Abstract
We present the clinical course, management, and final outcome of spontaneous suprachoroidal hemorrhage (SSCH) in an age-related macular degeneration (AMD) patient—a 64-year-old male receiving antiplatelet therapy who developed SSCH during the Valsalva maneuver. In addition to our case study, we discuss the results of a systemic review of the literature and reference lists of retrieved studies published from January 2001 to December 2013. Among a total of 31 patients (32 eyes), acute secondary glaucoma was a complication in 87.5% of the cases, and over half of the cases (20 eyes, 62.5%) received surgery. Twenty cases (64.5%) were characterized by systemic hypertension (HTN), followed by cardiovascular or cerebral vascular disease in 17 cases (54.8%). The Valsalva maneuver was performed in five cases (16.1%) prior to the episode. Twenty-three cases (74.2%) had abnormal hemostasis, including use of anticoagulants or thrombolytic agents (18 cases), chronic renal failure (CRF, 5 cases), and blood dyscrasia (3 cases). AMD was the most common (17 eyes of 16 patients, 53.1%) ocular disease. Visual acuity was classified as hand motion (HM) or worse in 20 eyes (of 28 eyes, 71.4%) at initial presentation and in 24 eyes (of 30 eyes, 80%) upon final examination. Anticoagulated patients with AMD should be informed of the risk of intraocular hemorrhage. Medical therapy usually fails in the treatment of glaucoma. Surgical intervention provides an option for the purpose of pain relief. Even so, the final visual prognosis is usually poor.
Keywords: choroidal, glaucoma, hemorrhage, sclerotomy, suprachoroidal
1. Introduction
Suprachoroidal hemorrhage, which commonly occurs during intraocular surgery, is infrequent and catastrophic.1,2 In extremely rare circumstances, spontaneous suprachoroidal hemorrhage (SSCH) occurs and presents with sudden-onset sight loss and acute angle-closure glaucoma. The associated systemic factors include old age,3,4,5 anticoagulant or thrombolytic therapy,6,7,8,9,10 systemic hypertension (HTN),11,12,13,14 atherosclerosis,3,15 diabetes mellitus (DM),10 blood dyscrasia,5,16,17 chronic renal disease,9,15,18 and the Valsalva maneuver.19 The associated ocular factors are age-related macular degeneration (AMD),6,20,21 glaucoma,20,22 and high myopia.23 Here, we present an AMD patient receiving antiplatelet therapy who developed SSCH during the Valsalva maneuver, and we review the literature on this topic regarding the predisposing factors, clinical course, and visual outcome, with the intent to prevent the occurrence of SSCH and provide appropriate management strategies.
2. Case Report
A 64-year-old man was referred due to severe ocular pain and a sudden loss of vision (no light perception) in the right eye 2 days prior to presentation while straining in a pull-up exercise. Two years previously, he experienced a visual disturbance. Visual acuity was 20/32 in the right eye and 20/25 in the left eye. Extrafoveal scarring was revealed in the right eye (Fig. 1, upper left), and no obvious leakage was disclosed by fluorescein angiography (Fig. 1, upper right). In addition to systemic hypertension (HTN), due to a brainstem stroke 3 years earlier the patient received clopidogrel bisulfate orally (75 mg/d, Plavix; Bristol-Myers Squibb Co. and Sanofi Aventis, Bridgewater, NJ, USA) in order to prevent platelets from clumping and forming blood clots.
Fig. 1.
