Abstract
Culex pipiens mosquitoes cause severe nuisance and transmit human diseases including West Nile. Vector control by insecticides is the main tool to prevent these diseases and diflubenzuron is one of the most effective mosquito larvicides used in many places. Here, high levels of resistance were identified in Cx. pipiens from Italy, with a Resistance Ratio of 128 fold. The phenotype was associated with mutations at amino acid I1043 (I1043M and I1043L) of the Chitin synthase gene, which showed significantly higher frequency in bioassay survivors. Both mutations have been introduced in the Drosophila melanogaster chitin synthase gene using the genome editing method CRISPR/Cas9 and validated to confer significant levels of resistance, although at different levels. The I→M mutation results in a Resistance Ratio >2,900 fold and the I→L mutation >20 fold. Two PCR based diagnostics were developed for monitoring of the resistant mutations in field populations. The findings are of major concern for public health given the importance of diflubenzuron in mosquito control in many places, the intensity of the resistance phenotype and the limited availability of alternative larvicides.
Introduction
Members of the Culex pipiens complex are among the most common mosquitoes in urban areas. In addition to biting nuisance, they are also the main vectors of several arboviruses, such as West Nile Virus (WNV), St. Louis encephalitis (SLE), Sindbis (SINV), Rift Valley fever (RVFV) and Japanese encephalitis (JEV)1, as well as primary vectors of lymphatic filariasis2.
Vector control is the main tool to prevent diseases transmitted by mosquitoes, particularly in the absence of effective vaccines, as is the case for all of the above pathogens except of JEV. A key part of these programs is the reduction of the vector’s population size by chemical control. This includes the use of mosquito adulticides either indoors or outdoors and application of larvicides to breeding sites. The latter are used extensively for the control of Cx. pipiens mosquitoes in urban and semi-urban environments, where the larval habitats are largely present. However, a limited number of mosquito larvicides are available,: (i) temephos, an organophosphate which has been used worldwide for several decades, is currently no longer available in Europe; (ii) microbial bacterial toxins Bacilus thuringiensis israelensis (Bti) and Bacillus (Lysinibacillus) sphaericus (Bs), currently used extensively against mosquito larvae in many regions, although their efficiency may be sometimes affected by certain ecological conditions3,4; (iii) hormonal insect growth regulators (methroprene and pyriproxyfen), which have been used to a lesser extent and (iv) the chitin biosynthesis inhibitor diflubenzuron, currently one of the most effective mosquito larvicides in Europe and other regions.
Diflubenzuron is a member of the Benzoyl(phenyl)urea family (BPUs-Group 15 based on the IRAC grouping system)5. It is an environmentally friendly insecticide that inhibits the chitin biosynthesis process affecting the insect’s growth. A recent study revealed the precise mode of action of BPUs including diflubenzuron, which is the direct interaction with chitin synthase 16.
The progressive reduction in the number of insecticides available for use in Public Health and their intense use can result in the rapid selection of insecticide resistance. Resistance development in Cx. pipiens mosquitoes has not proceeded at the pace observed in other vectors, such as Anopheles and Aedes species7,8. However, a number of low-moderate resistance cases have been reported, against pyrethroids9, temephos10, as well as Bacillus toxins11–13.
Insecticide resistance mechanisms involve mutations at the target site of insecticides, which render them less sensitive to inhibition by decreasing their affinity for the insecticide molecules, as well as detoxification enzymes, which metabolize or sequester the insecticide molecules keeping them away from their target14. Pyrethroid and organophosphate resistance have been associated with both target site mutations and detoxification enzymes in Cx. pipiens9,15, while pyriproxyfen resistance has been associated with the upregulation of P450 oxidases in other mosquito species16.
Diflubenzuron resistance has not been found yet in mosquitoes. However, diflubenzuron resistance was recently reported and characterized in the major agricultural pest Plutella xylostella (Lepidoptera), where a mutation on the C-terminal region of chitin synthase, namely I1042M (P. xylostella numbering), was associated with striking levels of resistance6.
Here, we report the identification of field caught Cx. pipiens populations with high levels of resistance against diflubenzuron and the presence of mutations strongly associated with the resistance phenotype. Given the limited availability of alternative larvicides, the findings are of major concern for public health.
