Abstract
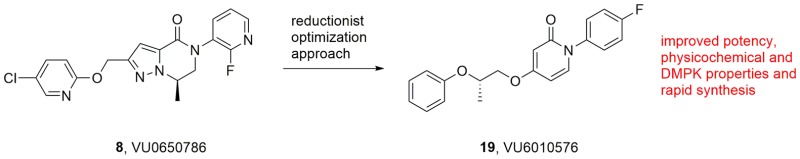
Herein, we detail the optimization of the mGlu3 NAM, VU0650786, via a reductionist approach to afford a novel, simplified mGlu3 NAM scaffold that engenders potent and selective mGlu3 inhibition (mGlu3 IC50 = 245 nM, mGlu2 IC50 > 30 μM) with excellent central nervous system penetration (rat brain/plasma Kp = 1.2, Kp,uu = 0.40). Moreover, this new chemotype, exemplified by VU6010572, requires only four synthetic steps and displays improved physiochemical properties and in vivo efficacy in a mouse tail suspension test (MED = 3 mg/kg i.p.).
Keywords: Negative allosteric modulator (NAM), metabotropic glutamate receptor 3 (mGlu3), depression, VU6010572, physiochemical properties
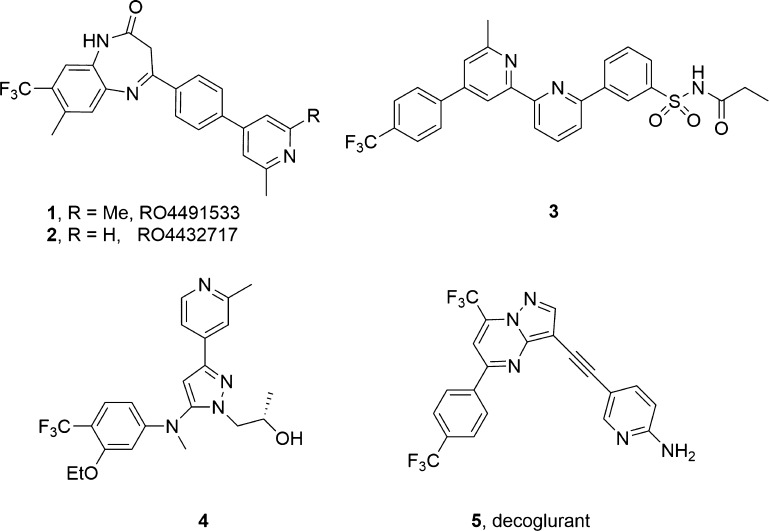
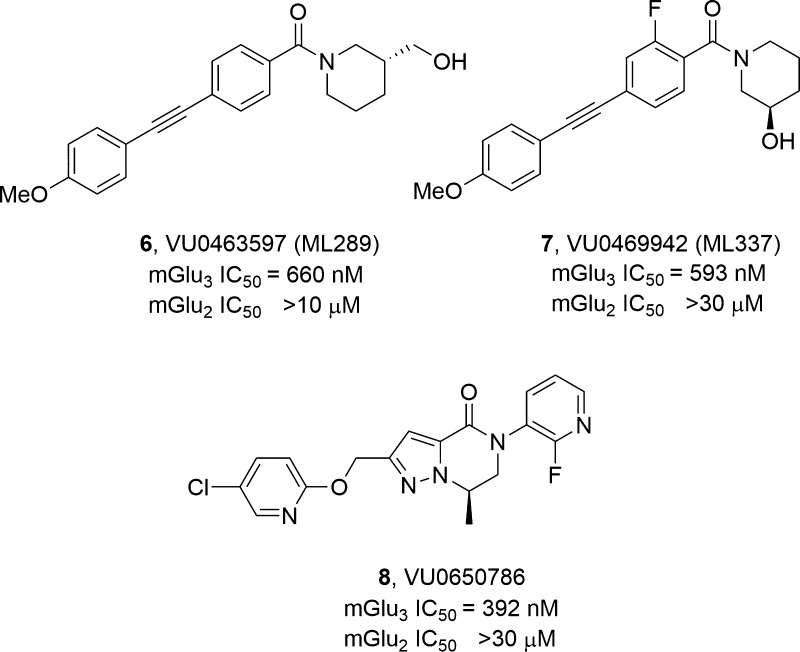
GRM3, the gene that encodes metabotropic glutamate receptor subtype 3 (mGlu3), represents a significant locus associated with schizophrenia, substance abuse disorders, and bipolar disorder; moreover, single-nucleotide polymorphisms (SNPs) within GRM3 are linked to cognitive deficits.1−6 Dual mGlu2/3 negative allosteric modulators (NAMs) 1–5 have demonstrated therapeutic potential in Alzheimer’s disease, anxiety, obsessive–compulsive disorder, autism spectrum disorders, and cognition (Figure 1).6−11 Moreover, the mGlu2/3 NAM decoglurant 5 advanced into human Phase II clinical trials for depression.12−14 Despite the therapeutic relevance and clinical interest, few highly selective mGlu3 NAMs exist to define the contribution of mGlu3 inhibition.15−19 Early mGlu3 NAM tool compounds 6 and 7 (Figure 2), derived from “molecular switches” within mGlu5 positive allosteric modulator (PAM) ligands,20,21 enabled study of selective mGlu3 inhibition and highlighted a key role for mGlu3 in the regulation of synaptic plasticity in medial prefrontal cortex (mPFC) as well as antidepressant and anxiolytic activity.22 In particular, VU0650786, 8, (mGlu2 IC50 > 30 μM, mGlu3 IC50 = 392 nM, rat brain/plasma Kp = 1.7; Kp,uu = 0.78) has emerged as a highly valuable mGlu3 NAM in vivo probe; however, it requires a nine-step synthesis.23 Thus, we hoped to simplify the VU0650786 chemotype and also improve upon physicochemical properties in a next generation mGlu3 NAM in vivo probe with a strong intellectual property (IP) position.
