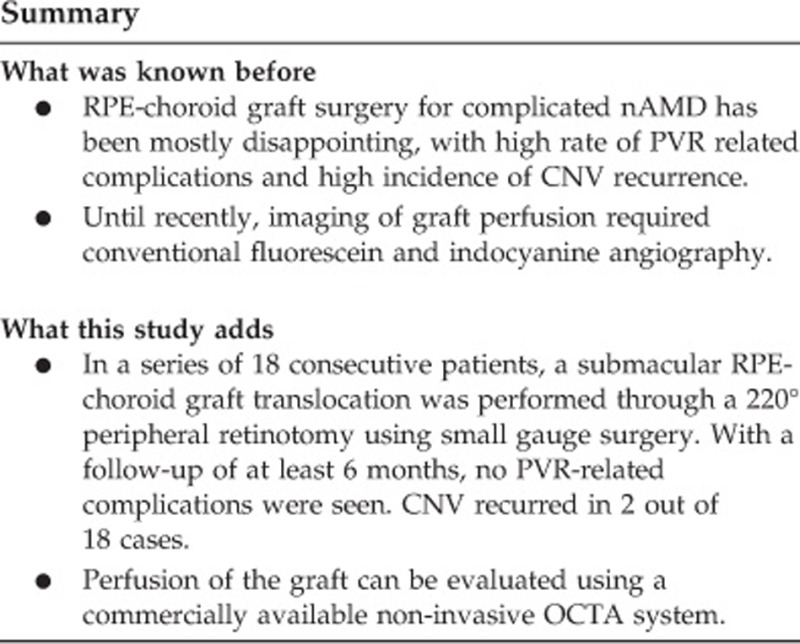
Abstract
Purpose
The purpose of this study is to investigate the reperfusion of translocated retinal pigment epithelium (RPE)-choroid graft in the treatment of patients with neovascular age-related macular degeneration (nAMD), using OCT angiography (OCTA), a novel non-invasive, high-resolution imaging modality.
Patients and methods
Eighteen eyes of 18 consecutive patients suffering from complicated nAMD underwent RPE-choroid patch graft translocation surgery using a peripheral retinotomy and flap-over technique. We analyzed functional and anatomical outcome using visual acuity, Spectral Domain OCT and OCTA.
Results
With a mean follow-up of 11 months, out of 18 patients, 15 gained vision, 1 remained stable, and 2 lost vision. Overall, the visual acuity improved with a mean of 30 letters. Perfusion of the graft tissue was confirmed in all patients. Two patients developed signs of a recurrent neovascular membrane during follow-up. No cases of proliferative vitreoretinopathy occurred in this series.
Conclusions
OCTA images show signs of perfusion in all grafts. Encouraging functional results and low risk of severe complications suggest that RPE-choroid graft translocation is a valid option in patients with complicated nAMD.
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in elderly in the industrialized world and the third most common cause of blindness worldwide. Although the neovascular subtype of AMD (nAMD) accounts for only 10–20% of the total AMD population, it is responsible for 80% of blindness caused by AMD.1 The introduction of anti-vascular endothelial growth factor (VEGF) therapy has improved the outcome of nAMD immensely. However, several severe complications of the disease such as a retinal pigment epithelial (RPE) tear, fibrotic contraction of the choroidal neovascular membrane (CNV), and submacular hemorrhage (SMH) can make the anti-VEGF treatment less effective. A RPE tear occurs in 12–20% of patients with nAMD after starting anti-VEGF injections.2, 3 The visual prognosis is worse if the fovea is included in the tear.2 Continued anti-VEGF treatment can stabilize the vision but improvement is rare.2, 3 For a SMH, there are different treatment options: anti-VEGF injections, intravitreal injection of tissue plasminogen activator (tPA) with or without gas, vitrectomy, and subretinal injection of tPA, all with uncertain success rates and different complication profiles.4, 5, 6, 7 Several characteristics of the SMH may have an impact on the outcome after treatment. These include thickness of subretinal blood, time delay to treatment, and condition of the outer retinal layers as seen on OCT. Above mentioned complicated cases can progress to atrophy and profound loss of vision very rapidly. In order to prevent this irreversible outer retinal damage, macular surgery can be considered.
Macular translocation surgery has shown some promising results in the past, but it has several drawbacks. Because of the limit to which the retina can be rotated around the optic disc, extensive pathology, involving the posterior pole and beyond, cannot be treated in this manner. In addition, a close collaboration with an experienced squint surgeon is necessary. Although functional results can be very good early on, long-term follow-up shows a risk of foveal atrophy in almost 50% of the patients and CNV recurrence in 30%.8
An autologous free RPE-choroid graft is another surgical option in such cases.9 Review of the literature shows mixed results with regard to visual improvement. In addition, several authors have reported a high complication rate, mostly related to development of proliferative vitreoretinopathy (PVR). This may be due, at least in part, to surgical technique. In particular, a central/macular retinotomy seems to be associated with a higher incidence of post-operative PVR.10, 11, 12, 13 Other reported problems include graft failure due to lack of reperfusion and recurrence of CNV.
OCT angiography (OCTA) is a non-invasive method that allows selective visualization of blood flow in the retinal layers and the choriocapillaris and is rapidly becoming an indispensible tool for the diagnosis and follow-up of CNV. We have recently treated 18 eyes of 18 patients suffering from complicated nAMD with RPE-choroid graft translocation. To our knowledge, this is the first report on the use of a commercially available OCTA system to demonstrate graft reperfusion.