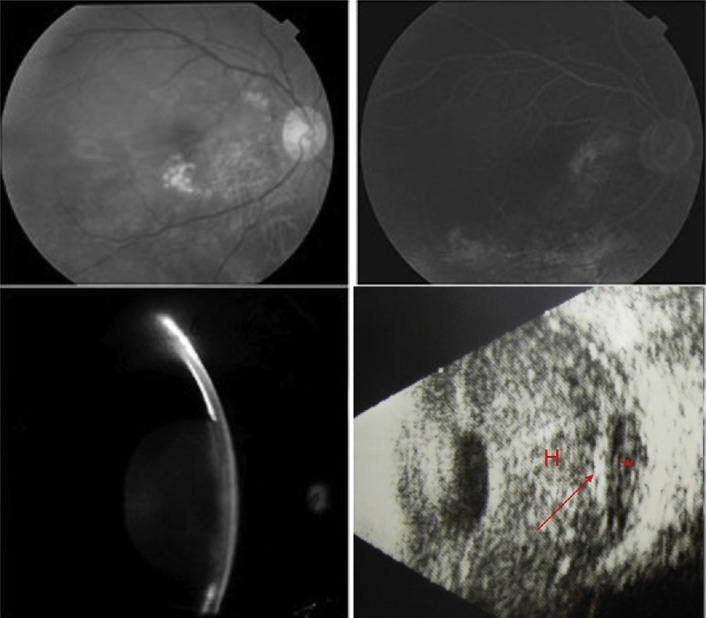
Fundus photography of the right eye showing numerous small-sized and confluent drusen (upper left), which were stained at the late stage without leakage using fluorescein angiography (upper right). These pictures were obtained 2 years prior to the episode. Slit-lamp examination shows forward displacement of the iris and lens with total iridocorneal touch (lower left). B-Scan ultrasonography of the right eye reveals a dome-shaped choroidal detachment (arrow) with hypo-echogenous content (asterisk) in addition to a dense vitreous hemorrhage (H) (lower right).
Marked injected conjunctiva, microcystic edematous cornea, and nearly 360° iridocorneal touch were present in the eye with a mid-dilated and fixed pupil (Fig. 1, lower left). The intraocular pressure (IOP) was 59 mmHg, and B-scan ultrasonography revealed a dense vitreous hemorrhage and dome-shaped choroidal detachment with relatively hypoechogenic content (Fig. 1, lower right). The left eye had a deep and widely open anterior chamber, and 20/32 vision could be achieved, despite the dry type of AMD (not shown).
Clopidogrel bisulfate was withheld. Medication was prescribed, including intravenous injection of mannitol, oral acetazolamide, and topical instillation of carteolol, pilocarpine, and prednisolone. Even following successful laser iridotomy (LI), the complete collapse of the anterior chamber and the ocular pain were not relieved. During the operation, rusty blood flooded into the anterior chamber following bridle traction on the superior rectus muscle. A large amount of old blood was removed through a limbal penetrance with Simcoe aspiration and was drained using a superiortemporal sclerotomy 3.75 mm away from the limbus until the eyeball became soft. One week later, a B-scan showed a V-shaped retinal detachment and residual vitreous hemorrhage. The pain was relieved although hyphema persisted, and blood-stained cornea was noted 2 months later. The IOP was 12 mmHg, but the eyeball was phthisical thereafter.
3. Methods
MEDLINE, PubMed, and Web of Science were searched during October 2013 (English-language results, no date restrictions) for combinations of the following terms: suprachoroidal, choroidal, hemorrhage, and spontaneous. Those cases related to injury or globular perforation, such as corneal ulceration, or those associated with ocular surgery were excluded.
4. Results
A total of 23 articles written since 2001 were reviewed and were meta-analyzed according to the clinical course, risk factors, diagnostic tools, management, and final outcomes.5,6,8,9,10,16,17,18,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34
4.1. Clinical presentation
Including our case, 15 men and 16 women (32 eyes) were analyzed. One case had bilateral involvement for 1 year. The ages of the patients ranged from 24 years to 90 years [mean ± standard deviation (SD) 69.7 ± 17.6 years]. The IOP on initial presentation ranged from 11 mmHg to 70 mmHg in 26 recorded eyes (mean ± SD 44.5 ± 15.3 mmHg), and only one of them did not experience acute glaucoma during the episode. Three patients did not have a high IOP on the first visit until the SSCH progressed. Among six eyes without IOP data, high IOP was mentioned in one case, one experienced pain, and one had a subsequent corneal perforation, which was assumed to result from glaucoma. In total, acute glaucoma was noted in 28 eyes (87.5%).
