Abstract
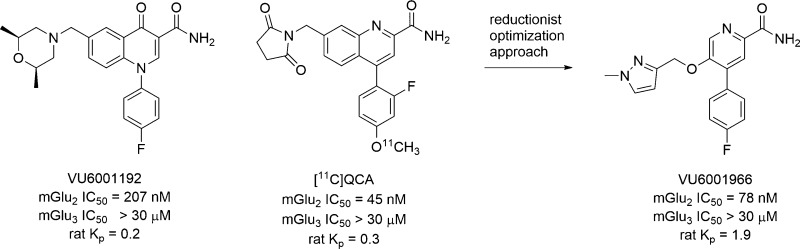
Herein, we detail the optimization of the mGlu2 negative allosteric modulator (NAM), VU6001192, by a reductionist approach to afford a novel, simplified mGlu2 NAM scaffold. This new chemotype not only affords potent and selective mGlu2 inhibition, as exemplified by VU6001966 (mGlu2 IC50 = 78 nM, mGlu3 IC50 > 30 μM), but also excellent central nervous system (CNS) penetration (Kp = 1.9, Kp,uu = 0.78), a feature devoid in all previously disclosed mGlu2 NAMs (Kps ≈ 0.3, Kp,uus ≈ 0.1). Moreover, this series, based on overall properties, represents an exciting lead series for potential mGlu2 PET tracer development.
Keywords: Negative allosteric modulator (NAM), metabotropic glutamate receptor 2 (mGlu2), depression, VU6001966, CNS penetration
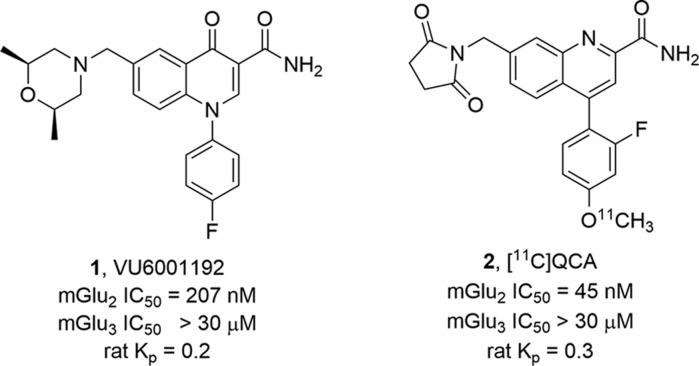
The group II metabotropic glutamate receptors, mGlu2 and mGlu3, signal through Gi/o to diminish cAMP production by the inhibition of adenylyl cyclase.1−8 These presynaptic receptors are widely expressed in the mammalian central nervous system (CNS; cerebral cortex, amygdala, hippocampus, and cerebellum).1−8 Utilizing dual mGlu2/3 orthosteric antagonists and negative allosteric modulators (NAMs), therapeutic relevance has been established in multiple neurodegenerative (chronic pain, Alzheimer’s disease (AD), Parkinson’s disease (PD), drug abuse) and psychiatric (schizophrenia, anxiety, and depression) disorders.9−14 However, the contribution due to selective mGlu2 or mGlu3 inhibition remains elusive; indeed, for the group II mGlu receptors, the field has only had selective mGlu2 PAMs15,16 and, only recently, selective mGlu3 NAM in vivo tool compounds.17−21 Furthermore, the only reported mGlu2 NAMs, 1 and 2 (Figure 1), are P-gp substrates with limited CNS penetration (Kps < 0.3), precluding their use as tools to dissect the role of selective mGlu2 inhibition in vivo or as mGlu2 PET tracers.22,23 The development of selective and highly CNS penetrant mGlu2 NAMs is clearly warranted, as mGlu2, by inhibiting glutamate release, has been proposed to protect neurons from excitotoxicity in key brain regions.24 For example, mGlu2 is overexpressed in the hippocampus of Alzheimer’s disease patients relative to age-matched controls.25 In this Letter, we will detail an optimization effort based on 1 and 2 in which we undertake a reductionist approach to develop a new, minimum pharmacophore-based series of potent and selective mGlu2 NAMs with exceptional CNS penetration (Kps > 1.5) suitable for use as in vivo probes and as leads for PET tracer development.
Figure 1.
Structures, pharmacology, and rat CNS exposure data for mGlu2 NAMs 1 and 2 reported in the primary literature.21,22
