Abstract
Background:
Gonadotropin-releasing hormone agonists (GnRH-a) was increasingly used for triggering oocyte maturationfor the prevention of ovarian hyperstimulation syndrome. Studies suggest that GnRH-a might be used as a better trigger agent since it causes both Luteinizing hormone and follicle stimulating hormone release from a physiologic natural cycle.
Objective:
The aim of this study was to evaluate the effect of dual-triggering in assisted reproductive technology outcomes.
Materials and Methods:
192 normal responder women aged ≤42 years and 18< Body Mass Index <30 kg/m2 enrolled in this single-blind randomized controlled trial. All participants received antagonist protocol. For final triggering, women randomly were divided into two groups. Group, I was triggered by 6500 IU human chorionic gonadotropin (hCG) alone, and group II by 6500 IU hCG plus 0.2 mg of triptorelin. The implantation, chemical, clinical and ongoing pregnancy, and abortion rates were measured.
Results:
The mean of retrieved oocytes and obtained embryos were statistically higher in the dual-trigger group (group I), but the implantation and pregnancy rates were similar in two groups.
Conclusion:
The results of our study did not confirm the favorable effect of dual-triggered oocyte maturation with a GnRH-a and a standard dosage of hCG as an effective strategy to optimize pregnancy outcome for normal responders in GnRH-antagonist cycles. We think that this new concept requires more studies before becoming a universal controlled ovarian hyperstimulation protocol in in vitro fertilization practice.
Key Words: Trigger, Gonadotropin-releasing hormone, Agonist, Antagonist, Normal responder, Human chorionic gonadotropin
Introduction
Reducing the rate of sever ovarian hyperstimulation syndrome (OHSS) is one of the ideal goals of controlled ovarian hyperstimulation (COH) in assisted reproduction technology (ART) programs (1, 2). Gonadotropin-releasing hormone agonists (GnRH-a) was used for triggering oocyte maturation for OHSS prevention increasingly. Initially, Gonen et al suggested that GnRH-a might be used as a better trigger since it causes both follicle stimulating hormone (FSH) and Luteinizing hormone (LH) release from a physiologic natural cycle (3). Several studies reported that GnRH-a decreased the implantation rate while it increased the abortion rate in comparison with human chorionic gonadotropin (hCG). This is probability attributed the luteal phase deficiency and decreased endometrial receptivity.
Therefore, modified luteal phase supports estradiol, progesterone and also a low dose of hCG either at oocyte retrieval or during the luteal phase was proposed in these cycles (3-9). The fact that gene expression pattern and downstream LH signaling receptors is different between hCG and GnRH-a triggered patients made several investigators to study the effect of co-administration of GnRH-a and hCG triggering to improve ART outcomes (4-6). Several studies showed significant improvement in in vitro fertilization (IVF) outcomes when a dual trigger was used without a significant increase in OHSS rate in high responders (6-9). Simultaneous administration of a standard dose of hCG and GnRH-a for triggering the final oocyte maturation has been studied in a limited number of studies (6-9).
Schachter et al initially proposed the dual trigger concept in normal responders (8). The investigators hypothesized that GnRH antagonist binding to endometrial GnRH receptors stimulates post-receptor events that reduces implantation rate. They suggested that administering preovulatory GnRH-a displaces bound GnRH antagonist from endometrial GnRH receptors, so that the blocked receptors will be activated and subsequent pre-implantation post-receptor events will be enabled. They demonstrated significantly improved ongoing pregnancy rate in the group who received hCG and GnRH-a for triggering. The other possible explanation in GnRH agonist-triggered cycles is the induction of FSH surge in addition to LH surge, which has been postulated to be instrumental in resumption of the oocyte’s meiotic processes, conferring some advantages for embryos development (8).
Regarding this promising results, we studied whether dual triggering for final oocyte maturation with a single dose of GnRH-a and a standard dose of hCG could improve pregnancy outcome in GnRH-antagonist IVF/ Intracytoplasmic sperm injection (ICSI) cycles in normal responders.
Materials and methods
All normal responder women by tubal or male infertility factor which has been referred to Yazd Research and Clinical Center for Infertility between April 2014 to February 2015 were enrolled in this single-blind randomized controlled trial.
