Abstract
Background
Atopic dermatitis (AD) is a common skin disorder with elevated prevalence. Cataract induced by AD rarely occurs in adolescent and young adult patients, which is also called atopic cataract. Using whole exome sequencing, we aimed to explore genetic alterations among AD and atopic cataract.
Result
We recruited a 19 year-old Chinese male with AD accompanied with cataracts, his father with AD and his mother without AD or cataract. Through analysis of the exomic sequence of the 3 individuals from the same family, we identified that with respect to AD, there were 162 genes mutated in both this patient and his father but not in his mother. In addition, we found 10 genes mutated in this patient only without in his parents according to cataract.
Conclusion
This research suggests that coinheritance of mutations in these genes may correlate with AD, and the pathogenesis of AD complicated with cataracts was related to genetic factors.
Keywords: atopic dermatitis, cataracts, mutation
INTRODUCTION
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disease with a worldwide prevalence of 8.7-18.1% in children [1] and 1.5-10.2% in adults [2]. It is characterized by continual itchiness, flares and sleep disturbance, negatively regulating the occupational activities and social relationships of patients, the quality of life of patients and their families [3]. Studies have convinced of a combination of genetic and environmental factors in the pathogenesis of AD. Genetic evidence depicts a complex network comprising epidermal barrier dysfunctions and dysregulation of innate and adaptive immunity in this disease. It has been accepted that mutations in the human filaggrin (FLG) gene are the most significant and well-replicated genetic mutations related to AD. Some other mutations such as SPINK5, SPRR3, and CLDN1 may also correlate with epidermal barriers linked to AD. Genetic variants are able to contribute to the abnormal innate and adaptive responses, such as mutations in IL-1 family cytokines and receptors genes, vitamin D pathway genes, Th2 cytokines genes [4]. A cataract is a clouding of the lens that reduces light transmission to the retina, and it decreases the visual acuity of the bearer. It is one of the severe ocular complications of AD manifested in the eyes. The general classification of cataract includes nuclear, cortical and posterior subcapsular subtypes. Here, we focused on a Chinese male with AD complicated with cataracts via the recently developed whole exome sequencing approach, which has been used to determine the genetic basis of rare diseases.
RESULTS
Clinical description
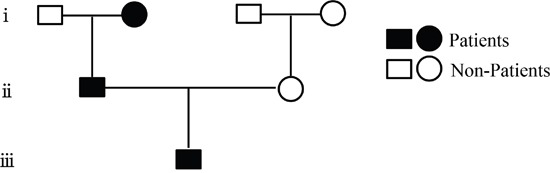
The patient was a 19 year-old Chinese male who was admitted to our hospital with the chief complaint of relapsing generalized skin rash and blurred vision in August, 2015. His rash began from 10 years ago, accompanied with diffuse red papules all over the body, white desquamation, and skin itchiness. He was diagnosed with AD, and treatment without corticosteroids was not effective. There were persistent skin lesions, with obvious itchiness. His skin became dry and flaking, and some area became hard and thick. Seven months ago, his binocular vision became gradually declined. When admitted, red papules and scratches were displayed on the face, neck, trunk, and four extremities, especially on the face and neck (Figure 1). Both eyelids were hard and thick. The right vision was 0.4, and the left vision was 0.1. Keratic precipitates were negative, but both lens were turbid (Figure 2), of which the right one was more severe than the left. Hemogram analysis revealed eosinophile granulocyte 0.8×109/l (12.1% in WBC), and immunological studies showed that expression of IgE was strongly elevated (>3000.00IU/ml). Other investigations such as expression of complements, immune complex, subpopulation of T cells, anti-double stranded DNA antibody, anti-nuclear antibody were normal. Serum allergen test indicated that combination of willow/poplar/elm, crab, shrimp, combination of dermatophagoides pteronyssinus/dermatophagoides culinae were positive allergens. He has a history of eczema and house dust allergy. Interestingly, his father and grandma were diagnosed with AD, respectively (Figure 3).
Figure 1. Appearance of red papules and scratches in the young patient.
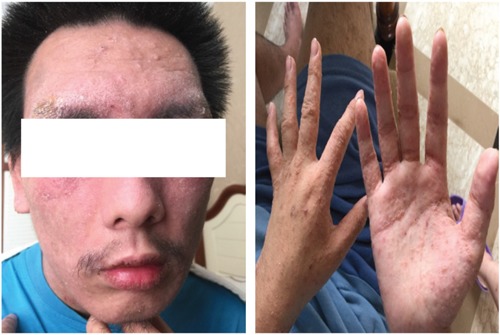
Figure 2. Anterior subcapsular cataracts and posterior subcapsular cataracts in both lens.
