Abstract
Studies have reported that cholecystectomy may increase the risk of cholangiocarcinoma. However, this association is controversial. Thus, we conducted a systematic review and meta-analysis to explore the relationship between cholecystectomy and the risk of cholangiocarcinoma. Relevant studies were identified by searching PubMed, EMBASE, ISI Web of Science published before February 2017. We used the random effects model proposed by DerSimonian and Laird to quantify the relationship between cholecystectomy and risk of cholangiocarcinoma. Publication bias was evaluated using funnel plots, Begg's and Egger's tests. Subgroup and sensitivity analyses were performed to validate the stability of the results. 16 articles, comprising 220,376 patients with cholecystectomy and 562,392 healthy controls, were included in our research. Our meta-analysis suggested that the risk of cholangiocarcinoma was significantly higher in the cholecystectomized patients in comparison with healthy controls, with heterogeneity among studies (summary odds ratio [OR] = 0.72; confidence interval [CI] = 0.55–0.90; I2 = 69.5%). Additionally, this association was also observed in cohort studies (OR = 0.83; 95% CI = 0.73–0.94) and case-control studies (OR = 0.60; 95% CI = 0.40–0.80). However, When the intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma were analyzed separately, the present study only indicated cholecystectomy was associated with increased the risk of extrahepatic cholangiocarcinoma (OR = 1.19; 95% CI = 0.32–2.05), rather than intrahepatic cholangiocarcinoma (OR = 1.19; 95% CI = 0.32–2.05). In conclusion, cholecystectomy was associated with a significant 54% increase in the risk of cholangiocarcinoma, especially in the extrahepatic cholangiocarcinoma.
Keywords: cholecystectomy, cholecystolithiasis, cholangiocarcinoma, biliary tract neoplasms, meta-analysis
INTRODUCTION
Gallstones are abnormal masses of a solid mixture of cholesterol crystals, mucin, calcium bilirubinate, and proteins [1]. Gallstone is the most common gastrointestinal disease. An estimated 10% of Europeans and Americans are carriers of gallbladder stones [2]. Furthermore, along with the improvement of living standards and population overall life extension, the incidence of cholecystolithiasis seems to be increasing [3, 4]. Additionally, gallstone is the most expensive gastrointestinal diseases, and become a global health burden [5]. For example, it costs of $6.5 billion approximately annually in the U.S [6]. Most of gallstones are silent. However, around 25% of gallstones are symptomatic and accompanied with severe complications, which need to remove the gallbladder by surgically, usually by laparoscopic cholecystectomy [7, 8]. An estimated 700,000 cholecystectomies are conducted annually in the US [3]. Over the past few decades, cholecystectomy has been reported to increase the risk of some types of cancer, including colorectal cancer, liver cancer and pancreatic cancer [9–14]. Recently, studies reported cholecystectomy may increase the risk of cholangiocarcinoma. However, this association is controversial [15–20].
Cholangiocarcinoma, which was first described by Durand-Fardel in 1840, is a malignant tumor originating from bile duct epithelium [21]. Cholangiocarcinoma is the second commonest primary liver cancer, as it accounts for 10%–25% of liver malignant tumors and 3% of all gastrointestinal neoplasms [22, 23]. Moreover, the incidence of cholangiocarcinoma still has been increasing over the past few decades. Somewhat surprisingly, the epidemiological characteristics between intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) are different, the incidence of ICC have been increasing; On the contrary, the incidence of ECC have been declining in some parts of the world, such as UK and USA [24]. In the United States, the age-adjusted incidence of ICC increased by 165%, whereas ECC declined by 14% during the past two decades [25]. Besides, the prognosis of cholangiocarcinoma is particularly poor. The overall 1-, 3- and 5-year relative survival rates are reportedly 25.0%, 9.7% and 6.8%, and almost no changes in recent decades [26]. Thus, to better understand the relationship between cholecystectomy and the risk of cholangiocarcinoma, we conducted a systematic review with meta-analysis of published observational studies.
RESULTS
Study selection and study characteristics
Figure 1 shows the process of selecting studies. We obtained 13291 articles through the initial search (8124 from PubMed, 1879 from EMBASE, 3288 from Web of Science), 3120 of which were duplicates. We excluded a further 10274 studies based on title and abstract review. Finally, four studies were further excluded due to providing insufficient information [27–30], we identified 16 eligible observational articles for our meta-analysis [15–20, 31–40].
Figure 1. The process of study selection for the meta-analysis.
