ABSTRACT
Bacterial biofilm formation is linked to several infections and foodborne disease outbreaks. To address this challenge, there is an unmet need to develop rechargeable antimicrobial materials that can provide continuous sanitation of contact surfaces, especially in the food industry. This study was aimed at evaluating a novel rechargeable antimicrobial polymer formed using poly(vinyl alcohol-co-ethylene) (PVA-co-PE) with halamine functionality to prevent biofilm formation with repeated exposure to high loads of bacteria and organic content and also to aid in inactivation of preformed biofilms upon contact with this novel material. The antibiofilm activity of this rechargeable antimicrobial material was evaluated using a combination of fluorescence and scanning electron microscopy techniques and biofilm metabolic activity analyses. The results determined on the basis of imaging and metabolic activity measurements demonstrated that halamine-functionalized polymer films significantly reduced Listeria innocua and Escherichia coli O157:H7 biofilm formation. This novel polymeric material maintained its antibiofilm activity with repeated cycles of extended exposure to high levels of bacterial load. These polymeric films were recharged using bleach and cleaned using mechanical sonication after each cycle of extended incubation with bacteria. Halamine-functionalized polymeric material also exhibited significant antibacterial activity against preformed biofilms on a model surface. In summary, our results demonstrate the potential of this antimicrobial material to provide continuous sanitation of surfaces and applications for inactivating preformed biofilms without extensive use of resources, including water and heat. This polymeric material may be used as a replacement for existing polymeric materials or as a coating on diverse materials.
IMPORTANCE Conventional sanitizers can have limited efficacy in inactivating biofilms in areas with limited accessibility and buildup of organic biomass. Furthermore, none of the current approaches provide continuous sanitation of surfaces. There is a significant unmet need to develop and validate materials that can prevent biofilm formation as well as inactivate preformed biofilms. In this study, the efficacy of a copolymer film containing N-halamine against biofilms of L. innocua and E. coli O157:H7 was evaluated. The polymer film showed strong inhibitory activity against pregrown biofilm or prevented the growth of a new biofilm. The polymer film also maintained its antibiofilm activity after multiple cycles of exposure to high titers of bacterial load with recharging of the polymer film using bleach at intermediate steps between the cycles. Overall, the results demonstrate the potential of a novel antimicrobial material to inhibit and treat biofilms in food industry applications.
KEYWORDS: antimicrobial polymers, biofilm, Escherichia coli O157:H7, halamines, Listeria innocua, sanitation
INTRODUCTION
Foodborne pathogens are linked to several million illnesses and hundreds of deaths each year in the United States alone (1). Biofilm formation has been suggested to be one of the main sources for cross-contamination in the food industry (2). Conveyor belts (3) and tanks and knives (4) are often identified as high-risk food contact surfaces with respect to contamination of food products. Currently, the disinfection of surfaces susceptible to biofilm contamination is carried out by employing conventional disinfectant agents such as sodium hypochlorite, sodium hydroxide, and benzalkonium chloride after a certain period of contact with nonsterile environments. In some cases, these conventional sanitizers can have limited efficacy for inactivation and eradication of biofilms due to the complexity of biophysical environments such as areas with limited accessibility and with buildup of organic biomass (5). Furthermore, none of the current approaches provide continuous sanitation of surfaces to reduce buildup of biofilms during the use of equipment and materials. Thus, there is a significant unmet need to develop and validate materials that can prevent biofilm formation during use as well as to inactivate preformed biofilms. Such materials can aid in both continuous sanitation of materials during their use in food and nonfood environments and improvement of current sanitation practices for inactivation and removal of biofilms.
Among diverse approaches designed to aid in sanitation and in reducing biofilm formation, significant research effort has been made to develop antimicrobial surfaces. In many of these approaches, antimicrobial functionalities are attached to or coated on the surface of selected materials such as polymeric materials. The examples of these antimicrobial functionalities include low-molecular-weight organic antimicrobials such as nisin (6, 7) and halamines (8), antimicrobial coatings based on metal ions such as copper and silver ions (9, 10), and coatings based on large biomacromolecules such as lysozyme or chitosan coatings (11, 12). Among these different approaches, the halamine-based antimicrobial coating is unique as the antimicrobial functionality can be easily regenerated upon treatment with bleach and halamine functionality can be introduced in diverse materials, including plastics (13) and metal surfaces (14), both in the form of surface coating and integrated with the bulk polymeric material (15).
In a recent study, we developed a novel copolymer of poly(vinyl alcohol-co-ethylene) (PVA-co-PE) with halamine functionality integrated within the polymer matrix (16). Our initial tests performed with this material achieved 3 to 5 log CFU reductions in the microbial plate count of planktonic cells in the presence of organic content within an hour of incubation. The material also exhibited excellent mechanical properties such as long-term durability and optical transparency (16).
It is widely recognized that formation of biofilms can significantly increase the antimicrobial resistance of bacterial cells and thus enhance the persistence of bacteria in complex biofilm structures (17). Thus, such biofilms represent the leading cause of cross-contamination of food and nonfood materials upon contact with contaminated surfaces. The focus of this study was to evaluate the efficacy of the novel copolymer film containing halamine against two bacterial biofilm models. The biofilms were formed with the Gram-positive species Listeria innocua (as a surrogate for Listeria monocytogenes) (18) and Gram-negative Escherichia coli strain O157:H7, which are two representative pathogens responsible for a significant fraction of food-related disease outbreaks originating from inanimate surfaces (19).
The efficacy of the antimicrobial material was evaluated based on inactivation of the preformed biofilms as well as prevention of biofilm formation upon extended exposure to target bacteria. The efficacy of the antimicrobial treatment was evaluated using a combination of metabolic activity assays and multimodal microscopy. The multimodal microscopy approach used was based on a combination of fluorescence microscopy and scanning electron microscopy (SEM) to characterize macroscale (millimeter-scale) and microscale (micrometer-scale) changes in biofilm ultrastructures. The ultrastructure was measured using staining of the extracellular matrix by calcofluor and fluorescence microscopy, and the ultrastructure was visualized using palladium coating of the fixed biofilm slides and SEM. Furthermore, the results of this study were also used to evaluate the rechargeability of the surface upon repeated exposure to biofilms. In summary, this study aimed to investigate the potential of this novel antimicrobial material to control, prevent, and treat biofilms under conditions of repeated exposure to bacteria and their biofilms. Successful demonstration of antimicrobial activity against preformed biofilms and active sanitation of surfaces to prevent biofilm formation can lead to multiple relevant applications in the food industry.