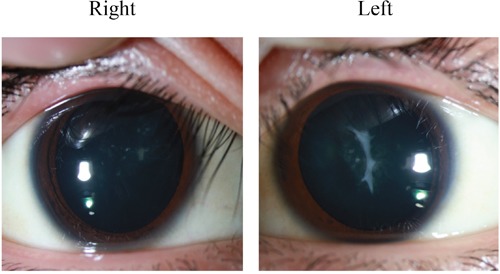
Figure 3. Family pedigree of the atopic dermatitis.
Genetic analysis
Due to the rarity of cataract occurred in this young male with AD, we hypothesized that an underlying genetic alteration might be present in this patient. We discussed the genetic relationship between the patient and his parents by whole exome sequencing. In order to discover the candidate mutations of AD, we searched for the genes both mutated in this patient and his father but not in his mother. Results showed that 162 genes were both mutated in this patient and his father but not in his mother (Table 1, Supplementary Table 1).
Table 1. List of 162 genes both mutated in the patient and his father by whole exome sequencing.
| Chromosome | Position | Gene | SNP | Chromosome | Position | Gene | SNP | ||
|---|---|---|---|---|---|---|---|---|---|
| chr1 | 93646190 | TMED5 | rs185712821 | C/T | chr10 | 75563726 | NDST2 | NA | C/T |
| chr1 | 45271238 | PLK3 | rs55654497 | G/A | chr11 | 5537592 | UBQLNL | rs142657773 | G/C |
| chr1 | 162569107 | UAP1 | rs190156359 | T/A | chr11 | 74915493 | SLCO2B1 | rs192050675 | C/A |
| chr1 | 214537946 | PTPN14 | rs200340171 | G/A | chr11 | 78369215 | TENM4 | rs185503085 | C/T |
| chr1 | 109260438 | FNDC7 | NA | T/C | chr11 | 123988461 | VWA5A | rs202202178 | A/T |
| chr1 | 158533225 | OR6P1 | NA | C/T | chr11 | 124266877 | OR8B3 | rs183842912 | A/C |
| chr1 | 16907303 | NBPF1 | rs681623 | C/T | chr11 | 10585620 | LYVE1 | NA | G/T |
| chr1 | 17083872 | MST1L | rs11545933 | G/A | chr11 | 118376389 | KMT2A | NA | C/T |
| chr1 | 17263277 | CROCC | rs200228265 | G/A | chr11 | 130060375 | ST14 | NA | C/T |
| chr1 | 181019227 | MR1 | NA | G/A | chr11 | 3726498 | NUP98 | NA | G/A |
| chr1 | 232561368 | SIPA1L2 | NA | C/A | chr11 | 6661388 | DCHS1 | rs147698268 | G/A |
| chr1 | 23637401 | HNRNPR | NA | C/T | chr11 | 71249529 | KRTAP5-8 | rs200162819 | G/A |
| chr1 | 248813827 | OR2T27 | rs1782241 | T/C | chr12 | 6669359 | NOP2 | rs142370738 | G/C |
| chr1 | 33237103 | KIAA1522 | NA | C/T | chr12 | 7475081 | ACSM4 | rs7485773 | C/T |
| chr1 | 57411713 | C8B | NA | G/C | chr12 | 105464439 | ALDH1L2 | rs199841702 | G/C |
| chr1 | 86355260 | COL24A1 | NA | C/G | chr12 | 109217071 | SSH1 | rs140582047 | T/A |
| chr1 | 9780232 | PIK3CD | NA | G/A | chr12 | 110221524 | TRPV4 | rs55728855 | C/T |
| chr2 | 90249249 | IGKV1D-43 | NA | T/C | chr12 | 112150408 | ACAD10 | rs145407775 | C/T |
| chr2 | 113343610 | CHCHD5 | rs199612227 | A/G | chr12 | 124097777 | DDX55 | rs117200049 | G/A |
| chr2 | 209108226 | IDH1 | rs186787509 | T/C | chr12 | 108956430 | ISCU | NA | G/C |
| chr2 | 233735070 | C2orf82 | rs200597442 | C/G | chr12 | 11183661 | TAS2R31 | NA | C/A |
| chr2 | 152484095 | NEB | NA | C/G | chr12 | 12966365 | DDX47 | NA | G/A |
| chr2 | 179466289 | TTN | NA | C/T | chr12 | 48104624 | ENDOU | NA | C/T |
| chr2 | 187627500 | FAM171B | NA | A/G | chr12 | 52885339 | KRT6A | rs199613662 | C/T |
| chr2 | 233675986 | GIGYF2 | NA | A/G | chr12 | 6950473 | GNB3 | NA | C/T |
| chr2 | 73315216 | RAB11FIP5 | NA | A/T | chr13 | 103419820 | TEX30 | rs200314758 | T/C |
| chr2 | 97877478 | ANKRD36 | rs10194525 | G/A | chr13 | 96242562 | DZIP1 | NA | T/G |
| chr3 | 7728055 | GRM7 | rs182447901 | C/T | chr14 | 45432003 | FAM179B | rs200775208 | C/T |
| chr3 | 33644578 | CLASP2 | rs117166070 | C/T | chr14 | 68241828 | ZFYVE26 | rs193244014 | G/C |
| chr3 | 49751251 | RNF123 | rs117758999 | G/A | chr14 | 105415264 | AHNAK2 | rs201041268 | G/A |