Results
Diflubenzuron bioassays
Two field populations were collected in 2015 and 2016 from Ravenna, Italy, an urban area where diflubenzuron had been used exclusively for mosquito control for several years with approximately 40-60 diflubenzuron based treatments, and tested for their susceptibility to diflubenzuron (Table 1). The “Ravenna 2015” population showed considerable resistance to diflubenzuron (RRLC50 = 32 fold), which increased after one year (“Ravenna 2016”) and approximately 5 diflubenzuron field applications, to RRLC50 = 128 fold, in 2016, exceeding the recommended WHO dosage of diflubenzuron in potable water containers (0.25 ppm)17. The estimated LC90 of the Ravenna population in 2016 is 8.51ppm, dramatically higher than the recommended WHO dosage, indicating a likely significant operational impact of resistance.
Table 1.
Log-dose probit-mortality data for diflubenzuron tested against third-fourth instar larvae of Culex pipiens. *Number of larvae tested.
| Mosquito population | N* | LC50 (95%CL)† | RRLC50‡ | Χ2 (df)§ |
|---|---|---|---|---|
| Benaki 2013 (lab strain) | 120 | 0.002 (0.001–0.003) | 1 | 8.52 (10) |
| Ravenna 2015 | 600 | 0.065 (0.026–0.396) | 32.5 | 16.838 (22) |
| Ravenna 2016 | 600 | 0.257 (0.146–0.619) | 128.5 | 11.860 (10) |
†Lethal concentration 50 in mg L−1 s± 95% Confidence Limits. ‡Resistance Ratio (calculated over the Benaki strain). §Chi-square testing linearity of dose-mortality response with degrees of freedom.
Identification of diflubenzuron resistance mutations
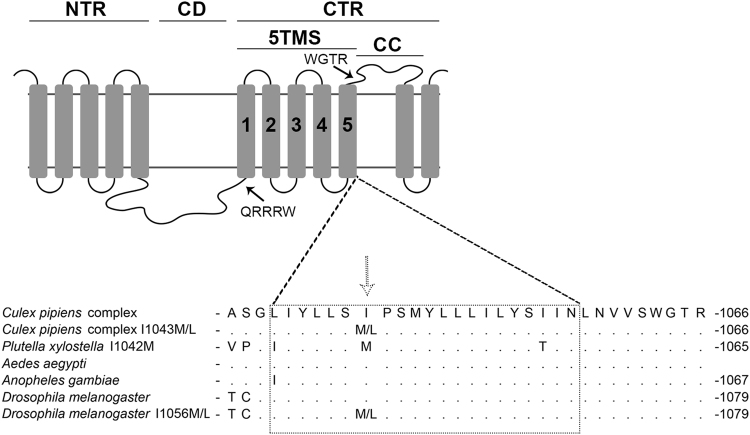
A sequence of 825 bp, part of the chitin synthase C-terminus, the putative binding site of diflubenzuron, spanning the 1043 position (Cx. pipiens numbering) was amplified from Cx. pipiens gDNA (Fig. 1) and examined for the presence of mutations. All individuals from the reference laboratory susceptible strain (Benaki) were homozygous for the wild type IIe (I) amino acid, i.e. the presumed susceptible allelic form6. However, 9 out of 34 individuals tested (26.4%) from the most resistant Ravenna 2016 population (RRLC50 128 fold), carried alleles with mutations at the 1043 site, either the previously characterized I1043M (I1042M based on P. xylostella numbering)6 or the novel I1043L. More specifically, 4 individuals were homozygotes for the mutated alleles (I1043M/I1043M or the combination I1043M/I1043L) and 5 were heterozygotes (I1043/I1043M or I1043/I1043L) (Table 2). The frequency of mutated alleles increased significantly (P value <0.0001, Fisher's test) in the bioassay survivors of the Ravenna population (bioassay doses 0.0187–0.468 ppm): 17 individuals out of 30 tested were homozygous for the mutation I1043M, 13 individuals were homozygous for the I1043L mutation and none had the susceptible allelic form, not even in heterozygote state.
Figure 1.