RESULTS
Assessment of biofilm formation on PVA-co-PE films by fluorescence microscopy.
Figure 1 shows calcofluor staining of biofilms formed on the surface of control (CTRL) or N-halamine-charged (HALA) films with three different thickness levels upon extended exposure to L. innocua or E. coli O157:H7. Increasing the thickness levels of the halamine-modified polymer corresponds to increasing total loading of activated chlorine (approximately equivalent to 200 ppm/g) in these materials while maintaining the same exposed surface for bacterial attachment. The imaging results reveal intense fluorescence signal based on calcofluor staining that indicates the formation of bacterial biofilm on the control surfaces. The mean fluorescence intensities corresponding to staining of biofilm on three different thicknesses of the control polymer films were 81.29 ± 5.93, 87.7 ± 7.18, and 106.05 ± 7.23 for L. innocua and 114.09 ± 4.56, 99.17 ± 16.54, and 99.98 ± 15.91 for E. coli O157:H7. In the case of the halamine-modified films, significant decreases in fluorescent intensity were observed. With increasing thickness levels of the halamine films, the mean fluorescence intensities were reduced by 11.11-fold, 18.5-fold, and 374.69-fold for L. innocua and by 30.15-fold, 28.47-fold, and 79.71-fold for E. coli O157:H7 compared to the fluorescence intensity observed for the CTRL films. These fold reductions indicate that neither a L. innocua biofilm nor an E. coli O157:H7 biofilm was sufficiently able to grow on the modified surfaces after 1 day of continuous exposure. The reduction in fluorescence intensity increased with increases in the thickness of the halamine films.
FIG 1.
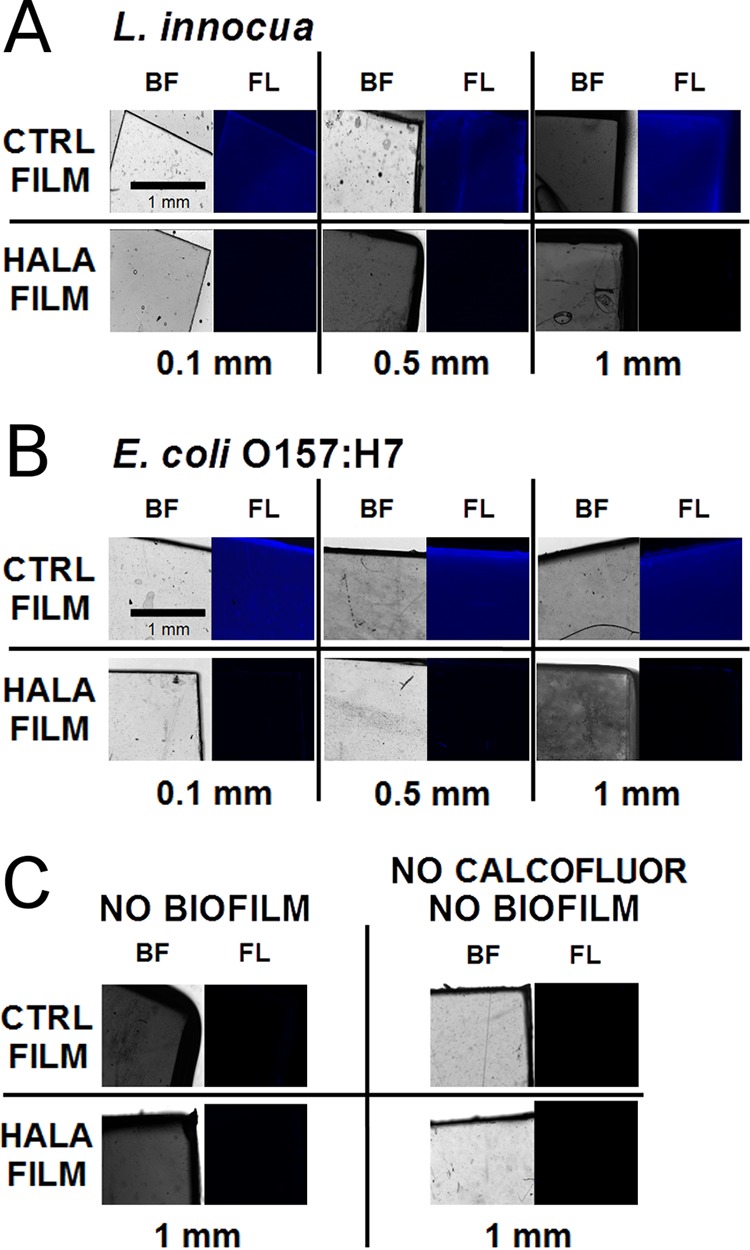
Fluorescence microscopy of bacterial biofilm growth on PVA-co-PE films. (A and B) Images show calcofluor staining of L. innocua (A) and E. coli O157:H7 (B) biofilms after 24 h of biofilm growth on the surface of control films (CTRL FILM; not charged) or N-halamine-charged films (HALA FILM) of 0.1-, 0.5-, or 1-mm thickness. Bright field (BF) and fluorescence (FL) images are shown for each sample. (C) Images of the 1-mm-thick films without biofilms and without calcofluor.
Metabolic activity of biofilm formed on PVA-co-PE films by resazurin reduction assay.
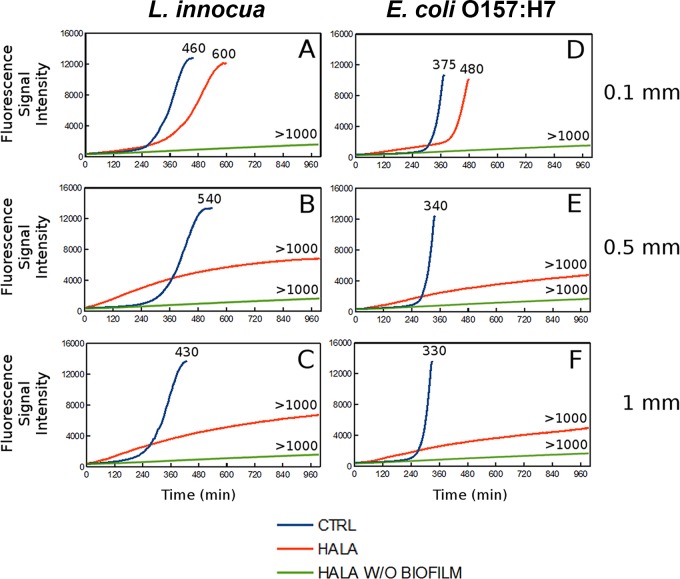
To complement fluorescence imaging measurements, the metabolic activity of L. innocua and E. coli O157:H7 biofilms on both the CTRL and the HALA films with three different thickness levels was measured using the resazurin assay after 1 day of biofilm growth. The biofilm metabolic activity status on these selected control and halamine-modified surfaces was assessed based on the time lag required to achieve peak fluorescence intensity. The extent of time lag in this assay is related to the antimicrobial properties of the selected surface for a biofilm. The results of this measurement (Fig. 2) illustrate that the halamine-modified surface showed an increase in the time lag required for the peak fluorescence intensity for all the selected thicknesses of the polymer films. The time lag increased by at least 2 orders of magnitude compared to the CTRL films for all halamine-modified films with a thickness of ≥0.5 mm (Fig. 2).