| chr3 | 112648174 | CD200R1 | rs188572017 | A/T | chr14 | 32256995 | NUBPL | NA | G/A |
| chr3 | 151107788 | MED12L | rs199780529 | T/C | chr14 | 70925106 | ADAM21 | NA | T/C |
| chr3 | 124351317 | KALRN | NA | G/A | chr15 | 45456025 | DUOX1 | rs186783799 | G/A |
| chr3 | 132319977 | CCRL1 | NA | G/A | chr15 | 89402346 | ACAN | rs188663484 | T/C |
| chr3 | 40442466 | ENTPD3 | rs140869368 | G/A | chr16 | 21994499 | UQCRC2 | NA | T/A |
| chr4 | 42119545 | BEND4 | rs187366202 | G/T | chr16 | 15761154 | NDE1 | rs147283674 | C/T |
| chr4 | 47788868 | CORIN | rs186748019 | C/A | chr16 | 55530864 | MMP2 | rs28730814 | G/A |
| chr4 | 52948557 | SPATA18 | rs184617860 | C/T | chr16 | 18849442 | SMG1 | NA | G/A |
| chr4 | 186291928 | LRP2BP | NA | C/T | chr16 | 2287576 | DNASE1L2 | NA | C/T |
| chr4 | 4190576 | OTOP1 | rs2215642 | C/G | chr16 | 28846489 | ATXN2L | NA | T/C |
| chr5 | 38451559 | EGFLAM | rs140968262 | A/G | chr16 | 30100451 | TBX6 | rs202193096 | G/A |
| chr5 | 94814011 | TTC37 | rs143227096 | C/A | chr16 | 456349 | DECR2 | NA | C/T |
| chr5 | 137722246 | KDM3B | rs184734460 | C/G | chr16 | 46637519 | SHCBP1 | NA | A/G |
| chr5 | 178507048 | ZNF354C | rs116562180 | C/G | chr16 | 67991689 | SLC12A4 | NA | G/A |
| chr5 | 128442753 | ISOC1 | NA | G/T | chr16 | 71163611 | HYDIN | NA | T/G |
| chr5 | 149357850 | SLC26A2 | NA | G/T | chr16 | 71961625 | IST1 | NA | C/G |
| chr5 | 171341357 | FBXW11 | NA | G/T | chr16 | 84213027 | TAF1C | NA | C/G |
| chr6 | 26056145 | HIST1H1C | rs79483116 | G/A | chr17 | 76166705 | SYNGR2 | NA | G/A |
| chr6 | 27277365 | POM121L2 | rs61736085 | G/A | chr17 | 36719794 | SRCIN1 | rs118094989 | C/A |
| chr6 | 39847207 | DAAM2 | rs139876341 | A/G | chr17 | 40714796 | COASY | rs200009135 | G/C |
| chr6 | 43017728 | CUL7 | rs146808129 | C/A | chr17 | 48916935 | WFIKKN2 | rs35300894 | G/A |
| chr6 | 83838955 | DOPEY1 | rs188246058 | A/C | chr17 | 55918596 | MRPS23 | rs117734846 | C/T |
| chr6 | 160485490 | IGF2R | rs8191859 | G/A | chr17 | 73096776 | SLC16A5 | rs116126425 | G/A |
| chr6 | 119628121 | MAN1A1 | NA | C/T | chr17 | 11461158 | SHISA6 | NA | A/G |
| chr6 | 143825320 | FUCA2 | NA | A/G | chr17 | 12920199 | ELAC2 | rs140665334 | G/A |
| chr6 | 34512160 | SPDEF | rs375427681 | G/A | chr17 | 14139300 | CDRT15 | rs11867613 | A/G |
| chr6 | 34802049 | UHRF1BP1 | rs368713702 | A/G | chr17 | 14204942 | HS3ST3B1 | NA | T/C |
| chr6 | 39893446 | MOCS1 | rs377167949 | G/A | chr17 | 26823582 | SLC13A2 | NA | G/A |
| chr7 | 75617513 | TMEM120A | rs372363121 | C/T | chr17 | 2966032 | OR1D5 | rs2676564 | C/G |
| chr7 | 141464509 | TAS2R3 | NA | T/C | chr17 | 5036211 | USP6 | rs201674756 | C/T |
| chr7 | 144096938 | NOBOX | NA | G/A | chr17 | 74869016 | MGAT5B | NA | G/A |
| chr7 | 148801869 | ZNF425 | NA | C/G | chr18 | 72913819 | ZADH2 | rs191356988 | A/G |
| chr7 | 2689594 | TTYH3 | NA | G/T | chr18 | 44584631 | KATNAL2 | NA | C/T |
| chr7 | 2962827 | CARD11 | rs3735133 | G/A | chr19 | 50028070 | FCGRT | rs374439544 | C/T |
| chr8 | 8748876 | MFHAS1 | rs201875377 | C/A | chr19 | 54743747 | LILRA6 | rs10403230 | C/G |
| chr8 | 17928855 | ASAH1 | rs11538152 | G/A | chr19 | 4504673 | PLIN4 | rs201143997 | G/A |
| chr8 | 21766971 | DOK2 | rs202013016 | G/A | chr19 | 15730502 | CYP4F8 | rs61746468 | C/T |
| chr8 | 42711517 | RNF170 | rs147488061 | T/C | chr19 | 15839677 | OR10H2 | NA | T/C |
| chr8 | 107691450 | OXR1 | rs200863692 | A/G | chr19 | 18119274 | ARRDC2 | NA | G/A |
