ABSTRACT
Fusarium graminearum (teleomorph: Ascomycota, Hypocreales, Gibberella, Gibberella zeae) is a destructive fungal pathogen that threatens the production and quality of wheat and barley worldwide. Controlling this toxin-producing pathogen is a significant challenge. In the present study, the commercially available strain Bacillus amyloliquefaciens (Bacteria, Firmicutes, Bacillales, Bacillus) FZB42 showed strong activity against F. graminearum. The lipopeptide bacillomycin D, produced by FZB42, was shown to contribute to the antifungal activity. Purified bacillomycin D showed strong activity against F. graminearum, and its 50% effective concentration was determined to be approximately 30 μg/ml. Analyses using scanning and transmission electron microscopy revealed that bacillomycin D caused morphological changes in the plasma membranes and cell walls of F. graminearum hyphae and conidia. Fluorescence microscopy combined with different dyes showed that bacillomycin D induced the accumulation of reactive oxygen species and caused cell death in F. graminearum hyphae and conidia. F. graminearum secondary metabolism also responded to bacillomycin D challenge, by increasing the production of deoxynivalenol. Biological control experiments demonstrated that bacillomycin D exerted good control of F. graminearum on corn silks, wheat seedlings, and wheat heads. In response to bacillomycin D, F. graminearum genes involved in scavenging reactive oxygen species were downregulated, whereas genes involved in the synthesis of deoxynivalenol were upregulated. Phosphorylation of MGV1 and HOG1, the mitogen-activated protein kinases of F. graminearum, was increased in response to bacillomycin D. Taken together, these findings reveal the mechanism of the antifungal action of bacillomycin D.
IMPORTANCE Biological control of plant disease caused by Fusarium graminearum is desirable. Bacillus amyloliquefaciens FZB42 is a representative of the biocontrol bacterial strains. In this work, the lipopeptide bacillomycin D, produced by FZB42, showed strong fungicidal activity against F. graminearum. Bacillomycin D caused morphological changes in the plasma membrane and cell wall of F. graminearum, induced accumulation of reactive oxygen species, and ultimately caused cell death in F. graminearum. Interestingly, when F. graminearum was challenged with bacillomycin D, the deoxynivalenol production, gene expression, mitogen-activated protein kinase phosphorylation, and pathogenicity of F. graminearum were significantly altered. These findings clarified the mechanisms of the activity of bacillomycin D against F. graminearum and highlighted the potential of B. amyloliquefaciens FZB42 as a biocontrol agent against F. graminearum.
KEYWORDS: fungus-bacterium interactions, Bacillus amyloliquefaciens, Fusarium graminearum, bacillomycin D, reactive oxygen species, cell death, mitogen-activated protein kinases
INTRODUCTION
The filamentous fungus Fusarium graminearum Schwabe (teleomorph: Gibberella zeae) can cause Fusarium head blight (FHB) on wheat and barley, stalk and ear rot diseases on maize, and seedling blight on maize and wheat (1). In addition to severe yield losses and reduction of grain quality, F. graminearum can produce several mycotoxins, such as deoxynivalenol (DON) and zearalenone, in infested grains. These metabolites represent a significant threat to the health of animals (2, 3). Despite the large economic and health impacts of FHB, no highly resistant varieties of wheat or barley are available (4, 5). In fact, FHB management relies heavily on synthetic antifungal agents, such as benzimidazole fungicides (6, 7). However, the continuous use of these synthetic antifungal agents has resulted in the emergence of resistant F. graminearum, which poses a potential risk to the environment and human health (7–9). Therefore, it is essential to develop alternative methods and agents, which show low toxicity and are environmentally friendly, for the efficient control of FHB. Among them, biocontrol agents that are friendly to the environment and ecosystems have attracted increasing attention worldwide (10).
Plant-growth-promoting rhizobacteria (PGPR), which live within or near plant roots, can suppress soilborne plant pathogens and promote plant growth. These beneficial biological activities have led to the development of many PGPR strains as commercial biocontrol agents that are able to control plant diseases (11). Among them, some species from the Bacillus genus, as typical representatives of PGPR, are considered the best candidates to develop as efficient biocontrol agents, because of their production of a wide range of bioactive compounds and their ability to form highly adversity-resistant endospores (10). Their protective effects might rely on different mechanisms that antagonize pathogen growth directly (12) or indirectly, via the stimulation of induced systemic resistance of plants and growth promotion (13). In addition, Bacillus species can compete with plant pathogens for nutrients, especially iron, which is another important factor in plant protection (13). Several Bacillus-based commercial products are available, such as Quantum-400 (Bacillus subtilis GB03), Serenade (B. subtilis QST713), and Rhizovital (Bacillus amyloliquefaciens FZB42) (14, 15).
B. amyloliquefaciens FZB42, now renamed as B. amyloliquefaciens subsp. plantarum FZB42, is the model strain of Gram-positive PGPR (16). FZB42 is known for its efficient rhizosphere colonization, its plant-growth-promoting properties, and its ability to suppress different plant pathogens (17, 18). Genome analysis revealed that 10 gene clusters in FZB42, covering nearly 10% of the whole genome, are responsible for the production of secondary metabolites with antimicrobial and nematocidal activities (10). Seven of these secondary metabolites, including three lipopeptides (surfactin, bacillomycin D, and fengycin) (17), three polyketides (macrolactin, bacillaene, and difficidin) (19, 20), and one siderophore (bacillibactin), are synthesized using a 4′-phosphopantetheinyl transferase (Sfp)-dependent nonribosomal mechanism (10). Bacilysin is produced via an Sfp-independent nonribosomal pathway (21). Plantazolicin and amylocyclicin are processed and modified peptides are synthesized by ribosomes (22, 23). These 10 compounds play important roles in the biocontrol properties of FZB42. Bacillomycin D and fengycin have antifungal activities, especially against filamentous fungi (17). The three polyketides, bacilysin, and amylocyclicin are associated with antibacterial action (19–21, 24). Plantazolicin has nematocidal activity (22). Bacillibactin, which acts as a siderophore, suppresses pathogens by competition for iron uptake (10). The lipopeptide surfactin has antiviral and anti-Mycoplasma activities and plays a key role in biofilm formation and root colonization (25).
In the present study, we found that FZB42 has strong antagonistic activity toward F. graminearum strain PH-1. Using mutants of FZB42, we demonstrated the involvement of bacillomycin D in this antagonism. Bacillomycin D, a member of the iturin family of lipopeptides, is a heptapeptide interlinked with a β-amino fatty acid that forms a cyclic ring structure (17, 26). Although many lipopeptides produced by Bacillus have antiphytopathogen actions, their mechanism of action remains poorly understood. The general opinion is that the lipopeptides might form a pore in the cell membrane of the microorganism, resulting in leakage of the cytoplasm. In the present study, to clarify the mechanism underlying the antifungal activity of bacillomycin D, we performed a detailed analysis of the effect exerted by bacillomycin D, because this lipopeptide is the main factor in the B. amyloliquefaciens-F. graminearum interaction.
RESULTS
Antagonistic activity toward F. graminearum of bacillomycin D produced by B. amyloliquefaciens FZB42.
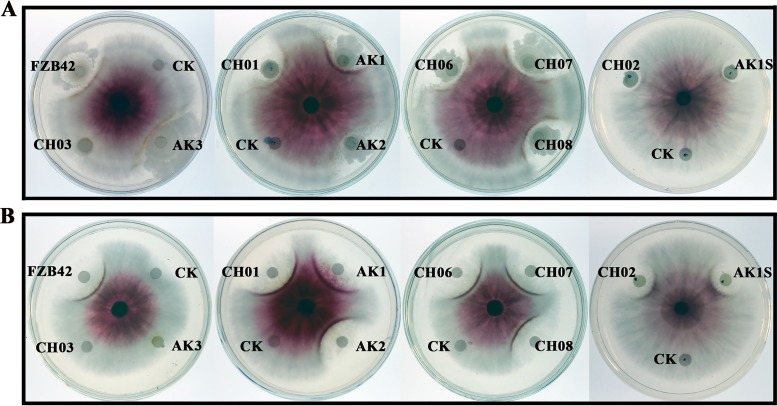
Antagonism assays showed that both FZB42 and a crude extract of its secondary metabolites could suppress the growth of F. graminearum. To identify the fungicidal agents acting against F. graminearum, corresponding mutants of FZB42 were used. In FZB42, the Sfp protein 4′-phosphopantetheinyl transferase acts as the peptidyl carrier protein and is essential for the production of three lipopeptides and three polyketides (10, 17). When the Sfp-deficient mutant CH03 was used in the assays, the strain and its secondary metabolites showed no antifungal activity (Fig. 1A and B). This finding indicated that lipopeptides and polyketides produced through the Sfp-dependent pathway were involved in the suppression of F. graminearum growth. Consequently, further mutant strains were used. Three polyketide-deficient mutants, namely, CH06 (deficient in bacillaene), CH07 (deficient in macrolactin), and CH08 (deficient in difficidin), together with CH01 (deficient in surfactin), showed antifungal activity similar to that of wild-type FZB42 (Fig. 1A and B). This finding indicated that these four non-ribosomally synthesized compounds had no antifungal activity. A common characteristic of these four mutants is that they all produce bacillomycin D and fengycin. When the bacillomycin D- and fengycin-deficient double mutant AK3 was tested, the antifungal activity was abolished. However, the mutants AK1 (surfactin and fengycin producer) and AK2 (surfactin and bacillomycin D producer), which can produce two lipopeptides, had antifungal activity similar to that of FZB42 (Fig. 1A and B). We then tested two double mutants, CH02 and AK1S. CH02 produces only one lipopeptide, bacillomycin D, and AK1S synthesizes only one lipopeptide, fengycin. Both mutants could suppress the growth of F. graminearum (Fig. 1A and B). Previously, surfactin was reported to have no antifungal activity (7, 17). Thus, the results indicated that both bacillomycin D and fengycin acted as fungicidal factors and were responsible for in vitro suppression of F. graminearum growth, confirming earlier findings obtained with Fusarium oxysporum (teleomorph: Ascomycota, Hypocreales, Nectriaceae) (17). Fengycin, which is a lipodecapeptide that has a β-hydroxy fatty acid in its side chain and has a different structure, compared with bacillomycin D (17), has attracted more attention (27, 28). Therefore, in this study we focus on the mechanism of action of bacillomycin D, which was shown previously to be the main antifungal agent produced by FZB42 (17).