Based on a reductionist approach to the optimization of an mGlu3 NAM 3 to provide 4 (Figure 2) with improved potency and physiochemical and DMPK properties,21 we elected to employ a similar strategy for development of a highly CNS penetrant tool compound from mGlu2 NAMs 1 and 2. Here, we again envisioned truncating the bicyclic core of 1 and 2 to a simple picolinamide scaffold with a suitably tethered western tail moiety, represented generically by 5.
Figure 2.
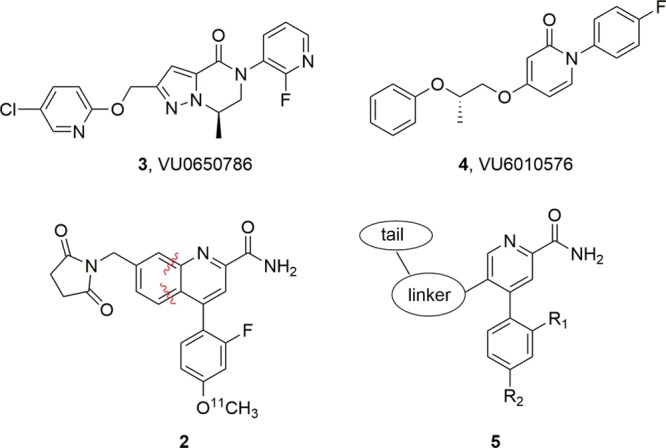
Structures of mGlu3 NAM 3 and the optimized mGlu3 NAM 4 via a reductionist approach to the minimum pharmacophore possessing the desired potency, selectivity, and CNS penetration. A similar strategy for 2 will ideally afford a simplified picolinamide-based mGlu2 NAM 5 with the desired potency, selectivity, and CNS penetration.21
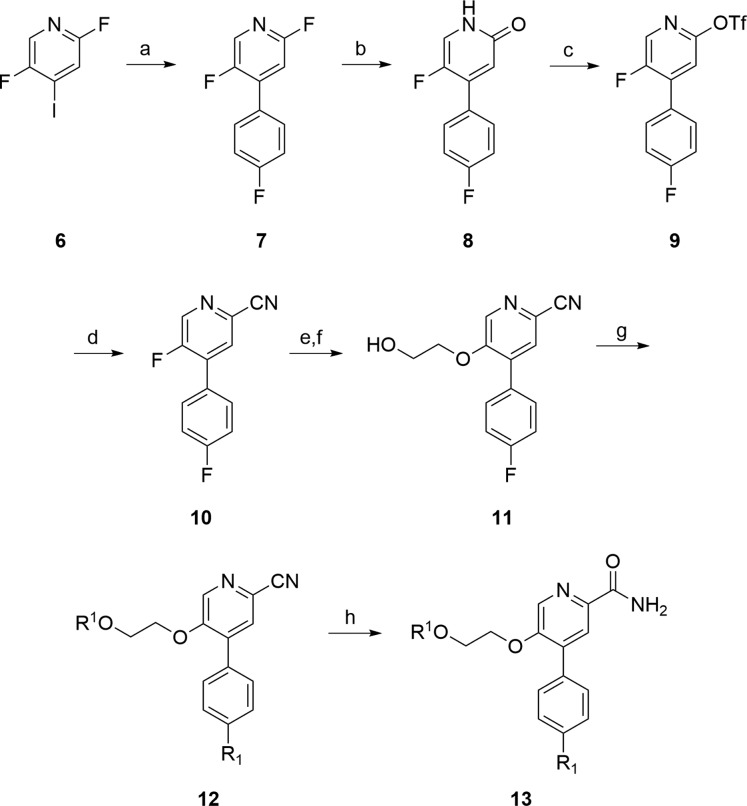
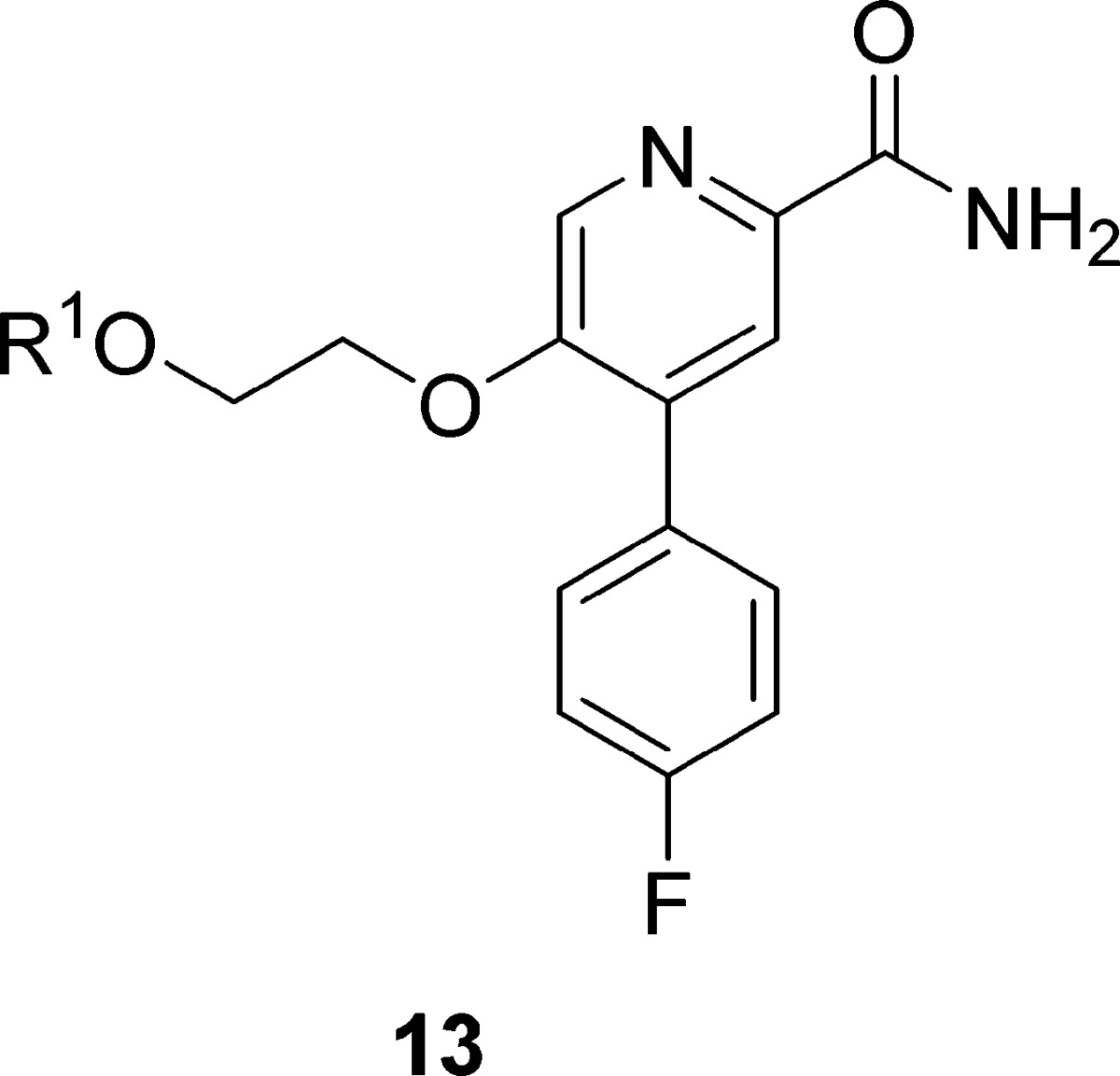
Using 1 and 2 as leads, our goal was to reduce molecular complexity and enhance CNS penetration in a next generation mGlu2 NAM suitable for in vivo studies and as a lead for PET tracer development to enable occupancy studies. Based on the success with mGlu3 NAM 4,21 we truncated 2 to a simple 4-phenylpicolinamide with a pendant 5-alkoxy linker to a diverse array of aryl and heteoraryl tails. The initial structure–activity relationship (SAR) exploration evaluated the ethylenedioxy linker of 2 while holding the 4-F phenyl moiety of 1 constant. The chemistry required to access analogues 13 required eight steps (Scheme 1).26 Starting from commercial 2,5-difluoro-4-iodopyridine 6, a chemoselective Suzuki coupling with 4-phenylboronic acid affords 7 in 92% yield. Acidic hydrolysis affords the pyridinone 8, which was smoothly converted to the corresponding triflate 9. A microwave-assisted, palladium-catalyzed cyanation reaction delivers 10 in good yield. An SNAr reaction with 2-(tetrahydro-2H-pyran-2-yloxy)ethanol and deprotection of the THP-ether provides alcohol 11. Finally, a Mitsunobu reaction with various hydroxypyridines and nitrile hydrolysis to the primary carboxamide gives putative mGlu2 NAM analogues 13.
Scheme 1. Synthesis of Analogues 13.
Reagents and conditions: (a) 4-fluorophenylboronic acid, 10 mol % PdCl2(dppf), 1 M aq. Na2CO3, DME, 100 °C, 92%; (b) AcOH, H2O, 130 °C, 99%; (c) N-phenyl triflimide, TEA, DCM, DMF, 0 °C, 93%; (d) Zn(CN)2, Pd(PPh3)4, DMF, microwave 140 °C, 15 min, 73%; (e) 2-(tetrahydro-2H-pyran-2-yloxy)ethanol, NaH, DMF, rt; (f) PTSA, DCM, EtOH, rt, 18 h, 65% for the two steps; (g) R1OH, PPh3, DtBAD, THF, rt,18 h, 9–35%; (h) KOSiMe3, THF, reflux, 85%.
