Abstract
Objective:
The HIPPO trial is a UK randomized Phase II trial of hippocampal sparing (HS) vs conventional whole-brain radiotherapy after surgical resection or radiosurgery in patients with favourable prognosis with 1–4 brain metastases. Each participating centre completed a planning benchmark case as part of the dedicated radiotherapy trials quality assurance programme (RTQA), promoting the safe and effective delivery of HS intensity-modulated radiotherapy (IMRT) in a multicentre trial setting.
Methods:
Submitted planning benchmark cases were reviewed using visualization for radiotherapy software (VODCA) evaluating plan quality and compliance in relation to the HIPPO radiotherapy planning and delivery guidelines.
Results:
Comparison of the planning benchmark data highlighted a plan specified using dose to medium as an outlier by comparison with those specified using dose to water. Further evaluation identified that the reported plan statistics for dose to medium were lower as a result of the dose calculated at regions of PTV inclusive of bony cranium being lower relative to brain.
Conclusion:
Specification of dose to water or medium remains a source of potential ambiguity and it is essential that as part of a multicentre trial, consideration is given to reported differences, particularly in the presence of bone. Evaluation of planning benchmark data as part of an RTQA programme has highlighted an important feature of HS IMRT dosimetry dependent on dose being specified to water or medium, informing the development and undertaking of HS IMRT as part of the HIPPO trial.
Advances in knowledge:
The potential clinical impact of differences between dose to medium and dose to water are demonstrated for the first time, in the setting of HS whole-brain radiotherapy.
INTRODUCTION
Radiotherapy trials quality assurance (RTQA) is an important component of multicentre radiotherapy trials, ensuring outcomes can be correlated with the intended trial intervention.1 An important feature of RTQA is acknowledgment of not only the inherent complexity associated with the radiotherapy pathway, but also the varying technical and practical aspects across different departments.
The uniform reporting of specified doses underpin radiotherapy trial outcomes. Given the broad range of radiotherapy planning systems and associated dose calculations, this has traditionally been considered as part of RTQA, including how planning system algorithms account for density heterogeneity.
Modern “Type B” dose calculations are now considered a required standard, particularly in the treatment of lung lesions, but there remains potential ambiguity in the reporting of dose from different algorithms and planning systems specified to either water or medium.2 Reported differences in soft tissues remain comparable in the order of a few percent; however, more significant differences are reported in bone ranging from 4% in soft bone to as high as 11% in cortical bone.3
The HIPPO trial is a UK randomized Phase II trial of hippocampal sparing (HS) vs conventional whole-brain radiotherapy (WBRT) after surgical resection or radiosurgery in patients with favourable prognosis with 1–4 brain metastases. The hypothesis behind HIPPO is that irradiation of the bilateral hippocampi may be responsible for much of the adverse neurocognitive effect of WBRT, and that reducing the radiotherapy dose to the hippocampi may help preserve neurocognitive function.
HS WBRT is a novel approach to WBRT requiring complex intensity-modulated radiotherapy (IMRT) planning and delivery. As part of the HIPPO trial, participating centres are required to undertake a dedicated RTQA programme (Figure 1), promoting the safe and effective delivery of HS IMRT in a multicentre trial setting. Analysis of pre-trial planning benchmark cases has highlighted important treatment-specific considerations for HS IMRT dependent on dose being specified to water or medium.
Figure 1.
HIPPO RTQA programme.
METHODS AND MATERIALS
Hippocampal-sparing whole-brain radiotherapy
It is acknowledged that the development and implementation of HS IMRT is technically challenging and that the associated outlining and planning represent relatively new and challenging tasks for most clinicians and radiotherapy departments.4
The hippocampi are small centrally located structures requiring accurate registration of a high-quality MRI scan for the purpose of delineation in combination with considered anatomical interpretation by outlining clinicians. The resultant planning volume provides the challenge of sparing the two central hippocampal structures whilst treating the clinical target volume (whole brain), which encases the hippocampal structures with the treatment prescription.