The inclusion criteria were 18< Body mass index <30 kg/m2, and age ≤42 yr with the history of infertility for at least 1 yr that were candidate for ART protocol. Our exclusion criteria were the presence of endocrine disorders such as diabetes mellitus, hyperprolactinemia, thyroid disorders, congenital adrenal hyperplasia, Cushing syndrome, polycystic ovary syndrome, congenital uterine anomaliesdisorders, repeated implantation failure, day-3 FSH concentration ≥10 IU/L or serum anti-Mullerian hormone ≤1.0 ng/mL, and azoospermia (10). In the next step, high or poor response to COH were excluded.The poor ovarian response was defined as serum estradiol (E2) level less than 500 pg/mL on the day of triggering or the number of retrieved oocytes less than three. The high ovarian response was defined as an E2 level higher than 3,000 pg/mL on the day of triggering or the number of retrieved oocytes more than 15.
Those who met the inclusion criteria, started ovarian stimulation with a flexible dosage of recombinant FSH (150-225 IU) on the second day of the menstrual cycle for 5 consecutive days. Once the leading follicle had reached 13 mm, co-treatment with the GnRH antagonist 0.25 mg/day, was initiated. Gonadotropins doses were further adjusted according to vaginal ultrasound measurements of follicular diameter. When at least two leading follicles reached 17 mm in diameter, women randomly were divided into two groups for final triggering according to a computer-generated randomization table: group I was triggered by 6500 I.U. hCG alone and group II by 6500 IU hCG plus 0.2 mg of triptorelin (Decapeptyl; Ferring, Switzerland). Women with high or poor response to COH were excluded in this step. Oocyte retrievals were performed under transvaginal ultrasound guidance 34-36 hr after triggering. Embryo transfers were performed 48-72 hr after oocyte retrieval.
The luteal phase was supported by 400 mg vaginal progesterone suppositories twice a day (Fertigest; Aburaihan pharmaceutical co., Iran) starting on the day of oocyte retrieval. Serum β-hCG was measured 14 days after embryo transfer, and a value above 50 IU/mL was considered to be a positive pregnancy. The luteal phase support was then continued until the 10th wk of gestation. The primary outcome was clinical pregnancy rate and the secondary outcome was implantation rate, chemical pregnancy, ongoing pregnancy, and abortion rate.
Power of the study
It is assumed that dual triggering will improve clinical pregnancy rate from 29-44%. Accordingly,the power was set at 80% and it was found that 100 cycles were needed in each group to detect this difference.
Ethical consideration
This study protocol was approved by Yazd Research and Clinical Center for Infertility ethics committee (Reference code: 315, dated 93.4.2). A midwife not involved in the study randomized women according to the randomization table on the day of triggering final oocyte maturation and the physicians were blinded. Oral informed consent was obtained from each participant.
Statistical analysis
Statistical Package for the Social Sciences, version 15.0, SPSS Inc, Chicago, Illinois, USA (SPSS) was used for all statistical calculations. Chi-squared test and the sample t-test test was used for comparing categorical data. P<0.05 was considered statistically significant.
Results
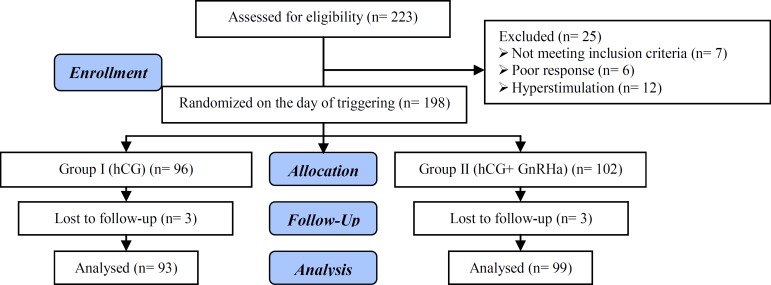
From 223 eligible women, totally 192 participants were enrolled in two groups (Figure 1). No significant differences were found in participants and cycles’ demographics characteristics between two groups (Table I, II). There were not statistically significant differences in the total recombinant FSH dose, duration of stimulation, duration of GnRH-antagonist treatment, serum E2 on the day of the trigger (Table II).
Figure 1.
Flowchart of the study design
Table I.
