Abstract
Since the description of cryptogenic organizing pneumonia in 1983 by Davison et al and the subsequent report on bronchiolitis obliterans organizing pneumonia by Epler et al, some reports have been published regarding the imaging features of organizing pneumonia (OP). In this pictorial review, we aimed to describe and illustrate different manifestations of OP on high-resolution CT (HRCT) accompanied by their histopathological correlations for a better comprehension of pathomechanism of the radiological findings. The main HRCT findings in OP include: consolidation, ground-glass opacification, perilobular opacity, reversed halo opacity, nodule or mass, parenchymal bands, bronchial wall thickening, bronchial dilatation, mediastinal lymphadenopathy and pleural effusion. In addition, we discuss the radiological differential diagnosis for each manifestation, as well as imaging evolution during patient follow-up, and two OP-related entities: the possibility of non-specific interstitial pneumonia development following OP and a relatively new rare entity related to OP called acute fibrinous and organizing pneumonia. For radiologists and physicians, a detailed knowledge of the potential radiological manifestations in OP is crucial for making a correct diagnosis and managing the patient properly. Moreover, some unnecessary lung biopsies will be avoided.
INTRODUCTION
Cryptogenic organizing pneumonia (COP) is considered as an acute/subacute idiopathic interstitial pneumonia (IIP), although it is not a true IIP, because it has an idiopathic nature and tends to be confused with other IIPs, particularly when it progresses to fibrosis.1
Since there is an identifiable cause on some occasions, the term “organizing pneumonia (OP)” is suggested for use instead of COP. The term may be modified with the secondary cause, e.g. OP associated with polymyositis.1 Therefore, the diagnosis of COP should be made only after the exclusion of any possible aetiology. The injuries that may lead to OP include: (1) viral infections including human immunodeficiency virus infection; (2) toxic gases such as nitrogen dioxide; (3) drugs; (4) radiation therapy; (5) free-base cocaine; (6) inflammatory bowel diseases; and (7) connective-tissue disorders such as rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis and polymyositis–dermatomyositis. There is no documented association with cigarette smoking, and most patients are non-smokers or ex-smokers.2
OP equally affects both genders, typically in their sixth decade of life. It usually has a subacute course with relatively short duration (median, less than 3 months). Patients may suffer from variable degrees of flu-like symptoms, dry cough and dyspnoea.1