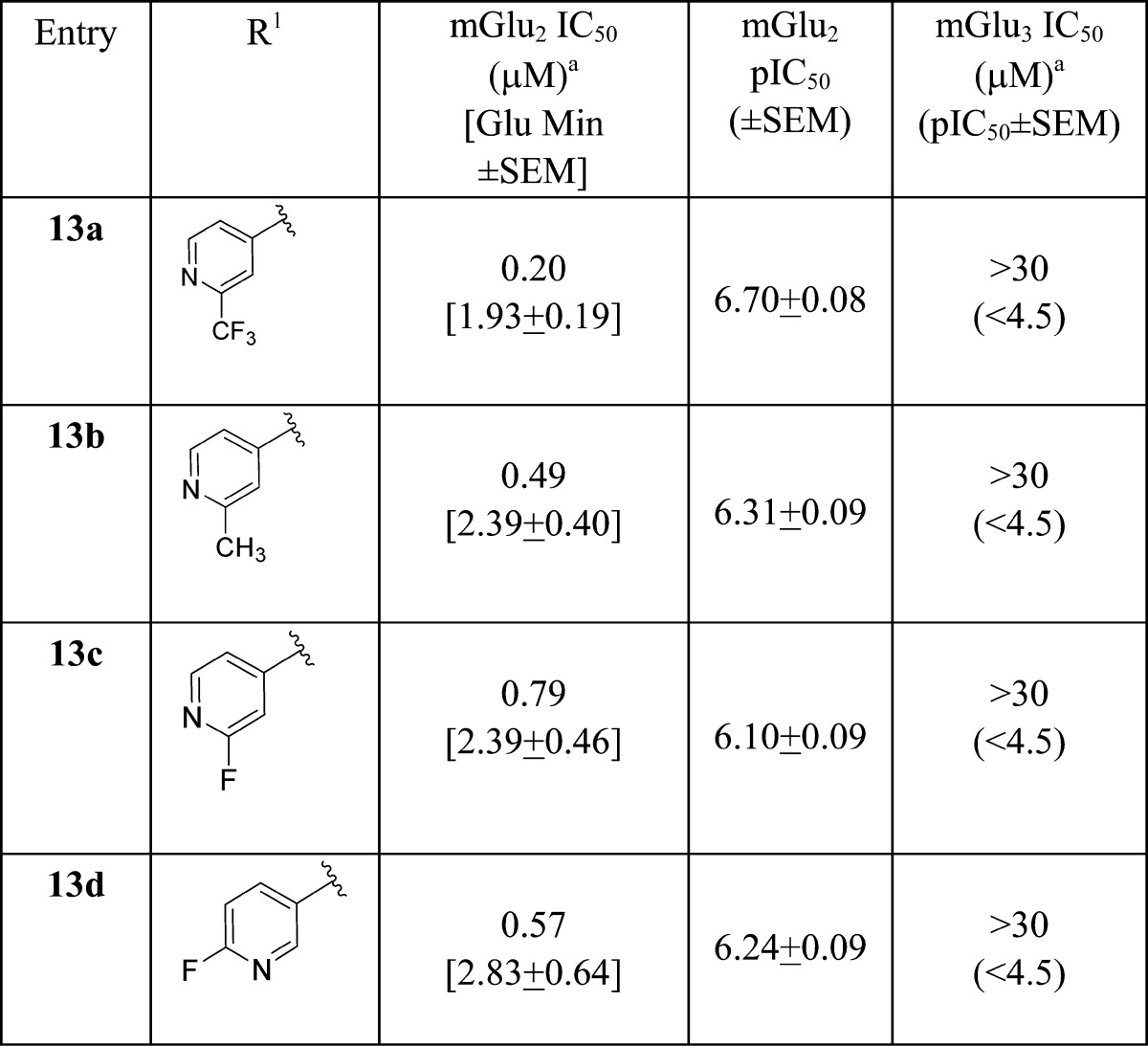
Gratifyingly, this strategy afforded potent mGlu2 NAMs 13a–d (Table 1), with high selectivity versus mGlu3 (IC50s > 30 μM). Whereas 1 and 2 possessed cLogPs in the 3.1 to 3.6 range, analogues 13 proved less lipophilic (cLogPs 2.1 to 2.7). Across the board, analogues 13 displayed good fraction unbound in rat plasma (fus 0.04 to 0.12) but moderate to high predicted hepatic clearance (rat CLhep = 36 to 64 mL/min/kg) and high brain homogenate binding (fu = 0.007).26 However, the most potent analogue in this series, 13a (mGlu2 IC50 = 200 nM), proved to be highly CNS penetrant with a rat brain/plasma partitioning coefficient (Kp) of 2.8 and a Kp,uu of 0.28, representing a ∼10-fold increase over historical mGlu2 NAMs 1 and 2 (Kps ≈ 0.2). Despite this exciting advance, we wished to further simplify the chemotype, reduce the number of synthetic steps, and further improve Kp,uu and disposition.
Table 1. Structures and Activities of Analogues 13a.
Calcium mobilization assays with rmGlu2/TREx/Ga15-HEKcells or rmGlu3/TREx/Ga15-HEK cells performed in the presence of an EC80 fixed concentration of glutamate; values represent means from three (n = 3) independent experiments performed in triplicate.