Figure 1.
Structures of reported dual mGlu2/3 NAMs 1–5 that have provided target validation for Group II mGlu inhibition in multiple CNS disorders.
Figure 2.
Structures and in vitro mGlu2/mGlu3 potencies of reported mGlu3 NAMs 6–8, all derived from mGlu5 PAM scaffolds via “molecular switches”.
Using 8 as a lead, our goal was to reduce molecular complexity and enhance physicochemical properties in a next generation mGlu3 NAM. We elected to deconstruct the heterobicyclic dihydropyrazolo[1,5-a]pyrazine-4(5H)-one core of 8 and replace it with an ethereal, aliphatic linker and either an N-aryl pyrimidine or N-arylpyridine head-piece (Figure 3) to provide greater conformational flexibility and rapid synthesis.
Figure 3.
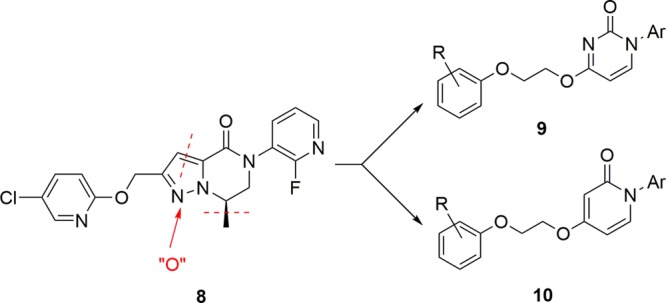
Optimization plan to deconstruct 8 into more flexible cores 9 and 10 with improved predicted physicochemical properties.
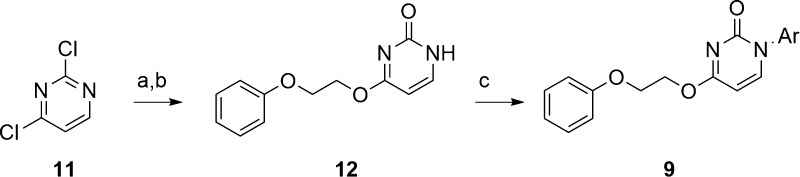
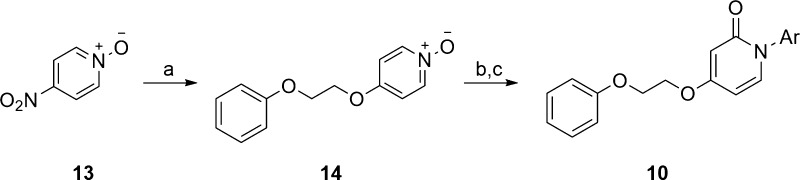
The chemistry to access scaffolds 9 and 10 proved straightforward.24 For scaffold 9 (Scheme 1), commercially available 2,4-dichloropyrimidine 11 underwent an SNAr reaction with 2-phenoxyethanol, followed by a second SNAr with water to afford pyrimidinone 12. Finally, a copper-mediated N-arylation step delivered analogues 9 in good yields in only three steps.24 Similarly, pyridine analogues 10 were all prepared in a three-step fashion (Scheme 2). Here, commercial 4-nitropyridine-1-oxide 13 undergoes an SNAr reaction with 2-phenoxyethanol to provide 14. N-Oxide migration provides the pyridine core, which is then N-alkylated under copper catalysis with aryl boronic acids to provide analogues 10.24 Variations on this scheme were used to generate analogues 10 where the unsubstituted phenyl moiety was replaced with functionalized aryl and heteroaryl moieties.
Scheme 1. Synthesis of Analogues 9.
Reagents and conditions: (a) 2-phenoxyethanol, NaH, DMF, 0 °C to rt, 46%; (b) K2CO3, DABCO, H2O, 1,4-dioxane, 70 °C, 83%; (c) 8-hydroxyquinoline, CuI, K2CO3, DMSO, microwave, 160 °C, 30 min, 16–45%.
Scheme 2. Synthesis of Analogues 10.
Reagents and conditions: (a) 2-phenoxyethanol, NaH, DMF, 0 to 100 °C, 81%; (b) Ac2O, microwave 140 °C, 60 min, then 1 N LiOH, 50 °C, 57%; (c) ArB(OH)2, Cu(OAc)2, pyridine, 4 Å MS, DCM, air, 44–65%.
A limited number of pyrimidine analogues 9 were prepared, as the structure–activity relationship (SAR) was steep and selectivity versus mGlu5 eroded, but Table 1 highlights key analogues in this series. While not productive en route to a new mGlu3in vivo probe, 9a was a potent mGlu3 NAM (IC50 = 295 nM) with no discernible activity at mGlu2 (IC50 > 30 μM). Relative to 8, potency was enhanced, molecular weight reduced, and fraction unbound in plasma doubled (rat fu,plasma = 0.16), all of which validated the reductionist approach.
Table 1. Structures and Activities of Analogues 9a.