Participants Demographics characteristics of study participants
| Group I (hCG +GnRHa) (n=99) | Group II (hCG)(n=93) | p-value | ||
|---|---|---|---|---|
| BMI | 24.13 ± 2.87 | 24.07 ±2.98 | 0.88* | |
| Age | 30.06 ± 5.30 | 30.49 ± 4.79 | 0.54 * | |
| Duration of infertility (yr) | 6.34 ± 3.85 | 6.23 ± 4.09 | 0.70 * | |
| Day-3 FSH level (IU/L) | 6.59 ± 2.76 | 6.14 ± 2.59 | 0.23 * | |
| Infertility type (%) | ||||
| Primary | 71.3 | 78.4 | 0.49** | |
| Secondary | 28.7 | 21.6 | ||
Data presented as Mean±SD.
BMI: Body Mass Index
Independent Sample t-test
Chi-Square test
Table II.
Comparison of cycles characteristics between two study groups
| Group I (hCG +GnRHa) (n=99) | Group II (hCG) (n=93) | p-value * | |
| Total dose of gonadotropins (IU) | 1753.81 ± 584.16 | 1739.33 ± 507.22 | 0.88 |
| Duration of stimulation (day) | 10.21 ± 1.69 | 10.25 ± 1.62 | 0.60 |
| Estradiol on trigger day(pg/mL) | 1506.01 ± 699.66 | 1508.20 ± 722.14 | 0.75 |
| No. oocytes retrieved | 10.85 ± 4.71 | 9.35 ± 4.35 | 0.009 |
| MII retrieved oocytes | 8.80 ± 3.99 | 7.98 ± 3.85 | 0.12 |
| embryos formed in each cycle | 6.86 ± 4.16 | 5.34 ± 3.80 | 0.007 |
| transferred embryos | 1.72 ± 0.86 | 1.66 ± 0.82 | 0.61 |
Data presented as Mean±SD.
Independent Sample t-test
The mean of retrieved oocytes and obtained embryos were statistically higher in the dual-trigger group (Table II), but the implantation and pregnancy rate were similar in two groups (Table III).
Table III.
Comparison of clinical outcomes between two study groups
| Group I (hCG +GnRHa) (n=99) | Group II (hCG) (n=93) | p-value * | |
|---|---|---|---|
| Implantation rate | 11 | 10 | 0.50 |
| Chemical pregnancy rate | (30/99) 30.3 | (24/93) 25.8 | 0.51 |
| Clinical pregnancy rate | (26/99) 26.3 | (21/93) 22.6 | 0.30 |
| Ongoing pregnancy rate | (24/99) 24.2 | (20/93) 22.9 | 0.77 |
| Abortion rate | (2/25) 8 | (4/24) 16.7 | 0.35 |
Data presented as n (%).
Chi-Square test
Discussion
Our results indicate that mean number of retrieved oocytes, mature metaphase II oocytes and formed embryos were higher in the dual-trigger group compared with the hCG. However, we found no significant differences in implantation and pregnancy rates. Ovulation has been preceded by a surge of LH and FSH during the menstrual cycle which triggers the oocyte maturation process. During the IVF cycle, hCG is given to mimic the LH surge, and the oocyte retrieval is generally performed 35-37 hr later.
However, hCG has no FSH receptor activity, unlike the GnRH-a, which releases an endogenous FSH (and LH) surge. FSH plays a role in oocyte maturation during the natural menstrual cycles and may have a benefit for patients with infertility treated with IVF. FSH has been shown to be important in in vitro maturation of oocytes and to induce ovulation independent of the LH surge in animal studies. The FSH surge induces LH receptor formation on luteinized granulosa cells and promotes oocyte maturation and cumulus expansion (12).
Decleer et al compared 5000 I.U. hCG with a combination of 5000 I.U. hCG and GnRH-a 36 hr prior to ovum pick-up in a randomized controlled trial in normal responder women. They showed no differences in a mean number of retrieved, mature oocytes, and even pregnancy rates. Also, their study showed the number of patients who received at least one excellent quality embryo and the number of cryopreserved embryos was significantly higher in dual trigger group (13).
