Introduction
Key Teaching Points.
-
•
The fetal diagnosis of long QT syndrome may be challenging, often relying on a constellation of rhythm abnormalities that may include sinus bradycardia or torsades de pointes ± second-degree atrioventricular block.
-
•
Genotype-phenotype correlation is generally not possible in utero; therefore multidrug therapy is required prior to delivery.
-
•
Aggressive postnatal management with cardiac pacing therapy and genotype-specific medical therapy can result in successful treatment of this possibly fatal condition.
Long QT syndrome (LQTS) is a disorder of cardiac repolarization that affects approximately 1 in 2500 individuals and is associated with syncope and sudden cardiac death. Over the past several decades, advances in clinical diagnosis—combined with gene-directed antiarrhythmic therapies and cardiac device management—have improved the outcome of this life-threatening disorder. Although early detection of the disorder is paramount, timely diagnosis by fetal echocardiography still remains a significant challenge. The present case characterizes the utility of fetal echocardiography for the diagnosis and subsequent management of congenital LQTS.
Case report
A 32-year-old gravid woman was referred for pediatric cardiologic evaluation at 26 weeks’ gestation for fetal bradycardia (atrial rate of 130 beats per minute [bpm]; ventricular rate of 65 bpm) with the referral diagnosis of congenital atrioventricular (AV) block. The fetal heart rate (FHR) prior to 26 weeks’ gestation had been reported as normal by routine prenatal ultrasound (Figure 1). The patient had given birth previously to 5 children, all of whom were in good health. She had a known history of gestational diabetes and was managed with insulin glargine subcutaneous injections during the current pregnancy. As part of an initial evaluation for AV block, laboratory testing for anti-SSA/SSB antibodies was performed, revealing an elevated anti-SSA antibody titer equal to 65 units.
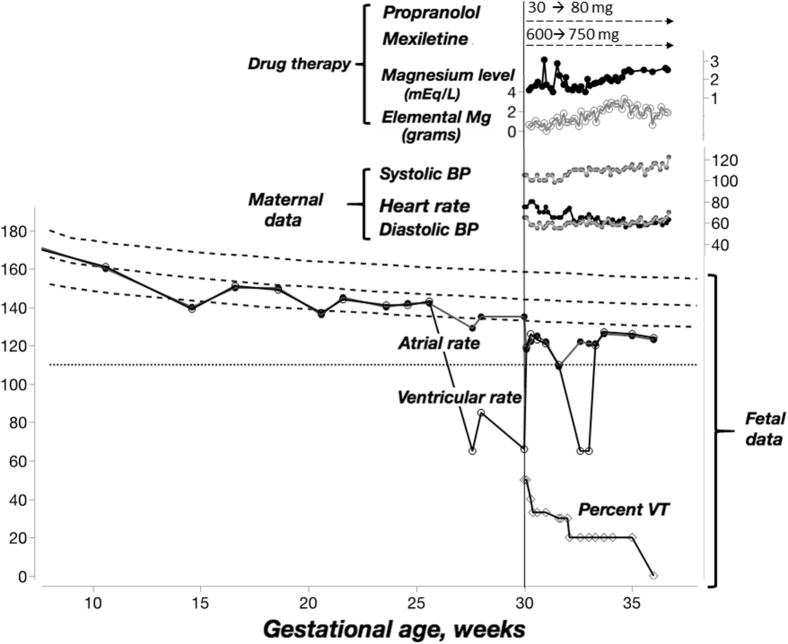
Figure 1.
Detailed timeline of fetal and maternal characteristics. The fetal heart rate is shown at the bottom half of the figure with superimposed 3rd, 50th, and 97th heart rate percentiles in hatched parallel lines (adapted from Mitchell and colleagues7). The horizontal dotted line represents the standard obstetric definition of fetal bradycardia (ie, 110 beats/min). The vertical line represents admission to the hospital for initiation of transplacental therapy. There is a marked reduction in fetal arrhythmia associated with therapy prior to eventual delivery at 37 weeks’ gestation. BP = blood pressure; VT = ventricular tachycardia.
Fetal echocardiography demonstrated normal intracardiac anatomy and preserved biventricular systolic function. There were frequent salvos of rapid ventricular tachycardia (VT) of variable cycle length with ventriculoatrial dissociation during the majority of the initial study (Figure 2) (see also supplemental video). A small pericardial effusion was also noted. Based on findings suggestive of polymorphic VT and intermittent 2:1 AV block, the working diagnosis of congenital LQTS was established. The patient was admitted to the inpatient service at 30 weeks’ gestation and transplacental therapy with propranolol of 30 mg/day was initiated.