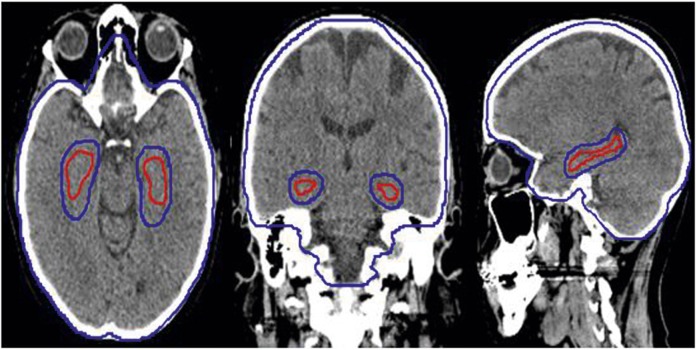
HIPPO prescribes a median or mean dose of 30 Gy in 10 fractions to the planning target volume (PTVhs_3000) defined as the clinical target volume (whole brain) expanded by 3 mm but excluding the hippocampal avoidance regions (hippocampi expanded by 5 mm) to allow for the necessary dose gradient between the hippocampus and whole brain (Figure 2).
Figure 2.
Blue = PTVhs_3000; red = hippocampi.
RESULTS
HIPPO planning benchmark case analysis
Submitted planning benchmark cases were reviewed using visualization for radiotherapy software (VODCA) evaluating plan quality and compliance in relation to the HIPPO radiotherapy planning and delivery guidelines.
Planning parameters are shown in Table 1 for benchmark case submissions from seven different radiotherapy centres in the trial. All planning submissions achieved the mandatory dose constraints to the PTVhs_3000 and all organs at risk (Appendix A).
Table 1.
Dose–volume histogram statistics and details of planning benchmark cases submitted
| Plan | Centre | Treatment planning system and algorithm | Technique | Dose specified to | PTVhs_3000 |
||||
|---|---|---|---|---|---|---|---|---|---|
| D98 | D95 | D90 | D2 | V95 | |||||
| 1 | 1 | Pinnacle, collapse cone | 2 coplanar arcs | Water | 23.5 | 28.1 | 29.3 | 32.3 | 93.4 |
| 2 | 2 | Hi-Art TomoTherapy superposition | n/a | Water | 24.9 | 28.4 | 29 | 32.1 | 94.9 |
| 3 | 3 | Hi-Art TomoTherapy superposition | n/a | Water | 22.4 | 27.4 | 29.1 | 31.2 | 93 |
| 4 | 4 | Eclipse, AAA | 3 non-coplanar arcs | Water | 28.2 | 29 | 29.3 | 30.9 | 97.4 |
| 5 | 5 | Eclipse, AAA | 2 coplanar arc | Water | 23.7 | 26.2 | 28 | 32.6 | 87.1 |
| 6 | 5 | Eclipse, AAA | 2 coplanar arc | Water | 24.4 | 27.2 | 28.3 | 32.7 | 88.3 |
| 7 | 5 | Eclipse, AAA | 2 coplanar arc | Water | 24.5 | 27.9 | 28.8 | 31.9 | 92.5 |
| 8 | 6 | Eclipse, AAA | 2 coplanar arc | Water | 23.7 | 27.2 | 29.2 | 30.8 | 92.6 |
| 9 | 6 | Eclipse, AAA | 2 coplanar arc | Water | 22.9 | 27.1 | 29.3 | 30.7 | 93.1 |
| 10w | 7 | Monaco, Monte Carlo | 3 non-coplanar arc | Water (converted) | 23.5 | 27.6 | 28.9 | 31.9 | 92.6 |
| 10m | 7 | Monaco, Monte Carlo | 3 non-coplanar arc | Medium | 23.4 | 26.7 | 27.7 | 31.5 | 82.3 |
D98, dose (Gy) received by 98% of volume; D95, volume (%) of PTV receiving 95% of the prescribed dose; D90, dose (Gy) received by 90% of volume; D2, dose (Gy) received by 2% of volume; V95, (%) of PTV receiving 95% of the prescribed dose; n/a, not applicable.