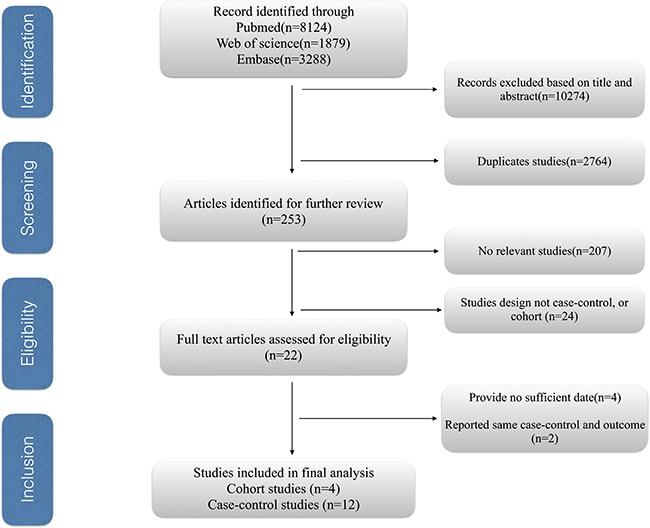
The main characteristics of the included studies are listed in Table 1. Six studies were performed in China, four in the USA, two in Denmark, one in Greece, one in Swedish, one in Korea and one in Taiwan. All included studies were observational studies and included 12 case-control studies and four cohort studies. The meta-analysis included 220,376 patients with cholecystectomy and 562,392 healthy controls to investigate the effect of cholecystectomy on the risk of cholangiocarcinoma. The data collected in the study ranged from 1965 to 2014. The NOS scores of the included studies ranged from 5 to 9, with 12 high quality studies and only four of medium quality (Supplementary Tables 1 and 2).
Table 1. The main characteristics of the included studies.
| Study/Years of Publication | Country | No. Case/control | Follow | Sources of Controls | Subtype of cancer | Subtype of study | Adjusted Factors | Adjusted OR/RR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Lee.2015 | korea | 276/452 | 2007–2013 | Hospital | CC | Case-control | Cigarette smoking, heavy alcohol consumption, obesity, choledocholithiasis, cholecystolithiasis, hepatolithiasis, ulcerative colitis, alcoholic liver disease, thyroid disease, chronic pancreatitis, pypertension, diabetes mellitus, HBV infection, HCV infection, liver fluke infestation | 1.38(0.67, 2.84) |
| Zhang.2014 | China | 127/254 | 1993–2013 | Hospital | ICC | Case-control | Age, sex, BMI, Smoking, Alcohol consumption, HBV infection, HCV, Liver cirrhosis. | 1.53(0.52, 4.49) |
| Chow.1999 | Denmark | 17715/42461 | 1977–1989 | Population | CC | Cohort | age and gender | 1.12(0.81, 1.43) |
| WELZEL.2007 | USA | 1084/102782 | 1993–1999 | Population | CC | Case-control | age, sex, race/ethnicity, cholecochal cysts, cholangitis, biliary cirrhosis, cholelithiasis, cholecystolithiasis, choledocholithiasis, liver flukes, alcoholic liver disease, nonspecific cirrhosis, HCV infection, diabetes mellitus type II, crohn’s disease, ulcerative colitis, duodenal ulcer, chronic pancreatitis, smoking, obesity | 5.4(3.9, 7.5) |
| Chen.2014 | Taiwan | 5850/62180 | 2000–2014 | Population | ECC | Cohort | sex, age and number of comorbidities | 2.22(0.91, 5.41) |
| Tao.2009 | China | 188/380 | 1998–2008 | Hospital | CC | Case-control | age, gender, diabetes mellitus | 3.6(0.9, 15.1) |
| WELZEL.2006 | Denmark | 764/3056 | 1978–1991 | Population | ICC | Case-control | Alcoholic liver diseases, nonspecific cirrhosis, cholangitis, choledocholithiasis, inflammatory bowel disease, diabetes, obesity | 1.56(0.65, 3.73) |
| Cai.2011 | China | 313/608 | 2000–2004 | Hospital | ECC1 | Case-control | choledocholithiasis, hepatolithiasis, cholecystolithiasis, biliary ascariasis, liver fluke and liver schistosomiasis were the risk factors for HC, while HBV infection, HCV infection, PSC, UC, alcoholic liver disease, type II diabetes mellitus, alcohol and smoking | 7.01(1.90, 25.95) |
| Zhou.2013 | China | 239/478 | 1999–2011 | Hospital | ECC | Case-control | sex, age (as continuous variable), liver cirrhosis, cholecystolithiasis, choledocholithiasis, hepatolithiasis, diabetes mellitus and family history of other cancer. | 4.04(1.58, 10.31) |
| CHALASANI.2000 | USA | 26/87 | 1991–1998 | Hospital | CC | Case-control | PSC and geographic location. | 7.11(2.71, 18.67) |
| Liu.2011 | China | 87/288 | 2000–2008 | Hospital | CC | Case-control | HBV infection, HCV infection, and liver fluke infestation, Diabetes mellitus, hypertension, alcohol, smoking, | 0.76(0.46, 1.24) |
| Kuper.2001 | Greece | 6/360 | 1995–1998 | Hospital | CC | Case-control | Years of schooling, tobacco smoking, excessive alcohol consumption or coffee drinking | 2.39(0.27, 21.22) |
| Shaib.2007 | USA | 248/236 | 1992–2002 | Hospital | CC | Case-control | race, age, gender, HCV, HBV markers, and mild/moderate alcohol drinking. | 1.1 (0.6, 2.2) |
| Nogueira.2014 | USA | 118/3681 | 1992–2005 | Population | CC | Cohort | age and gender | 1.19(0.98, 1.43) |
| Nordenstedt.2012 | Swedish | 192960/345251 | 1965–2008 | Population | CC | Cohort | age, sex and gender | 1.28(1.14, 1.43) |
| Peng.2011 | China | 98/126 | 2002–2009 | Hospital | ICC | Case-control | HBV infection, cirrhosis, hepatolithiasis, choledocholithiasis, cholecystolithiasis, and liver fluke infestation, Diabetes mellitus, Hypertension | 1.08 (0.42, 2.81) |
ICC, intrahepatic cholangiocarcinoma. ECC, extrahepatic cholangiocarcinoma. HBV, hepatitis B virus. HCV, hepatitis C virus. RR, relative risk. OR, odds ratio. CI, confidence interval.