FIG 1.
Antagonistic activities against F. graminearum PH-1 of FZB42 and its mutants (A) and of the secondary metabolite extract (B). FZB42 and its mutants are described in Materials and Methods. CK, control (LB medium or methanol).
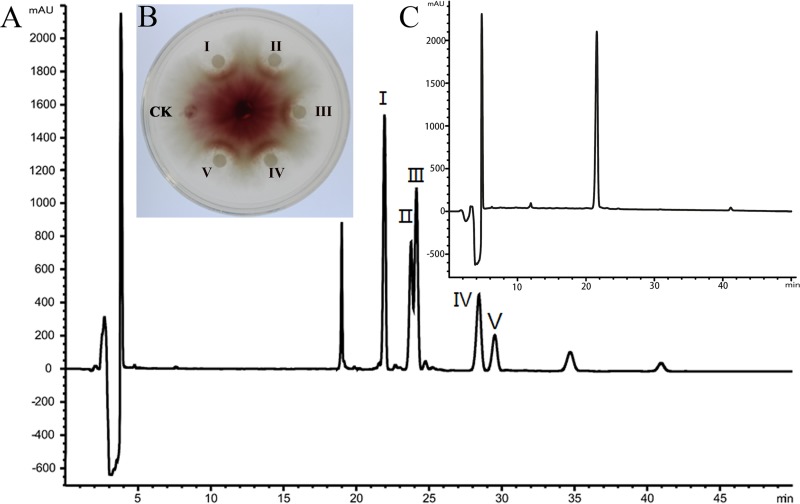
To identify the mechanism of action of bacillomycin D, the CH02 mutant, which produces only bacillomycin D, was grown and used for purification. High-performance liquid chromatography (HPLC) analysis showed five peaks between 21 min and 30 min (Fig. 2A). The substances extracted from these five peaks all showed inhibitory activity against F. graminearum (Fig. 2B). Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) analysis showed that the major component in those five peaks was bacillomycin D. There were molecular ion peaks [(M+H)+] for C16 bacillomycin D at m/z 1,059.6, ion peaks [(M+K)+] for C17 bacillomycin D at m/z 1,111.6, and ion peaks [(M+Na)+] for C14 to C18 bacillomycin D at m/z 1,053.5, 1,067.5, 1,081.7, 1,095.6, and 1,109.6 (see Fig. S1 in the supplemental material). Among these molecules, all containing the same ion, there were 14-Da differences in molecular mass, suggesting the presence of fatty acid chains of different lengths within bacillomycin D (CH2 is 14 Da). These results were identical to a previous report (17). The purity of bacillomycin D collected from peak I, which was used for further study, was determined to be 96.6% (Fig. 2C). We tested a range of concentrations to determine the 50% effective concentration (EC50) of purified bacillomycin D against F. graminearum. The results showed that the inhibitory activity increased with the concentration of purified bacillomycin D (Fig. S2), and the EC50 was calculated as approximately 30 μg/ml.
FIG 2.
Purification and characterization of bacillomycin D from B. amyloliquefaciens CH02. (A) Bacillomycin D was purified from B. amyloliquefaciens CH02 using reverse-phase HPLC. (B) Five fractions (I to V) were separated and collected by reverse-phase HPLC and were used for the analysis of antagonistic activity. 45% acetonitrile (vol/vol) served as the control (CK). (C) The purity of bacillomycin D collected from peak I was analyzed by HPLC.
Microscopic and ultrastructural changes to F. graminearum hyphae and conidia caused by bacillomycin D.
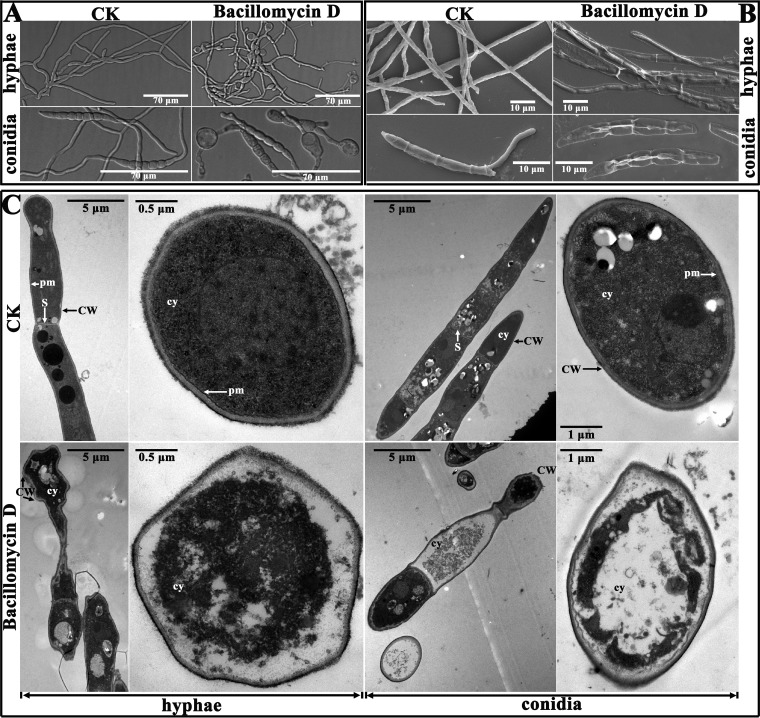
The morphological variations of fungal mycelia and spores treated with bacillomycin D at a relatively low concentration of 9 μg/ml were revealed clearly by microscopic observation. The structure of untreated F. graminearum mycelia was well organized, while structures appeared swollen in the presence of bacillomycin D (Fig. 3A). These swollen structures were observed both at the tip and in the central part of the treated hyphae. A similar phenomenon was also found in the conidia (Fig. 3A). The untreated conidia germinated and appeared normal. With bacillomycin D treatment, the germination of conidia was inhibited, and the conidia that were produced appeared swollen (Fig. 3A). However, these swollen structures were gradually reduced as the concentration of bacillomycin D was increased (Fig. S3). In further experiments, scanning electron microscopy and transmission electron microscopy were used to detect the damage to F. graminearum, at the ultrastructural level, caused by a relatively high concentration of bacillomycin D (30 μg/ml).
FIG 3.
Effect of bacillomycin D on the morphology of F. graminearum PH-1 hyphae and conidia. (A) Effect of 9 μg/ml bacillomycin D on the morphology of F. graminearum PH-1 hyphae and conidia observed with a light microscope. (B and C) Ultrastructural effects of 30 μg/ml bacillomycin D on F. graminearum PH-1 after 12 h, as determined by scanning electron microscopy (B) and transmission electron microscopy (C). CW, cell wall; cy, cytoplasm; pm, plasma membrane; S, septum. In all of the experiments, 6.67% (vol/vol) methanol served as the control (CK).
The results of scanning electron microscopy showed that the untreated control hyphae and conidial spores of F. graminearum appeared regular, intact, and plump, with a column-like trunk, and were typically multiseptate on the outer surface. The germ tubes generated from the conidia were visible (Fig. 3B). Upon exposure to bacillomycin D, substantial exterior destruction of hyphae and conidia was found, such as irregular shapes, loose cell walls, surface subsidence, and shriveled trunks (Fig. 3B). This finding indicated that the cytoplasm was leaking out of the cells as a result of treatment with bacillomycin D. To verify this observation, transmission electron microscopy was used. In the absence of bacillomycin D, the hyphae and conidia showed a distinct cell wall, intact plasma membranes and septum, and a uniformly distributed, electrodense, and clearly visible cytoplasm (Fig. 3C). In contrast, the cell walls of the treated hyphae and conidia showed an irregular structure, with no discernible layers, uneven thickness, and even gapped structures (Fig. 3C). Bacillomycin D treatment also resulted in plasmolysis and caused fragmentation of the plasma membranes. Furthermore, the damage to the cell wall and membranes finally resulted in leakage of the cytoplasm, as indicated by the higher electron density in the treated cells versus the control cells.