DISCUSSION
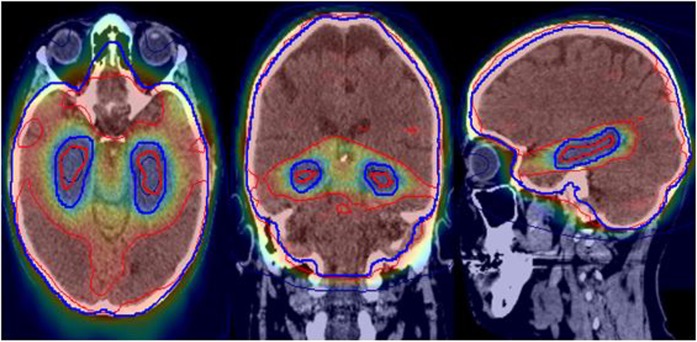
Careful optimization is an important feature of HS IMRT, in order to ensure optimal coverage of the PTV relative to the steep dose gradient necessary to spare the hippocampi. It is evident that three plans appear as outliers by comparison with the other planning submissions, with 5 , 6 and 10 m not achieving equivalent coverage of the PTVhs_3000 with 95% of the prescribed dose, despite meeting the dose constraints. Review of the dosimetry for Plans 5 and 6 identified a relative compromise to coverage of the central component of the PTV surrounding the hippocampal avoidance regions (Figure 3), prompting request for resubmission of these plans as part of the HIPPO QA programme.
Figure 3.
Example of Plan 6 dosimetry blue = PTVhs_3000; red = hippocampi (95% isodose displayed).
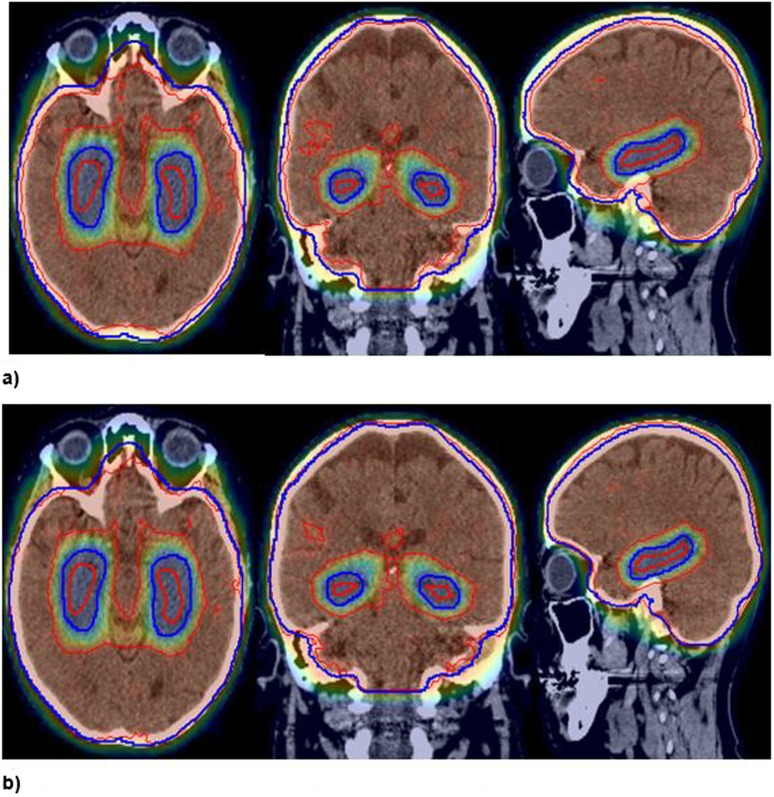
In contrast, the associated dosimetry for Plan 10m displays reasonable coverage and conformity of the central region of the PTVhs_3000 relative to the hippocampal avoidance regions, despite the reported PTVhs_3000 values being equivalent to Plans 5 and 6 (Figure 4a).
Figure 4.