The majority of patients gain full recovery with oral corticosteroids; however, relapse is common. A few reports have described a subgroup of patients with OP who do not completely recover despite prolonged treatment.1
Histopathologic findings of OP include: (1) intraluminal organizing fibroblastic tissue (i.e. plugs of granulation tissue, also known as Masson bodies) in distal air spaces; (2) mild interstitial inflammation with mononuclear cells; and (3) mild intra-alveolar cellular desquamation. Typically, the lung architecture is preserved. All the granulation tissues have the same age and contain few inflammatory cells.2,3
In this pictorial review, we aim to describe and illustrate different imaging manifestations of OP on high-resolution CT (HRCT) accompanied by their histopathological correlations. In addition, radiological differential diagnosis, HRCT evolution during patient follow-up and two OP-related entities will be discussed.
COMMON HIGH-RESOLUTION CT FINDINGS WITH HISTOPATHOLOGICAL CORRELATION
Consolidation
Consolidation is the most common OP manifestation, seen in 80–95% of patients either alone or as part of a mixed pattern. Consolidations are usually bilateral and asymmetric. They have a patchy appearance with no zonal or anteroposterior predominance; however, predominant involvement of one lung zone is seen in most individual patients (Figure 1). Four main patterns of distribution have been described: (1) predominantly subpleural, also referred to as reverse batwing appearance (most common); (2) predominantly peribronchovascular; (3) both subpleural and peribronchovascular; and (4) band-like. In addition, a few patients may present with an extensive central disease, while the lung peripheries are almost completely spared. Bubbly lucencies (representing unaffected aerated alveoli) and air bronchograms may be seen within the areas of consolidation. Migratory behaviour of consolidations may be evident in follow-up studies.3–7
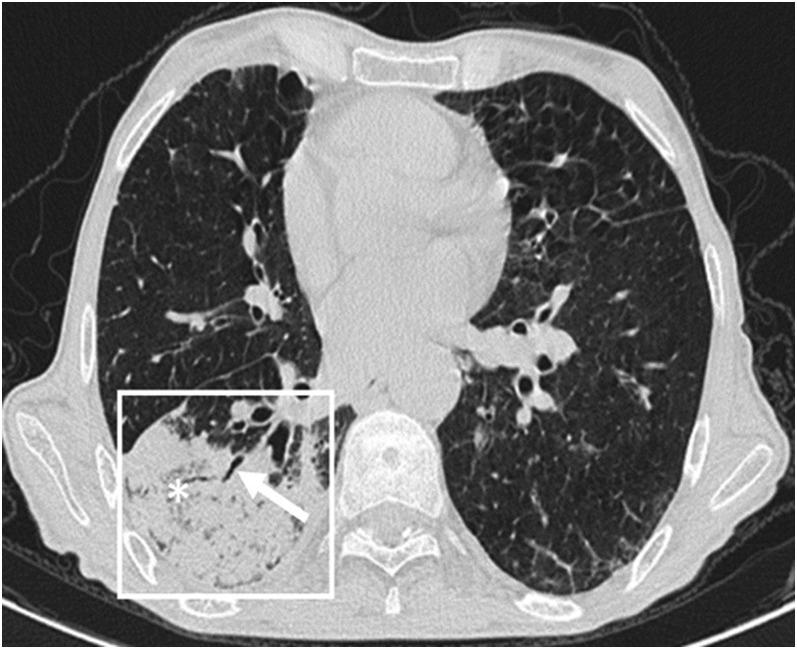
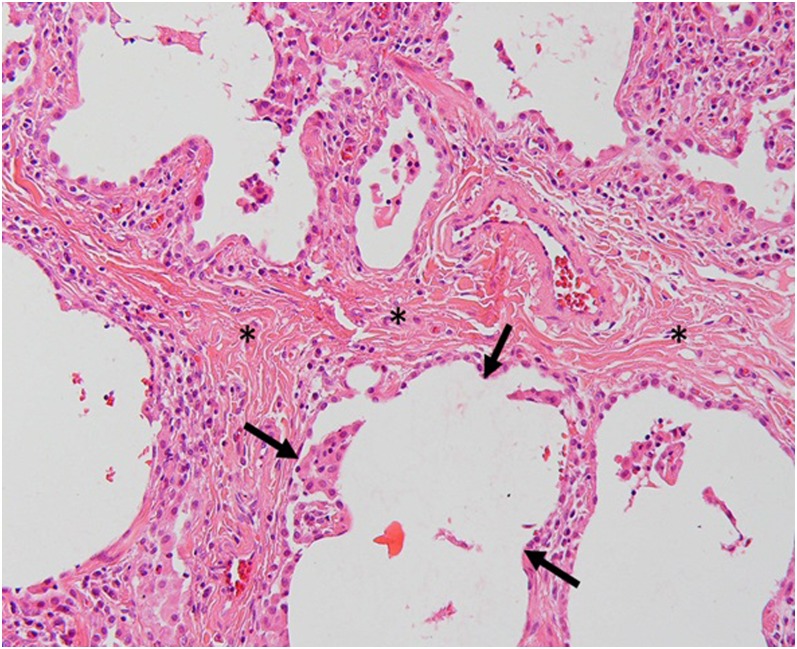
Figure 1.
Consolidation in organizing pneumonia: peripheral consolidation (within the box) with air bronchogram (arrow) and areas of bubbly lucencies (asterisk) is noted in right lower lobe.
Consolidation is more common in patients who are immunocompetent than in those who are immunocompromised.5 Histopathologically, consolidation is caused by intra-alveolar fibroblastic granulation tissues, also known as Masson bodies (Figure 2).2
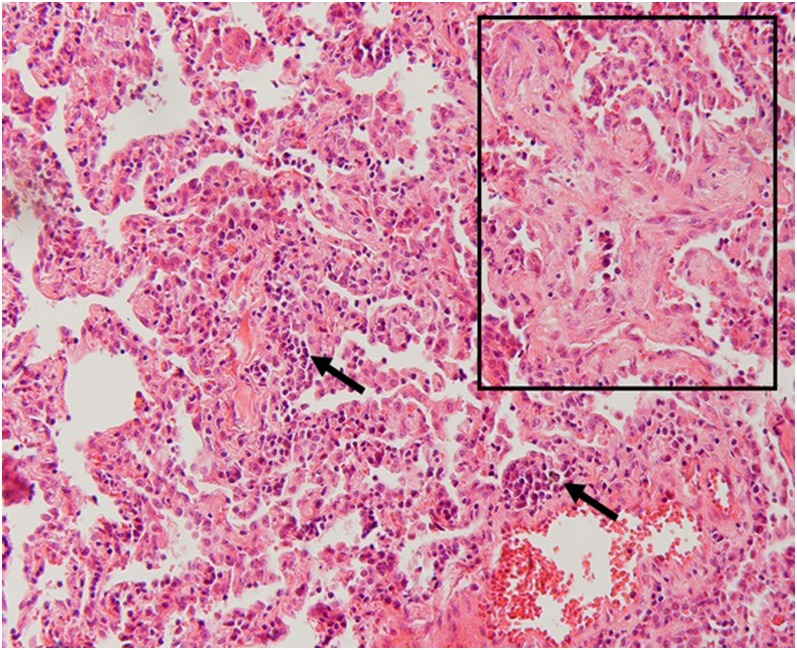
Figure 2.
Histopathologic correlation for Figure 1: a Masson body is seen in the intra-alveolar space (within the box). There is also mild infiltration of inflammatory cells in the interlobular septa (arrows) (haematoxylin and eosin stain, ×200).