4.2. Diagnosis
In 15 cases (48.3%), dome-shaped hemorrhagic retinal detachments were revealed with an ophthalmoscope in the absence of dense vitreous hemorrhage and edematous cornea. B-Mode ul-trasonography confirmed SSCH in 71.9% (23/32) of the eyes. Five patients had received head computer tomography examinations, and one had a magnetic resonance imaging (MRI) examination. An ultrasound biomicroscopic examination demonstrated one case with a swollen ciliary body and ciliochoroidal detachment in all four quadrants.
4.3. Risk factors
Fig. 2 summarizes the systemic factors associated with the development of SSCH. Hypertension was the most frequent systemic disease (20 cases, 64.5%), followed by cardiovascular or cerebrovascular disease (17 cases, 54.8%), DM (7 cases, 22.6%), and atherosclerosis (6 cases, 19.4%). The Valsalva maneuver was noted in five cases (16.1%) prior to the episode. Chronic renal failure (CRF, 5 cases), decompensated liver disease (1 case), disseminated intravascular coagulation (1 case), and chronic myelogenous leukemia (1 case) might have attributed to abnormal hemostasis (the previous 3 cases are not shown in Fig. 2). Anticoagulants, anti-platelet, and thrombolytic agents were used in 18 cases (58.1%; Fig. 3), including warfarin (10 cases), aspirin (5 cases), heparin (4 cases), clopidogrel (3 cases), and a tissue plasminogen activator (TPA, 2 cases). A combination of two anticoagulants was noted in five cases. Fig. 4 shows the associated ocular diseases. The most common factor was AMD (17 eyes, 53.1%), followed by glaucoma (3 eyes, 9.4%) and proliferative diabetic retinopathy (PDR, 2 eyes, 6.3%). High myopia (1 eye, 3.1%), retinal detachment (1 eye, not shown in Fig. 4), and corneal inflammatory disease (1 eye, not shown in Fig. 4) were seldom associated.
Fig. 2.
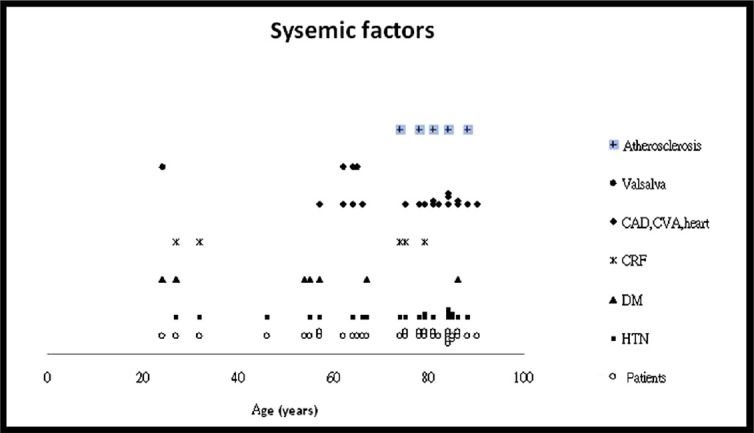
The common factors related to systemic associations in the 31 cases with SSCH. CAD = carotid arterial disease; CRF = chronic renal failure; CVA = cerebrovascular accident; DM = diabetes mellitus; HTN = systemic hypertension; SSCH = spontaneous suprachoroidal hemorrhage.
Fig. 3.
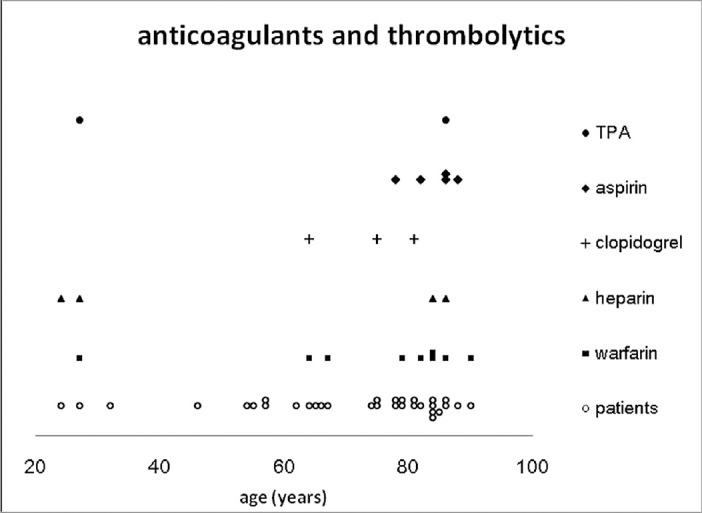
The use of anticoagulants, antiplatelets, and thrombolytic agents in the cases of SSCH in the literature. The use of medication was associated with hemostasis in 18 cases. SSCH = spontaneous suprachoroidal hemorrhage; TPA = tissue plasminogen activator.