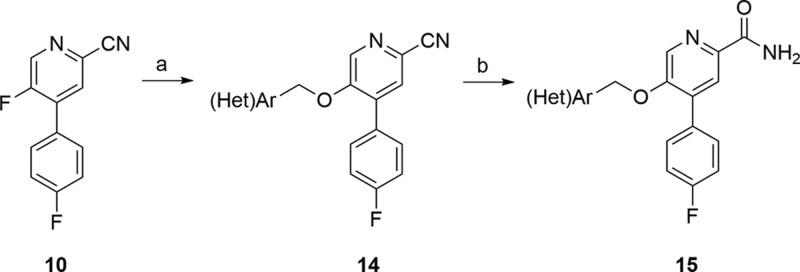
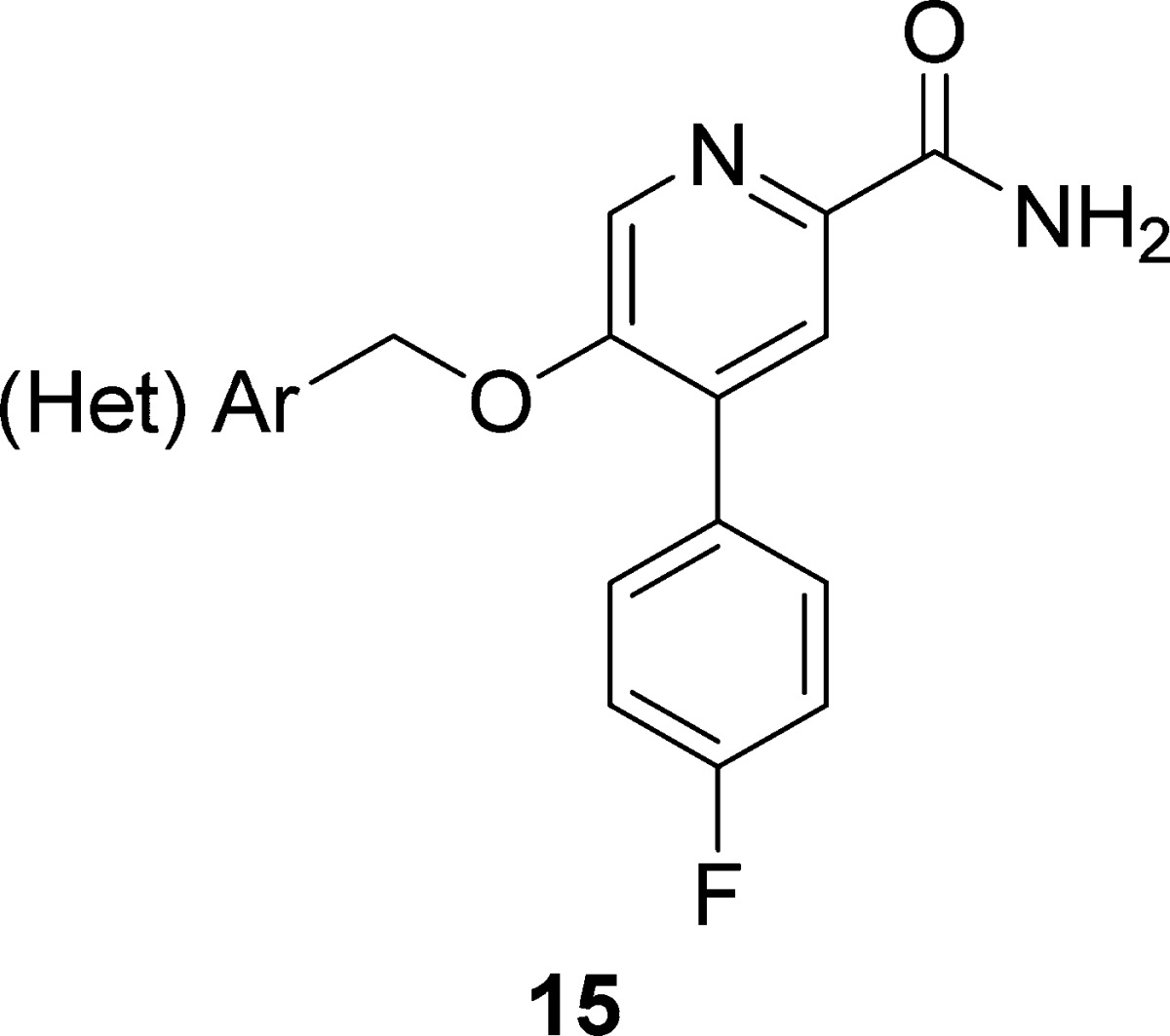
Next, we sought to evaluate if the ethylenedioxy linker of 13 could be contracted to a simple benzyloxy linker. If tolerated, this modification would expedite the synthesis of putative mGlu2 NAMs, while providing a more diverse array of functionality to modulate disposition. The synthesis of analogues 15 began with advanced intermediate 10 (Scheme 2). An SNAr reaction sequence with diversely functionalized benzyl and heteroaryl methyl alcohols delivered 14. In some cases, due to the presence of adventitious water, 15 was also produced in small amounts. Hydrolysis of nitrile 14 smoothly provided the desired analogues 15 in good overall yield.
Scheme 2. Synthesis of Analogues 15.
Reagents and conditions: (a) (Het)ArCH2OH, NaH, DMF, rt, 48–99%; (b) KOSiMe3, THF, reflux, 80–90%.
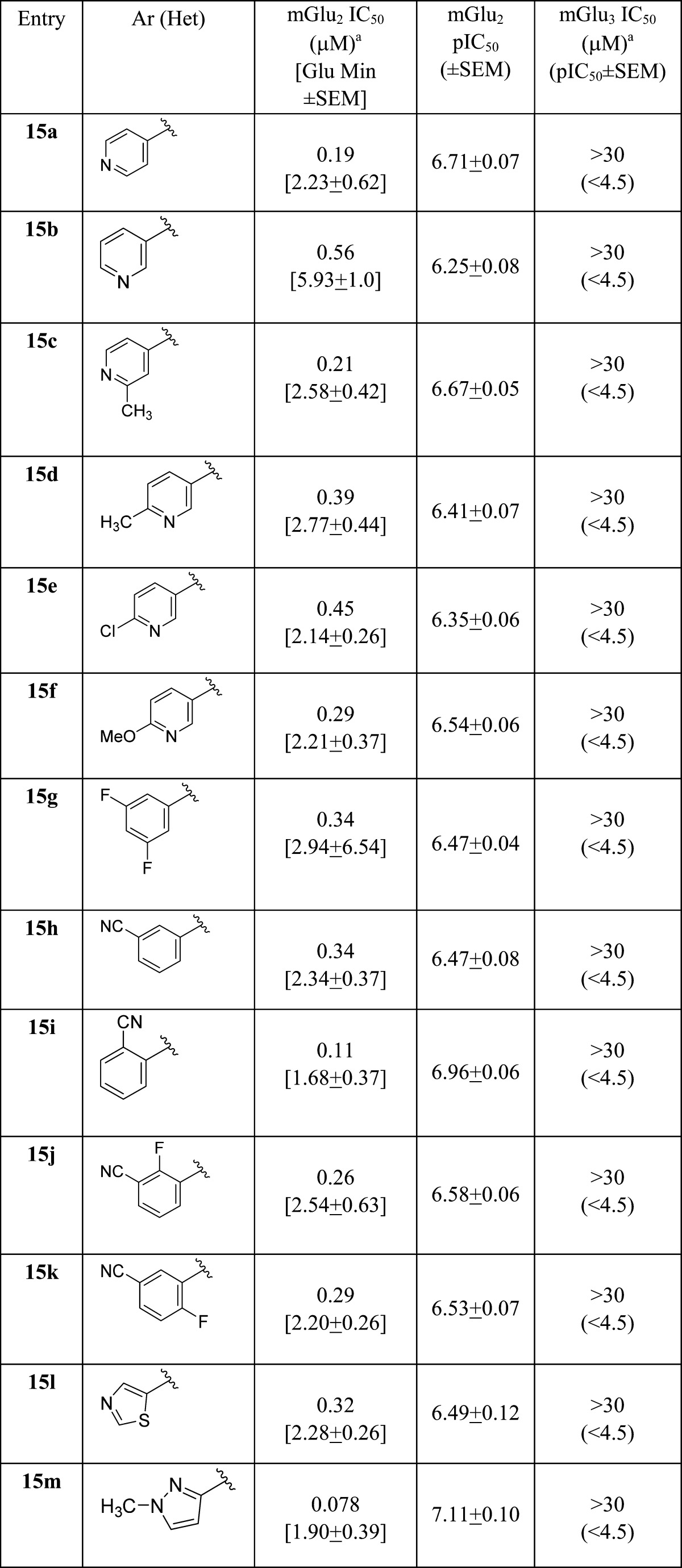
As shown in Table 2, all analogues 15 were potent mGlu2 NAMs (IC50s = 78 to 560 nM) with no activity at mGlu3 (IC50s > 30 μM), demonstrating significant tolerability for diverse substituents. Calculated physiochemical properties were also highly favorable (cLogPs 1.9 to 3.1, TPSAs 75–90 Å2, and molecular weights averaging 372). These analogues 15 displayed good fraction unbound in rat plasma (fus = 0.04 to 0.20), but moderate to high predicted hepatic clearance (rat CLhep = 48 to 65 mL/min/kg) and good brain homogenate binding (fu = 0.046 to 0.72).26 Of these, 15m (VU6001966) emerged as an mGlu2 NAM (mGlu2 IC50 = 78 nM, pIC50 = 7.11 ± 0.10 and 1.90 ± 0.39 Glu min) without activity at mGlu3 (IC50 > 30 μM) worthy of further evaluation. Thus, when evaluated against the full mGlu receptor family, 15m was completely selective versus mGlu1,3,4,5,6,7,8 in our standard 10 μM fold-shift assay.26 We also explored broader ancillary pharmacology beyond the mGlus in a Eurofins radioligand binding panel of 68 GPCRs, ion channels, transporters, and nuclear hormone receptors and found no significant activities (no inhibition >50% @ 10 μM).26,27 Calculated physiochemical properties were also highly favorable (cLogP 1.92, TPSAs 83 Å2, and molecular