Ground-glass opacity
Areas of ground-glass attenuation are present in 60–90% of patients, usually as part of a mixed pattern. They are bilateral and random in distribution (Figure 3).3,5,7 There is no significant difference in the occurrence between patients who are immunocompetent and those who are immunocompromised.5 Histopathologically, ground-glass opacity (GGO) corresponds to an area of alveolar septal inflammation and intra-alveolar cellular desquamation with a small amount of granulation tissue in the terminal air spaces (Figure 4).3
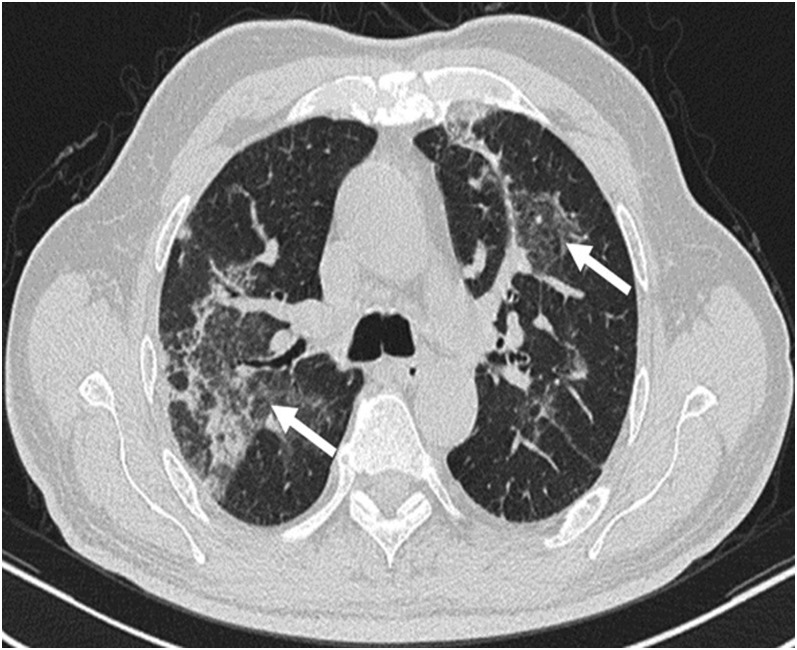
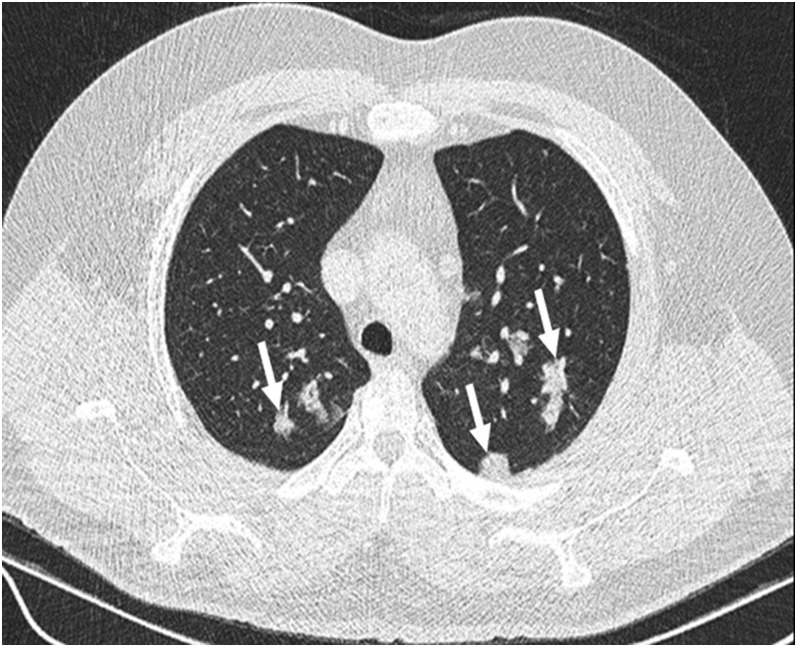
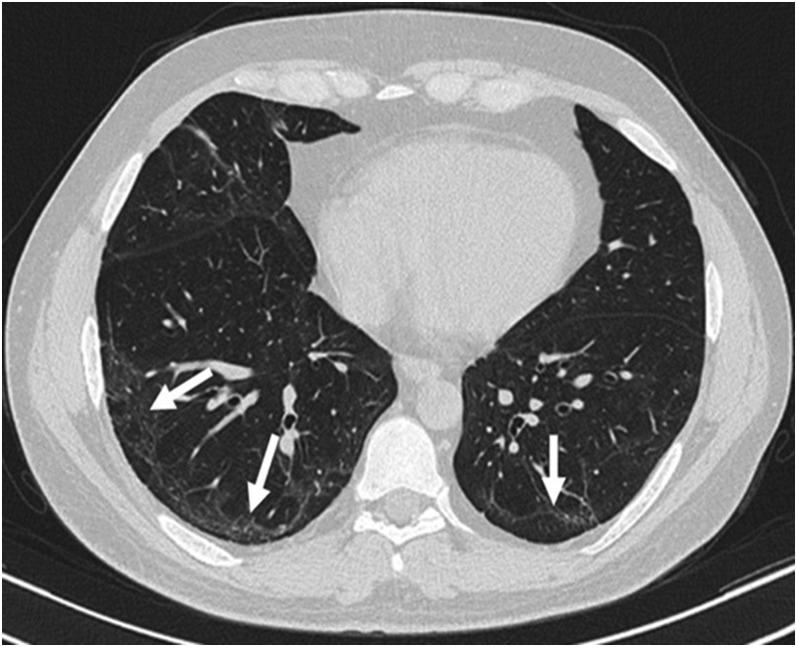
Figure 3.
Ground-glass opacity (GGO) in organizing pneumonia: bilateral patchy GGOs (arrows) are seen in parahilar regions.
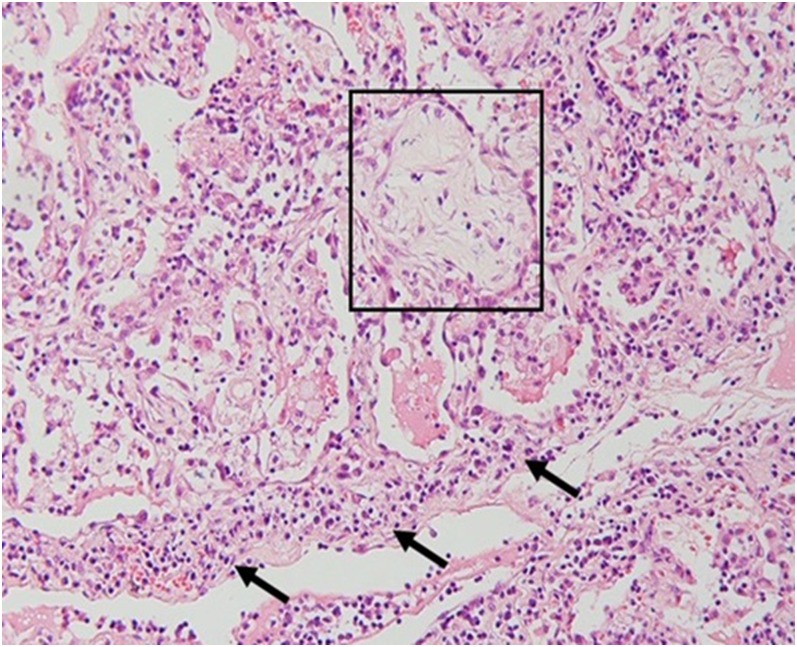
Figure 4.