Schematic representation of the Chitin Synthase and the position of the diflubenzuron resistance mutations. NTR, N-terminal region; CD, catalytic domain; CTR, C-terminal region; 5TMS, cluster of five transmembrane segments; and CC, coiled-coil motif. Rectangular boxes represent transmembrane domains. Arrows point to signature sequences QRRRW (catalytic domain) and WGTR (N-terminal region). Lower part: Aligned amino acid sequences of the fifth helix of the 5TMS (gray rectangular) from different insects. An arrow points to the position I1043 (Cx. pipiens numbering), where the diflubenzuron resistance mutations I1043M and I1043L are found (adapted from ref.25).
Table 2.
Screening of Culex pipiens individuals for the presence of mutations at the I1043 site of the chitin synthase.
| Population | Sample size* (N) | Genotype at the chitin synthase position 1043 | |||||
|---|---|---|---|---|---|---|---|
| Homo† (II) | Homo ‡(MM) | Homo§ (LL) | Hetero(IM) | Hetero(IL) | Hetero(ML) | ||
| Benaki 2013 | 14 | 14 | — | — | — | — | — |
| Ravenna 2016 | 34 | 25 | 3 | — | 2 | 3 | 1 |
| Ravenna 2016 (Survivors) | 30 | — | 17 | 13 | — | — | — |
*Number of individuals tested. †I:Isoleucine amino acid (wild type). ‡M: Methionine amino acid. §L:Leucine amino acid.
Functional characterization of the I1043L mutation
Embryos of the nos.Cas9 Drosophila strain were injected with a gRNA/donor plasmid mix and their progeny was screened in pools for the presence of genome-modified alleles. Out of the 29 flies (G0) that gave progeny, 12 were positive for the presence of genome modified alleles in G1 pools. G1 individuals from the 12 different original G0 lines were outcrossed to the yw strain and then screened to test whether they carried the genome modified allele. G2 individuals from positive G1 parents derived from five original (G0) lines were selected for generating homozygous lines by crossing to balancer flies and screening to identify positive heterozygotes. Several independent homozygous lines were established after balancing and verified by sequencing to be homozygous for the I1056L (equivalent to the I1043L in Cx. pipiens) allele. One of these, the Dif4 line was used to perform toxicity assays with diflubenzuron.
Toxicity assays with diflubenzuron were conducted using the Dif4 line and the parental nos.Cas9 strain as a susceptible control. The previously established diflubenzuron resistant Px39 line harboring mutation I1056M6 was also used, as a positive control. The LC50 value obtained for the nos.Cas9 strain possessing the wild type allele was 0.34 ppm, while the LC50 for the Dif4 line was 7.52 ppm resulting in a resistance ratio of approximately 22-fold (Table 3). The positive (resistant) control strain Px39 did not show any mortality even at the 1,000 ppm dose, which was the highest dose tested in this study.
Table 3.
Bioassay results with diflubenzuron in genome-modified Drosophila.
| Strain | LC50 in ppm (95% CL) | Resistance Ratio (95% CL) |
|---|---|---|
| nos.Cas9 | 0.34 (0.31–0.37) | 1 |
| Dif4 (I1056L) | 7.52 (6.73–8.19) | 22.1 (19.7–24.5) |
| Px39 (I1056M) | >1,000 | >2,941 |
LC50 values (in ppm, mg/L) for diflubenzuron are shown for the control flies (nos.Cas9) and the genome modified flies Dif4 (I1056L) and Px39 (I1056M) with the respective 95% confidence limits. The resistance ratio is calculated over the control flies.
Diagnostic assays
A PCR-RFLP diagnostic assay using the NlaIII endonuclease (5′-CATG-3′) was developed to identify the I1043M diflubenzuron resistance mutation at the C-terminus of the Cx pipiens chitin synthase. The PCR product (124 bp) from the susceptible allele or any other allele without the I1043M mutation is not cleaved by the enzyme. In contrast the I1043M mutation (ATC → ATG) creates a restriction site at the middle (64 bp) of the amplified product producing two fragments of 60 and 64 bp respectively. Thus, homozygous susceptible individuals show a single intact band of 124 bp, homozygous for the mutation individuals show a single band consisting of the 60 and 64 bp fragments and heterozygous individuals show both the 124 bp undigested band and the band consisting of the 60 bp and 64 bp fragments (Fig. 2A, Supplementary Fig. S1).
Figure 2.
