ABSTRACT
Biofilm-associated infections are a clinical challenge, in part because a hydrated matrix protects the bacterial community from antibiotics. Herein, we evaluated how different osmotic compounds (maltodextrin, sucrose, and polyethylene glycol [PEG]) enhance antibiotic efficacy against Acinetobacter baumannii biofilm communities. Established (24-h) test tube biofilms (strain ATCC 17978) were treated with osmotic compounds in the presence or absence of 10× the MIC of different antibiotics (50 μg/ml tobramycin, 20 μg/ml ciprofloxacin, 300 μg/ml chloramphenicol, 30 μg/ml nalidixic acid, or 100 μg/ml erythromycin). Combining antibiotics with hypertonic concentrations of the osmotic compounds for 24 h reduced the number of biofilm bacteria by 5 to 7 log (P < 0.05). Increasing concentrations of osmotic compounds improved the effect, but there was a trade-off with increasing solution viscosity, whereby low-molecular-mass compounds (sucrose, 400-Da PEG) worked better than higher-mass compounds (maltodextrin, 3,350-Da PEG). Ten other A. baumannii strains were similarly treated with 400-Da PEG and tobramycin, resulting in a mean 2.7-log reduction in recoverable bacteria compared with tobramycin treatment alone. Multivariate regression models with data from different osmotic compounds and nine antibiotics demonstrated that the benefit from combining hypertonic treatments with antibiotics is a function of antibiotic mass and lipophilicity (r2 > 0.82; P < 0.002), and the relationship was generalizable for biofilms formed by A. baumannii and Escherichia coli K-12. Augmenting topical antibiotic therapies with a low-mass hypertonic treatment may enhance the efficacy of antibiotics against wound biofilms, particularly when using low-mass hydrophilic antibiotics.
IMPORTANCE Biofilms form a barrier that protects bacteria from environmental insults, including exposure to antibiotics. We demonstrated that multiple osmotic compounds can enhance antibiotic efficacy against Acinetobacter baumannii biofilm communities, but viscosity is a limiting factor, and the most effective compounds have lower molecular mass. The synergism between osmotic compounds and antibiotics is also dependent on the hydrophobicity and mass of the antibiotics. The statistical models presented herein provide a basis for predicting the optimal combination of osmotic compounds and antibiotics against surface biofilms communities.
KEYWORDS: Acinetobacter baumannii, biofilm, osmotic agent, viscosity, biphasic response, Acinetobacter, antibiotic resistance, antibiotic treatment, hypertonic, infection
INTRODUCTION
Bacterial biofilms consist of compact bacterial communities that are enclosed in a polymeric matrix (1). Biofilms can adhere to inert or living surfaces, and the bacterial communities within biofilms express phenotypes that are distinct from free-floating cells, including increased tolerance to antibiotics (2–4). In fact, bacteria within biofilms can be up to 1,000 times more resistant to antimicrobial agents than those in planktonic cultures (5). Acinetobacter baumannii has emerged as an important nosocomial pathogen (6) that can cause urinary tract infections, secondary meningitis, wound and burn infections, and pneumonia (7). A. baumannii is a prolific biofilm producer, which partly explains its resistance to antibiotics, its survival in hospital environments, and its disease potential. A. baumannii can form biofilms on both abiotic (polystyrene and glass) and biotic (e.g., epithelial cells and fungal filaments) surfaces (8, 9). It has also been isolated from deep blast wound infections among military personnel in combat zones (10–12). New strategies are needed to eliminate A. baumannii biofilms from wound surfaces.
Standard antimicrobial treatments typically fail to eradicate biofilm infections, leading to chronic infections and the need for surgical removal of afflicted tissues. Several studies suggest that combinations of different antibiotics may work more effectively against biofilm-associated infections, but it is debatable if this is a prudent practice (13–15). Other investigators have studied the effect of osmotic stress on biofilms as a secondary means to attack these communities. In one study, 6 M NaCl reduced the number of CFU of a dual-species biofilm consisting of Enterococcus faecalis and Pseudomonas aeruginosa by 6 log after 72 h of exposure to the osmotic treatment (16). In another study, 0.4 M NaCl reduced the biomass and changed the gene expression of Streptococcus mutans biofilms (17). Combining maltodextrin with vancomycin improved antibiotic efficacy against Staphylococcus aureus biofilms (18). Sultana et al. (19) also demonstrated that a hydrogen peroxide-producing electrochemical scaffold was more effective against biofilms when operated in the presence of a hypertonic concentration of maltodextrin.
For the present study, we evaluated the efficacies of different antibiotics and osmotic compounds (maltodextrin, sucrose, and polyethylene glycol [PEG]) against A. baumannii biofilm communities. Sucrose and maltodextrin are nontoxic carbohydrates that are considered safe for general use and thus may be suitable for clinical applications (20). PEG is commonly used for different clinical purposes, including for dermatological preparations (21). Biofilms were cultured for 24 h before treatment, and the effects were evaluated by comparing the number of recoverable bacteria from untreated and treated biofilms. We found that the most effective treatments against A. baumannii biofilms combined hypertonic concentrations of lower-mass osmotic compounds with lower-mass hydrophilic antibiotics.
RESULTS
Effect of osmotic compounds on A. baumannii culture.
To assess the potential of osmotic compounds to alter bacterial growth, we determined if the optical density of A. baumannii culture (ATCC 17978) differed in the presence of these compounds. The least-squares mean difference in optical density at 600 nm (OD600) for culture alone compared with culture and osmotic compound was significantly higher for 20 mM maltodextrin (OD600, 0.299) than for sucrose (OD600, 0.055), PEG 3350 (OD600, −0.046), and PEG 400 (OD600, −0.031). These results are consistent with A. baumannii using maltodextrin as a carbon source, and this could be a confounding factor in our analysis and in clinical applications.
Maltodextrin restores antibiotic efficacy against A. baumannii preformed biofilms.
