrence was 5.6%: 3.6% of early and 19% of advanced stage cases recurred. Early stage patients had primarily local recurrences and advanced stage cases recurrences were mostly distant (Table 2; Fig. 3B). There was a striking difference in the rate of recurrence in EECs for the different MMR subtypes when evaluating by stage. Nearly half of the women with advanced stage (stages III/IV) epigenetic MMR deficient EECs recurred (7/15) compared to only 1/29 (3.4%) in the advanced stage MMR proficient group (Fig. 3B). Adjuvant therapy usage did not differ between the two groups.
Table 2.
Recurrences among 379 endometrioid histology cases stratified by MMR class
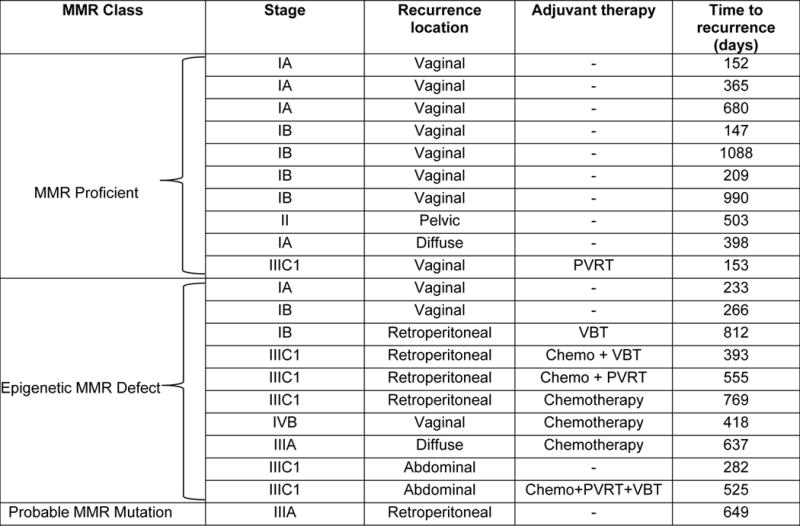
|
Abbreviations: MMR, mismatch repair; VBT, vaginal brachytherapy; PVRT, pelvic radiation therapy
Among early stage EECs (stages I/II, n=330) recurrence rates were similar for the MMR proficient and epigenetic MMR defect groups, 3.4% and 5.2%, respectively. In contrast to the advanced stage cases, the epigenetic MMR defect group received more adjuvant treatment in these early stage cases than the MMR proficient group (15.8% versus 8.9%). Most of the early stage recurrences were in patients who did not receive adjuvant therapy (11 of 12 events, 92%).
Discussion
In this study we confirmed prior reports that defective MMR and, in particular the epigenetic MMR deficient group is associated with poor prognostic features [11,21,22,23,24]. For the first time, we report that tumors with epigenetic MMR defects are larger (higher uterine tumor volumes) with a corresponding higher rate of lymph node involvement. Furthermore, these advanced stage women with epigenetic MMR deficient tumors had higher rates of recurrence than the advanced stage MMR proficient patients.
Tumor size is an established prognostic factor. Algorithms for risk assessment of lymph node involvement and thus decision for lymph node evaluation during surgery include intrauterine tumor size [25]. The significantly different rates of lymphadenectomy between the MMR proficient (61%) and the epigenetic MMR defect (80.5%) groups in our cohort illustrate the impact that an intraoperative assessment of tumor volume may have had in surgical decision making. Intrauterine tumor volume may also influence molecular findings that rely on analysis of primary specimens. Studies that have focused on frozen tumor specimens, such as TCGA, tend to have the highest rates of MMR defects, 30–36% [9,10,20]. We speculate that sampling for larger tumors enriches for cases with defective MMR.