Molecular diagnostic assays for the detection of diflubenzuron resistance mutations in Culex pipiens. (A) Diagnostic PCR-RFLP for the mutation I1043M. Upper part: Schematic representation of the assay. The position and sequence of primers used to amplify the 124 bp fragment are presented. The position of the NlaIII restriction site created by the mutation (CATC → CATG) is depicted as a pair of scissors at the middle (64 bp) of the PCR product. Below is shown a representative agarose gel (3%) were the NlaIII digested PCR products are shown. M: HyperLadder V 25pb (Bioline,UK), Lanes 1, 2, 3 individuals homozygous for the wild type allele (II), Lanes 4, 5, 6 individuals heterozygous for the mutated allele (IM), Lanes 7, 8, 9 individuals homozygous for the mutated allele (MM), M2 100 bp DNA ladder. (B) Allele specific PCR for the mutation I1043L.Upper part: Schematic representation of the assay. The position of the four primers used is shown. External_F and External_R primers pair and produce a common 352 bp band. External_F and ATC_ R pair and produce a susceptible (Ile) specific 135 bp band and CTC_F and External_R pair and produce a resistant (Leu) specific 260 bp band. Lower part: Representative agarose gel (2%) with obtained PCR products. M 100 bp ladder, Lane 1, 2 products from homozygous susceptible individuals (II), Lanes 3, 4 products from heterozygous individuals (IL) and Lane 5 product from a homozygous mutated individual (LL). White line denotes the part of the gel that has been cropped and re-grouped. Full-length gels are presented in Supplementary Fig. S1.
For the mutation I1043L, an allele specific PCR was designed to identify the mutated allele. Four primers are used in a single PCR reaction: two common, external primers flanking the site of the mutation asymmetrically, a reverse primer specific for the wild type (susceptible) allele (having at its 3′OH a T) and a forward primer specific for the mutated allele (having at its 3′OH a C). In all reactions a common, control band of 352 bp is produced from the External_F and External_R primers. The ATC_R primer binds specifically to the wild type allele and amplifies with the External_F primer a fragment of 135 bp, while the CTC_F primer binds specifically to the mutated allele, pairs with the External_R primer and produces a 260 bp band. In heterozygotes both bands (135 and 260 bp) are observed (Fig. 2B, Supplementary Fig. S1).
Εach diagnostic is designed to detect the presence of one mutation, either the I1043M or the I1043L (Supplementary Fig. S2). Thus, both have to be applied to gain a clear picture about the presence of mutations associated with diflubenzuron resistance in field populations.
Discussion
High levels of resistance against diflubenzuron, a very important mosquito larvicide for many places in the world (like Europe), was detected in Cx. pipiens, the major vector of WNV, in field populations from Ravenna, in Northern Italy. The presence and frequency of mutations at the aminoacid I1043 (I1043M and I1043L) in the CpCHS gene, an equivalent position to the BPU resistance mutation previously found in P. xylostella, was highly correlated with the resistance phenotype, as well as the ability of Cx. pipiens individuals to survive high dosages of diflubenzuron.
The introduction of I1056L in D. melanogaster, by the genome modification approach CRISPR/Cas9 coupled with homology-directed repair (HDR), showed a significant resistance phenotype (>20-fold) against diflubenzuron, albeit of lower intensity compared with the I1056M mutation, which conferred >15,000-fold diflubenzuron resistance6. Cx. pipiens individuals homozygous for either the mutations I1043M or I1043L survived at diflubenzuron concentrations exceeding the recommended WHO dose (i.e. 0.25 ppm of diflubenzuron, under optimum spraying conditions), which indicates that this resistance can dramatically affect diflubenzuron performance in the field. Indeed this has already been observed following quality control treatment, regularly conducted in the frame of the Emilia-Romagna mosquito control plan (data not published).
The identification of the resistant mutations with an apparent very strong effect on the phenotype, and the subsequent development of molecular diagnostics should substantially facilitate detection and monitoring of resistance in the field. This should delay the spread of the phenotype, particularly at this early stage, where the phenomenon seems very restricted in a small geographical area, based on available bioassay data around the globe, which have indicated a general susceptibility to diflubenzuron in both Culex and Aedes vectors18,19.