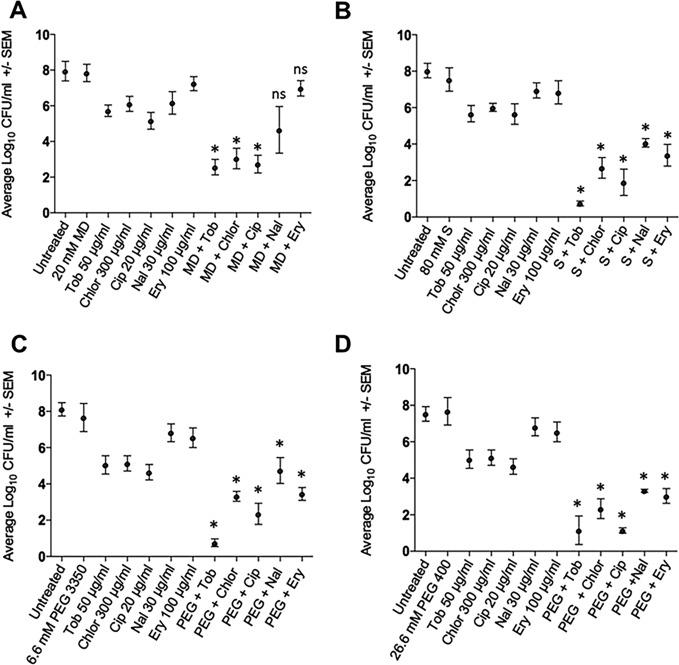
We next determined if a combinatorial treatment of maltodextrin (20, 40, or 60 mM) and tobramycin (50 μg/ml) would reduce the viability of A. baumannii biofilms more than treatment with antibiotic alone. Maltodextrin alone at all concentrations had no measurable effect on the A. baumannii biofilm population compared to the untreated control (Fig. 1A, P > 0.05). Treatment with tobramycin alone reduced cell recovery by 2.2 log (P < 0.05), but the combination of tobramycin with 20 mM maltodextrin reduced cell counts by ∼5.5 log. At higher maltodextrin concentrations (40 and 60 mM), the effect was reversed (Fig. 1A). Relative to individual antibiotic treatment alone, the enhanced efficacy was also evident for 20 mM maltodextrin combined with chloramphenicol and ciprofloxacin (P < 0.05) but not for nalidixic acid and erythromycin (P ≥ 0.13) (Fig. 2A). The enhanced antibiotic effect was lost between tobramycin and higher concentrations of maltodextrin, consistent with either confounded results from compensatory growth in higher concentrations of maltodextrin or reduced antibiotic diffusion with increasing viscosity of the solution. Consistent with the latter supposition, room temperature measurements indicated a >2-fold increase in viscosity between 20 and 60 mM maltodextrin (Table 1).
FIG 1.
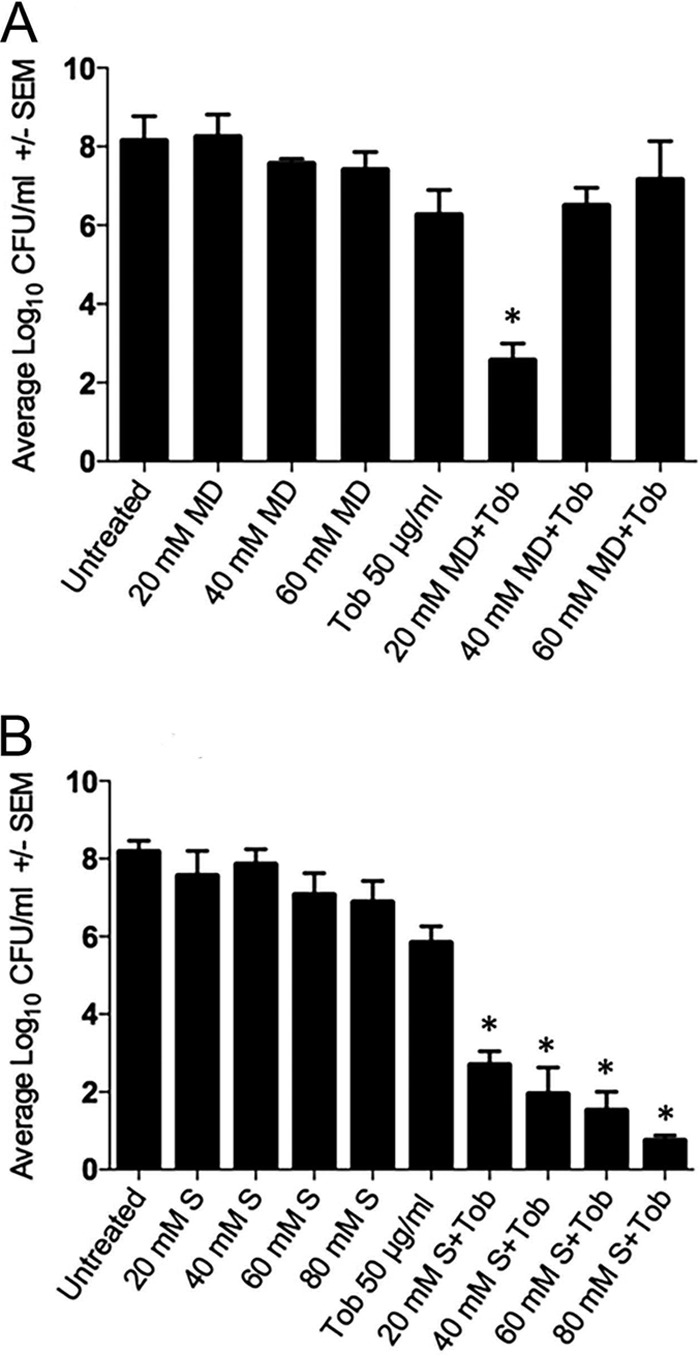
Effects of osmotic compounds maltodextrin (MD) (A) and sucrose (S) (B) with or without the antibiotic tobramycin (Tob) against Acinetobacter baumannii preformed biofilms. Error bars represent the standard errors of the means (SEM) calculated from three biological replicates. Statistical significance was calculated using a one-way ANOVA and Tukey's test for tobramycin with or without the osmotic compound (*, P < 0.05).
FIG 2.
Acinetobacter baumannii biofilm treated with antibiotics alone or in combination with maltodextrin (MD, 20 mM) (A), sucrose (80 mM) (B), 3,350-Da PEG (6.6 mM) (C), or 400-Da PEG (26.6 mM). Antibiotics included tobramycin (Tob; 50 μg/ml), chloramphenicol (Chlor; 300 μg/ml), ciprofloxacin (Cip; 20 μg/ml), nalidixic acid (Nal; 30 μg/ml), and erythromycin (Ery; 100 μg/ml). Error bars represent the standard errors of the means calculated from three biological replicates. Statistical significance was calculated using one-way ANOVA with paired comparisons (Tukey's test) for individual antibiotics with or without osmotic agent (*, P < 0.05; ns, not significant).
TABLE 1.
Osmotic agents used in this study
| Osmotic agent | Mass (Da; g/mol) | Concn |
Viscosity (cP)a | Osmotic pressure (atm)b | |
|---|---|---|---|---|---|
| mM | mg/ml | ||||
| Maltodextrin | 2,555 | 20.0 | 51.1 | 3 | 0.48 |
| 40.0 | 102.2 | ND | 0.97 | ||
| 60.0 | 153.3 | 7 | 1.46 | ||
| Sucrose | 342 | 20.0 | 6.84 | ND | 0.48 |
| 40.0 | 13.68 | ND | 0.97 | ||
| 60.0 | 20.52 | ND | 1.46 | ||
| 80.0 | 27.36 | 2 | 1.95 | ||
| 260.0 | 889.2 | 3 | 6.35 | ||
| PEG 3350 | 3,350 | 6.6 | 22.11 | 1 | 0.48 |
| 52.8 | 176.88 | 5 | 3.87 | ||
| PEG 400 | 400 | 26.6 | 10.64 | 2 | 1.95 |
| 345.8 | 1,383.2 | 3 | 24.9 | ||
cP, centipoise, a unit of viscosity. ND, not determined.
atm, atmosphere, a unit of osmotic pressure.