Materials and methods
Patient selection
We retrospectively analyzed the results of 18 eyes of 18 consecutive patients who underwent an RPE-choroid graft translocation procedure between June 2014 and August 2016. The age of the patients was between 55 and 88 years, with a mean of 75 years.
Twelve out of 18 cases were treated for large SMH (>500 μm thick and or extending to the arcades). Based on the history the SMH was longstanding (>2 weeks) in 7 of these patients so no tPA/gas injection was considered. In five other SMH cases tPA and gas injection was performed before the graft surgery.
In two patients, a star-like subretinal fold that was best demonstrated on enface OCT developed, indicating contraction of the CNV. Both patients were losing vision progressively despite fluid resolution after anti-VEGF injection. Two patients were considered non-responsive to anti-VEGF treatment. One of these patients had received four injections of aflibercept. Although changing the treatment to another anti-VEGF drug would have been a valid option, we decided to proceed to surgery, because the patient was losing vision. The second patient had previously received 12 injections of ranibizumab but had an enormous pigment epithelial detachment (height >1000 μm) with persistent subretinal fluid not responding to the injections. Two patients had a RPE tear exposing the fovea.
Patients presenting with complete atrophy of the outer retina at the fovea as documented on OCT were excluded. The general health of the patient had to allow safe general anesthesia and the discontinuation/bridging of any anti-clotting agents. No other criteria were set for exclusion. The patients consented to the surgery only after the risks and the potential benefits had been thoroughly explained.
This study was approved by the institutional review board of the ZNA Middelheim Hospital Antwerp (ethical committee approval number 4882).
Surgery
All procedures were performed by a single surgeon (MV) in ZNA Middelheim Hospital Antwerp.
If the eye was phakic (11 out of 18), phaco-emulsification with intraocular lens implantation was performed at the beginning of the surgery. The pars plana vitrectomy was performed using the Alcon Constellation platform (Alcon, Forth Worth, TX, USA), through 23/25 gauge-valved cannulas. Meticulous vitreous base shaving was aided by repeated injections of diluted triamcinolone. No epimacular or internal limiting membrane (ILM)peeling was performed. A 41-gauge subretinal infusion with BSS was used to detach the temporal quadrants, after which a 220° temporal retinotomy was made at the ora, using the 25-gauge cutter and/or 23-gauge ovali curved spatula scissors (DORC, Zuidland, The Netherlands). Perfluoro-octaline (PFO; DORC) was used to expose the subretinal space. If present, the submacular blood (clot) was removed using active backflush, scissors, and/or vitreous cutter. The CNV was peeled with an end-gripping forceps, after which diathermy was applied if necessary. The full thickness RPE-choroid patch graft was taken from the temporal midperiphery. The size of the graft was based on the RPE defect present after CNV removal. In order to maximize the likelihood of reperfusion, we aimed for a large graft so that at least 180° of its perimeter would be in direct contact with the healthy recipient RPE and choroid that remained after CNV extraction. The patch graft was excised using the 25 g cutter and/or curved or hooked scissors. In 2 out of 18 eyes, a connection with the donor site was preserved (flap graft). Diathermy and raising the intraocular pressure were used to minimize the bleeding. Finally, the graft was translocated bimanually using two end-gripping forceps to a position estimated to be subfoveal. The subretinal PFO was then removed, while simultaneously injecting PFO on the vitreous side to re-apply the retina. Two rows of soft laser burns were applied to the retinotomy edge. At the end of the surgery, direct PFO to silicone oil (Siluron 2000, Fluoron GmbH, Neu-Ulm, Germany) exchange was performed. The silicone oil was removed on average after 9 weeks.
Pre- and postoperative evaluation
At each post-op visit, best corrected visual acuity was measured in Snellen lines. Complete ophthalmic examination also included OCT and OCTA (Angiovue by Optovue, Fremont, CA, USA).
Fluorescein angiography (FA) and indocyanin angiography (ICGA) using the Heidelberg device (Heidelberg Engineering, Heidelberg, Germany) was only performed in selected cases when CNV recurrence was suspected.
All patients were at least seen 1 day, 2 weeks, 6 weeks, and 6 months postoperatively.