Calcium mobilization assays with mGlu3/Gqi5-CHO cells performed in the presence of a EC80 fixed concentration of glutamate; values represent means from three (n = 3) independent experiments performed in triplicate.
However, as alluded to above, 9a, while selective versus mGlu1,2,4,6,7,8, was a mGlu5 PAM (EC50 = 1.2 μM, 97% Glu max). Interestingly, 9c was a more potent mGlu5 PAM (EC50 = 427 nM, 83% Glu max) than mGlu3 NAM (IC50 = 1.02 μM). These findings were not entirely unexpected, as eliminating mGlu5 PAM activity was a major facet of the optimization effort that delivered 8.23 Would deletion of a single nitrogen atom in 9 yield analogues 10 and eliminate the mGlu5 PAM activity while maintaining all of the other favorable properties?
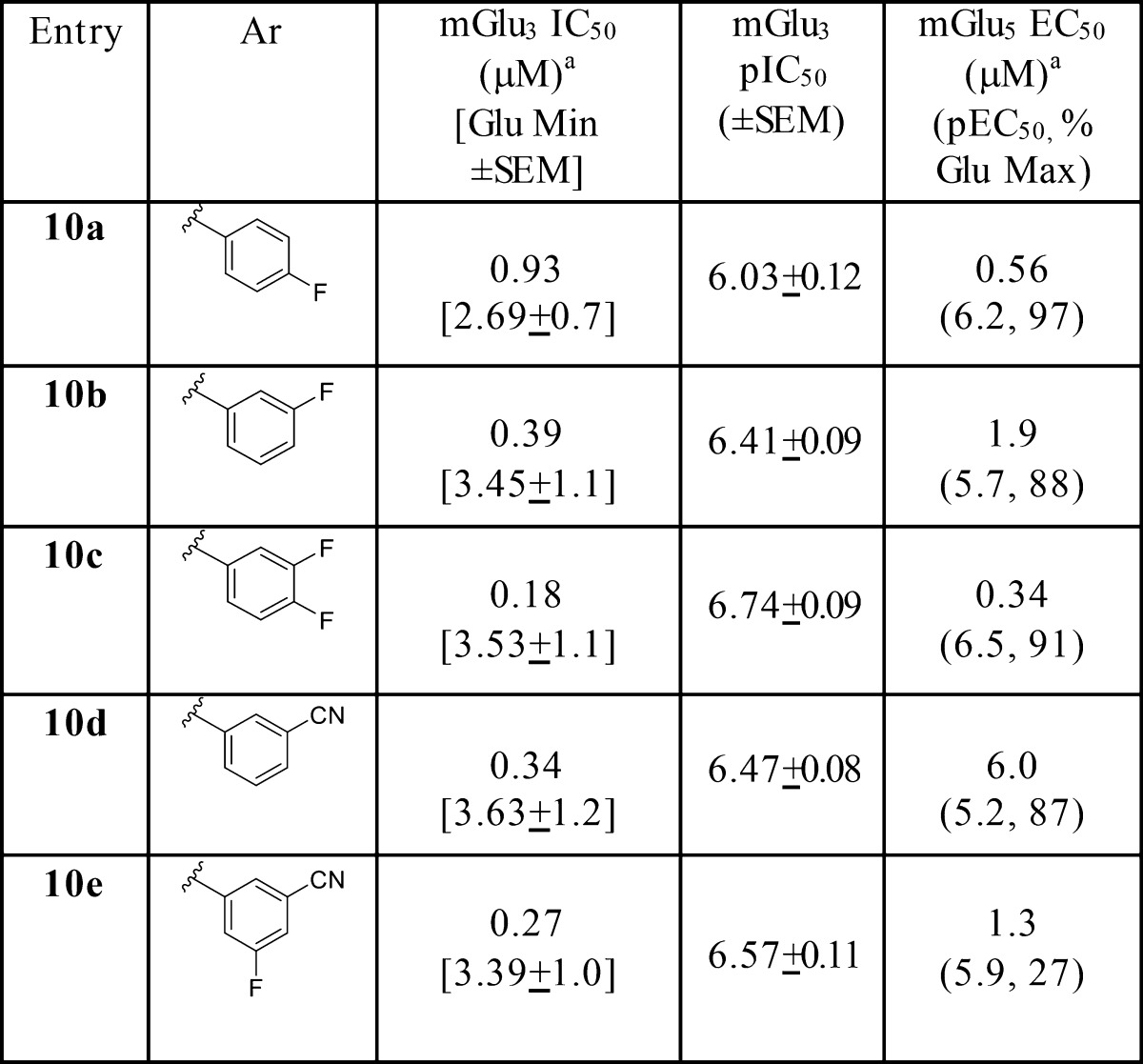
Table 2 highlights SAR for the pyridinone analogues 10, which proved more robust than NAMs 9, with direct analogues of 9b and 9c being significantly more potent (10b and 10c) mGlu3 NAMs. Relative to 8, potency was enhanced, molecular weight reduced, and fraction unbound in plasma doubled (rat fu,plasma = 0.12 to 0.16), all of which further validated the reductionist approach. Moreover, all were highly selective versus mGlu2 (IC50s > 30 μM), and mGlu5 PAM activity diminished (mGlu5 EC50s in the 400 nM to 6 μM ranges). However, analogues such as 10a and 10c emerged with unique, dual mGlu3 NAM/mGlu5 PAM pharmacological profiles; in contrast, 10d displayed ∼18-fold selectivity as a mGlu3 NAM versus mGlu5 PAM activity.