(a) Example of Plan 10 m dosimetry. (b) Example of plan 10 w dosimetry. Blue = PTVhs_3000; red = hippocampi (95% isodose displayed).
It was evident that the feature of the plan dosimetry that contributed to the plan values appearing as a relative outlier was associated with coverage at the perimeter of PTVhs_3000, consisting of the cranium. The 95% isodose appeared to conform to the whole brain and not the PTVhs_3000, suggestive that the plan had been optimized as such. In reality this reflected the dose being specified to medium, with dose calculated in the regions of bone cooler than that in the brain and therefore the reported PTVhs_3000 values appearing as an outlier in comparison with the other planning submissions, which were calculated to water.
Plan 10w is the same plan converted to water with the dose reported to regions inclusive of bone more comparable with the brain (Figure 4b), therefore not appearing as an outlier with regard to coverage of the PTV.
The literature reports differences ranging between 3% and 6% to the dose (Gy) received by 98% of volume and dose (Gy) received by 2% of volume for a given PTV reporting dose to water or dose to medium in prostate and head and neck treatment plans, with no reported comparison in brain radiotherapy plans.4 These reported differences provide a basis for comparison and serve to highlight the treatment-specific characteristics for HS IMRT in HIPPO.
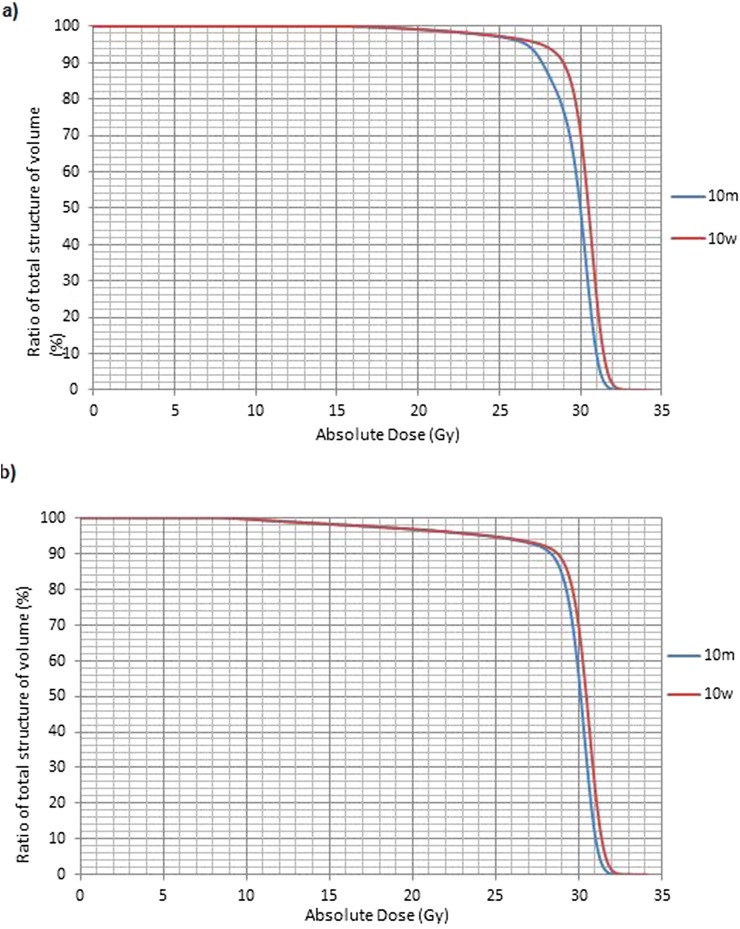
The difference reported in the dose (Gy) received by 98% of volume and dose (Gy) received by 2% of volume for Plans 10m and 10w is reasonably small at approximately 1–2%. Figure 5a shows plans 10m and 10w reporting to the whole-brain volume with a systematic difference of approximately 1% evident, reflecting the homogeneous and relatively water-equivalent composition of the brain.5 In contrast, in Figure 5b, the dose–volume histogram curves for the PTVhs_3000 highlight a difference in the volume (%) of PTV receiving 95% of the prescribed dose–dose (Gy) received by 80% of volume of approximately 3–5%, reflecting a volume now inclusive of cranium with higher mass stopping power ratio (water/bone).