Accumulation of reactive oxygen species and cell death of F. graminearum caused by bacillomycin D.
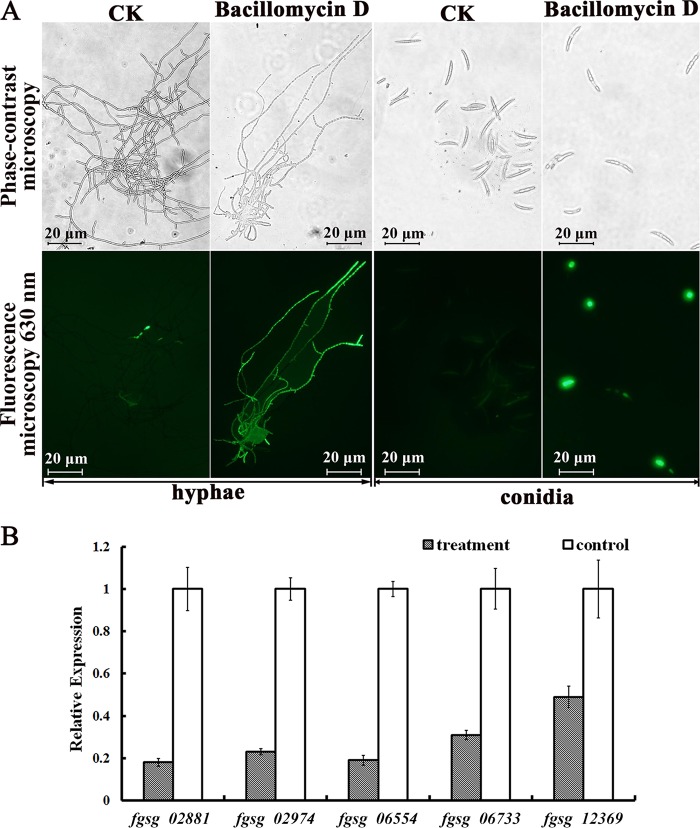
High concentrations of reactive oxygen species (ROS) are harmful to cells and result in cell death (29). To investigate whether F. graminearum cells accumulate ROS as a result of bacillomycin D treatment, a ROS assay kit was used. The treated hyphae and conidia of F. graminearum showed stronger green fluorescence than did those of the control. In particular, for the hyphae, nearly the entire mycelium showed green fluorescence (Fig. 4A). Subsequent flow cytometry assays confirmed that the treated conidia had stronger fluorescence intensity than did the controls (Fig. S4). F. graminearum contains five putative extracellular ROS-scavenging enzymes, i.e., three putative catalases (FGSG_02881, FGSG_06554, and FGSG_06733) and two putative peroxidases (FGSG_02974 and FGSG_12369) (30). The results of quantitative real-time PCR (qRT-PCR) analysis showed that all five genes in F. graminearum were downregulated after treatment with bacillomycin D, especially those for two catalases (FGSG_02881 and FGSG_06554) (Fig. 4B).
FIG 4.
Effect of 30 μg/ml bacillomycin D on ROS production by F. graminearum. (A) Detection of ROS was based on DCFH-DA staining after treatment with bacillomycin D for 5 h. (B) Quantitative real-time PCR analysis of the expression of five genes (fgsg_02881, fgsg_02974, fgsg_06554, fgsg_06733, and fgsg_12369) in F. graminearum PH-1 in response to bacillomycin D treatment. Values were normalized to the levels of the actin gene as an internal reference. The y axis represents the mean expression values ± standard deviations (SDs), relative to the control. The experiments were repeated independently three times. In all of the experiments, 6.67% (vol/vol) methanol served as the control (CK).
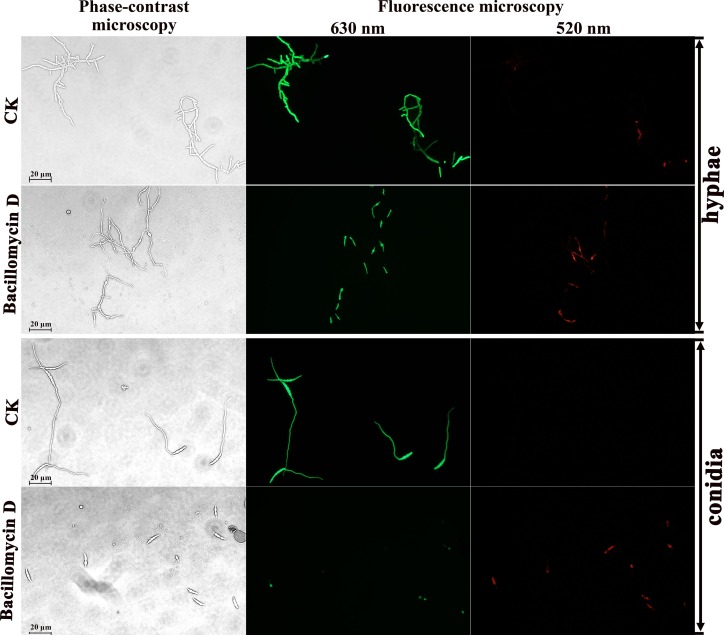
Therefore, to further detect whether cell death occurred, fluorescein diacetate and propidium iodide double fluorescence staining was used to analyze live and dead cells, in combination with phase-contrast and fluorescence microscopy. Fluorescein diacetate is an enzyme activity probe that is recognized by nonspecific esterases. This recognition releases green fluorescence once the compound enters living cells, and the compound thus serves as an indicator of live cells. Propidium iodide fluoresces red in response to membrane damage and is used as an indicator of dead cells. As shown in Fig. 5, the untreated hyphae and conidia had few dead cells (red fluorescence). Accordingly, their typical shape, outlined by green fluorescence (live cells), could be identified easily. In contrast, after 12 h of exposure to 30 μg/ml bacillomycin D, the proportion of cells with red fluorescence in the hyphae and conidia increased, and the green fluorescence showed blurred shapes (Fig. 5). Combined with the results of scanning and transmission electron microscopy, these observations indicated that bacillomycin D could destroy the membranes as well as the cell wall, thus inhibiting the growth of F. graminearum.
FIG 5.
Detection of F. graminearum viability based on fluorescein diacetate and propidium iodide staining after treatment with bacillomycin D for 12 h. Live fungal cells with intact membranes show green fluorescence, and fungal cells with damaged membranes show red fluorescence; 6.67% (vol/vol) methanol served as the control (CK).
Bacillomycin D inhibition of the formation and germination of F. graminearum conidia.
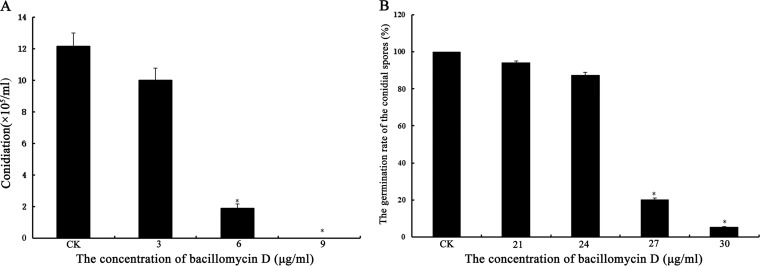
The formation and germination of conidial spores are important for F. graminearum infection of plants. When the mycelia were cultured in carboxymethyl cellulose (CMC) medium containing 3 to 9 μg/ml bacillomycin D, the formation of F. graminearum conidial spores was inhibited significantly. At concentrations of bacillomycin D above 9 μg/ml, almost no conidia were observed (Fig. 6A). In the control, mycelia could form conidia normally (Fig. 6A). Furthermore, the germination of F. graminearum conidia was inhibited by bacillomycin D in a dose-dependent manner (Fig. 6B); with 30 μg/ml bacillomycin D, the germination rate was only 5.44%.
FIG 6.
Analyses of F. graminearum conidiation (A) and conidial spore germination (B) for cultures treated with different concentrations of bacillomycin D. Data are expressed as means ± SDs. *, significant difference, compared with the control (P < 0.01). The experiment was repeated independently three times, and 6.67% (vol/vol) methanol served as the control (CK).
Bacillomycin D reduction of the pathogenicity of F. graminearum on plants and induction of the DON production on wheat kernels.