Should the molecular diagnostics developed in this study be successfully validated across geographical regions, they will be among the few available for monitoring insecticide resistance alleles, with direct links to operational significant levels of resistance in any mosquito species. Although a large number of molecular diagnostics are available to monitor field mosquito populations for the presence of insecticide resistance alleles (target site mutations or detoxification enzymes), the quantitative contribution of these molecular markers alone or in combination in resistance, and especially their operational significance, is often unclear20. Thus, many researchers would rather use bioassays (standard diagnostic protocols and/or intensity bioassays) to monitor the presence of resistant mosquitoes, their frequency in the population and the levels of resistance. However, bioassays have also practical limitations, such as the number of live mosquitoes required and integral variations. In this particular case, the application of the molecular diagnostics seems operationally more practical than bioassays. In addition the ability to detect heterozygotes, putatively susceptible and not detectable by bioassays based on the recessive nature of the resistant mutation in other species6, is very important for decision making, as it offers the opportunity to manage the phenotype at early stages.
The selection of resistance to diflubenzuron seems to be associated with the extensive use of this larvicide in the Emilia-Romagna region over the past ten years to control Ae. albopictus in road drains, a larval habitat exploited by Cx. pipiens as well. The potential for resistance selection could be high, since within only one year of systematic diflubenzuron applications, resistance increased from 32- to 128-fold in Ravenna.
The findings are of major concern for public health, as diflubenzuron is used in many places for the control of Cx.pipiens mosquitoes, transmitting West Nile and Aedes arbovirus vectors transmitting Dengue, Chikungunya and Zika. This is particularly the case in regions such as Europe, where neurotoxic insecticides have been banned from use in mosquito breeding sites. Screening of Cx.pipiens and Aedes mosquito populations from several geographical regions for possible resistant CHS1 alleles must be conducted to guide appropriate resistance management strategies and ensure the sustainability of control interventions.
Due to the limited number of alternative mosquito larvicides available on the market, resistance is an important consideration for many countries, where diflubenzuron is in use, as the main mosquito larvicide, like Italy and several other European countries. Our finding raises further serious concerns regarding the reduction of reliable biocides for routine and emergency mosquito control, especially in cases of new invasive mosquito species. The development of additional mosquito larvicides and the re-consideration of old active ingredients, such as temephos might need to be strongly supported.
Materials and Methods
Culex pipiens populations
Cx. pipiens mosquitoes were collected during summer 2015 and 2016 as egg rafts in gravid traps in Ravenna, Italy, where mosquito control programs have been conducted with diflubenzuron for ten years. A Cx. pipiens laboratory strain (Benaki) that has not been exposed to insecticides for more than 20 years was included in the study as a reference susceptible strain18. Egg rafts were transferred to the laboratory and reared in climatic rooms at 28 ± 1 °C, 80% RH and 14:10 light:dark photoperiod.
Bioassays with diflubenzuron
Standard WHO larval bioassays21 were performed using third-fourth instar larvae and diflubenzuron (DEVICE SC-15, diflubenzuron 13.9%, Arysta LifeScience, UK) doses ranging from 0.00015 to 0.468 ppm (dilutions made in water). For each concentration, four replicates of 25 larvae were used and emergence inhibition was measured daily until complete mortality or adult emergence was observed. Bioassays were conducted in climatic rooms as specified above. The data were subjected to probit regression analysis (Finney, 1971) using POLO-PC (LeOra Software POLO-PC, Berkeley, CA, USA).
Genomic DNA extraction and analysis of chitin synthase sequences
Genomic DNA was extracted from individual Cx. pipiens adults using the Cethyl Trymethyl Ammonium Bromide (CTAB) method described in22. The DNA pellet was dissolved in 20 μl of sterile water.
A 825 bp fragment of the Cx.pipiens chitin synthase, spanning the 1043 position (numbering based on Cx.pipiens sequence), was amplified in a PCR reaction (25 µl final volume) using genomic DNA from individual mosquitoes, 0.4 μM primers (CHSseqF and CHSseqR) (Supplementary Table S1), 0.4 mM dNTPs, 2.5 µl of 10X buffer and 2 U of Kappa Taq DNA Polymerase (Kappa Biosystems, Inc., Wilmington, MA, U.S.A). The PCR conditions were 95 °C for 5 min followed by 30 cycles of 94 °C for 30 sec, 60 °C for 30 sec, 72 °C for 1 min and a final extension of 72 °C for 10 min. PCR products were purified using a PCR purification kit (Macherey Nagel, Dueren, Germany) and sent for sequencing (Macrogen Sequencing Facility, Amsterdam) using the primer 5′-ACGTTTGCGGGTGTGATGTC-3′.