Figure 5.
(a) Dose–volume histogram (DVH) curve of Plans 10m and 10w to the whole brain. (b) DVH curve of Plans 10m and 10w to the PTVhs_3000.
CONCLUSION
Evaluation of HS IMRT as part of the pre-trial component of the HIPPO RTQA programme has highlighted an important feature of HS IMRT dosimetry dependent on dose being specified to water or medium.
It is important to acknowledge that conversion of a plan from dose to medium to dose to water serves here only for the purpose of comparison. Conversion from medium to water for a specified algorithm is unlikely to represent an accurate means for comparing and evaluating dose constraints in clinical practice and may introduce greater uncertainties.2,6 The challenge remains to interpret these findings in the context of the HIPPO trial and therefore the optimal features of HS IMRT dosimetry and treatment delivery.
One approach is to normalize the plan to achieve improved coverage. However, with relative high-dose regions within the brain increasing as a result, this would not appear consistent with the principles of HS IMRT, as defined by the HIPPO trial. These are dictated by not only achieving optimal coverage of the PTV and sparing of the hippocampi, but also ensuring relative homogeneity of dose within the brain, which may influence neurocognitive function. With good correlation in dose reported to the whole brain between Plan 10m and Plan 10w, the rationale for reoptimizing the plan based on the lower dose to regions of bone when calculating dose to medium must be carefully considered.7 In addition, the similarity between the dose to water plan and other submissions suggests that there is good consistency between centres in what is being delivered, but differences in what the planning system is reporting when using dose to medium.
An important feature of HS IMRT as part of the HIPPO trial is daily treatment verification with online treatment position correction. With the centre calculating dose to medium undertaking correction to within 1 mm, it could be inferred that the potential implications of the relative cooler region of PTV surrounding the whole brain will be limited. This must also be considered in the context of optimizing dose to these regions, whereby associated geometric errors or uncertainties would increase dose to the brain.
Therefore, whilst it is essential to acknowledge that the characteristics of the planned dosimetry and reported values for plans calculated using dose to medium will be different from those specified to water, it is not to say this is wrong.7–9 The purpose of RTQA within this trial setting is to ensure safe and effective HS IMRT, with dose to brain and organs at risk consistent across different radiotherapy centres and with different planning systems. It is not necessary to mandate dose specification to water for all centres to achieve this purpose as demonstrated in this report, and a more reasoned approach is to consider alternative metrics, constraints and/or means of evaluating HS IMRT plans when specified using dose to medium, which ensure equivalence with plans calculated using dose to water. In this way, RTQA achieves its purpose of assuring safe, effective and consistent plan generation and development within a multicentre setting.
Acknowledgments
ACKNOWLEDGMENTS
Thank you to all the radiotherapy centres taking part in the HIPPO trial.
Appendix A
Table A1.
HIPPO trial dose constraints
| Volume (%) | PTVhs_3000 |
Mandatory (Gy) |
|---|---|---|
| Optimal dose required (Gy) | ||
| 98 | 23.5 | 22.5 |
| 95 | 26.0 | 25.0 |
| 90 | 27.5 | 26.5 |
| 50 | 30.0 | 30.0 |
| 2 | 33.0 | 35.0 |
| OAR | Optimal dose constraint (Gy) | Mandatory (Gy) |
|---|---|---|
| Hippocampus_L | Mean dose < 10 | Mean dose < 11 |
| Hippocampus_R | Mean dose < 10 | Mean dose < 11 |
| Hippocampus_L | D2% < 15 | D2% < 15 |
| Hippocampus_R | D2% < 15 | D2% < 15 |
| OpticNerve_L_3 | D2% < 33 | D2% < 35 |
| OpticNerve_R_3 | D2% < 33 | D2% < 35 |
| Chiasm_3 | D2% < 33 | D2% < 33 |
| Lens_L | Dmax < 8 | Dmax < 10 |
| Lens_R | Dmax < 8 | Dmax < 10 |
| Eye_L | Dmax < 20 | Dmax < 20 |
| Eye_R | Dmax < 20 | Dmax < 20 |
Dmax, maximum dose received by volume; OAR, organ at risk.