We next investigated whether bacillomycin D could influence the pathogenicity of F. graminearum on plants. The results showed that corn silks treated with different concentrations of bacillomycin D exhibited significant reductions in F. graminearum virulence, relative to the controls. The lengths of reddish-brown lesions on the corn silks caused by F. graminearum decreased remarkably, and the effect was most pronounced at 90 μg/ml bacillomycin D (Fig. 7A; also see Fig. S5). Similar results were obtained when wheat seedlings were treated with the same concentrations of bacillomycin D (Fig. 7B). These results indicated that 90 μg/ml bacillomycin D could reduce the pathogenicity of F. graminearum on corn silks and wheat seedlings markedly. When wheat heads were treated with this concentration, the wheat heads were covered with fewer mycelia and remained green; 45% of the grains were healthy (Fig. 7C; also see Table S1). By comparison, the untreated wheat heads were almost completely covered with mycelia and turned completely gray; nearly all of the grains also turned gray (Fig. 7C). Thus, bacillomycin D could reduce the pathogenicity of F. graminearum in plants.
FIG 7.

Bacillomycin D effects on F. graminearum PH-1 infection of corn silks, wheat seedlings, and wheat heads. (A) Corn silks were inoculated with a 0.6-cm-diameter plug containing F. graminearum PH-1 mycelia and then were treated with 30 to 90 μg/ml bacillomycin D; 6.67% (vol/vol) methanol served as the control. White arrows show the reddish-brown discoloration in the corn silks. (B) Wheat seedlings were inoculated with conidial suspensions of F. graminearum PH-1 and then were treated with 30 to 90 μg/ml bacillomycin D. A conidial suspension (106 conidia/ml) with 2% (wt/vol) gelatin and 6.67% (vol/vol) methanol served as the control. Black arrows show the black discoloration in the wheat seedlings. (C) Wheat heads were point inoculated with conidial suspensions of F. graminearum PH-1 and then were treated with 90 μg/ml bacillomycin D. A conidial suspension with 6.67% (vol/vol) methanol served as the control.
To characterize the effect of bacillomycin D on the biosynthesis of DON in F. graminearum, we inoculated the fungus on sterilized wheat kernels treated with or without 30 μg/ml bacillomycin D. After 20 days of incubation, we found that bacillomycin D could stimulate the accumulation of DON on wheat kernels significantly (Fig. 8A). In F. graminearum, the DON biosynthetic enzymes and direct regulators are encoded by 15 tri genes (31). To further determine the molecular mechanism underlying the regulation of DON production by bacillomycin D, the tri5, tri6, tri10, and tri12 expression levels were analyzed. As shown in Fig. 8B, bacillomycin D induced the expression of tri5, tri6, and tri10 dramatically. These results suggested that, at the stated dose, bacillomycin D influences DON biosynthesis positively.
FIG 8.
Effects of bacillomycin D on deoxynivalenol (DON) biosynthesis (A) and the expression of DON-related genes (B) of F. graminearum. Data are expressed as means ± SDs. *, significant difference, compared with controls (P < 0.01). In the quantitative real-time PCR analysis, values were normalized to the levels of the actin gene, as an internal reference; gene expression values are reported as means ± SDs, relative to control values. The experiments were repeated independently three times.
Involvement of bacillomycin D in the phosphorylation of HOG1 and MGV1 of F. graminearum.
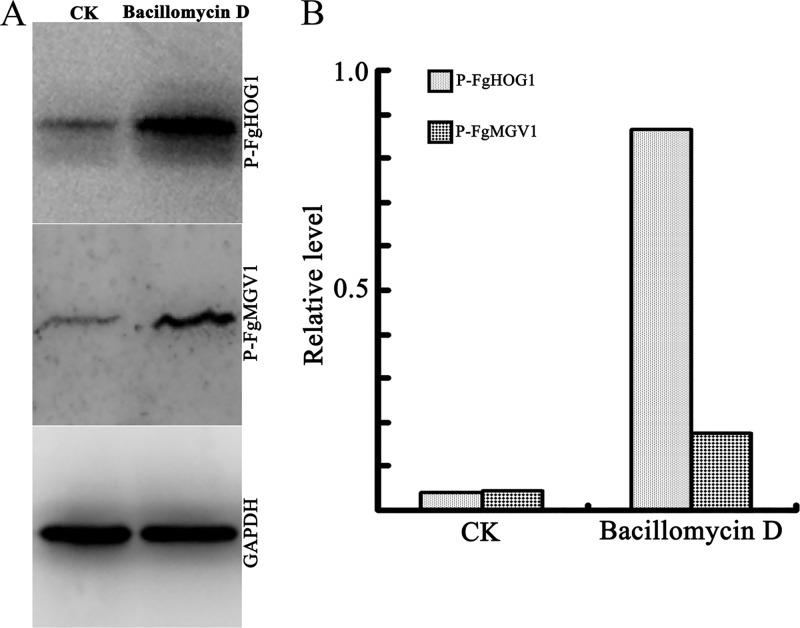
In Saccharomyces cerevisiae, the cell wall integrity (CWI) and high osmolarity glycerol (HOG) signaling pathways are involved in cell wall stress responses and the regulation of high osmotic stress responses, respectively. Phosphorylation of two mitogen-activated protein kinases (MAPKs), i.e., MPK1 and HOG1, are required for these two signaling pathways to respond to multiple environmental stresses (32, 33). In the current study, 30 μg/ml bacillomycin D, which could suppress the growth of one-half of the F. graminearum mycelia, could be regarded as an environmental stress for F. graminearum. Therefore, the phosphorylation of FgMGV1 and FgHOG1, which are the orthologs of MPK1 and HOG1, respectively, from S. cerevisiae (34, 35), was analyzed. As indicated in Fig. 9, significantly increased levels of phosphorylated FgMGV1 and FgHOG1 were observed in the strain treated with bacillomycin D for 2 h. These results indicated that, at the stated concentration, bacillomycin D induced the activation of the CWI and HOG signaling pathways in F. graminearum.
FIG 9.
Phosphorylation of FgHOG1 and FgMGV1 in F. graminearum after exposure to bacillomycin D. (A) Western blotting assay. (B) Bar graphs representing quantification of the levels of phosphorylated FgHOG1 or FgMGV1, relative to the GAPDH reference. Prepared conidia of F. graminearum PH-1 were incubated in PDB with 30 μg/ml bacillomycin D, and 6.67% (vol/vol) methanol was used as the control (CK). Anti-phospho-p44/42 MAPK, anti-phospho-p38 MAPK, and anti-GAPDH were used as antibodies.
DISCUSSION
In the present study, we showed that the bacillomycin D synthesized by FZB42 inhibited F. graminearum growth strongly (Fig. 1). Until now, the mechanism of the activity of bacillomycin D was poorly understood. Elucidation of the mechanism of the antifungal activity of bacillomycin D in biocontrol Bacillus strains is necessary for the efficient application of such compounds in agriculture.
Bacillomycin D is produced by several Bacillus strains and has antagonistic activities against different microorganisms, such as Candida species (teleomorph: Ascomycota, Saccharomycetales, Debaryomycetaceae) (36), Aspergillus flavus (teleomorph: Ascomycota, Eurotiales, Aspergillaceae) (37), Sclerotinia sclerotiorum (teleomorph: Ascomycota, Helotiales, Sclerotiniaceae) (38), and Botrytis cinerea (teleomorph: Ascomycota, Helotiales, Sclerotiniaceae) (39). In our previous studies, we also showed that bacillomycin D could inhibit the growth of Monilinia fructicola (teleomorph: Ascomycota, Helotiales, Sclerotiniaceae) and F. oxysporum (teleomorph: Ascomycota, Hypocreales, Nectriaceae) (17, 40). Here, we used scanning electron microscopy and transmission electron microscopy to better understand the mechanism of bacillomycin D. The results showed that bacillomycin D disrupted the plasma membranes of the hyphae and conidia of F. graminearum, resulting in the leakage of cytoplasm and plasmolysis (Fig. 3). These results were consistent with those reported for other lipopeptides. Surfactin induces the leakage and lysis of lipid membranes using POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine) vesicles (41). Fengycin and iturin cause cell lysis and cytoplasm extravasation of M. fructicola and Verticillium dahlia (anamorph: Hyphomycetes, Moniliales, Verticillium), respectively (40, 42). Accordingly, using interfacial monolayers as biomimetic membranes, Nasir and Besson suggested that the alcohol group of sterol was involved in the bacillomycin D-sterol interaction (43). Sterol is a major constituent of the fungal plasma membrane and is vital for the survival of a fungus. In addition, transmission electron microscopy revealed that the cell walls of the hyphae and conidia were damaged to some extent by bacillomycin D, with an irregular appearance, large depressions, loss of turgidness, and even gapped structures (Fig. 3C). This result differed from a previous report that showed no visible alterations in the Podosphaera fusca (teleomorph: Ascomycota, Erysiphales, Podosphaera) cell wall upon treatment with B. subtilis lipopeptides (44). The fungal cell wall protects the cell from changes in osmotic pressure and other environmental stresses. Taken together, our results and previous results suggested that bacillomycin D causes severe damage to the plasma membranes and alterations to the cell walls of F. graminearum hyphae and conidia, resulting in cell death.