Fig. 4.
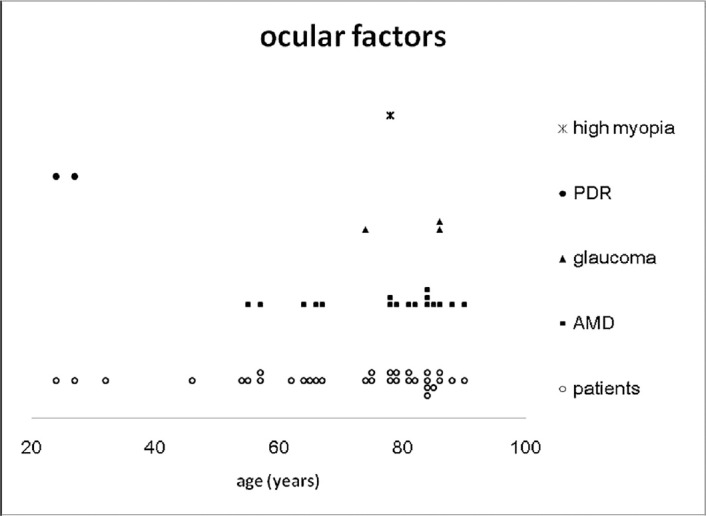
The possible risk factors for ocular associations [not including retinal detachment (1 case) and corneal inflammation (1 case)]. dAMD = age-related macular degeneration; PDR = proliferative diabetic retinopathy.
4.4. Treatment
In addition to medication and LI, 20 eyes (62.5%) received surgical intervention. Blood drainage with sclerotomy or vitrectomy was performed in 16 eyes (50.0%) on the 2nd day to over 30 days after the episode. Three cases (9.4%) had subsequent global perforations, and two of them received evisceration. The other three eyes underwent evisceration or enucleation due to pain or recurrent SSCH following drainage.
4.5. Prognosis
Fair visual acuity on presentation was noted in two cases only (20/40/→20/40 and 20/80→20/16 following medical therapy). At initial examination, visual acuity was hand motion (HM) or worse in 20 of the 28 recorded eyes (71.4%), including eight eyes (28.6%) presenting with no light perception. At the end, final visual acuity was HM or worse in 24 eyes (of 30 eyes, 80%), and no light perception was found in 19 eyes (63.3%), including seven phthitic eyes and five anophthalmic cases. Two cases (aged 27 years and 32 years, with hypertension and renal failure without AMD) experienced significant visual improvement after vitrectomy (20/2000→/20/25, and counting fingers→/20/40).
5. Discussion
The associated factors possibly attributing to SSCH can be divided into categories based on the mechanisms.18 First, fragile choroidal or posterior ciliary vasculature plays a role, which occurs in patients with atherosclerosis,15,18 hypertension,12 diabetic mellitus, old age,35 high myopia,23 and even in AMD cases with an inactive lesion.12,18,20,21 Second, mechanical forces tear the fragile vessels in the eye, resulting from fluctuations in ocular pressure, such as is exhibited in the Valsalva maneuver,19,36 and uncontrolled hypertension.26 A surge in systemic pressure results in an increase in episcleral vascular resistance and IOP, which is transmitted to the vulnerable choroidal vessels.37 The last category, blood dyscrasia or use of anticoagulants and antiplatelet agents, impairs hemostasis and precipitates minor bleeding to massive hemorrhaging, which erupts through all layers of the retina and flows into the vitreous. In our review, iatrogenic intervention of hemostasis was found in 18 cases, although a normal international normalized ratio was present in some patients. In a literature review of 83 patients with AMD, massive subretinal or vitreous hemorrhage occurred in 19% of the patients taking warfarin or aspirin.12 Since the mechanism of SSCH is multifactorial, those who have multiple risk factors may be more vulnerable.18,28 Our case had inactive AMD, the Valsalva maneuver, hypertension, and the patient was receiving an anti-platelet agent. However, one case with prostate carcinoma had only the Valsalva maneuver,19 and another patient aged 46 years had a hypertensive crisis only.26 It is difficult to score the importance or associated significance of these risk factors.
