Abstract
Venus kinase receptors (VKR) are a subfamily of invertebrate receptor tyrosine kinases, which have only recently been discovered. They contain an intracellular tyrosine kinase domain and an extracellular Venus FlyTrap domain. VKRs have been functionally and pharmacologically characterized in only two invertebrate species, namely the human parasite Schistosoma mansoni and the mosquito Aedes aegypti, where they play a crucial role in oogenesis. Here, we report the characterization of a VKR in the desert locust, Schistocerca gregaria. We performed an in-depth profiling study of the SgVKR transcript levels in different tissues throughout the female adult stage. Using the RNA interference technique, the possible role of SgVKR was investigated. SgVKR knockdown had significant effects on ovarian ecdysteroid levels and on the size of oocytes during the vitellogenic stage. SgVKR is probably involved in the complex cross-talk between several important pathways regulating female reproductive physiology. Contrary to A. aegypti and S. mansoni, we cannot conclude that this receptor is essential for reproduction, since silencing SgVKR did not affect fecundity or fertility. Considering the evolutionary distance between A. aegypti and S. gregaria, as well as the differences in regulation of their female reproductive physiology, this article constitutes a valuable asset in better understanding VKRs.
Introduction
In 2003, a novel receptor tyrosine kinase (RTK) was discovered in the human parasite, Schistosoma mansoni 1. This membrane receptor was designated as the venus kinase receptor (VKR), since it possesses an extracellular ligand-binding Venus FlyTrap (VFT) domain that is connected via a single membrane spanning segment with the intracellular tyrosine kinase (TK) domain. VKR-encoding genes have since then been identified in genomes of several other invertebrate species2. In most of these species, only one vkr gene was found, but the genomes of some Trematoda, such as Schistosoma 3, and Lepidoptera contain two vkr genes (vkr1 and vkr2). It should also be noted that no vkr genes were found in two major invertebrate research models, Drosophila melanogaster and Caenorhabditis elegans. Phylogenetic analysis evinced that all putative VKRs are clustering as a monophyletic group and likely evolved from a common ancestor2.
The TK domain of VKR closely resembles that of insulin-like peptide receptors (InR) and contains all motifs that are crucial for tyrosine kinase activity, such as the ATP binding site (GxGxxG), the catalytic loop implicated in the phosphotransfer (HRDxAxRN), and the two putative autophosphorylation sites (YY)4. Using recombinant VKRs and in vitro kinase assays, it was demonstrated that VKRs are functional tyrosine kinase receptors that can form dimers and autophosphorylate4,5. Phylogenetic and structural analysis of the VFT module (VFTM) of VKRs and other VFTM-containing receptors resulted in the identification of a monophyletic VKR clade which is closely resembling the γ-aminobutyric acid type B receptors (GABABR)4. VFTMs of class C G protein-coupled receptors (GPCRs), including GABABR, bind small ligands such as Ca2+, GABA and other amino acid transmitters, and small sugars6. Class C GPCRs that bind amino acids contain a consensus motif of 8 residues in their VFTM, which can be found in most VKRs2. In vitro kinase assays have shown that the VKR of Apis mellifera and SmVKR1 are activated by L-Arg, while SmVKR2 is activated by Ca2+ 3,4. Moreover, for the amino acid recognition by VKRs the serine and arginine residues of the conserved 8 residue motif are required2. Very recently, the VKR of Aedes aegypti was also deorphanized and surprisingly AaVKR was shown to bind to the rather ‘large’ ovary ecdysteroidogenic hormone (OEH)5. The OEH gene encodes a 149-residue preprohormone that is processed into an 86-residue peptide, which belongs to the neuroparsin family7.
Little is known about the role of VKRs, probably due to the absence of VKRs in D. melanogaster, C. elegans and vertebrates. Very recently, two papers were published implicating a role for VKRs in female reproductive physiology. Vanderstraete and coworkers8 have shown that SmVKRs control female reproduction in S. mansoni, since RNA interference (RNAi)-mediated silencing of SmVKR resulted in disorganization of the ovary and defective egg formation. Also in A. aegypti VKR seems to be required for egg formation5. In mosquitoes, juvenile hormone (JH) is responsible for preparing the fat body for production of vitellogenin. After a blood meal, OEH stimulates ecdysteroid production by the ovaries, which in turn stimulates vitellogenin production by the fat body. RNAi-mediated knockdown of AaVKR resulted in significantly lower ecdysteroid and vitellogenin production, leading to disabled egg formation. Another proof of AaVKR being the OEH receptor, was the lack of vitellogenin biosynthesis in the dsVKR treated females upon injection of OEH5.
This chapter focuses on the role of SgVKR in the adult female desert locust, S. gregaria. This ravenous swarm-forming phytophagous pest insect is a serious threat to the agricultural production in some of the world’s poorest countries, as swarms may contain thousands of millions of individuals eating everything on their path9. Contrary to A. aegypti, vitellogenin biosynthesis in S. gregaria is solely under the regulation of JH. Furthermore, neuroparsins act as anti-gonadotropic factors in S. gregaria, while in A. aegypti the neuroparsin-like OEH exerts a gonadotropic role. Given these differences in the regulation of female reproductive physiology between A. aegypti and S. gregaria, this study represents a valuable asset to better understand the role of the VKR subfamily throughout evolution. Here we report the cloning of SgVKR, as well as an in-depth tissue distribution of this receptor in adult female locusts, using qRT-PCR. Furthermore, using RNAi, we investigate the possible cross-talk of this receptor with several major hormonal pathways involved in female reproductive physiology, as well as its role in the maturation of oocytes, copulation behavior and post-copulation events.
Materials and Methods
Rearing of animals
The desert locusts, S. gregaria, were reared under crowded conditions (>200 locusts/cage) at constant temperature (32 ± 1 °C), constant day/night cycle (13:11 h photoperiod) and ambient relative humidity between 40% and 60%. The locusts were fed daily ad libitum with fresh cabbage leaves, supplemented with dry oat flakes. Following mating, females deposited their eggs in pots filled with a slightly moistened sand mixture (7 parts sand, 3 parts peat and 1 part water). Once a week these pots were collected and set apart in empty cages, where eggs were allowed to hatch into first instar larvae. In the described experiments, locusts were synchronized on the day of ecdysis into the adult stage. For the RNA interference experiments, locusts were injected one day after ecdysis, boost injections were given five, nine and thirteen days after ecdysis. Some locusts were dissected 12 days after ecdysis, while the others were observed for copulation behavior and post-copulation effects. Different experimental groups (distinctly labelled) were kept together in the same cage.
Tissue collection
The locust tissues of interest were dissected under a binocular microscope and rinsed in locust Ringer solution (1 L: 8.766 g NaCl; 0.188 g CaCl2; 0.746 g KCl; 0.407 g MgCl2; 0.336 g NaHCO3; 30.807 g sucrose; 1.892 g trehalose). Tissues were immediately pooled in MagNA Lyser Green Beads Tubes (Roche) or RNase-free Screw Cap Microcentrifuge tubes and snap-frozen in liquid nitrogen to prevent RNA degradation. Tissues for the tissue and temporal expression profile of SgVKR were collected in three independent pools consisting of five or six animals each. For the RNA interference experiments, tissues were collected in five independent pools consisting of three animals each. Tissues were stored at −80 °C until further processing.
