ABSTRACT
Microbiota-modulating strategies, including probiotic administration, have been tested for the treatment of chronic gastrointestinal diseases despite limited information regarding their mechanisms of action. We previously demonstrated that patients with active celiac disease have decreased duodenal expression of elafin, a human serine protease inhibitor, and supplementation of elafin by a recombinant Lactococcus lactis strain prevents gliadin-induced immunopathology in the NOD/DQ8 mouse model of gluten sensitivity. The commensal probiotic strain Bifidobacterium longum NCC2705 produces a serine protease inhibitor (Srp) that exhibits immune-modulating properties. Here, we demonstrate that B. longum NCC2705, but not a srp knockout mutant, attenuates gliadin-induced immunopathology and impacts intestinal microbial composition in NOD/DQ8 mice. Our results highlight the beneficial effects of a serine protease inhibitor produced by commensal B. longum strains.
IMPORTANCE Probiotic therapies have been widely used to treat gastrointestinal disorders with variable success and poor mechanistic insight. Delivery of specific anti-inflammatory molecules has been limited to the use of genetically modified organisms, which has raised some public and regulatory concerns. By examining a specific microbial product naturally expressed by a commensal bacterial strain, we provide insight into a mechanistic basis for the use of B. longum NCC2705 to help treat gluten-related disorders.
KEYWORDS: probiotic, microbiota, gluten, serpin, celiac, commensal
INTRODUCTION
Microbiota-modulating therapies have been tested for the treatment of chronic gastrointestinal diseases and disorders with inconsistent findings. Probiotics are live microorganisms which, when administered in adequate amounts, confer a health benefit to the host (57). Specific strains have shown modest efficacy in treatment of irritable bowel syndrome (IBS) (1); of complications of inflammatory bowel disease (IBD), such as pouchitis (2); and of celiac disease (CeD), a chronic enteropathy caused by ingestion of gluten-containing cereals in genetically susceptible individuals (3). In particular, a number of strains belonging to the genus Bifidobacterium have been proposed as beneficial supplements for a wide range of health conditions (4). Depletions in bifidobacteria have been noted in patients with CeD (5), and attempts have been made to supplement some strains as a therapy for CeD (3, 6). However, despite great public interest in the clinical use of specific probiotic strains for intestinal disorders, there is insufficient mechanistic insight to rationalize consistent recommendations. Investigating therapeutic effects of specific molecules produced by probiotic strains may help bridge this gap.
Dysregulated proteolytic balance has been described in several gastrointestinal disorders (7–11). We have previously shown that expression of the human serine protease inhibitor (serpin) elafin is decreased in the duodenum of patients with active CeD (10, 12). Recombinant Lactococcus lactis expressing elafin (L. lactis-elafin) has been shown (i) to be protective in several murine colitis models (10) and (ii) to prevent gluten immunopathology in the NOD/DQ8 mouse model of gluten sensitivity (12). However, given the concerns raised with the clinical application of such genetically modified organisms (GMOs), we investigated the effect of a commensal bacterium that naturally expresses an elafin-like serpin. The role of serpins produced by bacteria is unknown, but they are thought to contribute to host-commensal mutualism as these serpins likely provide protection from host proteases (13, 14). Although eukaryotic serpins such as elafin are known to possess anti-inflammatory properties (8), bacterially produced serpins have not been explored for their therapeutic capacity in vivo. The infant-derived commensal probiotic strain Bifidobacterium longum NCC2705 (B. longum srp+) produces a serpin (Srp) encoded by the BL0108 (srp) gene in a nonconstitutive manner. Expression of srp is induced in the murine intestinal tract, and Srp may exhibit anti-inflammatory properties as it inhibits both pancreatic elastase and neutrophilic elastase in vitro (13). We tested the hypothesis that administration of the commensal B. longum srp+ prevents immunopathology in the NOD/DQ8 mouse model of gluten sensitivity.
We show that both the wild-type B. longum srp+ strain and a recombinant strain constitutively expressing srp [B. longum srp(Con)] prevent gliadin-induced immunopathology in NOD/DQ8 mice, whereas an srp knockout strain (B. longum Δsrp) does not. These results clearly suggest that the beneficial effect of B. longum srp+ is mediated by Srp. This warrants clinical investigation of commensal B. longum srp+ in managing CeD and nonceliac gluten/wheat sensitivity (NCG/WS) or chronic gastrointestinal conditions associated with proteolytic imbalance.
RESULTS
B. longum srp+ and L. lactis-elafin are equally effective in preventing gliadin immunopathology in mice.
We initially compared the efficacy of B. longum srp+ treatment with the efficacy of elafin delivery by recombinant L. lactis, previously shown to prevent intraepithelial lymphocytosis in NOD/DQ8 mice sensitized with gliadin (Fig. 1A) (12). Mice treated with L. lactis-elafin or B. longum srp+ had lower CD3+ intraepithelial lymphocyte (IEL) counts in the small intestine than mice receiving vehicle and gliadin (P < 0.05) (Fig. 1B and C).
FIG 1.
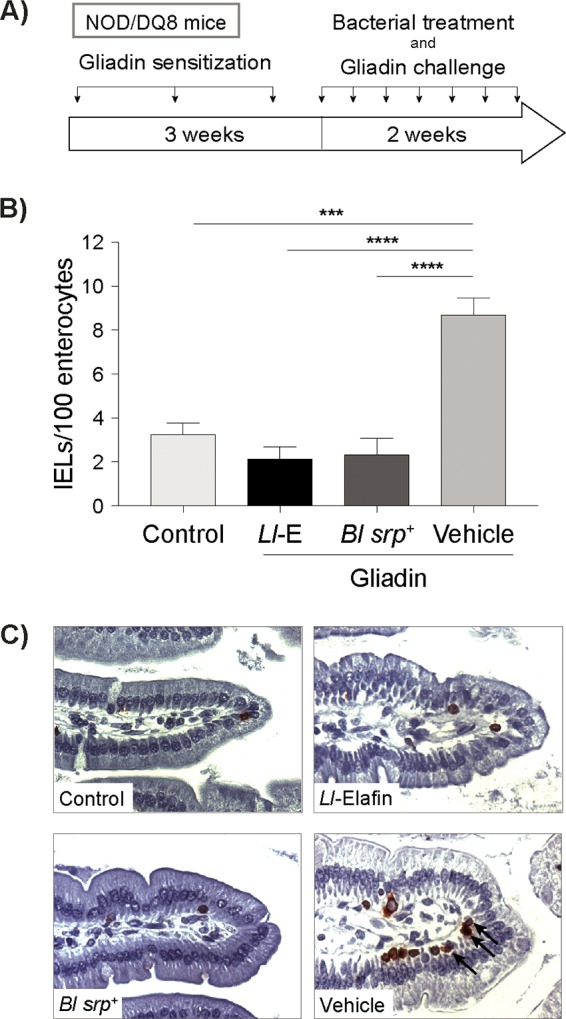
B. longum srp+ and L. lactis-elafin are equally effective in preventing gliadin immunopathology in mice. (A) NOD/DQ8 mice were sensitized with cholera toxin and pepsin-trypsin-digested gliadin 1× per week for 3 weeks. Nonsensitized mice (controls) received cholera toxin alone. Subsequently, mice were treated daily with B. longum srp+, L. lactis-elafin, or PBS–20% glycerol and simultaneously challenged with gliadin 3× per week for 2 weeks. Control mice received no bacterial treatment. (B) CD3+ intraepithelial lymphocytes in small-intestinal villus tips were quantified and expressed as the number of IELs per 100 enterocytes. Mice treated with srp-expressing B. longum srp+ had significantly lower numbers of IELs than gliadin-sensitized mice receiving no bacterial treatment. Further, B. longum srp+ treatment resulted in numbers of IELs similar to those seen with mice treated with L. lactis expressing elafin. ***, P < 0.001; ****, P < 0.0001. (C) Representative images were captured at ×40 magnification. Data are shown as means ± standard errors of the means (SEM). Statistical significance determinations were performed by ANOVA followed by Bonferroni post hoc analysis. Control, nonsensitized, no treatment; Ll-E, gliadin plus L. lactis expressing elafin; Bl srp+, gliadin plus wild-type (WT) B. longum NCC2705; Vehicle, gliadin, no treatment (n = 3 to 6/group).