Sucrose combined with antibiotics.
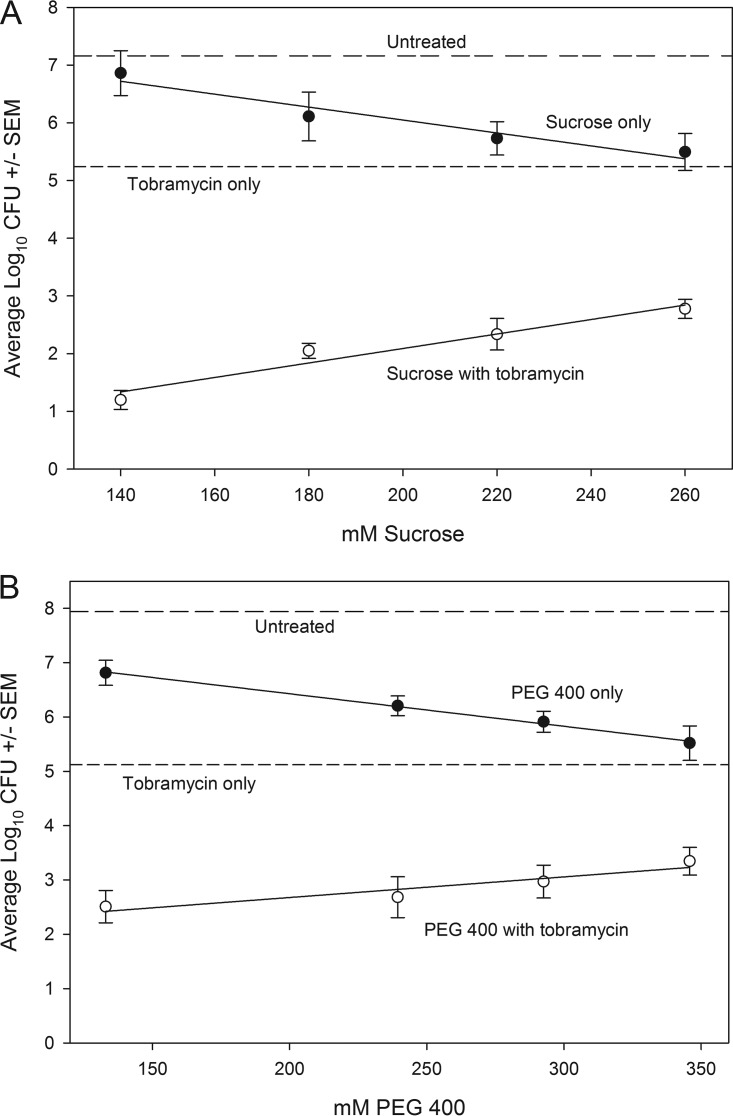
Because viscosity may be a limiting factor, we next determined if a lower-mass osmotic compound (sucrose, 342 Da) would alleviate the viscosity interference that we observed with maltodextrin (2,555 Da). The addition of 20 mM sucrose (0.48 atm) with 50 μg/ml tobramycin produced a significant reduction in the number of A. baumannii bacteria (∼3 log) that improved as the concentration was increased to 80 mM (∼5 log) (Fig. 1B). We subsequently treated biofilms with 80 mM sucrose and observed increased efficacies for all antibiotics that were tested (Fig. 2B). Further increased concentrations of sucrose, as expected, eventually resulted in decreasing effectiveness that is likely attributable to increasing viscosity (Fig. 3A and Table 1). Thus, the biphasic response observed with maltodextrin (Fig. 1A) was evident with sucrose (Fig. 1B and 3A), but the dynamic range of synergy with antibiotic efficacy encompassed a larger concentration range for sucrose.
FIG 3.
Acinetobacter baumannii biofilms treated with tobramycin (Tob; 50 μg/ml) and increasing concentrations of sucrose (A) or 400-Da PEG (B). Log10 CFU for untreated biofilm or biofilm treated with tobramycin are depicted as horizontal lines. The other lines depict concentration effects for sucrose (A) or PEG 400 (B) alone or with tobramycin. Error bars represent the standard errors of the means calculated from three independent replicates.
PEG combined with antibiotics.
We selected 3,350-Da PEG (PEG 3350) as an alternative osmotic compound because it has a mass similar to that of maltodextrin but is not metabolized. For comparative purposes, we used 6.6 mM PEG 3350 because it should produce an osmotic pressure equivalent to 20 mM maltodextrin (Table 1). PEG 3350 alone had no effect on cell recovery from the A. baumannii biofilm, but in combination with antibiotics, there was a clear synergistic effect (P < 0.05; Fig. 2C). Increasing the concentration of PEG 3350 to 52.8 mM was sufficient to demonstrate decreasing antibiotic efficacy (similar to maltodextrin), presumably due to increasing viscosity (Table 1).
We also characterized the effect of PEG 400 because of the similarity in mass with 342-Da sucrose. Because 80 mM sucrose was effective with different antibiotics, we used 26.6 mM PEG 400 to normalize osmotic pressure for comparative purposes (Table 1). As with other osmotic agents, antibiotic efficacy was enhanced significantly in the presence of PEG 400 (P < 0.05; Fig. 2D). Like sucrose, further increases in PEG 400 concentration eventually resulted in a diminishing benefit (Fig. 3B), but the dynamic range of effective concentrations was much greater than that of maltodextrin (Fig. 1A). To determine if these findings were applicable to a diversity of A. baumannii strains, we applied the PEG 400 and 50 μg/ml tobramycin treatment to 10 additional strains (Table 2). Log reductions were evident for all strains, with a mean reduction of 2.72 log compared with tobramycin treatment alone (paired t test, P < 0.0001). Three of the strains were positive for pellicle formation, as defined by Nait Chabane et al. (22), but there was no significant difference in log reduction between pellicle-forming (n = 3) and non-pellicle-forming (n = 7) strains (t test, P = 0.18) (Table 2).
TABLE 2.