We demonstrate a significant relationship between MMR class and RFS. The prognostic and predictive utility of MMR status in colorectal cancers has been well described. Our finding that patients with epigenetic silencing of MLH1 have poorer outcomes is unlike what has been reported in colorectal cancer. Multiple studies as well as a meta-analysis have demonstrated that colorectal cancers with MSI have more favorable outcomes than MMR proficient tumors [26,27,28]. The relationship between MMR classification and EC, however, has not been fully established [11,29]. The GOG210 study of over 1000 cases using the same MMR classification system suggested that the epigenetic MMR defect group had reduced progression-free survival. Our analysis confirms women with epigenetic MMR defects have reduced RFS. While the GOG210 outcomes difference was not significant in multivariate analysis, the most profound difference in recurrence rates in our cohort was among women with advanced stage (III/IV) EEC. The women with EEC and epigenetic MMR deficiency had a recurrence rate approaching 50%, while there was only one recurrence in the advanced stage EEC MMR proficient group. The GOG210 analysis did not assess recurrence rates by MMR class stratified by stage.
In contrast to the dramatic differences in recurrence rates for advanced stage cases, women with early stage EEC (I/II) had similar low recurrence rates (~3–5%) for the both the epigenetic defect and MMR proficient molecular groups. A possible explanation for why there was no difference in recurrence rates between MMR classes in early stage cases is that the epigenetic MMR deficient cases were almost twice as likely to have received adjuvant radiation therapy. The higher rate of adjuvant radiation therapy is explained by the fact that these patients are more likely to fall into the GOG99 and PORTEC high-intermediate risk classes [15,16] due to advanced age, presence of LVSI, grade 2 or 3 tumors, and myometrial invasion of the outer half (Supplemental Table 1). High-intermediate risk cases can be treated with pelvic radiation or vaginal brachytherapy in an effort to reduce local recurrence [15,16]. In our series, the early stage recurrences were primarily vaginal in women who had not received adjuvant treatment (Table 2). Local recurrences such as those seen our cohort are frequently salvaged with radiation therapy (~80%) [30].
The observed high rate of recurrence for the advanced stage epigenetic MMR deficient group may point to a molecular class:treatment interaction. It has been reported MMR deficient tumors have increased sensitivity to radiation therapy [24]. Several in vitro studies have suggested platinum resistance in MMR deficient cell lines [31,32,33,34]. Our data indicate that epigenetic MMR deficient tumors are less responsive to platinum-based chemotherapy, the standard of care for advanced stage EEC [35]. Recently, the MAGIC Trial in gastric cancer reported that MMR deficiency was a negative prognostic indicator in patients treated with chemotherapy [36]. Additionally, in colon cancer, fluorouracil-based adjuvant chemotherapy has limited value in MSI-high tumors (MMR deficient), whereas it substantially improves outcomes for those patients with MMR proficient tumors [37]. Previous work from our group suggested the possibility of chemoresistance in advanced stage MMR deficient patients [38]. However, methylation analysis was not performed and the epigenetic (methylated) and probable mutation cases were considered together [38]. To our knowledge no prospective evaluation of MMR deficiency and chemoresistance in endometrial cancer has been reported.
The high recurrence rate in the MLH1 methylated (epigenetic) group strongly suggests that the primary therapy for advanced stage EEC was ineffective for the group. Although our data are not sufficiently mature to evaluate overall survival at this time, we anticipate the cancer-specific survival will be reduced for the epigenetic MMR defect group, because these distant recurrences are unlikely to be cured with further traditional cytotoxic therapy. Immune checkpoint blockade (PD-L1 and PD-1 inhibitors) and potentially demethylating agents may prove valuable in this subset of patients. The benefit of PD-1 immunotherapy in MMR deficient tumors has been demonstrated [39]. The study by Le and colleagues [39] included a small number of MMR deficient EEC cases with striking responses. More recent findings for a PD-1 inhibitor trial in MMR deficient recurrent endometrial cancer have suggested significant therapeutic benefit with 56% overall response rate and 89% clinical benefit rate [40]. Together these data suggest that immunotherapy as part of the primary treatment for advanced stage MMR deficient EC is an attractive approach to improve patient outcomes. The high prevalence of epigenetic MMR defects (20%) in our cohort, with almost a quarter of these patients presenting at an advanced stage, highlights the fact that a large fraction of patients may benefit from alternative treatment strategies.
Limitations
Our study is limited by the modest number of recurrences, retrospective clinical record review and limited follow up time. It is nonetheless, the largest single institution experience with comprehensive MMR testing, and uniform utilization of adjuvant therapy for both early and advanced stage cases that has been reported to date.