Association between cholecystectomy and the risk of cholangiocarcinoma
Four cohort and 12 case-control studies were included to investigate the relationship between cholecystectomy and the risk of cholangiocarcinoma. Six studies reported significantly higher risk of cholangiocarcinoma in patients who had cholecystectomies in comparison with the healthy controls. Only one studies reported cholecystectomy was associated with a decreased risk of cholangiocarcinoma. The remaining of the studies did not show a relationship. The pooled estimate was significant (OR = 1.54; 95% CI = 1.15–1.94), with significant heterogeneity (I2 = 69.5%; p = 0.006) (Figure 2). The present study indicated a 54% increase in the risk for cholangiocarcinoma among the cholecystectomized patients in comparison with healthy controls. However, this relationship was only observed in ECC (OR = 2.31; 95% CI = 1.34–3.28, I2 = 86.3%), rather than ICC (OR = 1.40; 95% CI = 0.94–1.87, I2= 68.2%) (Table 2).
Figure 2. Forrest plot showing the relationship between cholecystectomy and the risk of cholangiocarcinoma.
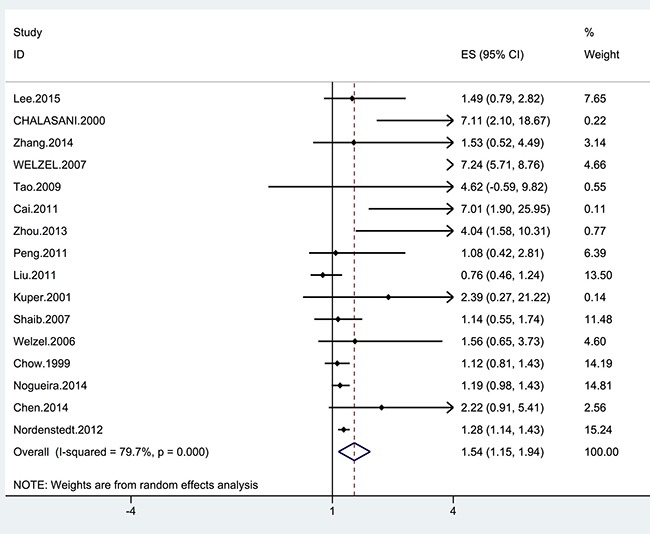
Points represent the risk estimates for each individual study. Horizontal lines represent 95% confidence intervals, and diamonds represent the summary risk estimates with 95% confidence intervals. ICC, intrahepatic cholangiocarcinoma. ECC, extrahepatic cholangiocarcinoma. CI, confidence interval. ES, effect size.
Table 2. Subgroup and sensitivity analyses of the effect of cholecystectomy and the risk of cholangiocarcinoma.