FIG 2.
Resazurin metabolic activity assay of bacterial biofilm on PVA-co-PE films. Graphs show the fluorescent intensity curves of resazurin reduction as a function of the time (in minutes) for L. innocua biofilms grown on CTRL (blue) or HALA (red) films of 0.1 (A)-, 0.5 (B)-, and 1 (C)-mm thickness and E. coli O157:H7 biofilms grown on CTRL or HALA films of 0.1 (D)-, 0.5 (E)-, and 1 (F)-mm thickness. Curves for HALA films without biofilm (green) are also shown. The numbers on the curves indicate the times (in minutes) required to reach the peak fluorescence signal intensity corresponding to resazurin reduction.
Antibiofilm properties of recharged HALA films with multiple cycles of biofilm growth.
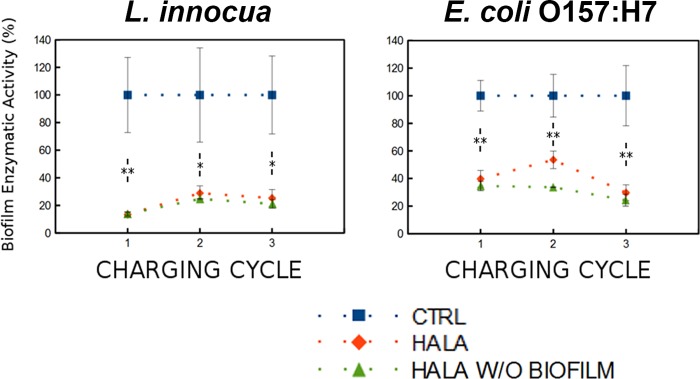
One of the key distinct advantages of halamine-modified surfaces is the ability to recharge the antimicrobial properties of the polymer surface upon exposure to bleach. To demonstrate the recharging potential of halamine-modified surfaces with repeated growth of biofilms on the polymer surfaces, this study evaluated both the metabolic activity of the biofilm and the structural properties. The metabolic activity in this part of the study was assessed based on the permeation and deesterification of fluorescein diacetate (FDA) dye. This approach is more suitable for rapid analysis of metabolic activity of biofilm than the resazurin assay, thus enabling repeat testing of biofilm growth on the same substrate. The results demonstrated significant reductions in metabolic activity of L. innocua and E. coli biofilms with repeated growth of biofilms on halamine-treated surfaces compared to control surfaces. In the case of L. innocua, the biofilm activity was reduced to levels of 13.4% ± 1.7%, 28.8% ± 5.2%, and 25.3% ± 6.1%, respectively, after the first, second, and third cycles of biofilm growth on a halamine-modified surface with recharging of the polymer films after each cycle of biofilm growth (Fig. 3).
FIG 3.
Antibiofilm properties of recharged HALA films with multiple cycles of biofilm growth assessed using the FDA enzymatic activity assay. Graphs show the relative fluorescent intensities (expressed as percentages) of the FDA deesterification product after 30 min of incubation to assess the enzymatic activity of bacterial cells in a biofilm. These measurements were conducted for three cycles of L. innocua (left) and E. coli O157:H7 (right) biofilm growth on CTRL (blue) or HALA (red) films of 1-mm thickness. The films were recharged with bleach after each cycle and reinoculated to grow biofilms. Percentages were calculated based on the fluorescence intensity corresponding to the enzymatic activity of the biofilm on the CTRL film for each cycle. Curves for HALA films without biofilm (green) are also shown, to report the background noise of the assay. Statistical significance was determined (*, P < 0.05; **, P < 0.01).
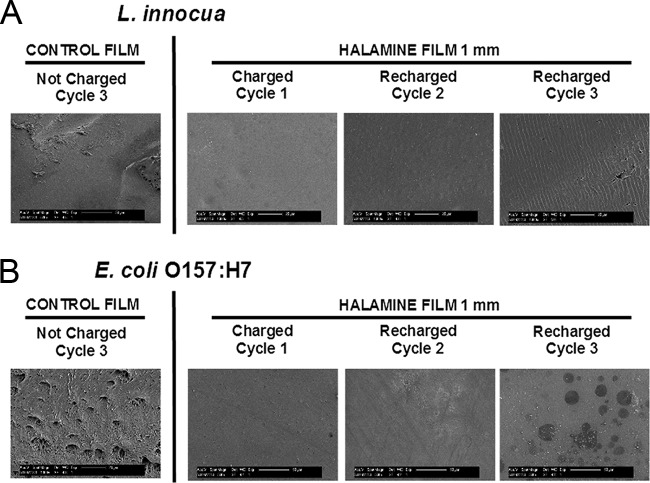
E. coli O157:H7 biofilm activity was also reduced by 50% to 70% during the three cycles of biofilm growth and recharging of the films (Fig. 3). The results showing the reductions in metabolic activity in the repeated biofilm growth assay on a surface were also validated using fluorescence staining as well as SEM imaging. The results of fluorescence staining of the films after each cycle of growth of biofilm and recharging of the polymer film surface with bleach demonstrate the lack of deposition of biofilm on the halamine-modified surfaces compared to the control (Fig. 4). Similarly, the results of SEM imaging reveal the lack of significant formation of biofilm after the 3 charging cycles of HALA films (Fig. 5).
FIG 4.