Table 2. Structures and Activities of Analogues 10a.
Calcium mobilization assays with mGlu3/Gqi5-CHO cells performed in the presence of a EC80 fixed concentration of glutamate; values represent means from three (n = 3) independent experiments performed in triplicate.
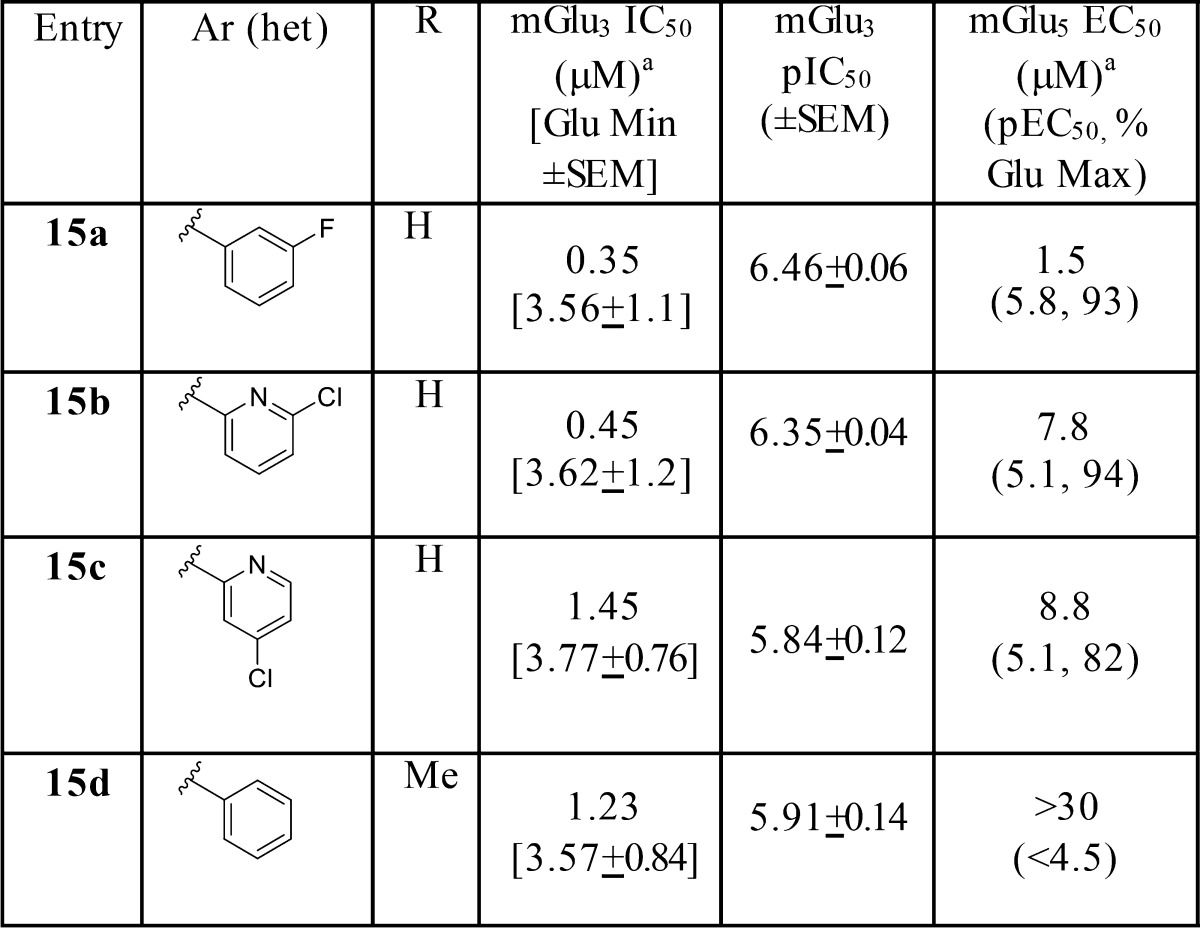
In an effort to eliminate mGlu5 PAM activity, we held the 4-fluorophenyl moiety of the pyridinone constant and surveyed replacements for the western phenyl moiety as well as a methyl substituent on the ether chain, generating analogues 15 (Table 3).24 While mGlu3 activity and selectivity versus mGlu2 was maintained (mGlu2 IC50s > 30 μM), mGlu5 PAM activity persisted in 15a–c (mGlu5 EC50s in the 1.7 to 9 μM ranges) but greatly diminished relative to analogues 10, with the pyridine ether moieties. However, the racemic methyl congener 15d proved exceptional. Compound 15d was a moderately potent mGlu3 NAM (IC50 = 1.2 μM), but proved to be selective versus both mGlu2 (IC50s > 30 μM) and mGlu5 (EC50s > 30 μM). In addition, 15d had a clean CYP450 inhibition profile (IC50 > 30 μM versus 3A4, 2D6, 2C9, and 1A2), good fraction unbound (fu,plasma = 0.04 (rat), 0.10 (human) and fu,brain = 0.05 (rat), was highly central nervous system (CNS) penetrant in rat (brain plasma Kp = 1.7, Kp,uu = 1.3), and showed moderate predicted hepatic clearance (rat CLhep= 43.2 mL/min/kg, human CLhep = 10.7 mL/min/kg; based on microsomal intrinsic clearance data).24,25 Thus, efforts focused on synthesizing and evaluating the discrete enantiomers of 15d.
Table 3. Structures and Activities of Analogues 15a.