Advanced age over 60 years is a well-known predisposing factor for suprachoroidal hemorrhage.3,4,5 Not surprisingly, most (14/16 cases) of the patients with AMD were older than this. In the case of patients aged over 60 years (24 eyes of 23 cases), the most common risk factor has been shown to be abnormal coagulopathy (19/23, 82.6%), including use of anticoagulants (16/23, 69.6%) due to cardiovascular or cerebrovascular diseases (15/23, 65.2%), followed by hypertension (14/23, 60.9%). However, among eight patients (25.8%) younger than 60 years,5,10,26,28,29,31,34 five cases (62.5%) had hypertension and five cases (62.5%) had DM [of them, PDR and taking anticoagulants in 2 cases (37.5%) and AMD in another 2 cases (37.5%)].
Following the occurrence of SSCH, bullous hemorrhagic choroidal detachment displaces the vitreous and moves the lens–iris diaphragm forward, resulting in secondary angle closure glaucoma,20 which was noted in most of the events (87.5%), and even subsequent global rupture was noted.5,22,31 In the absence of hyphema, such glaucoma may be diagnosed as a pupillary block and be mistreated with pilocarpine, as in our case. Due to a mass effect, SSCH-associated glaucoma is rarely responsive to medication and peripheral iridotomy.34,38 The diagnosis should be revised, and B-scan ultrasonography is necessary if treatment is still in vain and if the presence of a deep anterior chamber is noted in the fellow eye.
Surgical drainage, including anterior chamber lavage, sclerotomy, and vitrectomy, is indicated for lens–cornea touch, worsening corneal edema, progressive angle-closure glaucoma, cataract formation, or appositional choroidal detachment.20 The optimal time for surgery is controversial. In reviewing 12 recorded data for 16 eyes receiving surgical drainage, four patients underwent operations within 1 week (including our case), and two of them had recurrent SSCH.18,29 Considering the risk of rebleeding and an incomplete lytic clot, the recommended time for drainage is at least 7–25 days following the episode.15,39,40 However, a 1-week lag did not prevent the risk of rebleeding.6,18,29 Two eyes rebled post-operatively although surgical drainage was performed on the 10th day and 3 months after the episode, separately. For the remaining cases who received surgical drainage at least 7 days or longer (mean 17.6 days) after the episode, only two patients exhibited visual improvement.28,29 It seems that surgical drainage is effective for IOP control but fails to preserve vision.18 For intractable pain or global perforation, evisceration or enucleation might be performed (5 eyes, 15.6%).6,16,22,30,31 Other surgical interventions for pain relief include retrobulbar alcohol injection or cyclocryotherapy.30
Except for a limited lesion at the very beginning, the fundus picture is usually veiled by corneal edema and dense vitreous hemorrhage. Compared with computer tomography and MRI, a B-mode ultrasonography examination is a more available method to confirm SSCH, to exclude pathological mass lesions in the posterior segment, such as choroidal melanoma,15 and especially for serial observation. When a blood clot liquefies completely, ultrasonog-raphy shows homogenous and low-reflective mobile opacities.15 If a choroidal melanoma is highly suspected, surgical drainage is inadvisable, considering tumor extension, and therefore, enucleation is the most appropriate therapeutic option.10
Characteristically, visual loss occurs suddenly, and may deteriorate over days to weeks, especially for the delayed type or in the case of a recurrence of SSCH. Even though the eyeball is preserved after the SSCH is resolved, the visual outcome is generally poor, because of the disruption of the intraocular structure and subsequent optic nerve baro-trauma resulting in elevation of IOP. Only four eyes had a final visual acuity measurement of 20/40 or better.19,23,28,29 Localized SSCH without macular involvement was assumed to be the reason.