| chr8 | 146067346 | ZNF7 | NA | A/G | chr19 | 22846981 | ZNF492 | NA | A/C |
| chr8 | 52733228 | PCMTD1 | rs73592211 | G/A | chr19 | 40743901 | AKT2 | NA | C/T |
| chr8 | 70541824 | SULF1 | NA | C/T | chr19 | 43420636 | PSG6 | rs370759098 | G/A |
| chr9 | 2719083 | KCNV2 | rs143382624 | G/C | chr19 | 58370766 | ZNF587 | rs77577775 | G/A |
| chr9 | 18776971 | ADAMTSL1 | rs117558542 | G/A | chr20 | 31685424 | BPIFB4 | NA | T/C |
| chr9 | 19345978 | DENND4C | rs145052586 | G/A | chr21 | 33690064 | URB1 | rs145519835 | C/T |
| chr9 | 84226764 | TLE1 | rs141959893 | C/T | chr21 | 37584306 | DOPEY2 | rs117132686 | C/A |
| chr9 | 139750000 | MAMDC4 | rs200545888 | T/C | chr21 | 19666690 | TMPRSS15 | NA | C/T |
| chr9 | 131670227 | LRRC8A | NA | C/T | chr22 | 22673302 | IGLV5-52 | NA | C/T |
| chr10 | 25314128 | THNSL1 | rs78131600 | C/T | chr22 | 20127408 | ZDHHC8 | rs200408305 | A/G |
| chr10 | 63810739 | ARID5B | rs201704836 | G/A | chr22 | 46725974 | GTSE1 | rs188655025 | C/G |
| chr10 | 128192832 | C10orf90 | NA | C/T | chrX | 2833605 | ARSD | rs111939179 | C/T |
SNP, single nucleotide polymorphism; NA, not available.
We used OMIM database and GeneCards Database to further interpret these genes and found that 4 genes among the 162 genes might have relationship with the predisposition and/or oncogenesis of AD (Figure 4). To find the candidate mutations of atopic cataracts, we searched for the genes only in this patient without in his parents. We found 10 genes mutated in this patient only without in his parents (Table 2, Supplementary Table 2). Intriguingly, we compared these genes in this special patient with the patients those had been diagnosed with cataracts and had genes mutation, so as to discuss whether these 10 genes are belonging to this special kind of disease. After analyzing the available evidence, we found no data that may suggest these genes have been reported to correlate with cataracts. It is possible that these genes may uniquely belong to AD complicated with cataracts.
Figure 4. Gene prediction scores of the four genes and residual variation intolerance score of the genes.
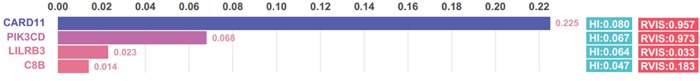
Table 2. List of 10 genes mutated in the patient without in his parents by whole exome sequencing.
| Chromosome | Position | Gene | SNP | |
|---|---|---|---|---|
| chr12 | 11183066 | TAS2R31 | rs138895028 | A/T |
| chr15 | 22473171 | IGHV4OR15-8 | NA | A/G |
| chr17 | 16068287 | NCOR1 | rs201932638 | A/T |
| chr19 | 33490566 | RHPN2 | rs74582927 | T/C |
| chr1 | 16890607 | NBPF1 | rs200783506 | G/A |
| chr22 | 22730788 | IGLV5-45 | NA | G/A |
| chr22 | 22730800 | IGLV5-45 | rs114116194 | A/C |
| chr2 | 90249202 | IGKV1D-43 | NA | G/A |
| chr2 | 90249205 | IGKV1D-43 | NA | A/C |
| chr5 | 140594470 | PCDHB13 | rs17844610 | G/A |
| chr7 | 142149078 | TRBV5-5 | NA | T/G |
| chr7 | 142149017 | TRBV5-5 | NA | G/C |
| chr7 | 142149029 | TRBV5-5 | NA | T/G |
| chr7 | 142149030 | TRBV5-5 | NA | C/G |
| chr7 | 142149058 | TRBV5-5 | NA | T/A |
| chr7 | 142149059 | TRBV5-5 | NA | T/C |
| chr7 | 142149060 | TRBV5-5 | NA | T/C |
| chr7 | 142149066 | TRBV5-5 | NA | A/C |
| chr7 | 142149071 | TRBV5-5 | NA | T/C |
| chr7 | 142149072 | TRBV5-5 | NA | G/A |
| chr7 | 142149075 | TRBV5-5 | NA | A/G |
| chr7 | 142149086 | TRBV5-5 | NA | G/A |
| chr7 | 142149092 | TRBV5-5 | NA | T/A |
| chr7 | 142149101 | TRBV5-5 | rs199978351 | A/G |
| chr9 | 33385750 | AQP7 | rs114484742 | C/T |
SNP, single nucleotide polymorphism; NA, not available.