| Subgroup | No. of studies | RR (95%CI) | I2 value(%) | P value |
|---|---|---|---|---|
| All studies | 16 | 1.54 (1.15, 1.94) | 86.3 | 0.001 |
| Subtype of cancer | ||||
| ECC | 9 | 2.31 (1.34, 3.28) | 86.3 | 0.001 |
| ICC | 10 | 1.40 (0.94, 1.87) | 68.2 | 0.001 |
| Geographic areas | ||||
| West | 8 | 1.71 (1.19, 2.23) | 88.8 | 0.001 |
| East | 8 | 1.17 (0.65, 1.69) | 16.7 | 0.298 |
| Study deign | ||||
| Cohort study | 4 | 1.24 (1.12, 1.35) | 0 | 0.618 |
| Case-control study | 12 | 2.31 (1.23, 3.39) | 84.7 | 0.001 |
| Adjustment for confoundersLiver fluke infestation | ||||
| Yes | 5 | 2.68 (0.53, 4.82) | 94.0 | 0.001 |
| No | 11 | 1.24 (1.13, 1.35) | 0 | 0.695 |
| Cholangitis | ||||
| Yes | 4 | 5.12 (0.64, 9.59) | 88.9 | 0.001 |
| No | 12 | 1.21 (1.10, 1.31) | 0 | 0.449 |
| Gallstone | ||||
| Yes | 6 | 3.09 (0.80, 5.39) | 89.7 | 0.001 |
| No | 10 | 1.17 (1.02, 1.32) | 17.6 | 0.281 |
| Smoking | ||||
| Yes | 6 | 2.83 (0.51, 5.16) | 92.5 | 0.001 |
| No | 10 | 1.24 (1.13, 1.35) | 0 | 0.610 |
| Alcohol intake | ||||
| Yes | 8 | 2.24 (0.96, 3.53) | 89.5 | 0.001 |
| No | 8 | 1.24 (1.13, 1.35) | 0 | 0.431 |
| Sensitive analyses | ||||
| High quality studies | 12 | 1.73 (1.19, 2.28) | 84.6 | 0.001 |
| Fixed-effects vs random-effects model method | ||||
| Fixed-effects model | 16 | 1.24 (1.13, 1.34) | 76.3 | 0.001 |
| Random-effects model | 16 | 1.54 (1.15, 1.94) | 79.7 | 0.001 |
ICC, intrahepatic cholangiocarcinoma. ECC, extrahepatic cholangiocarcinoma. RR, relative risk; CI, confidence interval
Subgroup and sensitivity analyses
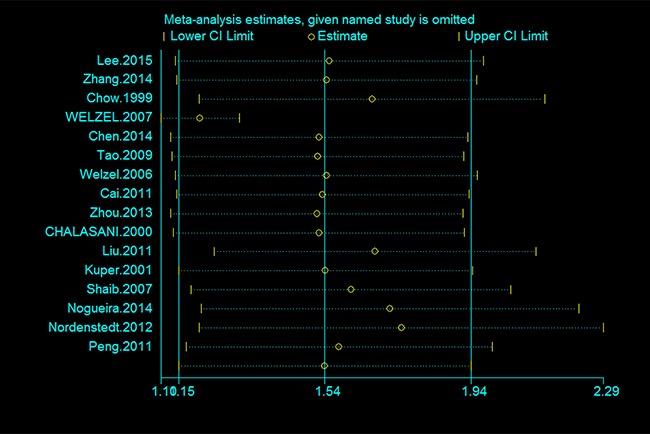
The results of the subgroup analyses and sensitivity analyses are shown in Table 2. When the studies from Western countries (USA, Denmark, Greece and Swedish) and Eastern countries (Taiwan, Korea and China) were analyzed, a significant difference was found between the two areas. Patients with cholecystectomy in western countries were more likely to develop cholangiocarcinoma compared to eastern countries (western countries: OR = 1.71; 95% CI = 1.19–2.23 and eastern countries: OR = 1.17; 95% CI = 0.65–1.69) (Table 2). According to the sensitivity analyses, despite excluding studies that the NOS sources were < 7, the relationship between cholecystectomy and the risk of cholangiocarcinoma remained stable (Table 2). Additionally, the overall results for the relationships of cholecystectomy to cholangiocarcinoma were maintained when the pooling model was altered (fixed-effects model: OR = 1.24; 95% CI = 1.13–1.34 and random-effects model: OR = 1.54; 95% CI = 1.15–1.94) (Table 2). Besides, when we sequentially excluded one study in one turn to assess the stability of the results, no study could possibly affect the pooled risk estimate (Figure 3).
Figure 3. Sensitivity analysis of the association between cholecystectomy and the risk of cholangiocarcinoma.
Publication bias
The funnel plot did not reveal substantial asymmetry. Additionally, Begg's and Egger's tests did not identify substantial publication bias (p > 0.05) (Figure 4).
Figure 4. Funnel plot of studies included in the meta-analysis of the relationships between cholecystectomy and the risk of cholangiocarcinoma.
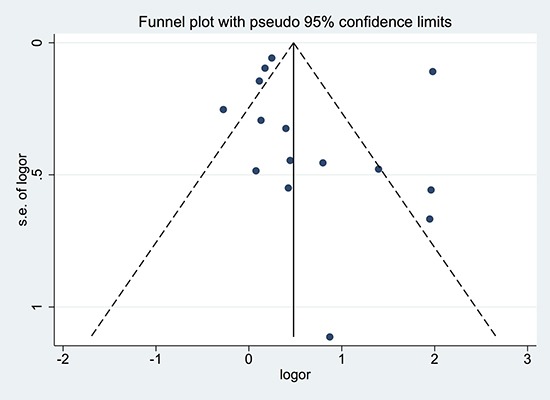
Logor: Log odds ratio. SE: standard error.