Histopathologic correlation for Figure 3: intra-alveolar granulation tissue and desquamated cells (within the box) along with interlobular septal inflammation (arrows) is seen (haematoxylin and eosin stain, ×200).
Perilobular opacity
The perilobular pattern consists of bowed or polygonal opacities with poorly defined margins around the interlobular septa. It is observed in about 55% of patients and occurs in all lung zones with a predominance in middle and lower zones. In most cases, the perilobular opacity abuts the pleural surface. They are surrounded by aerated lung parenchyma. Perilobular opacities are always accompanied by consolidation and/or GGO in the same lung zone.6
In the active phase of disease, the entire lobule is filled with granulation tissue and in the healing phase, the lobule is cleared centripetally. According to this pathomechanism, a variety of diseases affecting mainly the alveoli can involve the perilobular area in the healing phase and mimic septal abnormalities on HRCT (i.e. subpleural coarse reticulations), although they are not associated histologically with real thickening of the interlobular septa.5,6
Reversed halo (atoll) sign
The reversed halo sign is defined as “a focal rounded area of GGO surrounded by a more or less complete ring of consolidation” in the glossary of terms for thoracic imaging (Figure 5).8 The central GGO corresponds histologically to the area of alveolar septal inflammation and cellular debris in the alveoli, whereas the ring-shaped or crescentic peripheral consolidation corresponds to the area of granulation tissue within the distal air spaces. This sign is seen in about 20% of patients with OP.3
Figure 5.
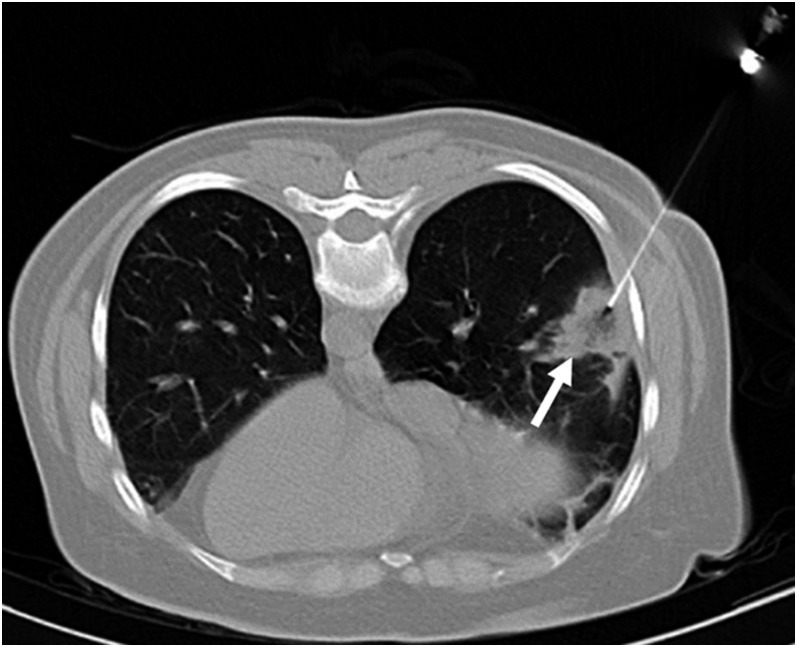
Reversed halo (Atoll sign) in organizing pneumonia (OP): a peripheral mass in right lung base with reversed halo sign (arrow). The mass was biopsied, and the histopathologic study revealed it to be OP.
Nodule or mass
Nodular opacities (1–10 mm in diameter) are seen in 15–50% of patients (Figure 6). These are randomly distributed, but more numerous along the bronchovascular bundles, and are usually part of a mixed pattern. Nodules are mostly bilateral. The margins are usually poorly defined, and they may be associated with an open bronchus sign. Also, they may cavitate.4,5,9 They are more commonly seen in patients who are immunocompromised. OP reaction after infection has been reported to appear as nodular foci, and this may be the case in some of the patients who are immunocompromised.5
Figure 6.
Nodular pattern of organizing pneumonia (OP): randomly distributed nodules (arrows) are seen in both lung fields. Histopathologic result was OP.
On histopathologic examination, parenchymal nodules represent granulation-impacted bronchioles surrounded by a localized zone of OP, separated from other involved areas by relatively normal parenchyma.4,5,9
Parenchymal bands
Parenchymal bands extend in a radial manner along the tract of a bronchus towards the pleura (Figure 7). It has been suggested that these lines are due to the involvement of the more proximal airways leading to linear atelectasis distal to the narrowed bronchus, or may be secondary to pleuroparenchymal fibrosis.9
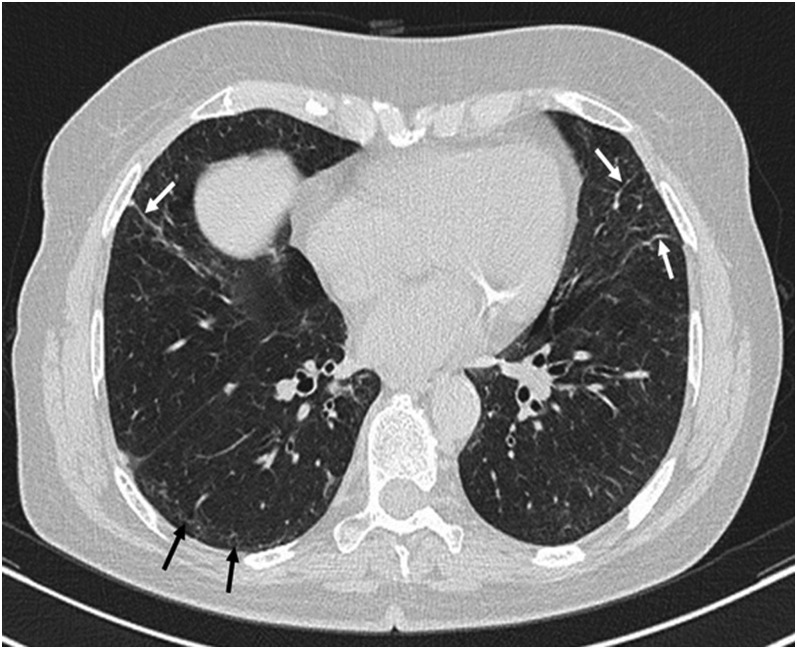
Figure 7.