Further study of the mechanism of action of bacillomycin D showed that it induced high ROS accumulation in F. graminearum hyphae and conidia (Fig. 4A). Additionally, bacillomycin D could induce the expression of the glutathione reductase and thioredoxin genes in F. graminearum, which are involved in the synthesis of ROS. In addition, five genes encoding putative ROS-scavenging enzymes, i.e., catalases (FGSG_02881, FGSG_06554, and FGSG_06733) and peroxidases (FGSG_02974 and FGSG_12369), were significantly downregulated when F. graminearum was treated with bacillomycin D (Fig. 4B). Low ROS concentrations act as intracellular messengers for many molecular events; however, large amounts of ROS are associated with cell death (29). Further experiments using double fluorescence staining with fluorescein diacetate and propidium iodide showed that bacillomycin D induced significant cell death of F. graminearum hyphae and conidia (Fig. 5). Similar results were observed for other lipopeptides. Recent reports indicated that bacillomycin D and surfactin could induce apoptosis and cell death in human cancer cells and fungi, respectively, via ROS generation (45, 46). The induction of ROS bursts by iturin was suggested to contribute to cell death in V. dahlia (42).
Conidial spore formation and germination represent the first steps triggering the asexual life cycle of F. graminearum and also play important roles in spreading the disease. In this study, both the formation and germination of conidial spores were inhibited strongly by bacillomycin D (Fig. 6). When the conidial spores of F. graminearum were used as the resource to infect wheat seedlings and wheat heads, the damage to the conidia and the inhibition of conidial germination caused by bacillomycin D resulted in markedly reduced disease symptoms in these two plant materials (Fig. 7). In addition, because of the negative effect of this compound on the hyphae, bacillomycin D could inhibit the infection of corn silks by F. graminearum hyphae (Fig. 7A). Therefore, the use of microorganisms producing antifungal compounds that inhibit hyphal growth and conidial germination should be considered a potential means for biocontrol of fungal diseases.
The MAPK signaling pathways have been well characterized in F. graminearum (47, 48). Phosphorylation of the MAPKs FgHOG1 and FgMGV1 regulates environmental stress responses and DON production positively (34, 35). Here, we observed that 30 μg/ml bacillomycin D was sufficient to stimulate DON biosynthesis in F. graminearum. Fengycin and surfactin were also reported to induce significant fumonisin production by Fusarium verticillioides (teleomorph: Ascomycota, Hypocreales, Nectriaceae) (28). Interestingly, bacillomycin D appeared to act as an activator for the phosphorylation of FgHOG1 and FgMGV1 (Fig. 9). The iturins were observed to induce HOG1 activation of V. dahlia (42). Thus, we hypothesized that bacillomycin D induces DON production via positive regulation of FgHOG1 and FgMGV1 in F. graminearum, although further experimental evidence is needed. In summary, we report that B. amyloliquefaciens FZB42 and its product bacillomycin D have potential as biocontrol agents against F. graminearum.
MATERIALS AND METHODS
Bacterial and fungal strains and growth conditions.
B. amyloliquefaciens FZB42 and its corresponding mutants that were tested for activity against F. graminearum PH-1 are described in Table 1. B. amyloliquefaciens strains were cultivated routinely in Luria-Bertani (LB) medium solidified with 1.5% agar and were fermented in Landy medium (17). When required, antibiotics were added at the following final concentrations: ampicillin, 100 μg/ml; chloramphenicol, 5 μg/ml; erythromycin, 10 μg/ml.
TABLE 1.
Fungal and bacterial strains used in this study
| Strain | Descriptiona | Source or reference |
|---|---|---|
| Fusarium graminearum | ||
| PH-1 | Wild type (lineage 7) | 30 |
| Bacillus amyloliquefaciens | ||
| FZB42 | Wild type; producer of lipopeptides (surfactin, bacillomycin D, and fengycin) and polyketides (macrolactin, bacillaene, and difficidin) | 17 |
| AK1 | FZB42 ΔbmyA::Emr; deficient in bacillomycin D synthesis | 17 |
| AK2 | FZB42 ΔfenA::Cmr; deficient in fengycin synthesis | 17 |
| AK3 | FZB42 ΔbmyA::Emr ΔfenA::Cmr; deficient in bacillomycin D and fengycin synthesis | 17 |
| AK1S | FZB42 ΔbmyA::Emr ΔsrfA::Cmr; deficient in bacillomycin D and surfactin synthesis | This study |
| CH01 | FZB42 ΔsrfA::Emr; deficient in surfactin synthesis | 17 |
| CH02 | FZB42 ΔfenA::Cmr ΔsrfA::Emr; deficient in fengycin and surfactin synthesis | 19 |
| CH03 | FZB42 Δsfp::Emr; deficient in lipopeptide (surfactin, bacillomycin D, and fengycin) and polyketide (macrolactin, bacillaene, and difficidin) synthesis | 19 |
| CH06 | FZB42 Δbae::Cmr; deficient in bacillaene synthesis | 19 |
| CH07 | FZB42 Δmln::Cmr; deficient in macrolactin synthesis | 19 |
| CH08 | FZB42 Δdfn::Emr; deficient in difficidin synthesis | 19 |
aCmr, chloramphenicol resistance; Emr, erythromycin resistance.
For conidial spore cultures, fresh mycelia of each strain (50 mg), taken from the periphery of a 3-day-old colony, were inoculated in a 50-ml flask containing 20 ml of mung bean liquid (MBL) broth (10 g of mung beans were boiled in 1 liter of water for 20 min and filtered through cheesecloth). After incubation at 25°C for 4 days in a shaker (180 rpm), the number of conidia in each flask was determined using a hemacytometer.
Construction of the AK1S mutant.
The AK1S mutant, which was deficient in the synthesis of both bacillomycin D and surfactin and produced one lipopeptide, fengycin, was constructed as follows. Genomic regions of about 600 bp upstream and downstream of the srfAA gene were amplified from B. amyloliquefaciens FZB42 chromosomal DNA. The two gel-purified double-stranded DNA fragments were linked by a chloramphenicol resistance cassette and then ligated into vector pMD-18T. The linearized plasmid was transformed into the AK1 mutant (erythromycin resistant and deficient in surfactin) and integrated into the genome by double-crossover recombination to produce the AK1S mutant. The specific primers used are listed in Table 2.
TABLE 2.
DNA primers used in this study
Antifungal activity assay and EC50 determination.
The antifungal activities of FZB42 and its mutants, extracts of their secondary metabolites, and purified bacillomycin D were analyzed as follows. The preparation of the secondary metabolite extracts and the purification of bacillomycin D are described below. Briefly, the antifungal activities were assessed on potato dextrose agar (PDA). A 0.6-cm-diameter plug containing mycelium was placed at the center of the PDA plates, 5 μl of bacterial suspension (optical density at 600 nm [OD600] of 2), or the corresponding secondary metabolite extract, was patched 3 cm from the fungus, and the plates were incubated at 25°C for 48 h; subsequently, the diameters of the inhibition zones were measured. The EC50 of purified bacillomycin D was evaluated as follows. A 0.6-cm-diameter plug containing mycelium was placed at the center of PDA plates containing different concentrations of bacillomycin D (12.5, 25, 50, and 100 μg/ml), and the plates were incubated at 25°C for 48 h. The diameters of the colonies were measured, and the EC50 was calculated using statistical analysis.
Purification of bacillomycin D from the CH02 mutant and MALDI-TOF MS analysis.
The CH02 mutant, which could produce only one lipopeptide, bacillomycin D, was used for purification of this compound. The use of CH02 excludes the possibility of contamination by other lipopeptides (surfactin and fengycin) in our preparation. A single CH02 colony was inoculated into 20 ml of LB medium in a 100-ml flask and cultured for 18 h at 37°C. Six milliliters of this culture was then inoculated into 200 ml of Landy medium in a 500-ml flask and cultured for 48 h at 30°C. The supernatant was collected following centrifugation at 12,000 × g for 20 min at 4°C (Beckman Coulter Avanti J-26S XP centrifuge with a JA-10 rotor). Lipopeptides in the supernatant were precipitated by adjusting the pH to 2 and centrifuging the mixture at 12,000 × g for 20 min at 4°C. The precipitates were redissolved in methanol and adjusted to pH 7.0 using 1.0 M NaOH (49). The supernatant was then passed through a silica gel column using different ratios of methanol and methylene chloride, termed MIX1 to MIX3 (the methanol/methylene chloride ratios in MIX1, MIX2, and MIX3 were 1:2, 3:1, and 5:1, respectively).