weight of 326). These properties translated into a soluble compound (kinetic solubility of 14.7 ± 1.1 μM in PBS buffer at pH 7.4, 24 h time point) and favorable disposition. NAM 15m displayed high fraction unbound in plasma (rat fu = 0.20; human fu = 0.11) and brain (rat brain fu = 0.07), but moderate to high predicted hepatic clearance (rat CLhep = 60 mL/min/kg and human CLhep = 16.8 mL/min/kg) with an acceptable CYP450 profile (3A4, 2D6, and 2C9 IC50s > 30 μM, 1A2 IC50 = 3.1 μM).26 In our standard rat plasma/brain level (PBL) cassette study, 15m demonstrated a rat Kp of 1.9, with a Kp,uu of 0.78, values much improved over 1 and 2, and an improved Kp,uu over 13a.26 A robust in vitro/in vivo correlation (IVIVC) was noted, with a rat in vivo PK study showing high clearance (CLp = 118 mL/min/kg), a short half-life (t1/2 = 20 min), and a good volume (Vss = 2.6 L/kg). Similarly, 15m showed favorable CNS penetration in mouse as well (Kp = 0.65, Kp,uu = 0.29), and similar PK (CLp = 136 mL/min/kg, t1/2 = 34 min, and Vss = 5.2 L/kg). However, while [11C]QCA, 2, was limited to autoradiography due to the low CNS penetration and rodent P-gp liabilities, 15m possesses an ideal profile for a PET tracer–high CNS penetration coupled with rapid clearance from plasma (following an IV route of administration) and desired physiochemical properties.
Table 2. Structures and Activities of Analogues 15a.
Calcium mobilization assays with rmGlu2/TREx/Ga15-HEKcells or rmGlu3/TREx/Ga15-HEK cells performed in the presence of an EC80 fixed concentration of glutamate; values represent means from three (n = 3) independent experiments performed in triplicate.
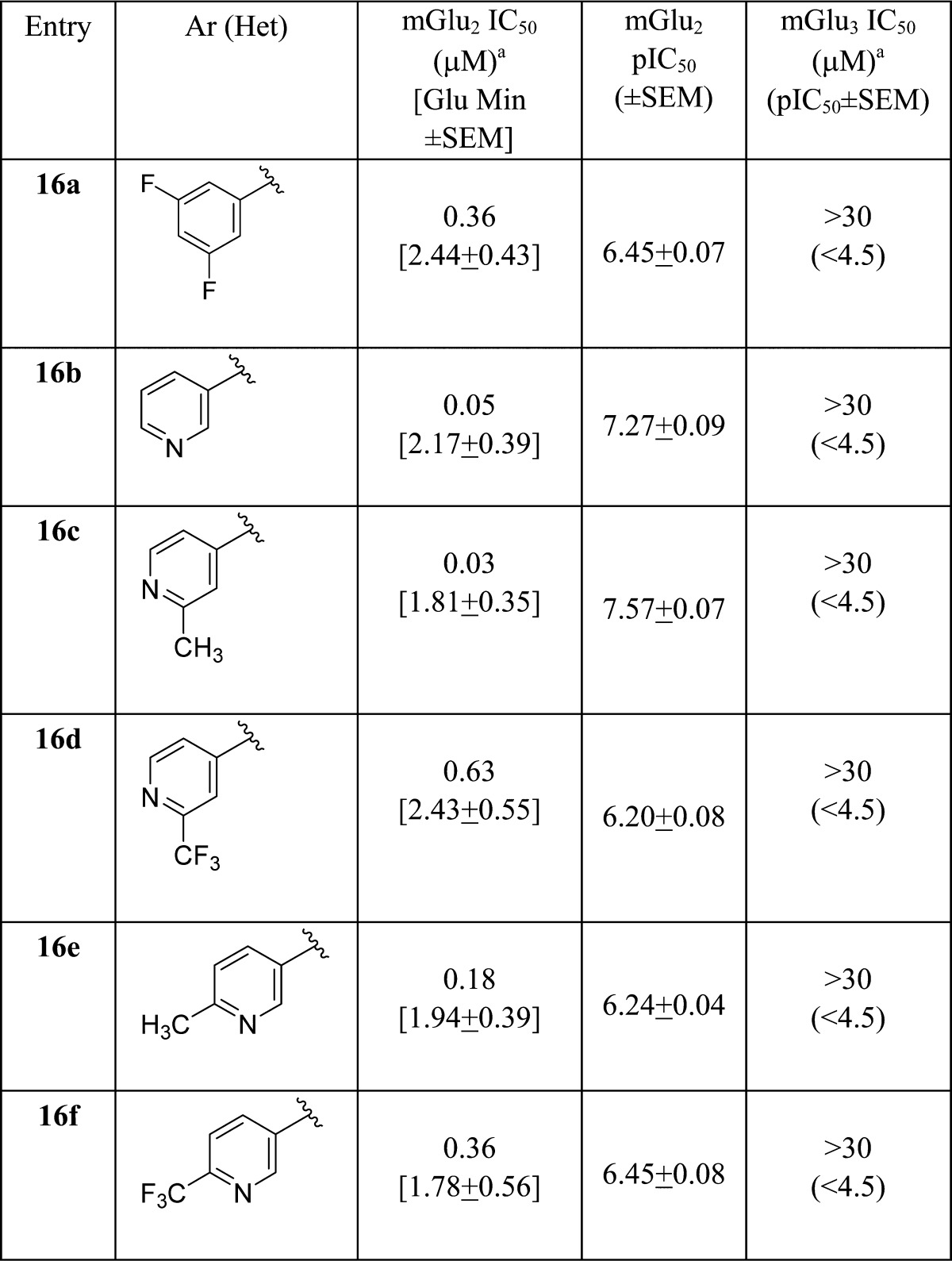
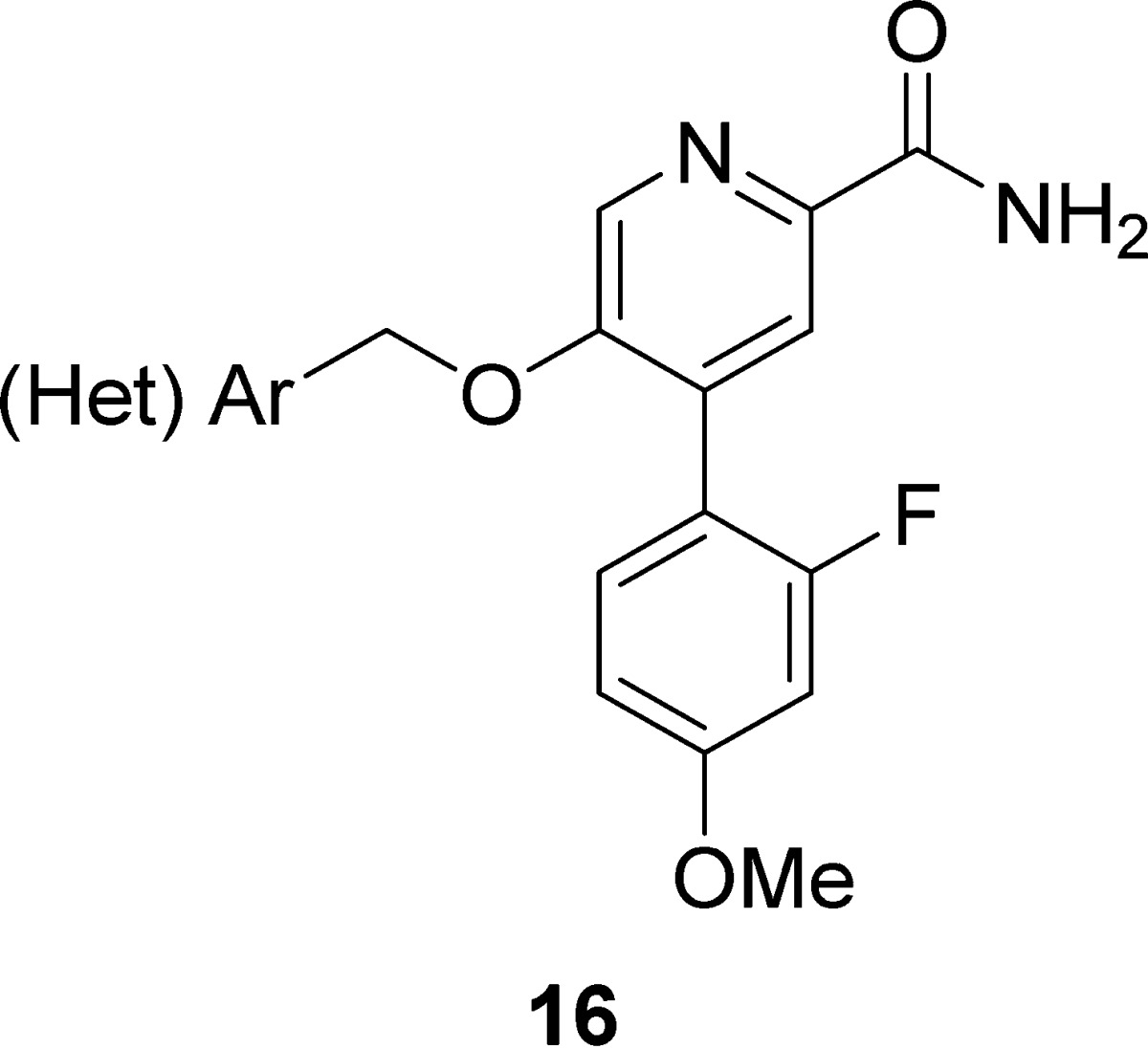
As this series began to position toward a candidate for PET tracer development rather than for robust in vivo tools suitable for proof-of-concept studies, we next evaluated the preferred aryl moiety of 2 (2-fluoro-4-methoxy phenyl) in the context of the simplified picolinamide scaffold and generated analogues 16 (Table 3) following a subtle variation of the route depicted in Scheme 2. Analogues 16 afforded the