Statistical analysis
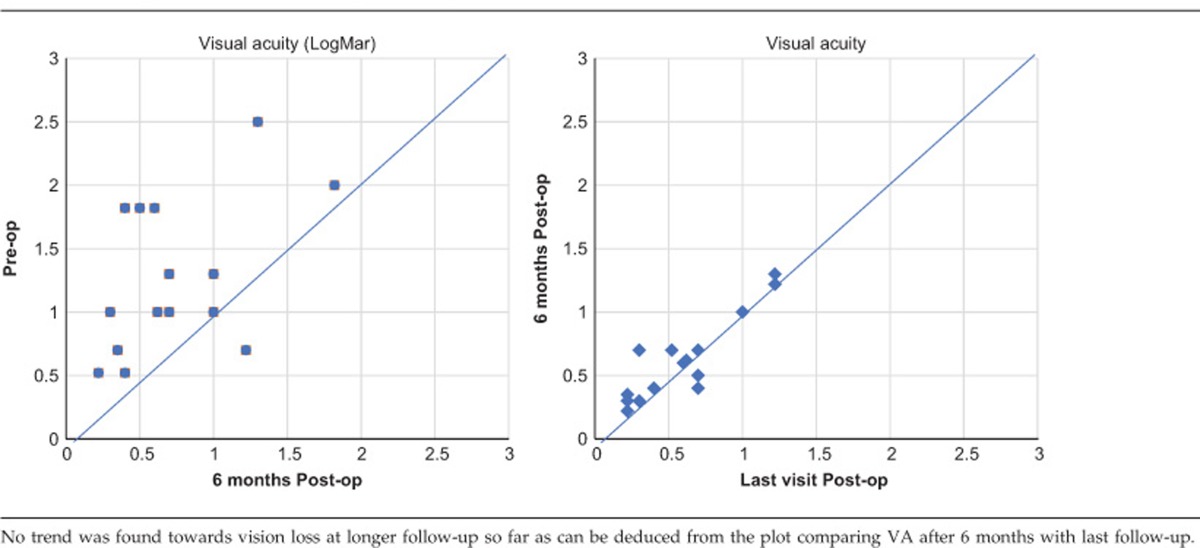
Statistical Package for Social Sciences software (version 21, SPSS, Inc., Chicago, IL, USA) was used to analyze the visual acuity data. According to the Kolmogorov–Smirnov and Shapiro–Wilk test, the VA data were not evenly distributed. Hence, the Wilcoxon signed-rank test was used to compare the VA pre-op to the VA after 6 months and at the last moment of follow-up. In addition, to assess the possibility of VA loss during follow-up, VA at last follow-up was compared with VA after 6 months.
Results
The mean follow-up was 11 months, with a minimum of 6 months. There was significant VA improvement at 6 months (P=0.001) postoperatively and at the last follow-up (P=0.001). No significant VA decline was observed during longer follow-up between 6 months postoperatively and the last follow-up (P=0.326.) Fifteen out of 18 patients gained visual acuity, with a mean of >7 lines on the logMar scale. In one patient the visual acuity was unaffected. Two patients suffered a drop in visual acuity. In all but two patients, central fixation on the graft, as tested with the slitlamp set to the smallest beam and with the fixation stimulus of the OCT machine, was present by 6 weeks post-op and this remained so during follow-up. Two patients did not regain foveal fixation. The surgery in the first patient was complicated by pronounced bleeding tendency and difficulty with positioning of the graft requiring extensive manipulation. This patient had a postoperative drop in VA (26 letters in the logMar scale). The second patient presented with a large bleed extending up to the equator. Although VA improved using eccentric fixation, central fixation did not recover. In both cases, postoperative structural OCT images show extensive atrophic changes in the outer retina at the fovea. The demographic data and VA results are summarized in Tables 1 and 2.
Table 1. Overview of cases.
| Gender | Age (years) | Indication | Delay (d) | VA pre | VA 6m | VA last follow-up | VA Diff | #Letters | FU | Phakic pre | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 84 | SMH | 15 | 1.30 | 0.70 | 0.52 | -0.78 | 39 | 30 | x |
| 2 | F | 75 | Fibrotic membrane | 7 | 0.70 | 0.35 | 0.22 | -0.48 | 24 | 14 | |
| 3 | M | 73 | SMH | 6 | 0.70 | 1.22 | 1.22 | 0.52 | -26 | 14 | x |
| 4 | F | 76 | SMH, RAP | 7 | 1.30 | 0.70 | 0.30 | -1.00 | 50 | 14 | x |
| 5 | M | 80 | SMH | 6 | 1.82 | 0.40 | 0.40 | -1.42 | 71 | 12 | |
| 6 | F | 67 | Anti-VEGF non-response, RAP | 20 | 1.82 | 0.60 | 0.60 | -1.22 | 61 | 14 | x |
| 7 | F | 81 | RIP bare fovea | 8 | 1.00 | 0.30 | 0.22 | -0.78 | 39 | 12 | x |
| 8 | F | 78 | SMH | 20 | 1.00 | 0.70 | 0.70 | -0.30 | 15 | 13 | x |
| 9 | F | 82 | SMH, RAP | 6 | 0.52 | 0.40 | 0.70 | 0.18 | -9 | 12 | |
| 10 | F | 70 | SMH | 13 | 1.30 | 1.00 | 1.00 | -0.30 | 15 | 9 | x |
| 11 | M | 84 | SMH, RAP | 13 | 2.00 | 1.82 | 1.82 | -0.18 | 9 | 12 | |
| 12 | F | 88 | SMH | 6 | 1.00 | 1.00 | 1.00 | 0.00 | 0 | 11 | |
| 13 | F | 70 | SMH | 36 | 2.52 | 1.82 | 1.30 | -1.22 | 61 | 8 | x |
| 14 | F | 69 | Fibrotic membrane | 7 | 0.52 | 0.22 | 0.22 | -0.30 | 15 | 7 | x |
| 15 | M | 55 | SMH, PCV | 16 | 1.82 | 0.50 | 0.70 | -1.12 | 56 | 7 | x |
| 16 | F | 74 | SMH | 6 | 2.50 | 1.22 | 1.22 | -1.28 | 64 | 6 | |
| 17 | F | 74 | RIP bare fovea | 15 | 1.00 | 0.30 | 0.30 | -0.70 | 35 | 6 | |
| 18 | F | 77 | Anti-VEGF non-response | 36 | 1.00 | 0.62 | 0.62 | -0.38 | 19 | 6 | x |
| Mean | 75.4 | 13.5 | 1.32 | 0.77 | 0.73 | -0.60 | 29.9 | 11.5 | 11 | ||
| P-value Wilcoxon signed-rank test, baseline=VA pre | 0.001 | 0.001 | |||||||||
| P-value Wilcoxon signed-rank test, baseline=VA 6m | 0.326 |
Abbreviations: #Letters, number of letters difference in vision before and after operation; 6m, 6 months after operation; d, days; Diff, difference in vision, expressed in LogMar scale; F, female; FU, follow-up in months; M, male; PCV, polypoidal choroidal vasculopathy; pre, before operation; RAP, retinal angiomatous proliferations; RIP, retinal pigment epithelial tear; SMH, submacular hemorrhage; VA, visual acuity; w, weeks.