In contrast to our results, Ming-Huei Lin et al found a significant increase in mature oocytes, implantation, and clinical pregnancy rate in dual trigger group. They used the dual triggering for final oocyte maturation by combining a single dose of GnRH-agonist with a 6500 I.U. hCG. However, their results in the mean number of retrieved oocytes were similar to our results (7).
Griffin et al evaluated the effect of the dual trigger (GnRHa and hCG 5,000 IU or 10,000 IU, 35-37 h) prior to oocyte retrieval in women with a previous history of >25% immature oocytes retrieved. Despite a significantly higher proportion of mature retrieved oocytes with the dual trigger, the observed IVF outcome remained poor, probably due to patients underlying oocyte dysfunction (12).
GnRH has been suggested to play multiple roles in the endometrial receptivity regulation and embryo implantation (14, 15). Not surprisingly, concerns have been raised regarding the impact of GnRH-antagonist exposure during the preimplantation stage (16). GnRH has been shown to modulate matrix metalloproteinases in the placental trophoblasts that mediate trophoblast cell invasion and extracellular matrix degradation (17, 18).
Rackow et al demonstrated that the expression of endometrial HOXA10, a modulator of endometrial receptivity, was significantly decreased in endometrial stromal cells of GnRH-antagonist IVF cycles when compared with either natural or GnRH-agonist IVF cycles (19). In a review, Orvieto evaluated the studies published about the different type of administration of GnRH-a in combination with hCG for final follicular maturation, aimed to clarify which is the best method for triggering. the review concluded that there was a comparable or even better oocyte/ embryos quality in patients, not at risk of OHSS with GnRH-a and hCG trigger as compared to hCG trigger.
The article proposed GnRH-a concomitant to the standard hCG trigger dose, to improve oocyte/embryo yield and quality. It suggested that concomitant administration of GnRH-a and hCG may be offered 34-37 hr prior to oocyte retrieval (dual trigger) in normal responder patients or 40 hr and 34 hr prior to oocyte retrieval (double trigger) in women with abnormal final follicular maturation, despite normal response to induction, to improve ART outcome (20).
Conclusion
The results of our study did not confirm the favorable effect of dual-triggered oocyte maturation with a GnRH-agonist and a standard dosage of hCG as an effective strategy to optimize pregnancy outcome for normal responders in GnRH-antagonist cycles. We think that this new concept requires more study before becoming a universal COH protocol in IVF practice.
Acknowledgments
The authors thank all of the staff and physicians at the Yazd research and clinical center for infertility for their help in accomplishing this research. The study has been supported by Yazd research and clinical center for infertility.
note
Registration ID in IRCT: IRCT2015031221420N2
Conflict of interest
The authors report no declarations of interest.
References
- 1.Fauser BC, de Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:709–715. doi: 10.1210/jcem.87.2.8197. [DOI] [PubMed] [Google Scholar]
- 2.Eftekhar M, MirajS , MortazavifarZ The effect of luteal phase gonadotropin-releasing hormone antagonist administration on IVF outcomes in women at risk of OHSS. Int J Reprod BioMed. 2016;14:507. [PMC free article] [PubMed] [Google Scholar]
- 3.Gonen Y, Balakier H, Powell W, Casper RF. Use of Gonadotropin-Releasing Hormone Agonist to Trigger Follicular Maturation for in Vitro Fertilization. J Clin Endocrinol Metab. 1990;71:918–922. doi: 10.1210/jcem-71-4-918. [DOI] [PubMed] [Google Scholar]
