Introduction
Key Teaching Points.
-
•
After coronary ischemia is ruled out, in the setting of new-onset ventricular arrhythmias, it is essential to consider all etiologies of ventricular arrhythmias.
-
•
Cardiac fluorodeoxyglucose–positron emission tomography computed tomography can be utilized to assist in diagnosis and monitor disease activity in cardiac sarcoidosis when magnetic resonance imaging cannot be performed.
-
•
Cardiac sarcoidosis can be an elusive diagnosis and can masquerade as arrhythmogenic right ventricular dysplasia when right ventricular involvement predominates.
Ventricular arrhythmia (VA) is most commonly caused by ischemic heart disease. However, alternative etiologies of VA, including channelopathies, congenital heart disease, other cardiomyopathies such as hypertrophic cardiomyopathy, drugs, electrolyte imbalances, and infiltrative diseases must always be considered on the differential.1 Arrhythmogenic right ventricular dysplasia (ARVD) and cardiac sarcoidosis (CS) are uncommon causes of VA and increase the risk for sudden cardiac death.2, 3 Both diagnoses are far distinct from each other, but they can be confused if a diagnosis is made and alternative diagnoses are not thoroughly considered. We present the case of a 66-year-old man who presented to our hospital in monomorphic ventricular tachycardia (VT) owing to CS mistaken as ARVD.
Case report
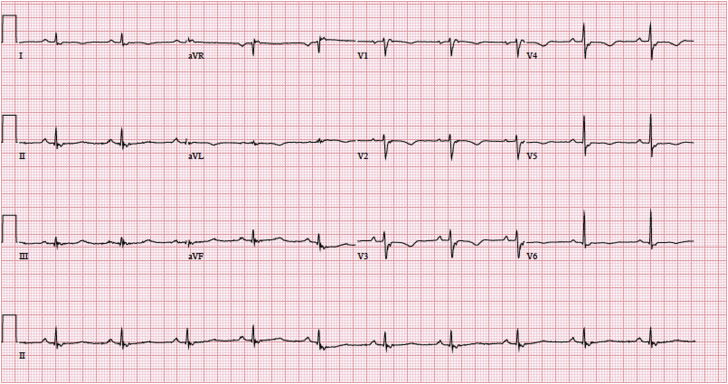
A 66-year-old white man presented to our Emergency Department with abrupt onset of dyspnea, palpitations, and lightheadedness. Prior to his presentation, the patient was in his normal, active state of health. In the Emergency Department, he was found to be in monomorphic VT (initial tracing unavailable); he had electric cardioversion, which resulted in normal sinus rhythm. His significant medical history included a cerebrovascular accident, Factor V Leiden and prothrombin gene mutations, hypertension, pulmonary nodules, and chronic kidney disease. Notably, he had no family history of sudden cardiac death and his medications included losartan and warfarin. Initial laboratory evaluation came back normal and cardiac catheterization ruled out ischemia. His electrocardiogram showed normal sinus rhythm without any evidence of ischemia. However, he was noted to have an epsilon wave in V1, a QRS of 140 ms, and T-wave inversions in V1–V5 (Figure 1). Subsequently, an echocardiogram showed preserved left ventricular function and no valvular heart disease but a markedly dilated and severely reduced right ventricle (RV) systolic function (Figure 2 and Supplemental Figure 1).
Figure 1.
Initial electrocardiogram tracing notable for an epsilon wave in V1, a QRS of 140 ms, and T-wave inversions in V1–V5, all consistent with the diagnosis of arrhythmogenic right ventricular dysplasia.
Figure 2.