B. longum constitutively expressing srp exhibits an increased inhibitory capacity with respect to HNE activity in vitro.
To characterize B. longum srp+, B. longum Δsrp, and B. longum srp(Con), we first measured the expression of srp in vitro. B. longum srp(Con) expressed 452-fold more srp mRNA in vitro than B. longum srp+, and no srp mRNA was detected in B. longum Δsrp (Fig. 2A). We then quantified the ability of B. longum srp+ and B. longum srp(Con) to inhibit human neutrophil elastase (HNE) activity in vitro, as pure Srp from B. longum srp+ was previously shown to inhibit HNE. B. longum Δsrp did not inhibit proteolysis of elastin by HNE, as levels of relative fluorescence units (RFU) produced from cleavage of fluorescein isothiocyanate-labeled elastin (FITC-elastin) were similar at all concentrations of added HNE. Compared to B. longum Δsrp, B. longum srp+ inhibited elastin degradation by HNE at 1.5 mU/ml (P < 0.01), resulting in a lower RFU value. B. longum srp(Con) inhibited HNE activity at all concentrations of HNE compared to B. longum Δsrp (P < 0.01 at 3.125, 6.25, and 50 mU/ml; P < 0.05 at 1.5, 12.5, and 25 mU/ml) (Fig. 2B).
FIG 2.
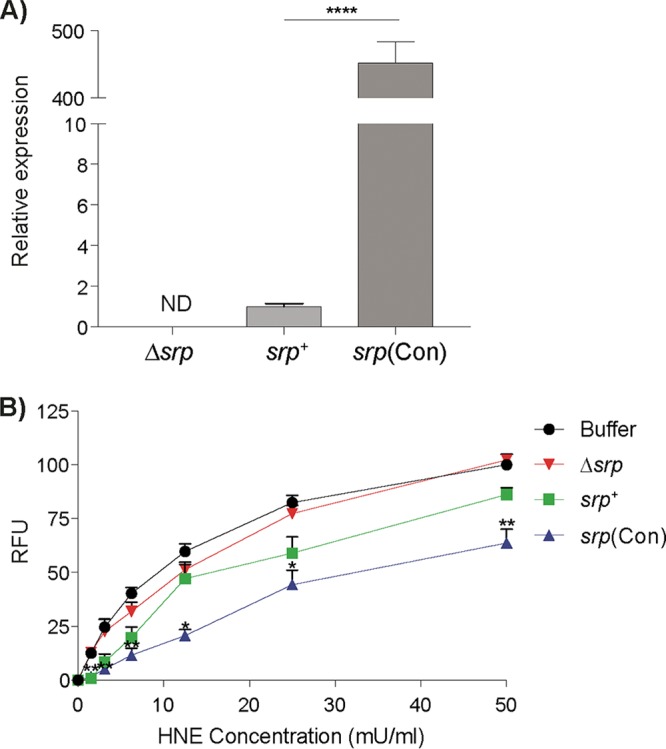
B. longum constitutively expressing srp inhibits human neutrophil elastase activity in vitro. (A) srp mRNA levels were quantified from various B. longum strains. Expression of srp was higher in B. longum srp(Con) than in B. longum srp+ (****, P < 0.0001). No srp mRNA was detected in B. longum Δsrp (n = 4). (B) Inhibitory capacities of various B. longum strains were tested in vitro, as measured by fluorescence produced via cleavage of FITC-elastin substrate at 1.5, 3.125, 6.25, 12.5, 25, and 50 mU/ml, expressed as relative fluorescence units (RFU) (n = 3/group). Compared to B. longum Δsrp, B. longum srp+ inhibited cleavage of elastin by human neutrophil elastase (HNE) in vitro at 1.5 mU/ml (P < 0.01), resulting in lower RFU. In the same assay, B. longum srp(Con) further inhibited HNE across all concentrations of HNE compared to B. longum Δsrp, resulting in lower RFU. As well, B. longum Δsrp did not inhibit HNE, as the levels of cleavage of FITC-elastin determined by RFU produced were not significantly different between B. longum Δsrp and buffer alone at any concentration of HNE added. Buffer, HNE alone; srp+, B. longum srp+; Δsrp, B. longum Δsrp; srp(Con), B. longum srp(Con). ND, not detectable. Data are shown as means ± SEM. Statistical significance determinations were performed using the Kruskal-Wallis test. *, P < 0.05 (versus B. longum Δsrp); **, P < 0.01 (versus B. longum Δsrp).
B. longum srp mediates the protective effect observed in mice.
We tested the capacity of B. longum strains to prevent gliadin immunopathology using B. longum srp+, B. longum Δsrp (positive control), and B. longum srp(Con). NOD/DQ8 mice sensitized to gliadin and treated with B. longum Δsrp had higher IEL counts than nonsensitized mice (controls; P < 0.0001) or mice receiving B. longum srp+ (P < 0.0001) or B. longum srp(Con) (P < 0.0001) (Fig. 3A). Mice receiving B. longum Δsrp had reduced villus-to-crypt (V:C) ratios compared with controls (P < 0.05) and B. longum srp(Con)-treated mice (P < 0.05) (Fig. 3B). Lastly, mice treated with B. longum Δsrp, but not mice receiving B. longum srp+ or B. longum srp(Con), had increased paracellular permeability in the proximal small intestine compared with controls (P < 0.05) (Fig. 3C).
FIG 3.
B. longum srp mediates the protective effect observed in mice. NOD/DQ8 mice were sensitized with cholera toxin and pepsin-trypsin-digested gliadin 1× per week for 3 weeks. Nonsensitized mice (controls) received cholera toxin alone. Subsequently, sensitized mice were treated daily with B. longum srp+ (srp+), B. longum Δsrp (Δsrp), or B. longum srp(Con) and simultaneously challenged with gliadin 3× per week for 2 weeks. Control mice received PBS–20% glycerol. (A) CD3+ intraepithelial lymphocytes in small-intestinal villus tips were quantified and expressed as IELs per 100 enterocytes. Mice treated with srp-expressing B. longum srp+ or B. longum srp(Con) had significantly lower numbers of IELs than B. longum Δsrp-treated mice. Representative images were captured at ×40 magnification (n = 10 to 11/group). (B) Small-intestinal sections were subjected to H&E staining, and villus (V) and crypt (C) lengths were measured via light microscopy, expressed as V:C ratios. B. longum srp(Con) treatment in gliadin-sensitized mice resulted in significantly higher V:C ratios than B. longum Δsrp treatment. Representative images were captured at ×10 magnification (n = 10 to 11/group). (C) Paracellular permeability was restored in sensitized NOD/DQ8 mice treated with B. longum strains expressing srp, i.e., B. longum srp+ and B. longum srp(Con). Proximal small-intestinal sections were mounted on Ussing chambers to measure ex vivo paracellular permeability, expressed as 51Cr-EDTA flux (n = 7 to 8/group). (“Hot sample” refers to the collection of mucosal buffer after addition of 51Cr-EDTA [100%]. All serosal samples collected afterwards are compared to the hot sample.) Data are shown as means ± SEM. Control, nonsensitized, no treatment; srp+, B. longum srp+; Δsrp, B. longum Δsrp; srp(Con), B. longum srp(Con). Statistical significance determinations were performed by ANOVA followed by Bonferroni post hoc analysis. ***, P < 0.001; *, P < 0.05.