Calcium mobilization assays with mGlu3/Gqi5-CHO cells performed in the presence of a EC80 fixed concentration of glutamate; values represent means from three (n = 3) independent experiments performed in triplicate.
The synthesis of the (R)- and (S)-enantiomers of 15d is shown in Scheme 3.24 Commercially available pyridine 16 is subjected to the standard copper-catalyzed arylation with 4-fluorophenyl boronic acid, followed by 10% Pd/C hydrogenation to provide 17. A Mitsunobu reaction with either (R)-2-phenoxypropan-1-ol or (S)-2-phenoxypropan-1-ol, both known compounds,24 delivers 18 and 19, respectively, in good overall yields and enantiopurity. When assessed in our assays, the (R)-enantiomer 18 was devoid of activity at both mGlu3 and mGlu2 (IC50s > 30 μM), demonstrating significant enantiopreference. In contrast (Figure 4), the (S)-enantiomer 19 proved to be a potent mGlu3 NAM (IC50 = 245 nM, pIC50 = 6.61 ± 0.12, 3.33 ± 0.31) with high selectivity versus not only mGlu2 (IC50 > 30 μM) and mGlu5 (EC50 > 30 μM), but all mGlu receptors (inactive at mGlu1,4,6,7,8).24 In terms of its DMPK profile, 19 displayed an attractive profile with no CYP450 inhibition liabilities (IC50s > 30 μM), good fraction unbound in plasma (fu,plasmas ≈ 0.18 for human, rat, and mouse), moderate predicted hepatic clearance across species, and, relative to 8, kinetic solubility (PBS buffer at pH 7.4) improved 4-fold (98 μM). In rat, 19 was highly CNS penetrant (brain/plasma Kp = 1.15, Kp,uu = 0.40), as well as in mouse (Kp = 1.17, Kp,uu = 0.26). Before any in vivo behavior was performed, we also explored broader ancillary pharmacology beyond the mGlus in a Eurofins radioligand binding panel of 68 GPCRs, ion channels, transporters, and nuclear hormone receptors.24,26 Gratifyingly, no significant activities were noted (no inhibition >50% @ 10 μM).
Scheme 3. Synthesis of Enantiomers 18 and 19.
Reagents and conditions: (a) 4-FPhB(OH)2, Cu(OAc)2, pyridine, 4 Å MS, DCM, air, 72%; (b) 10% Pd/C, H2 (1 atm), MeOH, 18 h, 99%; (R)-2-phenoxypropan-1-ol or (S)-2-phenoxypropan-1-ol, PPh3, DtBAD, THF, rt, 18 h, 72–75%.
Figure 4.
Structure, molecular pharmacology, and DMPK profile of 19.
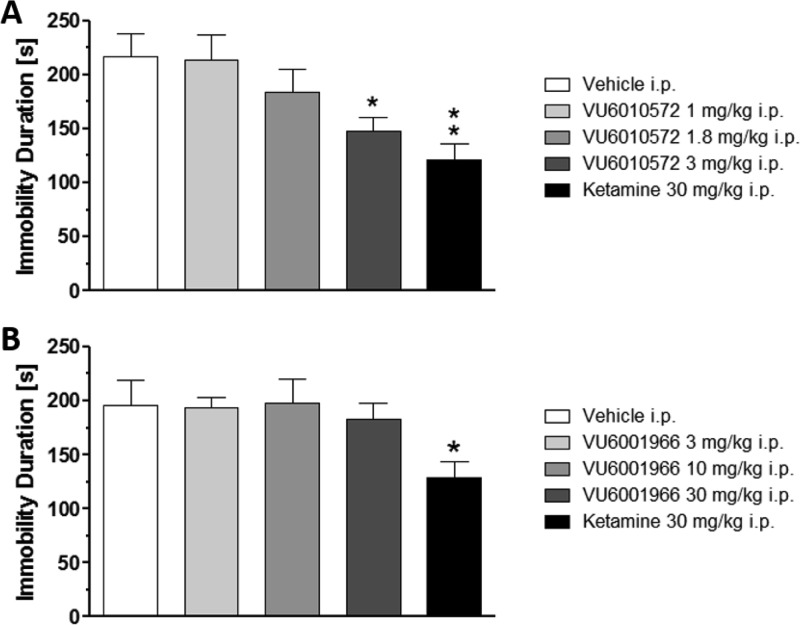
With interest in Group II NAMs for depression-related behaviors, and as potentially novel antidepressants, we evaluated 19 in a mouse tail suspension study27,28 side-by-side with a new CNS penetrant mGlu2 NAM (20, VU6001966)24,29−32 to dissect the role of the individual Group II mGluRs in this paradigm. The two NAMs were administered i.p. and compared relative to a 30 mg/kg i.p. dose of ketamine (Figure 5). The mGlu3 NAM 19 (VU6010572) showed robust efficacy in this model at 3 mg/kg (roughly comparable to the effect elicited by ketamine at 30 mg/kg), while the mGlu2 NAM 20 (VU6001966) was inactive up to 30 mg/kg i.p. Exposure was measured from these studies, and 19 achieved total brain levels of ∼1.2 μM (Kp = 1.2, Kp,uu = 0.27 for this mouse PK/PD study) at the 3 mg/kg dose (∼5-fold above the mGlu3 IC50), while 20 achieved total brain levels of ∼14 μM (∼180-fold above the mGlu2 IC50) at the highest dose tested (30 mg/kg i.p.). For the majority of our allosteric modulator programs, total brain exposure, and not free brain levels, is the best correlate of in vivo efficacy.22,23,33−35 These early data support a greater contribution of mGlu3 inhibition for the antidepressant effects of dual mGlu2/3 NAMs (and in agreement with previous studies with 8),23 but studies are underway in other antidepressant behavioral paradigms and in both mice and rats to strengthen these preliminary findings.