Acinetobacter baumannii strains used in this study
| Strain IDa | BEI designationb | Isolate informationc | Log10 CFUd |
Differencee | ||
|---|---|---|---|---|---|---|
| Untreated | Tobramycin only | Tobramycin and PEG 400 | ||||
| ATCC 17978 | NA | NA | 7.53 ± 0.13 | 5.24 ± 0.30 | 1.15 ± 0.26 | 4.09 |
| ATCC 19606 | NA | Urine isolate, 1948 | 8.19 ± 0.12 | 5.70 ± 0.12 | 1.92 ± 0.17 | 3.78 |
| Isolate 9 | NR-13382 | Blood isolate 2008 | 8.10 ± 0.14 | 6.17 ± 0.18 | 3.05 ± 0.12 | 3.12 |
| 3-137 (OIFC137) | NR-17777 | Catheter isolate, 2003 | 8.02 ± 0.13 | 5.89 ± 0.20 | 2.95 ± 0.36 | 2.94 |
| 5-032 (OIFC132)f | NR-17778 | Wound isolate, 2003 | 8.04 ± 0.22 | 5.46 ± 0.14 | 3.64 ± 0.15 | 1.82 |
| 5-143 (OIFC134)f | NR-17781 | Wound isolate, 2003 | 8.09 ± 0.06 | 6.65 ± 0.18 | 3.80 ± 0.13 | 2.85 |
| H72721 | NR-9667 | Sputum isolate, 2006 | 7.83 ± 0.10 | 5.46 ± 0.14 | 1.77 ± 0.09 | 3.69 |
| 5-189 (OIFC189) | NR-17782 | Human isolate, no yr | 7.94 ± 0.08 | 6.17 ± 0.11 | 4.50 ± 0.17 | 1.67 |
| BC-5 | NR-17783 | Nosocomial isolate, 2007 | 8.20 ± 0.18 | 5.76 ± 0.15 | 2.11 ± 0.08 | 3.65 |
| Naval-18f | NR-17785 | Wound isolate, 2006 | 7.97 ± 0.12 | 5.81 ± 0.17 | 3.80 ± 0.25 | 2.01 |
| IS-123 | NR-17787 | Wound isolate, 2009 | 8.11 ± 0.09 | 6.15 ± 0.19 | 4.46 ± 0.19 | 1.69 |
ATCC strains were obtained from the American Type Culture Collection (www.atcc.org). All others were obtained from BEI Resources, NIAID, NIH (www.beiresources.org). ID, identification. All strains were resistant to ampicillin and sensitive to tobramycin in broth culture.
Strain designation from BEI Resources.
Basic information about strains as supplied by vendors.
Log10 CFU expresses the log10-transformed average number of CFU (± standard error of the mean) recovered from untreated biofilms and those treated with 50 μg/ml tobramycin or 50 μg/ml tobramycin and PEG 400 (26.6 mM).
Difference is the log10 CFU for biofilms treated only with tobramycin minus the log10 CFU for biofilms treated with tobramycin and PEG 400 (mean, 2.72 log10 CFU), which was significantly greater than zero (t = 10.027, df = 9, P < 0.0001). The difference for ATCC 17978 was not used for statistical comparisons.
Strains that form a pellicle according to the methods of Nait Chabane et al. (22). The treatment effect was not different between pellicle-forming (n = 3; mean, 2.23 log10 CFU difference) and non-pellicle-forming (n = 7; mean, 2.9 log10 CFU) strains (t = −1.83, df = 6.5, P = 0.18).
Factors affecting antibiotic efficacy under hypertonic conditions.
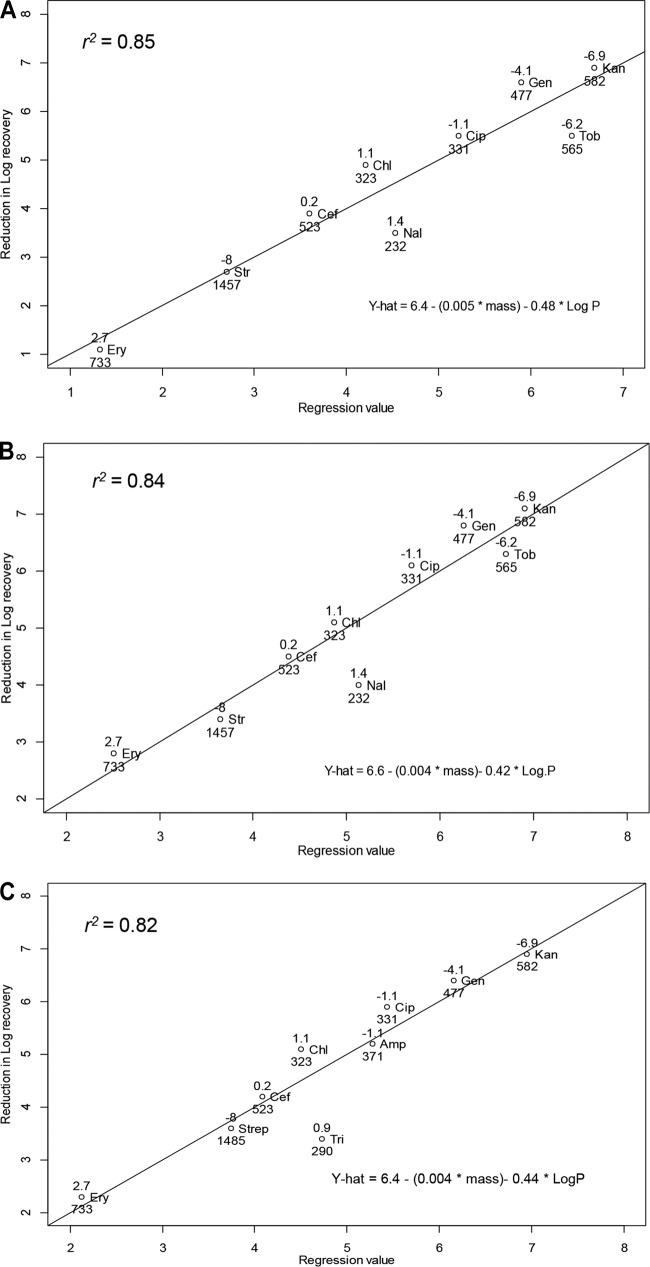
To determine if there are characteristics of individual antibiotics that affect the magnitude of the synergy with osmotic compounds, we evaluated the difference in log reduction for antibiotic alone compared with that under antibiotic and hypertonic conditions (20 mM maltodextrin or 26.6 mM PEG 400). A variety of chemical properties were collated for different antibiotics, including mass, charge, complexity, and lipophilicity (partial coefficient, log P). The difference in log reduction could not be explained by any single variable (P > 0.05), but a multivariate regression model with mass and log P (Table 3) produced a statistically significant fit for the maltodextrin (r2 = 0.85, P = 0.001; Fig. 4A) and PEG 400 conditions (r2 = 0.84, P = 0.001; Fig. 4B). As an independent assessment of the robustness of this relationship, we used the same methods to assess the impact of PEG 400 and antibiotic efficacy against E. coli K-12 biofilms and found a similar robust relationship between efficacy and mass and log P (r2 = 0.82, P = 0.002; Fig. 4C). The interaction terms were not significant for these models (and were excluded). All three multivariate regression models had remarkably similar regression coefficients for these experiments (Fig. 4A to C).