Gliadin treatment and B. longum srp expression shift fecal microbiota profiles in mice.
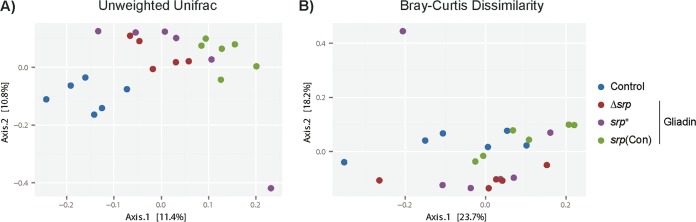
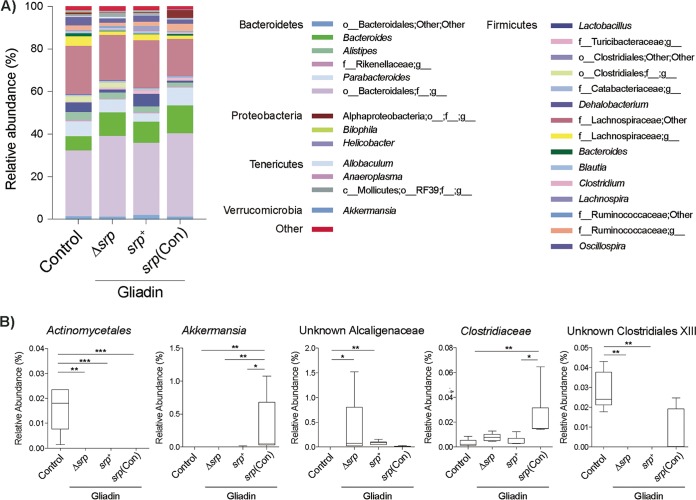
Both the small-intestinal and fecal contents of controls and gliadin-sensitized B. longum-treated NOD/DQ8 mice were sequenced using 16S Illumina technology. The small-intestinal microbiota profiles were similar for all groups (see Fig. S1 and S2 in the supplemental material). However, in both weighted (Bray-Curtis dissimilarity) and unweighted (Unifrac) β-diversity parameters, shifts in fecal microbiota profiles were observed between controls and all gliadin-sensitized mice (Fig. 4) (Fig. S3). Moreover, gliadin-sensitized mice treated with B. longum srp+ and B. longum srp(Con) clustered separately from mice receiving B. longum Δsrp, and this difference in β-diversity levels was significant (Fig. 4). Relative abundances of Actinomycetales were lower in all gliadin-sensitized mice than in controls. B. longum srp(Con) administration was associated with elevated levels of Akkermansia. The level of an unknown Clostridiaceae species was increased in mice treated with B. longum srp(Con) compared with those given B. longum srp+ or no probiotic. The relative abundance of an unknown Clostridiales family XIII member was increased in gliadin-treated mice given B. longum Δsrp and B. longum srp+ (Fig. 5A and B).
FIG 4.
Treatment with gliadin and srp-expressing B. longum shifts fecal microbiota profiles. The figure presents principal-coordinate analysis plots of 16S data in NOD/DQ8 mice. (A) Gliadin induces a shift in β-diversity as calculated using the Unifrac unweighted distance method (P < 0.001). Microbial compositions were different between mice receiving B. longum Δsrp and those receiving B. longum srp+ (P < 0.05); those receiving B. longum Δsrp and those receiving B. longum srp(Con) (P < 0.001); and those receiving B. longum srp+ and those receiving B. longum srp(Con) (P < 0.001) (n = 5 to 6/group). (B) Gliadin also shifted β-diversity as assessed using Bray-Curtis dissimilarity parameters (P < 0.005). Microbial compositions are significantly different between B. longum Δsrp and B. longum srp+ (P < 0.05); between B. longum Δsrp and B. longum srp(Con) (P < 0.005); and between B. longum srp+ and B. longum srp(Con) (P < 0.005). Each circle represents an individual fecal sample (n = 5 to 6/group). Control, nonsensitized, no treatment; srp+, B. longum srp+; Δsrp, B. longum Δsrp; srp(Con), B. longum srp(Con). Statistical analyses were performed via PERMANOVA in QIIME. Plots were constructed in R.
FIG 5.
Fecal genera affected by B. longum expressing srp. (A) Results of examinations of genus-level composition of fecal microbiota after B. longum treatment in NOD/DQ8 mice are depicted as an average percentage corresponding to each group in stacked column charts. (B) Genera significantly differing in relative abundances between groups. Data are shown as box and whisker plots (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Control, nonsensitized, no treatment; srp+, B. longum srp+; Δsrp, B. longum Δsrp; srp(Con), B. longum srp(Con) (n = 5 to 6/group). Statistical analyses were performed via Kruskal-Wallis testing followed by FDR (q < 0.05).
B. longum and B. longum srp-expressing strains were detected in the gastrointestinal tract of treated mice.
We next determined whether B. longum srp+ and the mutant strains, B. longum srp(Con) and B. longum Δsrp, were present in the small-intestinal lumen of treated mice via PCR amplification. There was no difference in the relative abundances of total Bifidobacteria in the small intestine between mice receiving vehicle or any of the B. longum strains, as measured by 16 Illumina sequencing (Fig. 6A). However, strain-specific primers for B. longum srp+ revealed that B. longum srp+ and its derivatives were present in the small intestine of treated mice (Fig. 6B). Furthermore, srp mRNA was detected in the feces and/or intestinal content of 2/4 mice treated with B. longum srp+ and 3/4 mice treated with B. longum srp(Con). In contrast, srp mRNA was not detected in any sample from controls or B. longum Δsrp-treated mice.
FIG 6.