Statistical analysis was based on Wilcoxon signed-rank test.
Table 2. VA improved in most of our patients by 6 months.
The mean delay between the pre-op examination and the actual surgery was 13 days, with a maximum of 36 days.
In the immediate postoperative period, some blood was present in all patients at the harvesting site, hemorrhages around the translocated graft were seen in four patients and cystoid macular edema occurred in three patients. In one patient, although no signs of PVR were present, the inferior retinotomy edge appeared only loosely adherent during the attempted oil removal 4 weeks after the graft surgery. Additional laser was applied and oil was re-injected for another 8 weeks, after which the retina was completely attached and the oil could be removed.
None of our patients developed PVR-related pathology. Despite the fact that ILM peeling was not performed in any of the eyes, no clinically relevant epiretinal fibrosis was seen on follow-up OCT images.
Blood remnants at the harvest site and those surrounding the subfoveal graft cleared spontaneously, and had all but disappeared completely by 6 months without notable consequences.
OCTA images suggest ample reperfusion of the translocated graft tissue in all 18 patients. Although reliable OCTA images could usually not be acquired at the 2-week visit, obvious signs of flow in the translocated choriocapillaris layer were present by 6 weeks and onward in all patients (Figures 1 and 2). Producing good en-face OCTA images of the elevated in situ graft bordered by flat recipient RPE can be facilitated by manual segmentation. In Figure 2, the anterior boundary of the en-face slab is based on the outer plexiform layer segmentation and the posterior boundary is based on the Bruch’s membrane segmentation, which is shifted posteriorly to include the choroid. AngioVue research software (Version 2016.200.0) was applied to reduce projection artifacts.
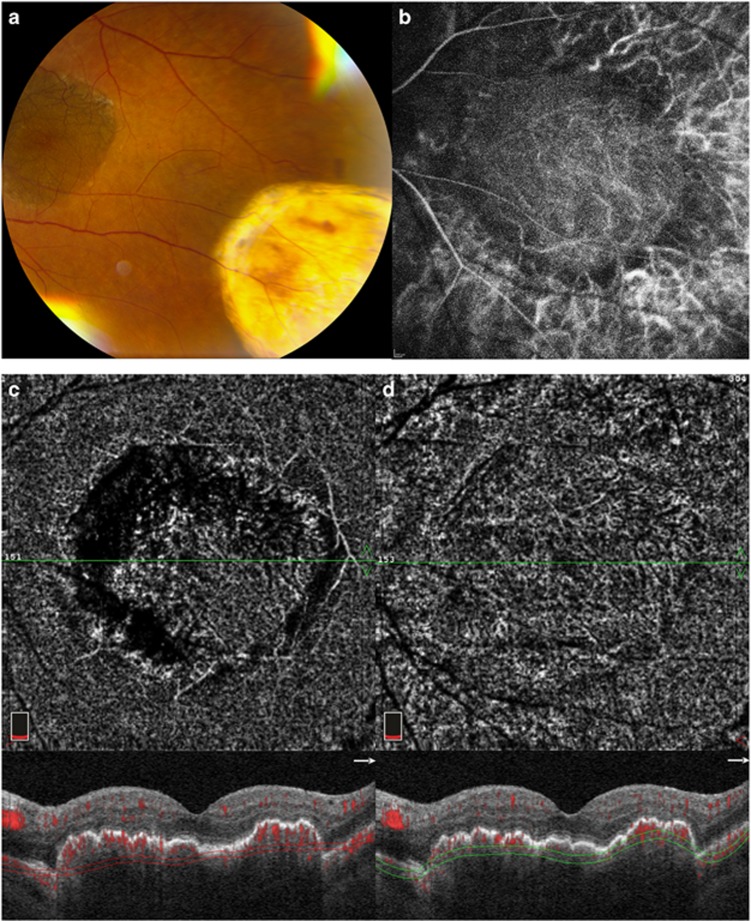
Figure 1.
Case number 2: 75 years old female. (a) Post-operative fundus photograph includes graft in situ and harvest site. (b) ICGA (30 s): dense pigmentation of graft RPE somewhat blocks ICGA picture of vessels in the graft. (c) AngioOCT image acquired 6 months post-op using automated segmentation of the choriocapillaris layer. (d) Manually adjusting segmentation to better fit the contour of the graft tissue results in an optimized en-face angioOCTimage of the graft choriocapillaris.