Functional characterization of I1056L using a genome-modified Drosophila line
For the generation of genome modified Drosophila flies, we used the strain y1 M{nos-Cas9.P}ZH-2A w*23, (nos.Cas9; stock no. 54591 at Bloomington Stock Center, Indiana, USA). For outcrossing and balancing we used strains yw and yw; TM3 Sb e/TM6B Tb Hu e, respectively. As positive controls in diflubenzuron toxicity bioassays, we used genome modified strain Px396. All strains were cultured at 25 °C temperature, 60–70% humidity, and 12/12h photoperiod on standard fly diet.
The CRISPR/Cas9 system coupled with homology-directed repair (HDR) was used to introduce the mutation I1056L to the krotzkopf verkehrt (kkv) gene of Drosophila melanogaster, which is equivalent to the I1043L mutation detected on the chitin synthase 1 of Cx. pipiens (CPIJ016255, Vector base). The genomic engineering strategy followed was similar to the one described in Douris et al.6. Injections were performed with a plasmid mix containing 75 ng/μL of each of the RNA expressing plasmids gRNA444 and gRNA65823, to express gRNAs targeting the relevant genomic positions (Supplementary Fig. S3) and 100 ng/μL of a new specific donor plasmid (I1056L donor) for HDR. The donor plasmid was generated by site-directed mutagenesis using as template the donor plasmid used to generate the I1056M mutation6, in a site directed mutagenesis kit (Quick change II Site directed mutagenesis kit)(Agilent, California, USA), following manufacturer’s instructions, and primers MutI1056L F/R (Supplementary Table S1) to replace M1056 with L1056. The successful replacement was validated with sequencing. A schematic representation of the genome modification strategy is provided in Supplementary Fig. S3.
To screen for genome modified flies DNA was extracted from their tissues as in ref.6, using the DNAzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions followed by PCR amplification using the Kappa Taq DNA polymerase (Kappa Biosystems, Inc., Wilmington, MA, U.S.A) (1 unit), the ‘generic’ DroCHS forward primer (0.4μΜ) and the mutant allele specific DroCHS reverse primer (0.4 μΜ) (Supplementary Table S1). The PCR conditions were: initial denaturation at 95 °C for 5 min followed by 30 cycles of 30 s at 94 °C, 30 s at 60 °C, 40 s at 72 °C and a final extension step at 72 °C for 5 min. A 418 bp specific product is amplified only in the presence of mutated alleles. Screening was performed as in6; initially in pools of circa 30 G1 flies to identify lines where successful CRISPR/Cas9 plus HDR had taken place in G0 (injected) flies and later in individual G1 flies after outcrossing to yw. The progeny of positive G1 flies was crossed to flies carrying a balancer 3rd chromosome (yw; TM3 Sb e/TM6B Tb e) and progeny flies carrying the I1043L mutation opposite to a balancer chromosome were intercrossed in order to generate homozygous lines bearing the genome modified allele. Allelic sequence was verified by sequencing the relevant PCR products (Macrogen Sequencing Facility, Amsterdam).
Second instar D. melanogaster larvae from control (nos.Cas9) and genome modified flies were transferred in batches of 15-20 in vials containing fly food supplemented with six different concentrations of diflubenzuron diluted in water (Dimillin [48% wt/vol diflubenzuron](Syngenta, Basel) causing mortality in the range of 0-100%. Each dose was performed in triplicates and control vials without insecticide were included. Dose-dependent molting and pupal eclosion were monitored for 10–12 days. LC50 values were calculated with Polo Plus (LeOra software).
Molecular diagnostic assays
Following the identification of two mutations at the 1043 site (I1043M and I1043L) all sequences obtained from the screened individuals were aligned using the BioEdit software (Ibis Biosciences, Carlsbad, CA, USA) to identify the presence of single nucleotide polymorphisms spanning the 1043 site and design accurate molecular diagnostic assays.