Except for an 84-year-old AMD case with bilateral SSCH, the fellow eyes of three patients (2 cases of AMD and 1 24-year-old case of PDR) had a macular hemorrhage or localized subretinal or choroidal hemorrhage.18,19,24,25 Of these, two AMD patients received both antiplatelet and anticoagulant agents. Since SSCH is extremely rare, AMD is not a contraindication for systemic anticoagulants or antiplatelet agents. However, it is important to warn AMD patients about the predisposing risk of SSCH, to check the prescription of anticoagulant/platelet agents, and to frequently follow up the posterior segment after the occurrence of an intraocular hemorrhage in one eye.
The limitations of our review include its retrospective nature, the lack of a control group, and the limited number of cases. The definite relative risk of each factor therefore cannot be clarified clearly.
The literature review presented in this work indicates that SSCH is highly associated with hypertension, systemic anticoagulation, and AMD. If the medication cannot be withheld, general practitioners or cardiologists should consult an ophthalmologist for a complete ophthalmic examination prior to treatment, and they should also inform their patients of the possible risk factors associated with SSCH. In the case of patients with intractable pain, surgery should be performed.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.tjo.2014.10.008.
Footnotes
Conflicts of interest: All authors declare no conflicts of interest.
References
- 1.Lakhanpal V. Experimental and clinical observations on massive suprachoroidal hemorrhage. Trans Am Ophthalmol Soc. 1993;91:545–652. [PMC free article] [PubMed] [Google Scholar]
- 2.Khawly JA, Ferrone PJ, Holck DE. Choroidal hemorrhage associated with systemic tissue plasminogen activator. Am J Ophthalmol. 1996;121:577–578. doi: 10.1016/s0002-9394(14)75438-8. [DOI] [PubMed] [Google Scholar]
- 3.Winslow RL, Stevenson W, 3rd, Yanoff M. Spontaneous expulsive choroidal hemorrhage. Arch Ophthalmol. 1974;92:33–36. doi: 10.1001/archopht.1974.01010010037009. [DOI] [PubMed] [Google Scholar]
- 4.Ophir A, Pikkel J, Groisman G. Spontaneous expulsive suprachoroidal hemorrhage. Cornea. 2001;20:893–896. doi: 10.1097/00003226-200111000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Srikanth K, Kumar MA. Spontaneous expulsive suprachoroidal hemorrhage caused by decompensated liver disease. Indian J Ophthalmol. 2013;61:78–79. doi: 10.4103/0301-4738.107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YY, Chen YY, Sheu SJ. Spontaneous suprachoroidal hemorrhage associated with age-related macular degeneration and anticoagulation therapy. JChinese Med Assoc. 2009;72:385–387. doi: 10.1016/S1726-4901(09)70393-4. [DOI] [PubMed] [Google Scholar]
- 7.Wong JS. Spontaneous suprachoroidal haemorrhage in a patient receiving low-molecular-weight heparin (fraxiparine) therapy. Aust N Z J Ophthalmol. 1999;27:433–434. doi: 10.1046/j.1440-1606.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- 8.Barsam A, Heatley CJ, Herbert L. Spontaneous suprachoroidal hemorrhage secondary to thrombolysis for the treatment of myocardial infarction. Clin Experiment Ophthalmol. 2006;34:177–179. doi: 10.1111/j.1442-9071.2006.01149.x. [DOI] [PubMed] [Google Scholar]