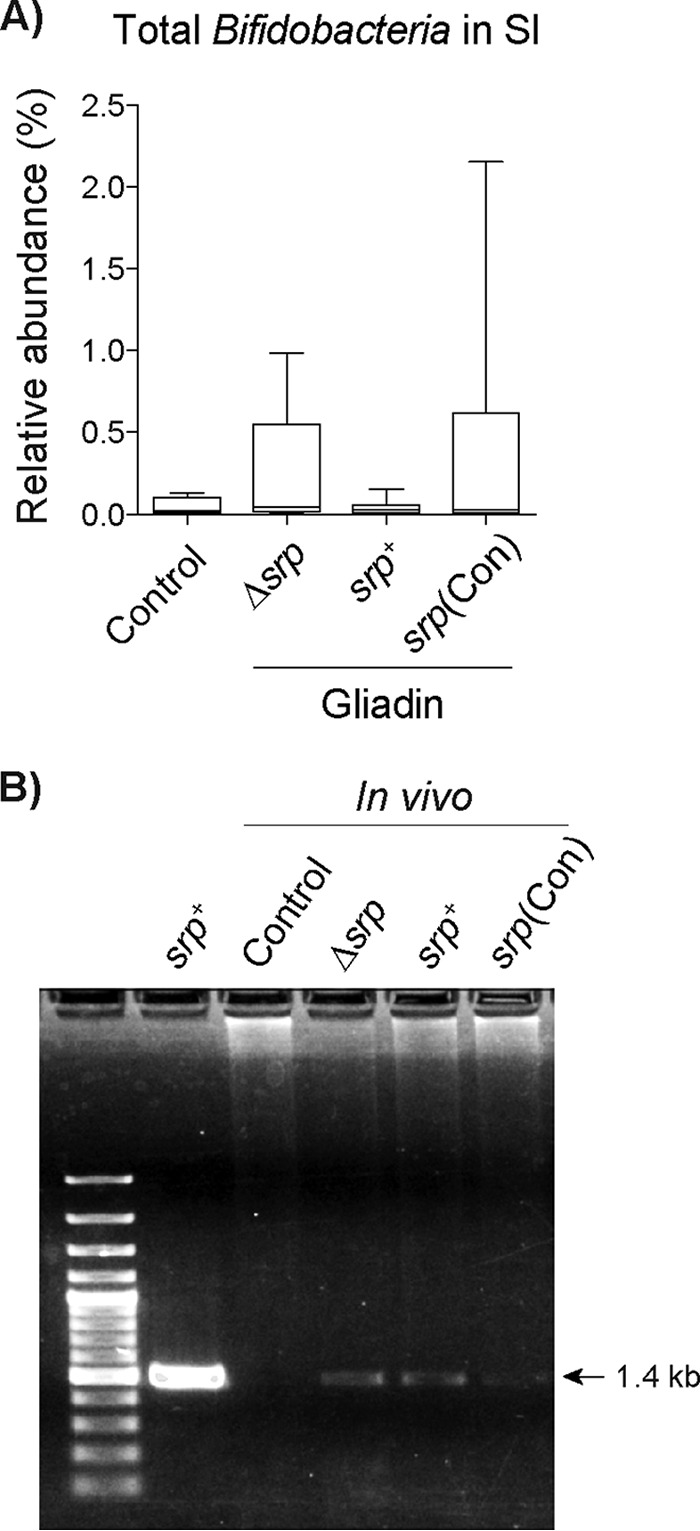
B. longum NCC2705 is detected in the gastrointestinal tract of treated mice. (A) The relative abundances of Bifidobacteria genus members as determined by 16S rRNA sequencing were similar for all groups of NOD/DQ8 mice (n = 5 to 6/group). SI, small intestine. (B) Strain-specific primers detected B. longum Δsrp, B. longum srp+, and B. longum srp(Con) in small-intestinal DNA extracted from all bacterially treated mice. Control, nonsensitized, no treatment; srp+, B. longum srp+; Δsrp, B. longum Δsrp; srp(Con), B. longum srp(Con) (n = 5/group). Statistical analyses were performed via Kruskal-Wallis testing followed by FDR determinations (q < 0.05).
DISCUSSION
There is a spectrum of clinical conditions caused by adverse reactions to gluten and to its constitutive proteins, such as gliadin. These include the well-characterized autoimmune enteropathy CeD and wheat allergy as well as NCG/WS, which overlaps symptomatically with IBS (15). The only effective management for CeD is a lifelong gluten-free diet (GFD), which has several limitations, including poor compliance, accidental contaminations, and slow resolution of mucosal inflammation (16). Patients with NCG/WS also improve symptomatically on a GFD, but it is unknown whether these patients could tolerate less restrictive avoidance or could be successfully treated with other therapies. Since patients with active CeD and nonresponders to a GFD have been found to harbor dysbiotic intestinal communities (5, 17–19), probiotics have been proposed as potential candidates to restore gut microbial homeostasis. Smecuol et al. found that administration of the Bifidobacterium probiotic Natren Life Start (NLS) attenuated symptoms in CeD patients on a gluten-containing diet (20), and administration of NLS was shown to modulate innate immunity in a follow-up study (3). In another clinical trial, children with newly diagnosed CeD that received encapsulated B. longum CECT 7347 showed moderate changes in inflammatory markers and microbiota but no symptomatic improvement beyond those achieved with the concomitant GFD (21). Although these studies showed the possibility that certain probiotics, adjuvant to the GFD, may be beneficial in CeD and perhaps other gluten-related disorders, their use was not guided by a pathophysiological rationale and the mechanisms of action remain unclear.
Our study addressed the efficiency of a specific bacterial serpin (Srp), expressed naturally by B. longum srp+, in the prevention of inflammation induced by gliadin in a genetically susceptible mouse model with previously determined well-defined endpoints (12, 22, 23). We have previously shown that the severity of gluten immunopathology in NOD/DQ8 mice is influenced by the microbiota with which these mice are colonized and that administration of recombinant L. lactis expressing elafin can attenuate the inflammatory response of the host to gluten (12, 23, 24). Serpins are produced by a wide range of organisms and play a key role in maintaining immune homeostasis (25, 26). In the gut, serpins are expressed at mucosal surfaces and are involved in regulating barrier function (27, 28). Srp inhibits eukaryotic serine proteases in vitro, including both neutrophilic elastase and pancreatic elastase. The inhibition of neutrophil elastase, which is a driver of intestinal tissue damage and a biomarker of intestinal inflammation (29), represents an immunomodulatory capacity for Srp that may be relevant in treating gastrointestinal inflammatory conditions (13). We confirmed that the recombinant B. longum srp(Con) expresses higher levels of srp than B. longum srp+ in vitro (Fig. 2A) and that srp expression is undetectable in the mutant strain B. longum Δsrp in vitro. Since purified Srp from B. longum srp+ has been demonstrated to inhibit HNE (13), we tested HNE inhibition by the three strains expressing srp at various levels in vitro. Indeed, B. longum strains expressing srp, but not B. longum Δsrp, inhibited HNE, suggesting that B. longum Srp has potential anti-inflammatory properties. Compared to B. longum Δsrp, B. longum srp(Con) inhibited HNE across all concentrations. Despite significant differences in srp expression between B. longum srp+ and B. longum srp(Con), both strains inhibited HNE in our method of elastase activity quantification. More-significant inhibition of HNE by B. longum srp(Con) may be observed using alternative incubation times and alternative concentrations of elastase and substrate. Further, it was previously shown that elastase was capable of inducing serpin mRNA levels in wild-type B. longum strains (14). Such induction could explain why only limited differences between the two strains [B. longum srp+ and B. longum srp(Con)] were observed in this experiment. The innate immune response is a key component in the development of atrophy in CeD (30, 31) and has been proposed to be involved in the pathogenesis of NCG/WS (32, 33). The influx and release of neutrophil components are increased in patients with CeD (34), and by inhibiting HNE activity, Srp may specifically target a mechanism that contributes to gluten-related disorders.
Using the NOD/DQ8 model of gluten sensitivity, we examined the therapeutic potential of B. longum srp+ in vivo. As a quality control, we confirmed the presence of B. longum in the small intestine of probiotic-treated mice (Fig. 6B) and confirmed srp expression only in mice receiving B. longum srp+ and B. longum srp(Con). Oral administration of B. longum srp+ and B. longum srp(Con) for 2 weeks protected mice from developing gliadin-induced immunopathology. Because these effects were not achieved in mice receiving B. longum Δsrp, srp expression is important for the protective mechanism. This may be related to immune regulation, maintenance of barrier function, overall beneficial shifts in gut microbiota, or inhibition of elastase released during inflammation (35, 36).