- 4.Casarini L, Lispi M, Longobardi S, Milosa F, La Marca A, Tagliasacchi D, et al. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signaling. PLoS One. 2012;7:e46682. doi: 10.1371/journal.pone.0046682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas J, Ophir L, Barzilay E, Yerushalmi GM, Yung Y, Kedem A, et al. GnRH agonist vs hCG for triggering of ovulation-differential effects on gene expression in human granulosa cells. PloS One . 2014;9:e90359. doi: 10.1371/journal.pone.0090359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas J, Zilberberg E, Dar Sh, Kedem A, Machtinger R, Orvieto R, et al. Co-administration of GnRH-agonist and hCG for final oocyte maturation (double trigger) in patients with low number of oocytes retrieved per number of preovulatory follicles-a preliminary report. J Ovarian Res. 2014;7:77. doi: 10.1186/1757-2215-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin MH, Wu FS, Lee RK, Li SH, Lin SY, Hwu YM. Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril. 2013;100:1296–1302. doi: 10.1016/j.fertnstert.2013.07.1976. [DOI] [PubMed] [Google Scholar]
- 8.Schachter M, Friedler S, Ron-El R, Zimmerman AL, Strassburger D, Bern O, et al. Can pregnancy rate be improved in gonadotropin-releasing hormone (GnRH) antagonist cycles by administering GnRH agonist before oocyte retrieval? A prospective, randomized study. Fertil Steril. 2008;90:1087–1093. doi: 10.1016/j.fertnstert.2007.07.1316. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Comparison of “triggers” using leuprolide acetate alone or in combination with low-dose human chorionic gonadotropin. Fertil Steril. 2011;95:2715–2717. doi: 10.1016/j.fertnstert.2011.03.109. [DOI] [PubMed] [Google Scholar]
- 10.Rosini R, Campisi E, De Chiara M, Tettelin H, Rinaudo D, Toniolo C, et al. Genomic analysis reveals the molecular basis for capsule loss in the group B Streptococcus population. PLoS One . 2015;10:e0125985. doi: 10.1371/journal.pone.0125985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpi TM, Silfverdal SA, Nilsson L, Syrjänen R, Belloni C, Desole M, et al. Immunogenicity and reactogenicity of two diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio virus-Haemophilus influenzae type b vaccines administered at 3, 5 and 11-12 months of age. Hum Vaccin. 2009;5:18–25. doi: 10.4161/hv.5.1.6369. [DOI] [PubMed] [Google Scholar]
- 12.Griffin D, Feinn R, Engmann L, Nulsen J, Budinetz T, Benadiva C. Dual trigger with gonadotropin-releasing hormone agonist and standard dose human chorionic gonadotropin to improve oocyte maturity rates. Fertil Steril. 2014;102:405–409. doi: 10.1016/j.fertnstert.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Decleer W, OsmanagaogluK , SeynhaveB , KolibianakisS , TarlatzisB , DevroeyP Comparison of hCG triggering versus hCG in combination with a GnRH agonist: a prospective randomized controlled trial. Facts Views Vis Obstet Gynecol. 2014;6:203–209. [PMC free article] [PubMed] [Google Scholar]
- 14.Casañ EM, Raga F, Bonilla-Musoles F, Polan ML. Human Oviductal Gonadotropin-Releasing Hormone: Possible Implications in Fertilization, Early Embryonic Development, and Implantation 1. J Clin Endocrinol Metab. 2000;85:1377–1381. doi: 10.1210/jcem.85.4.6503. [DOI] [PubMed] [Google Scholar]
- 15.Eftekhar M, Dehghani Firouzabadi R, Karimi H, Rahmani E. Outcome of cryopreserved-thawed embryo transfer in the GnRH agonist versus antagonist protocol. Iran J Reprod Med. 2012;10:297–302. [PMC free article] [PubMed] [Google Scholar]
- 16.Kol S. Embryo implantation and GnRH antagonists GnRH antagonists in ART: lower embryo implantation? Hum Reprod. 2000;15:1881–1882. doi: 10.1093/humrep/15.9.1881. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Cao B, Li YX, Wu XQ, Wang YL. GnRH I and II up-regulate MMP-26 expression through the JNK pathway in human cytotrophoblasts. Reprod Biol Endocrinol. 2010;8:1. doi: 10.1186/1477-7827-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki K, Norwitz ER. Gonadotropin-releasing hormone/gonadotropin-releasing hormone receptor signaling in the placenta. Curr Opin Endocrinol Diabetes Obes. 2011;18:401–408. doi: 10.1097/MED.0b013e32834cd3b0. [DOI] [PubMed] [Google Scholar]
- 19.Rackow BW, Kliman HJ, Taylor HS. GnRH antagonists may affect endometrial receptivity. Fertil Steril. 2008;89:1234–1239. doi: 10.1016/j.fertnstert.2007.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orvieto R. Triggering final follicular maturation-hCG, GnRH-agonist or both, when and to whom? J Assist Reprod Genet. 2016;33:1415–1416. doi: 10.1007/s10815-016-0775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]