most potent mGlu2 NAMs to date within this series. For example, 16c was a potent mGlu2 NAM (mGlu2 IC50 = 26 nM, pIC50 = 7.57 ± 0.07, 1.81 ± 0.35 Glu min) as was 16b (mGlu2 IC50 = 54 nM, pIC50 = 7.27 ± 0.09, 2.17 ± 0.39 Glu min), and both were without activity at mGlu3 (IC50 > 30 μM). In addition, both showed high fraction unbound in plasma (16b, rat fu = 0.08, human fu = 0.10; 16c, rat fu = 0.06, human fu = 0.06), but, as with 15m, moderate to high predicted hepatic clearance (16b, rat CLhep = 61 mL/min/kg and human CLhep = 16.0 mL/min/kg; 16c, rat CLhep = 67 mL/min/kg and human CLhep = 15.8 mL/min/kg). Finally, both demonstrated high CNS penetration (Kps of 0.34 and 1.38 and Kp,uus of 0.15 and 0.45 for 16b and 16c, respectively; therefore, like 15m, both represent attractive leads as in vivo PET tracers for mGlu2 and overcome CNS penetration issues associated with 2.
Table 3. Structures and Activities of Analogues 16a.
Calcium mobilization assays with rmGlu2/TREx/Ga15-HEKcells or rmGlu3/TREx/Ga15-HEK cells performed in the presence of an EC80 fixed concentration of glutamate; values represent means from three (n = 3) independent experiments performed in triplicate.
Before leaving 15m, we elected to assess a dose-range mouse PBL study via intraperitoneal (IP) dosing to determine if more meaningful exposure could be achieved by bypassing first-pass metabolism. Thus, 15m was dosed IP in male CD-1 mice at 3, 10, and 30 mg/kg (in 10% Tween80/H2O vehicle), and plasma and brain levels were determined at a 25 min time point. As shown in Figure 3, excellent 15m exposure was achieved in both plasma and brain across this dose-range with consistent Kps (Kp = 1.05 @ 3 mg/kg; Kp = 1.2 @ 10 mg/kg; Kp = 1.3 @ 30 mg/kg). Total brain concentrations at the highest dose in this study were 14 μM (@30 mg/kg IP), which corresponds to ∼180-fold above the mGlu2 IC50 of 15m, and 1.1 μM free brain (∼14-fold above the mGlu2 IC50 of 15m). Even at 10 mg/kg IP, free brain levels were 3-fold above the mGlu2 IC50 of 15m. Thus, 15m could serve as an excellent tracer candidate with IV dosing and as an in vivo proof of concept for mGlu2 NAM via IP dosing.