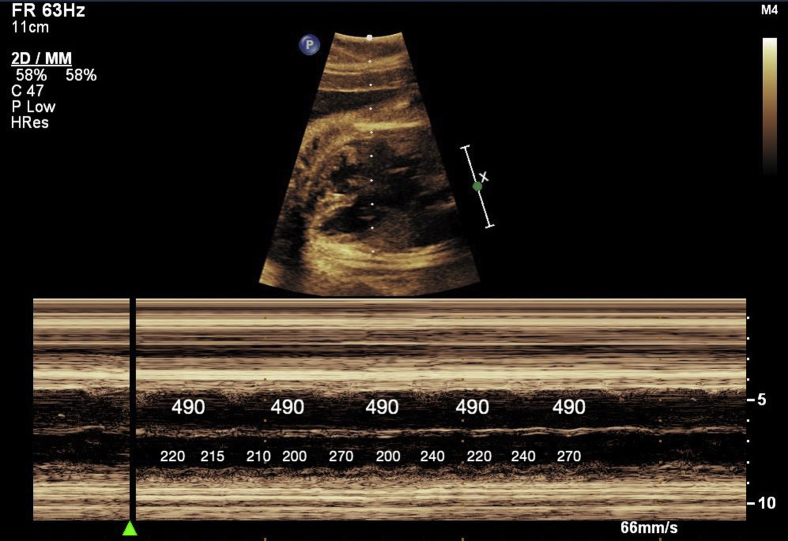
Figure 2.
Fetal echocardiogram M-mode still image demonstrating an episode of ventricular tachycardia of variable cycle length, with ventriculoatrial dissociation and a nonvariable, normal atrial rate. The interval between each beat is labeled in milliseconds.
Follow-up fetal echocardiography obtained 4 hours after initiation of therapy showed nonsustained VT during 50%–60% of the study, which was unchanged the following day. Mexiletine therapy (600 mg/day) was added, and echocardiography obtained 6 hours after first dose showed a modest reduction in frequency and shorter salvos of VT. Magnesium was then added approximately 12 hours later and the rhythm after 24 hours of the above regimen (associated with magnesium level of 1.6 mEq/L) showed a noticeable improvement in VT burden, now totaling approximately 33% of the study. Given evidence of decreasing ventricular arrhythmia in response to therapy, the mexiletine and propranolol doses were optimized (propranolol 80 mg/day and mexiletine 750 mg/day, respectively) and were maintained throughout pregnancy. Magnesium levels were checked twice daily and, in addition to scheduled oral and parenteral magnesium, intravenous magnesium sulfate boluses were given 1–3 times a day in response to the level to maintain a goal of greater than 2.0 mEq/L. The rhythm was assessed by serial fetal echocardiography intermittently throughout the rest of the pregnancy, and the VT burden was noted to decrease progressively, with the final echocardiogram demonstrating near-absence of ventricular arrhythmia (Figure 1).
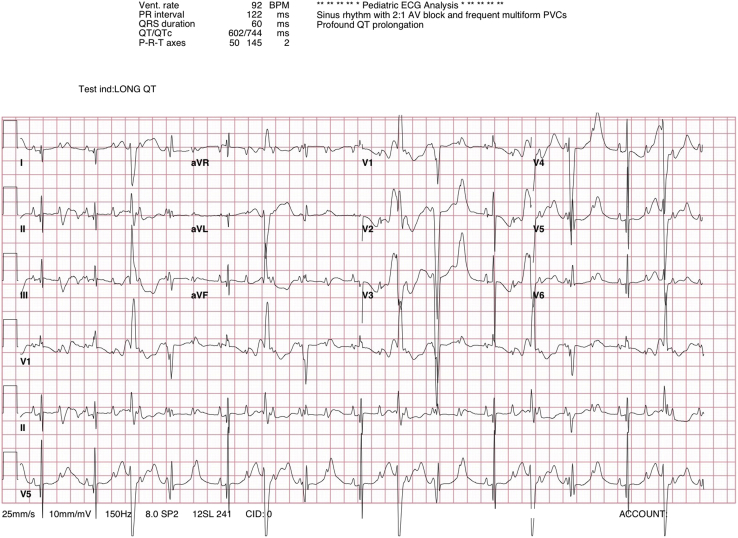
A cesarean delivery was planned owing to concerns about potentially inadequate cardiac monitoring during vaginal delivery. The infant was delivered at 37 weeks’ gestation, and immediately upon delivery the rhythm was noted to fluctuate between normal sinus rhythm (110 bpm) and 2:1 AV block. Central access was obtained and the patient was intubated. A 12-lead surface electrocardiogram confirmed the diagnosis of significant QT prolongation (corrected QT interval 680 ms) with 2:1 AV block and frequent multiform premature ventricular contractions (Figure 3). Lidocaine was initiated at 20 mcg/kg/min. However, as the rhythm was predominantly 2:1 AV block, a decision was made to place an epicardial dual-chamber pacemaker. The infant was started on a combination of oral propranolol and mexiletine therapy with dual-chamber pacing.