Parenchymal bands in organizing pneumonia (OP): parenchymal bands (white arrows) are seen in both lungs projecting from the visceral pleura towards the lung parenchyma in the follow-up high-resolution CT of a patient with OP. These bands are usually up to 5 cm in length and 1–3 mm in thickness. In addition, subpleural band-like reticulations (black arrows) are noted in right lung base, which may be suggestive of non-specific interstitial pneumonia development.
Bronchial wall thickening and dilatation
Bronchial wall thickening and dilatation are seen on HRCT in the patients with extensive consolidation and are usually limited to these areas (Figures 8 and 9). They are non-specific findings, seen in many inflammatory and fibrotic processes.
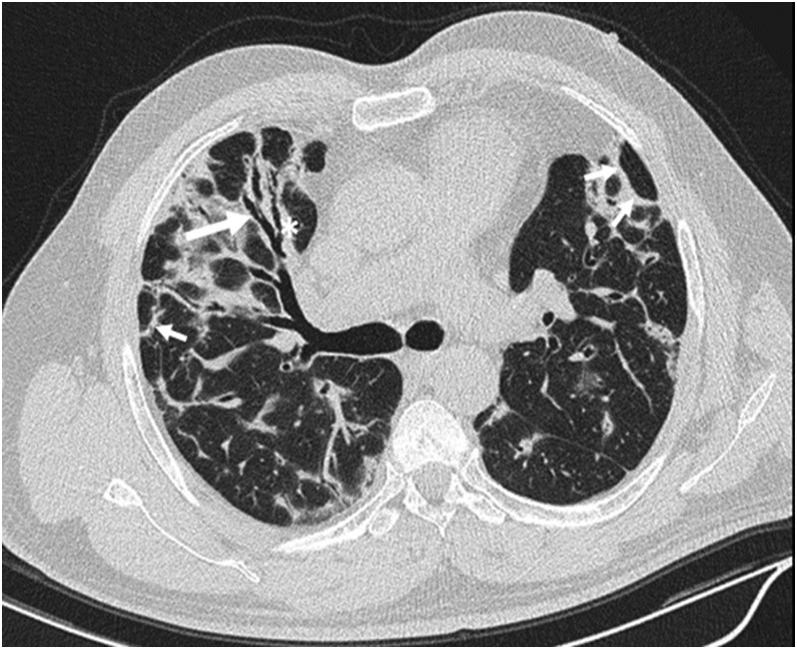
Figure 8.
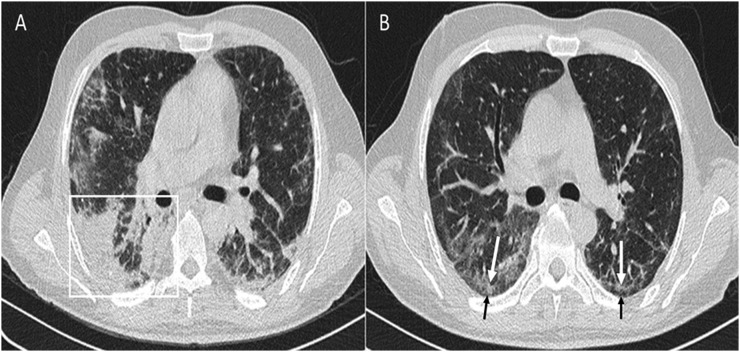
Bronchial wall thickening, bronchial dilatation and perilobular opacity in organizing pneumonia: peribronchial wall thickening (asterisk) and bronchial dilatation (long arrow) is noted in right middle lobe within areas of consolidation and ground-glass opacity. Also, perilobular opacities (short arrows) are evident in the subpleural regions.
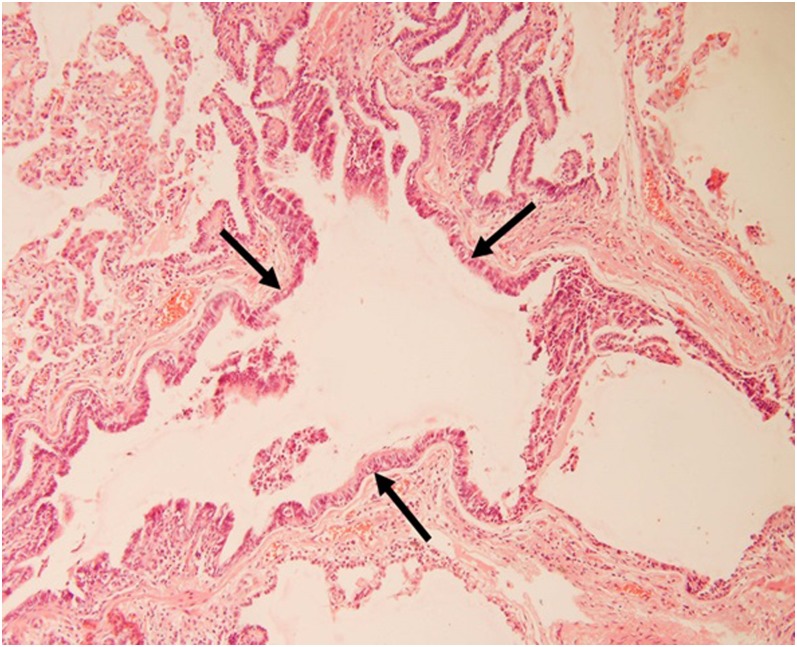
Figure 9.