RNA extraction and cDNA synthesis
Depending on the tissue, different RNA extraction methods were used. Brain and optic lobes, fat body, Malpighian tubules, male reproductive system (testes + accessory glands), female gonads (ovaria) and gut were transferred to MagNA Lyser Green Beads Tubes (Roche) and homogenized using a MagNa Lyser instrument (1 min, 6500 rpm; Roche). Subsequently, total RNA was extracted from these tissue homogenates using the RNeasy Lipid Tissue Kit (Qiagen) according to the manufacturer’s protocol. A DNase treatment (RNase-Free DNase set, Qiagen) was performed to eliminate potential genomic DNA contamination. Because of the relatively small size of the prothoracic glands (PG), corpora allata (CA) and corpora cardiaca (CC), and the ventral nerve cord (VNC), total RNA from these tissues was extracted using the RNAqueous-Micro Kit (Ambion) according to the manufacturer’s protocol. The manufacturer’s recommended DNase step was subsequently performed. Purity and concentration of the resulting RNA samples were checked using a Nanodrop spectrophotometer (Nanodrop ND-1000, Thermo Fisher Scientific, Inc.). For each RNA sample, cDNA was synthesized by reverse transcription of 500 ng of RNA with the PrimeScript™ RT reagent Kit (Perfect Real Time) (Takara, Invitrogen Life Technologies), using both random hexamer primers and oligo(dT) primers, according to the manufacturer’s protocol. The 10 µL reaction was diluted sixteen-fold with Milli-Q water (Millipore).
Molecular cloning of SgVKR
The full length sequence for the S. gregaria orthologue of VKR was present in our in-house (unpublished) S. gregaria transcriptome database. The complete sequence was amplified by PCR using adult S. gregaria fat body cDNA of 12-day-old adult female locusts, primers (listed in Table 1) and Q5® High-Fidelity DNA Polymerase (New England BioLabs® Inc.). Following thermal cycling profile was applied: 94 °C for 3 min; 35 cycles of 94 °C for 30 s, 64 °C for 30 s and 72 °C for 2,5 min; 72 °C for 10 min. The amplification products were separated using horizontal agarose gel electrophoresis and visualized using UV. The band of the expected size was cut out and further purified using the GenElute™ Gel extraction Kit (Sigma-Aldrich Co.). The resulting DNA fragment was cloned into a pCR4-TOPO vector using the TOPO® TA Cloning Kit (Invitrogen). Finally the sequence of the insert was confirmed using Sanger sequencing. The protein architecture was analyzed using the SMART software (http://smart.embl-heidelberg.de/).
Table 1.
Oligonucleotide sequences for primers used in cloning and sequence analysis of SgVKR.
| Forward primer | Reverse primer | |
|---|---|---|
| SgVKR | 5′-CTCCAGGAGCAGGACATCAC-3′ | 5′-TCAAAGAATGGAGTCCACTTGCTTAATTC-3′ |
Abbreviations: VKR = venus kinase receptor.
Quantitative real-time PCR (qRT-PCR)
Primers used in the qRT-PCR profiling are given in Table 2. These primers were validated by designing relative standard curves for gene transcripts with serial dilutions (5x) of female gonads, fat body or corpora allata/corpora cardiaca (CA/CC) complex cDNA. All qRT-PCR reactions were performed in duplicate in 96-well plates on a StepOne System (ABI Prism, Applied Biosystems). Each reaction contained 5 µL Fast SYBR® Green Master Mix (Applied Biosystems), 0.5 µL Forward and Reverse primer (10 µM) and 4 µL previously diluted cDNA. Following thermal cycling profile was used: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Finally, a dissociation protocol was performed, allowing melt curve analysis to check for primer dimers. Only a single melting peak was found for all transcripts. Additionally, amplification products were analyzed using horizontal agarose gel electrophoresis and visualized using UV. Only a single band could be seen which was further cloned and sequenced (TOPO® TA cloning kit for sequencing, Invitrogen) to confirm target specificity. Suitable reference genes were selected from a pool of candidate reference genes by means of the geNorm software10,11. “No template control” reactions confirmed there was no contamination. All qRT-PCR results were normalized to the relative quantity of the selected reference genes (indicated in figure legends) and calculated relative to the transcript level of a calibrator sample according to the comparative Ct method10. qRT-PCR was used to determine the tissue and temporal distribution of SgVKR in the adult stage. The cDNA samples were derived from adult female locusts, with exception of the male reproductive system. Moreover, qRT-PCR was performed to determine the knockdown efficiency of SgVKR as well as the effect on several genes of interest. GraphPad Prism 6 (GraphPad Software Inc.) was used to test the statistical significance of the observed differences for the RNAi experiments. Normalized relative quantities were log-transformed to allow the use of parametric statistical tests.
Table 2.
Oligonucleotide sequences for primers used in qRT-PCR.
| Reference genes | Forward primer | Reverse primer |
|---|---|---|
| Target genes | Forward primer | Reverse primer |
| α-tubulin1A | 5′-TGACAATGAGGCCATCTATG-3′ | 5′-TGCTTCCATACCCAGGAATGA-3′ |
| CG13220 | 5′-TGTTCAGTTTTGGCTCTGTTCTGA-3′ | 5′-ACTGTTCTCCGGCAGAATGC-3′ |
| Ubi | 5′-GACTTTGAGGTGTGGCGTAG-3′ | 5′-GGATCACAAACACAGAACGA-3′ |
| GAPDH | 5′-GTCTGATGACAACAGTGCAT-3′ | 5′-GTCCATCACGCCACAACTTTC-3′ |
| RP49 | 5′-CGCTACAAGAAGCTTAAGAGGTCAT-3′ | 5′-CCTACGGCGCACTCTGTTG-3′ |
| β-actin | 5′-AATTACCATTGGTAACGAGCGATT-3′ | 5′-TGCTTCCATACCCAGGAATGA-3′ |
| EF1α | 5′-GATGCTCCAGGCCACAGAGA-3′ | 5′-TGCACAGTCGGCCTGTGAT-3′ |
| SgVKR | 5′-GCATCTTGGCATTGATTTGCTA-3′ | 5′-GGAATCTCCCATTTGTCAAGAGTT-3′ |
| SgSpo | 5′-CAACATCTTCACCAGCTACATGTG-3′ | 5′-GGGTCGTCGTAGTCGAAGGA-3′ |
| SgPhm | 5′-CGCAGAGCCCGGACAAC-3′ | 5′-CGAACATGTCGGCCATGA-3′ |
| SgDib | 5′-TAGCTGGAATGGACACAACATC-3′ | 5′-CTGGGTCTGGAAAATACTCTGG-3′ |
| SgSad | 5′-ATCGTGGCCGAGATTACGAA-3′ | 5′-AGCACCATCTCCGGATCCT-3′ |
| SgShd | 5′-CCGCCGTCATTGACTTCATA-3′ | 5′-GTGAGCTCCCAAGCGTGG-3′ |
| SgVg1 | 5′-CCGCTGAACATCACTGCAAT-3′ | 5′-ACTTGGGCCAAATGGATGAG-3′ |
| SgVg2 | 5′-GCTACCCGCAATCTGTAAAATACA-3′ | 5′-CGACTGTGAAAGGGCATTGA-3′ |
| SgKr-h1 | 5′-CTCCAAGACGTTCATCCAGAG-3′ | 5′-TGCTTGGAGCAGGTGAAG-3′ |
| SgJHAMT | 5′-CGGAGCAAAGGCAAGCA-3′ | 5′-CCACTTCACCGCCTGGTTT-3′ |
| SgCYP15A1 | 5′-AAAGCAACTTCATCATTCACAGATG-3′ | 5′-CAGAGCCAGCCATGAACAAA-3′ |
Abbreviations: Ubi = ubiquitin conjugating enzyme 10, GAPDH = glyceraldehyde-3-phosphate dehydrogenase, RP49 = ribosomal protein 49, EF1α = elongation factor 1 alpha, VKR = venus kinase receptor, Spo = spook, Phm = phantom, Dib = disembodied, Sad = shadow, Shd = shade, Vg = vitellogenin, Kr-h1 = Krüppel-homolog 1, JHAMT = juvenile hormone acid methyltransferase, CYP15A1 = methyl farnesoate epoxidase.