Conclusions
MMR testing that includes MLH1 methylation analysis defines a subset of tumors that have worse prognostic features including higher tumor volume and lymph node involvement. Women with EEC and MLH1 methylation have reduced recurrence-free survival and highlight a high risk population, notably advanced stage cases. Our data illustrate the potential utility of MMR testing and classification beyond standard LS screening and identify a group of patients whom may benefit from radiation therapy and/or alternative treatment strategies such immunotherapy.
Supplementary Material
Supplemental Table 1 (S1 Table). Clinicopathologic characteristics of endometrioid cases by MMR class.
Highlights.
MLH1 methylated endometrial tumors have poor prognostic features including larger size
Tumor volume and mismatch repair class are associated with lymph node involvement
Women with MLH1 methylated tumors have reduced recurrence-free survival
Recurrence rate by MMR class differed dramatically in advanced stage endometrial cancer
MMR defective tumors with MLH1 methylation may exhibit chemoresistance
Acknowledgments
Funding/Support: The Ohio Colorectal Cancer Prevention Initiative (OCCPI) is supported by a grant from Pelotonia (Hampel). Additional funding provided from the Ohio State University Division of Gynecologic Oncology, Pelotonia and the National Cancer Institute (P30 CA016058) supporting the Biostatistics shared resource at the Ohio State University Comprehensive Cancer Center (Goodfellow).
Footnotes
Conflict of interest disclosure: None of the authors have a conflict of interest to disclose.
Author Contributions: Concept and design (CC, PG, HH, FB); Acquisition, analysis, or interpretation of data (all authors); Drafting of the manuscript (CC, PG, FB); Critical revision of the manuscript for important intellectual content (all authors); Statistical and data analysis (CC, JM, PG); Obtained funding (DC, PG, HH); Administrative technical or material support (DC, WF, DJ, AS, WZ, WC, RS, LC, DO, JF, AY, AC); Study supervision (PG, FB, CC, HH, DC).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner MJ. Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215; Bethesda, MD: 2007. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001. [Google Scholar]
- 3.Lancaster JM, Powell CB, Chen LM, Richardson DL. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136:3–7. doi: 10.1016/j.ygyno.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, De La Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. International Journal of Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Hampel H, Stephens JA, Pukkala E, Sankila R, Aaltonen LA, Mecklin JP, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129:415–421. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 6.SGO Clinical Practice Statement: Screening for Lynch Syndrome in Endometrial Cancer. 2014 [Google Scholar]
- 7.Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, Lau MW, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015;33:209–217. doi: 10.1200/JCO.2014.58.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backes FJ, Leon ME, Ivanov I, Suarez A, Frankel WL, Hampel H, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecol Oncol. 2009;114:486–490. doi: 10.1016/j.ygyno.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Zighelboim I, Goodfellow PJ, Gao F, Gibb RK, Powell MA, Rader JS, et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007;25:2042–2048. doi: 10.1200/JCO.2006.08.2107. [DOI] [PubMed] [Google Scholar]
- 10.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMeekin DS, Tritchler DL, Cohn DE, Mutch DG, Lankes HA, Geller MA, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: An NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34:3062–3068. doi: 10.1200/JCO.2016.67.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchanan DD, Tan YY, Walsh MD, Clendenning M, Metcalf AM, Ferguson K, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol. 2014;32:90–100. doi: 10.1200/JCO.2013.51.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 14.Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863–875. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 16.Nout RA, Smit VT, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 17.Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. 2017;3:464–471. doi: 10.1001/jamaoncol.2016.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton K, Jorgensen NM, Wallace AJ, Buchanan DD, Lalloo F, McMahon RF, et al. Tumour MLH1 promoter region methylation testing is an effective prescreen for Lynch Syndrome (HNPCC) J Med Genet. 2014;51:789–796. doi: 10.1136/jmedgenet-2014-102552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: An NRG Oncology and Gynecologic Oncology Group Study. J Clin Oncol. 2015;33:4301–4308. doi: 10.1200/JCO.2015.63.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McConechy MK, Talhouk A, Li-Chang HH, Leung S, Huntsman DG, Gilks CB, et al. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol. 2015;137:306–310. doi: 10.1016/j.ygyno.2015.01.541. [DOI] [PubMed] [Google Scholar]