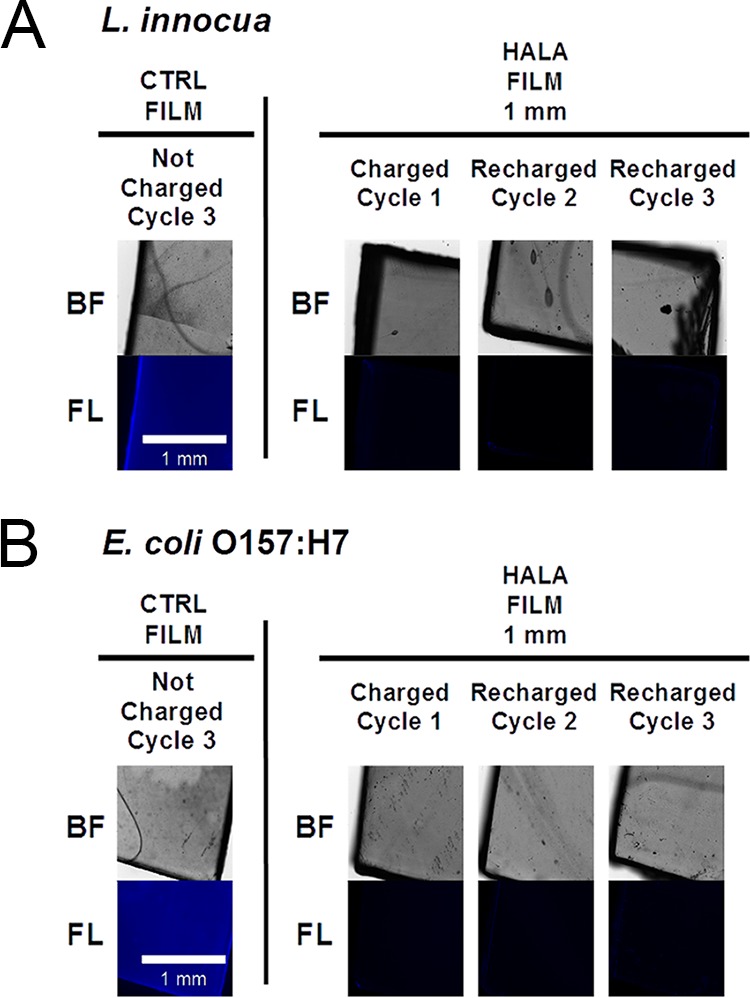
Fluorescence microscopy of bacterial biofilm growth on recharged HALA films with multiple cycles of biofilm growth. The calcofluor staining of L. innocua (A) and E. coli O157:H7 (B) biofilms after 24 h of biofilm growth on the surface of CTRL or recharged HALA film of 1-mm thickness is shown. Bright field (BF) and fluorescence (FL) images are shown for each sample.
FIG 5.
SEM images of bacterial biofilm growth on recharged HALA films with multiple cycles of biofilm growth. The figure shows images of L. innocua (A) and E. coli O157:H7 (B) biofilms on the surface of CTRL or recharged HALA film of 1-mm thickness.
Inhibition of the activity of biofilm on a plastic surface by contact with the HALA film.
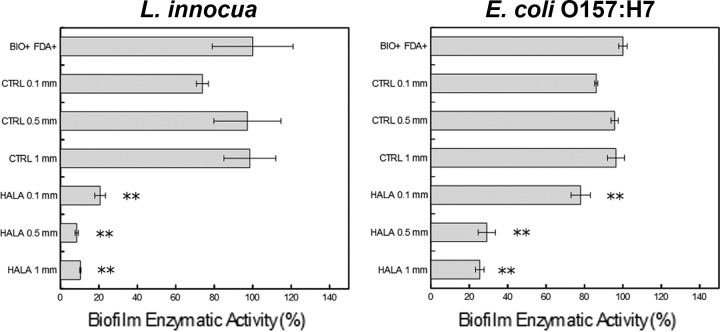
The activity of biofilm grown on a polystyrene surface and treated by contact with HALA film for 1 h was assessed based on the enzymatic activity assay using FDA dye (Fig. 6). The results showed that for L. innocua biofilms, contact with CTRL films of three different levels of thickness did not result in a significant reduction in the enzymatic activity of the biofilm. With HALA films of 0.1-mm thickness, the enzymatic activity of L. innocua biofilms was reduced to 20.6% ± 2.78% of that seen with the untreated biofilm. Similarly, contact with the HALA films with 0.5- and 1-mm thicknesses resulted in a reduction of over 90% in the enzymatic activity of L. innocua biofilms. In the case of E. coli O157:H7 biofilms, treatment with 0.1-mm-thick HALA film showed an approximately 20% reduction in enzymatic activity compared to the untreated control, while treatment with the 0.5- and 1-mm-thick HALA films reduced the enzymatic activity of the treated biofilms by approximately 75% compared to the control.
FIG 6.
Inhibition of the enzymatic activity of biofilm on a plastic surface by contact with HALA films. Graphs show the relative fluorescent intensities (expressed as percentages) of the FDA deesterification product after 30 min of incubation of the L. innocua (left) and E. coli O157:H7 (right) biofilms on a plastic surface upon contact with CTRL or HALA films of 3 different thickness levels (0.1, 0.5, and 1 mm) for 1 h. Percentages were calculated on the basis of the fluorescence intensity corresponding to the enzymatic activity of the biofilm on a plastic surface not treated with any film contact. Statistically significant differences relative to the positive-control sample (BIO+ FDA+) were determined (**, P < 0.01).
The SEM imaging analysis of treated biofilms (Fig. 7) showed that L. innocua biofilm on plastic surface was not affected by contact for 1 h with both the 0.1- and 1-mm-thick CTRL films. Contact with HALA 0.1-mm-thick film and with 1-mm-thick HALA film did not result in a morphological modification of the L. innocua biofilms. The SEM analysis of E. coli O157:H7-treated biofilm showed similar results in the cases of the biofilm contact with the 0.1- and 1-mm-thick CTRL films and with the HALA 0.1-mm-thick film, which did not dramatically affect the biofilm morphology. However, in the case of the E. coli O157:H7 biofilm in contact with the HALA 1-mm-thick film, the images showed morphological changes in the biofilm but without a removal of the biofilm matrix after washing steps were performed before fixation. In summary, these results demonstrate that N-halamine-modified polymer films can effectively inactive bacteria in a biofilm environment upon contact.
FIG 7.