DISCUSSION
The causes of cholangiocarcinoma remain poorly understood. Only a few risk factors for the disease have been identified. These include primary sclerosing cholangitis, hepatolithiasis, bile-duct cysts and parasitic infections [41]. Many recent meta-analyses have identified additional factors that may affect the risk of cholangiocarcinoma, including hepatitis B or C, obesity, diabetes mellitus, cirrhosis, alcohol consumption, smoking [42–46]. To our knowledge, this is the first comprehensive meta-analysis to investigate the relationship between cholecystectomy and the risk of cholangiocarcinoma. 16 studies were identified to examine the effect of cholecystectomy on the risk of cholangiocarcinoma and found that the risk of cholangiocarcinoma was significantly higher in 220,376 patients with cholecystectomy compared with 562,392 healthy populations (OR = 0.72; 95% CI = 0.55–0.90), with significant heterogeneity among studies. This effect also was observed in cohort and case-control studies. When the analysis was stratified by geographic area, this effect was more pronounced in Eastern countries in comparison with Western countries. However, When the ICC and ECC were analyzed separately, the present study only indicated cholecystectomy was associated with increased risk of ECC (OR = 1.19; 95% CI = 0.32–2.05), rather than ICC (OR = 1.19; 95% CI = 0.32–2.05).
Our study only demonstrated an association between cholecystectomy and an increased risk of cholangiocarcinoma; the data cannot establish a causative role for cholecystectomy in this regard. However, if such a causative role is present, possible mechanisms could be the following. First, several authors consider it as the effect of gallstones, rather than the ensuing cholecystectomy, which results in cancer. Early study indicated gallstones may increase the risk of cholangiocarcinoma, especially in ECC [15, 47, 48]. Second, removal of the gallbladder leads to the accumulation of bile and secondary bile acids, and secondary bile acids was associated with increased in the presence of gallstones [49–51]. Additionally, previous studies also reported secondary bile acids can enhance tumor formation in the liver [52]. Because both cholangiocytes and hepatocytes differentiate from the same progenitor cells, similar to the carcinogenesis of hepatocytes [53], secondary bile acids might induce carcinogenesis in cholangiocytes through the same mechanism.
Our study has several strengths. First, it is the first meta-analysis with a large sample size (220,376 patients with cholecystectomy and 562,392 healthy populations) to evaluate the effect of cholecystectomy on the risk of cholangiocarcinoma. Therefore, the findings may provide us insight into the relationship between cholecystectomy and the risk of cholangiocarcinoma, and these results are of potential interest to the field of cholangiocarcinoma research. Secondly, subgroup and sensitivity analyses were performed to determine the factors that may affect results. It makes our findings more reliable. Third, we performed a comprehensive literature search of the PubMed, EMBASE and Web of Science databases to identify potential studies to investigate the relationships between cholecystectomy and the risk of cholangiocarcinoma. In addition, most of the studies included in our meta-analysis were of high quality. All of these characteristics make the conclusions of our study more convincing.
There are several limitations that should be considered. First, most of the studies included in our meta-analysis were case-control studies, which was prone to generate recall and selection biases. Additionally, the heterogeneity among studies was significant because of different study designs and demographic characteristics inconsistency. Second, the present study only investigated the risk of cholangiocarcinoma in patients with cholecystectomy compared with healthy population. As a result of the restricted number of included studies in the analysis, the risk of cholangiocarcinoma in patients with cholecystectomy compared with gallstone patients was not explored. Third, what is being observed is just an association, which is subject to confounding bias. The established risk factors for cholangiocarcinoma include primary sclerosing cholangitis, hepatolithiasis, bile-duct cysts and parasitic infections [41]. However, only a few studies adjusted it in their models. Besides, the results of the present study are subject to diagnostic bias. Patients had cholecystectomies are more likely to undergo physical examination and thus might be more likely to have cholangiocarcinoma detected early. Finally, the length of time necessary following a cholecystectomy for any carcinogenic effect to have occurred remains unknown. It is likely that some cases of cholangiocarcinoma included in this research occurred too soon after cholecystectomy [39, 40].
In summary, our meta-analysis indicated that the risk of cholangiocarcinoma was associated with a 54% increase in patients who had cholecystectomies in comparison with healthy controls, and the relationship was also demonstrated in cohort and case-control studies. However, When the ICC and ECC were analyzed separately, the present study only indicated cholecystectomy was associated with increased the risk of ECC, rather than ICC. More prospective studies and basic research are still needed to validate the association of cholecystectomy and cholangiocarcinoma risk and the potential mechanisms.
MATERIALS AND METHODS
Data sources and search strategy
We searched published reports in the PubMed, EMBASE and Web of Science databases using the following keywords: (“gallstone” OR “cholelithiasis” OR “cholecystolithiasis” OR “choledocholithiasis” OR “cholecystectomy” OR “gallbladder surgery”) and (“biliary tract cancer” OR “bile duct cancer” OR “biliary tract neoplasms” OR “cholangiocarcinoma”). We placed no restrictions on the language or date of publication.
Eligibility criteria for study selection
The eligibility criteria were as follows: study design (case control or cohort); cholecystectomy as the exposure factor and cholangiocarcinoma or bile duct cancer or biliary tract cancer as the outcome; and odds ratio (OR)/risk ratio (RR) values and corresponding 95% confidence intervals available or sufficient information to calculate them. If two studies reported the same data, we selected the study with the larger sample.