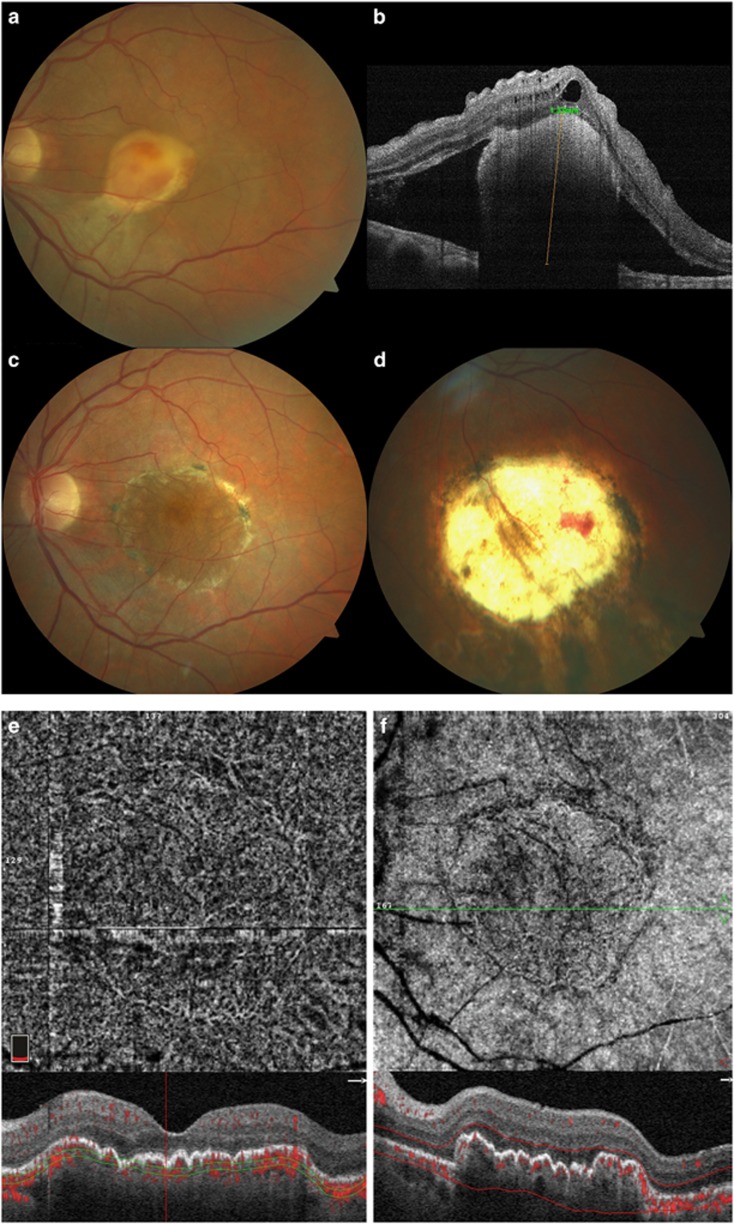
Figure 2.
Case number 14: 72-year-old female. Pre-op visual acuity was 0.50 (logMar). Nine months after the operation, visual acuity had improved to 0.22. (a and b). Pre-operative fundus photograph and OCT image suggest a fibrotic CNV with contracted clot. (c) Post-operative fundus photograph of RPE-choroid graft in situ. (d) Fundus photograph showing that the size of the graft in situ is well preserved when compared with the collection site. (e) AngioOCT and SD-OCT image, 3 months post-operative. Manually adjusting segmentation to better fit the contour of the graft tissue improves the en-face angioOCT image, despite the presence of motion artifact. (f) Image resulting from additional software processing to eliminate motion and projection artefacts (Projected retinal vessels appear dark).
CNV recurrence during the follow-up occurred in 2 eyes. One patient was managed conservatively, as there was no loss of vision and the intraretinal fluid was minimal on OCT. The other patient has been treated successfully with anti-VEGF injections. Figure 3 shows the evolution in this patient (patient number 9 in Table 1) from a well-perfused graft with no signs of CNV at 6 months to an image suggestive of CNV recurrence 1 year after surgery. OCTA images as well as FA and indocyanine green angiography (ICGA) confirm a RAP-type CNV.