Figure 5.
Mouse tail suspension test in CD-1 mice with (A) mGlu319 and (B) mGlu2 NAM 20. The MED for mGlu319 is 3 mg/kg i.p., while the mGlu2 NAM 20 is without effect up to 30 mg/kg in this paradigm. Ketamine (the positive control) displays efficacy at 30 mg/kg in this paradigm. Vehicle: 10% Tween 80 in H2O (10 mL/kg), n = 10–14 mice per dose group. *p < 0.05 vs vehicle, **p < 0.01 versus vehicle.24
In summary, we have discovered the next generation of highly selective and CNS penetrant mGlu3 NAMs by a reductionist strategy that lowered molecular weight and improved physicochemical and DMPK properties, while also reducing the synthetic route by 50%, relative to 8. Moreover, a head-to-head comparison of highly selective and CNS penetrant mGlu2 and mGlu3 NAMs in a mouse tail suspensions test, to assess potential antidepressant phenotype, indicated the mGlu3 inhibition is the dominant mGlu subtype responsible for efficacy. Further antidepressant paradigms in both mice and rats are underway, and results will be reported in due course.
Acknowledgments
The authors would also like to thank William K. Warren, Jr. and the William K. Warren Foundation who funded the William K. Warren, Jr. Chair in Medicine (to C.W.L.), and the National Institute of Mental Health for funding R01MH099269 (to K.A.E.).
Glossary
ABBREVIATIONS
- mGlu
metabotropic glutamate receptor
- PAM
positive allosteric modulator
- NAM
negative allosteric modulator
- mGlu3
metabotropic glutamate receptor subtype 3
- PBL
plasma/brain level
- Kp
plasma/brain partitioning coefficient
- Kp,uu
unbound plasma/unbound brain partitioning coefficient
- DCM
dichloromethane
- DABCO
1,4-diazabicylco[2.2.2]octane
- MED
minimum effective dose
- CYP
cytochrome P450
- PK/PD
pharmacokinetic/pharmacodynamics
- DMPK
drug metabolism and pharmacokinetics
- DtBAD
di-tert-butyl azodicarboxylate
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00249.
General methods for the synthesis and characterization of all compounds, and methods for the in vitro and in vivo DMPK protocols and supplemental figures (PDF)
Author Present Address
∥ Department of Pharmaceutical Sciences, UNT System College of Pharmacy, University of North Texas Health Science Center, 3500 Camp Bowie Boulevard, Fort Worth, Texas 76107, United States.
Author Contributions
C.W.L. wrote the manuscript and oversaw the medicinal chemistry. K.A.E. designed compounds, and J.L.E., K.A.B., M.F.L., M.B.M., and S.R.B. performed chemical synthesis. P.J.C., A.L.R., and C.M.N. performed and analyzed molecular pharmacology data. S.C., R.D.M., T.M.B., and A.L.B. oversaw and analyzed in vitro and in vivo DMPK data. C.K.J. and M.B. performed and analyzed the mouse tail suspension assay/data. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Egan M. F.; Straub R. E.; Goldberg T. E.; Yakub I.; Callicott J. H.; Hariri A. R.; Mattay V. S.; Bertolino A.; Hyde T. M.; Shannon-Weickert C.; Akil M.; Crook J.; Vakkalanka R. K.; Balkissoon R.; Gibbs R. A.; Kleinman J. E.; Weinberger D. R. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 12604–12609. 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J.; Lyon L.; Sartorius L. J.; Burnet P. W.; Lane T. A. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): Expression, function and involvement in schizophrenia. J. Psychopharmacol. 2008, 22, 308–322. 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- Tan H.-Y.; Chen Q.; Sust S.; Buckholtz J. W.; Meyers J. D.; Egan M. F.; Mattay V. S.; Meyer-Lindenberg A.; Weinberger D. R.; Callicott J. H. Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 12536–12541. 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandaswamy R.; McQuillin A.; Sharp S. I. Genetic association, mutation screening, and functional analysis of a Kozak sequence variant in the metabotropic glutamate receptor 3 gene in bipolar disorder. JAMA Psychiatry 2013, 70, 591–598. 10.1001/jamapsychiatry.2013.38. [DOI] [PubMed] [Google Scholar]
- O’Brien N. L.; Way M. J.; Kandaswamy R.; Fiorentino A.; Sharp S. I.; Quadri G.; Alex J.; Anjorin A.; Ball D.; Cherian R.; Dar K.; Gomez A.; Guerrini I.; Heydtmann M.; Hillman A.; Lankappa S.; Lydall G.; O’Kane A.; Patel S.; Quested D.; Smith I.; Thomson A. D.; Bass N.; Morgan M. Y.; Curtis D.; McQullin A. The functional GRM3 Kozak sequence variant rs148754219 affects the risk of schizophrenia and alcohol dependence as well as bipolar disorder. Psychiatr. Genet. 2014, 24, 277–278. 10.1097/YPG.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering T. J.; Wichmann J.; Goetschi E.; Knoflach F.; Ballard T. M.; Huwyler J.; Gatti S. Synthesis and characterization of 1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives: Part 4. In vivo active potent and selective non-competitive metabotropic gluta mate receptor 2/3 antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 6969–6974. 10.1016/j.bmcl.2010.09.125. [DOI] [PubMed] [Google Scholar]