Data abstraction and quality assessment
Two researchers (Y.W. and A.W.) independently extracted the required information from the selected studies in a standardized manner. We collected the following information from each article: first author's name, year of publication, country of origin, study design (case-control or cohort), number of participants, duration of follow-up, sources of controls, adjustment for confounding variables, and OR/RR values and 95% CIs.
The Newcastle-Ottawa Scale (NOS) [54] was used to evaluate the quality of the included studies. We assigned quality categories according to the scores of each study. Specifically, NOS scores of <4, 4–6, and 7–9 indicated low-, medium-, and high-quality studies, respectively [55]. The maximum total score was 9 points. We resolved discrepancies by consensus.
Statistical analyses
The OR/RR values and corresponding 95% CIs were used to evaluate the risk of cholangiocarcinoma in with a history of cholecystectomy. We treated hazard ratios as equivalent to RRs. We used the random effects model proposed by DerSimonian and Laird to quantify the relationship between cholecystectomy and the risk of cholangiocarcinoma [56].
The I2 statistic was used to quantify the heterogeneity between studies, and I2 values of 25%, 50%, and 75% represented low, medium, and high heterogeneity, respectively [57]. P values less than 0.1 indicated that clear heterogeneity existed. Publication bias was qualified with funnel plot and Begg's [58] and Egger's [59] tests, and funnel plot asymmetry and P values less than 0.05 indicated the presence of bias.
We also performed subgroup analyses by subtype of cancer, geographic areas, study design and whether liver fluke infestation, cholangitis, gallstones, alcohol intake or smoking were adjusted for in the models. Sensitivity analysis was conducted to assess the stability of the results by sequentially excluding one study in one turn. Additionally, sensitivity analyses were also performed by changing the pooling model (random-effects model or fixed-effects model) and excluded studies that the NOS sources were < 7.
All statistical analyses were performed using STATA version 12.0 (Stata).
SUPPLEMENTARY MATERIALS TABLES
Abbreviations
- ICC
intrahepatic cholangiocarcinoma
- ECC
extrahepatic cholangiocarcinoma
Footnotes
Author contributions
J.X. designed the study and wrote this manuscript. Y.W. and A.W. searched database and reviewed studies. H.H., J.B, J.L, Y.Z, Y.X., and X.S. collected and analyzed data. X.L. and H.Z. coordinated and provided financial support for this work. All of the authors have read and approved the final manuscript. H.Z. is the guarantor for this study.
CONFLICTS OF INTEREST
Declaration of personal interests: None
FUNDING
This work was supported by International Science and Technology Cooperation Projects (2015DFA30650 and 2016YFE0107100), The Capital Special Research Project for the clinical application (Z151100004015170), Capital Special Research Project for Health Development (2014-2-4012), Beijing Nature Science Foundation for Young Scholars Project (7164293), Program for New Century Excellent Talents in University (NCET-11-0288).
REFERENCES
- 1.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–239. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 2.Kratzer W, Mason RA, Kachele V. Prevalence of gallstones in sonographic surveys worldwide. J Clin Ultrasound. 1999;27:1–7. doi: 10.1002/(sici)1097-0096(199901)27:1<1::aid-jcu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Lammert F, Sauerbruch T. Mechanisms of disease: the genetic epidemiology of gallbladder stones. Nat Clin Pract Gastroenterol Hepatol. 2005;2:423–433. doi: 10.1038/ncpgasthep0257. [DOI] [PubMed] [Google Scholar]
- 4.Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res. 2009;50:S406–S411. doi: 10.1194/jlr.R800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 6.Shaffer EA. Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–996. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Ransohoff DF, Gracie WA, Wolfenson LB, Neuhauser D. Prophylactic cholecystectomy or expectant management for silent gallstones. A decision analysis to assess survival. Ann Intern Med. 1983;99:199–204. doi: 10.7326/0003-4819-99-2-199. [DOI] [PubMed] [Google Scholar]
- 8.Stinton LM, Shaffer EA. Epidemiology of Gallbladder Disease: Cholelithiasis and Cancer. Gut Liver. 2012;6:172–187. doi: 10.5009/gnl.2012.6.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giovannucci E, Colditz GA, Stampfer MJ. A meta-analysis of cholecystectomy and risk of colorectal cancer. Gastroenterology. 1993;105:130–141. doi: 10.1016/0016-5085(93)90018-8. [DOI] [PubMed] [Google Scholar]