Probiotic-based therapies have been advocated to restore the balance of a “dysbiotic” or disease-promoting microbiota (6). Overgrowth of Proteobacteria in the small intestine has been reported in patients with active CeD and in those with persistent symptoms after gluten withdrawal (16, 23). We have shown that experimental expansion of levels of Proteobacteria in the small intestine of NOD/DQ8 worsens gluten immunopathology (16, 23). We therefore measured small-intestinal and fecal microbial β-diversity levels and relative abundances of bacterial groups (Fig. 4 and 5) (see Fig. S1 and S2 in the supplemental material). We found no significant shifts in the small-intestinal microbiota between the separate groups, suggesting that Srp from B. longum is unlikely to act through modification of compositional changes of the upper gastrointestinal tract microbiota (37–40). On the other hand, mild shifts in β-diversity were observed in fecal microbiota of mice treated with B. longum srp+ and B. longum srp(Con) compared to B. longum Δsrp. Although most differences in relative abundances of genera between groups are difficult to interpret, the levels of Akkermansia spp. were exclusively increased in B. longum srp(Con)-treated mice compared to all other groups. The commensal Akkermansia muciniphila is considered to be anti-inflammatory and beneficial for the intestinal mucus layer and barrier integrity in some models of inflammatory disorders (41, 42), and decreased levels of A. muciniphila have been observed in patients with IBD and metabolic disorders (43, 44). This suggests the hypothesis that a significant level of Srp delivery, such as that provided by B. longum srp(Con), may improve the overall performance of the mucosal barrier and immune function of the gut, in part through increases in the levels of Akkermansia species. Because the role of Akkermansia is somewhat controversial based on a recent study (45), the implications of this finding in our model must be further tested to draw conclusions.
In conclusion, B. longum srp+ is a commensal bacterium that expresses a serpin, in a nonconstitutive manner, that is effective in preventing gliadin-induced immunopathology in NOD/DQ8 mice. As a commensal bacterium, B. longum srp+ circumvents the controversy surrounding the use of GMOs for the delivery of anti-inflammatory molecules, which may facilitate its translation for human consumption. This report provides mechanistic insights and pathophysiological rationales to explore the efficacy of the use of B. longum srp+ as an adjunctive therapy in gluten-related disorders or under other gastrointestinal inflammatory conditions associated with proteolytic imbalance. Future studies should address the host mechanisms behind the protection from gluten-induced pathology provided by protease inhibitors or behind exacerbation due to the presence of excess luminal proteases.
MATERIALS AND METHODS
Construction of bacterial strains.
B. longum NCC2705 (B. longum srp+) was isolated at the Nestlé Research Center from the feces of a healthy infant (46). The strain is well-characterized at the molecular and biochemical levels. The full genome of 2.26 Mb has been sequenced, and it was demonstrated that srp, previously known as Bl0108, encodes a bona fide serine protease inhibitor with affinity and inhibitory activity against eukaryotic elastases (13).
Upstream and downstream sequences (3 kb) of the srp gene of B. longum srp+ were amplified by PCR and cloned into the pJH101 vector. The pJH101 vector is available at the German Collection of Microorganisms and Cell Cultures (DSMZ) and was initially designed for the construction of integrable plasmids in Bacillus subtilis. pJH101 contains a chloramphenicol resistance gene and does not contain an origin of replication for B. subtilis or B. longum. The resulting plasmid (pMDY24) containing no coding sequences of srp was introduced into B. longum srp+. Transformation was performed as described previously (47). Five transformants were obtained by plating on De Man, Rogosa, and Sharpe (MRS) medium supplemented with 0.05% cysteine (MRS-cys) containing chloramphenicol, and integration was confirmed by Southern blotting. Transformants were cultivated for 100 generations on MRS-cys without chloramphenicol to clear the antibiotic resistance gene. Twelve chloramphenicol-sensitive isolates were confirmed to be srp knockout strains. One isolate (B. longum Δsrp) was included in the Nestlé Culture Collection under B. longum NCC 9035.
Plasmid pMDY25 was constructed by inserting a constitutive promoter from B. longum NCC 2705, pr-BL1363 (promoter of gene Bl1363, coding for a glyceraldehyde 3-phosphate dehydrogenase), in front of the srp gene in the pMDY23 plasmid which encodes spectinomycin resistance (48). In this recombinant strain [B. longum srp(Con)], the level of synthesis of Srp no longer depends on any kind of induction.
The L. lactis food-grade strain was engineered to express recombinant human elafin (L. lactis-elafin), whose expression was driven by a nisin-inducible promoter, as described in detail previously (10). Strains and plasmids used in the manuscript are outlined in Table 1.
TABLE 1.
Bacterial strains used and plasmids employed to construct them
| Strain or plasmid | Description (reference or source) |
|---|---|
| Strains | |
| L. lactis-elafin | Recombinant Lactococcus lactis strain expressing elafin (1) |
| B. longum srp+ | Commensal probiotic strain Bifidobacterium longum NCC 2705 expressing serpin (2) |
| B. longum srp(Con) | Recombinant Bifidobacterium longum strain derived from NCC 2705 (NCC 2705 pMDY25), constitutively producing serpin (this work) |
| B. longum Δsrp | Genetically modified Bifidobacterium longum strain derived from NCC 2705 (NCC 9035) without serpin coding sequences (this work) |
| Plasmids | |
| pJH101 | Commercially available plasmid (DSMZ) initially designed for the construction of integrable plasmids in Bacillus subtilis; contains a chloramphenicol resistance gene and does not contain origin of replication for either Bacillus subtilis or Bifidobacterium longum |
| pMDY24 | Plasmid derived from pJH101 containing upstream and downstream sequences of the BL108 serpin gene and containing chloramphenicol resistance gene (this work) |
| pMDY23 | Versatile reporter plasmid based on Bifidobacterium longum cryptic plasmid and Escherichia coli gusA gene and containing spectinomycin resistance gene (3) |
| pMDY25 | Plasmid derived from pMDY23 containing a constitutive promoter in front of the BL108 serpin gene (this work) |
Preparation of B. longum biomass.
B. longum srp+ and B. longum Δsrp strains were inoculated at 2% from a fresh overnight culture in MRS-cys and grown anaerobically at 37°C for 16 h. Bacteria were harvested by centrifugation, resuspended in sterile phosphate-buffered saline (PBS) containing 20% glycerol (PBS–20% glycerol), and stored in aliquots at −80°C. Viable counts for the B. longum srp+ and B. longum Δsrp preparations were equal to 6.6 × 109 CFU/ml and 4.4 × 109 CFU/ml, respectively. B. longum srp(Con) was cultured and further processed as described above in the presence of 100 μg/ml of spectinomycin and grown for 48 h at 37°C. Levels of viable bacteria were equal to 1.5 × 109 CFU/ml.
In vitro inhibitory activity of B. longum strains against elastase.
The enzymatic activity of human neutrophil elastase (HNE) was determined by cleavage of FITC-labeled elastin (FITC-elastin). Concentrations of HNE (1.5, 3.125, 6.25, 12.5, 25, and 50 mU/ml) were incubated with 108 CFU of B. longum srp+, B. longum srp(Con), or B. longum Δsrp and 40 μl of buffer solution (50 mM Tris-HCl, 1 mM CaCl2, 50 mM NaCl, 0.25% Triton X-100; pH 8.0) at 37°C for 30 min. FITC-elastin substrate (50 μl) was added, and fluorescence was measured at an excitation wavelength of 530 nm using a spectrophotometer (SpectraMax; Molecular Devices, San Leandro, CA).
Animals.
All experiments were conducted with approval from the McMaster University Animal Care Committee. Female and male 8-to-12-week-old NOD/DQ8 transgenic mice (22) were fed a gluten-free diet for 2 generations (Harlan Laboratories, Indianapolis, IN) and housed in a specific-pathogen-free colony at McMaster University. These mice lack all endogenous mouse major histocompatibility complex class II (MHC II) molecules and express the DQ8 human transgene on a NOD background (22). Oral sensitization of NOD/DQ8 mice with peptic-tryptic (PT) digestion of gliadin, one of the main protein fractions in gluten, and subsequent gliadin challenge induce moderate enteropathy, intraepithelial lymphocytosis, and barrier dysfunction (Fig. 1A) as described previously (22).
Mucosal delivery of B. longum and gliadin sensitization.