TABLE 3.
Antibiotics used in this studyc
| Antibiotic | Class | Log10 CFUa |
Mass (g/mol) | Log Pb | |||
|---|---|---|---|---|---|---|---|
| Maltodextrin |
PEG 400 |
||||||
| Untreated | Treated | Untreated | Treated | ||||
| Tobramycin | Aminoglycoside | 8.14 ± 0.18 | 2.56 ± 0.14 | 7.53 ± 0.13 | 1.15 ± 0.26 | 565 | −6.2 |
| Gentamicin | Aminoglycoside | 8.07 ± 0.12 | 1.4 ± 0.15 | 8.31 ± 0.16 | 1.5 ± 0.04 | 477 | −4.1 |
| Kanamycin | Aminoglycoside | 8.17 ± 0.12 | 1.25 ± 0.34 | 8.21 ± 0.13 | 1.1 ± 0.24 | 582 | −6.9 |
| Ciprofloxacin | Fluoroquinolone | 8.27 ± 0.16 | 2.73 ± 0.16 | 7.24 ± 0.2 | 1.14 ± 0.05 | 331 | −1.1 |
| Chloramphenicol | Amphenicol | 7.8 ± 0.09 | 3.04 ± 0.19 | 7.5 ± 0.03 | 2.34 ± 0.18 | 323 | 1.1 |
| Ceftiofur | Cephalosporin | 7.92 ± 0.16 | 4.2 ± 0.05 | 7.89 ± 0.08 | 3.35 ± 0.26 | 523 | 0.2 |
| Nalidixic acid | Quinolone | 8.27 ± 0.19 | 4.75 ± 0.01 | 7.65 ± 0.01 | 3.62 ± 0.03 | 232 | 1.4 |
| Streptomycin | Aminoglycoside | 8.22 ± 0.2 | 5.46 ± 0.03 | 7.96 ± 0.03 | 4.51 ± 0.23 | 1,457 | −8 |
| Erythromycin | Macrolide | 8.21 ± 0.16 | 7.18 ± 0.14 | 8.18 ± 0.19 | 5.3 ± 0.13 | 733 | 2.7 |
| Ampicillin | Beta-lactam | ND | ND | 7.79 ± 0.15 | 4.44 ± 0.17 | 371 | −1.1 |
| Trimethoprim | Miscellaneous | ND | ND | 7.61 ± 0.16 | 4.11 ± 0.32 | 290 | 0.9 |
Log10 CFU expresses the log10-transformed average number of cells recovered (± standard error of the mean) from untreated biofilms and number of cells recovered from biofilms that were treated with antibiotic and maltodextrin (20 mM) or PEG 400 (26.6 mM).
Log P is the partial coefficient between n-octanol and water. A high log P value indicates greater lipophilicity, whereas a low value indicates greater hydrophilicity.
Log P and mass values were obtained from PubChem Compound (https://www.ncbi.nlm.nih.gov/pccompound/). ND, not determined.
FIG 4.
The log reduction in recoverable CFU from A. baumannii and E. coli K-12 biofilms can be predicted from a linear model with antibiotic mass (lower number) and log P (upper number). A very similar linear relationship was evident for 20 mM maltodextrin (A) and 26.6 mM 400-Da PEG (B) and 26.6 mM 400-Da PEG (C) used for E. coli K-12 biofilm. Antibiotics included tobramycin (Tob; 50 μg/ml), chloramphenicol (Chlor; 300 μg/ml), ciprofloxacin (Cip; 20 μg/ml), nalidixic acid (Nal; 30 μg/ml), erythromycin (Ery; 100 μg/ml), streptomycin (Strep; 140 μg/ml), kanamycin (Kan; 30 μg/ml), gentamicin (Gent; 160 μg/ml), and ceftiofur (Cef; 100 μg/ml). The same antibiotics were used to treat E. coli K-12 biofilms, except nalidixic acid was replaced with trimethoprim (Tri; 40 μg/ml) and tobramycin was replaced with ampicillin (Amp; 160 μg/ml).
The trade-off between hydrophilicity (negative log P) and lipophilicity (positive log P) could influence how an antibiotic penetrates into a biofilm or how it interacts with bacterial membranes. To assess how it interacts with bacterial membranes, we compared the effect of two antibiotics having different log P values (kanamycin, log P = −6.9, hydrophilic; erythromycin, log P = 2.7, lipophilic). When A. baumannii was cultured in the presence of 0.5× the MIC of antibiotic (1.9 μg/ml kanamycin or 6.3 μg/ml erythromycin) with or without the addition of 26.6 mM PEG 400, there was no significant difference in the OD600 at 24 h (paired t test, t = 1.018, df = 3, P = 0.42, and t = 2.53, df = 2, P = 0.13, respectively). That is, under hyperosmotic conditions, the benefit of more hydrophilic antibiotics (Fig. 4) is likely due to better penetration of these antibiotics into the extracellular polymeric substance (EPS) matrix of biofilms rather than how these antibiotics interact with the bacteria per se.
DISCUSSION
Antibiotic treatment alone is often inadequate to overcome biofilm infections (23, 24). Treatment with hypertonic concentrations of osmotic compounds is a potential alternative therapy with and without antibiotics (16, 17, 25), although the mechanism of effect remains speculative, and the effect is rarely evaluated in combination with different antibiotics. We tested hypertonic concentrations of four osmotic compounds, and while we observed no benefit using the osmotic compounds alone, in all cases, we observed a significant reduction in recoverable biofilm cell counts when an “optimal” concentration of osmotic compound was combined with antibiotics. A validation experiment (10 A. baumannii strains) that combined PEG 400 and tobramycin demonstrated reductions in total CFU that were 45- to 6,000-fold (1.67 to 3.78 log) more effective than when using tobramycin alone (Table 2).
Our findings showed that the increased efficacy from combining hypertonic treatment with antibiotics was dependent on the mass of the osmotic compound and the mass and lipophilicity of the antibiotic that was used. Larger-mass osmotic compounds (maltodextrin and PEG 3350) exhibited a sharp decrease in synergistic effect with relatively small increases in concentration of these compounds (i.e., a “U”-shaped response). This concentration-dependent effect is likely due to the increasing viscosity of the solution. Higher viscosity reduces diffusivity of antibiotics within the treatment solution and therefore might reduce antibiotic penetration into biofilms. This is consistent with the findings of a previous study (18) confirming decreased diffusivity at higher concentrations of a high-mass compound, such as maltodextrin. This also suggests that larger-mass osmotic compounds may be less effective when treating wounds, because changing fluid characteristics in wound beds over time (26) will make it difficult to control the concentration of the osmotic compounds in proximity to the wound surface. Lower-mass osmotic compounds (e.g., PEG 400 and sucrose) appear to be inherently less sensitive to this problem. For example, in the presence of 50 μg/ml tobramycin, increasing the concentration of maltodextrin from 20 mM to 40 mM resulted in a much higher CFU count (≈3 log; Fig. 1A), whereas increasing the concentration of PEG 400 by 212 mM resulted in a ≈1-log increase (Fig. 3B).