- 9.De Marco R, Aurilia P, Mele A. Massive spontaneous choroidal hemorrhage in a patient with chronic renal failure and coronary artery disease treated with Plavix. Eur J Ophthalmol. 2009;19:883–886. doi: 10.1177/112067210901900534. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen HN, Nork TM. Massive spontaneous suprachoroidal hemorrhage in a young woman with cystic fibrosis and diabetes mellitus on anticoagulants. Retin Cases Brief Rep. 2012;6:216–218. doi: 10.1097/ICB.0b013e3182378c1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyakawa RT, Michels RG, Blase WP. Vitrectomy for nondiabetic vitreous hemorrhage. Am J Ophthalmol. 1983;96:517–525. doi: 10.1016/s0002-9394(14)77916-4. [DOI] [PubMed] [Google Scholar]
- 12.el Baba F, Jarrett WH, 2nd, Harbin TS, Jr, Fine SL, Michels RG, Schachat AP, et al. Massive hemorrhage complicating age-related macular degeneration. Clinicopath-ologic correlation and role of anticoagulants. Ophthalmology. 1986;93:1581–1592. doi: 10.1016/s0161-6420(86)33540-1. [DOI] [PubMed] [Google Scholar]
- 13.Butner RW, McPherson AR. Spontaneous vitreous hemorrhage. Ann Ophthalmol. 1982;14:268–270. [PubMed] [Google Scholar]
- 14.Wolter JR, McWilliams JR. Rupture of disciform macular degeneration causing massive retroretinal hemorrhage. Am J Ophthalmol. 1965;59:1044–1047. [PubMed] [Google Scholar]
- 15.Chu TG, Green RL. Suprachoroidal hemorrhage. Surv Ophthalmol. 1999;43:471–486. doi: 10.1016/s0039-6257(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 16.Lim LT, Agarwal PK, Rotchford A. Angle-closure glaucoma due to supra-choroidal hemorrhage secondary to disseminated intravascular coagulation. Semin Ophthalmol. 2011;26:59–60. doi: 10.3109/08820538.2011.559517. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh A, Parulekar M, James B. Acute suprachoroidal haemorrhage with acute angle closure glaucoma as a presenting sign of chronic myelomonocytic leukemia. Eye. 2002;16:651–653. doi: 10.1038/sj.eye.6700114. [DOI] [PubMed] [Google Scholar]
- 18.Yang SS, Fu AD, McDonald HR, Johnson RN, Ai E, Jumper JM. Massive spontaneous choroidal hemorrhage. Retina. 2003;23:139–144. doi: 10.1097/00006982-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Hammam T, Madhavan C. Spontaneous suprachoroidal haemorrhage following a valsalva manoeuvre. Eye. 2003;17:261–262. doi: 10.1038/sj.eye.6700298. [DOI] [PubMed] [Google Scholar]
- 20.Alexandrakis G, Chaudhry NA, Liggett PE, Weitzman M. Spontaneous supra-choroidal hemorrhage in age-related macular degeneration presenting as angle-closure glaucoma. Retina. 1998;18:485–486. doi: 10.1097/00006982-199805000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Knox FA, Johnston PB. Spontaneous suprachoroidal haemorrhage in a patient with age-related macular degeneration on excessive anticoagulation therapy. Eye. 2002;16:669–670. doi: 10.1038/sj.eye.6700109. [DOI] [PubMed] [Google Scholar]
- 22.Goldsmith C, Rene C. Massive spontaneous expulsive suprachoroidal haemorrhage in a blind glaucomatous eye treated with chronic topical steroid. Eye. 2003;17:439–440. doi: 10.1038/sj.eye.6700372. [DOI] [PubMed] [Google Scholar]
- 23.Chak M, Williamson TH. Spontaneous suprachoroidal haemorrhage associated with high myopia and aspirin. Eye. 2003;17:525–527. doi: 10.1038/sj.eye.6700388. [DOI] [PubMed] [Google Scholar]
- 24.Garrott HM, Haynes RJ. Blindness from suprachoroidal haemorrhage in two patients with age-related macular degeneration on systemic anticoagulation therapy or an antiplatelet agent. Med J Aust. 2010;192:346–347. doi: 10.5694/j.1326-5377.2010.tb03536.x. [DOI] [PubMed] [Google Scholar]
- 25.Chalasani R, Qureshi S. Anticoagulation and intraocular haemorrhage in age-related macular degeneration: a probable link? Med J Aust. 2010;192:228–229. doi: 10.5694/j.1326-5377.2010.tb03485.x. [DOI] [PubMed] [Google Scholar]