Mice were subjected to oral gavage with 500 μg PT-gliadin plus 25 μg cholera toxin as an adjuvant (Sigma-Aldrich) once a week for 3 weeks. Nonsensitized mice (controls) were subjected to gavage with PBS plus 25 μg cholera toxin. Sensitized mice were then treated daily by oral gavage (109 CFU, 200 μl/mouse) for 2 weeks with B. longum srp+ or B. longum Δsrp or B. longum srp(Con) suspended in PBS–20% glycerol. During the probiotic treatment period, sensitized mice were orally challenged with gliadin (2 mg/mouse) dissolved in 0.02 M acetic acid (vehicle) three times per week. Vehicle-treated mice were simultaneously subjected to gavage with PBS–20% glycerol during the challenge period. Control mice were maintained on a gluten-free chow diet and subjected to gavage with PBS–20% glycerol and 0.02 M acetic acid (Fig. 1A).
Detection of B. longum strains.
Primers associated with specificity for B. longum srp+ and derivatives, targeting the previously described insertion sequence ISBlo1b (49), were used to amplify DNA extracted from proximal small-intestinal tissue and contents (Fig. 6B). The primers were as follows: forward, 5′-TCCAGATCATTTCCGATTCC-3′; reverse, 5′-CGGCGTATTTCTATCGCATC-3′. The primers were amplified as previously described (49).
srp mRNA expression in B. longum strains.
B. longum srp+, B. longum srp(Con), and B. longum Δsrp were cultured as described above for 8 h, and cells were collected by centrifugation. Total RNA was extracted using an RNeasy minikit (Qiagen) with additional DNase treatment. Purity and quality were checked using QIAxcel RNA quality control kit v2.0 (Qiagen). RNA level was quantified using a SuperScript III Platinium SYBR green One-Step quantitative real-time PCR (qRT-PCR) kit (Invitrogen) and the standard PCR conditions described in the kit instructions. The srp primers were as follows: forward, 5′-ACCAATCGCTGCTAAGTTCG-3′; reverse, 5′-TCGCTGGCAAGAGAGTAGTC-3′. The lactate dehydrogenase (ldh) housekeeping gene was used for standardization. The following primers were used for ldh: forward, 5′-CGAACGCCATCTACATGCTC-3′; reverse, 5′-AAGATCTGGTTCTCTTGCAG-3′. Fold changes of srp mRNA levels were calculated using the Pfaffl method (50).
srp detection in vivo.
srp mRNA levels were measured in small-intestinal contents and feces of B. longum srp+-treated mice. Samples were collected fresh and flash frozen in liquid nitrogen. RNA was extracted from these samples using a PowerMicrobiome RNA isolation kit (catalog no. 26000-50; MoBio Laboratories Inc., Carlsbad, CA). RNA quality was checked using an Agilent 2100 Bioanalyzer system with an Agilent RNA 6000 Nano kit. RNA was quantified using a Quant-it Ribogreen RNA kit. srp mRNA levels were measured by qRT-PCR in two steps. RNA transcription to single-stranded cDNA was performed using 1 μg of RNA in a total reaction mixture of 20 μl by the use of qScript cDNA supermix and the following PCR conditions: 25°C for 5 min; 42°C for 30 min; 85°C for 5 min; a 4°C hold. srp was further amplified by real-time PCR using the following primers and probe: forward primer, 5′-ACCAATCGCTGCTAAGTTCG-3′, reverse primer, 5′-TCGCTGGCAAGAGAGTAGTC-3′; probe, 5′-6-carboxyfluorescein (FAM)-CCGAGATGAGCGCCGCGAACT-black hole quencher (BHQ [Microsynth])-3′. cDNA (100 ng) was used in a total reaction mixture of 20 μl with the TaqMan Universal PCR master mix and the following cycle: 50°C for 2 min; 95°C for 10 min; 95°C for 15 s; 60°C for 1 min (repeated for 40 cycles).
Evaluation of small-intestinal immunopathology.
Small-intestinal cross sections were fixed in 10% buffered formalin for 48 h and embedded in paraffin, as previously described (22). Immunohistochemistry experiments were performed on formalin-fixed, paraffin-embedded sections of proximal small intestine to visualize CD3+ cells as described previously (22, 51). Slides were examined at ×20 magnification using light microscopy in a blind fashion. The number of CD3+ IELs per 20 enterocytes in five randomly chosen villous tips was counted by an observer in a blind fashion as described above, and data were expressed as numbers of IELs/100 enterocytes (51). Paraffin-embedded sections were stained with hematoxylin and eosin (H&E) for histological evaluation of tissue morphology under conditions of light microscopy (Olympus, Ontario, Canada). Using Image-Pro 6.3 software (Mediacybernetics, MD, USA), enteropathy was quantified in a blind fashion by measuring villus-to-crypt (V:C) ratios as previously described (22). Intestinal paracellular permeability was evaluated ex vivo by the use of the Ussing chamber technique as previously described (World Precision Instruments, Sarasota, FL) (22). Paracellular permeability of proximal small-intestinal samples was evaluated by measuring the mucosa-to-serous flux of the inert paracellular probe 51Cr-EDTA. 51Cr-EDTA was quantified in samples using a liquid scintillation counter and expressed as percent recovery of 51Cr-EDTA flux per square centimeter per hour.
Microbiota compositional analysis.
Fecal and small-intestinal contents were collected and flash frozen on dry ice. DNA was extracted from samples as previously described (52) and amplified for the hypervariable V3 region of the 16S rRNA gene for sequencing on an Illumina MiSeq platform (Illumina, San Diego, CA). Analysis of data was performed as previously described (52). Briefly, sequences were trimmed using Cutadapt software (version 1.2.1) (53) and aligned using PANDAseq software (version 2.8) (54). Operational taxonomic units selected using AbundantOTU (55) were assigned taxonomy according to the Greengenes reference database (56). Principal-coordinate analysis (PCoA) plots were generated using R (R Foundation for Statistical Computing, Vienna, Austria). Pairwise UniFrac distances were calculated among microbial communities, and both relative abundance data (weighted) and presence/absence information (unweighted) were determined.
Statistical analysis.
Data were analyzed using GraphPad Prism 6.0, QIIME, R and SPSS software. Normal data were analyzed by analysis of variance (ANOVA) followed by Bonferroni post hoc analysis. Statistical analyses of HNE inhibition compared to buffer results were performed using the Mann-Whitney test. Statistical analyses of microbiota β-diversity were performed using permutational multivariate analysis of variance (PERMANOVA). Microbiota abundances were analyzed in SPSS via Kruskal-Wallis testing followed by false-discovery-rate (FDR) determinations (q < 0.05). All significant genera presented passed FDR testing.
Supplementary Material
ACKNOWLEDGMENTS
The mutant and recombinant bifidobacterial strains were constructed under the skillful leadership of F. Arigoni. We thank A. Bruttin for preparations of bacterial strains and T. Nunes for critical reading of the manuscript. We also thank the staff of the Farncombe Metagenomics Facility.
This project was supported by a CIHR grant (MOP#142773) to E.F.V. and Nestec SA. J.L.M. holds a Boris Family PhD award. J.D. holds an MSc student CIHR award. A.C. holds a Canadian Celiac Association YIA and a Farncombe Institute Award. S.D., M.S., M.D., A.M., and G.B. are employees of Nestec SA. E.F.V. holds a Canada Research Chair in inflammation, microbiota, and nutrition.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01323-17.