RNA interference experiments
Production of dsRNA
dsRNA constructs for SgVKR were produced using the MEGAscript® RNAi Kit (Ambion) according to the manufacturer’s protocol. This procedure is based on the high-yield transcription reaction of a user-provided linear transcript with a T7 promoter sequence. Forward and reverse primers flanked by the T7 promoter sequence were (Table 3) used in a PCR reaction with REDTaq® DNA polymerase (Sigma-Aldrich Co.) to amplify a fragment of the target gene (453 or 530 bp). PCR products were analyzed using horizontal agarose gel electrophoresis and visualized using UV. Only a single band could be seen which was further cloned and sequenced (TOPO® TA cloning kit for sequencing, Invitrogen) to confirm target specificity. Since only one band was observed after gel electrophoresis, the PCR product could directly be used in the RNA polymerase transcription reaction. The purity and concentration of the produced dsRNA was determined by means of spectrophotometry (Nanodrop ND-1000). To confirm dsRNA integrity, a small amount of the reaction product was checked on an agarose gel.
Table 3.
Oligonucleotide sequences for primers used in dsRNA construct design. Underlined sequences are the T7 promoter sequences.
| Target genes | Forward primer | Reverse primer |
|---|---|---|
| SgVKR1 | 5′-TAATACGACTCACTATAGGGAGAAAGGGTTGCTGCATTGACTG-3′ | 5′-TAATACGACTCACTATAGGGAGATCCACACTGCATCATAGGCA-3′ |
| SgVKR2 | 5′-GAAATTAATACGACTCACTATAGGGCCCACTGTGTATGGAGGAGAA-3′ | 5′-GAAATTAATACGACTCACTATAGGGCCTCAAACATTGGCCTTGTC-3′ |
| GFP | 5′-TAATACGACTCACTATAGGGAGA AAGGTGATGCTACATACGGAA-3′ | 5′-TAATACGACTCACTATAGGGAGA ATCCCAGCAGCAGTTACAAAC-3′ |
Abbreviations: VKR = venus kinase receptor, GFP = green fluorescent protein.
RNAi experiment
Adult female locusts were injected in their abdomen one, five, nine and thirteen days after final ecdysis with 200 ng (in 10 µL Ringer solution) of dsRNA against SgVKR. Control locusts were injected with a GFP dsRNA construct (200 ng in 10 µL locust Ringer solution) following the same treatment plan. A first group of locusts was dissected 12 days after ecdysis to check the knockdown efficiency and the effect of the knockdown on the oocyte size, ecdysteroid titers in the hemolymph, ecdysteroid levels in the gonads, and the transcript levels of other genes of interest. A second group of locusts was used to observe copulation behavior and post-copulation events.
Ecdysteroid measurements using an enzyme immunoassay (EIA)
Ecdysteroid titers in S. gregaria hemolymph and ecdysteroid levels in the female gonads were measured using an enzyme immunoassay, modified from Porcheron et al.12 and discussed by Pascual et al.13 and Lafont et al.14. In this protocol a peroxidase conjugate of 20-hydroxy-ecdysone (20E) was used as tracer together with rabbit L2 polyclonal antibodies. This L2 antiserum has a strong affinity for ecdysone (E), 3-deoxyecdysone and 2-deoxyecdysone and a 6- to 8-fold lower affinity for 20E. Both serum and tracer were very kindly given by Prof. J.P. Delbecque (Université de Bordeaux, France). Hemolymph samples were collected from 12-day-old adult female locusts by piercing the insect’s cuticle behind its hind leg and holding a capillary to the wound. 10 µL of hemolymph was collected from each animal, which was immediately transferred to 90 µL of cold ethanol (100%) and stored at −20 °C until further processing, as described by Marchal et al.15. For the sampling of the gonads, one ovarium was dissected from each 12-day-old adult female locust and transferred to 500 µL of cold ethanol (100%) and stored at −20 °C until further processing as described by Van Wielendaele et al.16. Both ‘free’ and total ecdysteroid levels were determined. ‘Free’ ecdysteroids are the non-conjugated ecdysteroids. The total ecdysteroid levels were determined after enzymatic conversion of the conjugated ecdysteroids in ‘free’ ecdysteroids. The standard curve used in all measurements was obtained with 20E, and therefore results are expressed as 20E equivalents. GraphPad Prism 6 (GraphPad Software Inc.) was used to test the statistical significance of the observed differences.
Copulation behavior and post-copulation effects
To investigate the effect of the RNAi-mediated knockdown of SgVKR on the first display of copulation behavior and fertility, the locusts were injected as described in ‘RNA interference experiments’. Until day 8 control and experimental female locusts were kept with non-treated male locusts of the same age. As of day 8 female locusts were kept in individual small transparent cages. Starting on day 10 the females were combined daily with mature virgin male locusts and allowed to mate. When mating occurred, the male stayed another 22 hours with the female. When no mating was observed, the males were removed from the cage. We only observed actual copulation, i.e. attachment of the male and female genitalia. After mating the females were supplied with pots filled with a humid sand/turf mixture, necessary for oviposition. These pots were checked daily for egg pods and the total number of eggs per egg pod was counted (to analyze fecundity). Eggs were allowed to hatch by placing them on the humid sand/turf mixture and covering them with approximately 1 cm of this mixture. Hatching was observed daily and the total number of hatched larvae was counted in order to determine the percentage of hatchlings (to analyze fertility).
Results
Cloning and sequence analysis of SgVKR
The complete SgVKR open reading frame (ORF) was found in the in-house S. gregaria transcriptome database and was fully confirmed by Sanger sequencing of the PCR amplicon acquired with the primers listed in Table 1. The SgVKR ORF (GenBank acc. no. KY273096) comprises 3834 nucleotides encoding a protein consisting of 1277 amino acids (Fig. 1). Based on similarities with previously predicted and identified VKRs2,4, we identified the typical VFTM and TK domains (Fig. 1, grey and black highlights). The VFTM contains the consensus motif of 8 residues (Fig. 1, red AA) that is known to participate in the binding of natural amino acids or their derivatives to class C GPCRs. Furthermore, the TK domain of SgVKR is composed of essential motifs (GxGxxG, VAVKx16E, HRDVxxRNxL, DFG, YY and PVRWMxPE) required for TK activity (Fig. 1, green AA)17.
Figure 1.