While viscosity is the most likely explanation for the U-shaped response observed in Fig. 1A, maltodextrin can be used as a carbon source by A. baumannii, and this may be a confounding factor in our analysis. For example, this might allow compensatory growth of A. baumannii, which in turn could diminish the efficacy of maltodextrin at higher concentrations (Fig. 1A). If this effect occurs, it is probably minimal relative to viscosity, because we observed a similar U-shaped response for high-molecular-mass PEG (which is not metabolized). It is also possible that a subpopulation of biofilm bacteria becomes quiescent in biofilms, and the introduction of maltodextrin might enhance metabolic activity, making the cells more susceptible to antibiotics (27, 28). In our system, however, this is unlikely to be the case, because the relationship between cell reduction and antibiotic properties was nearly identical for maltodextrin and the nonnutrient osmotic compound PEG 400 when tested with multiple classes of antibiotics (Fig. 4A to C).
PEG is commonly used as a component of pharmaceutical products because it can enhance drug uptake in vivo (21). For example, Du et al. (29) conjugated PEG (5,000 Da) to tobramycin and reported increased efficacy against in vivo Pseudomonas aeruginosa biofilm. Another study showed that ciprofloxacin could be conjugated to PEG (2,000 Da), and this enhanced antibiotic efficacy against both Gram-negative and -positive bacteria (30). Our experiments did not involve conjugation between the osmotic agents and antibiotics, although this is probably requisite for successful applications in vivo. While it is also probable that the mechanisms underlying our findings and those reported for PEG-conjugated antibiotics are different, the work on PEG-conjugated antibiotics highlights the fact that PEG is compatible with antibiotic applications and likely preferable to using maltodextrin, or even sucrose.
We included a diversity of antibiotics in this study as a means to identify characteristics suitable for predicting how the efficacy of antibiotics might change under hypertonic conditions. For the concentrations of osmotic compounds reported in Fig. 4, the magnitude of cell reduction was reliably predicted (r2 ≥ 0.84) as a linear function of antibiotic mass and log P (Fig. 4). Several investigators (25, 31) have surmised that hypertonic treatments increase diffusivity by damaging the biofilm structure and reducing the distance that antibiotics must diffuse to reach the bulk of the biofilm cells that are typically located near the bottom of the biofilm. All things being equal, mass is directly related to diffusivity (32), and thus, larger-mass antibiotics may not reach biofilm cells as quickly or in sufficient concentration relative to lower-mass antibiotics. This is somewhat evident by the limited reduction in cell count for large-mass antibiotics, such as streptomycin (1,457 Da) and erythromycin (733 Da), but this mass-only relationship was a poor predictor for other antibiotics, including nalidixic acid (232 Da) and kanamycin (582 Da) (Fig. 4).
Lipophilicity is an important property for crossing cell membranes (33), but by itself, this parameter was a poor predictor of cell recovery after treatment. All of the antibiotics that we tested must pass through or interact with the bacterial cell membrane, and thus, lipophilicity might influence how well these antibiotics function once they reach cells within the biofilms. Our planktonic culture data, however, indicated that the addition of PEG 400 had no effect on bacterial growth when combined with either a hydrophilic (kanamycin, at 0.5× the MIC) or lipophilic (erythromycin, at 0.5× the MIC) antibiotic. That is, in the presence of a hypertonic solution, the hydrophilicity of the antibiotic is important for penetration into biofilms rather than for penetration of the bacterial cells themselves.
The utility of the multivariate models presented in Fig. 4 can be illustrated when considering how polymyxin treatment might be improved when combined with osmotic compounds. Polymyxin is frequently used as an antibiotic against A. baumannii infections (34). This antibiotic has a relatively high mass (1,203 Da) and low lipophilicity (log P = −2.5), suggesting that combining polymyxin with a low-mass osmotic compound will improve performance, although the benefit will be less than that with other antibiotics (estimated 2.8-log reduction, based on Fig. 4B). Importantly, given that the composition and potentially the degree of hydrophobicity of biofilms may vary between species (35), the relationship that we found may vary depending on the pathogen of interest. As a means to partially address this limitation, we included an analysis of PEG 400 synergy with antibiotics against E. coli K-12 biofilms (Fig. 4C) and found a relationship very similar to that in our findings with A. baumannii (Fig. 4A and B). Empirical evaluation of other bacteria is still needed, but the E. coli findings are consistent with the possibility that the findings from this work can be generalized to other Gram-negative bacteria.
We were able to demonstrate a clear synergy between hypertonic treatment with osmotic compounds and antibiotics. The effective concentration was limited for large-mass compounds (e.g., maltodextrin), but small-mass compounds provide a much greater range of effective concentrations. Given the advantages of small-mass compounds and because PEG is already commonly used in pharmaceutical products, we surmise that a combination of PEG 400 and hydrophilic antibiotics shows considerable promise for more effective removal of A. baumannii biofilms, particularly as a topological application against A. baumannii biofilms in infected wound beds. This relationship may hold for other biofilm infections, but additional testing is warranted.
MATERIALS AND METHODS
Bacterial strain and reagents.
Eleven Acinetobacter baumannii strains were used in this study (Table 2), although strain ATCC 17978 was used for most experiments. Escherichia coli K-12 (ATCC 29947) was used for comparative purposes. Luria-Bertani (LB; Becton Dickinson and Company, Sparks, MD) agar and broth were used for bacterial culture. Ampicillin, tobramycin, chloramphenicol, ciprofloxacin, nalidixic acid, erythromycin, kanamycin, gentamicin, ceftiofur, streptomycin, trimethoprim, maltodextrin, sucrose, 3,350-Da PEG, and 400-Da PEG were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Biofilm assay.