DISCUSSION
Here, we presented a rare case of AD with cataract, and familial analysis by whole exome sequencing suggested that the pathogenesis of AD was related to genetic factors. Atopic cataract was firstly described in detail in 1936, where the author demonstrated the association of juvenile cataract with AD in 10 out of 101 AD patients, the mean age was 22 year-old, similar to our findings [5]. From 1940 to 1953, an ophthalmological check in 1,158 AD patients showed typical atopic cataract in 136 patients (11.7%) including 79 cases of visual disturbance [6]. To date, literatures describing cataracts in AD are mainly from Asian populations, including the Japanese population reporting the incidence of atopic cataracts around 10-15% [7], Filipino population [8] and Singapore population without Chinese population. Based on this, it seems that a greater interest may exist in Asians, or the prevalence and significance of this disease is greater in these populations. We firstly reported cataracts in a Chinese patient with AD with cataract. Interestingly, his father and his grandma are also AD patients.
It is known that cataract may develop as a result of aging, metabolic disorders, trauma, or heredity. Location of the cataract in the lens regulates visual acuity. There are two types of cataracts in AD patients in subcapsular region, anterior subcapsular cataracts (ASCs) and posterior subcapsular cataracts (PSCs). The literatures about ASCs or PSCs development in AD patients are inconsistent. Disease onset of ASCs is typically rapid, shieldlike bilateral visual impairment [4, 9], therefore, presentation of ASCs seems to be the “classic” cataract because ASCs in the absence of AD is not common [9]. On the contrary, some investigations showed that PSCs may be more common in AD patients [9–12]. In a 29 year-old male, AD presented with bilateral ASCs [13]. Histopathologic analysis of the ASCs tissues indicated a fibrous and amorphous mass, most likely extracellular matrix owing to the presence of irregularly arranged bundled strands of fibrils, typical of collagen. Lens epithelial cells (LECs) at the plaque were densely packed and myofibroblast-like and immunoreactive for alpha-smooth muscle actin. Similarly, a 6 year-old African American girl presented with an uncontrolled flare of AD, and her medical history was significant for asthma and allergic rhinitis with a family history of AD [14]. This was in agreement with our study that the male patient's father and grandmother were also AD. Our results showed that ASCs and PSCs were both existed in the left and right lens of the patient (Figure 2).
Although the pathogenesis of AD complicated with cataract has not been clearly elucidated, severe lesions of AD located over the face may be a critical factor in the development of atopic cataracts. In addition, AD complicated with cataracts may correlate with prolonged usage of corticosteroids and repetitive periorbital scratching [11]. Physical examination of the present patient showed a scratch on the face, neck, trunk, and four extremities, especially on the face and neck, suggesting that AD complicated with cataract in this patient may correlate with scratching. However, several studies reported that the presence of cataracts (both ASCs and PSCs) were not correlated with the disease onset, severity, or duration of AD [15, 16], and the clinical features of AD patients who developed cataracts were similar to the patients who did not have. It is notable that cataract was seen in some patients with only mild facial involvement [16, 17]. On the other hand, systemic corticosteroids are known to cause ocular complications. It is reported that incidence of cataract is dose and treatment duration dependent, where the patients received the equivalent of prednisone, 10 to 15 mg/d for at least 1 year displayed the greatest risk [18]. However, Niwa, et al discussed the incidence of cataract among 3 groups of AD patients [11]. The patients were treated with topical corticosteroids, or treated with both topical and systemic corticosteroids, or corticosteroid-naive patients, respectively. The authors found no difference among these groups. Interestingly, there are 37 patients developed cataract, by which 86% showed posterior cataract [11]. This finding was similar to our study, where the patient had no history of corticosteroids. Tatham, et al reported two boys about 10 year-old diagnosed with widespread AD of the face, neck, trunk and limbs. After diagnosis and treatment with topical steroids for 2 years, both of them complained of gradual onset of blurred vision in both eyes, ophtalmic testing found PSCs in these patients, suggesting that AD and topical corticosteroids may be associated with cataracts in children [19]. Together, whether usage of corticosteroids and scratching may be susceptible factors to AD complicated with cataract is still needed to be clarified in the future with large scales of patients.