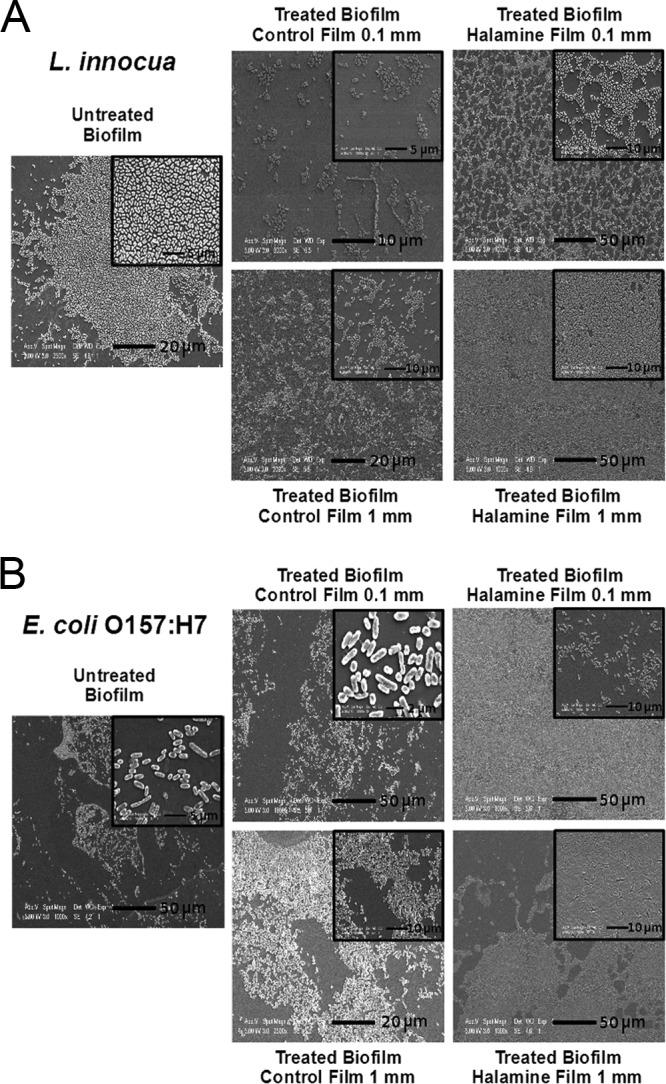
SEM images of bacterial biofilm on a plastic surface after contact with HALA film. The figure shows images of L. innocua (A) and E. coli O157:H7 (B) biofilms on a plastic surface after contact with CTRL or HALA films of 0.1- and 1-mm thickness for 1 h. Insets show zoomed sections of the images.
DISCUSSION
Using a combination of structural and biochemical approaches, this study demonstrated efficacy of the HALA films for inhibition of biofilm formation. The extent of inhibition was observed to be dependent on the overall thickness of the polymer film. The films with thickness greater than 0.5 mm were significantly more effective in reducing biofilm formation than films of 0.1-mm thickness. This trend indicates that biofilm inhibitory activity is dependent on the total fraction of halamine groups in the polymer film, including the modified groups on the surface as well as within the polymer matrix. The bacteria can be quickly inactivated once they come in contact with the HALA film and the active chlorine on the film surface is consumed. However, the chlorine bonded inside the polymer film would be further slowly released according to its dissociation mechanism and would be diffused to the surface to achieve an extended contact-killing effect (20). This sustained action of release of chlorine would also be enhanced by increasing the film thickness.
These results indicate that during charging of the films, the halamine groups present on both the surface and the inner side of the polymer matrix can be charged with chlorine. These activated chlorine molecules may be passively released from the inner side of the film or may diffuse to the surface as a function of time. Previous studies have already showed the efficacy of films containing halamine groups against bacterial biofilm in polyester paints (21) or against the biofilm growth of bacteria and fungi in polyurethane polymer (22). In our study, the PVA associate N-halamine showed similar results in achieving an antimicrobial effect against biofilm. Fluorinated N-halamine has already been shown to be poorly effective against bacteria, especially because of lack of compatibility with polymer matrices (23); for this reason, chlorinated N-halamine polymers have been extensively used for antimicrobial purposes (24). The stability of the N-halamine structure depends notably on the amine halamine structure in the polymer, which has the lowest dissociation constant in water (20), thus implying the good stability of the resultant HALA film (16). Further studies are needed to characterize dynamics of halamine groups in the polymer films and their influence on the release of chlorine. In summary, the results of this study demonstrate that films of greater thickness (equal to or greater than 0.5 mm) can be more effective in reducing biofilm formation.
Inhibition of biofilms can be assessed based on multiple biological parameters. In this study, biofilm inhibition was characterized based on calcofluor staining and the metabolic activity of the cells. Calcofluor staining indicates the presence of biofilm matrix based on staining of glucans that have beta-glycosidic bonds (25). The results of calcofluor staining were assessed based on wide-field fluorescence microscopy. Metabolic activity indicates the presence of viable bacteria on the surface. This measurement was based on reduction of resazurin or on intracellular uptake and activation of the FDA dye in cells. The combination of fluorescence staining and metabolic activity assessment can be a comprehensive approach to independently detect the presence of biofilm matrix and of viable cells. This combination of approaches also enables us to compare the relative sensitivities of the biochemical assays. The results of calcofluor staining demonstrated the lack of presence of biofilm matrix on all the HALA-modified films, while significant calcofluor staining was detected on the control films, indicating the formation of biofilm matrix. This indicates that biofilm formation was significantly reduced on HALA-modified films.
The results of the metabolic activity measurements indicate a similar trend for the inhibition of biofilm growth in the case of HALA film compared to controls, but the extent of inhibition was observed to be dependent on the thickness of the polymer film. Together, these results indicate that even though biofilm matrix deposition is significantly reduced by HALA films, certain fractions of bacterial cells can be viable on HALA polymers with thickness less than 0.5 mm. These results also indicate the relative differences in sensitivity of the biochemical assays in detecting the presence of viable bacteria and the extracellular matrix.
Ability to inhibit biofilm formation during multiple cycles of use is a critical unmet need. The key issues include the challenge of maintaining antimicrobial potential with multiple recharging of the polymer films and inhibiting deposition of biopolymers that can interfere with recharging of the polymer films as well as promoting binding and deposition of microbes. Some recent studies have evaluated the potential of halamine-based coatings to inhibit biofilm formation (26, 27); however, to the best of our knowledge, none of the prior studies evaluated the efficacy of this approach with multiple cycles of exposure to bacteria. This is a realistic simulation of the environmental conditions in food facilities where the contact surfaces are repeatedly exposed to microbes and are repeatedly sanitized with bleach or other sanitizers. In previous experiments performed with planktonic cells, HALA film of the type described in this report was able to inactivate bacteria after multiple charging (16).