The eluates from MIX2 and MIX3 were collected and used to purify bacillomycin D by preparative HPLC, using a microbore 1100 HPLC system (Agilent Technologies, Santa Clara, CA, USA) with a VP 250/21 Nucleodur C18 HTec 5-μm column (Macherey-Nagel). Mobile phase A was acetonitrile with 0.1% (vol/vol) trifluoroacetic acid (TFA), and mobile phase B was Milli-Q water with 0.1% (vol/vol) TFA. Purification was performed using a solvent containing 45% mobile phase A and 55% mobile phase B, at a flow rate of 8 ml/min. For detection, the UV absorption at 207 nm was recorded. The purity of bacillomycin D collected from different peaks was detected using a 1200 HPLC system (Agilent Technologies) with an Agilent Eclipse XDB-C18 5-μm column. Only one large peak appeared at running times between 10 min and 40 min. Based on the area of the peak, we calculated the purity of bacillomycin D, which was used in our further studies, as 96.6%.
The elution components from different peaks were tested for their antifungal activity and analyzed by MALDI-TOF MS in a Bruker Daltonik Reflex MALDI-TOF instrument containing a 337-nm nitrogen laser for desorption and ionization (50). α-Cyano-4-hydroxycinnamic acid was used as the matrix.
Light microscopic, scanning electron microscopic, and transmission electron microscopic observation of hyphal and conidial morphologies.
To observe the morphological changes of hyphae and conidia caused by bacillomycin D, light microscopy and electron microscopy were used. For light microscopy, hyphae and conidia were treated with different concentrations of bacillomycin D. Twelve hours after treatment, the hyphae and conidia were observed using an Olympus BX43 microscope, and findings were analyzed using cellSens standard software. Scanning electron microscopy and transmission electron microscopy were used to determine, at the ultrastructural level, the effects of bacillomycin D (30 μg/ml) on hyphae and conidia of F. graminearum. For scanning electron microscopy, hyphae and conidia treated with bacillomycin D were centrifuged and prefixed with 2.5% glutaraldehyde. Fixed cells were rinsed three times for 10 min with 100 mM phosphate buffer, postfixed for 3 h in 1% osmium tetroxide, and dehydrated through an ethanol gradient. The samples were then coated with gold and analyzed with a Hitachi S-3000N scanning electron microscope (Hitachi, Tokyo, Japan). For transmission electron microscopy, samples were embedded in Epon 812, sectioned using an ultramicrotome, and examined with a Hitachi H-600 transmission electron microscope.
Live/dead fungus viability staining.
The cell viability assay was performed using a green fluorescent fluorescein diacetate stain and a red fluorescent propidium iodide stain (27). When the stains are used in an appropriate mixture, live fungal cells with intact membranes show green fluorescence, while fungal cells with damaged membranes show red fluorescence. The hyphae and conidia of F. graminearum PH-1 that had been treated with 30 μg/ml bacillomycin D for 12 h were centrifuged at 1,000 × g for 10 min and resuspended in 10 mM sodium phosphate buffer (pH 7.4). Then, 10 μl of the fluorescein diacetate and propidium iodide molecular probes, prepared as recommended by the manufacturer, was added, and the cell suspensions were incubated for 15 min at 25°C in the dark. The samples were viewed using an Olympus BX43 microscope, and findings were analyzed using cellSens standard software.
Reactive oxygen species detection.
To detect whether reactive oxygen species (ROS) in the cells of F. graminearum hyphae and conidia accumulated after treatment with bacillomycin D, the probe dichlorodihydrofluorescein diacetate (DCFH-DA) (JianCheng Bioengineering Institute, Nanjing, China) and fluorescence microscopy were used. The F. graminearum hyphae and conidia were treated with 30 μg/ml bacillomycin D for 5 h, centrifuged at 1,000 × g for 10 min, and resuspended in 10 mM sodium phosphate buffer (pH 7.4). The samples were then incubated with 10 μM DCFH-DA for 30 min at 19 to 21°C (51). The samples were viewed with an Olympus BX43 microscope (excitation, 488 nm; emission, 535 nm), and findings were analyzed using cellSens standard software.
Formation and germination of conidia.
To elevate the formation of the conidia, CMC medium (15 g sodium carboxymethyl cellulose, 1 g yeast extract, 1 g NH4NO3, 1 g KH2PO4, 0.5 g MgSO4·7H2O, and 1 liter water) was used. Five fresh mycelial plugs of F. graminearum, taken from the periphery of a 3-day-old colony, were inoculated into 50-ml flasks containing 20 ml of CMC medium with 6.67% (vol/vol) methanol and different amounts of bacillomycin D (3, 6, or 9 μg/ml). Twenty milliliters of CMC medium with 6.67% (vol/vol) methanol served as the control. After incubation at 25°C for 4 days in a shaker (180 rpm), the number of conidia in each flask was determined using a hemacytometer. Three repeats were performed. For the assay of conidial spore germination, 1 ml of conidial suspension (103 conidia/ml) containing 6.67% (vol/vol) methanol and different amounts of bacillomycin D (21, 24, 27, or 30 μg/ml) was incubated at 25°C for 24 h in a shaker (180 rpm) before counting. One milliliter of conidial suspension (103 conidia/ml) with 6.67% (vol/vol) methanol served as the control. Three repeats were performed.
Plant infection and DON production assay.
F. graminearum can infect corn silks, wheat seedlings, wheat heads, and wheat kernels and causes the corresponding symptoms of the disease. Therefore, these plant materials were used to evaluate whether bacillomycin D could influence the pathogenicity of F. graminearum. For corn silks, the method described by Seong et al. was used (52). Fresh corn silks were collected from young corn ears of hybrid suyu20 and sliced into 6-cm fragments. The young corn ears had been fertilized and were in the grain filling stage. Four pieces of corn silk fragments were aligned with each other evenly, as a bundle, over Whatman no. 1 filter paper soaked with sterile distilled water, in square plates (side length, 9 cm). The lower ends of the corn silks were covered with a 0.6-cm-diameter block of F. graminearum mycelium. Each plate received four bundles of corn silks. Then, 5 ml of 30, 60, or 90 μg/ml bacillomycin D in 6.67% (vol/vol) methanol was added to the plates. Five milliliters of 6.67% (vol/vol) methanol served as the control. Seven days later, reddish-brown discoloration on the corn silks was observed. The lengths of lesions were measured and analyzed statistically. The experiment was repeated independently three times.
To infect wheat seedlings, the method described by Li et al. was used (53). Seeds of cultivar Annong 8455 were placed on filter paper that had been soaked with adequate sterile distilled water and were cultivated in an illuminated incubator (12-h day and night shifts) at 25°C. After 7 days of cultivation, wheat seedlings were obtained. Then, 2 μl of conidial suspension (106 conidia/ml) with 0.2% (wt/vol) gelatin, 6.67% (vol/vol) methanol, and 30, 60, or 90 μg/ml bacillomycin D was injected into the tip of the wheat seedlings. Two microliters of conidial suspension (106 conidia/ml) with 2% (wt/vol) gelatin and 6.67% (vol/vol) methanol served as the control. Each treatment used three wheat seedlings. Black discoloration in the stems toward the base of the seedling was recorded after 7 days. The experiment was repeated three times.
For wheat heads, the method described by Gu et al. was used (48). Cultivar Annong 8455 was cultivated in a glasshouse. When the wheat was in the anthesis stage, the flowering wheat heads were drop inoculated, at the sixth spikelet from the base of the spike, with 10 μl of conidial suspension (105 conidia/ml) containing 90 μg/ml bacillomycin D. Ten microliters of conidial suspension with 6.67% (vol/vol) methanol served as the control. For each treatment, three wheat heads were inoculated. After inoculation, the plants were kept under 100% humidity at 22 ± 2°C for 2 days and then maintained in the glasshouse. Symptomatic spikelets were examined and counted after 14 days. The experiment was repeated three times.
For the DON production assay, 50 g of healthy wheat kernels (wet weight) was sterilized without added water, inoculated with 1 ml of conidial suspension (106 conidia/ml) containing 30 μg/ml bacillomycin D and 6.67% (vol/vol) methanol, and incubated at 25°C for 20 days. One milliliter of conidial suspension (106 conidia/ml) with 6.67% (vol/vol) methanol served as the control. Three repeats were performed. DON extraction and quantification were performed as described previously (54).
Western blotting.