REFERENCES
- 1.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. 2010. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 2.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot I, Nicholls R, Gionchetti P, Campieri M, Kamm M. 2004. Once daily high dose probiotic therapy (VSL# 3) for maintaining remission in recurrent or refractory pouchitis. Gut 53:108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto-Sanchez MI, Smecuol EC, Temprano MP, Sugai E, Gonzalez A, Moreno ML, Huang X, Bercik P, Cabanne A, Vazquez H, Niveloni S, Mazure R, Maurino E, Verdu EF, Bai JC. 15 September 2016. Bifidobacterium infantis NLS super strain reduces the expression of alpha-defensin-5, a marker of innate immunity, in the mucosa of active celiac disease patients. J Clin Gastroenterol doi: 10.1097/MCG.0000000000000687. [DOI] [PubMed] [Google Scholar]
- 4.Tojo R, Suarez A, Clemente MG, de los Reyes-Gavilan CG, Margolles A, Gueimonde M, Ruas-Madiedo P. 2014. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol 20:15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golfetto L, de Senna FD, Hermes J, Beserra BT, Franca Fda S, Martinello F. 2014. Lower bifidobacteria counts in adult patients with celiac disease on a gluten-free diet. Arq Gastroenterol 51:139–143. doi: 10.1590/S0004-28032014000200013. [DOI] [PubMed] [Google Scholar]
- 6.Quagliariello A, Aloisio I, Bozzi Cionci N, Luiselli D, D'Auria G, Martinez-Priego L, Perez-Villarroya D, Langerholc T, Primec M, Micetic-Turk D, Di Gioia D. 2016. Effect of Bifidobacterium breve on the intestinal microbiota of coeliac children on a gluten free diet: a pilot study. Nutrients 8:E660. doi: 10.3390/nu8100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustos D, Negri G, De Paula JA, Di Carlo M, Yapur V, Facente A, De Paula A. 1998. Colonic proteinases: increased activity in patients with ulcerative colitis. Medicina (B Aires) 58:262–264. [PubMed] [Google Scholar]
- 8.Gecse K, Roka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztoczy A, Izbeki F, Fioramonti J. 2008. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 9.Róka R, Rosztóczy A, Leveque M, Izbéki F, Nagy F, Molnár T, Lonovics J, Garcia-Villar R, Fioramonti J, Wittmann T, Bueno L. 2007. A pilot study of fecal serine-protease activity: a pathophysiologic factor in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 5:550–555. doi: 10.1016/j.cgh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Motta J, Bermúdez-Humarán L, Deraison C, Martin L, Rolland C, Rousset P, Boue J, Dietrich G, Chapman K, Kharrat P, Vinel J, Alric L, Mas E, Sallenave J, Langella P, Vergnolle N. 2012. Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci Transl Med 4:158ra144. doi: 10.1126/scitranslmed.3004212. [DOI] [PubMed] [Google Scholar]
- 11.Annaházi A, Molnár T, Farkas K, Rosztóczy A, Izbéki F, Gecse K, Inczefi O, Nagy F, Földesi I, Szűcs M, Dabek M, Ferrier L, Theodorou V, Bueno L, Wittmann T, Róka R. 2013. Fecal MMP-9: a new noninvasive differential diagnostic and activity marker in ulcerative colitis. Inflamm Bowel Dis 19:316–312. doi: 10.1002/ibd.22996. [DOI] [PubMed] [Google Scholar]
- 12.Galipeau HJ, Wiepjes M, Motta JP, Schulz JD, Jury J, Natividad JM, Pinto-Sanchez I, Sinclair D, Rousset P, Martin-Rosique R, Bermudez-Humaran L, Leroux JC, Murray JA, Smecuol E, Bai JC, Vergnolle N, Langella P, Verdu EF. 2014. Novel role of the serine protease inhibitor elafin in gluten-related disorders. Am J Gastroenterol 109:748–756. doi: 10.1038/ajg.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov D, Emonet C, Foata F, Affolter M, Delley M, Fisseha M, Blum-Sperisen S, Kochhar S, Arigoni F. 2006. A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. J Biol Chem 281:17246–17252. doi: 10.1074/jbc.M601678200. [DOI] [PubMed] [Google Scholar]
- 14.Turroni F, Foroni E, Motherway MOC, Bottacini F, Giubellini V, Zomer A, Ferrarini A, Delledonne M, Zhang Z, van Sinderen D. 2010. Characterization of the serpin-encoding gene of Bifidobacterium breve 210B. Appl Environ Microbiol 76:3206–3219. doi: 10.1128/AEM.02938-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdu EF, Armstrong D, Murray JA. 2009. Between celiac disease and irritable bowel syndrome: the “no man's land” of gluten sensitivity. Am J Gastroenterol 104:1587–1594. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wacklin P, Kaukinen K, Tuovinen E, Collin P, Lindfors K, Partanen J, Mäki M, Mättö J. 2013. The duodenal microbiota composition of adult celiac disease patients is associated with the clinical manifestation of the disease. Inflamm Bowel Dis 19:934–941. doi: 10.1097/MIB.0b013e31828029a9. [DOI] [PubMed] [Google Scholar]
- 17.D'Argenio V, Casaburi G, Precone V, Pagliuca C, Colicchio R, Sarnataro D, Discepolo V, Kim SM, Russo I, Del Vecchio Blanco G, Horner DS, Chiara M, Pesole G, Salvatore P, Monteleone G, Ciacci C, Caporaso GJ, Jabri B, Salvatore F, Sacchetti L. 2016. Metagenomics reveals dysbiosis and a potentially pathogenic N. flavescens strain in duodenum of adult celiac patients. Am J Gastroenterol 111:879–890. doi: 10.1038/ajg.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mårild K, Ye W, Lebwohl B, Green PH, Blaser MJ, Card T, Ludvigsson JF. 2013. Antibiotic exposure and the development of coeliac disease: a nationwide case-control study. BMC Gastroenterol 13:109. doi: 10.1186/1471-230X-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decker E, Engelmann G, Findeisen A, Gerner P, Laass M, Ney D, Posovszky C, Hoy L, Hornef MW. 2010. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics 125:e1433–e1440. doi: 10.1542/peds.2009-2260. [DOI] [PubMed] [Google Scholar]
- 20.Smecuol E, Hwang HJ, Sugai E, Corso L, Chernavsky AC, Bellavite FP, Gonzalez A, Vodanovich F, Moreno ML, Vazquez H, Lozano G, Niveloni S, Mazure R, Meddings J, Maurino E, Bai JC. 2013. Exploratory, randomized, double-blind, placebo-controlled study on the effects of Bifidobacterium infantis natren life start strain super strain in active celiac disease. J Clin Gastroenterol 47:139–147. doi: 10.1097/MCG.0b013e31827759ac. [DOI] [PubMed] [Google Scholar]
- 21.Olivares M, Castillejo G, Varea V, Sanz Y. 2014. Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum CECT 7347 in children with newly diagnosed coeliac disease. Br J Nutr 112:30–40. doi: 10.1017/S0007114514000609. [DOI] [PubMed] [Google Scholar]
- 22.Galipeau H, Rulli N, Jury J, Huang X, Araya R, Murray J, David C, Chirdo F, McCoy K, Verdu E. 2011. Sensitization to gliadin induces moderate enteropathy and insulitis in nonobese diabetic-DQ8 mice. J Immunol 187:4338–4346. doi: 10.4049/jimmunol.1100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galipeau HJ, McCarville JL, Huebener S, Litwin O, Meisel M, Jabri B, Sanz Y, Murray JA, Jordana M, Alaedini A. 2015. Intestinal microbiota modulates gluten-induced immunopathology in humanized mice. Am J Pathol 185:2969–2982. doi: 10.1016/j.ajpath.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caminero A, Galipeau HJ, McCarville JL, Johnston CW, Bernier SP, Russell AK, Jury J, Herran AR, Casqueiro J, Tye-Din JA, Surette MG, Magarvey NA, Schuppan D, Verdu EF. 2016. Duodenal bacteria From patients with celiac disease and healthy subjects distinctly affect gluten breakdown and immunogenicity. Gastroenterology 151:670–683. doi: 10.1053/j.gastro.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 25.Heit C, Jackson BC, McAndrews M, Wright MW, Thompson DC, Silverman GA, Nebert DW, Vasiliou V. 2013. Update of the human and mouse SERPIN gene superfamily. Hum Genomics 7:22. doi: 10.1186/1479-7364-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiserman D, Whisstock JC, Bird PI. 2006. Mechanisms of serpin dysfunction in disease. Expert Rev Mol Med 8:1–19. doi: 10.1017/S1462399406000184. [DOI] [PubMed] [Google Scholar]