Sequence and structure of SgVKR. Left: Schematic representation of a VKR, composed of different domains: VFTM = Venus FlyTrap module, TM = transmembrane domain and TK = tyrosine kinase domain (adapted from Ahier et al.)4. Right: Amino acid sequence of SgVKR. The complete SgVKR sequence (GenBank acc. no. KY273096) was found in the in-house S. gregaria transcriptome database (our unpublished data) and was confirmed with PCR and Sanger sequencing. The VFTM is highlighted in grey, the TM domain is highlighted in yellow and the TK domain is highlighted in black. Underlined residues are conserved in most VKR sequences and those also in bold and red correspond to the amino-acid binding motif of VFTM29. The residues in green, bold and underlined are consensus sequences required for TK activity17.
Transcript level studies
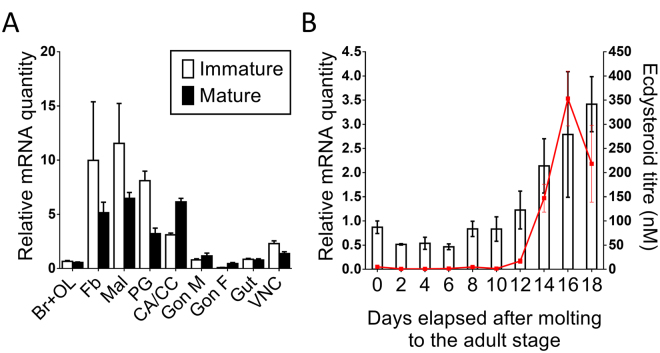
Tissue and temporal transcript profiles of SgVKR were determined using qRT-PCR. The tissue distribution profile has been studied in immature and mature female tissues, as well as in the male reproductive system (Fig. 2A), while the temporal distribution profile has been studied in the female gonads, every other day throughout the first reproductive cycle (Fig. 2B).
Figure 2.
Tissue and temporal distribution of SgVKR. (A) Tissue distribution of SgVKR in immature (white) and mature (black) adult locusts. Relative transcript levels of SgVKR were measured in different adult tissues using qRT-PCR. All tissues, except the male reproductive system (Gon M), were dissected from immature, 3-day-old female locusts or mature female locusts with an oocyte size between 3 and 6 mm. The male reproductive system was dissected from immature, 3-day-old and mature, 12-day-old male locusts. The data represent mean ± S.E.M. of three independent pools of five animals, run in duplicate and normalized to α-tubulin1A, CG13220 and ubiquitin transcript levels. Abbreviations X-axis: Br + OL: brain + optic lobes; Fb: fat body; Mal: Malpighian tubules; PG: prothoracic glands; CA + CC: corpora allata + corpora cardiaca; Gon M: male reproductive system (testes + accessory glands); Gon F: female gonads (ovaries); Gut: for-, mid- and hindgut; VNC: ventral nerve cord. (B) Temporal distribution profile of SgVKR in the female gonads during the first reproductive cycle. Using qRT-PCR, relative transcript levels of SgVKR were measured every other day in the female gonads, starting on the day of molting to the adult stage (day 0). Data represent mean ± S.E.M. of three independent pools of six animals, run in duplicate and normalized to CG13220 and ubiquitin transcript levels. The ecdysteroid titre (red line), expressed in nM, throughout the first reproductive cycle was measured with an EIA. The data represent mean ± S.E.M. of 6-18 hemolymph samples taken from different animals.
The tissue distribution of SgVKR (Fig. 2A) shows a wide profile, with the highest transcript levels in the fat body, the Malpighian tubules, the prothoracic glands and the CA/CC complex. The transcript levels of SgVKR are significantly higher in the prothoracic glands and the ventral nerve cord of immature females compared to mature females, while they are significantly lower in the CA/CC complex and gonads of immature females compared to mature females.
Given the significantly higher expression of SgVKR in mature female gonads, and the fact that some VKRs play an important role in female reproductive physiology of other invertebrate species5,8, we investigated the temporal expression profile of the SgVKR transcript in the female gonads throughout the first reproductive cycle (Fig. 2B). We observed an increase in transcript levels towards the end of the first gonadotrophic cycle, suggesting a possible role for this receptor in locust reproduction.
RNA interference of SgVKR
To investigate the role of SgVKR in the female reproductive physiology, the RNAi technique was used to silence SgVKR. To eliminate possible off-target effects two separate dsRNA constructs were designed for SgVKR, one in the VFTM and one in the TK domain. Similar results were obtained with both constructs. Female locusts were injected with 200 ng dsRNA against SgVKR or GFP (control) one, five, nine and thirteen days after molting to the adult stage. One group of locusts was sacrificed on day 12 to investigate the knockdown efficiency and the effect of the knockdown on the oocyte size, ecdysteroid biosynthesis and the transcript levels of several genes of interest. The other group of locusts was used to investigate the effect of the knockdown on copulation behavior and fertility.
Knockdown efficiency
The knockdown efficiency of SgVKR was investigated in different tissues of 12-day-old adult female locusts, using qRT-PCR (Suppl. Figure 1). A reduction of 80%, 69% and 65% in relative mRNA levels of SgVKR could be measured in respectively the ovaries, the fat body and the CA/CC complex. From these significant reductions it can be concluded that SgVKR was successfully silenced.
Effect on reproductive physiology
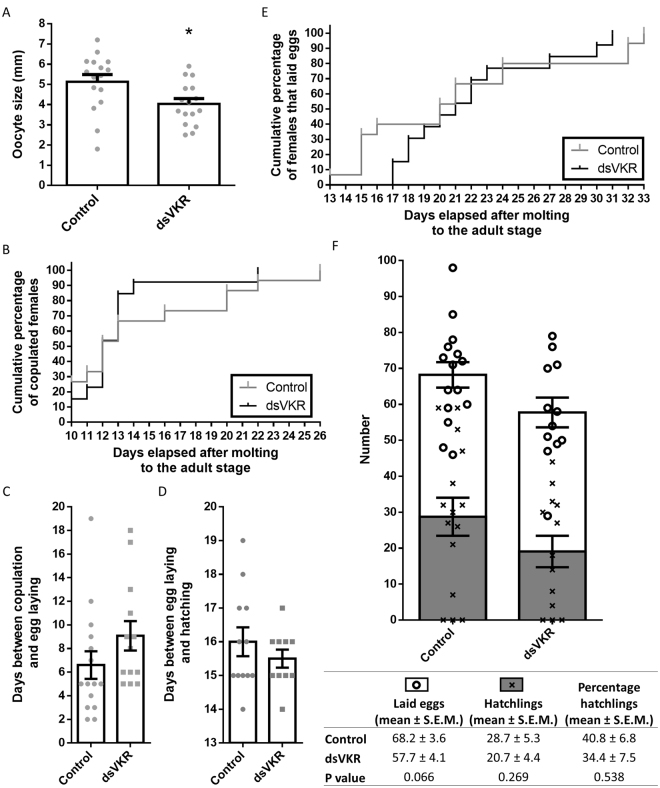
Knockdown of SgVKR resulted in significantly smaller oocytes in 12-day-old adult locusts (Fig. 3A). The average oocyte size of dsVKR-treated animals was 4.0 mm, while the control animals had an average oocyte size of 5.1 mm. We further investigated if copulation behavior, fecundity and fertility were affected after silencing SgVKR. Therefore, we observed the timing of actual copulation with a virgin male, egg laying and hatching, as well as the numbers of eggs and hatchlings. No effects of the SgVKR knockdown could be observed on copulation behavior, fecundity or fertility (Fig. 3B-F).
Figure 3.