In this experiment, complete medium (CM) (10 g glucose, 2 g peptone, 1 g yeast extract, 1 g Casamino Acids, nitrate salts, trace elements, 0.01% vitamins, 10 g agar, and 1 liter water [pH 6.5]) was used (55). Six mycelial plugs were inoculated into 150 ml liquid CM and incubated at 25°C in a shaker (200 rpm) for 36 h, after which bacillomycin D was added to the flask to a final concentration of 30 μg/ml and incubation was continued for another 2 h or 6 h; methanol served as the control. Mycelia were harvested, washed with deionized water, and ground in liquid nitrogen. Approximately 200 mg of finely ground mycelia was resuspended in 1 ml of extraction buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 5 mM EDTA, 1% Triton X-100, and 2 mM phenylmethylsulfonyl fluoride [PMSF]) with 10 μl of protease inhibitor cocktail (Sangon, Shanghai, China). After homogenization using a vortex shaker, the lysate was centrifuged in a microcentrifuge at 14,000 × g for 20 min at 4°C. The resulting proteins were separated by SDS-polyacrylamide gel electrophoresis (10% denaturing gel) and transferred to an Immobilon-P transfer membrane (Millipore, Billerica, MA, USA), using a Bio-Rad electroblotting apparatus. Antibodies against phospho-p44/42 MAPK (product no. 9101; Cell Signaling Technology, Boston, MA, USA) and phospho-p38 MAPK (product no. 4511; Cell Signaling Technology) were used at a dilution of 1:2,000, to detect phosphorylated FgMGV1 and FgHOG1, respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was detected in the samples using anti-GAPDH antibodies (product no. EM1101; Huabio, Hangzhou, China), at a dilution of 1:10,000, as a reference. Incubation with a horseradish peroxidase (HRP)-conjugated anti-rabbit IgG secondary antibody (product no. SC2004; Santa Cruz Biotechnology, Santa Cruz, CA, USA), at a dilution of 1:2,000, was performed as described previously (48). The experiment was performed three times independently.
RNA extraction and qRT-PCR analysis.
To extract total RNA, mycelia of F. graminearum PH-1 were inoculated in potato dextrose broth (PDB) and cultured for 2 days at 25°C in the dark. Bacillomycin D was then added to the flask at a final concentration of 30 μg/ml for another 2 h, with methanol serving as the control. Mycelia were harvested by filtration using two layers of Miracloth and were washed with sterilized water. Harvested mycelia were then lyophilized and ground in liquid nitrogen. Total RNA was extracted from the mycelia using the TaKaRa RNAiso reagent (TaKaRa Biotechnology Co., Dalian, China), according to the manufacturer's instructions. First-strand cDNA was synthesized using reverse transcriptase (TaKaRa) with random hexamer primers. The resulting cDNA was used as the template for subsequent PCR amplification. qRT-PCR was performed using SYBR Premix Ex Taq (TaKaRa) in a 7500 fast real-time PCR detection system. The actin gene was used as an internal reference for normalization. Primers for these genes are listed in Table 2.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Key R&D Program of China (grants 2017YFD0200400 and 2017YFD0201101), the Natural Science Foundation of Jiangsu Province, China (grant BK20160719), the Special Fund for the Fundamental Research Funds for Central Universities (grant KYZ201404), and the National Natural Science Foundation of China (grants 31100056 and 31471811).
We thank the native English-speaking scientists of Elixigen Company (Huntington Beach, CA, USA) for editing our manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01075-17.
REFERENCES
- 1.Goswami RS, Kistler HC. 2004. Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol 5:515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 2.McMullen M, Jones R, Gallenberg D. 1997. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis 81:1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- 3.Pestka JJ, Smolinski AT. 2005. Deoxynivalenol: toxicology and potential effects on humans. J Toxicol Environ Health B Crit Rev 8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- 4.Mesterhazy A. 1995. Types and components of resistance to Fusarium head blight. Plant Breed 114:377–386. doi: 10.1111/j.1439-0523.1995.tb00816.x. [DOI] [Google Scholar]
- 5.Anand A, Zhou T, Trick HN, Gill BS, Bockus WW, Muthukrishnan S. 2003. Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J Exp Bot 54:1101–1111. doi: 10.1093/jxb/erg110. [DOI] [PubMed] [Google Scholar]
- 6.Liu N, Fan F, Qiu D, Jiang L. 2013. The transcription cofactor FgSwi6 plays a role in growth and development, carbendazim sensitivity, cellulose utilization, lithium tolerance, deoxynivalenol production and virulence in the filamentous fungus Fusarium graminearum. Fungal Genet Biol 58–59:42–52. doi: 10.1016/j.fgb.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Sun HY, Zhu YF, Liu YY, Deng YY, Li W, Zhang AX, Chen HG. 2014. Evaluation of tebuconazole for the management of Fusarium head blight in China. Australas Plant Pathol 43:631–638. doi: 10.1007/s13313-014-0309-4. [DOI] [Google Scholar]
- 8.Duan Y, Zhang X, Ge C, Wang Y, Cao J, Jia X, Wang J, Zhou M. 2014. Development and application of loop-mediated isothermal amplification for detection of the F167Y mutation of carbendazim-resistant isolates in Fusarium graminearum. Sci Rep 4:7094. doi: 10.1038/srep07094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou Y, Luo Q, Chen C, Zhou M. 2013. Application of tetra primer ARMS-PCR approach for detection of Fusarium graminearum genotypes with resistance to carbendazim. Australas Plant Pathol 42:73–78. doi: 10.1007/s13313-012-0162-2. [DOI] [Google Scholar]
- 10.Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Süssmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R. 2007. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25:1007–1014. doi: 10.1038/nbt1325. [DOI] [PubMed] [Google Scholar]
- 11.Lugtenberg B, Kamilova F. 2009. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- 12.Ongena M, Jacques P. 2008. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Arguelles-Arias A, Ongena M, Halimi B, Lara Y, Brans A, Joris B, Fickers P. 2009. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb Cell Fact 8:63. doi: 10.1186/1475-2859-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brannen P, Kenney DS. 1997. Kodiak®: a successful biological control product for suppression of soil-borne plant pathogens of cotton. J Ind Microbiol Biotechnol 19:169–171. doi: 10.1038/sj.jim.2900439. [DOI] [Google Scholar]
- 15.Ngugi H, Dedej S, Delaplane K, Savelle A, Scherm H. 2005. Effect of flower-applied Serenade biofungicide (Bacillus subtilis) on pollination-related variables in rabbiteye blueberry. Biol Control 33:32–38. doi: 10.1016/j.biocontrol.2005.01.002. [DOI] [Google Scholar]
- 16.Chowdhury SP, Hartmann A, Gao XW, Borriss R. 2015. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42: a review. Front Microbiol 6:780. doi: 10.3389/fmicb.2015.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koumoutsi A, Chen XH, Henne A, Liesegang H, Hitzeroth G, Franke P, Vater J, Borriss R. 2004. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J Bacteriol 186:1084–1096. doi: 10.1128/JB.186.4.1084-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury SP, Dietel K, Rändler M, Schmid M, Junge H, Boriss R, Hartmann A, Grosch R. 2013. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS One 8(7):e68818. doi: 10.1371/journal.pone.0068818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen XH, Vater J, Piel J, Franke P, Scholz R, Schneider K, Koumoutsi A, Hitzeroth G, Grammel N, Strittmatter AW, Gottschalk G, Süssmuth RD, Borriss R. 2006. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB42. J Bacteriol 188:4024–4036. doi: 10.1128/JB.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider K, Chen XH, Vater J, Franke P, Nicholson G, Borriss R, Süssmuth RD. 2007. Macrolactin is the polyketide biosynthesis product of the pks2 cluster of Bacillus amyloliquefaciens FZB42. J Nat Prod 70:1417–1423. doi: 10.1021/np070070k. [DOI] [PubMed] [Google Scholar]
- 21.Chen XH, Scholz R, Borriss M, Junge H, Mogel G, Kunz S, Borriss R. 2009. Difficidin and bacilysin produced by plant-associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. J Biotechnol 140:38–44. doi: 10.1016/j.jbiotec.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Budiharjo A, Wang P, Shi H, Fang J, Borriss R, Zhang K, Huang X. 2013. The highly modified microcin peptide plantazolicin is associated with nematicidal activity of Bacillus amyloliquefaciens FZB42. Appl Microbiol Biotechnol 97:10081–10090. doi: 10.1007/s00253-013-5247-5. [DOI] [PubMed] [Google Scholar]