The results of this study demonstrate the significant potential of the HALA polymers to inhibit the formation of biofilms under conditions of multiple cycles of exposure to biofilm formation and recharging of the polymer films. We also observed that although HALA polymers inhibited biofilm formation, the deposition of inactivated cells on the polymer surface restricted recharging of the polymer films with bleach (data not shown). This was expected, as the organic content of the inactivated cells can scavenge bleach and can also inhibit the chargeability of the halamine groups on the polymer surface. As already stated in the literature, bacterial cells in biofilm can be killed but not removed by most disinfection procedures, potentially leading to recolonization of surfaces (28). To overcome this limitation, sonication in the presence of surfactant (SDS) was used before recharging of the polymer films. The efficacy of this mechanical approach to remove cell debris was validated by SEM imaging. These results indicated that the current design of HALA-modified films may need to be further modified with polymer functionalities that reduce adhesion of biomass to the surface. In future work, the effect of antibiofilm deposition would be enhanced also by the use of antifouling components which might effectively reduce the bacterial attachment, as already demonstrated in other studies (29–31).
The food process industry currently uses significant amounts of water and sanitizers to inactivate and remove biofilms from contact surfaces. This leads to a significant waste of water resources as well as contamination of discharge water with excessive concentrations of sanitizers that can influence the ecosystem (32). This study evaluated the potential of using HALA films for contact-mediated inhibition of preformed biofilms on contact surfaces. Success in this approach would reduce the consumption of resources, particularly of the water used for sanitation of food contact surfaces. The results demonstrate that 1-mm-thick HALA film was able to achieve around 90% inactivation of the metabolic activity of L. innocua biofilm and 70% inactivation of the metabolic activity of E. coli O157:H7 biofilm. However, the biofilm structure was not removed after the washing step before fixation. It is possible that mechanical agitation approaches such as ultrasound or shear stress generated with circulation of water without sanitizers may aid in further removal of the inactivated biofilms.
The results of this study demonstrate a novel approach to prevent biofilm formation upon exposure to high concentrations of bacteria for extended periods of time. Using a combination of imaging and metabolic assays, the results illustrate that halamine-functionalized polymer films with thickness greater than or equal to 0.5 mm can effectively inhibit biofilm formation. The results also highlight the potential of this approach to maintain continuous sanitation of contact surfaces upon repeated exposure to bacteria. To maintain continuous sanitation, the halamine-functionalized polymer films need to be cleaned using a mechanical method and recharged with bleach. This study also demonstrated that halamine-functionalized polymer films can inactivate bacterial cells within biofilms upon contact. Overall, the antibiofilm activity of these novel polymeric materials is desirable for several applications in the food industry.
MATERIALS AND METHODS
Antimicrobial N-halamine films.
An uncharged polymerized high-strength poly(vinyl alcohol-co-ethylene) (PVA-co-PE) grafting film was prepared as previously described (16) in 3 different thicknesses of 0.1, 0.5, and 1 mm and used as a negative-control (CTRL) film for the following experiments examining antimicrobial activity. The CTRL films of different thicknesses were then chlorinated to achieve a N-halamine-incorporated poly(vinyl alcohol-co-ethylene) (HALA) film as already described (16), assessing a 200-ppm concentration of active chlorine per g of film for all the three type of films. The films were then cut to form 1-by-1-cm samples and were used for the following antibacterial assays.
Bacterial cultures.
A rifampin (RIF)-resistant induced Escherichia coli strain, O157:H7 (ATCC 700728; ATCC, Manassas, VA) (33), was cultured in tryptic soy broth (TSB) (Sigma-Aldrich, St. Louis, MO, USA) with RIF (50 μg/ml) and grown at 37°C at 150 rpm. A bacterial culture with an absorbance at 600 nm of 1.5 (7 × 108 CFU/ml assessed by plate count) was used for the further experiments. A RIF-resistant Listeria innocua mutant (ATCC 33090; ATCC, Manassas, VA, USA) provided by Trevor Suslow's laboratory (University of California, Davis) was grown as described above until it reached a level of absorbance at 600 nm of 1.5 (1 × 109 CFU/ml assessed by plate count) and was used for the following experiments.
Biofilm growth inhibition assays on N-halamine-incorporated poly(vinyl alcohol-co-ethylene) film.
A 1-by-1-cm sample of film (CTRL or HALA film) representative of each of the three thicknesses was inserted into a well of a sterile 24-well polystyrene plate (Corning, Corning, NY, USA). A culture of Listeria innocua or E. coli O157:H7 was diluted to a final concentration of 1 × 107 CFU/ml in 10% (vol/vol) water–1× M9 medium with minimal salts (Sigma-Aldrich, St. Louis, MO, USA)–0.4% glucose (Fisher Scientific, Hampton, NH, USA)–0.4% tryptone (Sigma-Aldrich, St. Louis, MO, USA). The bacterial suspension was then aliquoted as a 1-ml volume for each well containing each film, and the film was allowed to sink to the bottom of the well. The plate was then incubated for 24 h at room temperature under dark conditions. The film was then recovered from the well and washed by 3 pulses of vortex mixing in 25 ml of sterile phosphate-buffered saline (PBS) (USB Co. Ltd., Cleveland, OH, USA) to remove planktonic cells.
The washed film was then incubated in 1 ml of 1:100 calcofluor white stain (Sigma-Aldrich, St. Louis, MO, USA)–water for 30 min under dark conditions at room temperature. After incubation, the film was washed in 25 ml of PBS, positioned on a microscope slide, and covered by a coverslip glass. The slide containing the film was observed on a fluorescence microscope (Olympus IX-7) to visualize with a 4× objective the fluorescence images with DAPI (4′,6-diamidino-2-phenylindole) filters (excitation, 358 nm; emission, 461 nm) using an exposure of 100-ms duration. Slides containing CTRL or HALA films representative of all the three thicknesses tested were observed. Image intensity was quantified by measuring the mean fluorescence intensity in the selected region of interest with NIH ImageJ software. To avoid contributions from scattering of light at the edge of the films, the selected regions of interest were 3 squares with a size of 250 μm2, with each on one corner of an equilateral triangle at the center of the image frame.