Genetic epidemiologic studies on monozygotic twins [20], and genetic association studies indicated a genetic susceptibility for AD [21]. In the present study, four genes including CORIN, CARD11, MMP2, DNASE1L, which were previously reported to be risk factors for AD [22–25], were also mutated in this patient and his father. CARD11 encodes CARMA1, an essential scaffold protein for lymphocyte activation via T cell receptor and B cell receptor signaling [26]. CARMA1 plays important roles in T cell differentiation, regulation of JunB, GATA3 and the subsequent generation of Th2 cell specific cytokines [27]. Mice that are homozygous for the mutation affecting CARMA1 showed gradual development of AD with high level of serum IgE [28]. Li, et al [29] showed that chronic loss of epidermal caspase-8 recapitulates many aspects of AD, such as a spongiotic phenotype whereby intercellular adhesion between epidermal keratinocytes is disrupted, adversely affecting tissue architecture and function. However, subcutaneous injection of matrix metalloproteinase-2 (MMP2) inhibitor strongly down-regulated the intercellular space found in the suprabasal layers of the epidermis [29]. Suppression of MMP2 also restored full length E-cadherin to normal levels and significantly decreased the amount of the cleaved E-cadherin C-terminal fragments product. Transepidermal water loss through the epidermis from caspase-8 conditional knockout mice treated with the MMP2 inhibitor was strongly reduced relative to controls, suggesting that suppression of MMP2 is able to abrogate the effect of caspase-8 knockout induced AD. In a whole exome sequencing study of early-onset AD from a Korean population, Heo, et al discussed family-specific candidate genetic variants from three separate families, and validated the possible genes in 112 AD patients and 61 controls. Results showed that three variants of the COL6A6 gene appeared in all three families and were in close proximity to AD related loci on chromosome 3q21 [30]. The homozygous frequency for the rs16830494 minor allele (AA) and the rs59021909 (TT) allele and the rs200963433 heterozygous (CT) frequency were all higher in AD cases compared to controls, suggesting that COL6A6 variants may be risk factors for AD.
Matsuda, et al [7] discovered that -56 T allele in the IFNGR1 promoter was significantly associated with an increased risk of ocular AD, especially of atopic cataracts. In our study, the whole exome sequencing revealed the -56 CT genotype in both the patient and his father, which contained -56 T allele, whereas his mother harbored -56 CC genotype. The IFNGR1 gene promoter construct that contained the -56 T allele showed higher transcriptional activity in LECs than did the construct with the -56 C allele after stimulation with IFN-γ, and there was higher IFNGR1 expression in the LECs in atopic than in senile cataracts [7], indicating that the -56 T allele in the IFNGR1 promoter leads to elevated IFNGR1 transcriptional activity and represents a genetic risk factor for atopic cataracts. Hori, et al [25] investigated the role of PAI-1, IFN-γ downstream molecule in the pathogenesis of atopic cataracts. They found that the IFN-γ, PAI-1 and TGF-β1 were involved in the pathophysiology of atopic cataracts.
According to the OMIM database and GeneCards Database, we found 4 genes including CARD11, PIK3CD, LILRB3, C8B, may correlate with the pathogenesis of AD. Among them, CARD11 had been reported to have relation with AD [24]. Phenolyzer were used to examine the association of these candidate genes with AD and we found that PIK3CD, LILRB3, C8B were in the same biosystem with CARD11 in the record of NCBI's Biosystem. According to the result of residual variation intolerance score (RVIS) [31], CARD11 had a RVIS score of-1.39 and a percentile of 4.33%, showing that it was amongst the 4.33% most intolerant of human gene (FDR = 1.87×10-6), and PIK3CD, the 2.72% most intolerant human gene (FDR = 8.11×10-6), had a score of -1.66, while LILRB3 and C8B with positive scores had more common functional variation. The normalized RVIS of CARD11 and PIK3CD was approximated to 1, indicating that these two genes were considered as “intolerant”. PIK3CD had a HI score of 0.607 [32], suggesting that haploinsufficiency of the PIK3CD gene may associate with the pathogenesis of AD (Figure 4).
In conclusion, this is the first report of familial AD with cataracts, and the family-based whole exome sequencing found that 162 genes were both mutated in the young patient and his father, while 10 genes were only mutated in the young patient of AD complicated with cataracts. Further studies with large scale need to discuss the functional role of these genes in AD, especially in AD complicated with cataracts.
MATERIALS AND METHODS
Subjects
There was a 19 year-old young male with AD accompanied with cataracts. His father was AD patient, while his mother was not AD or cataracts patient. All of them were recruited in this study. The grandma was also AD, because of impossibility, the grandma was not included. Patients were collected from the Department of Dermatology of the West China Hospital Sichuan University. AD patients met the diagnostic criteria of Hanifin and Rajka [33]. Data about demographic and clinical features were collected from hospital records or by questionnaire and reviewed by experienced physicians. All subjects gave their written consent to participate before study. This study was approved by the ethics committee of the Sichuan University.