Effect of RNAi-mediated knockdown of SgVKR on the oocyte size in 12-day-old S. gregaria and on copulation behavior, fecundity and fertility. Locusts were injected with 200 ng of dsRNA against SgVKR or GFP (control) one, five, nine and thirteen days after molting to the adult stage. (A) For each 12-day-old female locust the average size of five oocytes was calculated. The data represent mean ± S.E.M. of the average oocyte sizes of sixteen individual locusts (bars), as well as the average oocyte size of the individual locusts (grey dots) (N = 16). The significant difference (p < 0.05) is indicated with an asterisk (*) (two-sided unpaired t-test). (B) Starting from day 10 of the adult stage the females were assayed for displaying mating behavior, by allowing them to mate with mature virgin males. The cumulative percentage of females that mated is presented (Log-rank (Mantel-Cox) test; N = 13-15; p-value = 0.5045). (C-E) Mated females were allowed to lay eggs. The days between copulation and egg laying (C), as well as between egg laying and hatching (D) were observed. The data represent mean ± S.E.M. (bars) (t-test; N = 13-15). (E) The cumulative percentage of females that laid eggs is displayed (Log-rank (Mantel-Cox) test; N = 13-15; p-value = 0.6993). (F) The number of eggs per egg pod was counted, as well as the number of hatchlings per egg pod. The data represent mean ± S.E.M. (bars), as well as the individual number of eggs (Ο) or hatchlings (x) per egg pod (t-test; N = 13-15).
Effect on ecdysteroid biosynthesis
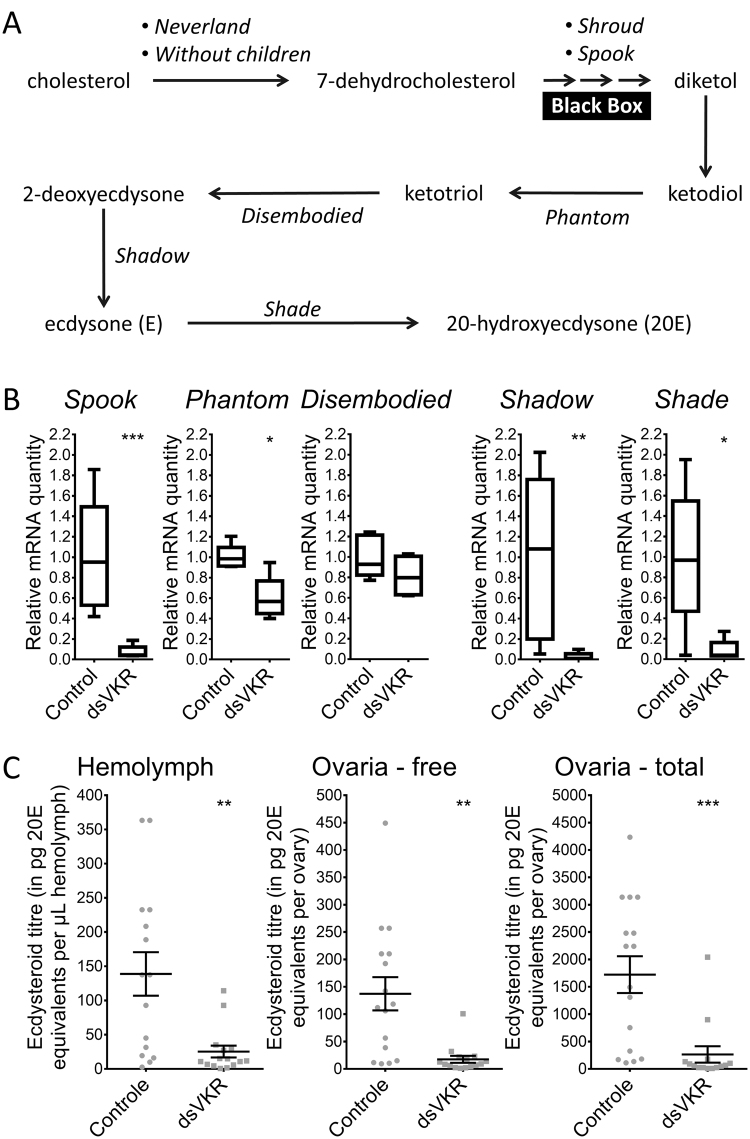
Since silencing of AaVKR affected the ecdysteroid biosynthesis5, we investigated if this was also the case in S. gregaria. We first checked the transcript levels of the known Halloween genes, Spook (SgSpo), Phantom (SgPhm), Disembodied (SgDib), Shadow (SgSad) and Shade (SgShd), which are involved in ecdysteroid biosynthesis by the female gonads (Fig. 4A). The relative mRNA levels of SgSpo, SgPhm, SgSad and SgShd, were significantly lower in 12-day-old adult females treated with dsRNA against SgVKR compared to control females (Fig. 4B). Most of the ecdysteroids produced by the gonads are incorporated in the growing oocytes as conjugates, while only a small fraction of the ecdysteroids is incorporated in the oocytes as ‘free’ ecdysteroids. Furthermore, a small fraction of the ovary-produced ecdysteroids will reach the hemolymph. We measured the ecdysteroid titer in the hemolymph, as well as the free and total ecdysteroid levels in the ovaries, of dsVKR-treated and control 12-day-old female locusts, and observed significantly lower ecdysteroid levels in the dsVKR-treated females compared to control females (Fig. 4C).
Figure 4.
Effect of RNAi-mediated knockdown of SgVKR on ecdysteroid biosynthesis in 12-day-old adult female S. gregaria. (A) Overview of the ecdysteroid biosynthesis pathway in larval D. melanogaster. (B) Relative transcript levels of the Halloween genes, SgSpo, SgPhm, SgDib, SgSad and SgShd, involved in ecdysteroid biosynthesis were measured in the gonads from control and dsVKR-treated 12-day-old female locusts, using qRT-PCR. The data represent box plots (min to max) of five independent pools of three locusts, run in duplicate and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and α-tubulin1A transcript levels. (C) Ecdysteroid titers in the hemolymph and ecdysteroid levels (free and total) in the gonads of 12-day-old control and dsVKR-treated female locusts. Ecdysteroid titers, expressed in pg 20E equivalents per µL hemolymph, and ecdysteroid levels, expressed in ng 20E equivalents per ovary, were measured with an EIA. The data represent mean ± S.E.M. of individual animals (N = 16), as well as the individual values (grey dots). Locusts were injected with 200 ng of dsRNA against SgVKR or GFP (control) one day, five days and nine days after molting to the adult stage. Significant differences (p < 0.05, p < 0.01 and p < 0.001) are indicated by (an) asterisk(s) (*, ** and *** respectively) (two-sided Welch’s t-test on log-transformed data).