- 10.Lin G, Zeng Z, Wang X, Wu Z, Wang J, Wang C, Sun Q, Chen Y, Quan H. Cholecystectomy and risk of pancreatic cancer: a meta-analysis of observational studies. Cancer Causes Control. 2012;23:59–67. doi: 10.1007/s10552-011-9856-y. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, He Y, Li T, Xie L, Wang J, Qin X, Li S. Risk of primary liver cancer associated with gallstones and cholecystectomy: a meta-analysis. PLoS One. 2014;9:e109733. doi: 10.1371/journal.pone.0109733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Y, Hu J, Feng B, Wang W, Yao G, Zhai J, Li X. Increased Risk of Pancreatic Cancer Related to Gallstones and Cholecystectomy: A Systematic Review and Meta-Analysis. Pancreas. 2016;45:503–509. doi: 10.1097/MPA.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 13.Ge ZM, Zhao CJ, Wang YM, Qian JB. Cholecystectomy and the risk of esophageal and gastric cancer. Saudi Med J. 2012;33:1073–1079. [PubMed] [Google Scholar]
- 14.Fall K, Ye WM, Nyren O. Risk for gastric cancer after cholecystectomy. Am J Gastroenterol. 2007;102:1180–1184. doi: 10.1111/j.1572-0241.2007.01169.x. [DOI] [PubMed] [Google Scholar]
- 15.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang GW, Lin JH, Qian JP, Zhou J. Identification of risk and prognostic factors for patients with clonorchiasis-associated intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2014;21:3628–3637. doi: 10.1245/s10434-014-3710-x. [DOI] [PubMed] [Google Scholar]
- 17.Liu ZY, Zhou YM, Shi LH, Yin ZF. Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study. Hepatobiliary Pancreat Dis Int. 2011;10:626–631. doi: 10.1016/s1499-3872(11)60106-9. [DOI] [PubMed] [Google Scholar]
- 18.Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016–1021. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 19.Peng NF, Li LQ, Qin X, Guo Y, Peng T, Xiao KY, Chen XG, Yang YF, Su ZX, Chen B, Su M, Qi LN. Evaluation of risk factors and clinicopathologic features for intrahepatic cholangiocarcinoma in Southern China: a possible role of hepatitis B virus. Ann Surg Oncol. 2011;18:1258–1266. doi: 10.1245/s10434-010-1458-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee BS, Park EC, Park SW, Nam CM, Roh J. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a case-control study in Korea. World J Gastroenterol. 2015;21:502–510. doi: 10.3748/wjg.v21.i2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167–179. doi: 10.1159/000077991. [DOI] [PubMed] [Google Scholar]
- 22.Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109–114. doi: 10.1055/s-2007-1007302. [DOI] [PubMed] [Google Scholar]
- 23.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 24.Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, Beck A, Khan SA, Elliott P, Thomas HC. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut. 2001;48:816–820. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Lepage C, Cottet V, Chauvenet M, Phelip JM, Bedenne L, Faivre J, Bouvier AM. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol. 2011;54:306–310. doi: 10.1016/j.jhep.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 27.Ioannou GN. Cholelithiasis, cholecystectomy, and liver disease. Am J Gastroenterol. 2010;105:1364–1373. doi: 10.1038/ajg.2009.737. [DOI] [PubMed] [Google Scholar]
- 28.Urbach DR, Swanstrom LL, Khajanchee YS, Hansen PD. Incidence of cancer of the pancreas, extrahepatic bile duct and ampulla of Vater in the United States, before and after the introduction of laparoscopic cholecystectomy. Am J Surg. 2001;181:526–528. doi: 10.1016/s0002-9610(01)00631-6. [DOI] [PubMed] [Google Scholar]
- 29.Huang YJ, Wu AT, Chiou HY, Chuang MT, Meng TC, Chien LN, Yen Y. Interactive role of diabetes mellitus and female sex in the risk of cholangiocarcinoma: A population-based nested case-control study. Oncotarget. 2017;8:6642–6651. doi: 10.18632/oncotarget.14254. http://doi.org/10.18632/oncotarget.14254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Songserm N, Promthet S, Sithithaworn P, Pientong C, Ekalaksananan T, Chopjitt P, Parkin DM. Risk factors for cholangiocarcinoma in high-risk area of Thailand: role of lifestyle, diet and methylenetetrahydrofolate reductase polymorphisms. Cancer Epidemiol. 2012;36:e89–94. doi: 10.1016/j.canep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, McCashland TM, Reddy KR, Zervos X, Anbari MA, Hoen H. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7–11. doi: 10.1002/hep.510310103. [DOI] [PubMed] [Google Scholar]
- 32.Tao LY, He XD, Cai L, Liu W, Ji WJ, Zhao L, Zhang SM. [Case-control study of risk factors in cholangiocarcinoma]. [Article in Chinese] Zhonghua Zhong Liu Aa Zhi. 2009;31:759–763. [PubMed] [Google Scholar]