Figure 3.
Electrocardiogram taken on the first day of life, demonstrating 2:1 atrioventricular block with frequent multiform premature ventricular contractions and a corrected QT interval of 680 ms. This study was performed before initiation of postnatal medical therapy.
Subsequent genetic testing revealed a heterozygous mutation in a highly conserved region of the voltage sensor for the cardiac sodium channel encoded by the SCN5A gene (R1623Q), consistent with a previously described genetic form of LQTS.1 Neither of the parents’ electrocardiograms demonstrated corrected QT interval prolongation. The infant was discharged to home at 1 month of life without further ventricular arrhythmia, and was thereafter followed as an outpatient on mexiletine and propranolol. At 10 months of age, after a 1-week history of gastroenteritis, the patient experienced an episode of syncope with documented polymorphic VT. The mexiletine level at that time was 0.8 mcg/mL. The patient underwent uneventful surgical upgrade to an epicardial implantable cardioverter-defibrillator and continues to do well, without further arrhythmia, at 1 year of age.
Discussion
A challenging diagnosis to establish in utero, LQTS requires the recognition of specific rhythm abnormalities unique to the fetus. In most cases of fetal LQTS, the only detectable rhythm abnormality is sinus bradycardia with an FHR < third percentile for age.2 In approximately 25% of fetuses, however, the more severe form of LQTS arrhythmia with torsades de pointes (TdP) and/or second-degree AV block is also observed. For fetuses with severe LQTS arrhythmia, the diagnosis may be unexpected owing to a higher probability of de novo mutations and, consequently, an unremarkable family history.3 The inheritance patterns of LQTS is complex and includes somatic mosaicism,4 autosomal recessive inheritance, and variable penetrance. Coexistence of common single nucleotide polymorphisms with LQTS-causing genes and/or unknown genes can also affect the phenotypic presentation. Therefore, although careful history taking for the outcomes of prior pregnancies and family history is paramount, it is important to recognize that the absence of family history of cardiac events should not eliminate suspicion for the disease.
In this case, the prenatal detection of maternal anti-SSA antibodies was misleading, as it suggested the diagnosis of antibody-related AV block, an entity that was not present. This patient’s constellation of fetal echocardiographic findings was more consistent with LQTS. In fact, sinus bradycardia (FHR < third percentile for age) was observed on 3 occasions (25% of recordings) prior to the referral (Figure 1) and could have prompted a more aggressive early approach. Timely recognition of this syndrome is imperative, as fetal demise may occur in the absence of appropriate therapy.4, 5 The classic echocardiographic findings demonstrated in the present report can help the practitioner identify the proper diagnosis in a timely fashion. Although fetal magnetocardiography can definitively diagnose prenatal LQTS, most centers in the country do not have access to this technology.
An understanding of the most common genotypes implicated in fetal LQTS, along with their previously reported treatments and outcomes, can be instructive in guiding the diagnosis and management of such fetal cases of LQTS. For fetuses that present with the signature LQTS arrhythmias of TdP and/or second-degree AV block, prior series have demonstrated a preponderance of de novo SCN5A and KCNH2 mutations, the former often involving the voltage sensor of the sodium channel and the latter most often involving the pore region of the potassium channel.2 SCN5A-R1623Q in particular has been detected frequently in fetuses with TdP and second-degree AV block, both in the form of case reports and in case series (as many as 7 unique cases exist in the literature).2, 4, 6, 7
Transplacental therapy generally consists of a combination of sodium channel and beta-blocker therapy, as well as aggressive magnesium supplementation. Sodium channel blockade, whether mexiletine6, 8 or lidocaine,9 has been shown to be effective in suppressing TdP for fetuses affected by LQTS related to SCN5A mutations. Beta-blocker therapy, most often in the form of propranolol because of its efficient transplacental passage, is also administered.2 In the present case, aggressive mexiletine administration was ultimately felt to contribute to the observed reduction in ventricular arrhythmia once therapeutic levels (0.5–2.5 mcg/mL) had been achieved. Using such an approach, aggressive management with complete suppression of fetal arrhythmia should be pursued in an effort to carry the fetus fully to term. In the present case, the fetus was ultimately delivered at 37 weeks’ gestation, given concern for the possibility of arrhythmia recurrence. Although this compares favorably with reported gestational ages of ≤35 weeks for fetuses with similar SCN5A-R1623Q mutations,2, 3, 7, 10 delivery should always be postponed as long as possible for the optimal neonatal outcome.