DNA extraction and whole exome sequencing
EDTA anti-coagulated venous blood (10ml) was collected from the young male and his parents. The genomic DNA was extracted using the TIANamp Genomic DNA Kit (Tiangen Biotech, Beijing, China) following the manufacturer's protocol. Whole exome enrichment was performed using Agilent SureSelect Human All Exon Kit 50M (Agilent Technologies, Santa Clara, CA, USA) and sequenced with the Illumina HiSeq 4000 System (HiSeq® 3000/4000 SBS Kit).
Sequence alignment, variant calling, and annotation
The sequenced reads were aligned to the hg19 human reference genome sequence using BWA aln and BWA sampe, and removed PCR duplicates with PICARD. Variations were called by GATK HaplotypeCaller with default parameters, after calling genotyping were jointed together by GATK CombineGVCFs/GenotypeGVCFs. Variants were retained considering reads depth DP>= 8, MQ >=20. Beyond that, variants were annotated by ANNOVAR, filtered by position (non-synonymous or gain/loss of stops), VAF < 0.005 (1000 genome project (2012) and HAPMAP), potential damaging effect (variants that were predicted as damaging variants by at least 2 databases, including SIFT, PolyPhen2 HDIV, PolyPhen2 HVAR, LRT, MutationTaste, MutationAssessor, FATHMM, GERP++, PhyloP and SiPhy).
SUPPLEMENTARY MATERIALS TABLES
Footnotes
CONFLICTS OF INTEREST
The authors report no declarations of interest.
GRANT SUPPORT
This work was supported by grants from the National Natural Science Foundation of China (81372504 and 81241068).
REFERENCES
- 1.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132–1138. doi: 10.1016/j.jaci.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 3.Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192–199. doi: 10.1111/j.1525-1470.2005.22303.x. [DOI] [PubMed] [Google Scholar]
- 4.Liang Y, Chang C, Lu Q. The Genetics and Epigenetics of Atopic Dermatitis-Filaggrin and Other Polymorphisms. Clin Rev Allergy Immunol. 2016;51:315–328. doi: 10.1007/s12016-015-8508-5. [DOI] [PubMed] [Google Scholar]
- 5.Brunsting LA. Atopic dermatitis (disseminated neurodermatitis) of young adults. Arch Derm Syphilol. 1936;34:935–957. [Google Scholar]
- 6.Brunsting LA, Reed WB, Bair HL. Occurrence of cataracts and keratoconus with atopic dermatitis. AMA Arch Dermatol. 1955;72:237–241. doi: 10.1001/archderm.1955.03730330017003. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda A, Ebihara N, Kumagai N, Fukuda K, Ebe K, Hirano K, Sotozono C, Tei M, Hasegawa K, Shimizu M, Tamari M, Namba K, Ohno S, et al. Genetic polymorphisms in the promoter of the interferon gamma receptor 1 gene are associated with atopic cataracts. Investigative ophthalmology & visual science. 2007;48:583–589. doi: 10.1167/iovs.06-0991. [DOI] [PubMed] [Google Scholar]
- 8.Mondzelewski L, Hagan C, White A. An 18-year-old male with severe bilateral cataracts and atopic dermatitis--a case report and review of the literature. Pediatric dermatology. 2009;26:583–586. doi: 10.1111/j.1525-1470.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RG. Cataract with atopic dermatitis; dermatologic aspects, with special reference to preoperative and postoperative care. Arch Derm Syphilol. 1950;61:433–441. doi: 10.1001/archderm.1950.01530100077010. [DOI] [PubMed] [Google Scholar]
- 10.Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129–132. doi: 10.1159/000308965. [DOI] [PubMed] [Google Scholar]
- 11.Niwa Y, Iizawa O. Abnormalities in serum lipids and leukocyte superoxide dismutase and associated cataract formation in patients with atopic dermatitis. Arch Dermatol. 1994;130:1387–1392. [PubMed] [Google Scholar]
- 12.Castrow FF., 2nd Atopic cataracts versus steroid cataracts. J Am Acad Dermatol. 1981;5:64–66. doi: 10.1016/s0190-9622(81)70079-3. [DOI] [PubMed] [Google Scholar]
- 13.Shu DY, Ong K, Lovicu FJ. Histopathology of Subcapsular Cataract in a Patient with Atopic Dermatitis. Optom Vis Sci. 2017;94:270–276. doi: 10.1097/OPX.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 14.Bair B, Dodd J, Heidelberg K, Krach K. Cataracts in atopic dermatitis: a case presentation and review of the literature. Arch Dermatol. 2011;147:585–588. doi: 10.1001/archdermatol.2010.411. [DOI] [PubMed] [Google Scholar]
- 15.Donshik PC, Hoss DM, Ehlers WH. Inflammatory and papulosquamous disorders of the skin and eye. Dermatol Clin. 1992;10:533–547. [PubMed] [Google Scholar]