We used a test tube biofilm assay that was described previously, with minor modifications (36, 37). LB broth (15 ml) supplemented with 100 μg of ampicillin was added into 50-ml conical tubes. Cultures were seeded with bacteria from one colony of each A. baumannii strain that was grown separately on an LB agar plate. After overnight incubation (37°C with shaking at 200 rpm), cultures at ∼1 × 108 CFU/ml were diluted 1:100 in fresh LB medium. An aliquot (3 ml) of each diluted culture was distributed into 16-ml polystyrene tubes and incubated at 37°C for 24 h without shaking. This procedure allowed A. baumannii to attach to the plastic and form a biofilm on the sides of the tube that was visually evident by a ring formed on the internal surface of the tube near the liquid-air interface (36). The culture supernatant from each tube was then decanted, and the adherent biofilm was washed three times with sterile phosphate-buffered saline (PBS) (pH 7.2) to remove detached cells. Biofilms were then considered ready for treatment. After treatment, the number of CFU was estimated. Biofilms were first harvested by adding LB broth (3 ml) with ∼10 sterilized glass beads, and then the tube was vortexed for 3 min to dislodge the biofilm and associated bacteria. CFU counts were then determined by serially diluting the cell suspension (1:10) in LB broth, and a 6 by 6 drop-plate protocol was used to enumerate bacteria (38). The exact same procedures were used to prepare E. coli K-12 biofilms and to enumerate the biofilm-associated bacteria. We followed the methods of Sampson et al. (34) to assess pellicle formation by A. baumannii strains.
Biofilm treatment.
We first determined the MIC of A. baumannii planktonic culture for different antibiotics, as described elsewhere (39). We subsequently selected a concentration of each antibiotic that was 10× the MIC when challenging biofilm communities. Biofilms were treated independently with a hyperosmotic compound (maltodextrin, sucrose, or PEG) or an antibiotic, and with a combination of osmotic compound and antibiotic. LB broth was used to dissolve or dilute osmotic compounds. Briefly, test tube biofilm models were treated by adding 4 ml of each test solution into tubes with ring biofilms, followed by incubation for 18 h at 37°C. Biofilms were then rinsed three times with autoclaved PBS and harvested for CFU enumeration, as described above. The same methods were used to estimate the MIC for E. coli K-12 culture and to assess the efficacy of different antibiotics in the presence of PEG 400. All treatments were evaluated by using three biological replicates.
Culture growth.
To determine if the osmotic compounds used in this study had any obvious effect on growth, we cultured A. baumannii overnight (37°C with shaking at 200 rpm) and then inoculated culture (1:100) into different dilutions of LB medium (1×, 0.5×, and 0.25×) that were supplemented with one of the osmotic compounds (20 mM maltodextrin, 80 mM sucrose, 6.6 mM PEG 3350 [3,350 Da], or 26.6 mM PEG 400). These concentrations were selected because they represented concentrations that appeared to enhance antibiotic efficacy (see Results). Growth curves were measured by optical density at 600 nm (OD600) for 24 h at 37°C.
Osmotic pressure and viscosity.
We estimated the osmotic pressure for different concentrations of the osmotic compounds by using a published equation to calculating osmotic pressure (40). A Couette rotational viscometer (Fann model 35; Fann Instrument Company, Houston, TX, USA) was used as described by the manufacturer to measure the viscosity (in centipoise units) of different solutions of osmotic compounds. Osmotic compounds were dissolved in LB broth, and viscosity was quantified by measuring the shear stress of the liquid.
Statistical analysis.
To compare the 24-h optical densities of A. baumannii cultures in the presence of different osmotic compounds, replicates (n = 3) were analyzed by using a two-factor analysis of variance (ANOVA) (compound, fixed; LB concentration, random) with a Tukey multiple-comparison test (α = 0.05). Least-squares mean values were reported for the difference between optical density with and without each osmotic compound. All bacterial counts (in CFU per milliliter) were log transformed prior to analysis, and every replicate (n = 3 per combination of compound × LB concentration) included an independent negative control (LB only) for comparison. ANOVA was used to analyze CFU data, and a Tukey test was used for post hoc pairwise comparisons (Sigma Plot, version 11.0). To assess the relative efficacies of different antibiotics in the presence of a fixed concentration of osmotic compound, the CFU (log transformed) from the osmotic compound-antibiotic treatment was subtracted from the osmotic compound-only-treated biofilm. This difference was then modeled as a function of antibiotic characteristics (e.g., mass, charge, etc.) by using multivariate regression [lm()function, R version 3.3.1].
ACKNOWLEDGMENTS
L. Orfe provided assistance with this project.
This work was partially funded by the Paul G. Allen School for Global Animal Health and a Department of Defense contract (grant DM110308). A.F. received financial support from the Libyan Ministry of Higher Education and Scientific Research (scholarship no. 700).
REFERENCES
- 1.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Coenye T, Van Acker H, Peeters E, Sass A, Buroni S, Riccardi G, Mahenthiralingam E. 2011. Molecular mechanisms of chlorhexidine tolerance in Burkholderia cenocepacia biofilms. Antimicrob Agents Chemother 55:1912–1919. doi: 10.1128/AAC.01571-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaddy JA, Actis LA. 2009. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol 4:273–278. doi: 10.2217/fmb.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Acker H, Van Dijck P, Coenye T. 2014. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol 22:326–333. doi: 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Furuno JP, Hebden JN, Standiford HC, Perencevich EN, Miller RR, Moore AC, Strauss SM, Harris AD. 2008. Prevalence of methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii in a long-term acute care facility. Am J Infect Control 36:468–471. doi: 10.1016/j.ajic.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergogne-Bérézin E, Decré D, Joly-Guillou ML. 1993. Opportunistic nosocomial multiply resistant bacterial infections–their treatment and prevention. J Antimicrob Chemother 32(Suppl A):39–47. [DOI] [PubMed] [Google Scholar]
- 7.Brossard KA, Campagnari AA. 2012. The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect Immun 80:228–233. doi: 10.1128/IAI.05913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jawad A, Seifert H, Snelling AM, Heritage J, Hawkey PM. 1998. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J Clin Microbiol 36:1938–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Baño J, Martí S, Soto S, Fernández-Cuenca F, Cisneros JM, Pachón J, Pascual A, Martínez-Martínez L, McQueary C, Actis LA, Vila J, Spanish Group for the Study of Nosocomial Infections (GEIH) . 2008. Biofilm formation in Acinetobacter baumannii: associated features and clinical implications. Clin Microbiol Infect 14:276–278. doi: 10.1111/j.1469-0691.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 10.Davis KA, Moran KA, McAllister CK, Gray PJ. 2005. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis 11:1218–1224. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuart TL, Mulvey M, Simor AE, Tien HC, Battad A, Taylor G, Vayalumkal JV, Weir C, Ofner M, Gravel D, Paton S. 2007. Acinetobacter baumannii in casualties returning from Afghanistan. Can J Infect Control 22:152–154. [PubMed] [Google Scholar]