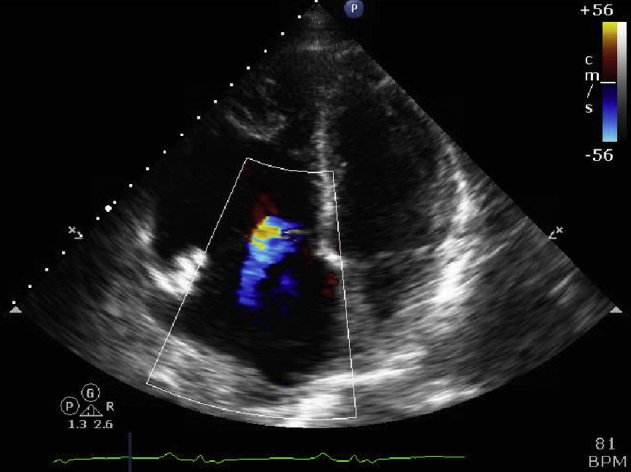
4 chamber view echocardiogram with color doppler demonstrating marked right atrial and ventricular enlargement. Tricuspid annular dilatation with secondary, moderate tricuspid regurgitation is also noted.
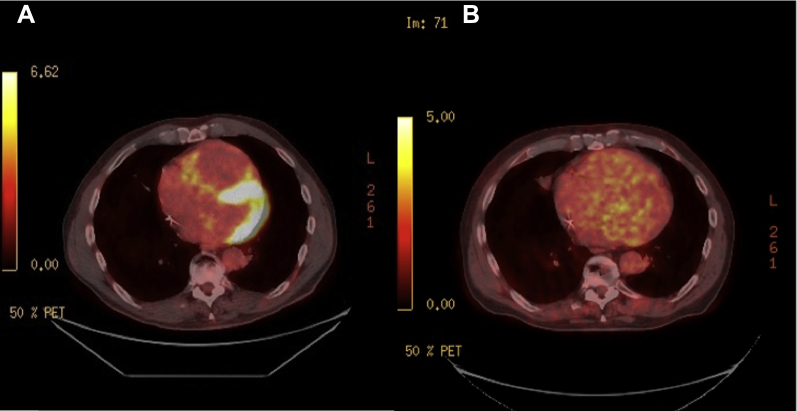
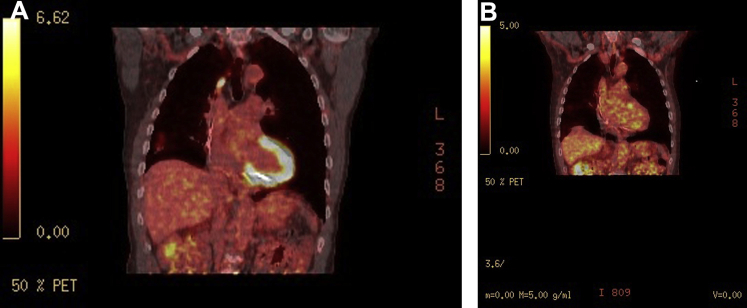
Based on these findings, ARVD was highly suspected. In fact, the patient met several major criteria for ARVD, including severe RV dilation/hypokinesia, RV outflow dimension greater than 32 mm, epsilon wave in V1–V3, and T-wave inversions in V1–V3 with a QRS > 110 ms.4 Cardiac magnetic resonance imaging (MRI) was indicated to further investigate these abnormalities but was not performed owing to the patient’s chronic kidney disease (baseline glomerular filtration rate 40–45 mL/min/1.73m2) and patient preference. Septal endomyocardial biopsy was considered, but owing to its classically poor yield (<25%) without image guidance or electroanatomic mapping, it was deferred.5 The patient was treated with intravenous/oral amiodarone and an implantable cardioverter-defibrillator (ICD) for secondary prevention of VAs. Because the patient had known pulmonary nodules, CS was still in our differential diagnosis. Therefore, after discharge, we obtained a fluorodeoxyglucose (FDG)-positron emission tomography (PET) computed tomography (CT), which noted uptake within multiple, patchy parenchymal pulmonary nodules with concurrent, nearly diffuse left ventricular and patchy right ventricular myocardial uptake (Figure 3A and Supplemental Figure 2A). These are sensitive and specific findings for CS,6 and the patient was started on oral prednisone (40 mg once a day). Despite steroid therapy and improvement of symptomatic dyspnea, the patient received an ICD shock 3 months after initiation of steroids. To monitor response on steroids, a repeat FDG-PET CT was performed after the firing of his ICD. It was notable for persistent cardiac uptake with improving pulmonary uptake. Given his refractory disease and ICD shock, 500 mg twice daily of mycophenolate was added to his 40 mg of prednisone daily.