Structures including suffix _3 denote an expansion of 3 mm from base structure.
Contributor Information
Daniel Megias, Email: daniel.megias@nhs.net, daniel.megias@gmail.com.
Mark Phillips, Email: mark.phillips@ucl.ac.uk.
Laura Clifton-Hadley, Email: l.clifton-hadley@ucl.ac.uk.
Elizabeth Harron, Email: Elizabeth.Harron@nuh.nhs.uk.
David J Eaton, Email: davideaton@nhs.net.
Paul Sanghera, Email: Paul.Sanghera@uhb.nhs.uk.
Gillian Whitfield, Email: gillian.whitfield@christie.nhs.uk.
FUNDING
The HIPPO trial is supported by Cancer Research UK [C47382/A16600], clinical trial number CRUK/13/017, and The Brain Tumour Charity. The HIPPO trial is sponsored by University College London (UCL) and co-ordinated by the Cancer Research UK and UCL Cancer Trials Centre, London.
REFERENCES
- 1.Melidis C, Bosch WR, Izewska J, Fidarova E, Zubizarreta E, Ishikura S, et al. Radiation therapy quality assurance in clinical trials—global harmonisation group. Radiother Oncol 2014; 111: 327–9. doi: https://doi.org/10.1016/j.radonc.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreo P. Dose to “water-like” media or dose to tissue in MV photons radiotherapy treatment planning: still a matter of debate. Phys Med Biol 2015; 60: 309–37. doi: https://doi.org/10.1088/0031-9155/60/1/309 [DOI] [PubMed] [Google Scholar]
- 3.Gladstone DJ, Kry SF, Xiao Y, Chetty IJ. Dose specification for NRG radiation therapy trials. Int J Radiat Oncol Biol Phys 2016; 95: 1344–5. doi: https://doi.org/10.1016/j.ijrobp.2016.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gondi V, Cui Y, Mehta M, Manfredi D, Xiao Y, Galvin JM, et al. Real-time pretreatment review limits unacceptable deviations on a cooperative group radiation therapy technique trial: quality assurance results of RTOG 0933. Int J Radiat Oncol Biol Phys 2014; 91: 564–70. doi: https://doi.org/10.1016/j.ijrobp.2014.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dogan N, Siebers JV, Keall PJ. Clinical comparison of head and neck and prostate IMRT plans using absorbed dose to medium and absorbed dose to water. Phys Med Biol 2006; 51: 4967–80. doi: https://doi.org/10.1088/0031-9155/51/19/015 [DOI] [PubMed] [Google Scholar]
- 6.Siebers JV, Keall PJ, Nahum AE, Mohan R. Converting absorbed dose to medium to absorbed dose to water for Monte Carlo based photon beam calculations. Phys Med Biol 2000; 45: 983–95. doi: https://doi.org/10.1088/0031-9155/45/4/313 [DOI] [PubMed] [Google Scholar]
- 7.Sterpin E. Potential pitfalls of the PTV concept in dose-to-medium planning optimization. Eur J Med Phys 2016; 32: 1103–10. doi: https://doi.org/10.1016/j.ejmp.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 8.Liu HH. Dm rather than Dw should be used in Monte Carlo treatment planning. For the proposition. Med Phys 2002; 29: 922–3. doi: https://doi.org/10.1118/1.1473137 [DOI] [PubMed] [Google Scholar]
- 9.Ma CM, Li J. Dose specification for radiation therapy: dose to water or dose to medium? Phys Med Biol 2011; 56: 3073–89. doi: https://doi.org/10.1088/0031-9155/56/10/012 [DOI] [PubMed] [Google Scholar]