Alternatively, the washed film with planktonic cells removed was inserted in a new sterile 24-well polystyrene plate with the addition of 1 ml of TSB–50 μM resazurin sodium salt (Sigma-Aldrich, St. Louis, MO, USA) to measure the metabolic activity of the biofilm formed on the film surface. The fluorescence intensity of the solution was then recorded with readings every 5 min for 16 h at room temperature using an excitation filter at 530 nm and an emission filter at 580 nm in a fluorescence reader (Spectrafluor Plus; Tecan, Durham, NC, USA).
Biofilm inactivation assay by contact with N-halamine-incorporated poly(vinyl alcohol-co-ethylene) film.
Bacterial biofilms were preformed on plastic surface and then inactivated upon contact with the HALA films. The culture of Listeria innocua or E. coli O157:H7 was diluted 1:100 in 1× M9 medium with minimal salts (Sigma-Aldrich, St. Louis, MO, USA) with the addition of 0.4% glucose and 0.4% tryptone, and 1 ml of each bacterial suspension was divided into aliquots, applied to the wells of a 24-well transparent polystyrene plate, and incubated at room temperature. The plate containing Listeria innocua was incubated for 24 h in the dark. The plate containing E. coli O157:H7 was incubated for 3 days; the medium was then removed, and 1 ml of fresh medium was divided into aliquots, applied to each well, and allowed to incubate for an additional 3 days. After incubation, the medium in the plates inoculated with Listeria innocua or E. coli O157:H7 was discarded and each well was washed twice with 2 ml of PBS. A 1-by-1-cm sample of each film was positioned on the bottom of each well containing the bacterial biofilm and incubated in the dark at room temperature for 1 h. After incubation, the film was carefully removed and discarded and the wells were washed once with 1 ml of PBS. The PBS was removed, and 1 ml of water containing 50 μM fluorescein diacetate (FDA) (Alfa Aesar, Ward Hill, MA, USA) was divided into aliquots and applied to each well.
The enzymatic activity of bacterial esterases was assessed by the permeation and deesterification of the nonfluorescent FDA to the fluorescent compound fluorescein. The plate was incubated for 30 min inside a fluorescence reader to record the fluorescence signal intensity from each well with the use of an excitation filter of 488 nm and an emission filter of 520 nm. Control experiments using biofilm without incubation with films and control experiments using films on the bottom of the well without biofilm were also carried out.
Recharging cycles of the N-halamine-incorporated poly(vinyl alcohol-co-ethylene) film.
A charged HALA film sample was incubated with Listeria innocua or E. coli O157:H7 in 10% (vol/vol) water–1× M9 medium with minimal salts–00.4% glucose–0.4% tryptone for 24 h as previously described. After washing, the film was incubated for 30 min with 1 ml of 50 μM FDA and the fluorescence signal intensities were recorded as described above. Then, the film was washed twice in water and incubated for 2 h in a volume of 20 ml with 1% NaOCl–0.05% SDS (pH 5). In addition, the film was subjected three times to 1 min of tip sonication using a Q55 sonicator (QSonica, Newtown, CT, USA) at 50% power before being washed twice in 50 ml water to remove the excess of NaOCl. The CTRL film was incubated in a 0.05% SDS solution at pH 5 without chlorine added. After charging, the HALA film and the CTRL film were placed on the wells of a 24-well polystyrene plate for incubation with Listeria innocua or E. coli O157:H7 for additional biofilm growth of 24 h. The entire procedure was repeated three times to achieve 3 cycles of biofilm growth assay/recharging of the HALA film.
Scanning electron microscopy (SEM) of biofilm after contact with N-halamine-incorporated poly(vinyl alcohol-co-ethylene) film.
Following contact treatment, the bottoms of the wells were washed with PBS and removed from the plate, and the biofilm on the surface was fixed at 4°C overnight with 2.5% glutaraldehyde–water. The following day, the plastic disks were soaked in 95% ethanol until processing was performed with SEM fixation. Subsequently, the plastic disks were soaked in a 1% OsO4 solution for 30 min and washed three times with 95% ethanol. Finally, the disk samples were placed on copper tape and coated with palladium prior to SEM analysis. The same fixation procedure was followed for the HALA films with biofilm on their surfaces.
Statistical analysis.
The means of results of triplicate biological experiments ± standard deviations are shown for data sets from the enzymatic activity assays. The Student t test from the GraphPad software was used to assess the significance of differences between means of the data from coupled data series or from the corresponding control at a confidence interval of 95% or 99%.
ACKNOWLEDGMENT
This project was supported by grant no. 2015-68003-23411 from the USDA-NIFA Program Enhancing Food Safety through Improved Processing Technologies (A4131). The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Souza EL, Meira QGS, de Medeiros Barbosa I, Athayde AJAA, da Conceição ML, de Siqueira Júnior JP. 2014. Biofilm formation by Staphylococcus aureus from food contact surfaces in a meat-based broth and sensitivity to sanitizers. Braz J Microbiol 45:67–75. doi: 10.1590/S1517-83822014000100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svanevik CS, Roiha IS, Levsen A, Lunestad BT. 2015. Microbiological assessment along the fish production chain of the Norwegian pelagic fisheries sector—results from a spot sampling programme. Food Microbiol 51:144–153. doi: 10.1016/j.fm.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Geornaras I, Kunene NF, von Holy A, Hastings JW. 1999. Amplified fragment length polymorphism fingerprinting of Pseudomonas strains from a poultry processing plant. Appl Environ Microbiol 65:3828–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcoran M, Morris D, De Lappe N, O'Connor J, Lalor P, Dockery P, Cormican M. 2014. Commonly used disinfectants fail to eradicate Salmonella enterica biofilms from food contact surface materials. Appl Environ Microbiol 80:1507–1514. doi: 10.1128/AEM.03109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooksey K. 2000. Utilization of antimicrobial packaging films for inhibition of selected microorganisms, p 17–25. In Food Packaging. American Chemical Society, Washington, DC. [Google Scholar]