- 16.Brandonisio TM, Bachman JA, Sears JM. Atopic dermatitis: a case report and current clinical review of systemic and ocular manifestations. Optometry. 2001;72:94–102. [PubMed] [Google Scholar]
- 17.Garrity JA, Liesegang TJ. Ocular complications of atopic dermatitis. Can J Ophthalmol. 1984;19:21–24. [PubMed] [Google Scholar]
- 18.Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin. 1992;10:505–512. [PubMed] [Google Scholar]
- 19.Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124. doi: 10.1186/1752-1947-2-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen FS, Holm NV, Henningsen K. Atopic dermatitis. A genetic-epidemiologic study in a population-based twin sample. J Am Acad Dermatol. 1986;15:487–494. [PubMed] [Google Scholar]
- 21.Takahashi N, Akahoshi M, Matsuda A, Ebe K, Inomata N, Obara K, Hirota T, Nakashima K, Shimizu M, Tamari M, Doi S, Miyatake A, Enomoto T, et al. Association of the IL12RB1 promoter polymorphisms with increased risk of atopic dermatitis and other allergic phenotypes. Hum Mol Genet. 2005;14:3149–3159. doi: 10.1093/hmg/ddi347. [DOI] [PubMed] [Google Scholar]
- 22.Naeem AS, Zhu Y, Di WL, Marmiroli S, O'Shaughnessy RF. AKT1-mediated Lamin A/C degradation is required for nuclear degradation and normal epidermal terminal differentiation. Cell Death Differ. 2015;22:2123–2132. doi: 10.1038/cdd.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh D, Ding L, Sivaprasad U, Geh E, Biagini Myers J, Bernstein JA, Khurana Hershey GK, Mersha TB. Multiple Transcriptome Data Analysis Reveals Biologically Relevant Atopic Dermatitis Signature Genes and Pathways. PLoS One. 2015;10:e0144316. doi: 10.1371/journal.pone.0144316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Sakashita M, Yamada T, Fujieda S, Tanaka S, Doi S, Miyatake A, Enomoto T, Nishiyama C, et al. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat Genet. 2012;44:1222–1226. doi: 10.1038/ng.2438. [DOI] [PubMed] [Google Scholar]
- 25.Hori K, Matsuda A, Ebihara N, Imai K, Mori K, Funaki T, Watanabe Y, Nakatani S, Okada K, Matsuo O, Murakami A. Involvement of plasminogen activator inhibitor-1 in the pathogenesis of atopic cataracts. Investigative ophthalmology & visual science. 2012;53:1846–1851. doi: 10.1167/iovs.11-8380. [DOI] [PubMed] [Google Scholar]
- 26.Hara H, Ishihara C, Takeuchi A, Xue L, Morris SW, Penninger JM, Yoshida H, Saito T. Cell type-specific regulation of ITAM-mediated NF-kappaB activation by the adaptors, CARMA1 and CARD9. J Immunol. 2008;181:918–930. doi: 10.4049/jimmunol.181.2.918. [DOI] [PubMed] [Google Scholar]
- 27.Blonska M, Joo D, Zweidler-McKay PA, Zhao Q, Lin X. CARMA1 controls Th2 cell-specific cytokine expression through regulating JunB and GATA3 transcription factors. J Immunol. 2012;188:3160–3168. doi: 10.4049/jimmunol.1102943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jun JE, Wilson LE, Vinuesa CG, Lesage S, Blery M, Miosge LA, Cook MC, Kucharska EM, Hara H, Penninger JM, Domashenz H, Hong NA, Glynne RJ, et al. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity. 2003;18:751–762. doi: 10.1016/s1074-7613(03)00141-9. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Lasse S, Lee P, Nakasaki M, Chen SW, Yamasaki K, Gallo RL, Jamora C. Development of atopic dermatitis-like skin disease from the chronic loss of epidermal caspase-8. Proc Natl Acad Sci U S A. 2010;107:22249–22254. doi: 10.1073/pnas.1009751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heo WI, Park KY, Jin T, Lee MK, Kim M, Choi EH, Kim HS, Bae JM, Moon NJ, Seo SJ. Identification of novel candidate variants including COL6A6 polymorphisms in early-onset atopic dermatitis using whole-exome sequencing. BMC medical genetics. 2017;18:8. doi: 10.1186/s12881-017-0368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrovski S, Wang Q, Heinzen EL, Allen AS, Goldstein DB. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 2013;9:e1003709. doi: 10.1371/journal.pgen.1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang N, Lee I, Marcotte EM, Hurles ME. Characterising and predicting haploinsufficiency in the human genome. PLoS Genet. 2010;6:e1001154. doi: 10.1371/journal.pgen.1001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohme M, Svensson A, Kull I, Wahlgren CF. Hanifin's and Rajka's minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785–792. doi: 10.1067/mjd.2000.110070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