Figure 3.
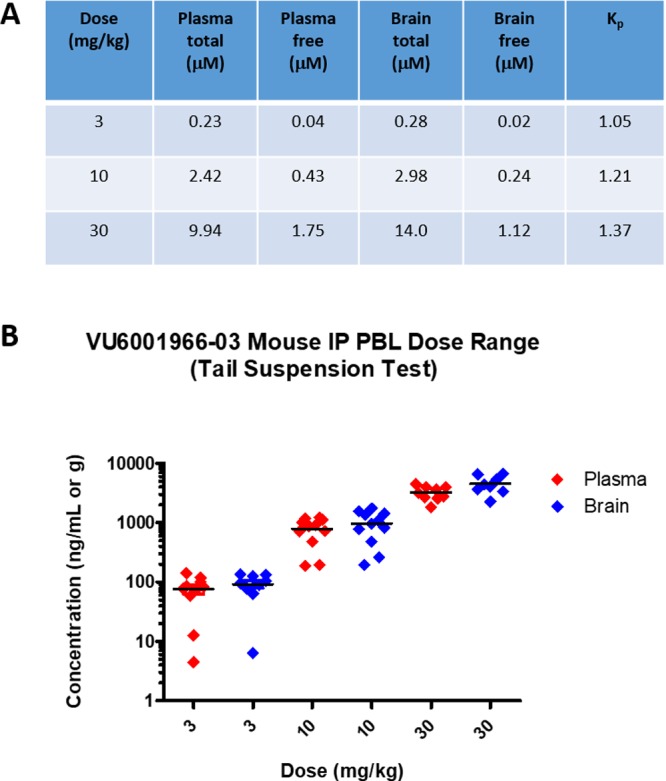
Exposures of 15m (VU6001966) in plasma and brain from an IP PBL dose range study in male CD-1 mice. (A) Free and total plasma and brain concentrations achieved (3, 10, and 30 mg/kg IP). (B) Plot of plasma and brain levels achieved in the three dose groups (8–10 mice/per group).
In summary, we have developed the next generation of highly selective mGlu2 NAMs by a reductionist strategy that also provided the first highly CNS penetrant mGlu2 NAMs in both mice and rats. Excitingly, these new mGlu2 NAMs possess profiles that render them attractive leads for mGlu2 PET tracer development via IV dosing paradigms and have utility as in vivo proof of concept compounds via IP dosing. Further refinements and progress toward mGlu2 NAM in vivo tool compounds and PET tracers are underway, and results will be reported in due course.
Acknowledgments
The authors would also like to thank William K. Warren, Jr. and the William K. Warren Foundation who funded the William K. Warren, Jr. Chair in Medicine (to C.W.L.), and the National Institute of Mental Health for funding R01MH099269 (to K.A.E.).
Glossary
ABBREVIATIONS
- mGlu
metabotropic glutamate receptor
- PAM
positive allosteric modulator
- NAM
negative allosteric modulator
- mGlu2
metabotropic glutamate receptor subtype 2
- PBL
plasma/brain level
- Kp
plamsa/brain partitioning coefficient
- Kp,uu
unbound plasma/unbound brain partitioning coefficient
- IP
intraperitoneal
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00279.
General methods for the synthesis and characterization of all compounds, methods for the in vitro and in vivo DMPK protocols, and supplemental figures (PDF)
Author Present Address
⊥ Department of Pharmaceutical Sciences, UNT System College of Pharmacy, University of North Texas Health Science Center, 3500 Camp Bowie Boulevard, Fort Worth, Texas 76107, United States.
Author Contributions
C.W.L. wrote the manuscript and oversaw the medicinal chemistry. K.A.E. designed compounds, and J.L.E., K.A.B., A.S.F., and C.J.B. performed chemical synthesis. P.J.C., A.L.R., H.P.C., and C.M.N. performed and analyzed molecular pharmacology data. R.L.W. performed molecular pharmacology assays. S.C. and A.L.B. oversaw and analyzed in vitro and in vivo DMPK data. C.K.J. and M.B. performed and analyzed the mouse tail suspension assay/data. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
This paper was published ASAP on August 18, 2017, with an error in the Supporting Information. The corrected version was reposted on August 25, 2017.
Supplementary Material
References
- Niswender C. M.; Conn P. J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa C. M.; Friberg I. K.; Weiss S. W.; Standaert D. G. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J. Comp. Neurol. 1998, 390, 5–19. . [DOI] [PubMed] [Google Scholar]
- Schoepp D. D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001, 299, 12–20. [PubMed] [Google Scholar]
- Ohishi H.; Shigemoto R.; Nakanishi S.; Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience 1993, 53, 1009–1018. 10.1016/0306-4522(93)90485-X. [DOI] [PubMed] [Google Scholar]
- Ohishi H.; Ogawa-Meguro R.; Shigemoto R.; Kaneko T.; Nakanishi S.; Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR2 and mGluR3, in rat cerebellar cortex. Neuron 1994, 13, 55–66. 10.1016/0896-6273(94)90459-6. [DOI] [PubMed] [Google Scholar]
- Ohishi H.; Neki A.; Mizuno N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci. Res. 1998, 30, 65–82. 10.1016/S0168-0102(97)00120-X. [DOI] [PubMed] [Google Scholar]
- Richards G.; Messer J.; Malherbe P.; Pink R.; Brockhaus M.; Stadler H.; Wichmann J.; Schaffhauser H.; Mutel V. Distribution and abundance of metabotropic glutamate receptor subtype 2 in rat brain revealed by [3H]LY354740 binding in vitro and quantitative radioautography: Correlation with the sites of synthesis, expression, and agonist stimulation of [35S]GTPγs binding. J. Comp. Neurol. 2005, 487, 15–27. 10.1002/cne.20538. [DOI] [PubMed] [Google Scholar]
- Wright R. A.; Johnson B. G.; Zhang C.; Salhoff C.; Kingston A. E.; Calligaro D. O.; Monn J. A.; Schoepp D. D.; Marek G. J. CNS distribution of metabotropic glutamate 2 and 3 receptors: transgenic mice and [3H]LY459477 autoradiography. Neuropharmacology 2013, 66, 89–98. 10.1016/j.neuropharm.2012.01.019. [DOI] [PubMed] [Google Scholar]
- O’Brien N. L.; et al. The functional GRM3 Kozak sequence variant rs148754219 affects the risk of schizophrenia and alcohol dependence as well as bipolar disorder. Psychiatr. Genet. 2014, 24, 277–278. 10.1097/YPG.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M.; Campo B.; Lambeng N.; Célanire S.; Schneider M.; Bessif A.; Royer-Urios I.; Parron D.; Legrand C.; Mahious N.; Girard F.; Le Poul E.. An mGluR2/3 negative allo-Steric modulator improves recognition memory assessed by natural forgetting in the novel object recognition test in rats. In Memory Consolidation and Reconsolidation: Molecular Mechanisms II; Proceedings of the 40th Annual Meeting of the Society for Neuroscience, San Diego, CA, Nov 13–17, 2010; Society for Neuroscience: Washington, DC, 2010; 406.9/MMM57.