- Yacoubi M. E.; Vaugeois J.-M.; Kalinichev M.; Célanire S.; Parron D.; Le Poul E.; Campo B.. Effects of a mGluR2/3 negative allosteric modulator and a reference mGluR2/3 orthosteric antagonist in a genetic mouse model of depression. In Behavioral Studies of Mood Disorders; Proceedings of the 40th Annual Meeting of the Society for Neuroscience, San Diego, CA, Nov 13–17, 2010; Society for Neuroscience: Washington, DC, 2010; 886.14/VV7. [Google Scholar]
- Goeldner C.; Ballard T. M.; Knoflach F.; Wichmann J.; Gatti S.; Umbricht D. Cognitive impairment in major depression and the mGlu2 receptor as a therapeutic target. Neuropharmacology 2013, 64, 337–346. 10.1016/j.neuropharm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Kalinichev M.; Campo B.; Lambeng N.; Célanire S.; Schneider M.; Bessif A.; Royer-Urios I.; Parron D.; Legrand C.; Mahious N.; Girard F.; Le Poul E.. An mGluR2/3 negative allosteric modulator improves recognition memory assessed by natural forgetting in the novel object recognition test in rats. In Memory Consolidation and Reconsolidation: Molecular Mechanisms II; Proceedings of the 40th Annual Meeting of the Society for Neuroscience, San Diego, CA, Nov 13–17, 2010; Society for Neuroscience: Washington, DC, 2010; 406.9/MMM57. [Google Scholar]
- Choi C. H.; Schoenfeld B. P.; Bell A. J.; Hinchey P.; Kollaros M.; Gertner M. J.; Woo N. H.; Tranfaglia M. R.; Bear M. F.; Zu-kin R. S.; McDonald T. V.; Jongens T. A.; McBride S. M. Pharmacological reversal of synaptic plasticity deficits in the mouse model of fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Res. 2011, 1380, 106–119. 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti McArthur S.; Saxe M.; Wichmann J.; Woltering T.. mGlu2/3 antagonists for the treatment of autistic disorders. PCT Int. Pat. Appl. WO 2014/064028 A1, May 1, 2014.
- Structure of decoglurant disclosed in Recommended International Nonproprietary Names (INN). WHO Drug Information; World Health Organization: Geneva, Switzerland, 2013; Vol. 27, no. (3), , p 150. [Google Scholar]
- ARTDeCo Study: A study of RO4995819 in patients with major depressive disorder and inadequate response to ongoing antidepressant treatment. https://www.clinicaltrials.gov/ct2/show/NCT01457677. [Google Scholar]
- Niswender C. M.; Conn P. J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp D. D.; Jane D. E.; Monn J. A. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology 1999, 38, 1431–1476. 10.1016/S0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Chaki S.; Ago Y.; Palucha-Paniewiera A.; Matrisciano F.; Pilc A. mGlu2/3 and mGlu5 receptors: Potential targets for novel antidepressants. Neuropharmacology 2013, 66, 40–52. 10.1016/j.neuropharm.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Palucha A.; Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol. Ther. 2007, 115, 116–147. 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Lindsley C. W.; Emmitte K. A.; Hopkins C. R.; Bridges T. M.; Gregory K. A.; Niswender C. M.; Conn P. J. Practical strategies and concepts in GPCR allosteric modulator discovery: Recent advances with metabotropic glutamate receptors. Chem. Rev. 2016, 116, 6707–6741. 10.1021/acs.chemrev.5b00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler D. J.; Wenthur C. J.; Bruner J. A.; Carrington S. J. S.; Vinson P. N.; Gogi K. K.; Blobaum A. L.; Morrison R. D.; Vamos M.; Cosford N. D. P.; Stauffer S. R.; Daniels J. S.; Niswender C. M.; Conn P. J.; Lindsley C. W. Development of a novel, CNS-penetrant, metabotropic glutamate receptor 3 (mGlu3) NAM probe (ML289) derived from a closely related mGlu5 PAM. Bioorg. Med. Chem. Lett. 2012, 22, 3921–3925. 10.1016/j.bmcl.2012.04.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthur C. J.; Morrison R.; Felts A. S.; Smith K. A.; Engers J. L.; Byers F. W.; Daniels J. S.; Emmitte K. A.; Conn P. J.; Lindsley C. W. Discovery of (R)–(2-fluoro-4-((−4-methoxy phenyl)ethynyl)-phenyl(3-hydroxypiperidin-1-yl)methanone (ML337), An mGlu3 selective and CNS penetrant negative allo steric modulator (NAM). J. Med. Chem. 2013, 56, 5208–5212. 10.1021/jm400439t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. G.; Wenthur C. J.; Xiang Z.; Rook J. M.; Emmitte K. A.; Niswender C. M.; Lindsley C. W.; Conn P. J. Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 1196–1201. 10.1073/pnas.1416196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers J. L.; Rodriguez A. L.; Konkol L. C.; Morrison R. D.; Thompson A. D.; Byers F. W.; Blobaum A. L.; Chang S.; Loch M. T.; Niswender C. M.; Daniels J. S.; Jones C. K.; Conn P. J.; Lindsley C. W.; Emmitte K. A. Discovery of VU0650786: A selective and CNS penetrant negative allosteric modulator of metabotropic glutamate receptor subtype 3 with antidepressant and anxiolytic activity in rodents. J. Med. Chem. 2015, 58, 7485–7500. 10.1021/acs.jmedchem.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See Supporting Information for full details.