- 27.Hasnain SZ, McGuckin MA, Grencis RK, Thornton DJ. 2012. Serine protease(s) secreted by the nematode Trichuris muris degrade the mucus barrier. PLoS Negl Trop Dis 6:e1856. doi: 10.1371/journal.pntd.0001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchiyama K, Naito Y, Takagi T, Mizushima K, Hirai Y, Hayashi N, Harusato A, Inoue K, Fukumoto K, Yamada S. 2012. Serpin B1 protects colonic epithelial cell via blockage of neutrophil elastase activity and its expression is enhanced in patients with ulcerative colitis. Am J Physiol Gastrointest Liver Physiol 302:G1163–G1170. doi: 10.1152/ajpgi.00292.2011. [DOI] [PubMed] [Google Scholar]
- 29.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. 2008. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 30.Jabri B, Sollid LM. 2009. Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol 9:858–870. doi: 10.1038/nri2670. [DOI] [PubMed] [Google Scholar]
- 31.Sollid LM, Jabri B. 2013. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat Rev Immunol 13:294–302. doi: 10.1038/nri3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araya RE, Gomez Castro MF, Carasi P, McCarville JL, Jury J, Mowat AM, Verdu EF, Chirdo FG. 2016. Mechanisms of innate immune activation by gluten peptide p31-43 in mice. Am J Physiol Gastrointest Liver Physiol 311:G40–G49. doi: 10.1152/ajpgi.00435.2015. [DOI] [PubMed] [Google Scholar]
- 33.Junker Y, Zeissig S, Kim S-J, Barisani D, Wieser H, Leffler DA, Zevallos V, Libermann TA, Dillon S, Freitag TL. 2012. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med 209:2395–2408. doi: 10.1084/jem.20102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hällgren R, Colombel JF, Dahl R, Fredens K, Kruse A, Jacobsen NO, Venge P, Rambaud JC. 1989. Neutrophil and eosinophil involvement of the small bowel in patients with celiac disease and Crohn's disease: studies on the secretion rate and immunohistochemical localization of granulocyte granule constituents. Am J Med 86:56–64. doi: 10.1016/0002-9343(89)90230-1. [DOI] [PubMed] [Google Scholar]
- 35.Nikolaus S, Bauditz J, Gionchetti P, Witt C, Lochs H, Schreiber S. 1998. Increased secretion of pro-inflammatory cytokines by circulating polymorphonuclear neutrophils and regulation by interleukin 10 during intestinal inflammation. Gut 42:470–476. doi: 10.1136/gut.42.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kessenbrock K, Fröhlich L, Sixt M, Lämmermann T, Pfister H, Bateman A, Belaaouaj A, Ring J, Ollert M, Fässler R. 2008. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J Clin Invest 118:2438–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. 2016. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med 8:52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimm V, Radulovic K, Riedel CU. 2015. Colonization of C57BL/6 mice by a potential probiotic Bifidobacterium bifidum strain under germ-free and specific pathogen-free conditions and during experimental colitis. PLoS One 10:e0139935. doi: 10.1371/journal.pone.0139935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behnsen J, Deriu E, Sassone-Corsi M, Raffatellu M. 2013. Probiotics: properties, examples, and specific applications. Cold Spring Harb Perspect Med 3:a010074. doi: 10.1101/cshperspect.a010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eloe-Fadrosh EA, Brady A, Crabtree J, Drabek EF, Ma B, Mahurkar A, Ravel J, Haverkamp M, Fiorino AM, Botelho C, Andreyeva I, Hibberd PL, Fraser CM. 2015. Functional dynamics of the gut microbiome in elderly people during probiotic consumption. mBio 6:e00231-15. doi: 10.1128/mBio.00231-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, de Vos WM, Satokari R. 2015. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol 81:3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derrien M, Belzer C, de Vos WM. 2017. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 106:171–181. doi: 10.1016/j.micpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Rajilić-Stojanović M, Shanahan F, Guarner F, de Vos WM. 2013. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis 19:481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 44.Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH. 2010. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 45.Seregin SS, Golovchenko N, Schaf B, Chen J, Pudlo NA, Mitchell J, Baxter NT, Zhao L, Schloss PD, Martens EC. 2017. NLRP6 protects IL-10−/− mice from colitis by limiting colonization of Akkermansia muciniphila. Cell Rep 19:733–745. doi: 10.1016/j.celrep.2017.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schell MA, Karmirantzou M, Snel B, Vilanova D, Berger B, Pessi G, Zwahlen MC, Desiere F, Bork P, Delley M, Pridmore DR, Arigoni F. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc Natl Acad Sci U S A 99:14422–14427. doi: 10.1073/pnas.212527599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Argnani A, Leer RJ, van Luijk N, Pouwels PH. 1996. A convenient and reproducible method to genetically transform bacteria of the genus Bifidobacterium. Microbiology 142:109–114. doi: 10.1099/13500872-142-1-109. [DOI] [PubMed] [Google Scholar]
- 48.Klijn A, Moine D, Delley M, Mercenier A, Arigoni F, Pridmore RD. 2006. Construction of a reporter vector for the analysis of Bifidobacterium longum promoters. Appl Environ Microbiol 72:7401–7405. doi: 10.1128/AEM.01611-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berger B, Moine D, Mansourian R, Arigoni F. 2010. HspR mutations are naturally selected in Bifidobacterium longum when successive heat shock treatments are applied. J Bacteriol 192:256–263. doi: 10.1128/JB.01147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biagi F, Luinetti O, Campanella J, Klersy C, Zambelli C, Villanacci V, Lanzini A, Corazza GR. 2004. Intraepithelial lymphocytes in the villous tip: do they indicate potential coeliac disease? J Clin Pathol 57:835–839. doi: 10.1136/jcp.2003.013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whelan FJ, Verschoor CP, Stearns JC, Rossi L, Luinstra K, Loeb M, Smieja M, Johnstone J, Surette MG, Bowdish DM. 2014. The loss of topography in the microbial communities of the upper respiratory tract in the elderly. Ann Am Thorac Soc 11:513–521. doi: 10.1513/AnnalsATS.201310-351OC. [DOI] [PubMed] [Google Scholar]
- 53.Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 54.Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD. 2012. PANDAseq: paired-end assembler for Illumina sequences. BMC Bioinformatics 13:31. doi: 10.1186/1471-2105-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye Y. 2011. Identification and quantification of abundant species from pyrosequences of 16S rRNA by consensus alignment. Proceedings (IEEE Int Conf Bioinformatics Biomed) 2010:153–157. doi: 10.1109/BIBM.2010.5706555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeSantis TZ Jr, Hugenholtz P, Keller K, Brodie EL, Larsen N, Piceno YM, Phan R, Andersen GL. 2006. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res 34:W394–W399. doi: 10.1093/nar/gkl244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Food and Agriculture Organization and World Health Organization Expert Consultation. 2001. Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Food and Agriculture Organization of the United Nations and World Health Organization, Córdoba, Argentina. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.