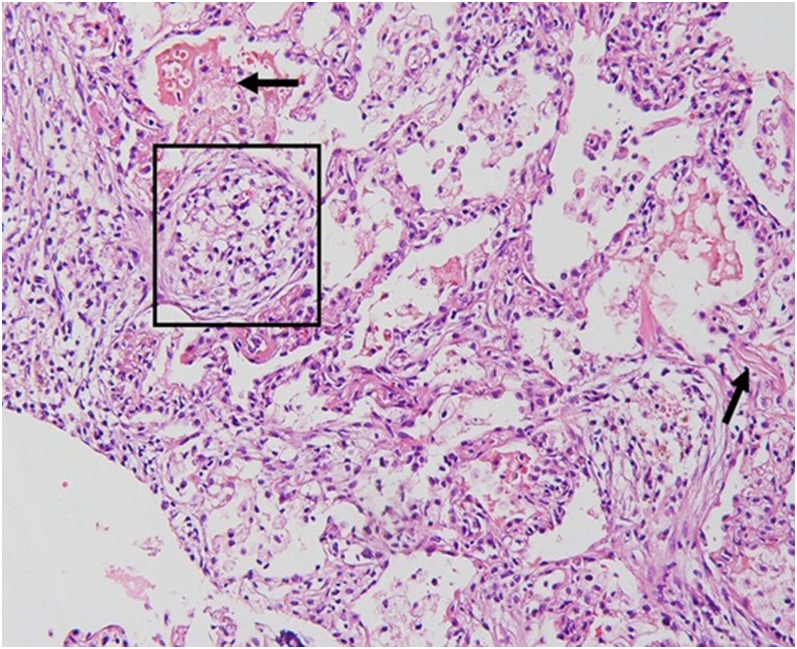
Histopathologic correlation for Figure 8: a dilated bronchus (between arrows) is evident (haematoxylin and eosin stain, ×200).
OTHER RADIOLOGICAL FINDINGS
Mediastinal lymphadenopathy
Mediastinal lymphadenopathy (nodes >1 cm in short-axis diameter) is observed in 20–30% of patients. They are mostly seen in the pulmonary hilum (Station 10), lower paratrachea (Station 4) and subcarina (Station 7).3,5
Pleural effusion
Small amounts of pleural effusion are seen in 10–35% of patients. They are usually bilateral and are more common in patients who are immunocompetent.5 Vasu et al reported pleural effusion in 60% of patients with secondary OP and in none of the patients with cryptogenic OP.10 Accordingly, pleural effusion seems to be related to the background disease rather than OP itself.
RADIOLOGICAL DIFFERENTIAL DIAGNOSIS
Since OP has a variety of manifestations on HRCT, there are several differential diagnoses. A pattern-based differential diagnosis is listed in Table 1.
Table 1.
Pattern-based radiological differential diagnosis of organizing pneumonia (OP)
| HRCT pattern | Prevalence in OP | Differential diagnosis | |
|---|---|---|---|
| Peripheral consolidation | 80–95% | Chronic eosinophilic pneumonia;
bronchoalveolar carcinoma; sarcoidosis; pulmonary contusion;
pulmonary infarct; aspiration pneumonia |
|
| Ground-glass opacity | 60–90% | Acute | Pulmonary oedema; pulmonary haemorrhage; atypical pneumonia; acute eosinophilic pneumonia; radiation pneumonitis |
| Chronic | Hypersensitivity pneumonitis; DIP; bronchoalveolar carcinoma; alveolar proteinosis; pulmonary haemosiderosis; sarcoidosis | ||
| Perilobular opacity | 55% | Typical and atypical pneumonia;
alveolar haemorrhage; alveolar proteinosis; amyloidosis;
interstitial lung diseases (including UIP, NSIP and
AIP) |
|
| Reversed halo sign | 20% | Invasive fungal infections (such as
angioinvasive aspergillosis and mucormycosis); Wegener's
granulomatosis; pulmonary infarct; tuberculosis;
lymphoproliferative disorders |
|
| Nodule and mass | 15–50% | Granulomatous infections; primary neoplasms; metastatic tumours; lymphoma; carcinoid and tumorlets; pulmonary hamartomas; arteriovenous malformations (e.g., in von Hippel–Lindau disease); sarcoidosis; Wegener's granulomatosis; rheumatoid arthritis; amyloidosis | |
AIP, acute interstitial pneumonitis; DIP, desquamative interstitial pneumonia; HRCT, high-resolution CT; NSIP, non-specific interstitial pneumonia; OP, organizing pneumonia; UIP, usual interstitial pneumonia.
HIGH-RESOLUTION CT EVOLUTION IN FOLLOW-UP
In Lee et al7 study, 73% of patients showed residual disease on follow-up CT scans (Figure 10). The most common findings on follow-up HRCTs were GGOs (in 73% of patients), followed by reticular pattern (in 50% of patients).