- Rook J. M.; Abe M.; Cho H. P.; Nance K. D.; Luscombe V. B.; Adams J. J.; Dickerson J. W.; Remke D. H.; Garcia-Barrantes P. M.; Engers D. W.; Engers J. L.; Chang S.; Foster J. J.; Blobaum A. L.; Niswender C. M.; Jones C. K.; Conn P. J.; Lindsley C. W. Diverse effects on M1 signaling and adverse effect liability with in a series of M1 ago-PAMs. ACS Chem. Neurosci. 2017, 8, 866–883. 10.1021/acschemneuro.6b00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeadProfilingScreen; (catalogue no. 68); Eurofins Panlabs, Inc.: Redmond, WA: (www.eurofinspanlabs.com). [Google Scholar]
- Ripoll N.; David D. J.; Dailly E.; Hascoet M.; Bourin M. Antide pressant-like effects in various mice strains in the tail suspension test. Behav. Brain Res. 2003, 143, 193–200. 10.1016/S0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Cryan J. F.; Mombereau C.; Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Walker A. G.; Wenthur C. J.; Xiang Z.; Rook J. M.; Emmitte K. A.; Niswender C. M.; Lindsley C. W.; Conn P. J. Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 1196–1201. 10.1073/pnas.1416196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger K. A.; Felts A. S.; Brassard C. J.; Engers J. L.; Weiner R. L.; Cho H. P.; Rodriguez A. L.; Bubser M.; Chang S.; Blobaum A. L.; Jones C. K.; Niswender C. M.; Emmitte K. A.; Conn P. J.; Lindsley C. W. Design of 4-aryl-5-(1-methyl-1H-pyrazol-3-yl)methoxy)picolinamides as highly selective and CNS penetrant metabotropic glutamate receptor subtype 2 (mGlu2) negative allosteric modulators. ACS Med. Chem. Lett. 2017, 10.1021/acsmedchemlett.7b00279. [DOI] [Google Scholar]; Companion paper.
- Felts A. S.; Rodriguez A. L.; Smith K. A.; Engers J. L.; Morrison R. D.; Byers F. W.; Blobaum A. L.; Locuson C. W.; Chang S.; Venable D. F.; Niswender C. M.; Daniels J. S.; Conn P. J.; Lindsley C. W.; Emmitte K. A. Design of 4-Oxo-1-aryl-1,4-dihydroquinoline-3-carboxamides as selective negative allosteric modulators of metabotropic glutamate receptor subtype 2. J. Med. Chem. 2015, 58, 9027–9040. 10.1021/acs.jmedchem.5b01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Kumata K.; Yamasaki T.; Cheng R.; Hatori A.; Ma L.; Zhang Y.; Xie L.; Wang L.; Kang J. H.; Sheffler D. J.; Cosford N. D. P.; Zhang M.-R.; Liang S. H. Synthesis and preliminary studies of a novel negative allosteric modulator, 7-((2,5-dioxopyrrolidin-1-yl)methyl)-4-(2-fluoro-4-[11C]methoxyphenyl) quinoline-2-carboxamide, for imaging of metabotropic glutamate receptor 2. ACS Chem. Neurosci. 2017, 10.1021/acschemneuro.7b00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers D. W.; Gogliotti R. D.; Cheung Y.-Y.; Salovich J. M.; Garcia-Barrantes P. M.; Daniels J. S.; Morrison R.; Jones C. K.; Blobaum A. L.; Macor J. E.; Bronson J. J.; Conn P. J.; Lindsley C. W.; Niswender C. M.; Hopkins C. R. Discovery, synthesis and pre-clinical characterization of N-(3-chloro-4-fluorophenyl)-1H-pyrazolo[4,3-b]pyridin-3-amine (VU0418506), a novel positive allosteric modulator of the metabotropic glutamate receptor 4 (mGlu4). ACS Chem. Neurosci. 2016, 7, 1192–1200. 10.1021/acschemneuro.6b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. R.; Noetzel M. J.; Melancon B. J.; Nance K. D.; Poslusney M. S.; Hurtado M. A.; Luscombe V. B.; Weiner R. L.; Rodriguez A. L.; Lamsal A.; Chang S.; Bubser M.; Blobaum A. L.; Engers D. W.; Niswender C. M.; Jones C. K.; Brandon N. J.; Wood M. W.; Duggan M. E.; Conn P. J.; Bridges T. M.; Lindsley C. W. Discovery of VU0467485: An M4 positive allosteric modulator evaluated as a preclinical candidate for the treatment of schizophrenia. ACS Med. Chem. Lett. 2017, 8, 233–238. 10.1021/acsmedchemlett.6b00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Ceide S.; Martin-Martin M. L.; Alcazar J.; Manka T.; Tong H. M.; Garcia-Barrantes P. M.; Lavreysen H.; Mackie C.; Vinson P. N.; Daniels S. J.; Menges A.; Niswender C. M.; Jones C. K.; Macdonald G. J.; Steckler T.; Conn P. J.; Stauffer S. R.; Bartolome-Nebreda J. M.; Lindsley C. W. Discovery of VU0409551/JNJ-46778212: An mGlu5 positive allosteric modulator clinical candidate targeting schizophrenia. ACS Med. Chem. Lett. 2015, 6, 716–720. 10.1021/acsmedchemlett.5b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.