- 7.Natrajan N, Sheldon BW. 2000. Efficacy of nisin-coated polymer films to inactivate Salmonella typhimurium on fresh broiler skin. J Food Prot 63:1189–1196. doi: 10.4315/0362-028X-63.9.1189. [DOI] [PubMed] [Google Scholar]
- 8.Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS. 2012. N-halamine copolymers for biocidal coatings. React Funct Polym 72:673–679. doi: 10.1016/j.reactfunctpolym.2012.06.018. [DOI] [Google Scholar]
- 9.Michels H, Noyce J, Keevil C. 2009. Effects of temperature and humidity on the efficacy of methicillin-resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Lett Appl Microbiol 49:191–195. doi: 10.1111/j.1472-765X.2009.02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grass G, Rensing C, Solioz M. 2011. Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye M, Neetoo H, Chen H. 2008. Effectiveness of chitosan-coated plastic films incorporating antimicrobials in inhibition of Listeria monocytogenes on cold-smoked salmon. Int J Food Microbiol 127:235–240. doi: 10.1016/j.ijfoodmicro.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Theapsak S, Watthanaphanit A, Rujiravanit R. 2012. Preparation of chitosan-coated polyethylene packaging films by DBD plasma treatment. ACS Appl Mater Interfaces 4:2474–2482. doi: 10.1021/am300168a. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Han Q. 2011. Designing N-halamine based antibacterial surface on polymers: fabrication, characterization, and biocidal functions. Appl Surf Sci 257:6034–6039. doi: 10.1016/j.apsusc.2011.01.115. [DOI] [Google Scholar]
- 14.Bastarrachea LJ, Goddard JM. 2013. Development of antimicrobial stainless steel via surface modification with N-halamines: characterization of surface chemistry and N-halamine chlorination. J Appl Polym Sci 127:821–831. doi: 10.1002/app.37806. [DOI] [Google Scholar]
- 15.Cao Z, Sun Y. 2009. Polymeric N-halamine latex emulsions for use in antimicrobial paints. ACS Appl Mater Interfaces 1:494–504. doi: 10.1021/am800157a. [DOI] [PubMed] [Google Scholar]
- 16.Si Y, Cossu A, Nitin N, Ma Y, Zhao C, Chiou B-S, Cao T, Wang D, Sun G. 2017. Mechanically robust and transparent N-halamine grafted PVA-co-PE films with renewable antimicrobial activity. Macromol Biosci 17(3). doi: 10.1002/mabi.201600304. [DOI] [PubMed] [Google Scholar]
- 17.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedly EC, Crandall PG, Ricke S, O'Bryan CA, Martin EM, Boyd LM. 2008. Identification of Listeria innocua surrogates for Listeria monocytogenes in hamburger patties. J Food Sci 73:M174–M178. doi: 10.1111/j.1750-3841.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 19.Ortega YR. 2008. Foodborne diseases. Emerg Infect Dis 14:1181. doi: 10.3201/eid1407.080346. [DOI] [Google Scholar]
- 20.Sun G, Worley SD. 2006. Halamine chemistry and its applications in biocidal textiles and polymers, p 81–89. In Modified fibers with medical and specialty applications. Springer, Dordrecht, Netherlands. [Google Scholar]
- 21.Paine MRL, Pianegonda NA, Huynh TT, Manefield M, MacLaughlin SA, Rice SA, Barker PJ, Blanksby SJ. 2016. Evaluation of hindered amine light stabilisers and their N-chlorinated derivatives as antibacterial and antifungal additives for thermoset surface coatings. Prog Org Coat 99:330–336. doi: 10.1016/j.porgcoat.2016.06.009. [DOI] [Google Scholar]
- 22.Sun X, Cao Z, Porteous N, Sun Y. 2012. N-halamine-based rechargeable antimicrobial and biofilm-controlling polyurethane. Acta Biomater 8:1498–1506. doi: 10.1016/j.actbio.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Jiang F, Wen J, Lv W, Porteous N, Deng Y, Sun Y. 2015. Fluorinated and un-fluorinated N-halamines as antimicrobial and biofilm-controlling additives for polymers. Polymer 68:92–100. doi: 10.1016/j.polymer.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren X, Jiang Z, Liu Y, Li L, Fan X. 2016. N-halamines as antimicrobial textile finishes, p 125–140. In Antimicrobial Textiles. Woodhead Publishing, Cambridge, United Kingdom. [Google Scholar]
- 25.Rahar S, Swami G, Nagpal N, Nagpal MA, Singh GS. 2011. Preparation, characterization, and biological properties of β-glucans. J Adv Pharm Technol Res 2:94–103. doi: 10.4103/2231-4040.82953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo J, Chen Z, Sun Y. 2006. Controlling biofilm formation with an N-halamine-based polymeric additive. J Biomed Mater Res A 77:823–831. doi: 10.1002/jbm.a.30689. [DOI] [PubMed] [Google Scholar]
- 27.Álvarez-Paino M, Muñoz-Bonilla A, Fernández-García M. 22 February 2017. Antimicrobial polymers in the nano-world. Nanomaterials (Basel) doi: 10.3390/nano7020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen P, Olesen BH, Nielsen PH, Nielsen JL. 2008. Quantification of lipids and protein in thin biofilms by fluorescence staining. Biofouling 24:241–250. doi: 10.1080/08927010802040255. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Zhang X, Zhang Y, Liu J, Zhang H. 2013. Development of a hydrophilic PES ultrafiltration membrane containing SiO2@N-Halamine nanoparticles with both organic antifouling and antibacterial properties. Desalination 326:69–76. doi: 10.1016/j.desal.2013.07.018. [DOI] [Google Scholar]
- 30.Wang X, Yuan S, Shi D, Yang Y, Jiang T, Yan S, Shi H, Luan S, Yin J. 2016. Integrated antifouling and bactericidal polymer membranes through bioinspired polydopamine/poly(N-vinyl pyrrolidone) coating. Appl Surf Sci 375:9–18. doi: 10.1016/j.apsusc.2016.01.198. [DOI] [Google Scholar]
- 31.Yan S, Song L, Luan S, Xin Z, Du S, Shi H, Yuan S, Yang Y, Yin J. 2017. A hierarchical polymer brush coating with dual-function antibacterial capability. Colloids Surf B Biointerfaces 149:260–270. doi: 10.1016/j.colsurfb.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Suslow TV, Oria MP, Beuchat LR, Garrett EH, Parish ME, Harris LJ, Farber JN, Busta FF. 2003. Production practices as risk factors in microbial food safety of fresh and fresh-cut produce. Compr Rev Food Sci Food Saf 2:38–77. doi: 10.1111/j.1541-4337.2003.tb00030.x. [DOI] [Google Scholar]
- 33.Moyne A-L, Sudarshana MR, Blessington T, Koike ST, Cahn MD, Harris LJ. 2011. Fate of Escherichia coli O157:H7 in field-inoculated lettuce. Food Microbiol 28:1417–1425. doi: 10.1016/j.fm.2011.02.001. [DOI] [PubMed] [Google Scholar]