Assay for the I1043M mutation
A PCR-RFLP diagnostic assay using the NlaIII (5′-CATG-3′) restriction endonuclease was developed to identify individuals carrying the I1043M mutation. A 124 bp PCR fragment flanking the I1043 site was amplified. The reaction was performed at a final volume of 25 ul, using 0.6 ul extracted gDNA, 2.5 μl of the 10X Kappa Taq Buffer A, 0.4 μM of each primer (Diagnostic I1043M_F and Diagnostic I1043M_R)(Supplementary Table S1), 0.2 mM dNTPs (Thermo Fisher Scientific, Waltham, MA, USA), 1.5U of Kappa Taq polymerase (Kappa Biosystems, Inc., Wilmington, MA, U.S.A) and water. The PCR reaction conditions were 5 min for 95 °C followed by 30 cycles of 30 sec at 95 °C, 30 sec at 60 °C, 30 sec at 72 °C and a final extension of 5 min at 72 °C. 5 μl of the PCR product were run on a 3% agarose gel to confirm successful amplification. The remaining 20 μl were digested with NlaIII (New England Biolabs, Ipswich, MA, USA) which selectively cleaves the resistant allele. The final volume of the reaction was 30 μl and included 5U of the restriction enzyme, 3 μl CutSmart Buffer (10X), 3 μl BSA (10 mg/ml) and water. The reaction was incubated for two hours at 37 °C followed by enzyme inactivation at 65 oC for 20 min. The reaction products were run on a 3% agarose gel.
Assay for the I1043L mutation
An allele specific PCR was designed to identify individuals carrying the I1043L mutation, based on the method described in24. The PCR reaction of 25 μl final volume included 0.6 μl gDNA, 0.3 μM of the External_F, External_R and ATCspecific_R primers, 0.6 μM of the CTCspecific_F primer (Supplementary Table S1), 0.2 mM dNTPs (Thermo Fisher Scientific, Waltham, MA, USA), 2.5 μl Kappa Buffer A, 1U Kappa Taq polymerase (Kappa Biosystems, Inc., Wilmington, MA, USA) and water. The PCR reaction conditions were 5 min for 95 °C followed by 28 cycles of 30 sec at 95 °C, 30 sec at 68 °C, 1 min at 72 oC and a final extension of 5 min at 72 °C. PCR products were run on a 2% agarose gel.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Ethics statement
No animals were used to conduct this research. In general, all research activities respect fundamental ethics principles, including those reflected in the Charter of Fundamental Rights of the European Union (2000/C 364/01). The research is compatible with EU and international law, as a number of entomological monitoring activities is contacted worldwide and in Europe (European Mosquito Control Association, http://www.emca-online.eu). The molecular work was carried out in full conformity with Greek regulations consisting of the Presidential Decree (160/91) and law (2015/92) which implement the directive 86/609/EEC from the European Union and the European Convention. The experiments were carried out in certified facilities (license EL91-BIOexp-02) and the protocols have been approved by the Prefecture of Crete (license number # 27290, 15/12/2014).
Electronic supplementary material
Acknowledgements
We thank Yannis Livadaras (Foundation for Research and Technology Heraklion-Crete, Greece) and Dimitra Tsakireli (University of Crete, Greece) for their help with the functional assays.
Author Contributions
Conceived and designed the experiments: J.V., L.G., V.D., R.B., K.M. Performed the experiments: L.G., A.P., M.M. Wrote the paper: J.V., R.B., L.G., V.D.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-23059-1.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12103-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farajollahi A, Fonseca DM, Kramer LD, Marm Kilpatrick A. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. 2011;11:1577–1585. doi: 10.1016/j.meegid.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Lymphatic filariasis, fact sheet. http://www.who.int/mediacentre/factsheets/fs102/en/ (2016).
- 3.Pusztai M, et al. The mechanism of sunlight-mediated inactivation of Bacillus thuringiensis crystals. Biochem J. 1991;273(Pt 1):43–47. doi: 10.1042/bj2730043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, et al. A new formulation of Bacillus thuringiensis: UV protection and sustained release mosquito larvae studies. Scientific reports. 2016;6:39425. doi: 10.1038/srep39425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verloop, A. & Ferrell, C. D. Benzoylphenyl ureas—A new group of larvicides interferingwith chitin deposition. Pesticide Chemistry in the 20th Century. ACS SymposiumSeries, ed Plummer JR (Am Chem Soc, Washington, DC)37, 237–270 (1977).