Figure 3.
A: Transverse fluorodeoxyglucose positron emission tomography computed tomography (FDG-PET CT) demonstrating diffuse uptake within the left ventricular myocardium and patchy uptake within the right ventricular myocardium. B: Repeat transverse FDG-PET CT imaging demonstrating nearly complete resolution of FDG uptake within the right and left ventricular myocardium after the addition of mycophenolate mofetil therapy.
On follow-up FDG-PET CT imaging 5 months later, the patient demonstrated drastic improvement in FDG uptake within the pulmonary parenchyma, as well as in the left and right ventricular myocardium (Figure 3B and Supplemental Figure 2B). Unfortunately, despite the improvement in active inflammation within the myocardium, the patient had recurrence of his VA requiring multiple shocks within 2 weeks of the scan, necessitating hospitalization. These events were investigated further with electroanatomic mapping with plans for VT ablation, which demonstrated a severely dilated RV with grossly abnormal voltages and diffuse late potentials throughout the RV consistent with scar. The patient underwent successful VT radiofrequency ablation via substrate modification of the RV outflow tract and lateral RV around the tricuspid annulus. The patient was continued on metoprolol tartrate, amiodarone, 2 g of mycophenolate, and prednisone. With 3 months of follow-up there has been no VA recurrence.
Discussion
Although rare, CS presenting as ARVD has been reported.7, 8, 9, 10 Similar to our case, a common theme among these cases is abnormal findings on initial imaging studies leading to ARVD as the diagnosis. However, indirect clues such as pulmonary nodules and lymphadenopathy on radiographic images should lead to the consideration of CS as an alternative diagnosis. Thus, it is important to keep a broad differential, and alternative diagnoses should be ruled out with confirmatory testing before committing to a rare final diagnosis. Despite similar initial treatments for secondary prevention of VAs with antiarrhythmic drug therapy (in this case, amiodarone) and ICD placement, the long-term treatment plans for CS and ARVD differ greatly, so appropriate diagnosis is critical.
According to the revised task force criteria for ARVD by Marcus and colleagues,11 multiple major and minor criteria may be used to diagnose ARVD, including imaging demonstrating right ventricular alterations, tissue diagnosis, repolarization abnormalities, depolarization/conduction abnormalities, documented VAs, and family history. To date, there is no specific ARVD targeting treatment other than antiarrhythmic agents and ICD.1 Once ARVD is diagnosed, screening of family members should be offered for early detection of the disease.1
On the other hand, CS is classically a pathologic diagnosis, but in clinical practice endomyocardial biopsies can be unreliable owing to its patchy involvement.5, 12 Retrospective studies demonstrate a 20%–50% rate of pathologic diagnosis with premortem endomyocardial biopsies.5, 12 The major diagnostic criteria for CS include advanced atrioventricular block, basal thinning of the ventricular septum, positive cardiac gallium uptake, and LV ejection fraction < 50%. However, in a meta-analysis by Youssef and colleagues,6 FDG-PET CT has been shown to have pooled sensitivity and specificity of 89% and 78%, respectively. FDG-PET CT is an imaging alternative in diagnosis when cardiac MRI is unable to be obtained. CS patients can be treated initially with prednisone and other steroid-sparing agents may be added, such as methotrexate, azathioprine, or mycophenolate.12, 13 In refractory cases, anti–tumor necrosis factor therapies, specifically infliximab, have been successful in achieving remission.14
Conclusion
Initial treatment is similar for all causes of VAs, but long-term management of the underlying cause is crucial. First, ischemia should be evaluated; but then other etiologies of VAs, including channelopathies, congenital heart disease, cardiomyopathies (such as hypertrophic cardiomyopathy), drugs, electrolyte imbalance, and infiltrative diseases should be considered.1 In this case, FDG-PET CT was used to monitor therapy and assist in the diagnosis of CS.