- 23.Scholz R, Vater J, Budiharjo A, Wang Z, He Y, Dietel K, Schwecke T, Herfort S, Lasch P, Borriss R. 2014. Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. J Bacteriol 196:1842–1852. doi: 10.1128/JB.01474-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu LM, Wu HJ, Chen L, Yu XF, Borriss R, Gao XW. 2015. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci Rep 5:12975. doi: 10.1038/srep12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao SF, Wu HJ, Wang WD, Yang Y, Xie SS, Xie YL, Gao XW. 2013. Efficient colonization and harpins mediated enhancement in growth and biocontrol of wilt disease in tomato by Bacillus subtilis. Lett Appl Microbiol 57:526–533. doi: 10.1111/lam.12144. [DOI] [PubMed] [Google Scholar]
- 26.Cawoy H, Debois D, Franzil L, De Pauw E, Thonart P, Ongena M. 2015. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb Biotechnol 8:281–295. doi: 10.1111/1751-7915.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong AD, Li HP, Yuan QS, Song XS, Yao W, He WJ, Zhang JB, Liao YC. 2015. Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS One 10(2):e0116871. doi: 10.1371/journal.pone.0116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blacutt AA, Mitchell TR, Bacon CW, Gold SE. 2016. Bacillus mojavensis RRC101 lipopeptides provoke physiological and metabolic changes during antagonism against Fusarium verticillioides. Mol Plant Microbe Interact 29:713–723. doi: 10.1094/MPMI-05-16-0093-R. [DOI] [PubMed] [Google Scholar]
- 29.Cadenas E, Davies KJ. 2000. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 30.Cuomo CA, Güldener U, Xu JR, Trail F, Turgeon BG, Di Pietro A, Walton JD, Ma LJ, Baker SE, Rep M, Adam G, Antoniw J, Baldwin T, Calvo S, Chang YL, Decaprio D, Gale LR, Gnerre S, Goswami RS, Hammond-Kosack K, Harris LJ, Hilburn K, Kennell JC, Kroken S, Magnuson JK, Mannhaupt G, Mauceli E, Mewes HW, Mitterbauer R, Muehlbauer G, Münsterkötter M, Nelson D, O'Donnell K, Ouellet T, Qi W, Quesneville H, Roncero MI, Seong KY, Tetko IV, Urban M, Waalwijk C, Ward TJ, Yao J, Birren BW, Kistler HC. 2007. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317:1400–1402. doi: 10.1126/science.1143708. [DOI] [PubMed] [Google Scholar]
- 31.Jonkers W, Dong Y, Broz K, Kistler HC. 2012. The Wor1-like protein Fgp1 regulates pathogenicity, toxin synthesis and reproduction in the phytopathogenic fungus Fusarium graminearum. PLoS Pathog 8(5):e1002724. doi: 10.1371/journal.ppat.1002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hohmann S. 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arroyo J, Bermejo C, García R, Rodríguez-Peña JM. 2009. Genomics in the detection of damage in microbial systems: cell wall stress in yeast. Clin Microbiol Infect 15(Suppl 1):44–46. doi: 10.1111/j.1469-0691.2008.02676.x. [DOI] [PubMed] [Google Scholar]
- 34.Zheng D, Zhang S, Zhou X, Wang C, Xiang P, Zheng Q, Xu JR. 2012. The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. PLoS One 7(11):e49495. doi: 10.1371/journal.pone.0049495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou Z, Xue C, Peng Y, Katan T, Kistler HC, Xu JR. 2002. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol Plant Microbe Interact 15:1119–1127. doi: 10.1094/MPMI.2002.15.11.1119. [DOI] [PubMed] [Google Scholar]
- 36.Tabbene O, Di Grazia A, Azaiez S, Ben Slimene I, Elkahoui S, Alfeddy MN, Casciaro B, Luca V, Limam F, Mangoni ML. 2015. Synergistic fungicidal activity of the lipopeptide bacillomycin D with amphotericin B against pathogenic Candida species. FEMS Yeast Res 15:fov022. doi: 10.1093/femsyr/fov022. [DOI] [PubMed] [Google Scholar]
- 37.Moyne AL, Shelby R, Cleveland TE, Tuzun S. 2001. Bacillomycin D: an iturin with antifungal activity against Aspergillus flavus. J Appl Microbiol 90:622–629. doi: 10.1046/j.1365-2672.2001.01290.x. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Saini S, Wray V, Nimtz M, Prakash A, Johri BN. 2012. Characterization of an antifungal compound produced by Bacillus sp. strain A5F that inhibits Sclerotinia sclerotiorum. J Basic Microbiol 52:670–678. doi: 10.1002/jobm.201100463. [DOI] [PubMed] [Google Scholar]
- 39.Kefi A, Ben Slimene I, Karkouch I, Rihouey C, Azaeiz S, Bejaoui M, Belaid R, Cosette P, Jouenne T, Limam F. 2015. Characterization of endophytic Bacillus strains from tomato plants (Lycopersicon esculentum) displaying antifungal activity against Botrytis cinerea Pers. World J Microbiol Biotechnol 31:1967–1976. doi: 10.1007/s11274-015-1943-x. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Zhou T, He D, Li XZ, Wu H, Liu W, Gao X. 2011. Functions of lipopeptides bacillomycin D and fengycin in antagonism of Bacillus amyloliquefaciens C06 towards Monilinia fructicola. J Mol Microbiol Biotechnol 20:43–52. doi: 10.1159/000323501. [DOI] [PubMed] [Google Scholar]
- 41.Heerklotz H, Seelig J. 2007. Leakage and lysis of lipid membranes induced by the lipopeptide surfactin. Eur Biophys J 36:305–314. doi: 10.1007/s00249-006-0091-5. [DOI] [PubMed] [Google Scholar]
- 42.Han Q, Wu F, Wang X, Qi H, Shi L, Ren A, Liu Q, Zhao M, Tang C. 2015. The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signalling pathways and mediate plant defence responses involved in pathogen-associated molecular pattern-triggered immunity. Environ Microbiol 17:1166–1188. doi: 10.1111/1462-2920.12538. [DOI] [PubMed] [Google Scholar]
- 43.Nasir MN, Besson F. 2012. Conformational analyses of bacillomycin D, a natural antimicrobial lipopeptide, alone or in interaction with lipid monolayers at the air-water interface. J Colloid Interface Sci 387:187–193. doi: 10.1016/j.jcis.2012.07.091. [DOI] [PubMed] [Google Scholar]
- 44.Romero D, Vicente AD, Olmos JL, Dávila JC, Pérez-García A. 2007. Effect of lipopeptides of antagonistic strains of Bacillus subtilis on the morphology and ultrastructure of the cucurbit fungal pathogen Podosphaera fusca. J Appl Microbiol 103:969–976. doi: 10.1111/j.1365-2672.2007.03323.x. [DOI] [PubMed] [Google Scholar]
- 45.Qi G, Zhu F, Du P, Yang X, Qiu D, Yu Z, Chen J, Zhao X. 2010. Lipopeptide induces apoptosis in fungal cells by a mitochondria-dependent pathway. Peptides 31:1978–1986. doi: 10.1016/j.peptides.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Hajare SN, Subramanian M, Gautam S, Sharma A. 2013. Induction of apoptosis in human cancer cells by a Bacillus lipopeptide bacillomycin D. Biochimie 95:1722–1731. doi: 10.1016/j.biochi.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Zhang S, Hou R, Zhao Z, Zheng Q, Xu Q, Zheng D, Wang G, Liu H, Gao X, Ma JW, Kistler HC, Kang Z, Xu JR. 2011. Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. PLoS Pathog 7(12):e1002460. doi: 10.1371/journal.ppat.1002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu Q, Zhang C, Yu F, Yin Y, Shim WB, Ma Z. 2015. Protein kinase FgSch9 serves as a mediator of the target of rapamycin and high osmolarity glycerol pathways and regulates multiple stress responses and secondary metabolism in Fusarium graminearum. Environ Microbiol 17:2661–2676. doi: 10.1111/1462-2920.12522. [DOI] [PubMed] [Google Scholar]
- 49.Lin SC, Minton MA, Sharma MM, Georgiou G. 1994. Structural and immunological characterization of a biosurfactant produced by Bacillus licheniformis JF-2. Appl Environ Microbiol 60:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vater J, Gao X, Hitzeroth G, Wilde C, Franke P. 2003. “Whole cell”-matrix-assisted laser desorption ionization-time of flight-mass spectrometry, an emerging technique for efficient screening of biocombinatorial libraries of natural compounds: present state of research. Comb Chem High Throughput Screening 6:557–567. doi: 10.2174/138620703106298725. [DOI] [PubMed] [Google Scholar]
- 51.Liu P, Luo L, Guo J, Liu H, Wang B, Deng B, Long CA, Cheng Y. 2010. Farnesol induces apoptosis and oxidative stress in the fungal pathogen Penicillium expansum. Mycologia 102:311–318. doi: 10.3852/09-176. [DOI] [PubMed] [Google Scholar]
- 52.Seong K, Hou Z, Tracy M, Kistler HC, Xu JR. 2005. Random insertional mutagenesis identifies genes associated with virulence in the wheat scab fungus Fusarium graminearum. Phytopathology 95:744–750. doi: 10.1094/PHYTO-95-0744. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Li B, Liu L, Chen H, Zhang H, Zheng X, Zhang Z. 2015. FgMon1, a guanine nucleotide exchange factor of FgRab7, is important for vacuole fusion, autophagy and plant infection in Fusarium graminearum. Sci Rep 5:18101. doi: 10.1038/srep18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji F, Xu JH, Liu X, Yin XC, Shi JR. 2014. Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu Province, China. Food Chem 157:393–397. doi: 10.1016/j.foodchem.2014.02.058. [DOI] [PubMed] [Google Scholar]
- 55.Correll JC, Klittich CJR, Leslie JF. 1987. Nitrate nonutilising mutants of Fusarium oxysporum and their use in vegetative compatibility tests. Phytopathology 77:1640–1646. doi: 10.1094/Phyto-77-1640. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.