Postnatally, management was guided by additional data obtained shortly after birth and during subsequent follow-up. Pacemaker implantation was recommended based on the early postnatal electrocardiographic findings of AV block as well as a strong suspicion for SCN5A mutation, situations where antibradycardia pacing may be beneficial. In addition, ongoing therapy with mexiletine and propranolol with verification of adequate therapeutic levels was prescribed after genetic confirmation, ultimately resulting in long-term rhythm control.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.hrcr.2017.04.007.
Appendix. Supplementary Data
Video clip demonstrating observed fetal tachyarrhythmias during clinical follow-up. The first video segment demonstrates an episode of presumptive polymorphic VT/VF, and the second segment demonstrates an episode of atrial tachyarrhythmia. While presumptive VT/VF was observed frequently, atrial tachyarrhythmia was only rarely observed by fetal echocardiography.
References
- 1.Yamagishi H., Furutani M., Kamisago M., Morikawa Y., Kojima Y., Hino Y., Furutani Y., Kimura M., Imamura S., Takao A., Momma K., Matsuoka R. A de novo missense mutation (R1623Q) of the SCN5A gene in a Japanese girl with sporadic long QT sydrome. Mutations in brief no. 140. Online. Hum Mutat. 1998;11:481. doi: 10.1002/(SICI)1098-1004(1998)11:6<481::AID-HUMU12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 2.Cuneo B.F., Etheridge S.P., Horigome H., Sallee D., Moon-Grady A., Weng H.Y., Ackerman M.J., Benson D.W. Arrhythmia phenotype during fetal life suggests long-QT syndrome genotype: risk stratification of perinatal long-QT syndrome. Circ Arrhythm Electrophysiol. 2013;6:946–951. doi: 10.1161/CIRCEP.113.000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuneo B.F., Strasburger J.F., Yu S., Horigome H., Hosono T., Kandori A., Wakai R.T. In utero diagnosis of long QT syndrome by magnetocardiography. Circulation. 2013;128:2183–2191. doi: 10.1161/CIRCULATIONAHA.113.004840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller T.E., Estrella E., Myerburg R.J., Garcia de Viera J., Moreno N., Rusconi P., Ahearn M.E., Baumbach L., Kurlansky P., Wolff G., Bishopric N.H. Recurrent third-trimester fetal loss and maternal mosaicism for long-QT syndrome. Circulation. 2004;109:3029–3034. doi: 10.1161/01.CIR.0000130666.81539.9E. [DOI] [PubMed] [Google Scholar]
- 5.Crotti L., Tester D.J., White W.M. Long QT syndrome-associated mutations in intrauterine fetal death. JAMA. 2013;309:1473–1482. doi: 10.1001/jama.2013.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horigome H., Nagashima M., Sumitomo N. Clinical characteristics and genetic background of congenital long-QT syndrome diagnosed in fetal, neonatal, and infantile life: a nationwide questionnaire survey in Japan. Circ Arrhythm Electrophysiol. 2010;3:10–17. doi: 10.1161/CIRCEP.109.882159. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell J.L., Cuneo B.F., Etheridge S.P., Horigome H., Weng H.Y., Benson D.W. Fetal heart rate predictors of long QT syndrome. Circulation. 2012;126:2688–2695. doi: 10.1161/CIRCULATIONAHA.112.114132. [DOI] [PubMed] [Google Scholar]
- 8.Wang D.W., Crotti L., Shimizu W., Pedrazzini M., Cantu F., De Filippo P., Kishiki K., Miyazaki A., Ikeda T., Schwartz P.J., George A.L., Jr. Malignant perinatal variant of long-QT syndrome caused by a profoundly dysfunctional cardiac sodium channel. Circ Arrhythm Electrophysiol. 2008;1:370–378. doi: 10.1161/CIRCEP.108.788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuneo B.F., Ovadia M., Strasburger J.F., Zhao H., Petropulos T., Schneider J., Wakai R.T. Prenatal diagnosis and in utero treatment of torsades de pointes associated with congenital long QT syndrome. Am J Cardiol. 2003;91:1395–1398. doi: 10.1016/s0002-9149(03)00343-6. [DOI] [PubMed] [Google Scholar]
- 10.Miura M., Yamagishi H., Morikawa Y., Matsuoka R. Congenital long QT syndrome and 2:1 atrioventricular block with a mutation of the SCN5A gene. Pediatr Cardiol. 2003;24:70–72. doi: 10.1007/s00246-002-0169-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video clip demonstrating observed fetal tachyarrhythmias during clinical follow-up. The first video segment demonstrates an episode of presumptive polymorphic VT/VF, and the second segment demonstrates an episode of atrial tachyarrhythmia. While presumptive VT/VF was observed frequently, atrial tachyarrhythmia was only rarely observed by fetal echocardiography.