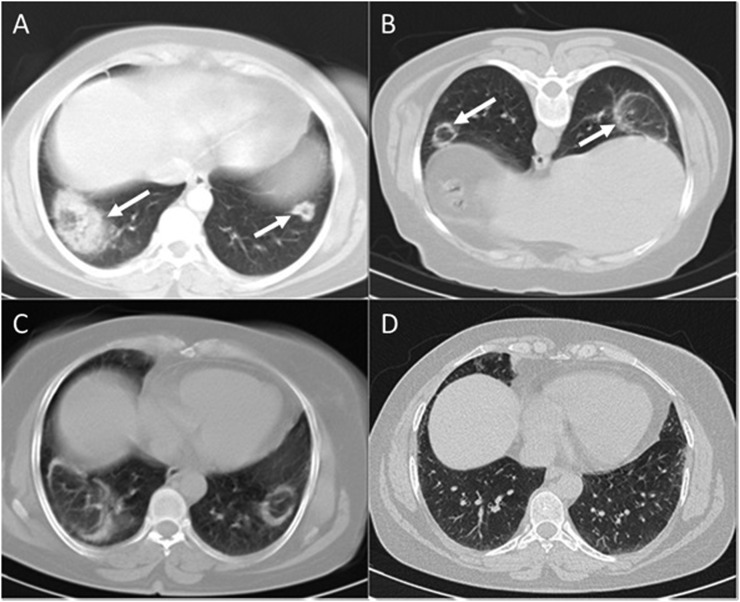
Figure 10.
Follow-up CT scans in organizing pneumonia: (a) the initial chest CT scan is revealing two round well-defined opacities with reversed halo appearance (arrows) in both lung bases. (b) 20 days later, the patient was referred for CT-guided transthoracic lung biopsy. At that stage, lesions had been resolved centrally revealing a typical “Atoll sign” (arrows). (c) 22 days after the second scan, another chest CT scan was performed. There was no significant improvement. (d) Approximately 100 days after the third scan, the follow-up high-resolution CT showed none of the lesions, and there was no evidence of any residual disease in both lungs.
Among the patients with residual disease, 63% of patients showed a pattern of lung abnormalities very similar to fibrotic non-specific interstitial pneumonia (NSIP). The principal findings in these patients were GGOs, interlobular/intralobular reticulation predominantly in subpleural and basal parenchyma and traction bronchiectasis.
13% of patients with residual disease revealed honeycombing with lower lung zone predominance on follow-up HRCTs, which was most similar to the usual interstitial pneumonia pattern.
MISCELLANEOUS ORGANIZING PNEUMONIA-RELATED ENTITIES
Organizing pneumonia and non-specific interstitial pneumonia
OP is characterized by granulation tissue-filled air spaces. However, some OP cases show more marked interstitial inflammation in the acute phase, and it seems there is an overlap between OP and cellular NSIP in these patients (Figures 11 and 12).1
Figure 11.
Organizing pneumonia (OP) and cellular non-specific interstitial pneumonia (NSIP): bilateral band-like interlobular/intralobular reticulations (arrows) parallel to the chest wall with subpleural sparing in a patient with prolonged OP in favour of cellular NSIP.
Figure 12.
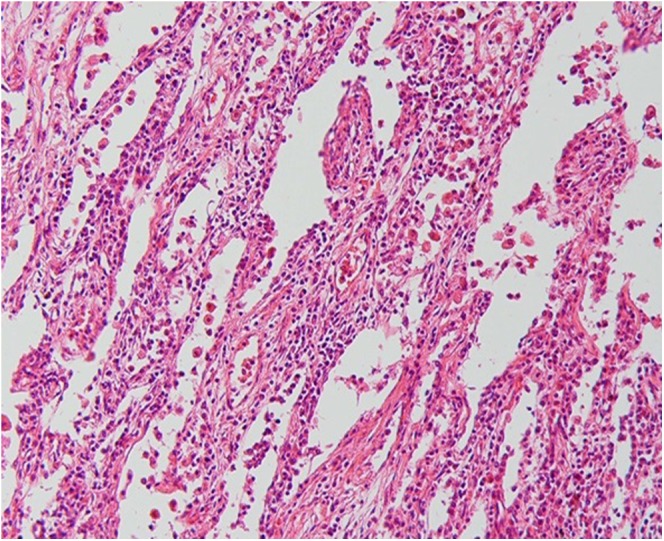
Histopathologic correlation for Figure 11: marked interstitial inflammation consistent with cellular non-specific interstitial pneumonia (haematoxylin and eosin stain, ×200).
In addition, OP does not resolve completely in some patients and progresses to interstitial fibrosis. It is likely that some cases of fibrotic NSIP are related to this variant of OP.7 In such patients, consolidation is prominent on HRCT, variably associated with reticular abnormalities (Figures 13 and 14). Some patients with this pattern of mixed fibrosis and OP may have underlying aetiologies, such as polymyositis or antisynthetase syndrome.1
Figure 13.
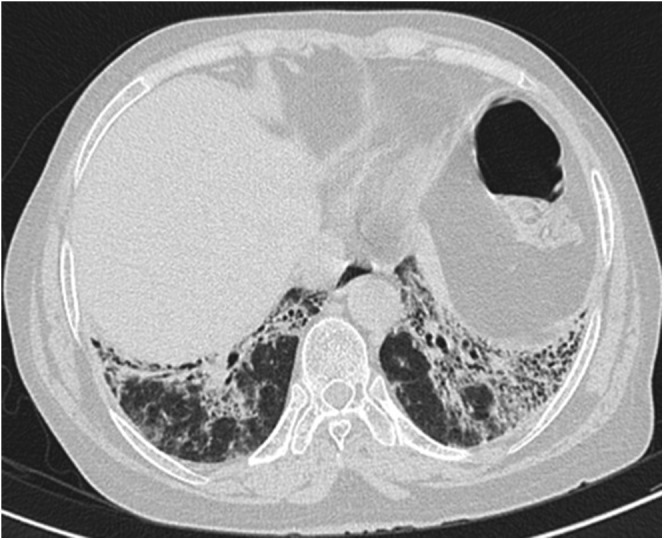
Organizing pneumonia and fibrotic non-specific interstitial pneumonia (NSIP): bibasilar subpleural interlobular/intralobular reticulation along with tractional bronchiolectasis in favour of fibrotic NSIP.