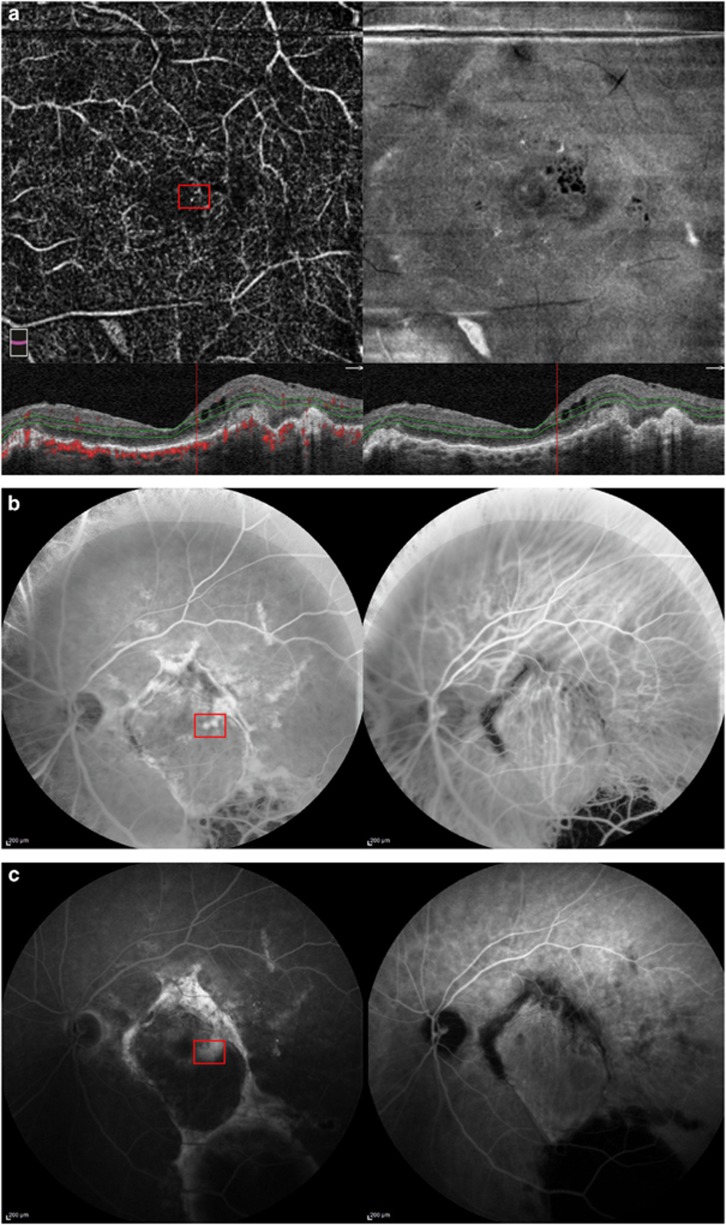
Figure 3.
Case number 9: 82-year-old female. The indication for the operation was a subfoveal hemorrhage, caused by a RAP lesion. Pre-op visual acuity was 0.52 (logMar). Six months after the operation visual acuity was 0.4 (logMar). One year after surgery, visual acuity drops to 0.7 (logMar) (a). AngioOCT image one year after translocation with matching OCT and superimposed Doppler signal below and en-face image on the right, at the level of the deep capillary plexus. On the OCT image, intraretinal fluid is present, suggestive of a recurrent CNV. At the level of the cysts, in the angioOCT image, two hyperintens dots are seen (marked with a red square) possible early signs of the RAP lesion. FA and ICGA images after 30 s (b) and after 10 min (c) acquired 1 year after surgery. On the FA, two hyperfluorescent dots are seen at the same location as in the angioOCT image, with leakage, confirming the diagnosis of recurrent RAP lesion.
Discussion
Autologous RPE-choroid graft translocation surgery has already been reported in the literature for >10 years but, overall, results have been disappointing. We here report our experience with this surgery in a selection of patients that have a poor prognosis even with adequate drug treatment including patients with a RPE-tear with exposed fovea, large and/or longstanding SMH, and anti-VEGF non-responders. These patients have been excluded from randomized drug trials and, to our knowledge, no evidence exists of another effective while less invasive treatment for these patients. Despite extensive reports, many uncertainties persist with regards to the optimal approach to SMH in nAMD. Subretinal tPA administration by pars plana vitrectomy combined with gas has not been convincingly shown to be superior to an intravitreal injection of tPA and gas. Moreover, recent reports suggest that anti-VEGF alone may be as successful in restoring vision in less extensive hemorrhages.14 Overall, results of tPA and gas SMH displacement suggest that very good outcome can be achieved in smaller hemorrhages, usually <500 μm thick, not extending beyond the arcades. In larger hemorrhages, the results are less encouraging.
tPA has the best effect when the hemorrhage is <2 weeks old.15 When a patient presents with a fresh SMH to our department, the standard approach includes intravitreal tPA/gas and anti-VEGF ASAP. In this series, seven patients who presented with a clear history of longstanding hemorrhage, were offered the RPE graft surgery, because OCT suggested the potential for macular function. In five patients with a large SMH, the duration of hemorrhage could not be reliably estimated from the history. tPA and gas displacement was performed in these patients as an intermediate salvage procedure, as the surgery had to be delayed due the use of anti-clotting agents.
Adding tPA to submacular surgery for SMH was not shown to have significant benefit in the SST trials.16 Nevertheless, reviewing our surgical videos, cases that had been pre-treated with tPA seemed to require less subretinal manipulation to extract/evacuate the blood. The numbers in this study are too small to show any significant benefit with regards to functional outcome.
A major concern regarding the functional outcome after translocation surgery is graft survival. For this, vascular re-anatomises of translocated tissue is vital. Maaijwee et al13 were the first to demonstrate revascularization of a free graft in a pig model. Several studies on this type of surgery in humans have since been published.10, 11, 12, 13, 17, 18, 19 In a retrospective series of 13 patients, Cereda et al17 performed dynamic ICGA. They confirmed graft perfusion based on a typical pattern of vessels that run parallel to one another in contrast to the otherwise radially arranged choroidal vascular system. More recently, direct flow in the free graft was demonstrated by van Zeeburg et al20 using an experimental Doppler OCT. We have been using the commercially available Optovue OCTA system since 2014. Although we initially also performed conventional angiography to study our graft patients, we found that this novel OCT technology, which can detect flow at a well-specified deeper layer, allowed us to study perfusion in a much more reproducible manner. Acquiring the images is completely non-invasive and proved not to be time consuming, allowing repeated investigations at every visit. By now, OCTA studies on many different retinal diseases have been published.21 Demonstrating the CNV in nAMD is one particularly promising application of OCTA.22
The ability of OCTA to reveal the laminar structure of the blood supply of the retina and choroid makes it exceptionally suited to study RPE-choroid graft patients. Interpreting the images can be challenging due to several different types of artifacts as was described by Spaide et al.23 We have found that both manually adjusting the segmentation to produce custom en-face images of the graft and carefully judging the Doppler signal superimposed on the structural OCT image are indispensible when evaluating the images in these eyes with a surgically induced anomalous anatomy (Figures 1 and 2).