- 26.Fukuchi T, Suda K, Matsuda H, Ueda J, Abe H. Secondary acute angle closure with spontaneous suprachoroidal hemorrhage suspected by ultrasound bio-microscopic examination. Jpn J Ophthalmol. 2009;53:661–663. doi: 10.1007/s10384-009-0742-z. [DOI] [PubMed] [Google Scholar]
- 27.Chandra A, Barsam A, Hugkulstone C. A spontaneous suprachoroidal haemorrhage: a case report. Cases J. 2009;2:185. doi: 10.1186/1757-1626-2-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tajika T, Yokozeki H, Ishimaru K, Naito T, Shiota H. Rare case of choroidal hemorrhage complicated with hypertension due to chronic renal failure. J Med Invest. 2008;55:151–155. doi: 10.2152/jmi.55.151. [DOI] [PubMed] [Google Scholar]
- 29.Saeed MU, Wong D, Heimann H, Gibran SK. Spontaneous progressive supra-choroidal haemorrhage in a patient undergoing haemodialysis. Graefes Arch Clin Exp Ophthalmol. 2007;245:1741–1742. doi: 10.1007/s00417-007-0653-y. [DOI] [PubMed] [Google Scholar]
- 30.Lee YJ, Kang SM, Kang IB. Acute angle-closure glaucoma from spontaneous massive hemorrhagic retinal detachment. Korean JOphthalmol. 2007;21:61–64. doi: 10.3341/kjo.2007.21.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudhir RR, Rao SK, Biswas J, Padmanabhan P. Spontaneous expulsive supra-choroidal hemorrhage. Cornea. 2002;21:632–633. doi: 10.1097/00003226-200208000-00026. [DOI] [PubMed] [Google Scholar]
- 32.Neudorfer M, Leibovitch I, Goldstein M, Loewenstein A. Massive choroidal hemorrhage associated with low molecular weight heparin therapy. Blood Coagul Fibrinolysis. 2002;13:257–259. doi: 10.1097/00001721-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Dandekar SS, Laidlaw DA. Suprachoroidal haemorrhage after addition of clar-ithromycin to warfarin. J R Soc Med. 2001;94:583–584. doi: 10.1177/014107680109401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen SN, Ho CL, Ho JD, Guo YH, Chen TL, Chen PF. Acute angle-closure glaucoma resulting from spontaneous hemorrhagic retinal detachment in age-related macular degeneration: case reports and literature review. Jpn J Ophthalmol. 2001;45:270–275. doi: 10.1016/s0021-5155(00)00382-8. [DOI] [PubMed] [Google Scholar]
- 35.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 36.Brody S, Erb C, Veit R, Rau H. Intraocular pressure changes: the influence of psychological stress and the Valsalva maneuver. Biol Psychol. 1999;51:43–57. doi: 10.1016/s0301-0511(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 37.Pollack AL, McDonald HR, Ai E, Johnson RN, Duqel PU, Folk J, et al. Massive suprachoroidal hemorrhage during pars plana vitrectomy associated with Valsalva maneuver. Am J Ophthalmol. 2001;132:383–387. doi: 10.1016/s0002-9394(01)01049-2. [DOI] [PubMed] [Google Scholar]
- 38.Pesin SR, Katz LJ, Augsburger JJ, Chien AM, Eagle RC., Jr Acute angle-closure glaucoma from spontaneous massive hemorrhagic retinal or choroidal detachment. An updated diagnostic and therapeutic approach. Ophthalmology. 1990;97:76–84. doi: 10.1016/s0161-6420(90)32639-8. [DOI] [PubMed] [Google Scholar]
- 39.Scott IU, Flynn HW, Jr, Schiffman J, Smiddy WE, Murray TG, Ehlies F. Visual acuity outcomes among patients with appositional suprachoroidal hemorrhage. Ophthalmology. 1997;104:2039–2046. doi: 10.1016/s0161-6420(97)30042-6. [DOI] [PubMed] [Google Scholar]
- 40.Lakhanpal V, Schocket SS, Elman MJ, Nirankari VS. A new modified vitreor-etinal surgical approach in the management of massive suprachoroidal hemorrhage. Ophthalmology. 1989;96:793–800. doi: 10.1016/s0161-6420(89)32819-3. [DOI] [PubMed] [Google Scholar]