Figure 14.
Histopathologic correlation for Figure 13: areas of interlobular fibrosis (asterisks) and histopathologic honeycombing (between arrows) is seen (haematoxylin and eosin stain, ×200).
Acute fibrinous and organizing pneumonia
Acute fibrinous and organizing pneumonia (AFOP) is a rare histologic pattern of lung injury with an acute/subacute clinical presentation, similar to either diffuse alveolar damage (DAD) or OP (Figure 15). It is characterized histologically by fibrin balls and Masson bodies within the alveoli, as well as inflammatory cells in the interstitium, and does not meet the criteria for either DAD or OP (Figure 16).1,11
Figure 15.
Acute fibrinous and organizing pneumonia: (a) confluent consolidation (within the box) and patchy ground-glass opacities (more in the right lung) with posterior predominance in a patient admitted in the intensive care unit. (b) Follow-up CT scan 15 days later shows partial resolution with bilateral subpleural intralobular reticulations (long white arrows) with a short distance of subpleural sparing (short black arrows).
Figure 16.
Histopathologic correlation for Figure 15: an intra-alveolar Masson body (within the box) and fibrin strands admixed with inflammatory cells (arrows) is seen (haematoxylin and eosin stain, ×200).
It should be emphasized that AFOP is a distinct histopathologic entity with no characteristic radiological features; however, the clinical course and radiological findings are well correlated. Patients whose disease course is rapid and progressive have imaging findings similar to DAD (diffuse but more basilar dependent consolidation and GGO), while those with a more subacute course show imaging findings consistent with OP.11
AFOP may be idiopathic or associated with connective tissue disorders, hypersensitivity pneumonitis or drug reaction. Since similar clinical and radiological manifestations may be seen in eosinophilic pneumonia, this diagnosis should be excluded by the absence of tissue and peripheral eosinophilia in the suspected cases of AFOP.11
CONCLUSION
We reviewed different manifestations of OP on HRCT accompanied by their histopathological correlations. While these imaging findings are not pathognomonic, in combination with patient history and clinical setting, they may be highly suggestive of OP. It is crucial for radiologists and physicians to be familiar with the spectrum of HRCT manifestations in patients with OP to make a correct diagnosis, which in part may obviate some unnecessary lung biopsies.
Contributor Information
Mohammad Zare Mehrjardi, Email: zare@sbmu.ac.ir.
Shahram Kahkouee, Email: shahramkah@yahoo.com.
Mihan Pourabdollah, Email: pourabdollah@sbmu.ac.ir.
REFERENCES
- 1.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–48. doi: https://doi.org/10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller-Mang C, Grosse C, Schmid K, Stiebellehner L, Bankier AA. What every radiologist should know about idiopathic interstitial pneumonias. RadioGraphics 2007; 27: 595–615. [DOI] [PubMed] [Google Scholar]
- 3.Kim SJ, Lee KS, Ryu YH, Yoon YC, Choe KO, Kim TS, et al. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR Am J Roentgenol 2003; 180: 1251–4. doi: https://doi.org/10.2214/ajr.180.5.1801251 [DOI] [PubMed] [Google Scholar]
- 4.Müller NL, Staples CA, Miller RR. Bronchiolitis obliterans organizing pneumonia: CT features in 14 patients. AJR Am J Roentgenol 1990; 154: 983–7. [DOI] [PubMed] [Google Scholar]
- 5.Lee KS, Kullnig P, Hartman TE, Müller NL. Cryptogenic organizing pneumonia: CT findings in 43 patients. AJR Am J Roentgenol 1994; 162: 543–6. doi: https://doi.org/10.2214/ajr.162.3.8109493 [DOI] [PubMed] [Google Scholar]
- 6.Greenberg-Wolff I, Konen E, Ben Dov I, Simansky D, Perelman M, Rozenman J. Cryptogenic organizing pneumonia: variety of radiologic findings. Isr Med Assoc J 2005; 7: 568–70. [PubMed] [Google Scholar]
- 7.Lee JW, Lee KS, Lee HY, Chung MP, Yi CA, Kim TS, et al. Cryptogenic organizing pneumonia: serial high-resolution CT findings in 22 patients. AJR Am J Roentgenol 2010; 195: 916–22. doi: https://doi.org/10.2214/AJR.09.3940 [DOI] [PubMed] [Google Scholar]
- 8.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: https://doi.org/10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 9.Akira M, Yamamoto S, Sakatani M. Bronchiolitis obliterans organizing pneumonia manifesting as multiple large nodules or masses. AJR Am J Roentgenol 1998; 170: 291–5. doi: https://doi.org/10.2214/ajr.170.2.9456931 [DOI] [PubMed] [Google Scholar]
- 10.Vasu TS, Cavallazzi R, Hirani A, Sharma D, Weibel SB, Kane GC. Clinical and radiologic distinctions between secondary bronchiolitis obliterans organizing pneumonia and cryptogenic organizing pneumonia. Respir Care 2009; 54: 1028–32. [PubMed] [Google Scholar]
- 11.Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002; 126: 1064–70. doi: https://doi.org/10.1043/0003-9985(2002)126<1064:AFAOP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]