- 21.Black D, Soslow RA, Levine DA, Tornos C, Chen SC, Hummer AJ, et al. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol. 2006;24:1745–1753. doi: 10.1200/JCO.2005.04.1574. [DOI] [PubMed] [Google Scholar]
- 22.Zighelboim I, Schmidt AP, Gao F, Thaker PH, Powell MA, Rader JS, et al. ATR mutation in endometrioid endometrial cancer is associated with poor clinical outcomes. J Clin Oncol. 2009;27:3091–3096. doi: 10.1200/JCO.2008.19.9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohn DE, Frankel WL, Resnick KE, Zanagnolo VL, Copeland LJ, Hampel H, et al. Improved survival with an intact DNA mismatch repair system in endometrial cancer. Obstet Gynecol. 2006;108:1208–1215. doi: 10.1097/01.AOG.0000239097.42987.0c. [DOI] [PubMed] [Google Scholar]
- 24.Bilbao C, Lara PC, Ramirez R, Henriquez-Hernandez LA, Rodriguez G, Falcon O, et al. Microsatellite instability predicts clinical outcome in radiation-treated endometrioid endometrial cancer. Int J Radiat Oncol Biol Phys. 2010;76:9–13. doi: 10.1016/j.ijrobp.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milam MR, Java J, Walker JL, Metzinger DS, Parker LP, Coleman RL. Nodal metastasis risk in endometrioid endometrial cancer. Obstet Gynecol. 2012;119:286–292. doi: 10.1097/AOG.0b013e318240de51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gryfe R, Kim H, Hsieh ETK, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. The New England Journal of Medicine. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 27.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 28.Lanza G, Gafa R, Santini A, Maestri I, Guerzoni L, Cavazzini L. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J Clin Oncol. 2006;24:2359–2367. doi: 10.1200/JCO.2005.03.2433. [DOI] [PubMed] [Google Scholar]
- 29.Diaz-Padilla I, Romero N, Amir E, Matias-Guiu X, Vilar E, Muggia F, et al. Mismatch repair status and clinical outcome in endometrial cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2013;88:154–167. doi: 10.1016/j.critrevonc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Huh WK, Straughn JM, Jr, Mariani A, Podratz KC, Havrilesky LJ, Alvarez-Secord A, et al. Salvage of isolated vaginal recurrences in women with surgical stage I endometrial cancer: a multiinstitutional experience. Int J Gynecol Cancer. 2007;17:886–889. doi: 10.1111/j.1525-1438.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 31.Strathdee G, MacKean MJ, Illand M, Brown R. A role for methylation of the hMLH1 promotor in loss of hMLH1 expression and drug resistance in ovarian cancer. Oncogene. 1999;18:2335–2341. doi: 10.1038/sj.onc.1202540. [DOI] [PubMed] [Google Scholar]
- 32.Aebi S, Kurdi-Haidar B, Gordon R, Cenni B, Zheng H, Fink D, et al. Loss of DNA mismatch repair in acquired resistance to Cisplatin. Cancer Research. 1996;56:3087–3090. [PubMed] [Google Scholar]
- 33.Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehme A, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Research. 1996;56:4881–4886. [PubMed] [Google Scholar]
- 34.Drummond JT, Anthoney A, Brown R, Modrich P. Cisplatin and adriamycin resistance are associated with MutLalpha and mismatch repair deficiency in an ovarian tumor cell line. J Biol Chem. 1996;271:19645–19648. doi: 10.1074/jbc.271.33.19645. [DOI] [PubMed] [Google Scholar]
- 35.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 36.Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2016.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resnick KE, Frankel WL, Morrison CD, Fowler JM, Copeland LJ, Stephens J, et al. Mismatch repair status and outcomes after adjuvant therapy in patients with surgically staged endometrial cancer. Gynecol Oncol. 2010;117:234–238. doi: 10.1016/j.ygyno.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fader AN, D LA, Armstrong DK, Tanner EJ, III, Uram J, Eyring A, Wang1 H, Fisher G, Greten T, Le D. Preliminary results of a phase II study: PD-1 blockade in mismatch repair–deficient, recurrent or persistent endometrial cancer. Annual Meeting for Women’s Cancer; San Diego, CA. 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 (S1 Table). Clinicopathologic characteristics of endometrioid cases by MMR class.