Effect on expression of neuroparsins and insulin-related peptide
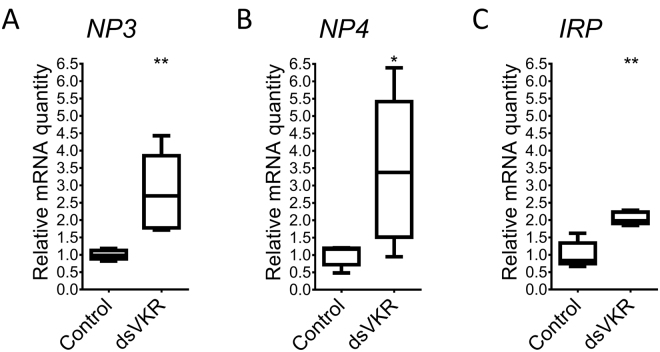
In vitro activation of AaVKR by OEH, a neuroparsin (NP)-like peptide, results in phosphorylation of Ak strain transforming factor (Akt/PKB), a Ser/Thr protein kinase which also functions downstream of the insulin receptor (InR)5. We reasoned that if SgVKR is indeed a receptor for locust NPs and/or affects processes that are also regulated by insulin-related peptide (IRP), we might observe changes in expression of NPs and/or IRP as a result of the RNAi-mediated downregulation of SgVKR. Our data show that silencing of SgVKR resulted in significantly higher relative mRNA levels of SgNP3, SgNP4 and SgIRP in the fat body of 12-day-old adult female locusts (Fig. 5). When compared to control animals, neuroparsin transcripts were about 3 times upregulated, while SgIRP mRNA levels were ca. 2-fold higher in the fat body of dsVKR-treated animals.
Figure 5.
Effect of RNAi-mediated knockdown of SgVKR on transcripts coding for neuroparsins and insulin-related peptide in 12-day-old adult female S. gregaria. Relative (A) SgNP3, (B) SgNP4 and (C) SgIRP transcript levels were measured in the fat body from control and dsVKR-treated 12-day-old female locusts, using qRT-PCR. Locusts were injected with 200 ng of dsRNA against SgVKR or GFP (control) one, five and nine days after molting to the adult stage. The data represent box plots (min to max) of five independent pools of three locusts, run in duplicate and normalized to GAPDH and ribosomal protein 49 (RP49) transcript levels. Significant differences (p < 0.05 and p < 0.01) are indicated by (an) asterisk(s) (* and ** respectively) (two-sided Welch’s t-test on log-transformed data).
Effect on vitellogenesis and JH biosynthesis
To investigate whether the smaller oocyte size was due to reduced vitellogenin biosynthesis, we analyzed the transcript levels of the vitellogenin 1 and 2 genes (SgVg1 and SgVg2) after silencing SgVKR (Suppl. Figure 2A,B). Furthermore, we also checked the transcript levels of the enzymes responsible for the last two steps in juvenile hormone (JH) biosynthesis as well as the JH response gene Krüppel-homologue 1 (SgKr-h1), since it is known that JH regulates vitellogenesis in S. gregaria (Suppl. Figure 2C-G). Although the relative mRNA levels of SgVg1 and SgVg2 seem lower (resp. 42% and 36%) in the fat body of 12-day-old adult females treated with dsVKR, they were not significantly different when statistically compared to control females. Similar results were obtained for the relative mRNA levels of the JH biosynthesis enzymes, SgJHAMT (juvenile hormone acid methyltransferase) and SgCYP15A1 (methyl farnesoate epoxidase), in the CA/CC complex (resp. 34% and 40% lower on average, but not significantly). When looking at the SgKr-h1 mRNA levels in the fat body, gonads and CA/CC complex, a reduction of 42%, 25% and 56% respectively could be calculated for dsVKR-treated females when compared to controls. The reduction of SgKr-h1 transcript levels was only significantly different in the ovaries and the CA/CC complex.
Discussion
Characteristics of SgVKR
VKRs are members of the receptor tyrosine kinase (RTK) family, which are transmembrane proteins involved in many fundamental cellular processes, such as cell proliferation and differentiation18. Like previously identified VKRs2,4, the SgVKR consists of a VFTM and a TK domain. The VFTM of the SgVKR contains the 8 residue motif crucial for binding of amino acids (Fig. 1)2,4. Vanderstraete et al.2 also confirmed the requirement of the serine and arginine residues in this motif for amino acid recognition by AmVKR and SmVKR1. These two residues are also present in the conserved motif of the VFTM of SgVKR, hence a natural amino acid or derivative might be the ligand of SgVKR. In spite of the presence of the serine and arginine residues in the conserved 8 residue motif in the VFTM of AaVKR, this mosquito receptor was only activated upon binding of OEH5. However, it cannot be excluded that a small molecule was already bound to the VFT module of this receptor, as discussed by Dissous19. Since OEH displays sequence similarities with neuroparsins, a deducible hypothesis could be that locust neuroparsins may act as natural agonists of SgVKR. An alternative hypothesis is that at some point in evolution a neuroparsin-like ligand may have been “hijacked” by a member of the VKR subfamily20. If so, then this evolutionary event has determined in which animal species a functional interaction between neuroparsins and VKRs is occurring. Since ligands of VKRs have only been demonstrated in A. mellifera, A. aegypti and S. mansoni, it is difficult to predict whether (an) amino acid(s) and/or (a) neuroparsin(s) can activate SgVKR. The TK domain contains all motifs crucial for TK activity, such as the G-rich motif GxGxxG important in ATP binding, the VAVKx16E motif necessary for ATP stabilization, the HRDxAxRN motif forming the catalytic loop implicated in phosphotransfer, the triplet DGF for Mg2+ binding, the consensus PVRWMxPE sequence and the two putative autophosphorylation sites (YY)2. We can thus conclude that SgVKR belongs to the RTK family and more specifically to the VKR subfamily.
Role of SgVKR in female locust reproductive physiology
VKRs play an important role in the female reproductive physiology of S. mansoni and A. aegypti, as RNAi of SmVKR1/2 and AaVKR resulted in disabled egg formation5,8. Silencing of AaVKR resulted in less yolk per oocyte and lower ecdysteroid production by the ovary, because of disabled OEH activity5. In this mosquito, a blood meal triggers the release of OEH by the brain, which then induces the ovarian production of ecdysteroids that subsequently stimulate the fat body to produce the vitellogenins that are packaged into the oocytes. On the other hand, as in cockroaches, JH stimulates the fat body in locusts to produce vitellogenins, which will be taken up by the oocytes, while ecdysteroids produced by the ovaries are mainly incorporated into the growing terminal oocytes and are only released to a limited extent into the hemolymph towards the end of the reproductive cycle, resulting in choriogenesis and egg laying (Hult et al.21; Marchal et al.,22–28; Martín et al.,23, our unpublished data). After RNAi-mediated knockdown of SgVKR, we only observed smaller oocytes in 12-day-old adult female locusts (Fig. 3), but did not observe an effect on copulation behavior and post-copulation events. The fecundity or fertility of the female locusts were not affected when silencing SgVKR. Thus, in S. gregaria VKR does not seem to play a role that is as crucial as it is the case for S. mansoni and A. aegypti. It should also be noted that the locusts used in these experiments were fed ad libitum and kept under optimal breeding conditions. Consequently, the regulation of oocyte growth in locusts reared in these laboratory conditions might be different from the situation occurring in nature. Contrary to A. aegypti, where a blood meal triggers the start of oogenesis, almost like a binary switch, production of mature oocytes in locusts that occur in natural conditions may take two weeks till a few months, depending on amongst others temperature, humidity and nutrient availability9. Therefore, it is not because VKR does not seem to play a crucial role in these laboratory locusts, which are kept under optimal breeding conditions, that this receptor might not play a more prominent role in locusts in their natural habitat, which are highly dependent on different input signals for initiating their reproductive cycle. Furthermore, one should also keep in mind that an efficient knockdown at mRNA level does not mean that protein levels are equally lowered. Hence, there might still be enough residual VKR protein to (partially) fulfill its role, making it harder to judge the necessity of this receptor in the reproductive process.