- 12.Calhoun JH, Murray CK, Manring MM. 2008. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res 466:1356–1362. doi: 10.1007/s11999-008-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes F, Teixeira P, Ceri H, Oliveira R. 2012. Evaluation of antimicrobial activity of certain combinations of antibiotics against in vitro Staphylococcus epidermidis biofilms. Indian J Med Res 135:542–547. [PMC free article] [PubMed] [Google Scholar]
- 14.Monzón M, Oteiza C, Leiva J, Amorena B. 2001. Synergy of different antibiotic combinations in biofilms of Staphylococcus epidermidis. J Antimicrob Chemother 48:793–801. doi: 10.1093/jac/48.6.793. [DOI] [PubMed] [Google Scholar]
- 15.Aaron SD, Ferris W, Henry DA, Speert DP, MacDonald NE. 2000. Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am J Respir Crit Care Med 161:1206–1212. doi: 10.1164/ajrccm.161.4.9907147. [DOI] [PubMed] [Google Scholar]
- 16.van der Waal SV, van der Sluis LWM, Özok AR, Exterkate RA, van Marle J, Wesselink PR, de Soet JJ. 2011. The effects of hyperosmosis or high pH on a dual-species biofilm of Enterococcus faecalis and Pseudomonas aeruginosa: an in vitro study. Int Endod J 44:1110–1117. doi: 10.1111/j.1365-2591.2011.01929.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Niu Y, Zhou X, Zhang K, Cheng L, Li M, Li Y, Wang R, Yang Y, Xu X. 2013. Hyperosmotic response of Streptococcus mutans: from microscopic physiology to transcriptomic profile. BMC Microbiol 13:275. doi: 10.1186/1471-2180-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiamco MM, Atci E, Khan QF, Mohamed A, Renslow RS, Abu-Lail N, Fransson BA, Call DR, Beyenal H. 2015. Vancomycin and maltodextrin affect structure and activity of Staphylococcus aureus biofilms. Biotechnol Bioeng 112:2562–2570. doi: 10.1002/bit.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sultana ST, Call DR, Beyenal H. 2016. Maltodextrin enhances biofilm elimination by electrochemical scaffold. Sci Rep 6:36003. doi: 10.1038/srep36003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grotz VL, Munro IC. 2009. An overview of the safety of sucralose. Regul Toxicol Pharmacol 55:1–5. doi: 10.1016/j.yrtph.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Jang HJ, Shin CY, Kim KB. 2015. Safety evaluation of polyethylene glycol (PEG) compounds for cosmetic use. Toxicol Res 31:105–136. doi: 10.5487/TR.2015.31.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nait Chabane Y, Marti S, Rihouey C, Alexandre S, Hardouin J, Lesouhaitier O, Vila J, Kaplan JB, Jouenne T, Dé E. 2014. Characterisation of pellicles formed by Acinetobacter baumannii at the air-liquid interface. PLoS One 9:e111660. doi: 10.1371/journal.pone.0111660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen I. 2015. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis 34:877–886. doi: 10.1007/s10096-015-2323-z. [DOI] [PubMed] [Google Scholar]
- 24.Barraud N, Buson A, Jarolimek W, Rice SA. 2013. Mannitol enhances antibiotic sensitivity of persister bacteria in Pseudomonas aeruginosa biofilms. PLoS One 8:e84220. doi: 10.1371/journal.pone.0084220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Almeida J, Hoogenkamp M, Felippe WT, Crielaard W, van der Waal SV. 2016. Effectiveness of EDTA and modified salt solution to detach and kill cells from Enterococcus faecalis biofilm. J Endod 42:320–323. doi: 10.1016/j.joen.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 26.James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, Costerton JW, Stewart PS. 2008. Biofilms in chronic wounds. Wound Repair Regen 16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 27.Allison KR, Brynildsen MP, Collins JJ. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amato SM, Fazen CH, Henry TC, Mok WWK, Orman MA, Sandvik EL, Volzing KG, Brynildsen MP. 2014. The role of metabolism in bacterial persistence. Front Microbiol 5:70. doi: 10.3389/fmicb.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du J, Bandara HMHN, Du P, Huang H, Hoang K, Nguyen D, Mogarala SV, Smyth HDC. 2015. Improved biofilm antimicrobial activity of polyethylene glycol conjugated tobramycin compared to tobramycin in Pseudomonas aeruginosa biofilms. Mol Pharm 12:1544–1553. doi: 10.1021/mp500846u. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Harmuth S, Barth ER, Wurm E, Fobbe R, Sickmann A, Krumm C, Tiller JC. 2015. Conjugation of ciprofloxacin with poly(2-oxazoline)s and polyethylene glycol via end groups. Bioconjug Chem 26:1950–1962. doi: 10.1021/acs.bioconjchem.5b00393. [DOI] [PubMed] [Google Scholar]
- 31.Williams HD, Behrends V, Bundy JG, Ryall B, Zlosnik JEA. 2010. Hypertonic saline therapy in cystic fibrosis: do population shifts caused by the osmotic sensitivity of infecting bacteria explain the effectiveness of this treatment? Front Microbiol 1:120. doi: 10.3389/fmicb.2010.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R, Sahore S, Kaur P, Rani A, Ray P. 2016. Penetration barrier contributes to bacterial biofilm-associated resistance against only select antibiotics, and exhibits genus-, strain- and antibiotic-specific differences. Pathog Dis 74:ftw056. doi: 10.1093/femspd/ftw056. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Deng L-Q, Chen J-X, Zhou C-Q, Chen W-H. 2015. Does lipophilicity affect the effectiveness of a transmembrane anion transporter? Insight from squaramido-functionalized bis(choloyl) conjugates. Org Biomol Chem 13:11761–11769. [DOI] [PubMed] [Google Scholar]
- 34.Sampson TR, Liu X, Schroeder MR, Kraft CS, Burd EM, Weiss DS. 2012. Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob Agents Chemother 56:5642–5649. doi: 10.1128/AAC.00756-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 36.Abdi-Ali A, Hendiani S, Mohammadi P, Gharavi S. 2014. Assessment of biofilm formation and resistance to imipenem and ciprofloxacin among clinical isolates of Acinetobacter baumannii in Tehran. Jundishapur J Microbiol 7:e8606. doi: 10.5812/jjm.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pour NK, Dusane DH, Dhakephalkar PK, Zamin FR, Zinjarde SS, Chopade BA. 2011. Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol Med Microbiol 62:328–338. doi: 10.1111/j.1574-695X.2011.00818.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen CY, Nace GW, Irwin PL. 2003. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J Microbiol Methods 55:475–479. doi: 10.1016/S0167-7012(03)00194-5. [DOI] [PubMed] [Google Scholar]
- 39.Andrews JM. 2001. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48(Suppl 1):S5–S16. [DOI] [PubMed] [Google Scholar]
- 40.Brown TL, LeMay HE, Bursten BE, Murphy CJ, Woodward PM. 1991. Chemistry: the central science, 12th ed. Pearson Prentice Hall, Glenview, IL. [Google Scholar]