High-quality OCTA images require good fixation and this was a problem at the first post-op examination making the images unreliable in many cases. Enlargement of choroidal vessel diameter and graft thickening has been suggested to be an early sign of ingrowth of afferent vessels.24 We documented this phenomenon on the structural SD-OCT images in all our patients by 2 weeks post-op. All patients had signs of flow on OCTA in the graft tissue by 6 weeks and a recent software update, which greatly reduces motion artifacts, now enables us to acquire good-quality OCTA images, even in the eyes with suboptimal fixation (data not shown). Ensuring a large contact area between graft and healthy appearing recipient tissue may be important for early ingrowth of vessels, as these seem more likely to originate from the relatively healthy choroidal borders than from the atrophic base. Further improvements of the quality of the early post-op OCTA images could help to confirm this hypothesis.
Other important concerns with this type of surgery include loss of function due to iatrogenic trauma to the outer retina and late complications such as PVR-related retinal detachment. Most authors describe a technique in which the donor tissue is harvested transretinally, after which positioning under the macula is accomplished through a paramacular retinotomy.10, 11, 12, 13 These studies report a high incidence of PVR as well as CNV recurrence. Our surgical approach, based on the technique described by Pertile and colleagues,17 uses a 220° peripheral retinotomy to expose the subretinal space, giving easy access. This allows the surgeon to remove the CNV and stop any bleeding in a more controlled manner, preserving the outer retinal tissue as much as possible and making manipulation of the donor tissue less traumatic. Furthermore, a large central retinotomy temporal to the fovea is avoided in this way. The lack of PVR formation and the encouraging visual outcome (taking case selection into account) in this series may be largely due to the modified surgical technique. The suggestion that a retinotomy at the ora, rather than to close to the macula, may reduce the risk of PVR, is also supported by the fact that no clinically relevant fibrotic response was seen on post-op SD-OCT images, despite the fact that no epimacular membrane or ILM peeling was performed in any of the eyes. In contrast to the technique described by Pertile and colleagues,17 the complete procedure was performed through valved cannulas in this series, allowing precise management of intraocular fluid flow and pressure, making the surgery even more controlled.
Recurrence of CNV is yet another concern after this surgery. In our series so far, two patients suffered a recurrence. Only one of these patients has so far needed anti-VEGF injections and has responded well. Other surgeons with extensive experience with RPE graft surgery have found that treatment with anti-VEGF is usually safe and effective once the graft is well perfused (personal communication).
Seven patients were pseudo-phakic at the time of surgery; in the other 11, phaco-emulsification with IOL implantation was combined with translocation procedure. This could very well have influenced the initial postoperative VA and is a limitation of the study. Nevertheless, no trend towards visual loss was found between the 6 months and last follow-up measurements.
This study suffers from several other limitations including retrospective data collection, small sample size, lack of routine conventional angiography, and microperimetry. In addition, this series contains a heterogeneous group of patients not according to strict inclusion criteria of large pharmacological trials. As a result, the statistical significance of our results needs to be interpreted with caution.
Conclusion
We present the results of 18 eyes of 18 consecutive patients that underwent RPE-choroid graft translocation surgery for complicated nAMD. In all patients, perfusion of the graft was documented with OCTA. This new imaging modality produces high-resolution structural SD-OCT in combination with layered en-face flow images, visualizing graft perfusion non-invasively. In addition, OCTA proved a reliable follow-up tool making early signs of recurrent CNV easily recognizable, as was demonstrated in two of our patients.
Based on the OCTA data from this series, graft failure due to non-perfusion seems to be an unlikely event. Surgical technique with a low complication rate that ensures a large graft-to-healthy-recipient contact area may have contributed to this. Our results suggest that autologous RPE-choroid graft translocation can effectively improve the visual acuity in selected complicated nAMD patients that do poorly with currently available drug treatments.
Acknowledgments
We thank Qienyuan Zhou, clinical affairs Optovue, for advice on image processing
Footnotes
The authors declare no conflict of interest.