Cross-talk between SgVKR and different hormonal pathways
This is the first report of an in-depth tissue distribution profile of SgVKR in insects. In A. aegypti, the expression of AaVKR was investigated in only three tissues, namely the fat body, the gut and the ovaries of female mosquitoes. While VKRs are highly expressed in the ovaries of female S. mansoni and A. aegypti 5,8, the relative mRNA levels of SgVKR are low in the locust ovaries, compared to several other tissues (Fig. 2B). Interestingly, when looking at the temporal expression profile of SgVKR in the female ovaries, an increase could be observed towards the end of the first reproductive cycle. This profile is similar to the temporal expression profile of two ecdysteroid biosynthesis enzymes, SgSpo and SgPhm 15 and correlates with the hemolymph ecdysteroid titre, suggesting this receptor could possibly regulate the ecdysteroid biosynthesis by the follicle cells surrounding the oocytes in the ovaries. When further investigating the involvement of SgVKR in the regulation of ecdysteroid biosynthesis, we observed that downregulation of SgVKR indeed resulted in lower ecdysteroid biosynthesis by the ovaries in 12-day-old adult female locusts (Fig. 4). Consequently, we hypothesize that SgVKR (directly or indirectly) regulates ecdysteroid biosynthesis in adult female S. gregaria. This result is similar to what was found in A. aegypti 5, but probably due to the highly divergent roles of ecdysteroids in both species, silencing VKR results in a very different effect on the female reproductive physiology. Although ecdysteroid biosynthesis is affected, no effect on choriogenesis was observed. One possible explanation for this lies in the fact that we did not perform a knockout but a knockdown. In case of a knockdown, dsRNA treatment effects may be transitory. Furthermore, compensatory effects cannot be excluded.
By affecting the ecdysteroid biosynthesis SgVKR appears to have a gonadotropic role, which is opposite to the anti-gonadotropic role of locust NPs24,25. Neuroparsin was initially discovered based on its inhibitory effects on vitellogenesis and oocyte growth, without directly affecting JH biosynthesis25. Recently the anti-gonadotropic role of NPs has also been confirmed in the shrimp Metapenaeus ensis 26. In A. aegypti on the other hand, the neuroparsin-like factor OEH has been demonstrated to act as a gonadotropic factor7. Based on sequence similarities with the insulin-like growth factor binding proteins (IGFBP), the hypothesis was postulated that locust NPs might act as humoral binding factors of IRP, which stimulates vitellogenesis and ecdysteroidogenesis. This hypothesis is supported by the observation of in vitro binding of a locust NP and IRP27. Therefore, locust NPs might act as negative modulators of the insulin signalling pathway, while the mosquito OEH acts in parallel with the insulin signalling pathway. Alternatively or additionally, as it is the case for several IGFBPs, NPs might also have direct functions of their own. For the latter option, one possible target could be SgVKR. However, if locust neuroparsins would indeed be physiological agonists of VKR, one would expect to observe identical phenotypic effects and this is not the case24. On the other hand, NPs could perhaps act as allosteric inhibitors of SgVKR in locusts, while the neuroparsin-like OEH acts as an activator of AaVKR in mosquitoes. The latter hypothesis might also explain the opposing roles of NPs and OEH in the female reproductive physiology of respectively S. gregaria and A. aegypti. Furthermore, it should also be noted that four NPs were identified in S. gregaria, but their specific roles are still unknown24. Hence, not all NPs might act in the same manner, which would generate a much more complex situation than in several other species. Given these different hypotheses, it will be necessary to pharmacologically characterize SgVKR.
One of the highest transcript levels of SgVKR was observed in the fat body. Since the fat body is known to be the major organ for controlling the insect’s energetic and nutritional homeostasis and for producing vitellogenins that are incorporated in maturing oocytes of adult females, it is not excluded that SgVKR plays a role as a nutrient sensor or more specifically an amino acid sensor. Studies in A. aegypti and S. mansoni have proven the involvement of VKRs in the activation of the PI3K/Akt/p70S6K and ERK/ MAPK pathways, which are known to act downstream of several RTKs, such as InRs, and which are involved in the control of protein synthesis, cell growth and proliferation5,8. To further analyse the possible links between SgVKR, SgNPs and IRP/InR, we examined if silencing SgVKR had an influence on transcript levels of SgNPs and SgIRP (Fig. 5). The data obtained for SgNP3, SgNP4 and SgIRP transcript levels indeed suggest that this receptor is involved in the regulation of these peptides. The smaller oocyte size observed in 12-day-old dsVKR-treated female locusts might perhaps be a consequence of the upregulation of SgNP3 and SgNP4, since NPs are known to have an anti-gonadotropic function24. The upregulation of SgIRP may compensate for this anti-gonadotropic effect of the SgNPs, but does not seem to be sufficient. These results again raise the question whether SgVKR acts as a NP receptor, as discussed in the previous paragraph.
Since smaller oocytes were observed in 12-day-old SgVKR knockdown female locusts, we investigated if this was due to lower vitellogenin biosynthesis by the fat body. Although we repeated this experiment several times, with both dsRNA constructs, no significant differences in SgVg1/2 transcript levels could be observed between dsVKR-treated and control female locusts (Suppl. Figure 2). As vitellogenesis in locusts is regulated by JHs, we also checked if the transcript levels of the last two enzymes in the JH biosynthetic pathway, SgJHAMT and SgCYP15a1 28, as well as the JH response gene Kr-h1, were altered in these dsVKR-treated female locusts (Suppl. Figure 2). Similarly as for SgVg1/2 transcript, no significant differences were observed for the JH biosynthesis enzymes. However, the Kr-h1 mRNA levels were significantly lower in the CA/CC complex and the gonads of 12-day-old dsVKR-treated female locusts, but not in the fat body. Based on these data, we cannot conclude that SgVKR affects JH biosynthesis in the CA or vitellogenin biosynthesis in the fat body. JH-mediated signaling via Kr-h1 seems to be reduced in some tissues of the SgVKR knockdown animals. These tissue-dependent effects might be caused by regulatory effects occurring downstream of JH biosynthesis and upstream of its signaling, for instance on JH degradation. The observation that SgVKR knockdown does not affect Kr-h1 transcript levels in the fat body is well in line with the data on SgVg1/2 expression. On the other hand, it should also be noted that the knockdown of SgVKR in the Fb and CA/CC complex was not as potent as in the ovaries. As such, it is possible that this resulted in absence of significant differences in fertility or in transcript levels of the vitellogenins and JH biosynthesis enzymes, when compared to the respective control conditions. Furthermore, it should also be noted that the dsRNA treatment in S. gregaria generates a systemic knockdown, so it is currently difficult to make a distinction between the direct and indirect in vivo roles of SgVKR.
Conclusion
We cloned the SgVKR from S. gregaria and investigated its in vivo role. By means of RNAi, we have shown a gonadotropic function for this receptor in adult female locusts. However, this locust VKR did not seem to be as crucial as the VKRs in S. mansoni and A. aegypti for controlling reproductive success in adult females. While SgVKR knockdown, as in A. aegypti, significantly reduced ovarian ecdysteroid biosynthesis, only a limited effect was observed on oocyte size and fertility was not affected. Although its mode of action is not yet entirely clarified, SgVKR does not show the same phenotypic effects as locust NPs. It is possible that SgVKR acts as a nutrient-dependent sensor in several tissues, where it may contribute to the complex coordination by different pathways that regulate the process of egg production in adult female S. gregaria.