- Choi C. H.; Schoenfeld B. P.; Bell A. J.; Hinchey P.; Kollaros M.; Gertner M. J.; Woo N. H.; Tranfaglia M. R.; Bear M. F.; Zu-kin R. S.; McDonald T. V.; Jongens T. A.; McBride S. M. Pharmacological reversal of synaptic plasticity deficits in the mouse model of fragile X syndrome by group II mGluR antagonist or lith-ium treatment. Brain Res. 2011, 1380, 106–119. 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti McArthur S.; Saxe M.; Wichmann J.; Woltering T.. mGlu2/3 antagonists for the treatment of autistic disorders. PCT Int. Pat. Appl. WO 2014/064028 A1, May 1, 2014.
- Structure of decoglurant disclosed in Recommended International Nonproprietary Names (INN). In WHO Drug Information; World Health Organization: Geneva, Switzerland, 2013; Vol. 27, no. (3), , p 150. [Google Scholar]
- ARTDeCo Study: A study of RO4995819 in patients with major depressive disorder and inadequate response to ongoing antidepressant treatment. ClinicalTrials.gov; U.S. National Institutes of Health: Bethesda, MD, 2011; https://www.clinicaltrials.gov/ct2/show/NCT01457677. [Google Scholar]
- Lindsley C. W.; Emmitte K. A.; Hopkins C. R.; Bridges T. M.; Gregory K. J.; Niswender C. M.; Conn P. J. Practical Strategies and Concepts in GPCR Allosteric Modulator Discovery: Recent Advances with Metabotropic Glutamate Receptors. Chem. Rev. 2016, 116, 6707–6741. 10.1021/acs.chemrev.5b00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabanco A. A.; Cid J. M. mGluR2 positive allosteric modulators: a patent review (2009-present). Expert Opin. Ther. Pat. 2013, 23, 629–647. 10.1517/13543776.2013.777043. [DOI] [PubMed] [Google Scholar]
- Sheffler D. J.; Wenthur C. J.; Bruner J. A.; Carrington S. J. S.; Vinson P. N.; Gogi K. K.; Blobaum A. L.; Morrison R. D.; Vamos M.; Cosford N. D. P.; Stauffer S. R.; Daniels J. S.; Niswender C. M.; Conn P. J.; Lindsley C. W. Development of a novel, CNS-penetrant, metabotropic glutamate receptor 3 (mGlu3) NAM probe (ML289) derived from a closely related mGlu5 PAM. Bioorg. Med. Chem. Lett. 2012, 22, 3921–3925. 10.1016/j.bmcl.2012.04.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthur C. J.; Morrison R.; Felts A. S.; Smith K. A.; Engers J. L.; Byers F. W.; Daniels J. S.; Emmitte K. A.; Conn P. J.; Lindsley C. W. Discovery of (R)–(2-fluoro-4-((−4-methoxy phenyl)ethynyl)-phenyl(3-hydroxypiperidin-1-yl)methanone (ML337), an mGlu3 selective and CNS penetrant negative allosteric modulator (NAM). J. Med. Chem. 2013, 56, 5208–5212. 10.1021/jm400439t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. G.; Wenthur C. J.; Xiang Z.; Rook J. M.; Emmitte K. A.; Niswender C. M.; Lindsley C. W.; Conn P. J. Metabotropic glutamate receptor 3 activation is required for long-term de-pression in medial prefrontal cortex and fear extinction. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 1196–1201. 10.1073/pnas.1416196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers J. L.; Rodriguez A. L.; Konkol L. C.; Morrison R. D.; Thompson A. D.; Byers F. W.; Blobaum A. L.; Chang S.; Loch M. T.; Niswender C. M.; Daniels J. S.; Jones C. K.; Conn P. J.; Lindsley C. W.; Emmitte K. A. Discovery of VU0650786: A selective and CNS penetrant negative allosteric modulator of metabotropic glutamate receptor subtype 3 with antidepressant and anxio lytic activity in rodents. J. Med. Chem. 2015, 58, 7485–7500. 10.1021/acs.jmedchem.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers J. L.; Bollinger K. A.; Rodriguez A. L.; Long M. F.; Breiner M. M.; Chang S.; Bubser M.; Jones C. K.; Morrison R. D.; Bridges T. M.; Blobaum A. L.; Niswender C. M.; Conn P. J.; Emmitte K. A.; Lindsley C. W.; Weiner R. L.; Bollinger S. R. Design and Synthesis of N-Aryl Phenoxyethoxy Pyridines as Highly Selective and CNS Penetrant mGlu3 NAMs. ACS Med. Chem. Lett. 2017, x. 10.1021/acsmedchemlett.7b00249. [DOI] [PMC free article] [PubMed] [Google Scholar]; Companion paper.
- Felts A. S.; Rodriguez A. L.; Smith K. A.; Engers J. L.; Morrison R. D.; Byers F. W.; Blobaum A. L.; Locuson C. W.; Chang S.; Venable D. F.; Niswender C. M.; Daniels J. S.; Conn P. J.; Lindsley C. W.; Emmitte K. A. Design of 4-Oxo-1-aryl-1,4-dihydroquinoline-3-carboxamides as selective negative allosteric modulators of metabotropic glutamate receptor subtype 2. J. Med. Chem. 2015, 58, 9027–9040. 10.1021/acs.jmedchem.5b01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Kumata K.; Yamasaki T.; Cheng R.; Hatori A.; Ma L.; Zhang Y.; Xie L.; Wang L.; Kang H. J.; Sheffler D. J.; Cosford N. D. P.; Zhnag M.-R.; Liang S. H. Synthesis and Preliminary Studies of a Novel Negative Allosteric Modulator, 7-((2,5- Dioxopyrrolidin-1-yl)methyl)-4-(2-fluoro-4-[11C]methoxyphenyl) quinoline-2-carboxamide, for Imaging of Metabotropic Glutamate Receptor 2. ACS Chem. Neurosci. 2017, 10.1021/acschemneuro.7b00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi P.; Rajput A.; Calon F.; Grégoire L.; Hornykiewicz O.; Rajput A. H.; Di Paolo T. Metabotropic Glutamate Receptor II in the Brains of Parkinsonian Patients. J. Neuropathol. Exp. Neurol. 2009, 68, 374–382. 10.1097/NEN.0b013e31819cabe4. [DOI] [PubMed] [Google Scholar]
- Lee H. G.; Zhu X.; O’Neill M. J.; Webber K.; Casadesus G.; Marlatt M.; Raina A. K.; Perry G.; Smith M. A. The role of metabotropic glutamate receptors in Alzheimer’s disease. Acta Neurobiol. Exp. 2004, 64, 89–98. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for full details.
- LeadProfilingScreen; (catalogue no. 68) Eurofins Panlabs, Inc.: Redmond, WA (www.eurofinspanlabs.com).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.