- 33.Cai WK, Sima H, Chen BD, Yang GS. Risk factors for hilar cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2011;17:249–253. doi: 10.3748/wjg.v17.i2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Zhou Q, Lin Q, Chen R, Gong Y, Liu Y, Yu M, Zeng B, Li K, Chen R, Li Z. Evaluation of risk factors for extrahepatic cholangiocarcinoma: ABO blood group, hepatitis B virus and their synergism. Int J Cancer. 2013;133:1867–1875. doi: 10.1002/ijc.28196. [DOI] [PubMed] [Google Scholar]
- 35.Kuper H, Lagiou P, Mucci LA, Tamimi R, Benetou V, Trichopoulos D. Risk factors for cholangiocarcinoma in a low risk Caucasian population. Soz Praventivmed. 2001;46:182–185. doi: 10.1007/BF01324254. [DOI] [PubMed] [Google Scholar]
- 36.Welzel TM, Mellemkjaer L, Gloria G, Sakoda LC, Hsing AW, El Ghormli L, Olsen JH, McGlynn KA. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer. 2007;120:638–641. doi: 10.1002/ijc.22283. [DOI] [PubMed] [Google Scholar]
- 37.Nordenstedt H, Mattsson F, El-Serag H, Lagergren J. Gallstones and cholecystectomy in relation to risk of intra- and extrahepatic cholangiocarcinoma. Br J Cancer. 2012;106:1011–1015. doi: 10.1038/bjc.2011.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen YK, Yeh JH, Lin CL, Peng CL, Sung FC, Hwang IM, Kao CH. Cancer risk in patients with cholelithiasis and after cholecystectomy: a nationwide cohort study. J Gastroenterol. 2014;49:923–931. doi: 10.1007/s00535-013-0846-6. [DOI] [PubMed] [Google Scholar]
- 39.Nogueira L, Freedman ND, Engels EA, Warren JL, Castro F, Koshiol J. Gallstones, cholecystectomy, and risk of digestive system cancers. Am J Epidemiol. 2014;179:731–739. doi: 10.1093/aje/kwt322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow WH, Johansen C, Gridley G, Mellemkjær L, Olsen JH, Jr JFF. Gallstones, cholecystectomy and risk of cancers of the liver, biliary tract and pancreas. Br J Cancer. 1999;79:640–644. doi: 10.1038/sj.bjc.6690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 42.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y, Zhao Y, Li B, Huang J, Wu L, Xu D, Yang J, He J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. 2012;12:289. doi: 10.1186/1471-2407-12-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Sheng YY, Dong QZ, Qin LX. Hepatitis B virus and hepatitis C virus play different prognostic roles in intrahepatic cholangiocarcinoma: A meta-analysis. World J Gastroenterol. 2016;22:3038–3051. doi: 10.3748/wjg.v22.i10.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Li J, Li P, Li H, Su T, Zhu R, Gong J. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. J Gastroenterol Hepatol. 2012;27:1561–1568. doi: 10.1111/j.1440-1746.2012.07207.x. [DOI] [PubMed] [Google Scholar]
- 46.Jing W, Jin G, Zhou X, Zhou Y, Zhang Y, Shao C, Liu R, Hu X. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev. 2012;21:24–31. doi: 10.1097/CEJ.0b013e3283481d89. [DOI] [PubMed] [Google Scholar]
- 47.Cai H, Kong WT, Chen CB, Shi GM, Huang C, Shen YH, Sun HC. Cholelithiasis and the risk of intrahepatic cholangiocarcinoma: a meta-analysis of observational studies. BMC Cancer. 2015;15:7. doi: 10.1186/s12885-015-1870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nordenstedt H, Mattsson F, El-Serag H, Lagergren J. Gallstones and cholecystectomy in relation to risk of intra- and extrahepatic cholangiocarcinoma. Br J Cancer. 2012;106:1011–1015. doi: 10.1038/bjc.2011.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pomare EW, Heaton KW. Bile salt metabolism in patients with gallstones in functioning gallbladders. Gut. 1973;14:885–890. doi: 10.1136/gut.14.11.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Linden W, Katzenstein B, Nakayama F. The possible carcinogenic effect of cholecystectomy. No postoperative increase in the proportion of secondary bile acids. Cancer. 1983;52:1265–1268. doi: 10.1002/1097-0142(19831001)52:7<1265::aid-cncr2820520722>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 51.Pomare EW, Heaton KW. The effect of cholecystectomy on bile salt metabolism. Gut. 1973;14:753–762. [PMC free article] [PubMed] [Google Scholar]
- 52.Cameron RG, Imaida K, Tsuda H, Ito N. Promotive effects of steroids and bile acids on hepatocarcinogenesis initiated by diethylnitrosamine. Cancer Res. 1982;42:2426–2428. [PubMed] [Google Scholar]
- 53.Tanaka M, Tanaka H, Tsukuma H, Ioka A, Oshima A, Nakahara T. Risk factors for intrahepatic cholangiocarcinoma: a possible role of hepatitis B virus. J Viral Hepatitis. 2010;17:742–748. doi: 10.1111/j.1365-2893.2009.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YP, Li WQ, Sun YL, Zhu RT, Wang WJ. Systematic review with meta-analysis: coffee consumption and the risk of gallstone disease. Aliment Pharmacol Ther. 2015;42:637–648. doi: 10.1111/apt.13328. [DOI] [PubMed] [Google Scholar]
- 56.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 57.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 58.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 59.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