Electronic supplementary material
Acknowledgements
The authors acknowledge Prof. Jean-Paul Delbecque (Université de Bordeaux, France) for his kind gifts of antiserum and tracer. A special thanks to Roger Jonckers and Evelien Herinckx for their technical assistance in the locust breeding. The authors are also very grateful to Dorien Cools for her practical assistance in the lab. The authors gratefully acknowledge the Agency for Innovation by Science and Technology (IWT), the Research Foundation of Flanders (FWO) and the Research Foundation of KU Leuven (GOA/11/02 and C14/15/050) for funding. CL was supported by a PhD fellowship of the IWT, RV was supported by a PhD fellowship of the FWO.
Author Contributions
Molecular cloning, dissections, transcript profiling and RNAi experiments were performed by C.L., J.P. and E.M. Transcriptome data assembly was performed by R.V. J.V. is the senior academic author. Guidance of the study, writing and correction of the manuscript were performed by C.L., E.M. and J.V.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-11434-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vicogne J, et al. An unusual receptor tyrosine kinase of Schistosoma mansoni contains a Venus Flytrap module. Mol. Biochem. Parasitol. 2003;126:51–62. doi: 10.1016/S0166-6851(02)00249-9. [DOI] [PubMed] [Google Scholar]
- 2.Vanderstraete M, et al. The venus kinase receptor (VKR) family: structure and evolution. BMC Genomics. 2013;14:361. doi: 10.1186/1471-2164-14-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouignard N, et al. Schistosoma mansoni: Structural and biochemical characterization of two distinct Venus Kinase Receptors. Exp. Parasitol. 2012;132:32–39. doi: 10.1016/j.exppara.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Ahier, A. et al. A new family of receptor tyrosine kinases with a venus flytrap binding domain in insects and other invertebrates activated by amino acids. PLoS One4 (2009). [DOI] [PMC free article] [PubMed]
- 5.Vogel KJ, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone requires a receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2015;112:9–10. doi: 10.1073/pnas.1501814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pin JP, Galvez T, Prézeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 2003;98:325–354. doi: 10.1016/S0163-7258(03)00038-X. [DOI] [PubMed] [Google Scholar]
- 7.Brown MR, et al. Identification of a steroidogenic neurohormone in female mosquitoes. J. Biol. Chem. 1998;273:3967–3971. doi: 10.1074/jbc.273.7.3967. [DOI] [PubMed] [Google Scholar]
- 8.Vanderstraete, M. et al. Venus kinase receptors control reproduction in the platyhelminth parasite Schistosoma mansoni. PLoS Pathog. 10 (2014). [DOI] [PMC free article] [PubMed]
- 9.Symmons, P. M. & Cressman, K. Desert Locust Guidelines. 42 (2001).
- 10.Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, research 0034.1–0034.11 (2002). [DOI] [PMC free article] [PubMed]
- 11.Van Hiel MB, et al. Identification and validation of housekeeping genes in brains of the desert locust Schistocerca gregaria under different developmental conditions. BMC Mol. Biol. 2009;10:56. doi: 10.1186/1471-2199-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porcheron P, Moriniere M, Grassi J, Pradelles P. Development of an enzyme immunoassay for ecdysteroids using acetylcholinesterase as label. Insect Biochem. 1989;19:117–122. doi: 10.1016/0020-1790(89)90081-4. [DOI] [Google Scholar]
- 13.Pascual N, Bellés X, Delbecque JP, Hua YJ, Koolman J. Quantification of ecdysteroids by immunoassay: comparison of enzyme immunoassay and radioimmunoassay. Zeitschrift für Naturforsch. C-A J. Biosci. 1995;50:862–867. doi: 10.1515/znc-1995-11-1219. [DOI] [PubMed] [Google Scholar]
- 14.Lafont, R., Dauphin–Villemant, C., Warren, J. T. & Rees, H. Ecdysteroid Chemistry and Biochemistry. In Comprehensive Molecular Insect Science (eds Gilbert, L. I., Iatrou, K. & Gill, S. S.) 125–195, doi:10.1016/B0-44-451924-6/00035-1 (Elsevier, 2005).
- 15.Marchal E, et al. Role of the Halloween genes, Spook and Phantom in ecdysteroidogenesis in the desert locust, Schistocerca gregaria. J. Insect Physiol. 2011;57:1240–1248. doi: 10.1016/j.jinsphys.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Van Wielendaele, P., Dillen, S., Marchal, E., Badisco, L. & Vanden Broeck, J. CRF-like diuretic hormone negatively affects both feeding and reproduction in the desert locust, Schistocerca gregaria. PLoS One7 (2012). [DOI] [PMC free article] [PubMed]
- 17.Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42 LP–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 18.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dissous C. Venus Kinase Receptors at the Crossroads of Insulin Signaling: Their Role in Reproduction for Helminths and Insects. Front. Endocrinol. (Lausanne). 2015;6:118. doi: 10.3389/fendo.2015.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nässel, D. R. & Vanden Broeck, J. Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci., doi:10.1007/s00018-015-2063-3 (2015). [DOI] [PMC free article] [PubMed]
- 21.Hult, E. F., Huang, J., Marchal, E., Lam, J. & Tobe, S. S. RXR/USP and EcR are critical for the regulation of reproduction and the control of JH biosynthesis in Diploptera punctata. J. Insect Physiol., doi:10.1016/j.jinsphys.2015.04.006 (2015). [DOI] [PubMed]
- 22.Marchal E, et al. Methoprene-tolerant (met) knockdown in the adult female cockroach, Diploptera punctata completely inhibits ovarian development. PLoS One. 2014;9:10–13. doi: 10.1371/journal.pone.0106737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martín D, Maestro O, Cruz J, Mané-Padrós D, Bellés X. RNAi studies reveal a conserved role for RXR in molting in the cockroach Blattella germanica. J. Insect Physiol. 2006;52:410–6. doi: 10.1016/j.jinsphys.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Badisco L, et al. RNA interference of insulin-related peptide and neuroparsins affects vitellogenesis in the desert locust Schistocerca gregaria. Peptides. 2011;32:573–580. doi: 10.1016/j.peptides.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Girardie J, Boureme D, Couillaud F, Tamarelle M, Girardie A. Anti-juvenile effect of neuroparsin A, a neuroprotein isolated from locust corpora cardiaca. Insect Biochem. 1987;17:977–983. doi: 10.1016/0020-1790(87)90106-5. [DOI] [Google Scholar]
- 26.Yang SP, He JG, Sun CB, Chan SF. Characterization of the shrimp neuroparsin (MeNPLP): RNAi silencing resulted in inhibition of vitellogenesis. FEBS Open Bio. 2014;4:976–986. doi: 10.1016/j.fob.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badisco L, et al. Purification and characterization of an insulin-related peptide in the desert locust, Schistocerca gregaria: Immunolocalization, cDNA cloning, transcript profiling and interaction with neuroparsin. J. Mol. Endocrinol. 2008;40:137–150. doi: 10.1677/JME-07-0161. [DOI] [PubMed] [Google Scholar]
- 28.Marchal E, et al. Final steps in juvenile hormone biosynthesis in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2011;41:219–227. doi: 10.1016/j.ibmb.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Acher FC, Bertrand HO. Amino acid recognition by venus flytrap domains is encoded in an 8-residue motif. Biopolym. - Pept. Sci. Sect. 2005;80:357–366. doi: 10.1002/bip.20229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.