References
- Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology 2008; 115: 116–126. [DOI] [PubMed] [Google Scholar]
- Gutfleisch M, Heimes B, Schumacher M, Dietzel M, Lommatzsch A, Bird A et al. Longterm visual outcome of pigment epithelial tears in association with anti-VEGF therapy of pigmentepithelial detachment in AMD. Eye 2011; 25: 1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimes B, Farecki Jr ML, Bartels S, Barrelmann A, Gutfleisch M, Spital G et al. Retinal pigment epithelial tear and anti-vascular endothelial growth factor therapy in exudative age-related macular degeneration. Retina 2016; 36: 868–874. [DOI] [PubMed] [Google Scholar]
- Stanescu-Segall D, Balta F, Jackson TL. Submacular hemorrhage in neovascular age-related macular degeneration: a synthesis of the literature. Surv Ophthalmol 2016; 61: 18–32. [DOI] [PubMed] [Google Scholar]
- Shin JY, Lee JM, Byeon SH. Anti-vascular endothelial growth factor with or without pneumatic displacement for submacular hemorrhage. Am J Ophthalmol 2015; 159: 904–914. [DOI] [PubMed] [Google Scholar]
- Hirashima T, Moriya T, Bun T, Utsumi T, Hirose M, Oh H. Optical coherence tomography findings and surgical outcomes of tissue plasminogen activator-assisted vitrectomy for submacular hemorrhage secondary to age-related macular degeneration. Retina 2015; 35: 1969–1978. [DOI] [PubMed] [Google Scholar]
- de Jong JH, van Zeeburg EJ, Cereda MG, van Velthoven ME, Faridpooya K, Vermeer KA et al. Intravitreal versus subretinal administration of recombinant tissue plasminogen activator combined with gas for acute submacular hemorrhages due to age-related macular degeneration: an Exploratory Prospective Study. Retina 2016; 36: 914–925. [DOI] [PubMed] [Google Scholar]
- van Romunde SH, Polito A, Bertazzi L, Guerriero M, Pertile G. Long-term results of full macular translocation for choroidal neovascularization in age-related macular degeneration. Ophthalmology 2015; 122: 1366–1374. [DOI] [PubMed] [Google Scholar]
- Neubauer AS, Liakopoulos S, van Meurs JC, Kirchhof B. Cost-effectiveness of autologous retinal pigment epithelium and choroid translocation in neovascular AMD. Int J Ophthalmol 2010; 3: 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeres S, Llacer H, Heussen FM, Weiss C, Kirchhof B, Joussen AM. Optical coherence tomography on autologous translocation of choroid and retinal pigment epithelium in age-related macular degeneration. Eye 2008; 22: 782–789. [DOI] [PubMed] [Google Scholar]
- Maaijwee K, Heimann H, Missotten T, Mulder P, Joussen A, van Meurs J. Retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: long-term results. Graefes Arch Clin Exp Ophthalmol 2007; 245: 1681–1689. [DOI] [PubMed] [Google Scholar]
- Chen FK, Uppal GS, MacLaren RE, Coffey PJ, Rubin GS, Tufail A et al. Long-term visual and microperimetry outcomes following autologous retinal pigment epithelium choroid graft for neovascular age-related macular degeneration. Clin Exp Ophthalmol 2009; 37: 275–285. [DOI] [PubMed] [Google Scholar]
- Maaijwee KJ, van Meurs JC, Kirchhof B, Mooij CM, Fischer JH, Mackiewicz J et al. Histological evidence for revascularisation of an autologous retinal pigment epithelium—choroid graft in the pig. Br J Ophthalmol 2007; 91: 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Cho HJ, SG Yoo, Kim JH, Han JI, Lee TG, Kim JW. Intravitreal anti-vascular endothelial growth factor monotherapy for large submacular hemorrhage secondary to neovascular age-related macular degeneration. Eye 2015; 29: 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeeburg EJ, van Meurs JC. Literature review of recombinant tissue plasminogen activator used for recent-onset submacular hemorrhage displacement in age-related macular degeneration. Ophthalmologica 2013; 229: 1–14. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Bressler SB, Childs AL, Haller JA, Hawkins BS, Lewis H et al. Submacular Surgery Trials (SST) Research Group. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings: SST report no. 13. Ophthalmology 2004; 111: 1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda MG, Parolini B, Bellesini E, Pertile G. Surgery for CNV and autologous choroidal RPE patch transplantation: exposing the submacular space. Graefes Arch Clin Exp Ophthalmol 2010; 248: 37–47. [DOI] [PubMed] [Google Scholar]
- Ma Z, Han L, Wang C, Dou H, Hu Y, Feng X et al. Autologous transplantation of retinal pigment epithelium-Bruch’s membrane complex for hemorrhagic age-related macular degeneration. Invest Ophthalmol Vis Sci 2009; 50: 2975–2981. [DOI] [PubMed] [Google Scholar]
- Degenring RF, Cordes A, Schrage NF. Autologous translocation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Acta Ophthalmol 2011; 89: 654–659. [DOI] [PubMed] [Google Scholar]
- van Zeeburg EJ, Braaf B, Cereda MG, van Meurs JC, de Boer JF. direct blood flow measurements in a free RPE-choroid graft with phase-resolved Doppler OCT. Transl Vis Sci Technol 2015; 13: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalam KV, Sambhav K. Optical coherence tomography angiography in retinal diseases. J Ophthalmic Vis Res 2016; 11: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscas GJ, Lupidi M, Coscas F, Cagini C, Souied EH. Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: a new diagnostic challenge. Retina 2015; 35: 2219–2228. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina 2015; 35: 2163–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeeburg EJ, Cereda MG, van der Schoot J, Pertile G, van Meurs JC. Early perfusion of a free RPE-choroid graft in patients with exudative macular degeneration can be imaged with spectral domain-OCT. Invest Ophthalmol Vis Sci 2011; 52: 5881–5886. [DOI] [PubMed] [Google Scholar]