CS is a rare but important cause of VA and should be pursued when the etiology of a VA is unclear. As demonstrated in this case and prior reports in the literature, treatable infiltrative disorders can mimic ARVD and should be ruled out with further imaging with cardiac MRI or FDG-PET CT.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.hrcr.2017.06.008.
Appendix. Supplementary Data
Supplementary Figure 1.
Supplementary Figure 2.
References
- 1.Koplan B.A., Stevenson W.G. Ventricular tachycardia and sudden cardiac death. Mayo Clin Proc. 2009;84:289–297. doi: 10.4065/84.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabib A., Loire R., Chalabreysse L. Circumstances of death and gross and microscopic observations in a series of 200 cases of sudden death associated with arrhythmogenic right ventricular cardiomyopathy and/or dysplasia. Circulation. 2003;108:3000–3005. doi: 10.1161/01.CIR.0000108396.65446.21. [DOI] [PubMed] [Google Scholar]
- 3.Mehta D., Mori N., Goldbarg S.H., Lubitz S., Wisnivesky J.P., Teirstein A. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol. 2011;4:43–48. doi: 10.1161/CIRCEP.110.958322. [DOI] [PubMed] [Google Scholar]
- 4.Romero J., Mejia-Lopez E., Manrique C., Lucariello R. Arrhythmogenic right ventricular cardiomyopathy (ARVC/D): a systematic literature review. Clin Med Insights Cardiol. 2013;7:97–114. doi: 10.4137/CMC.S10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel B., Shah M., Gelaye A. A complete heart block in a young male: a case report and review of literature of cardiac sarcoidosis. Heart Fail Rev. 2017;22:55. doi: 10.1007/s10741-016-9585-0. [DOI] [PubMed] [Google Scholar]
- 6.Youssef G., Leung E., Mylonas I. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–248. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- 7.Chia P.L., Subbiah R.N., Kuchar D., Walker B. Cardiac sarcoidosis masquerading as arrhythmogenic right ventricular cardiomyopathy. Heart Lung Circ. 2012;21:42–45. doi: 10.1016/j.hlc.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Mohsen A., Panday M., Wetherold S., Jimenez A. Cardiac sarcoidosis mimicking arrhythmogenic right ventricular dysplasia with high defibrillation threshold requiring subcutaneous shocking coil implantation. Heart Lung Circ. 2012;21:46–49. doi: 10.1016/j.hlc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Yared K., Johri A.M., Soni A.V. Cardiac sarcoidosis imitating arrhythmogenic right ventricular dysplasia. Circulation. 2008;118:e113–e115. doi: 10.1161/CIRCULATIONAHA.107.755215. [DOI] [PubMed] [Google Scholar]
- 10.Steger C.M., Hager T., Antretter H. Cardiac sarcoidosis mimicking arrhythmogenic right ventricular dysplasia. BMJ Case Rep. 2009;8:2204. doi: 10.1136/bcr.08.2009.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus F.I., McKenna W.J., Sherrill D. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur Heart J. 2010;121:1533–1541. [Google Scholar]
- 12.Dubrey S.W., Falk R. Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis. 2010;52:336–346. doi: 10.1016/j.pcad.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Birnie D.H., Nery P.B., Ha A.C., Beanlands R.S.B. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 14.Uthman I., Touma Z., Khoury M. Cardiac sarcoidosis responding to monotherapy with infliximab. Clin Rheumatol. 2007;26:2001–2003. doi: 10.1007/s10067-007-0614-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.