- 6.Douris V, et al. Resistance mutation conserved between insects and mites unravels the benzoylurea insecticide mode of action on chitin biosynthesis. Proc Natl Acad Sci USA. 2016;113:14692–14697. doi: 10.1073/pnas.1618258113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vontas J, et al. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic Biochem Phys. 2012;104:126–131. doi: 10.1016/j.pestbp.2012.05.008. [DOI] [Google Scholar]
- 8.Ranson H, Lissenden N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Scott JG, Yoshimizu MH, Kasai S. Pyrethroid resistance in Culex pipiens mosquitoes. Pestic Biochem Physiol. 2015;120:68–76. doi: 10.1016/j.pestbp.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Ben Cheikh H, Pasteur N. Resistance to temephos, an organophosphorous insecticide, in Culex pipiens from Tunisia, North Africa. J Am Mosq Control Assoc. 1993;9:335–337. [PubMed] [Google Scholar]
- 11.Paul A, Harrington LC, Zhang L, Scott JG. Insecticide resistance in Culex pipiens from New York. J Am Mosq Control Assoc. 2005;21:305–309. doi: 10.2987/8756-971X(2005)21[305:IRICPF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen-Leroux C, et al. Resistance to Bacillus sphaericus involves different mechanisms in Culex pipiens (Diptera:Culicidae) larvae. J Med Entomol. 1997;34:321–327. doi: 10.1093/jmedent/34.3.321. [DOI] [PubMed] [Google Scholar]
- 13.Darboux I, Charles JF, Pauchet Y, Warot S, Pauron D. Transposon-mediated resistance to Bacillus sphaericus in a field-evolved population of Culex pipiens (Diptera: Culicidae) Cell Microbiol. 2007;9:2022–2029. doi: 10.1111/j.1462-5822.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 14.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 15.Weill M, et al. Comparative genomics: Insecticide resistance in mosquito vectors. Nature. 2003;423:136–137. doi: 10.1038/423136b. [DOI] [PubMed] [Google Scholar]
- 16.Yunta C, et al. Pyriproxyfen is metabolized by P450s associated with pyrethroid resistance in An. gambiae. Insect Biochem Mol Biol. 2016;78:50–57. doi: 10.1016/j.ibmb.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Guidelines for Drinking-Water Quality 3rd edition including 1st and 2nd addenda (2008).
- 18.Kioulos I, Kampouraki A, Morou E, Skavdis G, Vontas J. Insecticide resistance status in the major West Nile virus vector Culex pipiens from Greece. Pest Manag Sci. 2014;70:623–627. doi: 10.1002/ps.3595. [DOI] [PubMed] [Google Scholar]
- 19.Bellinato DF, et al. Resistance Status to the Insecticides Temephos, Deltamethrin, and Diflubenzuron in Brazilian Aedes aegypti Populations. BioMed research international. 2016;2016:8603263. doi: 10.1155/2016/8603263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weetman D, Donnelly MJ. Evolution of insecticide resistance diagnostics in malaria vectors. Trans R Soc Trop Med Hyg. 2015;109:291–293. doi: 10.1093/trstmh/trv017. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides. Geneva: World Health Organization DoVBaC (1981).
- 22.Navajas M, Lagnel J, Fauvel G, De Moraes G. Sequence variation of ribosomal internal transcribed spacers (ITS) in commercially important phytoseiidae mites. Exp Appl Acarol. 1999;23:851–859. doi: 10.1023/A:1006251220052. [DOI] [PubMed] [Google Scholar]
- 23.Port F, Chen HM, Lee T, Bullock SL. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc Natl Acad Sci USA. 2014;111:E2967–2976. doi: 10.1073/pnas.1405500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Torres D, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 25.Van Leeuwen T, et al. Population bulk segregant mapping uncovers resistance mutations and the mode of action of a chitin synthesis inhibitor in arthropods. Proc Natl Acad Sci USA. 2012;109:4407–4412. doi: 10.1073/pnas.1200068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).